Submitted:
06 December 2024
Posted:
10 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Resveratrol: Structure, Biological Properties, and Synthetic Factors
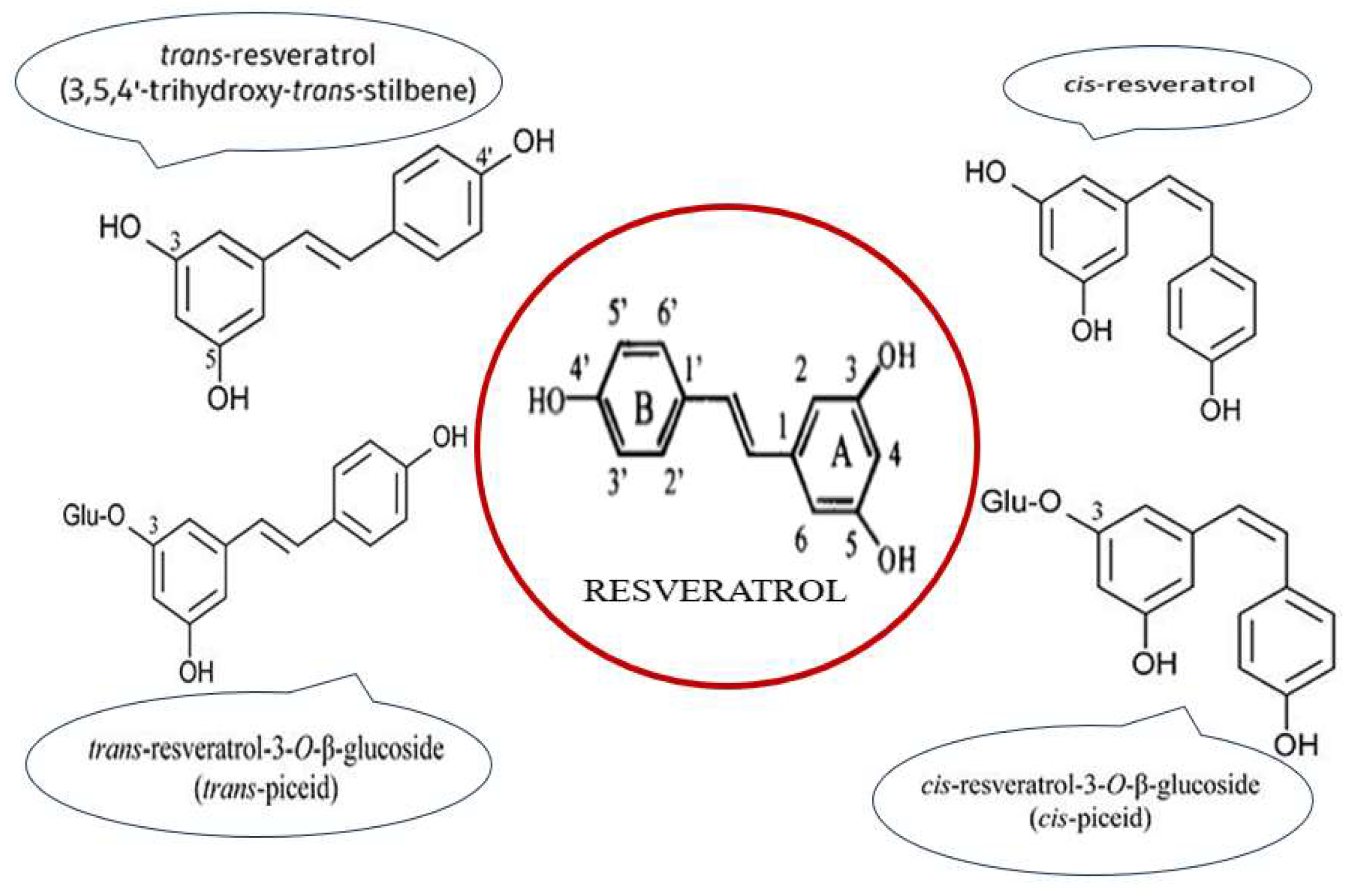
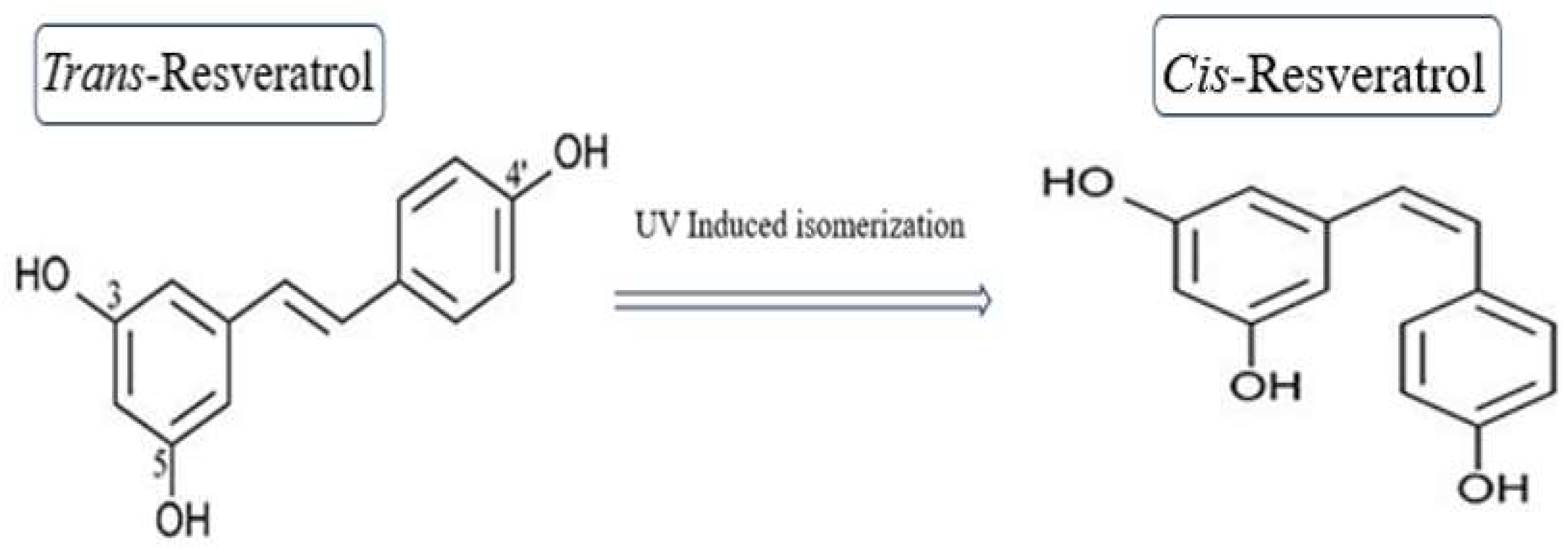
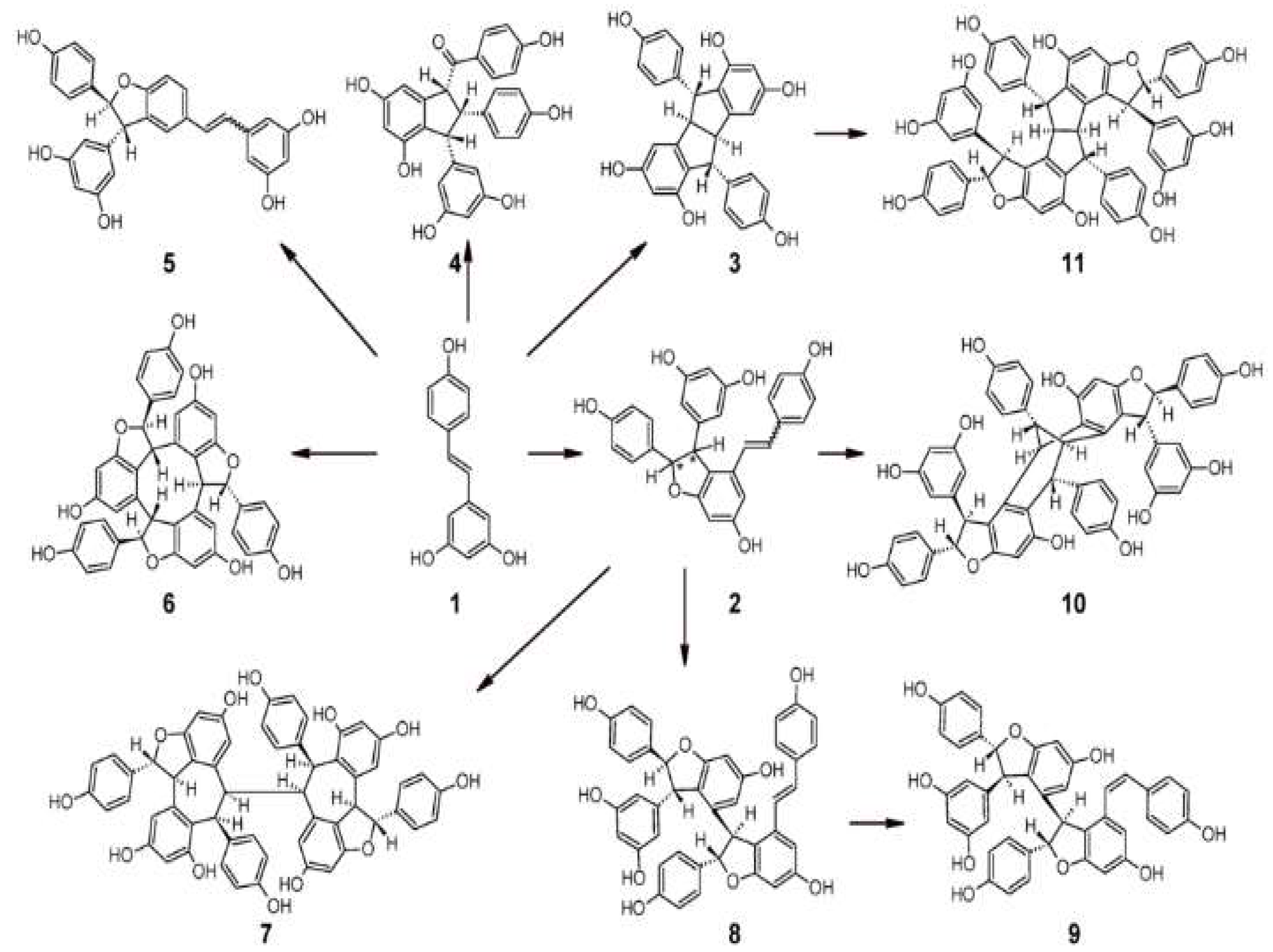
2.1. Chemical Structure and Isomers
2.2. Factors Influencing Resveratrol Synthesis and Content in Grapevine
| Abiotic factors | References |
|---|---|
| Variety | [33,34,35] |
| Climatic conditions in the growing areas | [25,36,37,38,39,40] |
| Grapevine health | [41] |
| Vineyard management | [42,43] |
| Winemaking techniques | [37,44,45,46] |
| Wine ageing method | [12,45,47,48] |
| Terroir effect | [49,50,51,52,53] |
| Harvest time | [11,54] |
| Treatment with ultrasound (US), light emitting diode (LED), ultraviolet (UV) irradiation or macronutrients and fungicides | [55,56,57,58] |
| Storage conditions | [8,59] |
2.3. Biological Properties
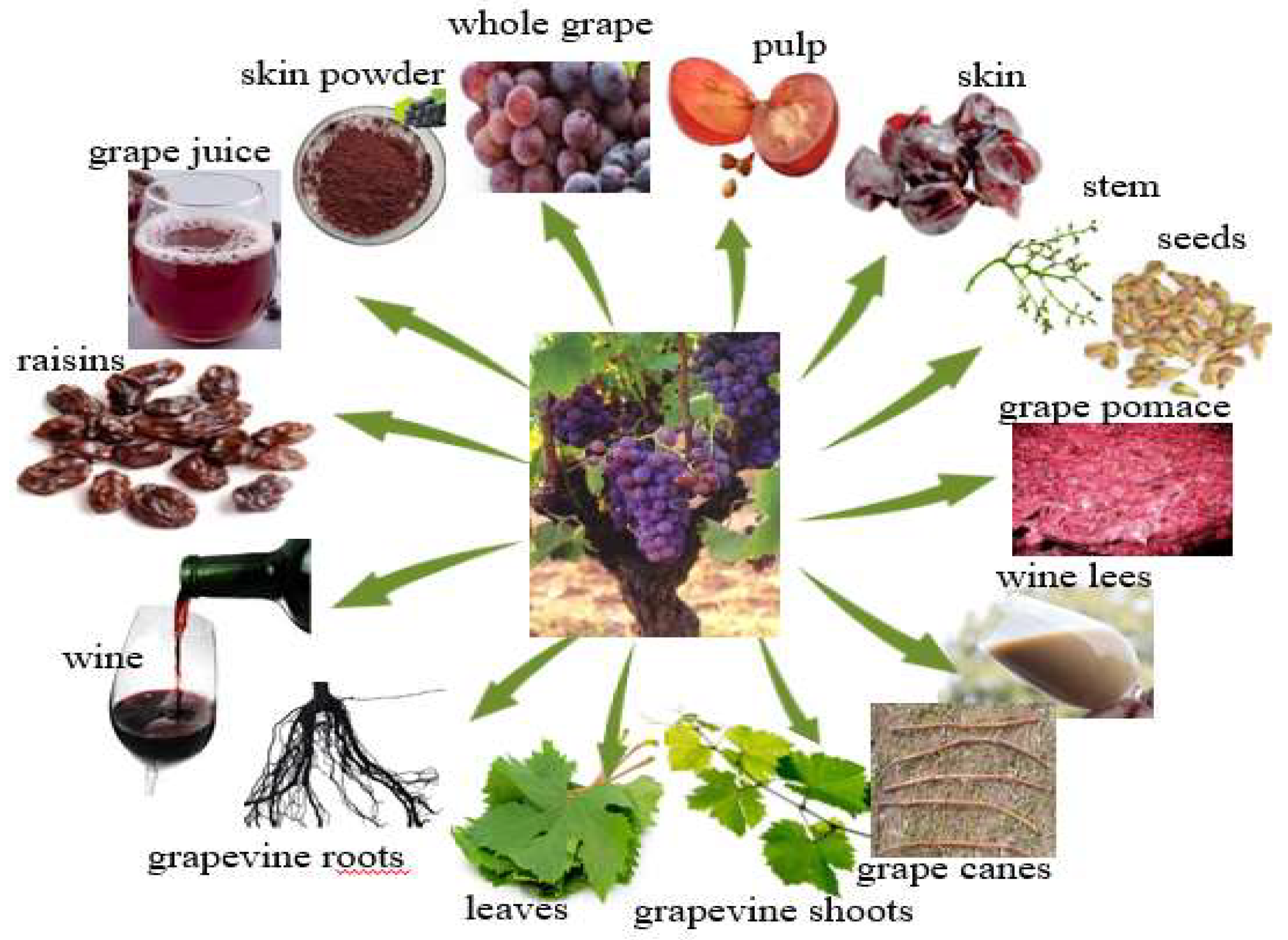
3. Sources of Resveratrol from the Grapevine
3.1. Sources of Resveratrol in Grapes and Other Grapevine Components
3.2. Sources of Resveratrol in Products and By-Products
4. Conclusions
References
- Valletta, A.; Iozia, L.M.; Leonelli, F. Impact of environmental factors on stilbene biosynthesis. Plants 2021, 10, 90. [Google Scholar] [CrossRef]
- Takaoka, M. Resveratrol, a new phenolic compound from Veratrum grandiflorum. J Chem Soc Jpn 1939, 60, 1090–1100. [Google Scholar]
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Kupe, M.; Karatas, N.; Unal, M.S.; Ercisli, S.; Baron, M.; Sochor, J. Nutraceutical and Functional Properties of Peel, Pulp, and Seed Extracts of Six ‘Köhnü’ Grape Clones. Horticulturae 2021, 7, 346. [Google Scholar] [CrossRef]
- Balanov, P.E.; Smotraeva, I.V.; Abdullaeva, M.S.; Volkova, D.A.; Ivanchenko, O.B. Study on resveratrol content in grapes and wine products. E3S Web Conf. EDP Sci. 2021, 247, 01063. [Google Scholar] [CrossRef]
- Zhang, K.; Li, W.; Ju, Y.; Wang, X.; Sun, X.; Fang, Y.; Chen, K. Transcriptomic and metabolomic basis of short-and long-term post-harvest UV-C application in regulating grape berry quality development. Foods 2021, 10, 625. [Google Scholar] [CrossRef]
- Eshghi, S.; Karimi, R.; Shiri, A.; Karami, M.; Moradi, M. Effects of polysaccharide-based coatings on postharvest storage life of grape: Measuring the changes in nutritional, antioxidant and phenolic compounds. J. Food Meas. Charact. 2022, 16, 1159–1170. [Google Scholar] [CrossRef]
- Houillé, B.; Besseau, S.; Courdavault, V.; Oudin, A.; Glévarec, G.; Delanoue, G.; Guérin, L.; Simkin, A.J.; Papon, N.; Clastre, M.; Giglioli-Guivarc’h, N.; Lanoue, A. Biosynthetic origin of E-resveratrol accumulation in grape canes during postharvest storage. J. Agric. Food Chem. 2015, 63, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Concenco, F.I.G.R.; Brotto, G.F.; Nora, L. Grape wine and juice: Comparison on resveratrol levels. Int. J. Adv. Res. Sci. Eng. Technol. 2019, 6, 368–386. [Google Scholar] [CrossRef]
- Crăciun, A.L.; Gutt, G. Study on kinetics of trans-resveratrol, total phenolic content, and antioxidant activity increase in vine waste during post-pruning storage. Appl. Sci. 2022, 12, 1450. [Google Scholar] [CrossRef]
- Brillante, L.; De Rosso, M.; Dalla Vedova, A.; Maoz, I.; Flamini, R.; Tomasi, D. Insights on the stilbenes in Raboso Piave grape (Vitis vinifera L.) as a consequence of postharvest vs on-vine dehydration. J. Sci. Food Agric. 2018, 98, 1961–1967. [Google Scholar] [CrossRef] [PubMed]
- Naiker, M.; Anderson, S.; Johnson, J.B.; Mani, J.S.; Wakeling, L.; Bowry, V. Loss of trans-resveratrol during storage and ageing of red wines. Aust. J. Grape Wine Res. 2020, 26, 385–387. [Google Scholar] [CrossRef]
- Jordão, A.M.; Correia, A.C.; DelCampo, R.; González SanJosé, M.L. Antioxidant capacity, scavenger activity, and ellagitannins content from commercial oak pieces used in winemaking. Eur. Food Res. Technol. 2012, 235, 817–825. [Google Scholar] [CrossRef]
- Fabjanowicz, M.; Płotka-Wasylka, J.; Namieśnik, J. Detection, identification and determination of resveratrol in wine. Problems and challenges. Trends Analyt Chem 2018, 103, 21–33. [Google Scholar] [CrossRef]
- Stoenescu, A.M.; Trandafir, I.; Cosmulescu, S. Determination of phenolic compounds using HPLC-UV method in wild fruit species. Horticulturae 2022, 8, 84. [Google Scholar] [CrossRef]
- Biţă, C.E.; Scorei, I.R.; Vreju, A.F.; Muşetescu, A.E.; Mogoşanu, G.D.; Biţă, A.; Dinescu, V.C.; Dinescu, Ş.C.; Criveanu, C.; Bărbulescu, A.L.; Florescu, A.; Ciurea, P.L. Microbiota-Accessible Boron-Containing Compounds in Complex Regional Pain Syndrome. Medicina 2023, 59, 1965. [Google Scholar] [CrossRef] [PubMed]
- Biţă, A.; Scorei, I.R.; Bălşeanu, T.A.; Ciocîlteu, M.V.; Bejenaru, C.; Radu, A.; Bejenaru, L.E.; Rău, G.; Mogoşanu, G.D.; Neamţu, J.; Benner, S.A. New Insights into Boron Essentiality in Humans and Animals. Int. J. Mol. Sci. 2022, 23, 9147. [Google Scholar] [CrossRef] [PubMed]
- Ciocîlteu, M.V.; Scorei, I.R.; Rău, G.; Niculescu, C.; Biţă, A.; Ene, V.L.; Simionescu, A.; Turcu-Ştiolică, A.; Dinescu, V.C.; Neamţu, J.; Mogoantă, L.; Mogoşanu, G.D. Zinc–Boron–PLGA biocomposite material: preparation, structural characterization, and in vitro assessment. Rom. J. Morphol. Embryol. 2023, 64, 567–577. [Google Scholar] [CrossRef]
- Salla, M.; Karaki, N.; El Kaderi, B.; Ayoub, A.J.; Younes, S.; Abou Chahla, M.N.; Baksh, S.; El Khatib, S. Enhancing the Bioavailability of Resveratrol: Combine It, Derivatize It, or Encapsulate It? Pharmaceutics 2024, 16, 569. [Google Scholar] [CrossRef]
- Moreno, J.; Peinado, R. Composition of Grape Must. In Enological Chemistry; Academic Press: Cambridge, MA, USA, 2012; pp. 13–22. [Google Scholar] [CrossRef]
- Nawaz, W.; Zhou, Z.; Deng, S.; Ma, X.; Li, C.; Shu, X. Therapeutic Versatility of Resveratrol Derivatives. Nutrients 2017, 9, 1188. [Google Scholar] [CrossRef]
- Akinwumi, B.C.; Bordun, K.A.M.; Anderson, H.D. Biological activities of stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef]
- Vitrac, X.; Bornet, A.; Vanderlinde, R.; Valls, J.; Tristan, R.; Delaunay, J.C.; Mérillon, J.M.; Teissédre, P.L. Determination of Stilbenes (delta-viniferin, trans-astringin, trans-piceid, cis- and trans-resveratrol, E-viniferin) in Brazilian Wines. J. Agric. Food Chem. 2005, 53, 5664–5669. [Google Scholar] [CrossRef]
- Lekli, I.; Ray, D.; Das, D.K. Longevity nutrients resveratrol, wines and grapes. Genes Nutr. 2010, 5, 55–60. [Google Scholar] [CrossRef]
- Căpruciu, R.; Cichi, D.D.; Mărăcineanu, L.C.; Costea, D.C. The resveratrol content in black grapes skins at different development stages. Sci Papers Ser B Hortic 2022, LXVI, 245–252. [Google Scholar]
- Mikulski, D.; Molski, M. Quantitative structure–antioxidant activity relationship of trans-resveratrol oligomers, trans-4,4′-dihydroxystilbene dimer, trans-resveratrol-3-O-glucuronide, glucosides: Trans-piceid, cis-piceid, trans-astringin and trans-resveratrol-4′-O-β-D-glucopyranoside. Eur J Med Chem 2010, 45, 2366–2380. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Harikumar, K.B.; Aggarwal, B.B. Resveratrol addiction: To die or not to die. Mol. Nutr. Food Res. 2009, 53, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Flamini, R.; Mattivi, F.; De Rosso, M.; Arapitsas, P.; Bavaresco, L. Advanced knowledge of three important classes of grape phenolics: anthocyanins, stilbenes and flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669. [Google Scholar] [CrossRef]
- Flamini, R.; Zanzotto, A.; de Rosso, M.; Lucchetta, G.; Dalla Vedova, A.; Bavaresco, L. Stilbene oligomer phytoalexins in grape as a response to Aspergillus carbonarius infection. Physiol. Mol. Plant. Pathol. 2016, 93, 112–118. [Google Scholar] [CrossRef]
- Gao, S.; Wei, D.; Pan, D.; Wang, F.; Kang, H.; Yao, Y. Melatonin Promotes Accumulation of Resveratrol and Its Derivatives through Upregulation of PAL, 4CL, C4H, and STS in Grape Seeds. Horticulturae 2024, 10, 65. [Google Scholar] [CrossRef]
- Latruffe, N.; Vervandier-Fasseur, D. Strategic syntheses of vine and wine resveratrol derivatives to explore their effects on cell functions and dysfunctions. Diseases 2018, 6, 110. [Google Scholar] [CrossRef]
- Šušnjar, A.; Šunjka, D.; Boškovic, D.; Lazic, S.; Vukovic, S. Resveratrol in grapes-benefits and potential use. Agrofor Int. J. 2022, 7, 110–122. [Google Scholar]
- Pajović, R.; Raičević, D.; Popović, T.; Sivilotti, P.; Lisjak, K.; Vanzo, A. Polyphenolic characterisation of Vranac, Kratosija and Cabernet Sauvignon (Vitis vinifera L. cv.) grapes and wines from different vineyard locations in Montenegro. S. Afr. J. Enol. Vitic 2014, 35, 134–143. [Google Scholar] [CrossRef]
- Tzanova, M.; Atanasov, V.; Yaneva, Z.; Ivanova, D.; Dinev, T. Selectivity of current extraction techniques for flavonoids from plant materials. Processes 2020, 8, 1222. [Google Scholar] [CrossRef]
- Ochoa-Hueso, R.; Cantos-Villar, E.; Puertas, B.; Aguiar del Rio, J.F.; Belda, I.; Delgado-Baquerizo, M.; Fernández, V.; Gallardo, A.; García-Morales, J.L.; Garde-Cerdán, T.; Gonzaga-Santesteban, L.; Lazcano, C.; Liberal, I.M.; Serrano-Grijalva, L.; Casimiro-Soriguer, R. Nature-based strategies to regenerate the functioning and biodiversity of vineyards. J. Sustainable Agric. Environ 2024, 3, e12088. [Google Scholar] [CrossRef]
- Geana, E.I.; Dinca, O.R.; Ionete, R.E.; Artem, V.; Niculescu, V.C. Monitoring trans-resveratrol in grape berry skins during ripening and in corresponding wines by HPLC. Food Technol. Biotechnol. 2015, 53, 73–80. [Google Scholar] [CrossRef]
- Raičević, D.; Božinović, Z.; Petkov, M.; Ivanova, V.; Kodžulović, V.; Mugoša, M.; Sanja, S.; Maraš, V. Polyphenolic content and sensory profile of Montenegrin Vranac wines produced with different oenological products and maceration. Maced. J. Chem. Chem. Eng. 2017, 36, 229–238. [Google Scholar] [CrossRef]
- Rocchetti, G.; Ferrari, F.; Trevisan, M.; Bavaresco, L. Impact of climatic conditions on the resveratrol concentration in blend of Vitis vinifera L. cvs. Barbera and Croatina grape wines. Molecules 2021, 26, 401. [Google Scholar] [CrossRef] [PubMed]
- Cichi, D.D.; Căpruciu, R.; Gheorghiu, N.; Stoica, F. Agrobiological and technological characteristics of table grapes varieties, grown in the temperatecontinental climate from southwestern Romania. Sci. Papers. Ser. B Hortic. 2023, LXVII, 302–308. [Google Scholar]
- Sun, Y.; Xi, B.; Dai, H. Effects of Water Stress on Resveratrol Accumulation and Synthesis in ‘Cabernet Sauvignon’Grape Berries. Agronomy 2023, 13, 633. [Google Scholar] [CrossRef]
- Constantin, O.E.; Stoica, F.; Rațu, R.N.; Stănciuc, N.; Bahrim, G.E.; Râpeanu, G. Bioactive Components, Applications, Extractions, and Health Benefits of Winery By-Products from a Circular Bioeconomy Perspective: A Review. Antioxidants 2024, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Bešlić, Z.; Pantelić, M.; Dabić, D.; Todić, S.; Natić, M.; Tešić, Ž. Effect of vineyard floor management on water regime, growth response, yield and fruit quality in Cabernet Sauvignon. Sci. Hortic. 2015, 197, 650–656. [Google Scholar] [CrossRef]
- Ingrà, C.; Del Frari, G.; Favole, M.; Tumminelli, E.; Rossi, D.; Collina, S.; Prati, M.; Ferreira, R.B.; Ferrandino, A. Effects of Growing Areas, Pruning Wound Protection Products, and Phenological Stage on the Stilbene Composition of Grapevine (Vitis vinifera L.) Canes. J. Agric. Food Chem. 2024, 72, 11465–11479. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Yan, Y. Bioproduction of Resveratrol. In Biotechnology of Natural Products; Schwab, W., Lange, B., Wüst, M., Eds.; Springer: Cham, 2018; pp. 61–79. [Google Scholar] [CrossRef]
- Tıraş, Z.Ş.E.; Okur, H.H.; Günay, Z.; Yıldırım, H.K. Different approaches to enhance resveratrol content in wine. Cienc. Tec. Vitivinic. 2022, 37, 13–28. [Google Scholar] [CrossRef]
- Ispiryan, A.; Kraujutiene, I.; Viskelis, J. Retaining Resveratrol Content in Berries and Berry Products with Agricultural and Processing Techniques. Processes 2024, 12, 1216. [Google Scholar] [CrossRef]
- Guld, Z.S.; Rácz, A.; Tima, H.; Kállay, M.; Sárdy, D.N. Effects of aging in oak barrels on the trans-resveratrol and anthocyanin concentration of red wines from Hungary. Acta Aliment. 2019, 48, 349–357. [Google Scholar] [CrossRef]
- Uysal, R.S.; Issa-Issa, H.; Sendra, E.; Carbonell-Barrachina, Á.A. Changes in anthocyanin pigments, trans-resveratrol, and colorimetric characteristics of Fondillón wine and other “Monastrell” wines during the aging period. Eur. Food Res. Technol. 2023, 249, 1821–1831. [Google Scholar] [CrossRef]
- Fernández-Marín, M.I.; Guerrero, R.F.; García-Parrilla, M.C.; Puertas, B.; Ramírez, P.; Cantos-Villar, E. Terroir and variety: Two key factors for obtaining stilbene-enriched grapes. J. Food Compost. Anal. 2013, 31, 191–198. [Google Scholar] [CrossRef]
- Cavallini, G.; Straniero, S.; Donati, A.; Bergamini, E. Resveratrol Requires Red Wine Polyphenols for Optimum Antioxidant Activity. J. Nutr. Health Aging. 2016, 20, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Lubin, B.C.R.; Inbar, N.; Pinkus, A.; Stanevsky, M.; Cohen, J.; Rahimi, O.; Anker, Y.; Shoseyov, O.; Drori, E. Ecogeographic conditions dramatically affect trans-resveratrol and other major phenolics’ levels in wine at a semi-arid area. Plants 2022, 11, 629. [Google Scholar] [CrossRef]
- Noviello, M.; Caputi, A.F.; Squeo, G.; Paradiso, V.M.; Gambacorta, G.; Caponio, F. Vine shoots as a source of trans-resveratrol and ε-Viniferin: A Study of 23 Italian varieties. Foods 2022, 11, 553. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Patra, J.K.; Kotseridis, Y.; Dimopoulou, M. Fermented beverages revisited: From terroir to customized functional products. Fermentation 2024, 10, 74. [Google Scholar] [CrossRef]
- Mucalo, A.; Maletić, E.; Zdunić, G. Positive Impact of Late Harvest Date on Polyphenolic Composition of Plavac Mali (Vitis vinifera L.) Wine Depends on Location. Foods 2024, 13, 2695. [Google Scholar] [CrossRef]
- Hasan, M.M.; Bae, H. An overview of stress-induced resveratrol synthesis in grapes: perspectives for resveratrol-enriched grape products. Molecules 2017, 22, 294. [Google Scholar] [CrossRef]
- Erte, E.; Vural, N.; Mehmetoğlu, Ü.; Güvenç, A. Optimization of an abiotic elicitor (ultrasound) treatment conditions on trans-resveratrol production from Kalecik Karası (Vitis vinifera L.) grape skin. J. Food Sci. Tehnol. 2021, 58, 2121–2132. [Google Scholar] [CrossRef]
- Candar, S. How abiotic stress induced by artificial wounding changes maturity levels and berry composition of Merlot (Vitis vinifera L.). Eur. Food Res. Technol. 2023, 249, 2611–2623. [Google Scholar] [CrossRef]
- Aleynova, O.A.; Ogneva, Z.V.; Suprun, A.R.; Ananev, A.A.; Nityagovsky, N.N.; Beresh, A.A.; Dubrovina, A.S.; Kiselev, K.V. The Effect of External Treatment of Arabidopsis thaliana with Plant-Derived Stilbene Compounds on Plant Resistance to Abiotic Stresses. Plants 2024, 13, 184. [Google Scholar] [CrossRef]
- Ferreyra, S.; Bottini, R.; Fontana, A. Temperature and light conditions affect stability of phenolic compounds of stored grape cane extracts. Food Chem. 2023, 405, 134718. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuw, R.; Kevers, C.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Antioxidant capacity and phenolic composition of red wines from various grape varieties: Specificity of Pinot Noir. J. Food Compos. Anal. 2014, 36, 40–50. [Google Scholar] [CrossRef]
- Yaman, Ü.R.; Adıgüzel, B.Ç.; Yücel, U.; Çetinkaya, N. Effect of vegetation time and climatic conditions on trans-resveratrol concentrations in Cabernet Sauvignon and Merlot wines from different regions in Turkey. S. Afr. J. Enol. Vitic. 2016, 37, 85–92. [Google Scholar] [CrossRef]
- Tříska, J.; Houška, M. Physical methods of resveratrol induction in grapes and grape products-a review. Czech J. Food Sci. 2012, 30, 489–502. [Google Scholar] [CrossRef]
- Filimon, R.V.; Bunea, C.I.; Bora, F.D.; Filimon, R.M.; Dunca, S.I.; Rózsa, S.; Ciurlă, L.; Patraș, A. Physico-Chemical Characterization, Phenolic Compound Extraction and Biological Activity of Grapevine (Vitis vinifera L.) Canes. Horticulturae 2023, 9, 1164. [Google Scholar] [CrossRef]
- Singh, C.K.; Liu, X.; Ahmad, N. Resveratrol, in its natural combination in whole grape, for health promotion and disease management. Ann. N. Y. Acad. Sci. 2015, 1348, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Alañón, M.E.; Alarcón, M.; Marchante, L.; Díaz-Maroto, M.C.; Pérez-Coello, M.S. Extraction of Natural Flavorings with Antioxidant Capacity from Cooperage By-Products by Green Extraction Procedure with Subcritical Fluids. Ind. Crop. Prod. 2017, 103, 222–232. [Google Scholar] [CrossRef]
- Venkat, R.; Verma, E.; Daimary, U.D.; Kumar, A.; Girisa, S.; Dutta, U.; Ahn, K.S.; Kunnumakkara, A.B. The Journey of Resveratrol from Vineyards to Clinics. Cancer Invest. 2022, 41, 183–220. [Google Scholar] [CrossRef] [PubMed]
- Mongioi, L.M.; La Vignera, S.; Cannarella, R.; Cimino, L.; Compagnone, M.; Condorelli, R.A.; Calogero, A.E. The role of resveratrol administration in human obesity. Int J Mol Sci 2021, 22, 4362. [Google Scholar] [CrossRef] [PubMed]
- Batista-Jorge, G.C.; Barala-Jorge, A.S.; Silveira, M.F.; Lelis, D.F.; Andrade, J.M.O.; de Paula, A.M.B.; Guimarães, A.L.S.; Santos, S.H.S. Oral resveratrol supplementation improves metabolic syndrome features in obese patients submitted to a lifestyle-changing program. Life Sci. 2020, 256, 117962. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, Y.; Gu, H.; Xu, Z.; Tang, Y.; He, H.; Peng, L.; Xiang, L. Adherence to the Paleolithic diet and Paleolithic-like lifestyle reduce the risk of colorectal cancer in the United States: a prospective cohort study. J. Transl. Med. 2023, 21, 482. [Google Scholar] [CrossRef] [PubMed]
- Wajima, C.S.; Pitol-Palin, L.; de Souza Batista, F.R.; Dos Santos, P.H.; Matsushita, D.H.; Okamoto, R. Morphological and biomechanical characterization of long bones and peri-implant bone repair in type 2 diabetic rats treated with resveratrol. Sci Rep 2024, 14, 2860. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.F.; Silva-Reis, R.; Ferreira, R.; Oliveira, P.A.; Faustino-Rocha, A.I.; Pinto, M.D.L.; Coimbra, A.M.; Silva, A.M.S.; Cardoso, S.M. The impact of resveratrol-enriched bread on cardiac remodeling in a preclinical model of diabetes. Antioxidants 2023, 12, 1066. [Google Scholar] [CrossRef]
- Meyer, C.; Brockmueller, A.; Buhrmann, C.; Shakibaei, M. Prevention and Co-management of breast cancer-related osteoporosis using resveratrol. Nutrients 2024, 16, 708. [Google Scholar] [CrossRef]
- Darby, J.R.T.; Saini, B.V.; Soo, J.Y.; Lock, M.C.; Holman, S.; Bradshaw, E.L.; McInnes, S.J.P.; Voelcker, N.H.; Macgowan, C.K.; Seed, M.; Wiese, M.D.; Morrison, J.L. Subcutaneous maternal resveratrol treatment increases uterine artery blood flow in the pregnant ewe and increases fetal but not cardiac growth. J. Physiol. 2019, 597, 5063–5077. [Google Scholar] [CrossRef]
- Novakovic, R.; Rajkovic, J.; Gostimirovic, M.; Gojkovic-Bukarica, L.; Radunovic, N. Resveratrol and Reproductive Health. Life 2022, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Gumus, R.; Ercan, N.; Özbilgin, A.; Moğulkoç, M.N.; İmik, H. The effect of dietary supplementation with natural antioxidants on growth performance, antioxidant capacity and intestinal microbial counts of broiler. J. Hellenic Vet. Med. Soc. 2024, 75, 7441–7450. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, J.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health benefits and molecular mechanisms of resveratrol: a narrative review. Foods 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Rivière, C.; Pawlus, A.D.; Mérillon, J.M. Natural stilbenoids: Distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317–1333. [Google Scholar] [CrossRef] [PubMed]
- Olivati, C.; de Oliveira Nishiyama, Y.P.; Teodoro de Souza, R.N.; Janzantti, S.; Mauro, M.A.; Gomes, E.; Hermosín-Gutiérrez, I.; da Silva, R.; Lago-Vanzela, E.S. Effect of the pre-treatment and the drying process on the phenolic composition of raisins produced with a seedless Brazilian grape cultivar. Food Res. Int. 2019, 116, 190–199. [Google Scholar] [CrossRef]
- Šuković, D.; Knežević, B.; Gašić, U.; Sredojević, M.; Ćirić, I.; Todić, S.; Mutić, J.; Tešić, Ž. Phenolic Profiles of Leaves, Grapes and Wine of Grapevine Variety Vranac (Vitis vinifera L.) from Montenegro. Foods 2020, 9, 138. [Google Scholar] [CrossRef]
- de Peredo, A.V.G.; Vázquez-Espinosa, M.; Piñeiro, Z.; Espada-Bellido, E.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. Development of a rapid and accurate UHPLC-PDA-FL method for the quantification of phenolic compounds in grapes. Food Chem. 2021, 334, 127569. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Stanisavljević, N.S.; Kostić, A.Ž.; Soković Bajić, S.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Lj Tešić, Ž.; Pešić, M.B. Phenolic compounds and biopotential of grape pomace extracts from Prokupac red grape variety. LWT 2021, 138, 110739. [Google Scholar] [CrossRef]
- Kavgacı, M.; Yukunc, G.O.; Keskin, M.; Can, Z.; Kolaylı, S. Comparison of Phenolic Profile and Antioxidant Properties of Pulp and Seeds of Two Different Grapes Types (Vitis vinifera L. and Vitis labrusca L.) Grown in Anatolia: The Amount of Resveratrol of Grape Samples. Chem. Afr. 2023, 6, 2463–2469. [Google Scholar] [CrossRef]
- Li, X.; Wu, B.; Wang, L.; Li, S. Extractable amounts of trans-resveratrol in seed and berry skin in Vitis evaluated at the germplasm level. J. Agric. Food Chem. 2006, 54, 8804–8811. [Google Scholar] [CrossRef] [PubMed]
- Casas, L.; Mantell, C.; Rodríguez, M.; de la Ossa, E.J.; Roldán, M.; De Ory, A.I.; Caro, I.; Blandino, A. Extraction of resveratrol from the pomace of Palomino fino grapes by supercritical carbon dioxide. J. Food Eng. 2010, 96, 304–308. [Google Scholar] [CrossRef]
- Barcia, M.T.; Pertuzatti, P.B.; Gómez-Alonso, S.; Godoy, H.T.; Hermosín-Gutiérrez, I. Phenolic composition of grape and winemaking by-products of Brazilian hybrid cultivars BRS Violeta and BRS Lorena. Food Chem. 2014, 159, 95–105. [Google Scholar] [CrossRef]
- Hua, L.H.; Stephen Inbaraj, B.; Chen, B.H. An improved analytical method for determination of trans-resveratrol and related stilbenes in grape skin by QuEChERS coupled with HPLC-PDA-MS. Int. J. Food Sci. Technol. 2021, 56, 6376–6387. [Google Scholar] [CrossRef]
- Takács, K.; Pregi, E.; Vági, E.; Renkecz, T.; Tátraaljai, D.; Pukánszky, B. Processing Stabilization of Polyethylene with Grape Peel Extract: Effect of Extraction Technology and Composition. Molecules 2023, 28, 1011. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kaur, H.; Kaur, R.; Garg, R.; Prasad, R.; Assouguem, A.; Kara, M.; Bahhou, J. A Review on the Nutritional Value and Health Benefits of Different Parts of Grape (Vitis vinifera L.). Trop. J. Nat. Prod. Res. 2023, 7, 3874–3880. [Google Scholar] [CrossRef]
- Fataliyev, H.; Lazgiyev, Y.; İmamguliyeva, M.; Haydarov, E.; Fataliyeva, S.; Huseynova, S.; Agayeva, S.; İsganderova, S.; Askarova, A.; Askarova, İ. Comparative evaluation and studing of some indigenous and introduced red grape varieties. Food Sci. Tehnol. 2023, 17, 2073–8684. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Hua, L.H.; Chen, B.H. Comparative study on inhibition of pancreatic cancer cells by resveratrol gold nanoparticles and a resveratrol nanoemulsion prepared from grape skin. Pharmaceutics 2021, 13, 1871. [Google Scholar] [CrossRef] [PubMed]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; Gonçalves, A.E.D.S.S.; Genovese, M.I.; Fett, R. Phenolic compounds and antioxidant activity of seed and skin extracts of red grape (Vitis vinifera and Vitis labrusca) pomace from Brazilian winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Mohammadparast, B.; Rasouli, M.; Eyni, M. Resveratrol Contents of 27 Grape Cultivars. Appl. Fruit Sci. 2024, 66, 1053–1060. [Google Scholar] [CrossRef]
- Anastasiadi, M.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.L.; Haroutounian, S.A. Grape stem extracts: Polyphenolic content and assessment of their in vitro antioxidant properties. LWT-Food Sci. Technol. 2012, 48, 316–322. [Google Scholar] [CrossRef]
- Jiménez-Moreno, N.; Volpe, F.; Moler, J.A.; Esparza, I.; Ancín-Azpilicueta, C. Impact of extraction conditions on the phenolic composition and antioxidant capacity of grape stem extracts. Antioxidants 2019, 8, 597. [Google Scholar] [CrossRef] [PubMed]
- Bzainia, A.; Dias, R.C.S.; Costa, M.R.P.F.N. A simple process to purify (E)-resveratrol from grape stems with a photo-molecularly imprinted sorbent. Food Bioprod. Process. 2023, 142, 1–16. [Google Scholar] [CrossRef]
- Timperio, A.M.; d’Alessandro, A.; Fagioni, M.; Magro, P.; Zolla, L. Production of the phytoalexins trans-resveratrol and delta-viniferin in two economy-relevant grape cultivars upon infection with Botrytis cinerea in field conditions. Plant Physiol. Biochem. 2012, 50, 65–71. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Miklaszewski, A.; Michniak-Kohn, B.; Cielecka-Piontek, J. The antioxidant potential of resveratrol from red vine leaves delivered in an electrospun nanofiber system. Antioxidants 2023, 12, 1777. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.D.M.; Feriani, A.; Gómez-Cruz, I.; Hfaiedh, N.; Harrath, A.H.; Romero, I.; Castro, E.; Tlili, N. Grapevine shoot extract rich in trans-resveratrol and trans-εagronomy-viniferin: evaluation of their potential use for cardiac health. Foods 2023, 12, 4351. [Google Scholar] [CrossRef]
- Hoseinpanahi, B.; Bahramnejad, B.; Majdi, M.; Dastan, D.; Ashengroph, M. The Effect of Different Elicitors on Hairy Root Biomass and Resveratrol Production in Wild Vitis vinifera. J. Appl. Biotechnol. Rep. 2020, 7, 25–31. [Google Scholar] [CrossRef]
- Zhou, D.D.; Li, J.; Xiong, R.G.; Saimaiti, A.; Huang, S.Y.; Wu, S.X.; Yang, Z.J.; Shang, A.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Bioactive compounds, health benefits and food applications of grape. Foods 2022, 11, 2755. [Google Scholar] [CrossRef] [PubMed]
- Leena, M.M.; Yoha, K.S.; Moses, J.A.; Anandharamakrishnan, C.J.F.B. Edible coating with resveratrol loaded electrospun zein nanofibers with enhanced bioaccessibility. Food Biosci. 2020, 36, 100669. [Google Scholar] [CrossRef]
- Visioli, F.; Panaite, S.A.; Tomé-Carneiro, J. Wine’s Phenolic Compounds and Health: A Pythagorean View. Molecules 2020, 25, 4105. [Google Scholar] [CrossRef] [PubMed]
- Bede, T.P.; Jesuz, V.A.; Souza, V.R.; Elias, M.B.; Oliveira, F.L.; Dias, J.F.; Teodoro, A.J.; Azeredo, V.B. Effects of grape juice, red wine and resveratrol on liver parameters of rat submitted high-fat diet. An. Acad. Bras. Cienc. 2020, 92, e20191230. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Carughi, A. Polyphenol content and health benefits of raisins. Nutrition Research 2010, 30, 511–519. [Google Scholar] [CrossRef]
- Abdel-Hamid, G.A.; Ayuob, N.N. Can raisins ameliorate the hypercholesterolaemia-induced cardiac affection? Folia Morphol. 2015, 74, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Weiter, K.M.; Christian, A.L.; Ritchey, M.B.; Bays, H.E. Raisins compared with other snack effects on glycemia and blood pressure: a randomized, controlled trial. Postgrad Med 2014, 126, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Management 2018, 72, 99–118. [Google Scholar] [CrossRef]
- Ghendov-Mosanu, A.; Cojocari, D.; Balan, G.; Patras, A.; Lung, I.; Soran, M.L.; Opriş, O.; Cristea, E.; Sturza, R. Chemometric Optimization of Biologically Active Compounds Extraction from Grape Marc: Composition and Antimicrobial Activity. Molecules 2022, 27, 1610. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.; Asim, R. The Pharmacological Properties of Red Grape Polyphenol Resveratrol: Clinical Trials and Obstacles in Drug Development. Nutrients 2023, 15, 4486. [Google Scholar] [CrossRef]
- Guler, A. Effects of different maceration techniques on the colour, polyphenols and antioxidant capacity of grape juice. Food Chem 2023, 404, 134603. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Merrell, C.; Yokoyama, W.; Nitin, N. Infusion of trans-resveratrol in micron-scale grape skin powder for enhanced stability and bioaccessibility. Food Chem 2021, 340, 127894. [Google Scholar] [CrossRef]
- Liu, F.; Lei, J.; Shao, X.; Fan, Y.; Huang, W.; Lian, W.; Wang, C. Effect of Pretreatment and Drying on the Nutritional and Functional Quality of Raisins Produced with Seedless Purple Grapes. Foods 2024, 13, 1138. [Google Scholar] [CrossRef]
- Kosović, E.; Topiař, M.; Cuřínová, P.; Sajfrtová, M. Stability testing of resveratrol and viniferin obtained from Vitis vinifera L. by various extraction methods considering the industrial viewpoint. Scientific Reports 2020, 10, 5564. [Google Scholar] [CrossRef]
- Negro, C.; Aprile, A.; Luvisi, A.; De Bellis, L.; Miceli, A. Antioxidant activity and polyphenols characterization of four monovarietal grape pomaces from Salento (Apulia, Italy). Antioxidants 2021, 10, 1406. [Google Scholar] [CrossRef] [PubMed]
- Sinrod, A.J.; Shah, I.M.; Surek, E.; Balanovarile, D. Uncovering the promising role of grape pomace as a modulator of the gut microbiome: An in-depth review. Heliyon 2023, 9, e20499. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Santos, P.; Nobre, C.; Rodrigues, R.M.; Genisheva, Z.; Botelho, C.; Teixeira, J.A. Extraction of phenolic compounds from grape pomace using ohmic heating: Chemical composition, bioactivity and bioaccessibility. Food Chem. 2024, 436, 137780. [Google Scholar] [CrossRef] [PubMed]
- Zemni, H.; Khiari, R.; Lamine, M.; Houimli, Y.; Chenenaoui, S.; Ben Salem, A. Grape Marc Skin Valorization: From Waste to Valuable Polyphenol Source. Chem. Afr. 2024, 7, 765–776. [Google Scholar] [CrossRef]
- Qsaib, S.; Mateus, N.; Ikbal, F.E.; Rifai, L.A.; De Freitas, V.; Koussa, T. Direct identification and characterization of phenolic compounds from crude extracts of buds and internodes of grapevine (Vitis vinifera cv Merlot). Nat Prod Commun 2014, 9, 1569–1572. [Google Scholar] [CrossRef]
- Zwingelstein, M.; Draye, M.; Besombes, J.L.; Piot, C.; Chatel, C. trans-Resveratrol and trans-ε-Viniferin in Grape Canes and Stocks Originating from Savoie Mont Blanc Vineyard Region: Pre-extraction Parameters for Improved Recovery. ACS Sustainable Chem. Eng. 2019, 7, 8310–8316. [Google Scholar] [CrossRef]
- Kiene, M.; Zaremba, M.; Fellensiek, H.; Januschewski, E.; Juadjur, A.; Jerz, G.; Winterhalter, P. In Silico-Assisted Isolation of trans-Resveratrol and trans-ε-Viniferin from Grapevine Canes and Their Sustainable Extraction Using Natural Deep Eutectic Solvents (NADES). Foods 2023, 12, 4184. [Google Scholar] [CrossRef] [PubMed]
- Noronha, H.; Silva, A.; Garcia, V.; Billet, K.; Dias, A.C.; Lanoue, A.; Gallusci, P.; Gerós, H. Grapevine woody tissues accumulate stilbenoids following bud burst. Planta 2023, 258, 118. [Google Scholar] [CrossRef]
- Besrukow, P.; Orbach, A.; Schweiggert, R. Modulating the Photostability of (E)-Resveratrol in Grape Cane Extract Formulations. ACS Food Sci. Technol. 2024, 4, 625–632. [Google Scholar] [CrossRef]
- Duarte, H.; Aliaño-González, M.J.; Cantos-Villar, E.; Faleiro, L.; Romano, A.; Medronho, B. Sustainable extraction of polyphenols from vine shoots using deep eutectic solvents: influence of the solvent, Vitis sp., and extraction technique. Talanta 2024, 267, 125135. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bedmar, Z.; Anter, J.; Alonso-Moraga, A.; Delgado de la Torre, P.; Luque de Castro, M.D.; Millán-Ruiz, Y.; Sánchez-Frías, M.; Guil-Luna, S. Red and White Wine Lees Show Inhibitory Effects on Liver Carcinogenesis. Mol. Nutr. Food Res. 2019, 63, 1800864. [Google Scholar] [CrossRef]
- Dujmić, F.; Kovačević Ganić, K.; Ćurić, D.; Karlović, S.; Bosiljkov, T.; Ježek, D.; Vidrih, R.; Hribar, J.; Zlatić, E.; Prusina, T.; Khubber, S.; Barba, F.J.; Brnčić, M. Non-thermal ultrasonic extraction of polyphenolic compounds from red wine lees. Foods 2020, 9, 472. [Google Scholar] [CrossRef] [PubMed]
- López-Fernández-Sobrino, R.; Margalef, M.; Torres-Fuentes, C.; Ávila-Román, J.; Aragonès, G.; Muguerza, B.; Bravo, F.I. Enzyme-assisted extraction to obtain phenolic-enriched wine lees with enhanced bioactivity in hypertensive rats. Antioxidants 2021, 10, 517. [Google Scholar] [CrossRef]
- Hassan, Y.I.; Kosir, V.; Yin, X.; Ross, K.; Diarra, M.S. Grape pomace as a promising antimicrobial alternative in feed: A critical review. J. Agric. Food Chem. 2019, 67, 9705–9718. [Google Scholar] [CrossRef] [PubMed]
- Chioru, A.; Chiselita, N.; Suhodol, N.; Boiştean, A.; Paladi, D.; Capcanari, T.; Chirsanova, A. physico-chemical and microbiological profile of wine lees of red wines from local grapes varieties. Food Nutr Sci 2023, 14, 1133–1148. [Google Scholar] [CrossRef]

| Fractions | Content in resveratrol | Method of analysis | References |
|---|---|---|---|
| whole grape | 3.2 ppm | HPLC-MS | [55] |
| 3.06 a ± 0.51 mg/kg | HPLC-ESI-MS/MS | [78] | |
| 4.86 ppm-resveratrol hexoxide; 3.66 ppm-RSV; 4.96-ppm RSV tetramer; 4.57-resveratrol dimer; 4.77-ppm RSV trimer | UHPLC-LTQ-MS | [79] | |
| 0.2–9.1 mg/L (PDA) 0.04–9.1 mg/L (FL) |
UPLC-PDA-FL | [80] | |
| 111.0 mg/kg DM | UHPLC-MS/MS | [81] | |
| 11.9 μg g−1 FW | HPLC | [7] | |
| de la 11.86 mg/100 g up to 101.89 mg/100 g depending on varieties | HPLC-UV | [82] | |
| skin | 50–100 μg/g | HPLC | [83] |
| 49.1 mgRSV/gDS | HPLC | [84] | |
| 48.99 ± 2.69 mg/kg | HPLC-DAD-ESI-MSn | [85] | |
| 0.967 μg mL−1 (t-RSV) 0.183 μg mL−1 (t-RSV) |
by QuEChERS method coupled with an HPLC-PDA-MS | [86] | |
| 57.7 mg/kg DM | UHPLC/MS | [81] | |
| 9,152 mg/L to 11,083 mg/L (trans-resveratrol depending on variety and period); from 7,119 mg/L to 8,071 mg/L (cis-resveratrol depending on variety and period) | HPLC | [25] | |
| 21.7 μg/mL | HPLC | [87] | |
| 1.17 to 12.96 μg g−1 | HPLC | [88] | |
| 0.75–8.25 mg/kg | HPLC | [89] | |
| 11.02 μg/mL | HPLC-MS | [90] | |
| seed | 8.3 mgRSV/gDS | HPLC | [84] |
| 3.75 ± 0.08 a mg/100 g dw (Isabel Variety) 1.11 ± 0.02 c mg/100 g dw (Sangiovese Variety) 1.42 ± 0.07 b mg/100 g dw (Negro Amaro Variety) |
HPLC | [91] | |
| 2.8 mg/kg DM | UHPLC-orbitrap MS4 | [81] | |
| 0.31–5.7 mg/kg | HPLC | [89] | |
| from 3.60 mg/100 g to 37.50 mg/100 g, depending on the variety | HPLC-UV | [82] | |
| 92,312.43 ± 2404.19 (Con3) | HPLC | [30] | |
| 4.97 mg Kg−1 FW | HPLC | [92] | |
| pulp | 4,50 mg/100 g | HPLC-UV | [82] |
| stem | 0.9 mgRSV/gDS | HPLC | [84] |
| 5–0.078 mg/L | HPLC/LC-MS-MS | [93] | |
| 122 ± 16 μg/g DM | HPLC-DAD | [94] | |
| 3700 mg/kg of dry weight | HPLC | [95] | |
| leaf | 180 pg/µl | HPLC-MS | [96] |
| 0.306 ± 0.009 µg/mg | HPLC | [97] | |
| 0.01–0.25 mg/kg | HPLC | [89] | |
| grapevine shoots | 27.4 ± 0.3 mg/g | HPLC–quadrupole time-of-flight (QTOF)–mass spectrometry (MS) | [98] |
| 90,74 μg g−1 DW | TLC and HPLC | [99] |
| Fractions | Content in resveratrol | Method of identification / determination | References | |
|---|---|---|---|---|
| Grapes product | wine | 6,9 to 12,6 mg/dm3 | HPLC | [5] |
| 64 μg/mL | HPLC | [109] | ||
| juice | De la 4,4 la 7,0 mg in grape juice / dm3; from 12,4 la 21,3 mg / dm3 in concentrated juice | HPLC | [5] | |
| 0,09 la 0,23 mg/100 g | HPLC | [110] | ||
| grape skin powder | 17.87 µg/mL | HPLC-MS | [90] | |
| 0.25 and 0.05 mg/g on a wet basis which | HPLC | [111] | ||
| 0.0313 wt% (Stir) 0.0044 wt% (Sox) 0.0178 wt% (LC-MS/MS) |
LC-MS/MS | [87] | ||
| raisins | 0.40 c ± 0.04 mg/kg | HPLC-ESI-MS/MS | [78] | |
| 8993 ± 391 b 16,544 ± 440 a 8798 ± 137 b |
UPLC-VION-IMS-QTOF-the physical pretreatment using a motorised rotating drum (PT) -the drying agent treatment group (DT) -In the control group (CK), the grape samples received no pretreatment |
[112] | ||
| By-products | grape pomace | 16.1 mgRSV / gDS | HPLC | [84] |
| 0.042–0.653 mg/L | HPLC-DAD/MS | [113] | ||
| 0.09 ± 0.04 a mg/g DW | HPLC/MS | [114] | ||
| 0.7–21.7 mg/kg DM | UHPLC-MS/MS | [81] | ||
| 26.3 ± 0.5 µg/g DW | HPLC | [115] | ||
| 2.38 ± 0.2 mg/L | HPLC | [116] | ||
| 0.80 mg/kg DM | UPLC | [117] | ||
| grape canes | 470 MV (resveratrol dimer) | LC-MS | [118] | |
| 3450 mg·kg−1 dw (Pinot Noir) 5361 mg·kg−1 dw (Gewurztraminer) |
HPLC-UV | [119] | ||
| 5298.1 mg kg−1 DW | HPLC | [52] | ||
| 419.01–425.60 a μg/g d.w. (Pinot Gris) 282.19 ± 4.14 b μg/g d.w. (Sauvignon Blanc) 425.60 ± 5.98 a (Cabernet Sauvignon) |
HPLC | [63] | ||
| 0.55–3.96 mg/g DW | UPLC (HPLC-ESI-MS) | [120] | ||
| 69.1 to 436.5 µg g DW−1 | HPLC-DAD | [121] | ||
| 3.7 ± 0.2 g/100 g | HPLC-DAD | [122] | ||
| 9.50 mg·L−1 | HPLC | [123] | ||
| wine lees | 104 ppm (Red wine lees) 30 ppm (White wine lees) |
HPLC-DAD | [124] | |
| 0.04 ± 0.00 mg/g d.m. (Merlot) 0.11 ± 0.01 mg/g d.m. (Vranac) |
HPLC-MS/MS | [125] | ||
| 2.95 ± 0.01 µg/g (RSV) 4.60 ± 0.02 (t-RSV) |
UHPLC | [126] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




