Submitted:
02 December 2024
Posted:
03 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
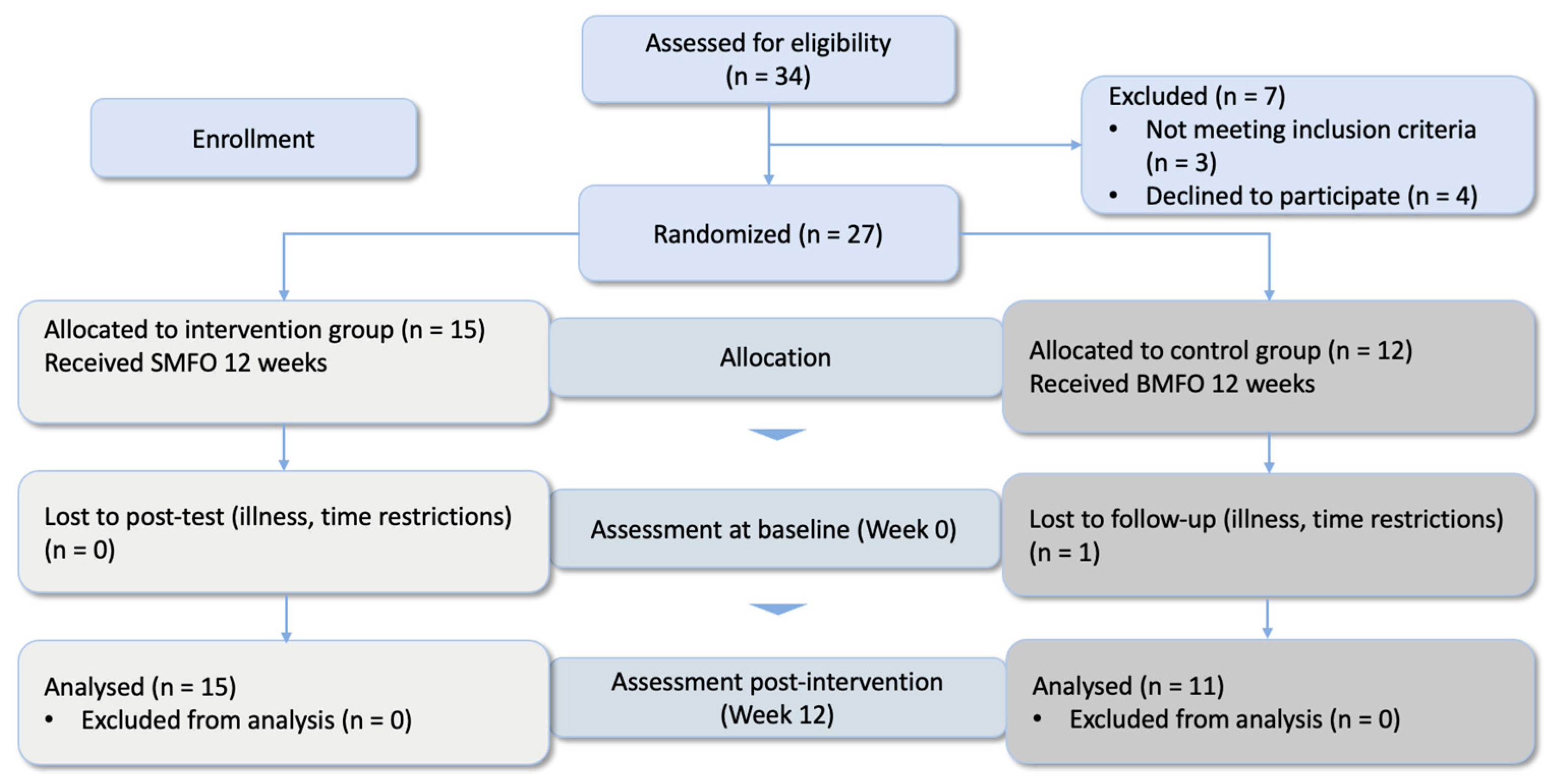
2.1. Study Design
2.2. Sample
- ●
- Age between 15 and 60 years
- ●
- Discomfort in the knee joint area during at least two weight-bearing activities (walking stairs, squatting, standing up) for at least three weeks: pain during these activities on most days in the last month that is ≥ 30 mm on a 100 mm VAS
- ●
- Indication (at least one diagnosis from the following list):
- ○
- Femoropatellar pain syndrome
- ○
- Chondropathia patellae up to grade 3 with pathological alignment and femoral antetorsion
- ○
- Runner’s knee, jumper’s knee
- ○
- Osteochondral defects, inflammation, and impingement of the Hoffa fat body
- ○
- Tendinopathies of the patellar or quadriceps tendon, patellofemoral osteoarthritis, plica syndrome
- ●
- Altered Q-angle [34] of the lower extremity/recognizable rotational abnormality of ankle joints, tibia, and femur during gait
- ●
- Foot deformity: Pes planus, Pes valgus, Pes planovalgus, Pes cavus, and Pes transversoplanus
- ●
- Medical history of knee joint arthroplasty or osteotomy
- ●
- Previous (surgical) treatment (< 12 months) in the ankle, knee, or hip joint
- ●
- X-ray evidence of fixed bone deformity or joint erosion
- ●
- Moderate or severe concomitant tibiofemoral OA (Kellgren and Lawrence grade ≥ 3 on anteroposterior radiograph [35])
- ●
- Underlying neurological pathology
- ●
- Known underlying rheumatic disease with drug treatment
- ●
- Previous treatment with orthopedic FO according to the above concepts while treating the given knee pain indication
- ●
- Acute muscle/ligament injury (< 4 weeks) with associated restriction of the musculoskeletal system
2.3. Procedure
2.4. Intervention with Foot Orthoses
2.5. Knee Pain, Effectiveness, and Comfort Rating
2.6. Statistical Analysis
3. Results
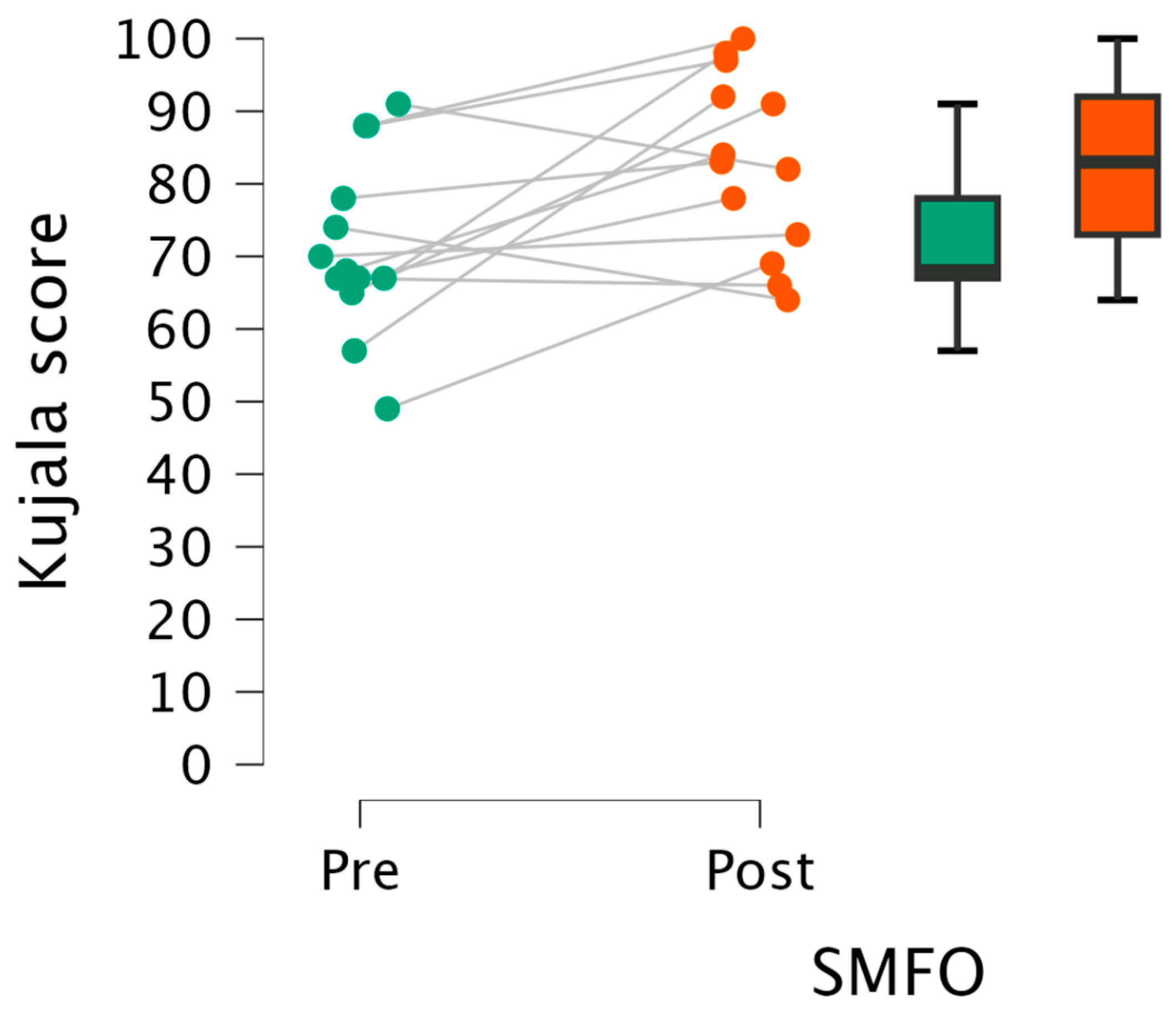
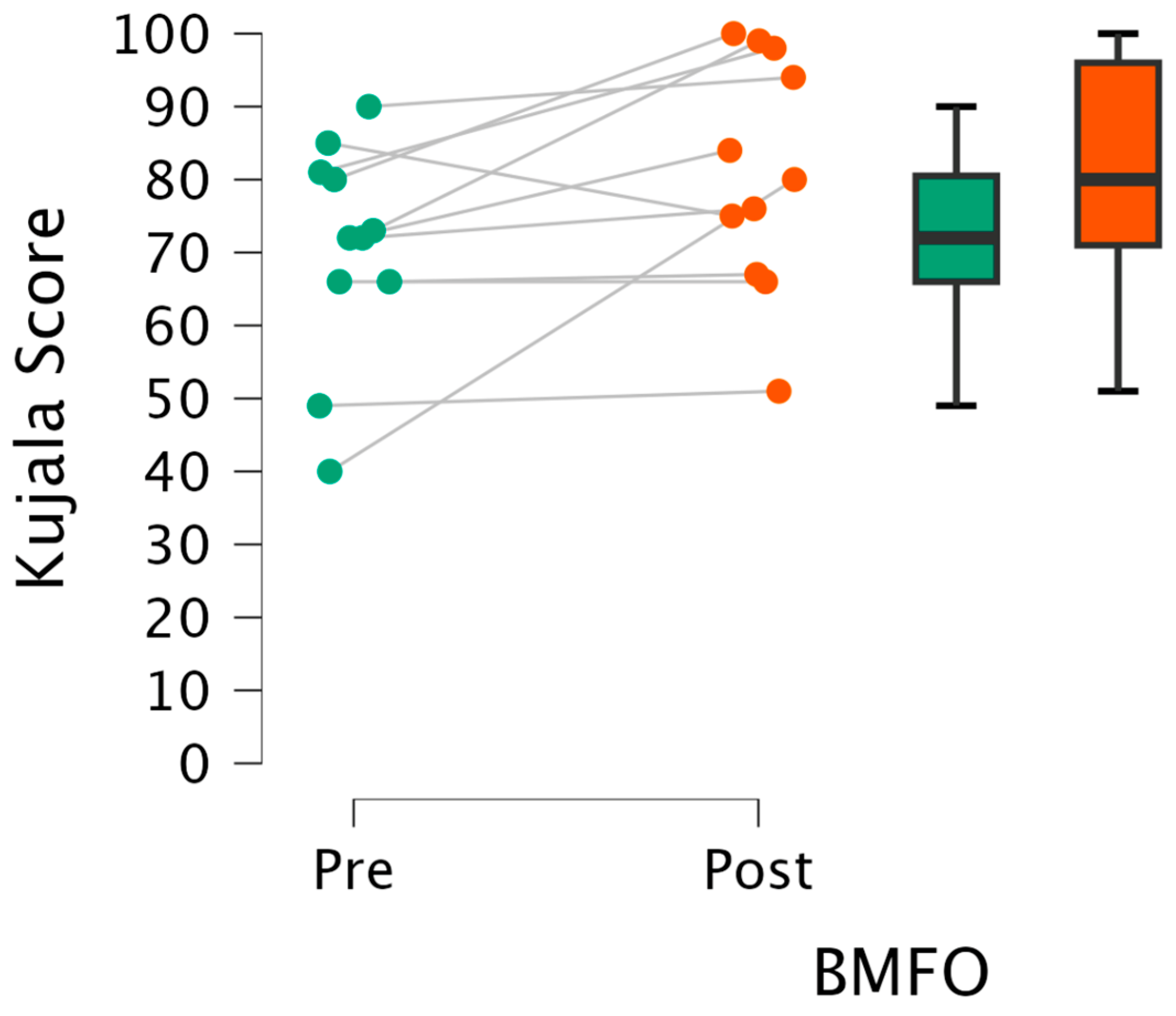
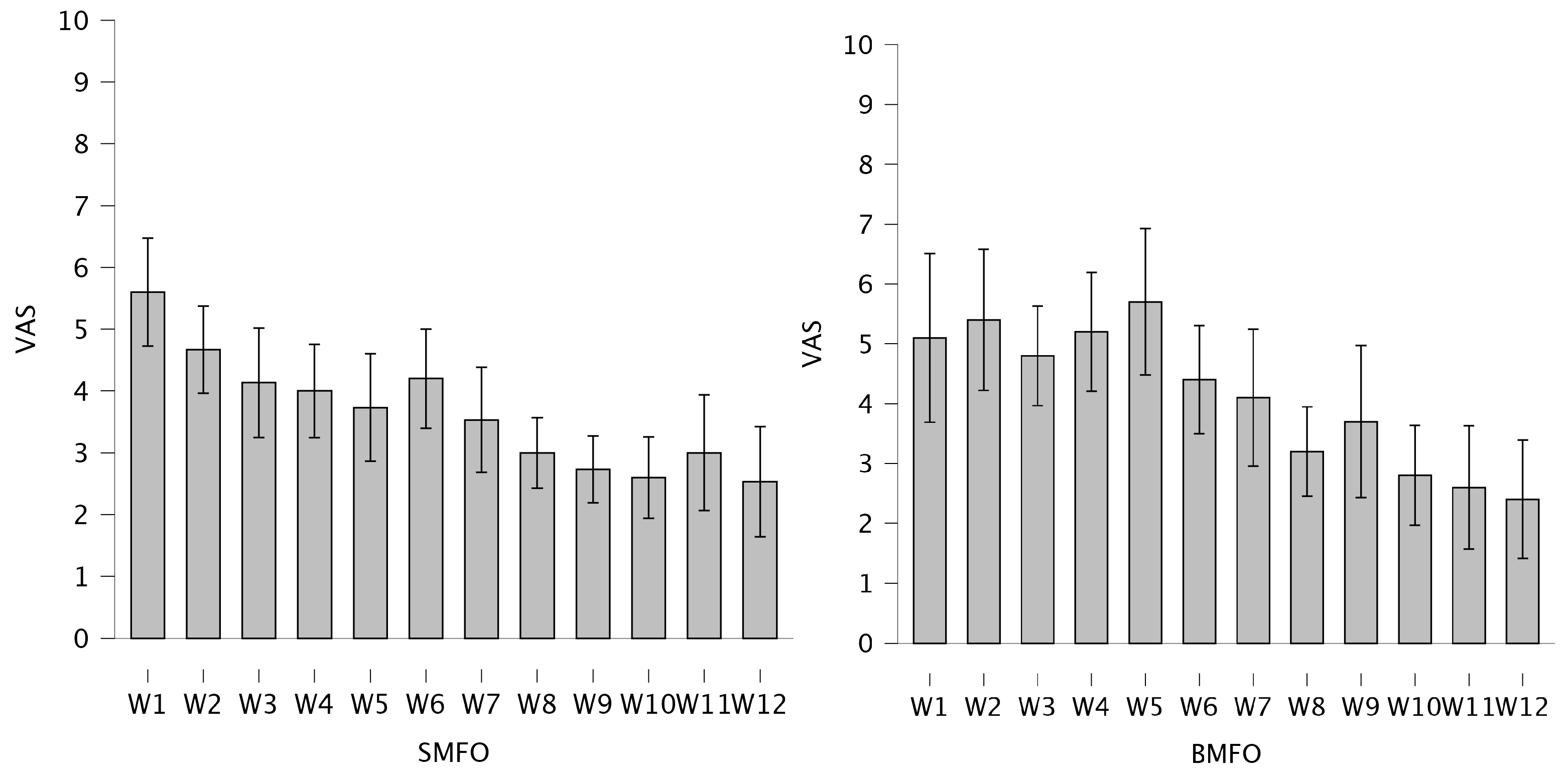
3.1. Perceived Knee Pain
3.1.1. Kujala Knee Pain Score
3.1.2. 12-Week Visual Analog Scales (VASs)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bazett-Jones, D.M.; Neal, B.S.; Legg, C.; Hart, H.F.; Collins, N.J.; Barton, C.J. Kinematic and kinetic gait characteristics in people with Patellofemoral Pain: a systematic review and Meta-analysis. Sports Medicine 2023, 53, 519–547. [Google Scholar] [CrossRef] [PubMed]
- Kellish, A.S.; Kellish, P.; Hakim, A.; Miskiel, S.; Shahi, A.; Kellish, A. What is the effect on kinesio taping on pain and gait in patients with patellofemoral pain syndrome? Cureus 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Crossley, K.M.; van Middelkoop, M.; Callaghan, M.J.; Collins, N.J.; Rathleff, M.S.; Barton, C.J. 2016 Patellofemoral pain consensus statement from the 4th International Patellofemoral Pain Research Retreat, Manchester. Part 2: recommended physical interventions (exercise, taping, bracing, foot orthoses and combined interventions). British journal of sports medicine 2016, 50, 844–852. [Google Scholar] [CrossRef]
- Coburn, S.L.; Barton, C.J.; Filbay, S.R.; Hart, H.F.; Rathleff, M.S.; Crossley, K.M. Quality of life in individuals with patellofemoral pain: a systematic review including meta-analysis. Physical Therapy in Sport 2018, 33, 96–108. [Google Scholar] [CrossRef]
- Fulkerson, J.P. Diagnosis and treatment of patients with patellofemoral pain. The American journal of sports medicine 2002, 30, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Utting, M.; Davies, G.; Newman, J. Is anterior knee pain a predisposing factor to patellofemoral osteoarthritis? The knee 2005, 12, 362–365. [Google Scholar] [CrossRef]
- Afzali, T.; Fangel, M.V.; Vestergaard, A.S.; Rathleff, M.S.; Ehlers, L.H.; Jensen, M.B. Cost-effectiveness of treatments for non-osteoarthritic knee pain conditions: A systematic review. PLoS One 2018, 13, e0209240. [Google Scholar] [CrossRef] [PubMed]
- Glaviano, N.R.; Kew, M.; Hart, J.M.; Saliba, S. Demographic and epidemiological trends in patellofemoral pain. International journal of sports physical therapy 2015, 10, 281. [Google Scholar] [PubMed]
- Aysin, I.K.; Askin, A.; Mete, B.D.; Guvendi, E.; Aysin, M.; Kocyigit, H. Investigation of the relationship between anterior knee pain and chondromalacia patellae and patellofemoral malalignment. The Eurasian journal of medicine 2018, 50, 28. [Google Scholar] [CrossRef] [PubMed]
- Fairbank, J.; Pynsent, P.; van Poortvliet, J.A.; Phillips, H. Mechanical factors in the incidence of knee pain in adolescents and young adults. The Journal of Bone & Joint Surgery British Volume 1984, 66, 685–693. [Google Scholar] [CrossRef]
- Powers, C.M.; Witvrouw, E.; Davis, I.S.; Crossley, K.M. Evidence-based framework for a pathomechanical model of patellofemoral pain: 2017 patellofemoral pain consensus statement from the 4th International Patellofemoral Pain Research Retreat, Manchester, UK: part 3. British journal of sports medicine 2017, 51, 1713–1723. [Google Scholar] [CrossRef]
- Stefanyshyn, D.J.; Stergiou, P.; Lun, V.M.; Meeuwisse, W.H.; Worobets, J.T. Knee angular impulse as a predictor of patellofemoral pain in runners. The American journal of sports medicine 2006, 34, 1844–1851. [Google Scholar] [CrossRef]
- Lankhorst, N.E.; Bierma-Zeinstra, S.M.; van Middelkoop, M. Risk factors for patellofemoral pain syndrome: a systematic review. Journal of orthopaedic & sports physical therapy 2012, 42, 81–94. [Google Scholar] [CrossRef]
- Powers, C.M. The influence of altered lower-extremity kinematics on patellofemoral joint dysfunction: a theoretical perspective. Journal of Orthopaedic & Sports Physical Therapy 2003, 33, 639–646. [Google Scholar] [CrossRef]
- Barton, C.J.; Bonanno, D.; Levinger, P.; Menz, H.B. Foot and ankle characteristics in patellofemoral pain syndrome: a case control and reliability study. Journal of Orthopaedic & Sports Physical Therapy 2010, 40, 286–296. [Google Scholar] [CrossRef]
- Hintermann, B.; Nigg, B.M. Pronation in runners: Implications for injuries. Sports medicine 1998, 26, 169–176. [Google Scholar] [CrossRef]
- Hoglund, L.T.; Hulcher, T.A.; Amabile, A.H. Males with patellofemoral pain have altered movements during step-down and single-leg squatting tasks compared to asymptomatic males: A cross-sectional study. Health Science Reports 2024, 7, e2193. [Google Scholar] [CrossRef] [PubMed]
- Duval, K.; Lam, T.; Sanderson, D. The mechanical relationship between the rearfoot, pelvis and low-back. Gait & posture 2010, 32, 637–640. [Google Scholar] [CrossRef]
- Skou, S.T.; Hojgaard, L.; Simonsen, O.H. Customized foot insoles have a positive effect on pain, function, and quality of life in patients with medial knee osteoarthritis. Journal of the American Podiatric Medical Association 2013, 103, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Collins, N.; Crossley, K.; Beller, E.; Darnell, R.; McPoil, T.; Vicenzino, B. Foot orthoses and physiotherapy in the treatment of patellofemoral pain syndrome: randomised clinical trial. Bmj 2008, 337. [Google Scholar] [CrossRef]
- Mündermann, A.; Nigg, B.M.; Humble, R.N.; Stefanyshyn, D.J. Foot orthotics affect lower extremity kinematics and kinetics during running. Clinical biomechanics 2003, 18, 254–262. [Google Scholar] [CrossRef]
- Saxena, A.; Haddad, J. The effect of foot orthoses on patellofemoral pain syndrome. Journal of the American Podiatric Medical Association 2003, 93, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Simon, S.; Mühlen, J.; Dindorf, C.; Fröhlich, M. Assessing the Subjective Effectiveness of Sensorimotor Insoles (SMIs) in Reducing Pain: A Descriptive Multicenter Pilot Study. Journal of Functional Morphology and Kinesiology 2023, 8, 66. [Google Scholar] [CrossRef]
- Gross, M.T.; Foxworth, J.L. The role of foot orthoses as an intervention for patellofemoral pain. Journal of Orthopaedic & Sports Physical Therapy 2003, 33, 661–670. [Google Scholar] [CrossRef]
- Barton, C.J.; Munteanu, S.E.; Menz, H.B.; Crossley, K.M. The efficacy of foot orthoses in the treatment of individuals with patellofemoral pain syndrome: a systematic review. Sports Medicine 2010, 40, 377–395. [Google Scholar] [CrossRef] [PubMed]
- Lewinson, R.T.; Wiley, J.P.; Humble, R.N.; Worobets, J.T.; Stefanyshyn, D.J. Altering knee abduction angular impulse using wedged insoles for treatment of patellofemoral pain in runners: a six-week randomized controlled trial. PloS one 2015, 10, e0134461. [Google Scholar] [CrossRef]
- Kayll, S.A.; Hinman, R.S.; Bryant, A.L.; Bennell, K.L.; Rowe, P.L.; Paterson, K.L. Do biomechanical foot-based interventions reduce patellofemoral joint loads in adults with and without patellofemoral pain or osteoarthritis? A systematic review and meta-analysis. British Journal of Sports Medicine 2023. [Google Scholar] [CrossRef]
- Ranker, A. Sensomotorische Schuheinlagen–Wirkprinzipien und Evidenz. manuelletherapie 2020, 24, 168–172. [Google Scholar] [CrossRef]
- Becker, S.; Ludwig, O.; Woltring, S.; Simon, S.; Fröhlich, M. Sensomotorische Einlagen: Grundlagen und Funktionen; Springer-Verlag, 2024. [Google Scholar]
- Greitemann, B.; Franzen, M.; Stinus, H.; Walther, M.; Dierolf, W.; Schievink, F.; Perick, H.; Stief, T.; Stumpf, J. DGOOC-Beratungsausschuss Orthopädieschuhtechnik. Orthopädie und Unfallchirurgie-Mitteilungen und Nachrichten 2016, 5, 283–286. [Google Scholar] [CrossRef]
- Kerkhoff, A.; Wagner, H.; Nagel, A.; Möller, M.; Peikenkamp, K. Effects of two different foot orthoses on muscle activity in female during single-leg landing. German Journal of Exercise and Sport Research 2017, 4, 305–314. [Google Scholar] [CrossRef]
- Kerkhoff, A.; Wagner, H.; Peikenkamp, K. Different effects of sensorimotor and soft bedding foot orthoses on muscle activity during single-leg landing in sports. Footwear Science 2019, 11, S37–S38. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behavior research methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Mizuno, Y.; Kumagai, M.; Mattessich, S.M.; Elias, J.J.; Ramrattan, N.; Cosgarea, A.J.; Chao, E.Y. Q-angle influences tibiofemoral and patellofemoral kinematics. Journal of orthopaedic research 2001, 19, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Schiphof, D.; Boers, M.; Bierma-Zeinstra, S.M. Differences in descriptions of Kellgren and Lawrence grades of knee osteoarthritis. Annals of the rheumatic diseases 2008, 67, 1034–1036. [Google Scholar] [CrossRef] [PubMed]
- Murley, G.S.; Menz, H.B.; Landorf, K.B. A protocol for classifying normal-and flat-arched foot posture for research studies using clinical and radiographic measurements. Journal of foot and ankle research 2009, 2, 1–13. [Google Scholar] [CrossRef]
- Queen, R.M.; Mall, N.A.; Hardaker, W.M.; Nunley, J.A. Describing the medial longitudinal arch using footprint indices and a clinical grading system. Foot & ankle international 2007, 28, 456–462. [Google Scholar]
- Cavanagh, P.R.; Rodgers, M.M. The arch index: a useful measure from footprints. Journal of biomechanics 1987, 20, 547–551. [Google Scholar] [CrossRef]
- Kujala, U.M.; Jaakkola, L.H.; Koskinen, S.K.; Taimela, S.; Hurme, M.; Nelimarkka, O. Scoring of patellofemoral disorders. Arthroscopy: The Journal of Arthroscopic & Related Surgery 1993, 9, 159–163. [Google Scholar] [CrossRef]
- Dammerer, D.; Liebensteiner, M.; Kujala, U.; Emmanuel, K.; Kopf, S.; Dirisamer, F.; Giesinger, J. Validation of the German version of the Kujala score in patients with patellofemoral instability: a prospective multi-centre study. Archives of Orthopaedic and Trauma Surgery 2018, 138, 527–535. [Google Scholar] [CrossRef]
- Crossley, K.M.; Bennell, K.L.; Cowan, S.M.; Green, S. Analysis of outcome measures for persons with patellofemoral pain: which are reliable and valid? Archives of physical medicine and rehabilitation 2004, 85, 815–822. [Google Scholar] [CrossRef]
- Bijur, P.E.; Silver, W.; Gallagher, E.J. Reliability of the visual analog scale for measurement of acute pain. Academic emergency medicine 2001, 8, 1153–1157. [Google Scholar] [CrossRef] [PubMed]
- Murley, G.S.; Landorf, K.B.; Menz, H.B. Do foot orthoses change lower limb muscle activity in flat-arched feet towards a pattern observed in normal-arched feet? Clinical Biomechanics 2010, 25, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Team, J. JASP. 2024.
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: New York, 1988; Volume 2, p. 567. [Google Scholar]
- Vicenzino, B.; Collins, N.; Cleland, J.; McPoil, T. A clinical prediction rule for identifying patients with patellofemoral pain who are likely to benefit from foot orthoses: a preliminary determination. British journal of sports medicine 2010, 44, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.S.; Vanderlei, F.M.; Pastre, E.C.; Martins, R.A.; Padovani, C.R.; Guaracy Filho, C. Comparison of two types of insoles on Musculoskeletal symptoms and plantar pressure distribution in a work environment: a randomized clinical trial. Clinical Medicine & Research 2016, 14, 67–74. [Google Scholar] [CrossRef]
- Ludwig, O.; Kelm, J.; Fröhlich, M. The influence of insoles with a peroneal pressure point on the electromyographic activity of tibialis anterior and peroneus longus during gait. Journal of foot and ankle research 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Burnfield, M. Gait analysis: normal and pathological function. Journal of Sports Science and Medicine 2010, 9, 353. [Google Scholar]
- Ohlendorf, D.; Natrup, J.; Niklas, A.; Kopp, S. Veränderung der Körperhaltung durch haltungsverbessernde, sensomotorische, Einlegesohlen. Manuelle Medizin 2008, 46, 93–98. [Google Scholar] [CrossRef]
- Chen, A.T.; Shrestha, S.; Collins, J.E.; Sullivan, J.K.; Losina, E.; Katz, J.N. Estimating contextual effect in nonpharmacological therapies for pain in knee osteoarthritis: a systematic analytic review. Osteoarthritis Cartilage 2020, 28, 1154–1169. [Google Scholar] [CrossRef]
- Mabuchi, A.; Kitoh, H.; Inoue, M.; Hayashi, M.; Ishiguro, N.; Suzuki, N. The biomechanical effect of the sensomotor insole on a pediatric intoeing gait. International Scholarly Research Notices 2012, 2012. [Google Scholar] [CrossRef]
- Ferreira, L.A.B.; Cimolin, V.; Neto, H.P.; Grecco, L.A.C.; Lazzari, R.D.; Dumont, A.J.L.; Galli, M.; Oliveira, C.S. Effect of postural insoles on gait pattern in individuals with hemiparesis: A randomized controlled clinical trial. Journal of Bodywork and Movement Therapies 2018, 22, 792–797. [Google Scholar] [CrossRef]
- Klein, T.; Lastovicka, O.; Janura, M.; Svoboda, Z.; Chapman, G.J.; Richards, J. The immediate effects of sensorimotor foot orthoses on foot kinematics in healthy adults. Gait & Posture 2021, 84, 93–101. [Google Scholar] [CrossRef]
- MacFarlane, C.; Hing, W.; Orr, R. Using the Edinburgh visual gait score to compare ankle-foot orthoses, sensorimotor orthoses and barefoot gait pattern in children with cerebral palsy. Children 2020, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Murley, G.S.; Menz, H.B.; Landorf, K.B. A protocol for classifying normal-and flat-arched foot posture for research studies using clinical and radiographic measurements. Journal of foot and ankle research 2009, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Salsich, G.B.; Perman, W.H. Tibiofemoral and patellofemoral mechanics are altered at small knee flexion angles in people with patellofemoral pain. Journal of Science and Medicine in Sport 2013, 16, 13–17. [Google Scholar] [CrossRef]

| Age (y) | Height (m) | Weight (kg) | BMI | NI links | NI rechts | AI links | AI rechts | ||
| SMFO | Mean | 27.27 | 1.76 | 75.40 | 24.30 | 0.17 | 0.17 | 0.22 | 0.22 |
| SD | 9.19 | 0.10 | 18.24 | 4.64 | 0.04 | 0.04 | 0.07 | 0.06 | |
| Max | 42.00 | 1.92 | 115.00 | 33.24 | 0.25 | 0.24 | 0.31 | 0.31 | |
| Min | 15.00 | 1.56 | 47.00 | 17.26 | 0.11 | 0.11 | 0.09 | 0.09 | |
| BMFO | Mean | 29.67 | 1.75 | 77.58 | 25.32 | 0.20 | 0.21 | 0.24 | 0.24 |
| SD | 13.39 | 0.09 | 20.22 | 6.53 | 0.06 | 0.06 | 0.06 | 0.02 | |
| Max | 54 | 1.97 | 120 | 42.52 | 0.33 | 0.33 | 0.30 | 0.27 | |
| Min | 16 | 1.64 | 50 | 16.14 | 0.13 | 0.13 | 0.10 | 0.21 |
| FO type | Manufacturer | Primary medical target | Elements | Materials |
| SMFO | Springer Aktiv AG | Stimulating M. tibialis posterior and M. peroneus longus and brevisStretching plantar fascia and toes | Medial spot (oriented toward M. tibialis posterior tendon at sustentaculum tali)Lateral spot (oriented toward M. peroneus longus and brevis tendon near Os cuboideum)Retrocapital bar (supporting the transversal arch and stretching plantar fascia)Toe bar (placing and stretching of toes) | EVA-material; Sandwich construction consisting of 35 Shore (outsole), 25 Shore (midsole), 35 Shore (top-layer) |
| BMFO | Hema Orthopädische Systeme GmbH | Medial arch supportTransversal arch support Pressure relief |
Heel padSupination wedgeMetatarsal pad (pelotte) | Injection molded foam25 Shore |
| FO effectiveness | Wearing comfort | Daily steps in FOs |
Wearing time/ day (h) |
||
| IG | Mean | 7.87 | 8.27 | 8360 | 9.68 |
| SD | 2.23 | 1.10 | 6464 | 4.10 | |
| CG | Mean | 6.45 | 7.91 | 8947 | 10.00 |
| SD | 2.94 | 1.87 | 4842 | 2.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





