Submitted:
28 November 2024
Posted:
29 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Metals
2.2. Fuel and Oxidizer for the Pulsed Detonation Gun
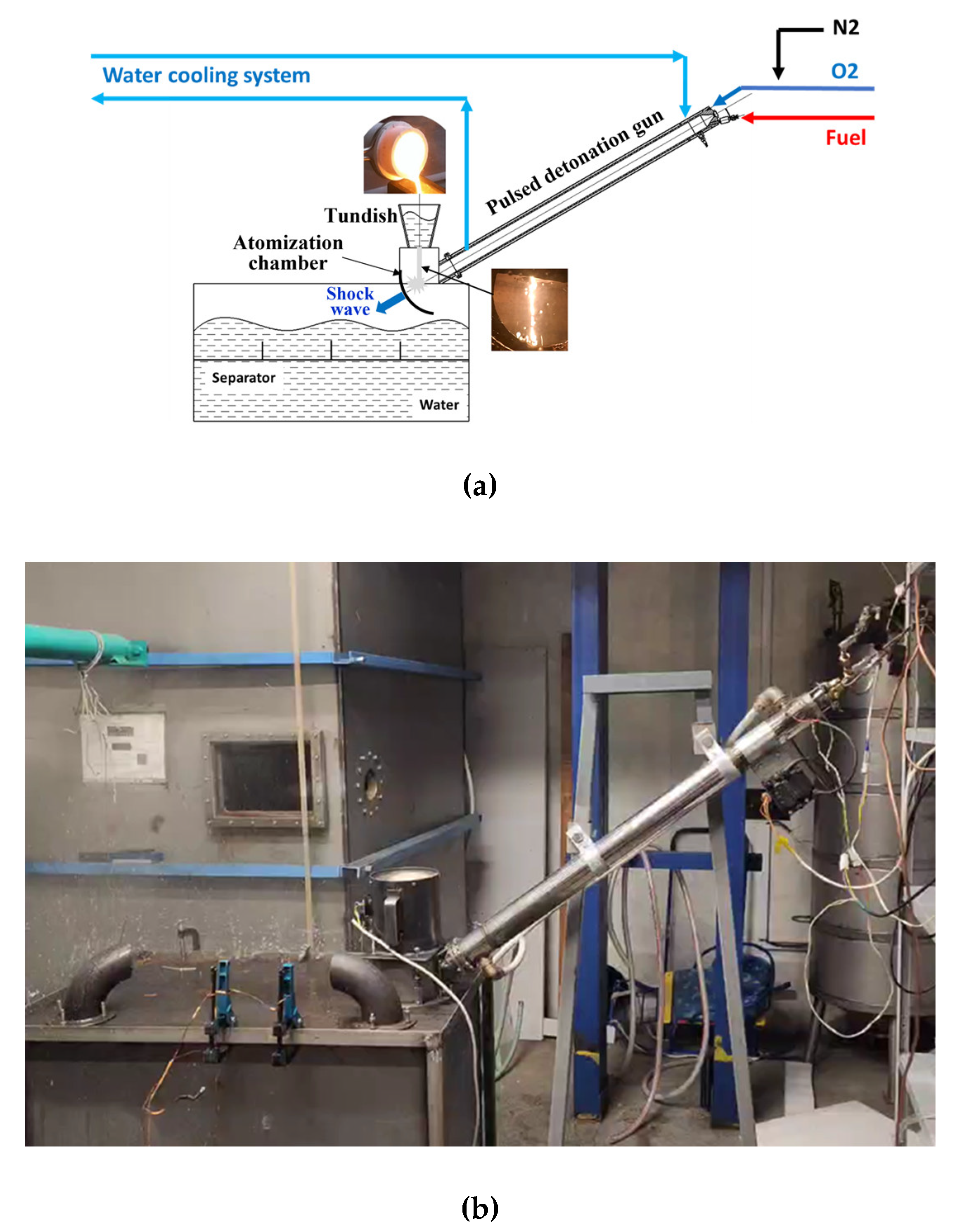
2.3. Experimental Setup
2.4. Measuring Tchniques
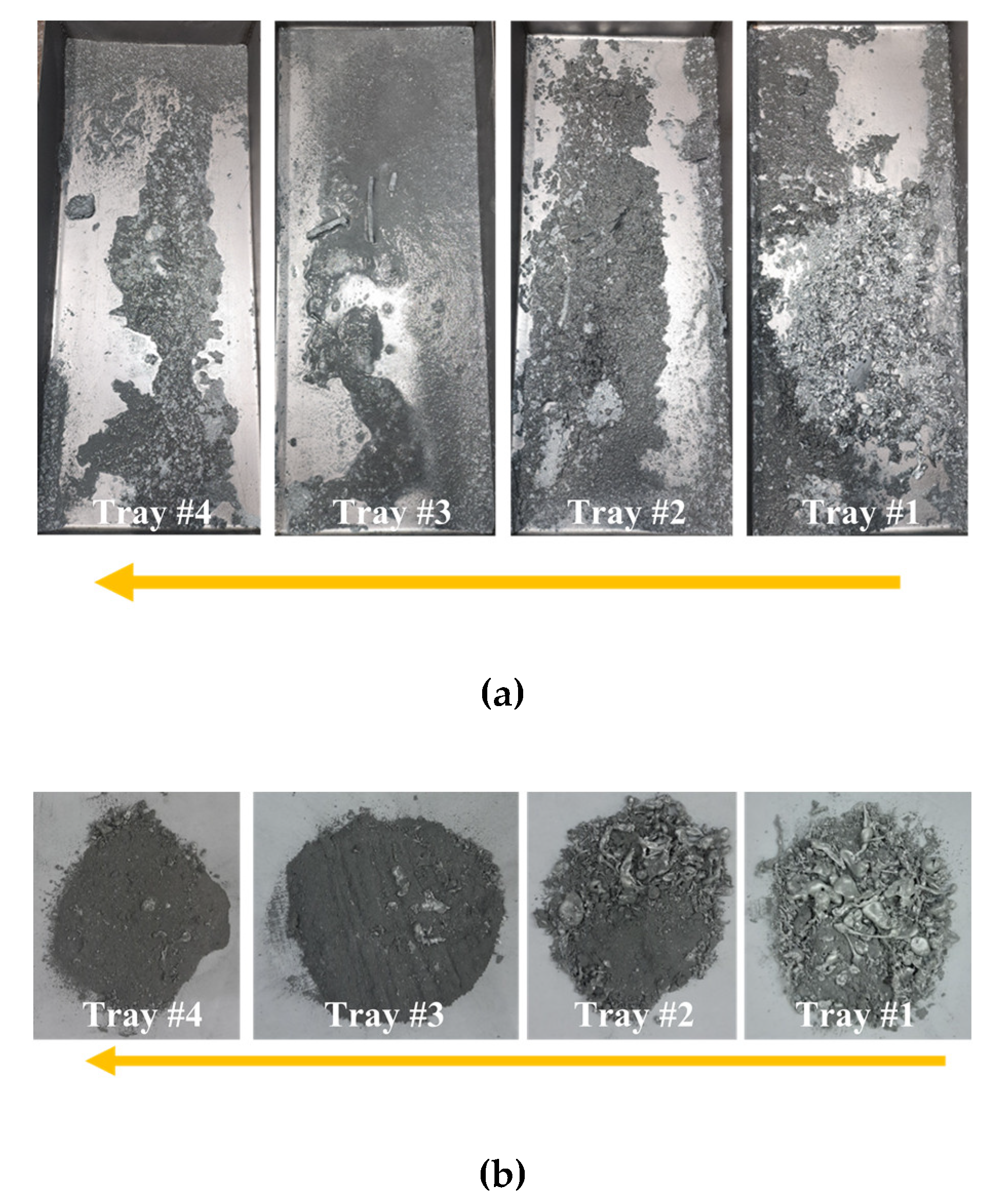
3. Results
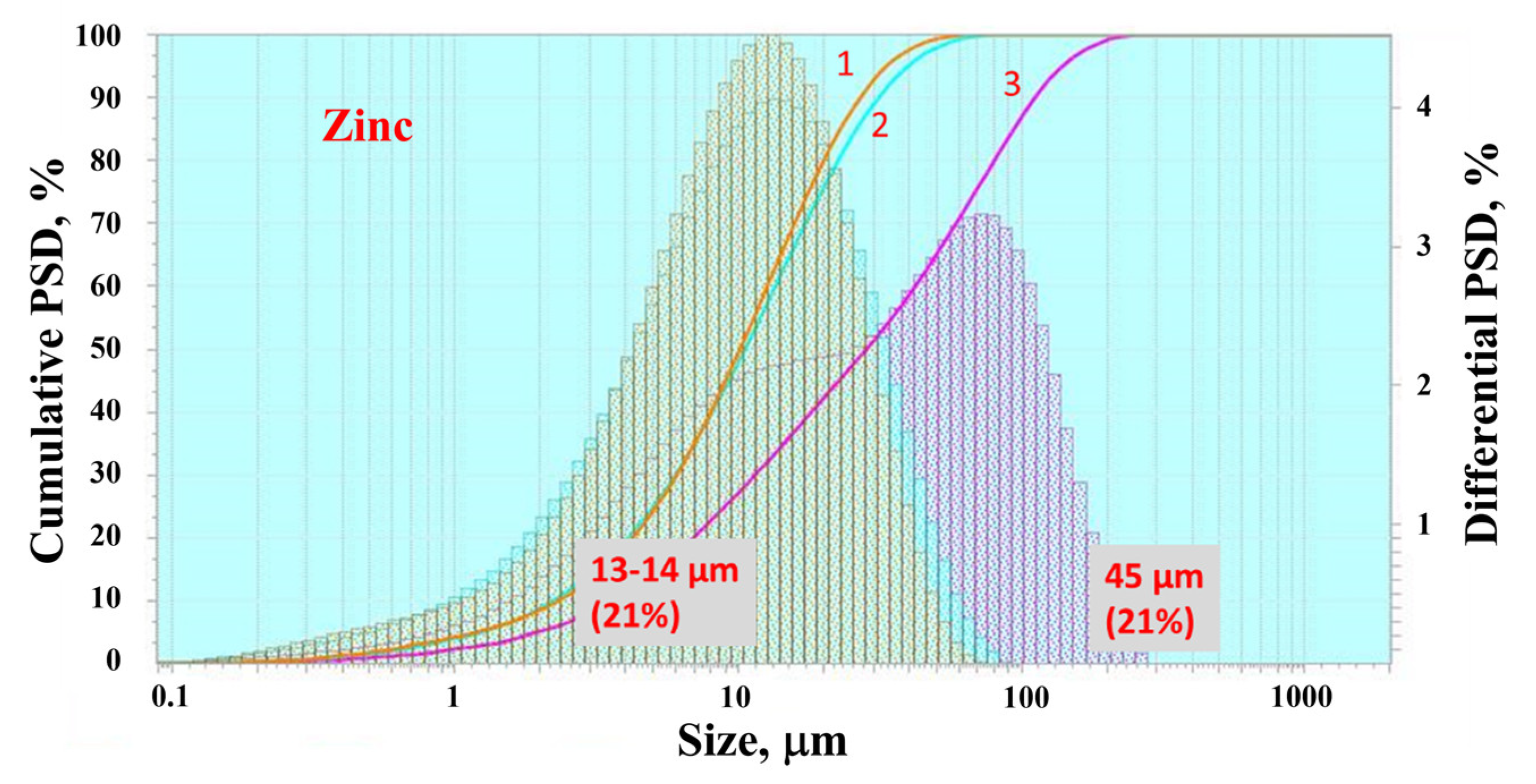
3.1. Zinc
3.2. Aluminum
3.3. Stainless Steel
4. Discussion
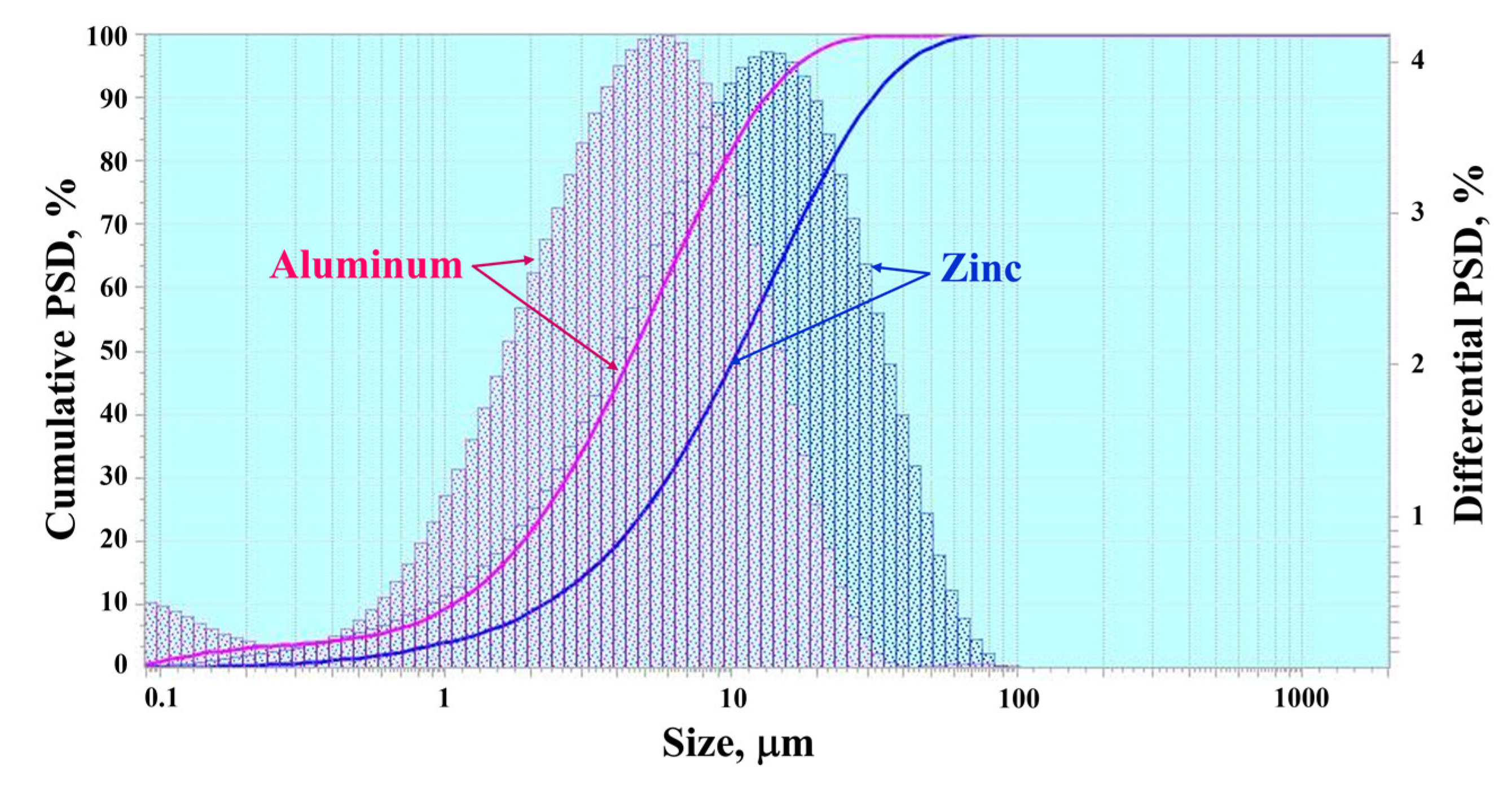
4.1. Comparison of Particle Size Distributions
4.2. Phenomenology of Melt Stream Atomization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, N.; Leu, M.C. Additive manufacturing: technology, applications and research needs. Front. Mech. Eng. 2013, 8, 215–243. [Google Scholar] [CrossRef]
- Zhou, L.; Miller, J.; Vezza, J.; Mayster, M.; Raffay, M.; Justice, Q.; Al Tamimi, Z.; Hansotte, G.; Sunkara, L.D.; Bernat, J. Additive manufacturing: A comprehensive review. Sensors 2024, 24, 2668. [Google Scholar] [CrossRef] [PubMed]
- Milewski, J.O. Additive Manufacturing of Metals: From Fundamental Technology to Rocket Nozzles, Medical Implants, and Custom Jewelry. Springer: Berlin/Heidelberg, Germany, 2017.
- El-Eskandarany, M.; Sherif; Aoki, K.; Sumiyama, K.; Suzuki, K. Mater. Sci. Forum 1992, 88, 801.
- El-Eskandarany, M.; Sherif; Saida, J.; Inoue, A. Acta mater. 2003, 51, 1481.
- Champagne, B.; Angers, R. REP atomization mechanism. Powder Metall. Int. 1984;16, 125–8.
- Bondarev, B.I.; Shmakov, Y.V. Technology of rapidly solidified Al alloy production. Moscow: All-Russian Institute of Light Alloys Publishers; 1997.
- Huo, S.H.; Qian, M.; Schaffer, G.B.; Crossin, E. Aluminium powder metallurgy. In: Fundamentals of Aluminium Metallurgy; Production, Processing and Applications, ed. by R. Lumley. Woodhead Publishing Limited, Cambridge, UK, 2011, 655-701.
- Xia, Y.; Fang, Z.Z.; Sun, P.; Zhang, Y.; Zhu, J. Novel method for making biomedical segregation-free Ti-30Ta alloy spherical powder for additive manufacturing. JOM 2018, 70, 364–369. [Google Scholar] [CrossRef]
- Singh, D.; Koria, S.C.; Dube, R.K. Study of free fall gas atomisation of liquid metals to produce powder. Powder Metall. 2001, 44, 177–184. [Google Scholar] [CrossRef]
- Lawley, A. Atomization of specialty alloy powders. JOM Orig. 2014, 33(1), 13–18. [Google Scholar] [CrossRef]
- Dawes, J.; Bowerman, R.; Trepleton, R. Introduction to the additive manufacturing powder metallurgy supply chain. Johnson Matthey Technol. Rev. 2015, 59, 243–256. [Google Scholar] [CrossRef]
- Ciftcia, N.; Ellendta, N.; Mladlera, L.; Uhlenwinkel, V. Impact of hot gas atomization on glass forming alloys. In: Compiled by European powder metallurgy association. Bellstone Shrewsbury, UK. Proceedings of PM 2016 world congress, Hamburg, Germany; 2016. p. 1–7.
- Lohner, H.; Czisch, C.; Schreckenberg, P.; Fritsching, U.; Bauckhage, K. Atomization of viscous melts. Atomiz. Spr. 2005, 15, 169–180. [Google Scholar] [CrossRef]
- Wolf, G.; Neoth, M.; Schubert, M.V.E.; Bergmann, H.W. Production and characterization of liquid gas atomized hard magnetic NdFeB alloy powders for bonded isotropic magnets. In: Proceedings of the powder metallurgy world congress, vol. 3. 1994. p. 1745–53.
- Wolf, G.; Lang, A.; Bergmann, H.W. Investigations on melt atomization with gas and liquefied cryogenic gas. In: Proceedings of international conference on spray deposition and melt forming. Bremen, Germany: Bremen Universit€at; 2000. p. 535–47.
- Dunkley, J.J. Atomization. In: ASM handbook, vol. 7. ASM International Publishers; 1998. p.
- Small, S.; Bruce, T.J. The comparison of characteristics of water and inert gas atomized powders. Int. J. Powder Metall. 1968, 4, 7–17. [Google Scholar]
- Cao, W.; Shu, J.; Chen, J. Enhanced recovery of high-purity Fe powder from iron-rich electrolytic manganese residue by slurry electrolysis. Int. J. Miner. Metall. Mater. 2024, 31, 531–538. [Google Scholar] [CrossRef]
- Rai, V.; Liu, D.; Xia, D.; Jayaraman, Y.; Gabriel, J.-C.P. Electrochemical approaches for the recovery of metals from electronic waste: A critical review. Recycling 2021, 6, 53–ff10.3390. [Google Scholar] [CrossRef]
- Dutta, B.; Babu, S.; Jared, B.H. Science, Technology and Applications of Metals in Additive Manufacturing. Elsevier Science, 2019.
- Smagorinski, M.E.; Tsantrizos, P.G. Production of spherical titanium powder by plasma atomization. In: Proceedings of 2002 world congress of PM and particulate materials, vol. 3. Orlando, FL: Metal Powder Industries Federation; 2002. p. 248–60.
- Alagheband, A.; Brown, C. Plasma atomization goes commercial. Met. Powder Rep. 1998, 53, 26–28. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, S.Y.; Tan, P.; Wang, J.; Xiang, C.S.; Tanga, H.P. A comparative study of Ti-6Al-4V powders for additive manufacturing by gas atomization, plasma rotating electrode process and plasma atomization. Powder Techn. 2018, 333, 38–46. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Amal, R.; Madler, L. Flame spray pyrolysis: an enabling technology for nanoparticles design and fabrication. Nanoscale 2010, 2, 1324–47. [Google Scholar] [CrossRef]
- Cui, C.; Stern, F.; Ellendt, N.; Uhlenwinkel, V.; Steinbacher, M.; Tenkamp, J.; Walther, F.; Fechte-Heinen, R. Gas atomization of duplex stainless steel powder for laser powder bed fusion. Materials 2023, 16, 435. [Google Scholar] [CrossRef]
- Mathias, L.E.T.; Andreoli, A.F.; Gargarella, P. Gas atomization of A2 tool steel: Effect of process parameters on powders’ physical properties. J. Alloys Compounds 2023, 960, 170696. [Google Scholar] [CrossRef]
- Cui, C.; Uhlenwinkel, V.; Schulz, A.; Zoch, H.-W. Austenitic stainless steel powders with increased nitrogen content for laser additive manufacturing. Metals 2020, 10, 61. [Google Scholar] [CrossRef]
- Fritsching, U. Spray Simulation: Modeling and Simulation of Sprayforming Metals. Cambridge: Cambridge University Press, 2004.
- Gibson, I.; Rosen, D.; Stucker, B.; Khorasani, M.; Gibson, I.; Rosen, D.; Stucker, B.; Khorasani, M. Materials for additive manufacturing. Addit. Manuf. Technol. 2021, Third volume, 379–428. [Google Scholar]
- Ciftci, N.; Ellendt, N.; Soares Barreto, E.; Mädler, L.; Uhlenwinkel, V. Increasing the amorphous yield of {(Fe0.6Co0.4)0.75B0.2Si0.05}96Nb4 powders by hot gas atomization. Adv. Powder Technol. 2018, 29, 380–385. [Google Scholar] [CrossRef]
- Goudar, D.M.; Srivastava, V.; Rudrakshi, G. Effect of atomization parameters on size and morphology of Al-17Si alloy powder produced by free fall atomizer. Eng. J. 2017, 21, 155–168. [Google Scholar] [CrossRef]
- See, J.B.; Johnston, G.H. Interactions between nitrogen jets and liquid lead and tin streams. Powder Technol. 1978, 21, 119–25. [Google Scholar]
- Haferkamp, L.; Haudenschild, L.; Spierings, A.; Wegener, K.; Riener, K.; Ziegelmeier, S.; Leichtfried, G.J. The influence of particle shape, powder flowability, and powder layer density on part density in laser powder bed fusion. Metals 2021, 11, 418. [Google Scholar] [CrossRef]
- Fereiduni, E.; Ghasemi, A.; Elbestawi, M. Characterization of composite powder feedstock from powder bed fusion additive manufacturing perspective. Materials 2019, 12, 3673. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, Sh.; Huang, B.; Liu, Z.; Du, Y. Densification behaviour of Al-Ni-Y powder containing amorphous and nanocrystalline phases. In: Proceedings PM2004 world congress, vol. 1. Wien, Austria: European Powder Metallurgy Association; 2004. p. 425–30.
- Lubanska, H. Correlation of spray ring data for gas atomization of liquid metals. J. Met. 1970, 22, 45–49. [Google Scholar] [CrossRef]
- Gärtner, E.; Jung, H.Y.; Peter, N.J.; Dehm, G.; Jägle, E.A.; Uhlenwinkel, V.; Mädler, L. Reducing cohesion of metal powders for additive manufacturing by nanoparticle dry-coating. Powder Technol. 2021, 379, 585–595. [Google Scholar] [CrossRef]
- Frolov, S.M.; Smetanyuk, V.A.; Nabatnikov, S.A.; Moiseev, A.V.; Andrienko, V.G.; Piletsky, V.G. Method of ultrafine atomization of liquid fuel and device for its implementation. Patent of the Russian Federation No. 2644422 dated 12.02. 2018.
- Hsiang, L.-P.; Faeth, G.M. Near-limit drop deformation and secondary breakup. In. J. Multiphase Flow 1992, 18, 635–652. [Google Scholar] [CrossRef]
- Guildenbecher, D.R.; Gao, J.; Chen, J.; Sojka, P.E. Characterization of drop aerodynamic fragmentation in the bag and sheet-thinning regimes by crossed-beam, two-view, digital in-line holography. Int. J. Multiphase Flow 2017, 94, 107–122. [Google Scholar] [CrossRef]
- Reuter, C.B.; Tuttle, S.G. Interactions between liquid sprays and shock waves in underexpanded flows. Proc. Combust. Inst. 2024, 40, 105244. [Google Scholar] [CrossRef]
- Chen, Y.; Wagner, J.L.; Farias, P.A.; DeMauro, E.P.; Guildenbecher, D.R. Galinstan liquid metal breakup and droplet formation in a shock-induced cross-flow. Int. J. Multiphase Flow 2018, 106, 147–163. [Google Scholar] [CrossRef]
- SDToolBox – Numerical tools for shock and detonation wave modeling, https://shepherd.caltechedu/SDT.
- Goodwin, D. G.; Moffat, H. K.; Schoegl, I.; Speth, R. L.; Weber, B. W. Cantera: An object-oriented software toolkit for chemical kinetics, thermodynamics, and transport processes, https://www.cantera.org, 2023, Version 3.0. [CrossRef]
- Pilch, M.; Erdman, C. Use of break-up time data and velocity history data to predict the maximum size of stable fragments for acceleration induced break-up of a liquid drop. Int. J. Multiphase Flow 1987, 13, 741–757. [Google Scholar] [CrossRef]
- Nigamtulin, R.I. Dynamics of multiphase media. Vol. 1. Moscow: Nauka Publ., 1987.
- Ranger, A.A.; Nicholls, J.A. Aerodynamic shattering of liquid drops. AIAA J. 1969, 7, 285–290. [Google Scholar]
- Chou, W.-H.; Faeth, G.M. Temporal properties of secondary drop breakup in the bag breakup regime. Int. J. Multiphase Flow 1998, 4, 889–912. [Google Scholar] [CrossRef]











| Metal | Purity/Composition |
| Zinc | 99,9% |
| Aluminum alloy AlMg5 | Аl (92–95%), Mg (5–6%), Mn (up to 0.8%) |
| Stainless steel AISI 304 | Fe(66–74%), Cr (17.5–20%), Ni (8–11%) |
| Metal | Density, kg/m3 |
Melting temperature, °C |
Boiling temperature, °C |
Melt surface tension, N/m |
Melt viscosity, mPa∙s |
|---|---|---|---|---|---|
| Zinc | 7100 | 420 | 906 | 0.78 | 2.1 |
| Aluminum alloy | 2700 | 660 | 2519 | 0.88 | 1.3 |
| Stainless steel AISI 304 | 7900 | 1400 | 2900–3200 | 1.7 | 6.0 |
| Compound | Content, vol.% |
| Heptane isomers | 71 |
| Methylcyclohexane | 14 |
| Cyclohexane | 8 |
| Hexane isomers | 4 |
| Octane isomers | 3 |
| Property | Value |
| Density at 20 °C | 0.700 kg/m3 |
| Flash Temperature [Method] | –9 °C [ASTM D-56] |
| Flammability limits (vol.% in air) | Lower flammability limit 0.8; Upper flammability limit 7.7 |
| Self-ignition temperature, °C | >200 °С |
| Boiling temperature, °C | 78–113 |
| Vapor pressure (air = 1 at 101 kPa) | > 1 |
| Saturated vapor pressure, kPa | 6.1 kPa at 20 °С; 14.5 kPa at 38°С; 23.1 kPa at 50 °С |
| Evaporation rate (n-buthylacetate = 1): | 3 |
| Kinematic viscosity | 0.49 mm2/s at 40 °С; 0.67 mm2/s at 25°С |
| Freezing temperature, °С | < –40 °С |
| Molecular mass, a.u. | Calc. 98 |
|
, m/s |
a, m/s |
a, K |
H2O b, vol.% |
CO2, vol.% |
CO, vol.% |
O2, vol.% |
H2, vol.% |
Other, vol.% |
| 2100 | 2342 | 2811 | 36.1 | 22.3 | 17.7 | 8.7 | 4.1 | 11.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





