1. Introduction
Deferred or delayed cord clamping (DCC) increases blood transfusion from the placenta to the newborn [
1,
2,
3] and improves hemodynamic stability and cardiovascular transition at birth. In preterm infants, DCC for at least 30-60 seconds has been shown to reduce mortality, risks of transfusion, inotropes for low blood pressure (BP), intraventricular hemorrhage (IVH), and improved hematocrit and BP after birth [
4,
5,
6,
7]. National organizations including the American College of Obstetricians and Gynecologists and American Academy of Pediatrics have recommended at least 30-60 seconds of DCC for preterm infants. International organizations including World Health Organization [
8], European Resuscitation Council [
9], Neonatal Resuscitation group of Spanish Society of Neonatology [
10], Canadian Fetus and Newborn Committee [
11] have recommended at least 60 seconds of DCC for preterm infants.
Early studies have shown that increasing the time of cord clamping from birth leads to incremental increases in the blood volume transfer from the placenta to the newborn. In term infants, the percentage of feto-placental blood volume in the infant is 67% at birth, which increases to 80% and 87% when the cord is clamped at 1 and 3 minutes after birth [
1]. Saigal et al have shown that with 5 minutes of DCC there is up to 50% increase in blood volume from birth in both term and preterm infants [
3]. However, the rate of placental transfusion with DCC is slower in preterm infants than in term infants; within the 1st minute of DCC, 75% of the placental transfusion occurs in term infants, whereas only 50% in preterm infants. This suggests that preterm infants may need a longer duration of DCC to maximize the benefits of placental transfusion. Randomized controlled trials (RCT) have shown improved preterm neonatal outcomes with up to 180 seconds of DCC. A recent network meta-analysis compared short (14-45 seconds), medium (45-120 seconds) and long (≥120 seconds) DCC in preterm infants and showed that long DCC duration (>/= 120s) has the highest reduction in mortality by 69% [
7] .
Our center implemented 30 seconds of DCC as the standard of care for preterm infants in 2007 and increased DCC duration to at least 60 seconds in 2011. We evaluated the neonatal outcomes in preterm infants who received 30-45 seconds vs. 60-75 seconds DCC and demonstrated that longer duration was associated with a reduction in hypothermia, transfusions, and intubation [
12]. In 2016, we increased the duration of DCC to at least 120 seconds to further increase placental transfusion. This study’s objective was to evaluate the effect of the longer duration of DCC on neonatal outcomes in very preterm infants born <33 weeks of gestational age (GA).
2. Methods
2.1. Study Design and Subjects
This is a single-center retrospective, observational study and data collection was approved by the institutional review board (IRB, IRB #11-033ER) as a quality improvement project.
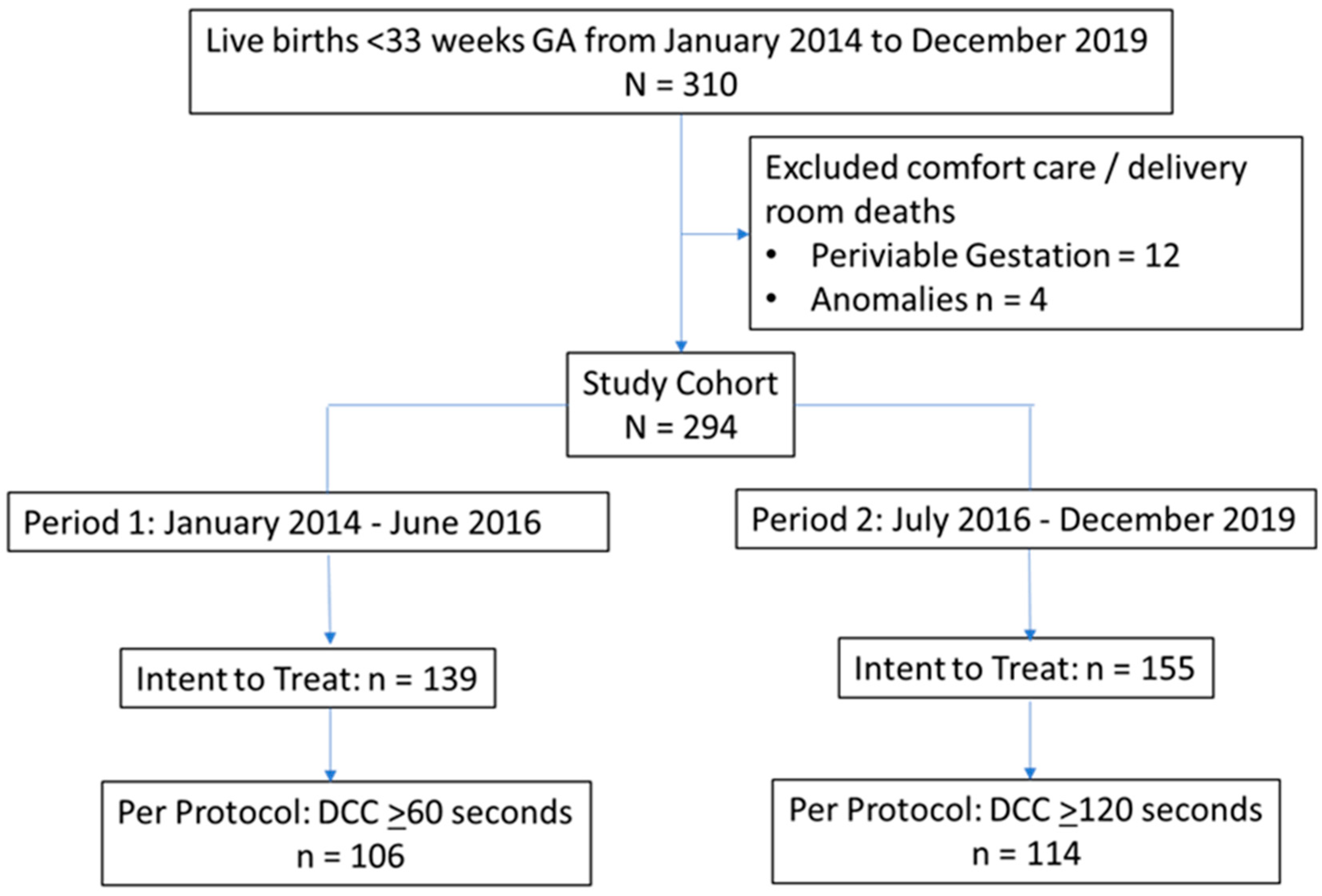
This study was performed at an AAP Level IV NICU in a California public safety-net hospital from January 2014 to December 2019. The intended duration of DCC at birth was ≥60 seconds from January 2014 to June 2016 (Period 1) and ≥120 seconds from July 2016 to December 2019 (Period 2). This study included preterm infants born at <33 weeks GA (
Figure 1). Infants with no intent to resuscitate or those who died in the delivery room were excluded.
2.2. Standardized Delivery Room Management Bundle for Very Preterm Infants
Our delivery room (DR) management bundle for very preterm infants includes pre-delivery huddles and role assignments with one provider designated to care for the baby during DCC in both vaginal and cesarean deliveries (CD). The details of this bundle have previously been published [
13]. Our center performed at least 60 seconds DCC as part of this standardized DR management bundle for all preterm infants since March 2011 [
12]. The intended DCC duration was incrementally increased to at least 120 seconds in July 2016 and 180 seconds in January 2018 for those infants who were breathing by 1 minute of life.
During DCC, the infant was placed on a portable warming mattress and gently stimulated by tactile stimulation. Oral and nasal airways were suctioned using a bulb syringe as needed. If the infant was breathing spontaneously or in response to stimulation and suctioning by 60 seconds, then DCC was continued to the intended duration. DCC was discontinued at any time if there were concerns for placental abruption with active bleeding per the assessment of the obstetrician at the delivery, placenta detaching, cord avulsion/tear or tight knot disrupting of placenta-infant circulation, if the infant appeared lifeless without tone or heart rate, or if the infant was persistently apneic despite stimulation and bulb suctioning. In 2016, cord milking was standardized when the intended duration of DCC was not performed. Cord milking was performed by the obstetric team when feasible. In June 2019, cord milking was excluded in extremely preterm infants <29 weeks GA based on the early evidence from a RCT that showed cord milking increased the risk of severe intraventricular hemorrhage (IVH) in these extremely preterm infants [
14].
After DCC was completed, all preterm infants started with continuous positive airway pressure (CPAP) in the DR as per standard of care. The FiO2 at the initiation of CPAP of 5 cmH2O was 30% for infants <29 weeks GA and 21% for infants born between 29-32 weeks GA. APGAR score was assigned by the NICU provider while the infant was still connected to the cord at 1 minute of life. We have a comprehensive standardized care bundle for very preterm infants in the NICU that includes enteral and parenteral nutrition guidelines such as exclusive human milk feeding until after 34 weeks post menstrual age (PMA), early colostrum oral care, indications for insertion and removal of central line, Intubation criteria and respiratory care with CPAP, non-invasive or invasive mechanical ventilation, caffeine, Vitamin A prophylaxis for prevention of chronic lung disease (CLD), IVH prevention bundle, high humidity protocol to maintain euthermia for the extremely preterm infants, criteria for blood transfusion, use of erythropoietin (EPO) for extremely preterm infants, phototherapy guidelines for hyperbilirubinemia, and conservative approach for evaluation and treatment of patent ductus arteriosus. The anemia prevention bundle was expanded to include routine use of EPO for infants >28 weeks PMA who were anemic in June 2016, along with at least 120 seconds of DCC to reduce packed red blood cell (RBC) transfusion risk. In April 2017, less invasive surfactant administration was implemented for those infants who were not intubated and needed surfactant.
2.3. Data Collection
Demographics, DR measures and interventions and outcomes during NICU stay were obtained from the NICU database. Hematocrit values were obtained retrospectively from medical records. Duration of DCC was documented in seconds throughout the study period. Cord milking and breathing before cord clamping were standardized as part of our documentation in 2016 and were obtained only for Period 2.
Demographic variables included GA, birthweight (BW), CD, antenatal steroids (ANS), and maternal magnesium; DR measures included completion of the intended duration of DCC (at least 60 seconds during Period 1 and at least 120 seconds during Period 2), DCC duration in seconds, chest compressions, and intubation; NICU interventions included early (within 72 hours of life) RBC transfusion (within 72 hours of life), any RBC transfusion during NICU stay, hematocrit values at 0-2 and 12-24 hours of life, and EPO use; and neonatal outcomes included severe IVH (grade 3 or 4), late onset sepsis (LOS, positive blood or cerebral spinal fluid culture at >72 hours of life), CLD (requiring oxygen/respiratory support at 36 weeks PMA), severe retinopathy of prematurity (ROP ≥stage 3, or plus disease, or received anti-VEGF/ laser treatment), necrotizing enterocolitis (NEC, Bell stage ≥20, survival without major morbidity (severe IVH, NEC, LOS, CLD, severe ROP), and death.
2.4. Analysis
Demographics, DR and NICU measures and interventions, and neonatal outcomes were compared between infants born during Periods 1 and 2 with intent to treat (ITT) analysis. Per protocol (PP) analysis was performed comparing infants who received at least 60 seconds of DCC from January 2014 to June 2016 and those who received at least 120 seconds of DCC from July 2016 to December 2019. Both ITT and PP analysis were performed to compare the 2 groups using generalized estimating equations. STATA 14.0 (Statacorp, Texas) was used for statistical analysis. A p-value <0.05 was considered significant. Risk adjusted analysis, adjusting for GA, was performed for outcomes that were different between the two periods.
3. Results
This study included 294 preterm infants born <33 weeks of gestation (Period 1: n=139, Period 2: n=155). There were no differences in baseline demographics between the two periods (
Table 1). The intended duration of DCC was successfully achieved in 220 infants (Period 1: n=106, Period 2: n=114) (
Figure 1).
3.1. Deferred Cord Clamping Implementation
Overall, 75% of infants received the intended duration of DCC; there was no difference in the percentages of infants who received the intended duration of DCC between Period 1 and 2 (
Table 2). Distribution of DCC duration is shown in
Table 2. The median duration was 60 seconds in Period 1 compared to 129 seconds in Period 2. While there was no difference in the percentage of infants who completed the intended duration of DCC, there were more infants who had DCC discontinued at <60 seconds in Period 1 compared to Period 2 (24% vs. 14%, p=0.04). The reasons for discontinuing DCC are shown in
Table 2. There were no differences in the reasons for discontinuing DCC between the two periods. In Period 2, 93% of infants were breathing before cord clamping.
3.2. Delivery Room and NICU Measures and Interventions
The DR and NICU measures and interventions are shown in
Table 3A. There were fewer infants with 1 minute APGAR <4 in Period 2 compared to Period 1, although there were no differences in the 5-minute APGAR scores. In the ITT and PP analysis, there were no differences in hypothermia, chest compressions, DR intubation or any intubation, hematocrit values; early or any PRBC transfusions between Period 1 and Period 2. There was an increase in the use of EPO in Period 2 compared to Period 1 in both the ITT (45% vs. 11%; p<0.001) and PP (43% vs. 9%; p<0.001) analysis.
3.3. Neonatal Outcomes
Neonatal mortality and morbidities are shown in
Table 3B. In both ITT and PP analysis, there were no differences in severe IVH, LOS, CLD, severe ROP, NEC, or death between Period 1 and Period 2. Survival without major morbidities increased from 76% in Period 1 to 88% in Period 2 (p=0.032) in PP analysis. This increase remained significant after adjusting for GA and EPO use (Odds ratio 8.6, 95% CI 1.6 to 45.7) (
Table 4).
4. Discussion
Our single-center observational cohort study showed the feasibility of extending the duration of DCC to at least 120 seconds in 75% of the very preterm infants born at <33 weeks GA. Extending the DCC duration to at least 120 seconds is associated with increased survival without major morbidities in these infants.
4.1. Outcomes with Longer Duration of Cord Clamping
The benefits of deferred cord clamping in very preterm infants have been shown in RCTs. However, the optimal duration of DCC has not yet been agreed upon. Cumulative evidence indicates that longer duration of DCC may provide more benefits. Early placental transfusion studies showed an incremental increase in newborn blood volume with delaying cord clamping up to 5 minutes [
2,
3]. DCC duration was extended to 2-3 minutes in multiple RCTs, showing an increase in hematocrit levels with no associated adverse effects in moderate preterm infants between 30 and 36 weeks GA [
15,
16,
17,
18]. A network meta-analysis compared different durations of DCC and showed that the long DCC ≥120s reduced mortality by 69% [
7]. This meta-analysis included 5 RCTs, 2 studies [
16,
17] excluded infants who needed resuscitation, and 3 studies [
19,
20,
21] had a trolley to provide positive pressure ventilation (PPV) if infant needed resuscitation during DCC. A recent RCT, VentFirst trial [
22], evaluated the effect of ventilation during DCC on the outcomes of extremely preterm infants. The infants were randomized to 60 seconds DCC with tactile stimulation or 120 seconds DCC with CPAP or PPV as needed. This study demonstrated that in the subgroup of infants who were not breathing well, the 120 seconds DCC with ventilatory support group had better APGAR scores at 5 minutes, lower DR intubation, and higher hematocrit values in the first 24 hours of life.
In our center, we increased the duration of DCC in a stepwise fashion over the last decade to optimize the outcome in preterm infants. We initiated 30-45 seconds of DCC in late 2007 and then increased it to 60-75 seconds when neonatal resuscitation program recommendations included 60 seconds of DCC in 2011. Previously, we have shown that at least 60 seconds of DCC compared to at least 30 seconds of DCC reduced the risk of admission hypothermia, intubations, and RBC transfusions [
12]. In 2016, to optimize the transition of preterm infants and placental transfusion, we deferred clamping of the cord to 120 seconds if the infant was breathing by 60 seconds. If the infant was not breathing by 60 seconds despite warmth, drying, stimulating and suctioning, then we clamped the cord as we did not have a system to provide PPV during DCC.
4.2. Feasibility
The duration of DCC in preterm RCTs has varied from 30 to 180 seconds. In our study, it was feasible to implement 60 to 120 seconds of DCC in 75% of the infants successfully without an increase in adverse effects in the delivery room including hypothermia, chest compressions, and DR intubation. This is similar to the large Australian Placental Transfusion study [
23], including <30 weeks GA preterm infants in which 74% of their cohort randomized to the DCC group received at least 60 seconds DCC. Two studies including more mature infants have shown that more than 85% of their infants received ≥120s DCC after excluding infants who required resuscitation, 86% in <34 weeks GA by Rana et al and 88% in 30-36 weeks GA by Ranjit et al. [
16,
17]. A recent individual patient data (IPD) network meta-analysis of 47 trials with 6094 preterm infants comparing the different durations of cord clamping (15-45 seconds short DCC, 45-120 seconds medium DCC, and ≥120 seconds long DCC) showed that the adherence to short and medium DCC intervention was 80% and long DCC was only 67%. There were two RCTs that included preterm infants <32 weeks GA receiving ≥120 seconds DCC and <30 weeks GA receiving ≥180 seconds DCC while providing immediate positive pressure support with intact cord in their intervention group and showing long DCC was successfully performed in 60% and 75% of the infants respectively [
19,
21]. It is interesting that only 60-75% of infants to received ≥120 seconds of DCC even with respiratory support before cord clamping. In our study, we show that in a non-clinical trial setting, with standardized guidelines on continuing DCC beyond 60 seconds in an infant with respirations, we were able to successfully perform at least 120 seconds of DCC in 75% of all preterm births <33weeks GA. The Vent First study[
22] has shown that only the infants who were not breathing well benefitted from providing ventilatory support during 120s DCC. Having a system to provide ventilatory support with intact cord for very preterm infants not breathing with tactile stimulation in the first 60 seconds will equip centers with the ability to provide ≥120s DCC in >85% of infants. Once there is an opportunity to provide respiratory support with intact cords for those infants who are not breathing well, maternal bleeding concerns will be the only limitation for a longer duration of DCC.
4.3. Safety
In our study, there was no difference in the DR measures of 5-minute APGAR, hypothermia, DR intubation, or chest compressions between the 2 periods. We developed a standard protocol with clearly defined indications for when to discontinue DCC. When there is a concern for maternal bleeding, the obstetricians can decide to clamp the cord at any time. If the infant is not breathing by 60 seconds with warmth, drying, and tactile stimulation, and suctioning, the pediatric providers can decide to stop DCC at any time. Our experience shows that prolonging the duration of DCC to at least 120 seconds did not have an adverse effect on the DR measures in our study.
4.4. Transfusion Benefit
Improved placental transfusion is one of the potential benefits of a longer duration of DCC. We have previously shown that 60-75 seconds of DCC is associated with an increase in hematocrit values and decreases in early (within 72 hours of life) or any RBC transfusion during NICU stay when compared to 30-45 seconds of DCC [
12]. In this study, we compared the 60-seconds DCC cohort to 120-second DCC cohort and showed that there is no difference in hematocrit values, although there is a suggestion of reduction in RBC transfusions. Previous RCTs and meta-analysis have shown that DCC 30 to 180 seconds compared to early cord clamping (ECC) is associated with higher hematocrit values and lower risk of RBC transfusion [
4,
5,
6,
15,
16,
17,
18,
24,
25]. However, the RCTs have not directly compared RBC transfusion between 60 and 120 seconds DCC. The recent IPD network meta-analysis comparing short, medium, and long DCC showed that short and medium DCC compared to ECC decreased the risk of transfusions, although the evidence for long DCC was inconclusive due to insufficient events for comparison.
4.5. Neonatal Outcomes
RCTs and meta-analysis of RCTs in preterm infants have shown that there is a significant reduction in mortality in those who receive DCC compared to ECC [
4,
5,
6,
7,
23], and reduction in IVH [
4]. The duration of DCC in these RCTs ranged from 30 to 120 seconds and comparisons have been made between DCC and ECC groups. In the Australian placental transfusion study, there was no difference between the ECC group and 60 seconds of DCC group in the composite outcome of survival without major morbidities [
23]. A 2018 meta-analysis, including 27 trails and 2384 preterm infants, performed subgroup analysis based on the duration of DCC (≥30-45 seconds, ≥45-60 seconds, ≥60-120 seconds, and ≥120 seconds) and did not see any differences in any of the neonatal outcomes, IVH, CLD, NEC, LOS, or ROP [
5]. However, a more recent 2023 IPD network meta-analysis, including 47 trials and 6094 infants, compared short (14-45 seconds), medium (45-120 seconds), and long (≥120 seconds) DCC showed that long DCC ranked highest in reducing mortality, but showed no difference in IVH. We performed both ITT and PP analyses to compare the outcomes between the two study periods and observed an increase in survival without major morbidities that was significant in the PP analysis. While increasing DCC duration from 60 to 120 seconds did not make a difference in individual neonatal outcomes, the composite outcome of survival without major morbidities improved. We had an increase in the use of EPO in the 120 seconds DCC cohort as part of our bundle for eliminating transfusions. However, this improvement remained after adjusting for the use of EPO. This is consistent with the finding of the RCT that using EPO in extreme preterm infants did not make a difference in death and neonatal outcomes expect for an improvement in the transfusion rate [
26]. Our study is an observational cohort study and could be at risk for selection bias although the rate of DCC was similar between the two study periods at 74-76%.
DCC promotes optimal cardiorespiratory transition in all newborns especially in preterm infants who may need longer time for this transition to happen. This allows the infant to remain hemodynamically stable and less likely to have major morbidities. Even though the individual morbidities were not different between the 60 seconds and 120 seconds DCC group, it had a favorable effect on the composite outcome of survival without major morbidities. DCC and other perinatal interventions including ANS, antenatal magnesium for neuroprotection, and preventing hypothermia on admission improves preterm neonatal outcomes. A Canadian neonatal network study showed the cumulative effect of ANS, magnesium for neuroprotection, DCC and normothermia on preterm outcomes: any two or more of these interventions increased survival without severe neurological injuries [
27]. During the study period, we have a high antenatal steroid use (>95%), maternal magnesium use (70-77%), and no hypothermia. We observed an improvement in survival without major morbidities with increasing the duration of DCC with optimal other perinatal interventions.
4.6. Strengths and Limitations
DCC was implemented in a clinical setting with multiple obstetric and pediatric providers and trainees at a county hospital. We have clearly defined criteria for when to discontinue DCC with a collaborative approach between obstetricians and pediatricians in the delivery room. This makes it adaptable to clinical settings and generalizable to the patient population with optimal perinatal interventions including ANS use. Study limitations include the study being performed at a single center with small sample size and being observational, which may be subject to selection bias. Study findings need to be further validated in larger patient population across multiple centers.
5. Conclusions
This study shows that longer duration of DCC for at least 120 seconds is feasible in majority of the very preterm births who are breathing by 60 seconds of life, and this long duration is associated with an increase in survival without major morbidities. A standardized protocol and collaborative approach between obstetric and pediatric team is essential to safely implement longer DCC to optimize very preterm neonatal outcomes.
Authors Contributions
DS, BG, PJ contributed to the conception and design of the study, AH, EB, PJ contributed to the acquisition of the data, PJ and MN contributed to the analysis of the data, PJ, DS, SRN, and MN contributed to the interpretation of the data, EB and PJ prepared the original draft of the manuscript, and all authors contributed to the revision and finalization of the manuscript.
Acknowledgments
Gratitude is expressed to our patients and families and for the dedicated staff at Santa Clara Valley Medical Center neonatal intensive care unit and labor and delivery, and Santa Clara County First Five and Valley Medical Center Foundation. We are grateful to Dr. James Byrne’s leadership in implementing delayed cord clamping and for Claudia Flores, BA for bibliography support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yao, A.C.; Moinian, M.; Lind, J. Distribution of blood between infant and placenta after birth. Lancet 1969, 2, 871-873.
- Yao, A.C.; Lind, J.; Tiisala, R.; Michelsson, K. Placetal transfusion in the premature infant with observation on clinical course and outcome. Acta Paediatr Scand 1969, 58, 561-566. [CrossRef]
- Saigal, S.; O'Neill, A.; Surainder, Y.; Chua, L.B.; Usher, R. Placental transfusion and hyperbilirubinemia in the premature. Pediatrics 1972, 49, 406-419. [CrossRef]
- Rabe, H.; Gyte, G.M.; Díaz-Rossello, J.L.; Duley, L. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev 2019, 9, CD003248. [CrossRef]
- Fogarty, M.; Osborn, D.A.; Askie, L.; Seidler, A.L.; Hunter, K.; Lui, K.; Simes, J.; Tarnow-Mordi, W. Delayed vs early umbilical cord clamping for preterm infants: a systematic review and meta-analysis. Am J Obstet Gynecol 2018, 218, 1-18. [CrossRef]
- Seidler, A.L.; Aberoumand, M.; Hunter, K.E.; Barba, A.; Libesman, S.; Williams, J.G.; Shrestha, N.; Aagerup, J.; Sotiropoulos, J.X.; Montgomery, A.A.; et al. Deferred cord clamping, cord milking, and immediate cord clamping at preterm birth: a systematic review and individual participant data meta-analysis. Lancet 2023, 402, 2209-2222. [CrossRef]
- Seidler, A.L.; Libesman, S.; Hunter, K.E.; Barba, A.; Aberoumand, M.; Williams, J.G.; Shrestha, N.; Aagerup, J.; Sotiropoulos, J.X.; Montgomery, A.A.; et al. Short, medium, and long deferral of umbilical cord clamping compared with umbilical cord milking and immediate clamping at preterm birth: a systematic review and network meta-analysis with individual participant data. Lancet 2023, 402, 2223-2234. [CrossRef]
- Guideline: Delayed Umbilical Cord Clamping for Improved Maternal and Infant Health and Nutrition Outcomes. Available online: https://www.ncbi.nlm.nih.gov/pubmed/26269880 (accessed on 11.29.2024).
- Madar, J.; Roehr, C.C.; Ainsworth, S.; Ersdal, H.; Morley, C.; Rüdiger, M.; Skåre, C.; Szczapa, T.; Te Pas, A.; Trevisanuto, D.; et al. European Resuscitation Council Guidelines 2021: Newborn resuscitation and support of transition of infants at birth. Resuscitation 2021, 161, 291-326. [CrossRef]
- Zeballos Sarrato, G.; Avila-Alvarez, A.; Escrig Fernandez, R.; Izquierdo Renau, M.; Ruiz Campillo, C.W.; Gomez Robles, C.; Iriondo Sanz, M.; en representacion del Grupo de Reanimacion Neonatal de la Sociedad Espanola de, N. [Spanish guide for neonatal stabilization and resuscitation 2021: Analysis, adaptation and consensus on international recommendations]. An Pediatr (Engl Ed) 2021. [CrossRef]
- McDonald, S.D.; Narvey, M.; Ehman, W.; Jain, V.; Cassell, K. Guideline No. 424: Umbilical Cord Management in Preterm and Term Infants. J Obstet Gynaecol Can 2022, 44, 313-322.e311. [CrossRef]
- Song, D.; Jegatheesan, P.; DeSandre, G.; Govindaswami, B. Duration of Cord Clamping and Neonatal Outcomes in Very Preterm Infants. PloS one 2015, 10, e0138829. [CrossRef]
- Manani, M.; Jegatheesan, P.; DeSandre, G.; Song, D.; Showalter, L.; Govindaswami, B. Elimination of admission hypothermia in preterm very low-birth-weight infants by standardization of delivery room management. Perm J 2013, 17, 8-13. [CrossRef]
- Katheria, A.; Reister, F.; Essers, J.; Mendler, M.; Hummler, H.; Subramaniam, A.; Carlo, W.; Tita, A.; Truong, G.; Davis-Nelson, S.; et al. Association of Umbilical Cord Milking vs Delayed Umbilical Cord Clamping With Death or Severe Intraventricular Hemorrhage Among Preterm Infants. JAMA 2019, 322, 1877-1886. [CrossRef]
- Salae, R.; Tanprasertkul, C.; Somprasit, C.; Bhamarapravatana, K.; Suwannarurk, K. Efficacy of Delayed versus Immediate Cord Clamping in Late Preterm Newborns following Normal Labor: A Randomized Control Trial. J Med Assoc Thai 2016, 99 Suppl 4, S159-165.
- Ranjit, T.; Nesargi, S.; Rao, P.N.; Sahoo, J.P.; Ashok, C.; Chandrakala, B.S.; Bhat, S. Effect of early versus delayed cord clamping on hematological status of preterm infants at 6 wk of age. Indian J Pediatr 2015, 82, 29-34. [CrossRef]
- Rana, A.; Agarwal, K.; Ramji, S.; Gandhi, G.; Sahu, L. Safety of delayed umbilical cord clamping in preterm neonates of less than 34 weeks of gestation: a randomized controlled trial. Obstet Gynecol Sci 2018, 61, 655-661. [CrossRef]
- Ultee, C.A.; van der Deure, J.; Swart, J.; Lasham, C.; van Baar, A.L. Delayed cord clamping in preterm infants delivered at 34 36 weeks' gestation: a randomised controlled trial. Archives of disease in childhood. Fetal and neonatal edition 2008, 93, F20-23. [CrossRef]
- Duley, L.; Dorling, J.; Pushpa-Rajah, A.; Oddie, S.J.; Yoxall, C.W.; Schoonakker, B.; Bradshaw, L.; Mitchell, E.J.; Fawke, J.A.; Group, C.P.T.C. Randomised trial of cord clamping and initial stabilisation at very preterm birth. Archives of disease in childhood. Fetal and neonatal edition 2018, 103, F6-F14. [CrossRef]
- Raina, J.S.; Chawla, D.; Jain, S.; Khurana, S.; Sehgal, A.; Rani, S. Resuscitation with Intact Cord Versus Clamped Cord in Late Preterm and Term Neonates: A Randomized Controlled Trial. The Journal of pediatrics 2023, 254, 54-60 e54. [CrossRef]
- Pratesi, S.; Montano, S.; Ghirardello, S.; Mosca, F.; Boni, L.; Tofani, L.; Dani, C. Placental Circulation Intact Trial (PCI-T)-Resuscitation With the Placental Circulation Intact vs. Cord Milking for Very Preterm Infants: A Feasibility Study. Front Pediatr 2018, 6, 364. [CrossRef]
- Fairchild, K.D.; Petroni, G.R.; Varhegyi, N.E.; Strand, M.L.; Josephsen, J.B.; Niermeyer, S.; Barry, J.S.; Warren, J.B.; Rincon, M.; Fang, J.L.; et al. Ventilatory Assistance Before Umbilical Cord Clamping in Extremely Preterm Infants: A Randomized Clinical Trial. JAMA Netw Open 2024, 7, e2411140. [CrossRef]
- Tarnow-Mordi, W.; Morris, J.; Kirby, A.; Robledo, K.; Askie, L.; Brown, R.; Evans, N.; Finlayson, S.; Fogarty, M.; Gebski, V.; et al. Delayed versus Immediate Cord Clamping in Preterm Infants. N Engl J Med 2017, 377, 2445-2455. [CrossRef]
- Kc, A.; Rana, N.; Målqvist, M.; Jarawka Ranneberg, L.; Subedi, K.; Andersson, O. Effects of Delayed Umbilical Cord Clamping vs Early Clamping on Anemia in Infants at 8 and 12 Months: A Randomized Clinical Trial. JAMA Pediatr 2017, 171, 264-270. [CrossRef]
- Garcia, C.; Prieto, M.T.; Escudero, F.; Bosh-Gimenez, V.; Quesada, L.; Lewanczyk, M.; Pertegal, M.; Delgado, J.L.; Blanco-Carnero, J.E.; De Paco Matallana, C. The impact of early versus delayed cord clamping on hematological and cardiovascular changes in preterm newborns between 24 and 34 weeks' gestation: a randomized clinical trial. Archives of gynecology and obstetrics 2024, 309, 2483-2490. [CrossRef]
- Juul SE, Comstock BA, Wadhawan R, Mayock DE, Courtney SE, Robinson T, Ahmad KA, Bendel-Stenzel E, Baserga M, LaGamma EF, Downey LC, Rao R, Fahim N, Lampland A, Frantz ID III, Khan JY, Weiss M, Gilmore MM, Ohls RK, Srinivasan N, Perez JE, McKay V, Vu PT, Lowe J, Kuban K, O'Shea TM, Hartman AL, Heagerty PJ; PENUT Trial Consortium. A Randomized Trial of Erythropoietin for Neuroprotection in Preterm Infants. N Engl J Med. 2020, 382, 233-243. [CrossRef]
- Rizzolo, A.; Shah, P.S.; Boucorian, I.; Lemyre, B.; Bertelle, V.; Pelausa, E.; St Hilaire, M.; Dahlgren, L.; Beltempo, M.; Canadian Neonatal, N.; et al. Cumulative effect of evidence-based practices on outcomes of preterm infants born at <29 weeks' gestational age. Am J Obstet Gynecol 2020, 222, 181 e181-181 e110. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).