Submitted:
05 December 2024
Posted:
06 December 2024
You are already at the latest version
Abstract
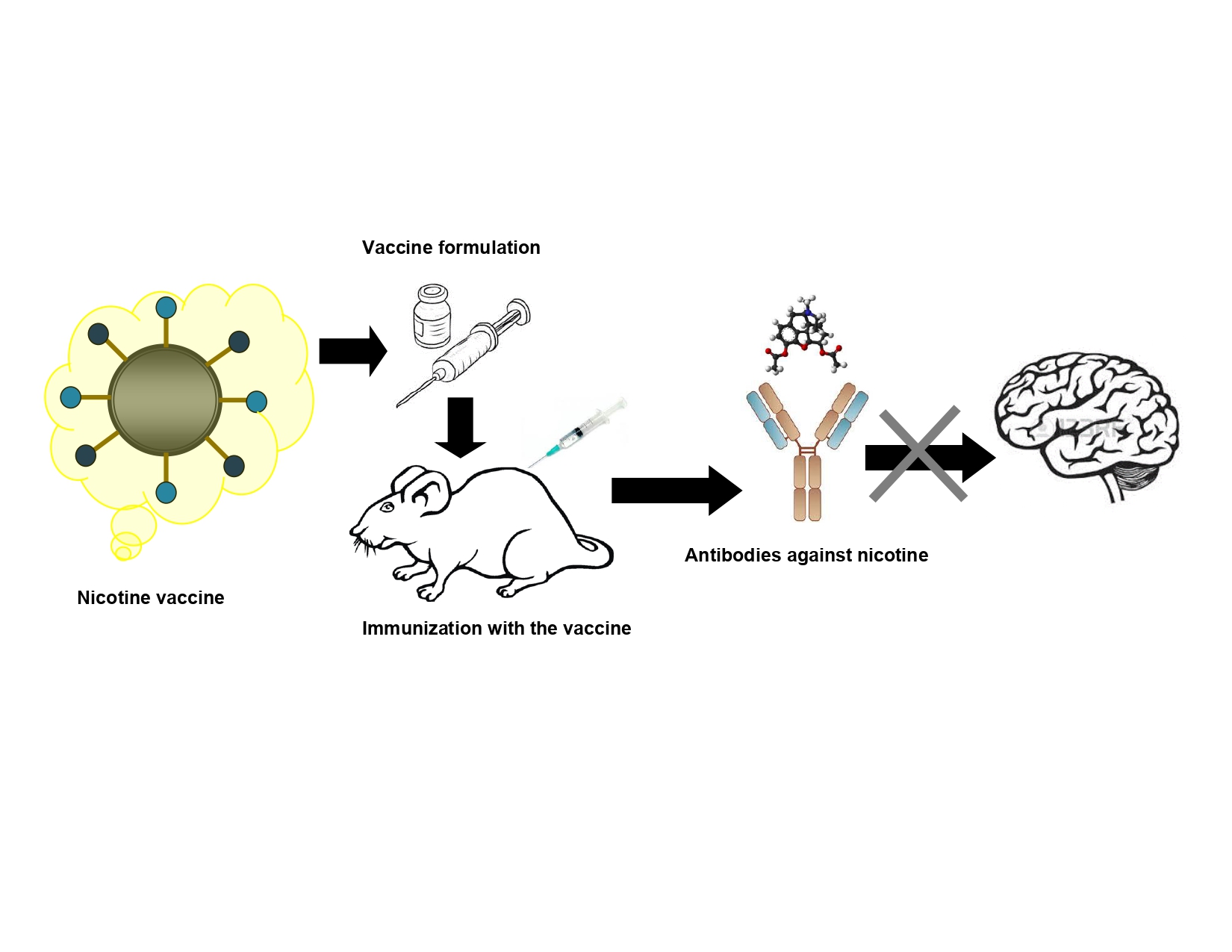
Keywords:
1. Introduction
2. Methods
2.1. Subjects
2.2. Drugs
2.3. Synthesis of the NIC-TT Vaccine
2.4. Immunization Schedule
2.5. Determination of Serum Antibody Titters via ELISA
2.6. Behavioral Procedure
2.6.1. Self-Administration Procedure
2.6.2. Behavioral Sensitization Procedure
2.7. Experimental Procedures
2.7.1. Experiment 1
2.7.2. Experiment 2
2.7.3. Experiment 3
2.8. Statistical Analysis
3. Results
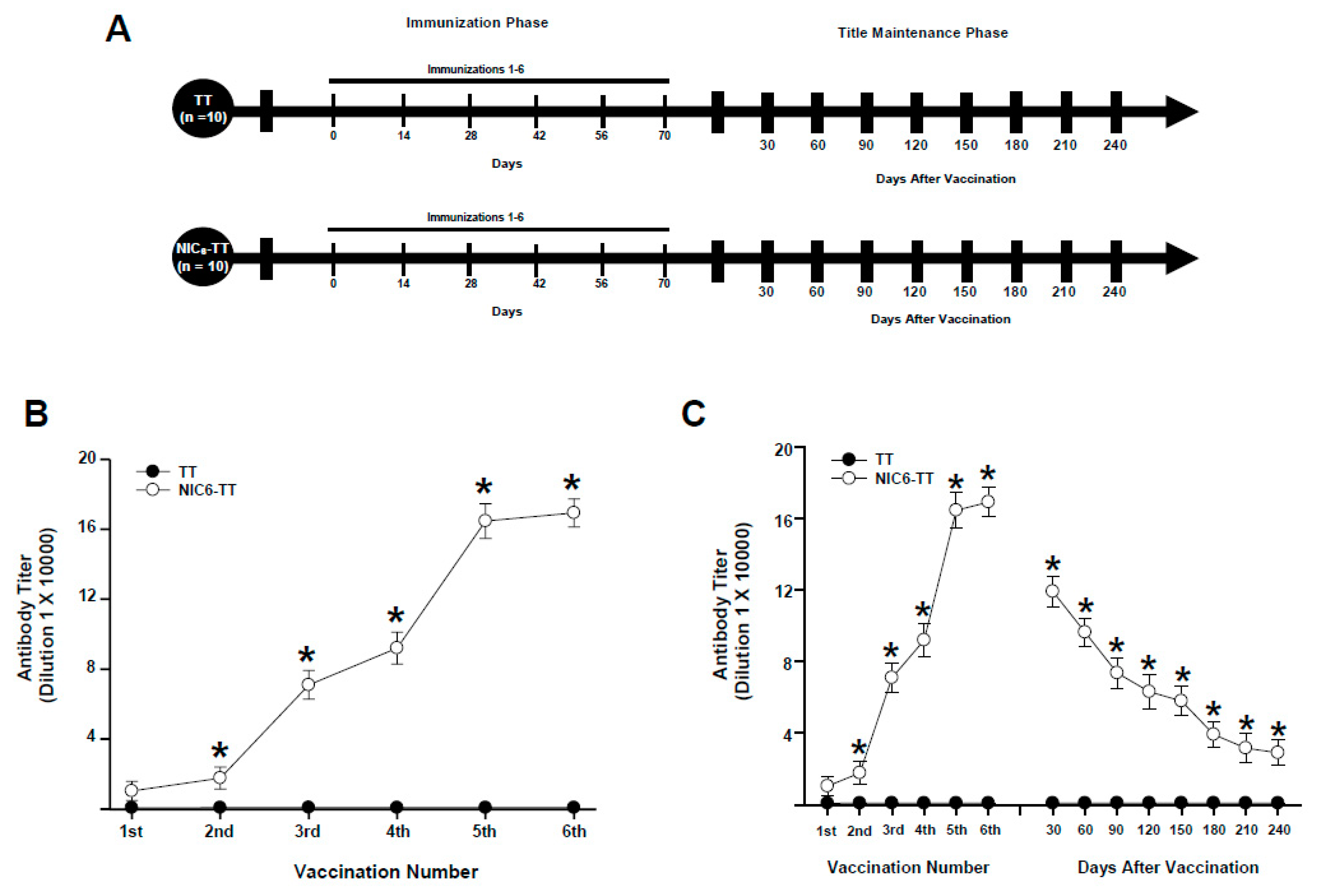
3.1. Experiment 1
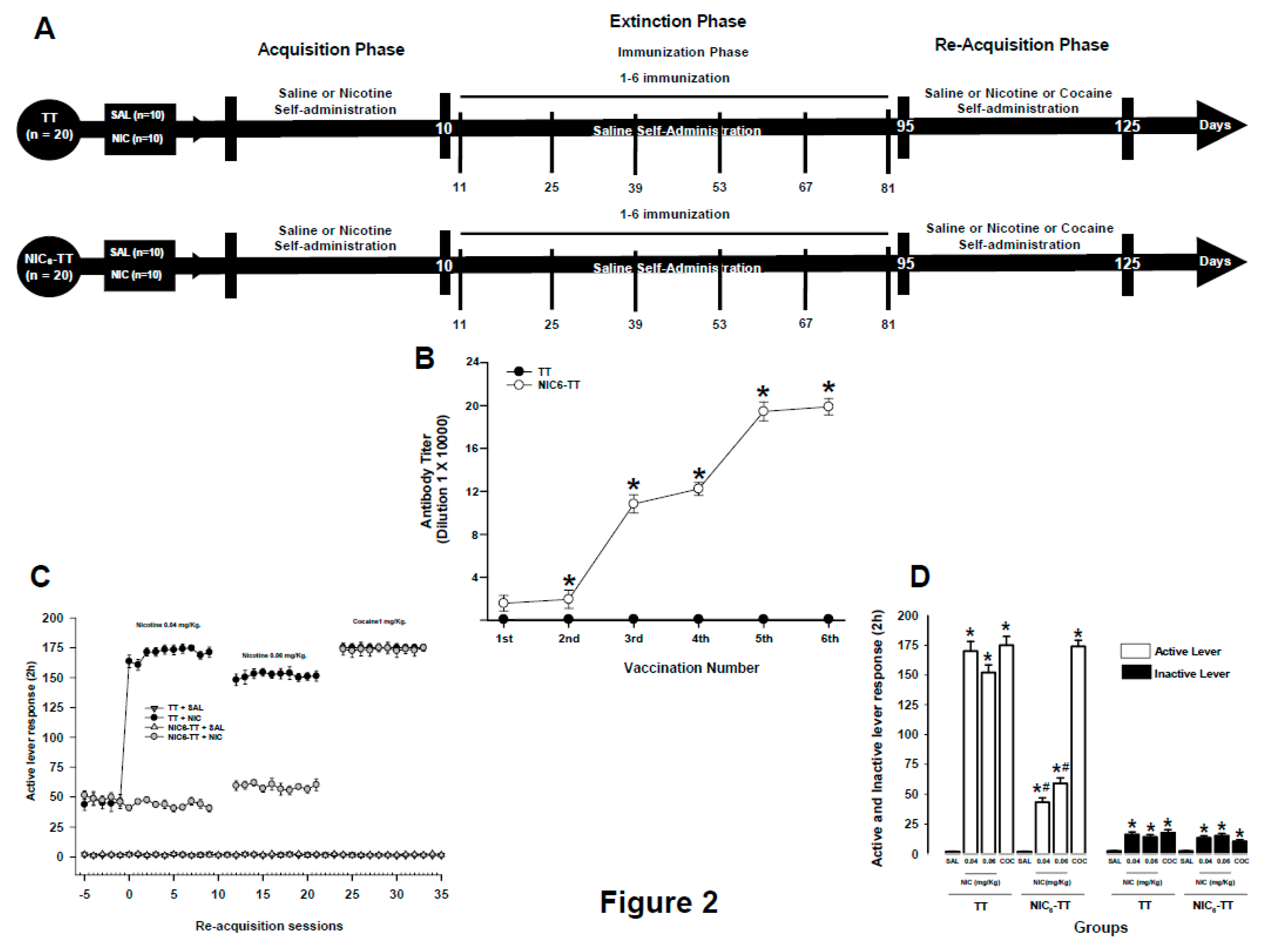
3.2. Experiment 2
Titters
Nicotine self-administration
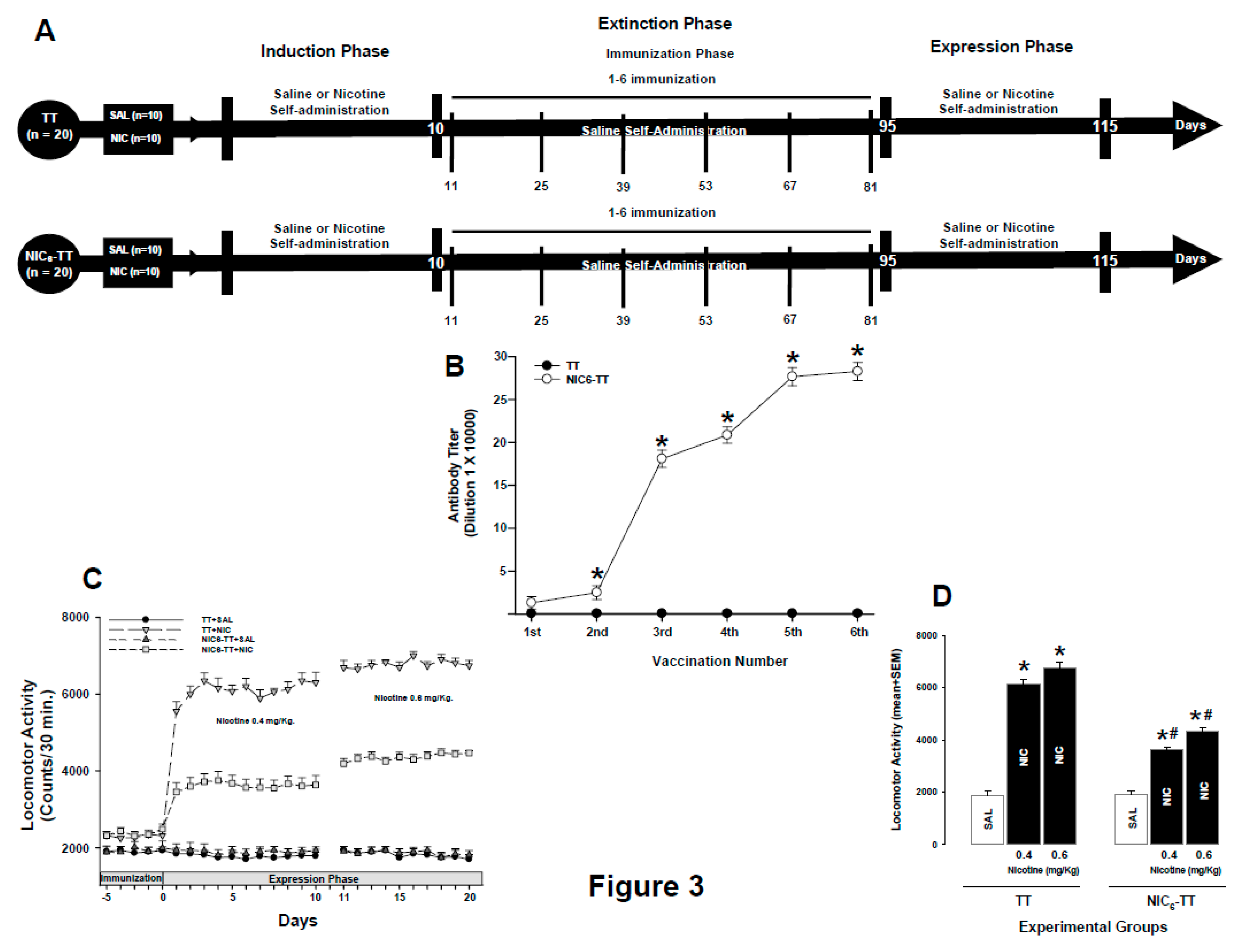
3.3. Experiment 3
Titters
Nicotine Locomotor Activity
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shirazi, A.; Radgoudarzi, N.; Brody, A.L. Adolescent Tobacco/Nicotine Use and the Potential Role of Contingency Management-based Interventions. J Addict Med. 2024, 18, 97-102. [CrossRef] [PubMed]
- Shevorykin, A.; Hyland, B.M.; Robles, D.; Ji, M.; Vantucci, D.; Bensch, L.; Thorner, H.; Marion, M.; Liskiewicz, A.; Carl, E.; Ostroff, J.S.; Sheffer, C.E. Tobacco use; trauma exposure and PTSD: a systematic review. Health Psychol Rev. 2024, 6, 1-32. [CrossRef] [PubMed]
- D'Souza, M.S.; Markou, A. Neuronal mechanisms underlying development of nicotine dependence: implications for novel smoking-cessation treatments. Addict Sci Clin Pract. 2011, 6, 4-16.
- Keyler, D.E.; Hieda, Y.; St Peter, J.; Pentel, P.R. Altered disposition of repeated nicotine doses in rats immunized against nicotine. Nicotine Tob Res. 1999 1,241-9. [CrossRef]
- Hieda, Y.; Keyler, D.E.; Vandevoort, J.T.; Kane, J.K.; Ross, C.A.; Raphael, D.E.; Niedbalas, R.S.; Pentel, P.R. Active immunization alters the plasma nicotine concentration in rats. J Pharmacol Exp Ther. 1997 283, 1076-81.
- Pentel, P.R.; Malin, D.H.; Ennifar, S.; Hieda, Y.; Keyler, D.E.; Lake, J.R.; Milstein, J.R.; Basham, L.E.; Coy, R.T.; Moon, J.W.; Naso, R.; Fattom, A. A nicotine conjugate vaccine reduces nicotine distribution to brain and attenuates its behavioral and cardiovascular effects in rats. Pharmacol Biochem Behav. 2000 65, 191-8. [CrossRef]
- Tuncok, Y.; Hieda, Y.; Keyler, D.E.; Brown, S.; Ennifar, S.; Fattom, A.; Pentel, P.R. Inhibition of nicotine-induced seizures in rats by combining vaccination against nicotine with chronic nicotine infusion. Exp. Clin Psychopharmacol. 2001 9, 228-34.
- de Villiers, S.H.; Lindblom, N.; Kalayanov, G.; Gordon, S.; Malmerfelt, A.; Johansson, A.M.; Svensson, T.H. Active immunization against nicotine suppresses nicotine-induced dopamine release in the rat nucleus accumbens shell. Respiration. 2002, 69, 247-53. [CrossRef] [PubMed]
- Lindblom, N.; de Villiers, S.H.; Kalayanov, G.; Gordon, S.; Johansson, A.M.; Svensson, T.H. Active immunization against nicotine prevents reinstatement of nicotine-seeking behavior in rats. Respiration. 2002, 69, 254-60. [CrossRef] [PubMed]
- LeSage, M.G.; Keyler, D.E.; Hieda, Y.; Collins, G.; Burroughs, D.; Le, C.; Pentel, P.R. Effects of a nicotine conjugate vaccine on the acquisition and maintenance of nicotine self-administration in rats. Psychopharmacology (Berl). 2006, 184, 409-16. [CrossRef] [PubMed]
- Moreno, A.Y.; Azar, M.R.; Warren, N.A.; Dickerson, T.J.; Koob, G.F.; Janda, K.D. A critical evaluation of a nicotine vaccine within a self-administration behavioral model. Mol Pharm. 2010, 7, 431-41. [CrossRef] [PubMed]
- Carrera, M.R.; Ashley, J.A.; Hoffman, T.Z.; Isomura, S.; Wirsching, P.; Koob, G.F.; Janda, K.D. Investigations using immunization to attenuate the psychoactive effects of nicotine. Bioorg Med Chem. 2004, 12, 563-70. [CrossRef] [PubMed]
- Cornish, K.E.; Harris, A.C.; LeSage, M.G.; Keyler, D.E.; Burroughs, D.; Earley, C.; Pentel, P.R. Combined active and passive immunization against nicotine: minimizing monoclonal antibody requirements using a target antibody concentration strategy. Int Immunopharmacol. 2011, 11, 1809-15. [CrossRef] [PubMed]
- Heading, C.E. NicVAX (Nabi Biopharmaceuticals). IDrugs. 2003, 6, 1178-81. [PubMed]
- Heading, C.E. Drug evaluation: CYT-002-NicQb; a therapeutic vaccine for the treatment of nicotine addiction. Curr Opin Investig Drugs. 2007, 8, 71-7.
- Trial watch: Xenova's TA-NIC vaccine shows promise. Expert Rev Vaccines. 2004, 386.
- Maurer, P.; Jennings, G.T.; Willers, J.; Rohner, F.; Lindman, Y.; Roubicek, K.; Renner, W.A.; Müller, P.; Bachmann, M.F. A therapeutic vaccine for nicotine dependence: preclinical efficacy; and Phase I safety and immunogenicity. Eur J Immunol. 2005, 35, 2031-40. [CrossRef] [PubMed]
- Hatsukami, D.K.; Rennard, S.; Jorenby, D.; Fiore, M.; Koopmeiners, J.; de Vos, A.; Horwith, G.; Pentel, P.R. Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clin Pharmacol Ther. 2005, 78, 456-67. [CrossRef]
- Wagena, E.J.; de Vos, A.; Horwith, G.; van Schayck, C.P. The immunogenicity and safety of a nicotine vaccine in smokers and nonsmokers: results of a randomized; placebo-controlled phase 1/2 trial. Nicotine Tob Res. 2008, 10, 213-8. [CrossRef]
- Cornuz, J.; Zwahlen, S.; Jungi, W.F.; Osterwalder, J.; Klingler, K.; van Melle, G.; Bangala, Y.; Guessous, I.; Müller, P.; Willers, J.; Maurer, P.; Bachmann, M.F.; Cerny, T. A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS One. 2008, 3, e2547. [CrossRef] [PubMed]
- Hatsukami, D.K.; Jorenby, D.E.; Gonzales, D.; Rigotti, N.A.; Glover, E.D.; Oncken, C.A.; Tashkin, D.P.; Reus, V.I.; Akhavain, R.C.; Fahim, R.E.; Kessler, P.D.; Niknian, M.; Kalnik, M.W.; Rennard, S.I. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Ther. 2011, 89, 392-9. [CrossRef]
- Hoogsteder, P.H.; Kotz, D.; van Spiegel, P.I.; Viechtbauer, W.; Brauer, R.; Kessler, P.D.; Kalnik, M.W.; Fahim, R.E.; van Schayck, O.C. The efficacy and safety of a nicotine conjugate vaccine (NicVAX®) or placebo co-administered with varenicline (Champix®) for smoking cessation: study protocol of a phase IIb; double blind; randomized; placebo-controlled trial. BMC Public Health. 2012, 12, 1052. [CrossRef] [PubMed]
- Bennett, L. Vaccines in trials for nicotine cessation therapy. JAAPA. 2013, 26,55-6. [CrossRef]
- Havermans, A.; Vuurman, E.F.; van den Hurk, J.; Hoogsteder, P.; van Schayck, O.C. Treatment with a nicotine vaccine does not lead to changes in brain activity during smoking cue exposure or a working memory task. Addiction. 2014, 109, 1260-7. [CrossRef] [PubMed]
- Hoogsteder, P.H.; Kotz, D.; van Spiegel, P.I.; Viechtbauer, W.; van Schayck, O.C. Efficacy of the nicotine vaccine 3'-AmNic-rEPA (NicVAX) co-administered with varenicline and counselling for smoking cessation: a randomized placebo-controlled trial. Addiction. 2014, 109, 1252-9. [CrossRef] [PubMed]
- Wolters, A.; de Wert, G.; van Schayck, O.; Horstman, K. Constructing a trial as a personal lifestyle change project: participants' experiences in a clinical study for nicotine vaccination. Soc Sci Med. 2014, 104, 116-23. [CrossRef]
- Tonstad, S.; Heggen, E.; Giljam, H.; Lagerbäck, P.Å.; Tønnesen, P.; Wikingsson, L.D.; Lindblom, N.; de Villiers, S.; Svensson, T.H.; Fagerström, K.O. Niccine®; a nicotine vaccine; for relapse prevention: a phase II; randomized; placebo-controlled; multicenter clinical trial. Nicotine Tob Res. 2013, 15, 1492-501. [CrossRef] [PubMed]
- Esterlis, I.; Hannestad, J.O.; Perkins, E.; Bois, F.; D'Souza, D.C.; Tyndale, R.F.; Seibyl, J.P.; Hatsukami, D.M.; Cosgrove, K.P.; O'Malley, S.S. Effect of a nicotine vaccine on nicotine binding to β2*-nicotinic acetylcholine receptors in vivo in human tobacco smokers. Am. J Psychiatry. 2013, 170, 399-407. [CrossRef] [PubMed]
- De, B.P.; Pagovich, O.E.; Hicks, M.J.; Rosenberg, J.B.; Moreno, A.Y.; Janda, K.D.; Koob, G.F.; Worgall, S.; Kaminsky, S.M.; Sondhi, D.; Crystal, R.G. Disrupted adenovirus-based vaccines against small addictive molecules circumvent anti-adenovirus immunity. Hum Gene Ther. 2013, 24, 58-66. [CrossRef]
- Hu, Y.; Zheng, H.; Huang, W.; Zhang, C. A novel and efficient nicotine vaccine using nano-lipoplex as a delivery vehicle. Hum Vaccin Immunother. 2014, 10,64-72. [CrossRef]
- Meijler, M.M.; Matsushita, M.; Altobell, L.J. 3rd.; Wirsching, P.; Janda, K.D. A new strategy for improved nicotine vaccines using conformationally constrained haptens. J Am Chem Soc. 2003, 125, 7164-5. [CrossRef]
- Zeigler, D.F.; Roque, R.; Clegg, C.H. Construction of an enantiopure bivalent nicotine vaccine using synthetic peptides. PLoS One. 2017, 12, e0178835. [CrossRef] [PubMed]
- Rosenberg, J.B.; De, B.P.; Hicks, M.J.; Janda, K.D.; Kaminsky, S.M.; Worgall, S.; Crystal, R.G. Suppression of nicotine-induced pathophysiology by an adenovirus hexon-based antinicotine vaccine. Hum Gene Ther. 2013, 24, 595-603. [CrossRef] [PubMed]
- Anton, B.; Leff, P. A novel bivalent morphine/heroin vaccine that prevents relapse to heroin addiction in rodents. Vaccine. 2006, 24, 3232–40. [CrossRef]
- Anton, B.; Salazar, A.; Flores, A.; Matus, M.; Marin, R.; Hernandez, J.A.; Leff, P. Vaccines against morphine/heroin and its use as effective medication for preventing relapse to opiate addictive behaviors. Hum Vaccine. 2009, 5, 214–29. [CrossRef]
- Barbosa-Méndez, S.; Matus-Ortega, M.; Hernandez-Miramontes, R.; Salazar-Juárez, A. Effect of the morphine/heroin vaccine on opioid and non-opioid drug-induced antinociception in mice. Eur J Pharmacol. 2021a, 891, 173718. [CrossRef] [PubMed]
- Barbosa-Méndez, S.; Matus-Ortega, M.; Hernandez-Miramontes, R.; Salazar-Juárez, A. The morphine/heroin vaccine decreased the heroin-induced antinociceptive and reinforcing effects in three inbred strains mouse. Int Immunopharmacol. 2021b, 98, 107887. [CrossRef] [PubMed]
- Barbosa-Méndez, S.; Matus-Ortega, M.; Hernandez-Miramontes, R.; Salazar-Juárez, A. The M3-TT vaccine decreases the antinociceptive effects of morphine and heroin in mice. Int J Ment Health & Addict. 2021c, 21, 783–802.
- Barbosa-Méndez, S.; Matus-Ortega, M.; Hernandez-Miramontes, R.; Salazar-Juárez, A. Synergistic immune and antinociceptive effects induced from the combination of two different vaccines against morphine/heroin in mouse. Hum Vaccin Immunother. 2021d, 17, 3515–3528. [CrossRef] [PubMed]
- Barbosa-Méndez, S.; Matus-Ortega, M.; Hernandez-Miramontes, R.; Salazar-Juárez, A. The effect of chronic stress on the immunogenicity and immunoprotection of the M6-TT vaccine in female mice. Physiol & Behav. 2023, 271, 114345.
- Barbosa-Méndez, S.; Matus-Ortega, M.; Hernandez-Miramontes, R.; Salazar-Juárez, A. COT-TT vaccine attenuates cocaine-seeking and cocaine-conditioned place preference in rats. Hum Vaccin Immunother. 2024, 20, 2299068. [CrossRef] [PubMed]
- Pravetoni, M.; Le Naour, M.; Harmon, T.M.; Tucker, A.M.; Portoghese, P.S.; Pentel, P.R. An oxycodone conjugate vaccine elicits drug-specific antibodies that reduce oxycodone distribution to brain and hot-plate analgesia. J Pharmacol Exp Ther. 2012, 341, 225-32. [CrossRef] [PubMed]
- Barbosa-Méndez, S.; Salazar-Juárez, A. Mirtazapine attenuates nicotine-seeking behavior in rats. J Psychopharmacol. 2018, 32, 1010-1017. [CrossRef] [PubMed]
- Salazar-Juárez, A.; Barbosa-Méndez, S.; Jurado, N.; Hernández-Miramontes, R.; Leff, P.; Antón, B. Mirtazapine prevents induction and expression of cocaine-induced behavioral sensitization in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2016, 68,15-24. [CrossRef]
- Barbosa-Méndez, S.; Jurado, N.; Matus-Ortega, M.; Martiñon, S.; Heinze, G.; Salazar-Juárez, A. Mirtazapine attenuates the expression of nicotine-induced locomotor sensitization in rats. Eur J Pharmacol. 2017, 812, 28-37. [CrossRef] [PubMed]
- de Villiers, S.H.L.; Lindblom, N.; Kalayanov, G.; Gordon, S.; Baraznenok, I.; Malmerfelt, A.; Marcus, M.M.; Johansson, A.M.; Svensson, T.H. Nicotine hapten structure; antibody selectivity and effect relationships: results from a nicotine vaccine screening procedure. Vaccine. 2010, 28, 2161-2168. [CrossRef]
- Chen, X.; Pravetoni, M.; Bhayana, B.; Pentel, P.R.; Wu, M.X. High immunogenicity of nicotine vaccines obtained by intradermal delivery with safe adjuvants. Vaccine. 2012, 31, 159-64. [CrossRef] [PubMed]
- Miller, K.D.; Roque, R.; Clegg, C.H. Novel Anti-Nicotine Vaccine Using a Trimeric Coiled-Coil Hapten Carrier. PLoS One. 2014, 9, e114366. [CrossRef] [PubMed]
- Hoogsteder, P.; van Merrebach, M.; Otters, M.; van Schayck, O.; Kotz, D. Exploring Smokers' Knowledge and Expectations Toward Nicotine Vaccination: A Qualitative Study. Subst Use Misuse. 2014, 49, 87-94. [CrossRef]
- Bayart, C.; Mularoni, A.; Hemmani, N.; Kerachni, S.; Jose, J.; Gouet, P.; Paladino, J.; Le Borgne, M. Tetanus toxin fragment C: structure; drug discovery research and production. Pharm (Basel). 2022, 15, 756. [CrossRef]
- Arora, R.; Haile, C.N.; Kosten, T.A.; Wu, Y.; Ramakrishnan, M.; Hawkins, L.D.; Orson, F.M.; Kosten, T.R. Preclinical efficacy of an anti-methamphetamine vaccine using E6020 adjuvant. Am. J Addict. 2019, 28, 119–126. [CrossRef] [PubMed]
- Barrientos, R.C.; Whalen, C.; Torres, O.B.; Sulima, A.; Bow, E.W.; Komla, E.; Beck, Z.; Jacobson, A.E.; Rice, K.C.; Matyas, G.R. Bivalent conjugate vaccine induces dual immunogenic response that attenuates heroin and fentanyl effects in mice. Bioconjug Chem. 2021, 32 ,2295–2306. [CrossRef] [PubMed]
- Zhao, Z.; Hu, Y.; Harmon, T.; Pentel, P.R.; Ehrich, M.; Zhang, C. Hybrid nanoparticle-based nicotine nanovaccines: Boosting the immunological efficacy by conjugation of potent carrier proteins. Nanomedicine. 2018, 14, 1655-1665. [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




