1. Introduction
Alzheimer's disease (AD) is a leading cause of neurodegenerative disorders characterized by dementia and the progressive loss of neurons, with the number of deaths increasing each year, making it a significant global public health issue [
1]. In recent years, disease-modifying therapies such as Aducanumab and Lecanemab have been developed, focusing on reducing amyloid-β plaque accumulation. However, these treatments have shown limited effectiveness in slowing cognitive decline, and the results from clinical trials have sparked controversy. This highlights the need for a more comprehensive approach targeting other pathological mechanisms, such as tau proteins and inflammation [
2].
In neurodegenerative diseases, including AD, a specific subtype of microglia called disease-associated microglia (DAM) is found [
3,
4]. Microglia, which belongs to the innate immune system in the central nervous system (CNS), is key to interpreting and propagating inflammatory signals [
5,
6,
7]. Activated microglia to proinflammatory phenotypes upregulate the expression of inflammatory mediators such as inducible nitric oxide synthase (iNOS) and TNFα, accelerating AD pathology [
8,
9,
10]. Cytokines such as TNF-α activate other immune cells, contributing to the development of neurodegenerative disorders [
11]. Microglial activity can be modulated by factors such as fractalkine (CX3CL1) [
12].
Recent studies have identified the CX3CL1-CX3CR1 interaction as a critical mechanism in microglial regulation. CX3CL1, highly expressed in the brain, interacts with fractalkine receptor (CX3CR1) on microglia to regulate their activation, suggesting a unique communication system between neurons and microglia [
13]. Furthermore, recently developed CX3CR1 inhibitors have shown that delayed administration of CX3CL1 can suppress proinflammatory microglia, indicating that CX3CR1 signaling may even inhibit reactive microglia [
14,
15,
16].
Regulatory T cells (Tregs) are crucial for suppressing autoimmune responses and maintaining immune homeostasis. These cells inhibit the production of inflammatory cytokines, modulate immune activity, and exert immune suppression, thereby preventing excessive immune responses and slowing the progression of inflammatory diseases [
17]. In the CNS, Tregs also play a crucial role in suppressing neuroinflammation. In AD and other neurodegenerative disorders, Tregs have been shown to inhibit the overactivation of microglia, providing neuroprotective effects. Tregs reduce the expression of proinflammatory cytokines and regulate the proinflammatory activation of microglia, helping to control neuroinflammatory processes [
18,
19].
Our previous study demonstrated that the administration of mouse Tregs into 3xTg-AD mice had neuroprotective effects on AD [
20]. Additionally, recent studies have shown that the administration of antigen-specific amyloid-beta (Aβ) positive Tregs can attenuate the progression of AD, suggesting that the bystander effect of Tregs could serve as a potential therapeutic strategy [
21,
22].
CX3CR1-expressing Tregs (CX3CR1
+ Tregs) have been studied for their ability to selectively migrate to sites of inflammation through the CX3CL1/CX3CR1 axis. In atherosclerosis models, CX3CR1
+ Tregs were shown to effectively migrate to inflamed areas and suppress immune-inflammatory responses, suggesting their potential as a therapeutic strategy for other inflammatory conditions, including neuroinflammatory diseases [
23].
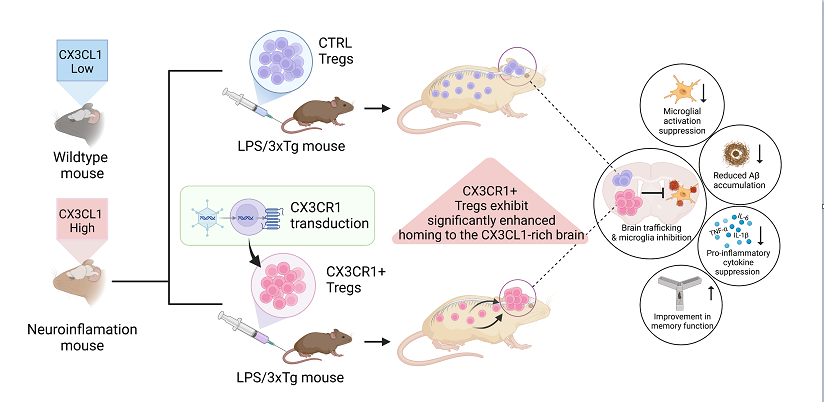
In this study, we aim to evaluate whether CX3CR1+ Tregs, which have enhanced trafficking to inflammatory sites through the CX3CL1/CX3CR1 axis, can exert stronger anti-inflammatory and neuroprotective effects in AD. CX3CR1+ Tregs are hypothesized to migrate more effectively to inflamed areas, where they can suppress the activation of proinflammatory microglia. First, we assessed the ability of CX3CR1+ Tregs to suppress the expression of inflammatory cytokines in an LPS-induced neuroinflammation model, and we then evaluated their effect on chronic neuroinflammation and cognitive function in the 3xTg-AD mouse model. Therefore, this study focuses on evaluating the potential of CX3CR1+ Tregs to modulate neuroinflammation and improve cognitive function in the context of AD pathology.
3. Discussion
Our study explores the therapeutic potential of CX3CR1+ Tregs in mitigating neuroinflammation associated with AD across both acute and chronic models. We utilized the LPS-induced model to mimic acute neuroinflammation and the 3xTg mouse model to assess chronic neurodegenerative processes, providing broad insights into how CX3CR1+ Tregs modulate neuroinflammatory pathways.
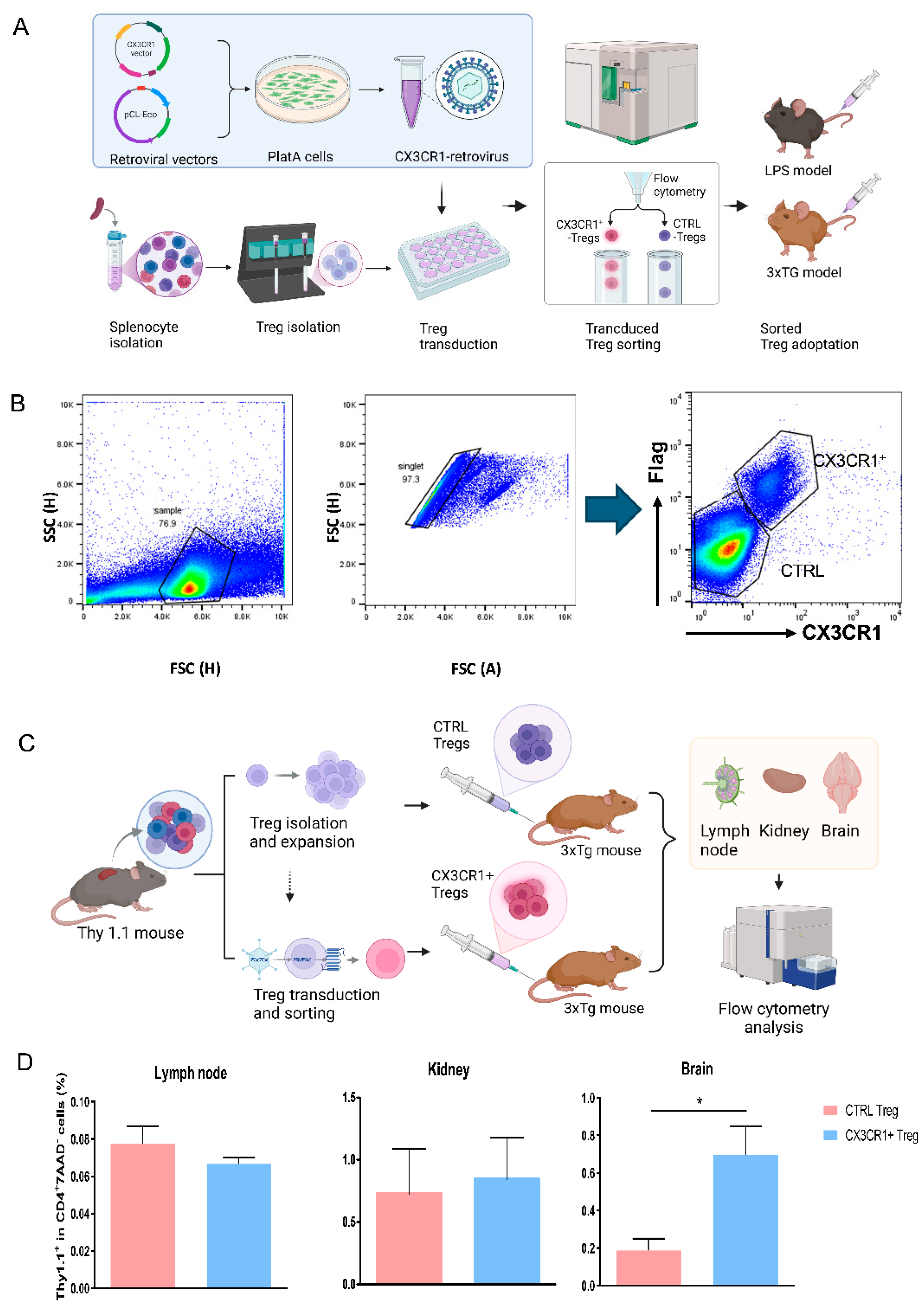
We introduced a CX3CR1 retrovirus into Tregs to optimize their migration and regulate the functional activity of microglia during neuroinflammation. The production of CX3CR1 on Tregs is crucial for attracting them to activated microglial sites and inducing adaptive immune responses, which are essential for recruiting these cells to sites of inflammation within the CNS [
22,
23]. Flow cytometry results from our Treg trafficking study demonstrated a significant presence of CX3CR1
+ Tregs in the brain, confirming their enhanced homing capabilities. This indicates that CX3CR1
+ Tregs not only reach sites of inflammation but also play a vital role in mitigating the inflammatory response, which is crucial for preventing the progression of neurodegenerative processes (
Figure 1D).
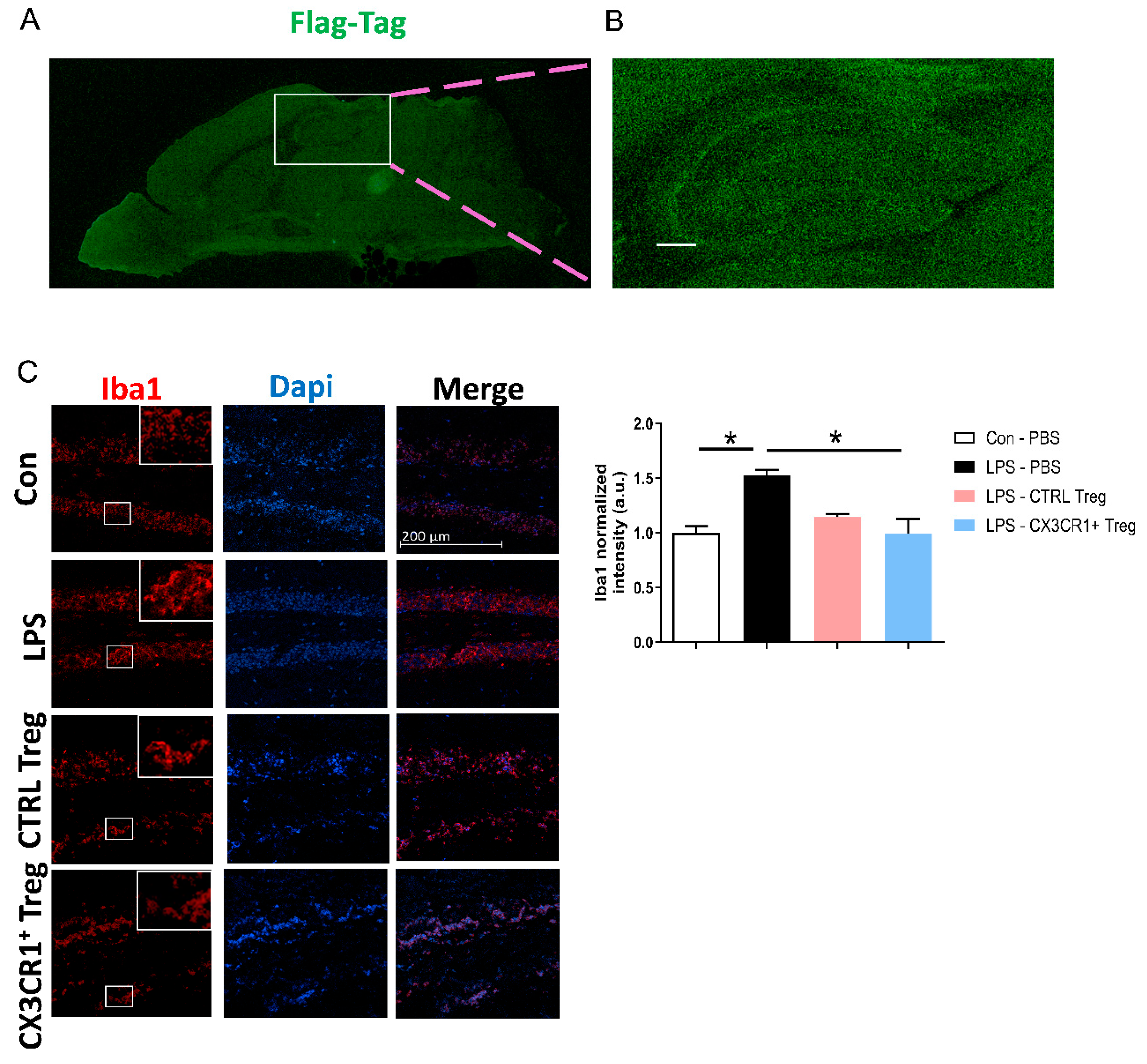
In brain tissues, CX3CL1 is primarily expressed in neurons, while microglia express CX3CR1, the unique receptor for CX3CL1. This interaction is pivotal in both health and disease, as neuroinflammation caused by the hyperactivity of microglia is associated with neurodegenerative diseases such as AD [
12,
24]. The homing of CX3CR1-transduced Tregs to the brain was confirmed through the analysis of paramedian sagittal sections of adoptively transferred CX3CR1
+ Treg mouse brains, utilizing confocal laser scanning microscopy with antibodies against the Flag-tag. Notably, strong Flag-tag expression was detected in forebrain regions, including the cortex and hippocampus (
Figure 3A,B).
Activated microglial cells, revealed by anti-Iba-1 staining in the hippocampal region, were suppressed by CX3CR1
+ Tregs (
Figure 3C). This suppression coincided with a reduction in the total mRNA and protein levels of proinflammatory markers post-LPS injection (
Figure 4A). Furthermore, we investigated a significant suppression effect of CX3CR1
+ Treg on inflamed microglia, supporting the idea that CX3CR1
+ Treg regulates the activation of microglia. The downregulation of proinflammation in microglia of LPS-induced mice was associated with reduced cytokine levels, TNF-α, IL-6, and IL-1β, demonstrating that CX3CR1
+ Treg is a potent regulator of locally activated microglia and might be used as a potential therapeutic target to control neurodegenerative diseases and other diseases related to inflammation (
Figure 5A,B).
In expanding our research to the 3xTg model, we observed that CX3CR1+ Tregs significantly contribute to reducing hallmark features of Alzheimer's disease, such as Aβ accumulation and microglial activation. The 3xTg mouse model is widely used in AD research due to its three mutations associated with familial Alzheimer's disease. This model exhibits progressive cognitive decline and neuroinflammation, making it a valuable tool for studying the chronic aspects of neurodegenerative diseases.
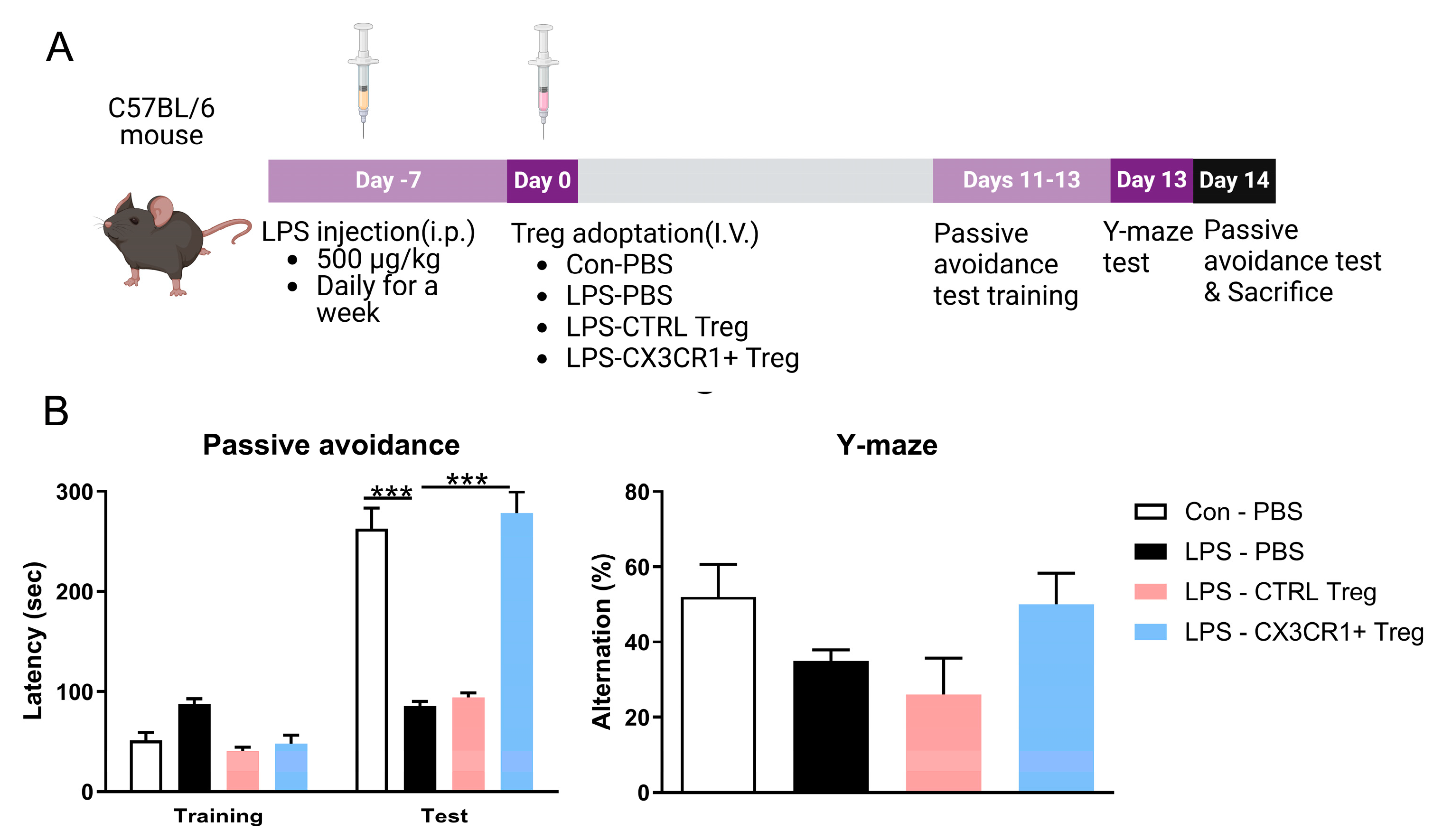
To evaluate the cognitive-enhancing effects of Tregs, we assessed learning and memory using the passive avoidance test PAT and Y-maze. CTRL-Tregs did not induce significant cognitive improvement, whereas the 3xTg mice receiving 1 x 10
5 CX3CR1
+ Tregs remained in the dark compartment significantly longer than PBS-treated 3xTg mice. Furthermore, the Y-maze results showed that CX3CR1
+ Tregs enhanced spontaneous alternation behavior, indicating an improvement in spatial working memory function compared to CTRL-Tregs (
Figure 6B).
To visualize the migration of retrovirus-induced Tregs to the brain, we analyzed paramedian sagittal sections of CX3CR1
+ Treg mice brains (day 6 post-injection) using confocal laser scanning microscopy and antibodies against the DYKDDDDK (Flag) tag. Strong Flag-Tag expression was notably detected in forebrain regions, such as the cortex and hippocampus (
Figure 7A).
Moreover, we assessed the therapeutic effects of CTRL-Tregs and CX3CR1
+ Tregs on AD pathology by staining the CA1 and DG of the hippocampus for Aβ. While CTRL-Tregs reduced Aβ accumulation, CX3CR1
+ Tregs induced a more dramatic reduction, further corroborating their therapeutic potential (
Figure 7B).
We also examined activated microglia using antibodies against Iba1. Immunofluorescence images revealed increased Iba1 reactivity in 3xTg mice compared to non-transgenic controls (WT). Notably, CX3CR1
+ Treg treatment significantly decreased Iba1 immunoreactivity in the CA1 region of the hippocampus compared to 3xTg mice, whereas CTRL-Treg treatment did not result in significant suppression (
Figure 7C).
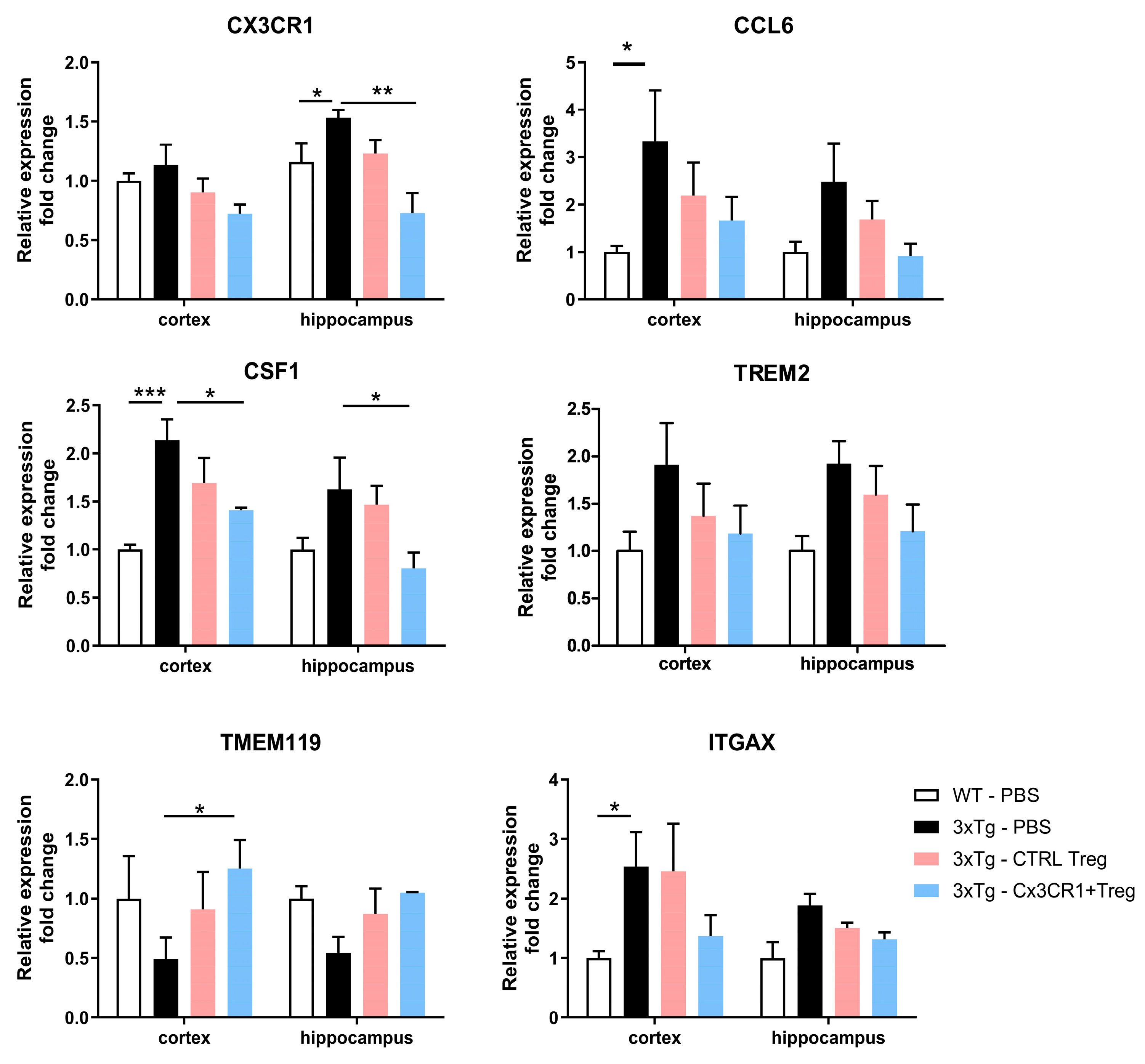
In addition to these findings, we characterized activated microglia in 3xTg mice by assessing the mRNA expression levels of several DAM markers in the cortex and hippocampus. Both CX3CR1 and CSF1, a growth factor essential for microglial proliferation and activation, exhibited increased expression in 3xTg mice. These elevated expressions were reduced by CX3CR1
+ Treg treatment, highlighting their regulatory effects on microglial activation. Furthermore, CX3CR1
+ Tregs specifically increased the expression of TMEM119, a gene associated with microglial phagocytic activity in the cortex, and also modulated the expression of CCL6 and TREM2, further demonstrating their role in reducing microglial activation (
Figure 8A).
These findings collectively illustrate that CX3CR1
+ Tregs not only improve cognitive functions in the 3xTg model but also play a critical role in modulating neuroinflammation and promoting a more favorable neuroinflammatory environment (
Figure 9).
In this study, we aimed to leverage the unique properties of CX3CR1-expressing Tregs, going beyond the general effects of regular Tregs. These special Tregs migrate effectively to inflammatory sites through the CX3CL1/CX3CR1 axis and suppress inflammatory responses. When both Control Tregs and CX3CR1+ Tregs were administered in a neuroinflammatory model, although some results were not statistically significant, most data showed that CX3CR1+ Tregs outperformed Control Tregs, with the differences being particularly noticeable in the Passive Avoidance behavioral tests. As illustrated in the graphic abstract, when the same number of cells was administered to mice, CX3CR1+ Tregs migrated predominantly to CX3CL1-enriched brain regions, demonstrating localized anti-inflammatory effects focused on inflamed areas of the brain. This suggests that CX3CR1+ Tregs can effectively suppress neuroinflammation and enhance neuroprotection relative to the number of cells administered.
Our previous studies have validated the dose-dependent therapeutic effects of Aβ-specific Tregs in the 5xFAD model, confirming that treatment efficacy can be achieved even at relatively low doses [
22]. This indicates that modified Tregs possess the ability to recognize Aβ with high target specificity and selectively migrate to inflammatory sites, thereby maximizing treatment efficiency relative to the number of cells used. Therefore, administering Tregs with high target specificity in appropriate doses could prevent side effects like Cytokine Release Syndrome (CRS) [
31] and precisely target inflammatory sites, potentially minimizing complications associated with systemic immunosuppression [
32]. Likewise, CX3CR1
+ Tregs could provide a more precise and effective therapeutic approach for treating inflammatory diseases compared to traditional Tregs.
However, this study has several important limitations. The specific molecular mechanisms by which CX3CR1+ Tregs regulate microglial activity have not yet been fully elucidated, and a clear understanding of these pathways is essential. Moreover, while this study primarily focused on short-term outcomes observed two weeks after Treg administration, neurodegenerative diseases generally progress over much longer periods. Long-term studies are crucial to evaluate the sustainability of treatment effects and the potential side effects of CX3CR1+ Tregs under chronic conditions. Future research should explore the detailed mechanisms through which CX3CR1+ Tregs exert their effects and assess the safety and efficacy of prolonged treatments. Adjustments based on cell dosage, administration intervals, and disease severity are also required to optimize treatment strategies. Lastly, translating these findings into human clinical trials based on preclinical evidence will be critical to confirm the therapeutic potential of CX3CR1+ Tregs in actual patient populations.
4. Materials and Methods
4.1. Animals
Male 3xTg mice harboring transgenes encoding APP (KM670/671NL), PS1 (M146V), and tau (P301L) proteins [B6;129-Psen1tm1MpmTg (APPSwe, tauP301L)1Lfa/J], non-transgenic-control mice [B6129SF2/J], Thy1.1 mice [B6.PL-Thy1a/CyJ] and C57BL/6 mice were obtained from Jackson Laboratory (Bar Harbor, Me, USA). All animals were housed with free access to food and water and a 12-h light/dark cycle. All animal experiments were conducted according to the rules for animal care and the guiding principles for experiments using animals and were approved by the University of Kyung Hee Animal Care and Use Committee [KHUASP(SE)-21-102]. All animal studies were ethically reviewed and performed in compliance with the ARRIVE guidelines.
4.2. Treg Transduction
Spleens from C57BL/6 mice (7-8 weeks) used to generate Tregs were homogenized and processed by mechanical disruption. The splenocytes were passed through a 40-μm cell strainer. CD4+CD25+ Tregs were isolated using magnetic-activated cell sorting (MACS) according to the manufacturer’s protocol (CD4+CD25+ Regulatory T Cell Isolation Kit, Miltenyi Biotec, Bergisch Gladbach, Germany). Tregs were cultured for 4 days. To generate retroviral vectors, one day prior to transfection, 5 x 105 platA cells/well were plated into a 6-well plate in 2mL of Dulbecco’s Modified Eagle’s Medium (DMEM) with high glucose L-glutamine and 10% fetal bovine serum, without antibiotics. Transient transfection of the platA was performed in Optimem (Gibco, Grand Island, NY, USA) medium with 1.6% Lipofectamine 3000 (Invitrogen, Waltham, MA, USA), 2 μg of packaging vector pCL-Eco (Novus, Centennial, CO, USA) in combination with a plasmid encoding for m5p-CX3CR1-2A-Flag for 48 h. Retrovirus was transduced to Tregs by adding 1/3 volume filtered supernatants of the transfected platA cells, along with fresh IL-2 and a final concentration of 6 mg/mL of protamine sulphate (Sigma Aldrich; Saint Louis, MO, USA). The Tregs were spun for 2 h at 1800 rpm at 32 ℃. Next day, CX3CR1- Flag- Tregs (CTRL-Tregs) and CX3CR1+ Flag+ Tregs (CX3CR1+ Tregs) were sorted using a S3e cell sorter (Bio-Rad; Hercules, CA, USA). CTRL and CX3CR1+ Tregs were cultured for three days in a complete medium supplemented with IL-2 (100 UI/mL).
4.3. Adoptive Transfer of Mouse Tregs
CX3CR1-Flag- and CX3CR1+Flag+ Tregs (1 x 105 cells/mouse) were adoptively transferred into LPS-induced mice and 5-month-old 3xTg-AD mice via tail vein injection. After 14 days, the mice were assessed with behavior tests and sacrificed.
4.4. Thy 1.1 Treg Trafficking
For Tregs trafficking, CX3CR1-Flag- and CX3CR1+Flag+ Tregs were produced from splenocytes of Thy1.1 mice. Five-month-old 3xTg-AD mice (Thy 1.2+) received adoptive transfer of 1x105 Thy 1.1+ Tregs. After 7 days, mice were sacrificed, and inguinal lymph nodes, kidneys, and brain were harvested. T cells were enriched by 30-70% Percoll (Cytiva, Marlborough, MA, USA) density gradient centrifugation from the kidney and brain. Adoptively transferred Tregs were analyzed by flow cytometry.
4.5. Behavior Tests
To confirm the effects of CX3CR1-transduced Treg injection, Y-maze and passive avoidance tests (PAT) were performed. Spatial working memory was examined with the Y-maze test, with the maze consisting of three identical arms at 120° from each other. Mice were placed in an arm facing the wall of the arm and allowed to navigate through all three arms for 5 min freely, and their behavior was recorded. The PAT was performed to estimate learning and memory capacity. The apparatus consisted of a lighted chamber and a dark chamber (20 cm x 20 cm x 30 cm) divided by a door. On the training day, mice were placed in a lighted chamber facing away from the door. Upon the mice entering the dark chamber, the door was closed, and foot shock (0.35 mA, 2 sec) was delivered. Thirty seconds after shock, the mouse was removed. The mice were given trials for two days. On the test day, the mice were placed in a lighted chamber, and the latencies to enter the dark chamber were recorded.
4.6. Immunofluorescence Staining
For immunofluorescence, mice were anesthetized with isoflurane isoflurane (Forane solution; ChoongWae Pharma, Seoul, South Korea) and were transcardially perfused with PBS. The cerebral tissues were removed, incubated overnight in 4% paraformaldehyde (PFA) in 0.1 M sodium phosphate buffer (PBS), and stored in a 30% sucrose solution. After being transferred to a 30% sucrose solution, the brains were cut into 30-μm-thick sections using a cryostat microtome (Leica CM 1850; Leica Microsystems, Wetzlar, Germany) and incubated at 37 °C overnight. After three 5 min washes with PBS (pH 7.4) and permeabilization was performed with heated in 10 mM sodium citrate buffer (pH 6.0) at 65°C for 20 min. The brains sections were blocked for 1 h in 1% bovine serum albumin (BSA) solution and overnight at 4°C with a 1:100 dilution of an antibody against DYKDDDDK tag (Flag-tag; Cell signaling Technology, Danvers, MA, USA), amyloid-beta (Aβ; Biolegend, San Diego, CA, USA), and ionized calcium-binding adapter protein 1 (Iba1; Millipore Corp, Billerica, MA, USA). On the following day, after the incubation with the primary antibodies, the sections were subsequently incubated for 2 h at RT with Alexa Fluor™ 488- or Alexa Fluor™ 594-conjugated IgG secondary antibodies (Thermo Fisher Scientific, Waltham, MA, USA) at room temperature and then washed with PBS three times for 10 min per wash. Then, the sections were stained with DAPI staining solution. Finally, fluorescence images were obtained using LSM 800 confocal laser-scanning microscope (Carl Zeiss, Oberkochen, Germany). The staining intensity was measured and quantified using ImageJ software 1.53e (
http://rsb.info.nih.gov/ij/, accessed on 17 June 2022 ). Staining images were split with an ImageJ plug-in to obtain a monochromatic version. The channel corresponding to the stain of interest was used for measurement.
4.7. RNA Extraction and RT–qPCR Assays
Total RNA was isolated from brain tissues (cortex and hippocampus) using an easy-BLUE RNA extraction kit (iNtRON Biotechnology, Seongnam, Korea, #17061), and cDNA was synthesized using Cyclescript reverse transcriptase (Bioneer, Deajeon, Korea). The samples were prepared for RT–qPCR using a SensiFAST SYBR no-Rox kit (Bioline, London, UK). The cycling conditions were 95 °C for 10 s, 55 °C for 10 s, and 72 °C for 10 s, and RT–qPCR was performed using a CFX Connect System (Bio-Rad). The expression of target mRNAs was analyzed by the ΔΔCt method, and mouse β-actin and GAPDH were used as an endogenous control. All fold changes were calculated relative to the control group. The base sequences of the primers are shown in
Table 1.
4.8. Western Blot Analysis
Brain tissues were lysed with PRO-PREP (iNtRON Biotechnology) in ice-cold conditions (4°C) for 20 min. The protein concentration of each sample was determined using the Bradford assay. Samples (30 μg of protein) were resolved on a 10% sodium dodecyl sulfate-polyacrylamide gel and transferred to a nitrocellulose membrane, which was blocked for 30 min in 5% skim milk at room temperature. After 30 min of blocking, the membrane was incubated overnight at 4°C with specific antibodies against CD86 (1:1000; Cell Signaling Technology), iNOS (1:2000; Santa Cruz Biotechnology, Dallas, TX, USA), or β-actin (1:5000; Santa Cruz Biotechnology). After washing with Tris-buffered saline containing 25 mM Tris-Cl, 150 mM NaCl, and 0.05% Tween-20, the membrane was incubated for 2 h with a horseradish peroxidase-conjugated secondary antibody (anti-rabbit or anti-mouse IgG). Immunoreactivity was visualized with a Western Blotting Detection Reagent Kit (Thermo, Waltham, MA, USA). Antibody binding was determined using a chemiluminescence detection system. Densitometric analysis was performed using ImageJ 1.53e.
4.9. ELISA
To measure TNF-α, IL-6 and IL-1β and in brain tissues, protein extracts were collected used and analyzed by ELISA according to the manufacturer’s protocol (R&D Systems, Minneapolis, MN, USA).
4.10. Statistical Analysis
GraphPad Prism 10.4.0 software (GraphPad Software Inc., San Diego, CA, USA) was used for statistical analyses. A one-way analysis of variance (ANOVA) was performed, followed by Tukey's multiple comparison tests. Two-tailed Student's unpaired test was performed for comparing the two groups. All experiments were performed in a blinded manner and were repeated independently under identical conditions. Data were expressed as the mean ± SEM. p < 0.05 was considered as significant.
Figure 1.
CX3CR1 retrovirus-transduced Tregs migrate to the brain. (A) Schematic Representation of Experimental Workflow, engineering of cx3cr1 Treg and subsequent administration into mice. (B) CX3CR1 expression in transduced Tregs by flow cytometry to confirm the wild type Treg isolation and transduction (CX3CR1+Flag+). Retrovirus transduced Tregs (CX3CR1-Flag- and CX3CR1+Flag+) were isolated for adoptive transfer. (C) Flow cytometry analysis was conducted to assess the migration of Tregs to the brain. Upon examining the lymph nodes, kidneys, and brains, a significantly higher presence of CX3CR1+Flag+ Tregs was observed in the brain tissues. Data are presented as the mean ± SEM. n = 5 per group; * p < 0.05, ** p < 0.01, *** p < 0.001.
Figure 1.
CX3CR1 retrovirus-transduced Tregs migrate to the brain. (A) Schematic Representation of Experimental Workflow, engineering of cx3cr1 Treg and subsequent administration into mice. (B) CX3CR1 expression in transduced Tregs by flow cytometry to confirm the wild type Treg isolation and transduction (CX3CR1+Flag+). Retrovirus transduced Tregs (CX3CR1-Flag- and CX3CR1+Flag+) were isolated for adoptive transfer. (C) Flow cytometry analysis was conducted to assess the migration of Tregs to the brain. Upon examining the lymph nodes, kidneys, and brains, a significantly higher presence of CX3CR1+Flag+ Tregs was observed in the brain tissues. Data are presented as the mean ± SEM. n = 5 per group; * p < 0.05, ** p < 0.01, *** p < 0.001.
Figure 2.
CX3CR1+ Treg improved the memory function of the LPS-treated mice. (A) Schematic representation of the workflow, including the timeline of the experiments and tests. 2 weeks after the injection, mice were performed behavior test and sacrificed for analysis of Treg homing. (B) In the passive avoidance test, significant intergroup differences were found in terms of the time taken to enter the dark compartment. (C) Spontaneous alternation behavior % of the mice during the Y-maze test. Data are presented as the mean ± SEM. n = 5 per group; ** p < 0.01, *** p < 0.001.
Figure 2.
CX3CR1+ Treg improved the memory function of the LPS-treated mice. (A) Schematic representation of the workflow, including the timeline of the experiments and tests. 2 weeks after the injection, mice were performed behavior test and sacrificed for analysis of Treg homing. (B) In the passive avoidance test, significant intergroup differences were found in terms of the time taken to enter the dark compartment. (C) Spontaneous alternation behavior % of the mice during the Y-maze test. Data are presented as the mean ± SEM. n = 5 per group; ** p < 0.01, *** p < 0.001.
Figure 3.
Homing of CX3CR1+ Treg and its effect on activated microglia expression. (A) Distribution of CX3CR1+Flag+ cells visualized by staining against Flag-Tag on paramedian sagittal brain section. (B) High resolution analysis of CX3CR1+Flag+ cells in hippocampus region. (C) Representative immunofluoresce+nce images and quantified histogram of Iba1 in the hippocampus DG region of the mice (5 mice/group). Scale bar = 200 μm. The expressed data are relative to the control. * p < 0.05.
Figure 3.
Homing of CX3CR1+ Treg and its effect on activated microglia expression. (A) Distribution of CX3CR1+Flag+ cells visualized by staining against Flag-Tag on paramedian sagittal brain section. (B) High resolution analysis of CX3CR1+Flag+ cells in hippocampus region. (C) Representative immunofluoresce+nce images and quantified histogram of Iba1 in the hippocampus DG region of the mice (5 mice/group). Scale bar = 200 μm. The expressed data are relative to the control. * p < 0.05.
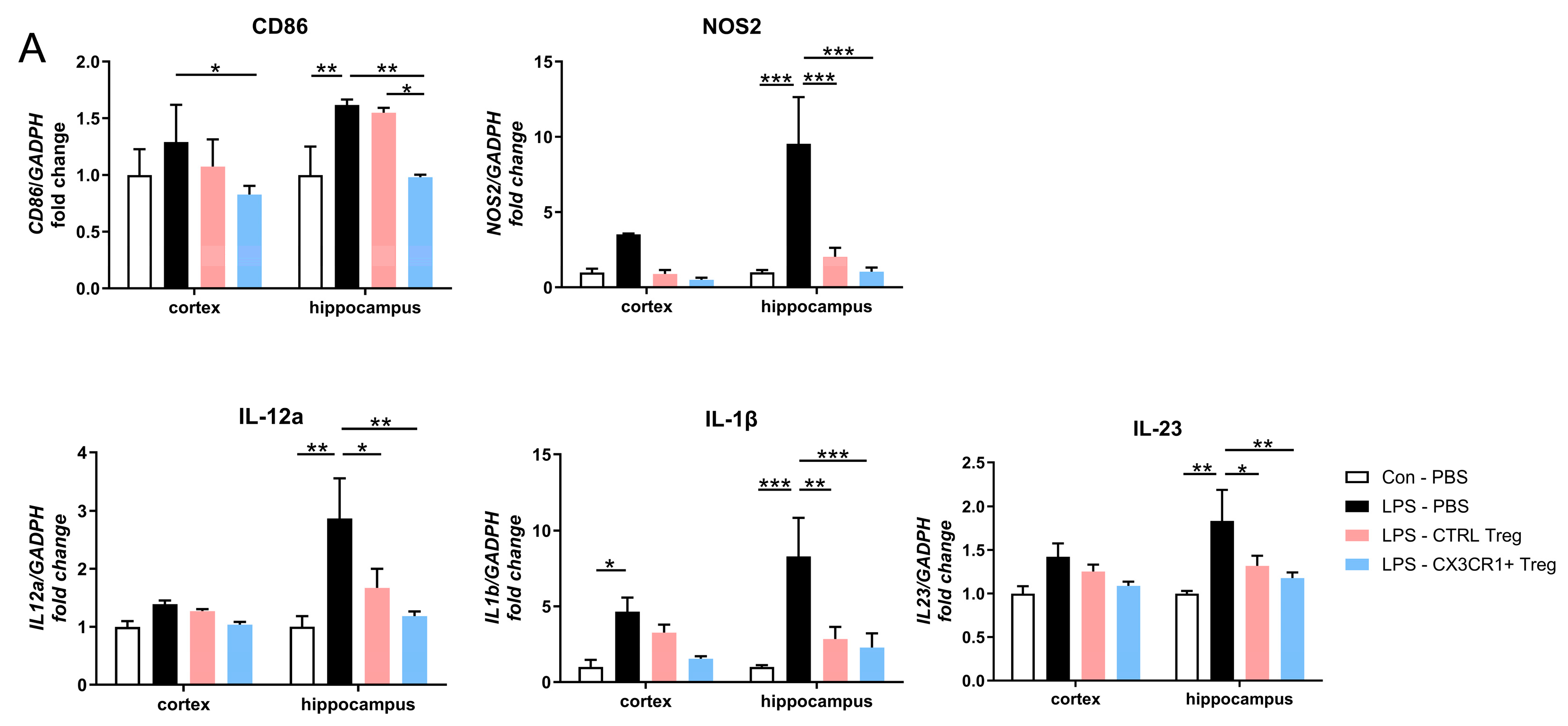
Figure 4.
Treg downregulates proinflammatory mRNA expression. (A) Quantitative RT-PCR analysis of genes encoding for proinflammatory markers, CD86, NOS2, IL12a, IL1b and IL23, in cortex and hippocampus compared to control and LPS-treated group. Relative fold changes indicate mean ± SEM from three independent experiments (5 mice/group). * p < 0.05, ** p < 0.01, *** p < 0.001.
Figure 4.
Treg downregulates proinflammatory mRNA expression. (A) Quantitative RT-PCR analysis of genes encoding for proinflammatory markers, CD86, NOS2, IL12a, IL1b and IL23, in cortex and hippocampus compared to control and LPS-treated group. Relative fold changes indicate mean ± SEM from three independent experiments (5 mice/group). * p < 0.05, ** p < 0.01, *** p < 0.001.
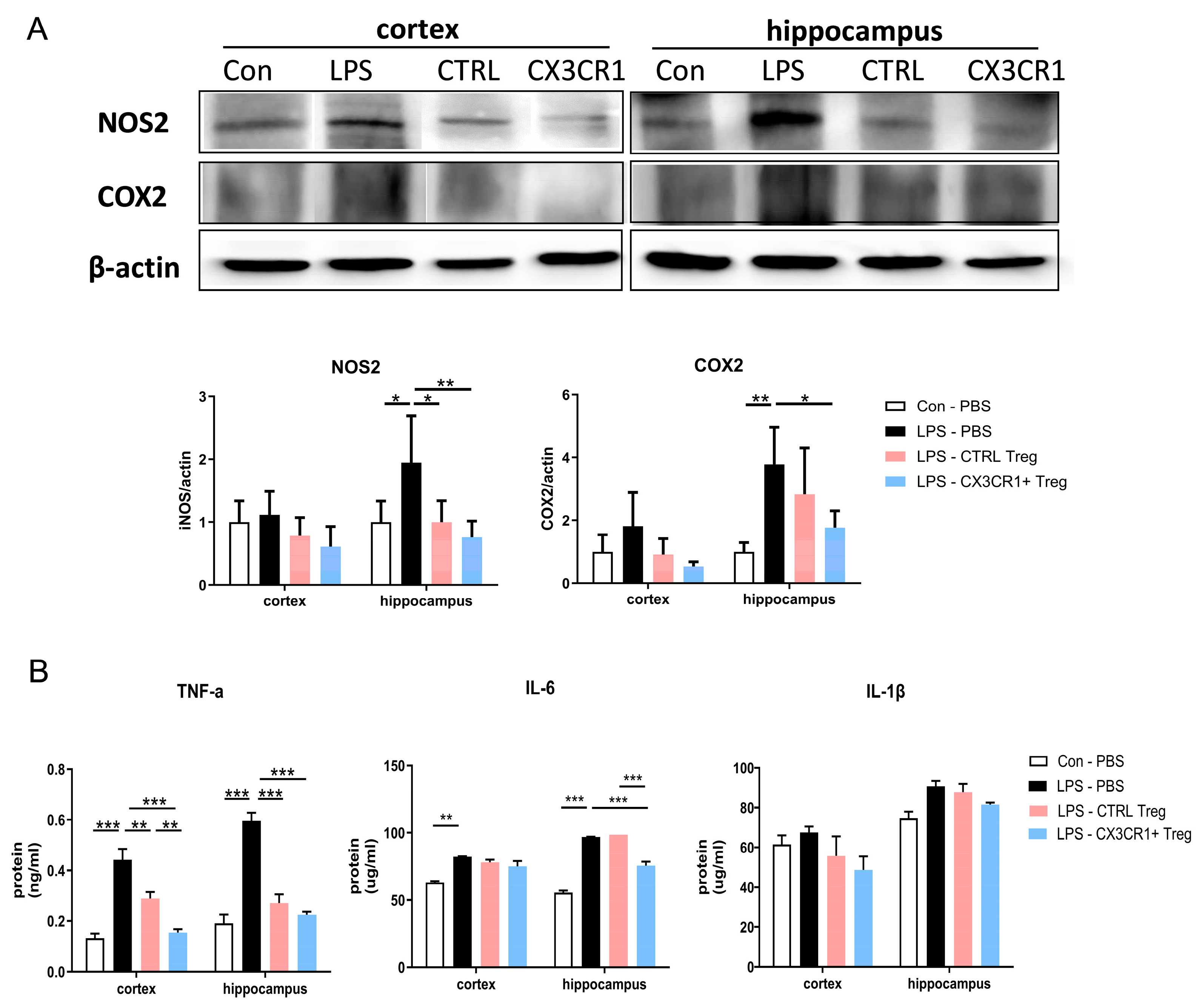
Figure 5.
CX3CR1+ Tregs effectively halted the activation of inflammatory pathways and subdued the expression of proinflammatory cytokines in an LPS-induced mouse model. (A) Western blotting of NOS2 and COX2 proteins. The expression differences in the cortex and hippocampus of mouse brains are represented by a histogram. β-Actin was used as a loading control. (B) The expression of the proinflammatory cytokines TNF-α, IL-6, and IL-1β in brain homogenates. The data are described as the mean ± SEM (5 mice/group). * p < 0.05, ** p < 0.01, *** p < 0.001.
Figure 5.
CX3CR1+ Tregs effectively halted the activation of inflammatory pathways and subdued the expression of proinflammatory cytokines in an LPS-induced mouse model. (A) Western blotting of NOS2 and COX2 proteins. The expression differences in the cortex and hippocampus of mouse brains are represented by a histogram. β-Actin was used as a loading control. (B) The expression of the proinflammatory cytokines TNF-α, IL-6, and IL-1β in brain homogenates. The data are described as the mean ± SEM (5 mice/group). * p < 0.05, ** p < 0.01, *** p < 0.001.
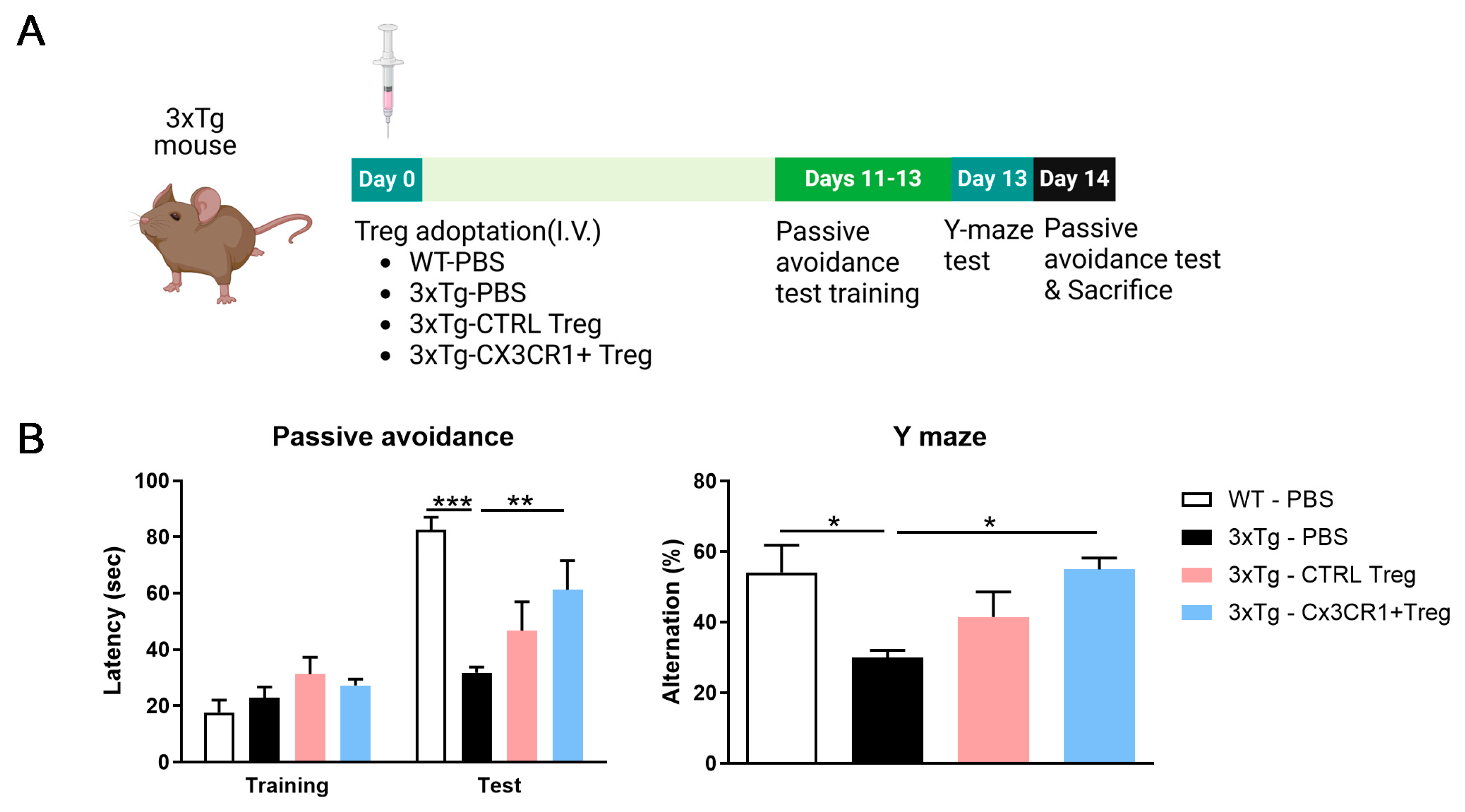
Figure 6.
CX3CR1+ Tregs Alleviate Cognitive Decline in 3xTg mouse model (A) Schematic representation of the workflow, including the timeline of the experiments and tests. 2 weeks after the injection, mice were performed behavior test and sacrificed for analysis of Treg homing. (B) In the passive avoidance test, 3xTg mice treated with 1 x 105 CX3CR1+ Tregs displayed a significantly longer latency to enter the dark compartment compared to PBS-treated 3xTg controls, indicating improved cognitive retention. (C) Y-maze testing revealed that CX3CR1+ Tregs enhanced spontaneous alternation behavior in 3xTg mice, suggesting improved spatial working memory. Unlike control Tregs, CX3CR1+ Tregs showed a clear cognitive benefit in this model of Alzheimer’s disease. Data are presented as the mean ± SEM. n = 5 per group; ; * p < 0.05, ** p < 0.01, *** p < 0.001.
Figure 6.
CX3CR1+ Tregs Alleviate Cognitive Decline in 3xTg mouse model (A) Schematic representation of the workflow, including the timeline of the experiments and tests. 2 weeks after the injection, mice were performed behavior test and sacrificed for analysis of Treg homing. (B) In the passive avoidance test, 3xTg mice treated with 1 x 105 CX3CR1+ Tregs displayed a significantly longer latency to enter the dark compartment compared to PBS-treated 3xTg controls, indicating improved cognitive retention. (C) Y-maze testing revealed that CX3CR1+ Tregs enhanced spontaneous alternation behavior in 3xTg mice, suggesting improved spatial working memory. Unlike control Tregs, CX3CR1+ Tregs showed a clear cognitive benefit in this model of Alzheimer’s disease. Data are presented as the mean ± SEM. n = 5 per group; ; * p < 0.05, ** p < 0.01, *** p < 0.001.
Figure 7.
CX3CR1+ Tregs home to sites of neuroinflammation and reduce activated microglial expression in a 3xTg mouse model. (A)Visualization of CX3CR1+ Tregs in Mouse Brain Subregions. Enhanced Flag-tag expression was predominantly observed in the hippocampal region, indicating targeted homing of these cells to areas of neuroinflammation. (B) Effect of Tregs on Aβ Accumulation in the Hippocampus. Immunostaining of the CA1 and dentate gyrus (DG) regions for Aβ revealed that while CTRL-Tregs led to a reduction in Aβ deposits, CX3CR1+ Tregs resulted in a more substantial decrease in these Alzheimer’s disease markers. (C) Representative immunofluorescence images and quantified histogram of Iba1 in the hippocampus DG and CA1 region of 3xTg Mice. Treatment with CX3CR1+ Tregs significantly reduced Iba1 reactivity in the hippocampal CA1 region, surpassing the effects seen with CTRL-Tregs, which showed no significant impact on microglial activation. Scale bar = 200 μm. The expressed data are relative to the control. Data are presented as the mean ± SEM. n = 5 per group; *p < 0.05, ** p < 0.01, *** p < 0.001.
Figure 7.
CX3CR1+ Tregs home to sites of neuroinflammation and reduce activated microglial expression in a 3xTg mouse model. (A)Visualization of CX3CR1+ Tregs in Mouse Brain Subregions. Enhanced Flag-tag expression was predominantly observed in the hippocampal region, indicating targeted homing of these cells to areas of neuroinflammation. (B) Effect of Tregs on Aβ Accumulation in the Hippocampus. Immunostaining of the CA1 and dentate gyrus (DG) regions for Aβ revealed that while CTRL-Tregs led to a reduction in Aβ deposits, CX3CR1+ Tregs resulted in a more substantial decrease in these Alzheimer’s disease markers. (C) Representative immunofluorescence images and quantified histogram of Iba1 in the hippocampus DG and CA1 region of 3xTg Mice. Treatment with CX3CR1+ Tregs significantly reduced Iba1 reactivity in the hippocampal CA1 region, surpassing the effects seen with CTRL-Tregs, which showed no significant impact on microglial activation. Scale bar = 200 μm. The expressed data are relative to the control. Data are presented as the mean ± SEM. n = 5 per group; *p < 0.05, ** p < 0.01, *** p < 0.001.

Figure 8.
Suppression of Disease-Associated Microglial Activation by CX3CR1+ Tregs (A) Quantitative RT-PCR Analysis of Disease-Associated Microglial (DAM) Markers in the Cortex and Hippocampus of 3xTg Mice. Markers assessed include CX3CR1 and CSF1, associated with microglial activation, and TMEM119, linked to phagocytic activity. CCL6 and TREM2, related to immune cell recruitment and neuroprotection, respectively, are shown alongside ITGAX, a marker involved in microglial activation. CX3CR1 and CSF1 expressions decreased, indicating reduced activation, while TMEM119 expression increased, reflecting improved phagocytic function. Expressions of CCL6, were evaluated to demonstrate the comprehensive impact of CX3CR1+ Tregs on neuroinflammation. Data are presented as the mean ± SEM. n = 5 per group; *p < 0.05, ** p < 0.01, *** p < 0.001.
Figure 8.
Suppression of Disease-Associated Microglial Activation by CX3CR1+ Tregs (A) Quantitative RT-PCR Analysis of Disease-Associated Microglial (DAM) Markers in the Cortex and Hippocampus of 3xTg Mice. Markers assessed include CX3CR1 and CSF1, associated with microglial activation, and TMEM119, linked to phagocytic activity. CCL6 and TREM2, related to immune cell recruitment and neuroprotection, respectively, are shown alongside ITGAX, a marker involved in microglial activation. CX3CR1 and CSF1 expressions decreased, indicating reduced activation, while TMEM119 expression increased, reflecting improved phagocytic function. Expressions of CCL6, were evaluated to demonstrate the comprehensive impact of CX3CR1+ Tregs on neuroinflammation. Data are presented as the mean ± SEM. n = 5 per group; *p < 0.05, ** p < 0.01, *** p < 0.001.
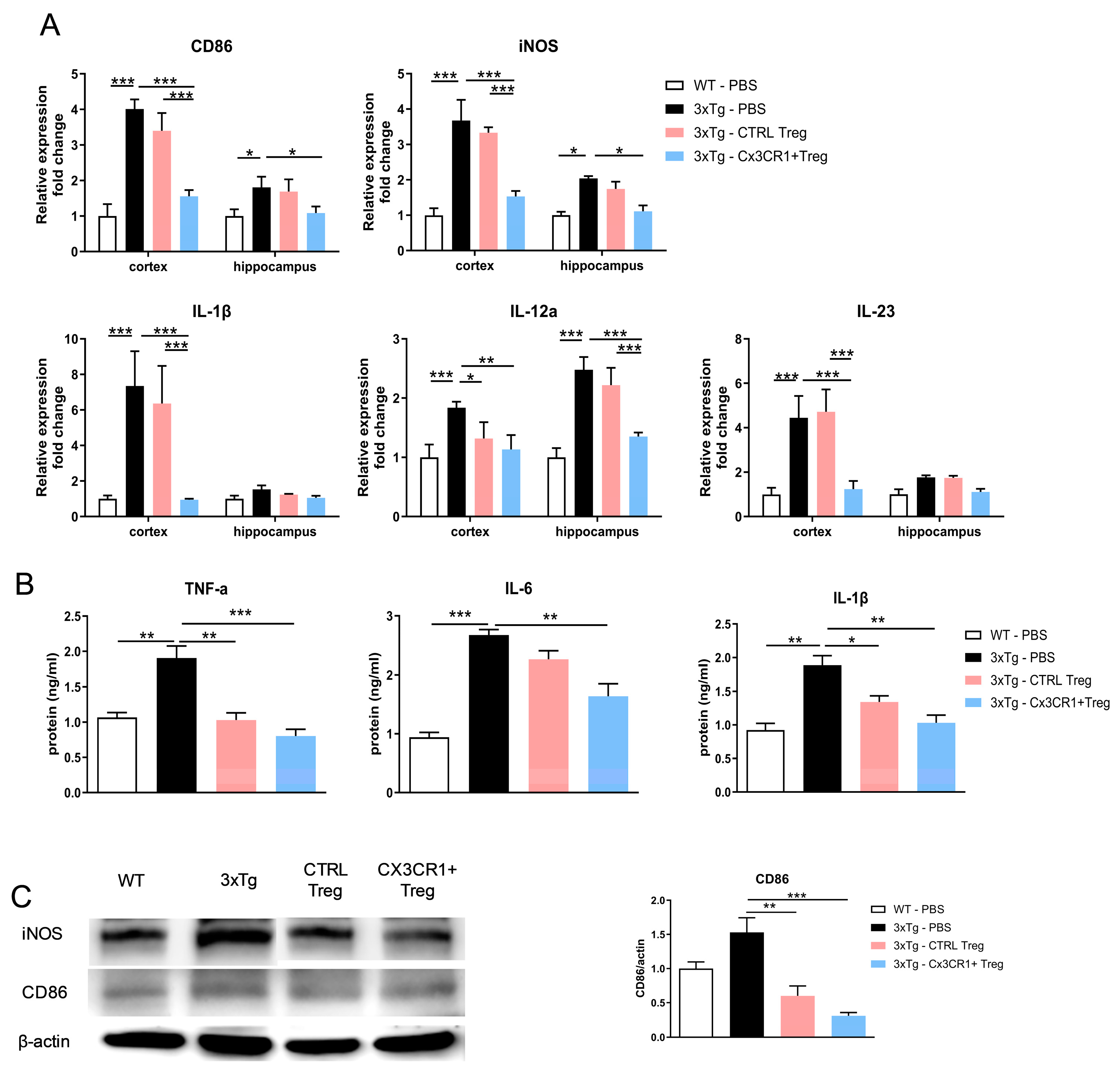
Figure 9.
Effectiveness of CX3CR1+ Tregs in suppressing inflammatory responses (A) RT-qPCR Analysis of Proinflammatory Gene Expression in the Cortex and Hippocampus. RT-qPCR was used to evaluate the expression of proinflammatory genes CD86, iNOS, IL-1β, IL-12a, and IL-23 in the cortex and hippocampus. β-Actin served as an internal control for calculating fold changes in gene expression. (B) ELISA Results of Proinflammatory Cytokines in 3xTg Mice. CX3CR1+ Tregs significantly decreased the levels of TNF-α, IL-6, and IL-1β. (C) Western Blot Analysis of CD86 and iNOS Protein Levels in the Brain. While CTRL-Tregs reduced their expression, CX3CR1+ Tregs achieved a greater decrease. Data are presented as the mean ± SEM. n = 5 per group; * p < 0.05, ** p < 0.01, *** p < 0.001.
Figure 9.
Effectiveness of CX3CR1+ Tregs in suppressing inflammatory responses (A) RT-qPCR Analysis of Proinflammatory Gene Expression in the Cortex and Hippocampus. RT-qPCR was used to evaluate the expression of proinflammatory genes CD86, iNOS, IL-1β, IL-12a, and IL-23 in the cortex and hippocampus. β-Actin served as an internal control for calculating fold changes in gene expression. (B) ELISA Results of Proinflammatory Cytokines in 3xTg Mice. CX3CR1+ Tregs significantly decreased the levels of TNF-α, IL-6, and IL-1β. (C) Western Blot Analysis of CD86 and iNOS Protein Levels in the Brain. While CTRL-Tregs reduced their expression, CX3CR1+ Tregs achieved a greater decrease. Data are presented as the mean ± SEM. n = 5 per group; * p < 0.05, ** p < 0.01, *** p < 0.001.
Table 1.
The base sequences of the primers.
Table 1.
The base sequences of the primers.
| Gene name |
Forward primer sequence (5`-3`) |
Reverse primer sequence (5`-3`) |
| β-Actin |
GTG CTA TGT TGC TCT AGA CTT CG |
ATG CCA CAG GAT TCC ATA CC |
| CX3CR1 |
GGA GAC TGG AGC CAA CAG AG |
TCT TGT CTG GCT GTG TCC TG |
| CD86 |
GAC CGT TGT GTG TGT TCT GG |
GAT GAG CAG CAT CAC AAG GA |
| iNOS |
CAG CTG GGC TGT ACA AAC CTT |
CAT TGG AAG TGA AGC GTT TCG |
| IL-1β |
AAG CCT CGT GCT GTC GGA CC |
TGA GGC CCA AGG CCA CAG G |
| IL-12a |
AAG CTC TGC ATC CTG CTT CAC |
GAT AGC CCA TCA CCC TGT TGA |
| IL-23 |
CCT TCT CCG TTC CAA GAT CCT |
ACT AAG GGC TCA GTC AGA GTT GCT |
| CCL6 |
ATG ATG AGA CAT TCC AAG ACT GC |
TCA AGC AAT GGC ACT GTT CCC AGA |
| ITGAX |
CTG GAT AGC CTT TCT TCT GCT G |
GCA CAC TGT GTC CGA ACT CA |
| CSF1 |
AGT ATT GCC AAG GAG GTG TCA G |
ATC TGG CAT GAA GTC TCC ATT T |
| TREM2 |
AGG GCC CAT GCC AGC GTG TGG T |
CCA GAG ATC TCC AGC ATC |
| TMEM119 |
GTG TCT AAC AGG CCC CAG AA |
AGC CAC GTG GTA TCA AGG AG |
| GAPDH |
CAA CTC CCA CTC TTC CAC CT |
GAG TTG GGA TAG GGC CTC TC |