3.1. Molecular Docking of G, G-6-P, 2-DG, and 2-DG-6-P with HKI
We employed the high-precision ligand docking technology DynamicBind to simulate the interactions of G, G-6-P, 2-DG, and 2-DG-6-P with HKI. Overlapping docking results revealed that all ligands shared a high degree of spatial overlap in the binding region of HKI with G, suggesting that they may affect HKI catalytic activity via competitive bindings (
Figure 1A). The binding modes of these small molecules at the catalytic site of HKI were shown in greater detail: G-6-P, 2-DG, and 2-DG-6-P exhibited binding patterns similar to the natural substrate, glucose, but with subtle differences that suggest variations in binding affinity with HKI (
Figure 1B).
The three-dimensional view showed that G engaged in interactions with several amino acid residues of HKI, including GLY 534, GLY 535, SER 603, ASP 657, GLU 742, and GLU 708. SER 603 and GLU 742 played a critical role in the phosphorylation reaction of G, potentially participating directly in substrate conversion. ASP 657, GLU 742, and GLU 708 are key in positioning ATP and transferring phosphate groups, reflecting the optimized binding state of glucose as the natural substrate. These residues were located within the binding region of the optimally docked ligands, further confirming the stable binding of G within the catalytic pocket of HKI. In addition to the residues interacting with G, G-6-P also interacted with VAL 678, TRP 709, and GLY 862 (
Figure 1C). These additional interactions may provide extra stability, accommodating its phosphorylated state and potentially leading to minor structural adjustments at the active site. Compared to the natural substrate glucose, 2-DG exhibited fewer close interactions at the docking site, lacking interactions with GLY 710 and GLN 739, which may reduce its binding affinity. 2-DG-6-P demonstrated a unique binding mode, forming new contacts with TYR 749 and GLY 862, potentially indicating stronger binding stability as an inhibitor (
Figure 1C). This may reflect the potential efficacy of 2-DG-6-P in inhibiting HKI activity. From a structural perspective, the interactions between different ligands and HKI revealed subtle changes in the enzyme’s adaptability to various substrates, which is crucial for understanding its catalytic mechanism and designing inhibitors. Particularly, the differential interactions of 2-DG and 2-DG-6-P at the active site of the kinase are noteworthy.
An exhaustive map of interactions at the active site of HKI with different ligands was provided in
Figure 1D, illustrating potential polar interactions, hydrophobic effects, electrostatic interactions, and hydrogen bonding. G, as the natural substrate, engages in interactions filled with polarity, hydrophobic effects, and electrostatic interactions, particularly with key residues such as ASP 657 and GLU 742. These interactions are crucial for its phosphorylation, facilitating the production of G-6-P and paving the way for the glycolytic process. In contrast, G-6-P interacts with additional residues such as GLY 534 and GLU 708, which may particularly adapt to the binding of the phosphate group. The interaction diagram for 2-DG, lacking key hydroxyl groups compared to G, shows a reduction in hydrogen bonding with GLY 710, potentially leading to weaker binding at the active site, though still sufficient for phosphorylation. The interaction pattern of 2-DG-6-P is more complex, involving additional residues such as GLY 534 and GLU 708, and offering a hydrogen bonding site at ASN 683 (
Figure 1D). Overall, the variations in these interaction patterns highlight the unique roles that different ligands may play in regulating HKI activity, providing important insights into the enzyme’s substrate specificity, catalytic mechanisms, and potential inhibitor design.
3.2. Conformation and Motion Analysis of the Complexes of G, G-6-P, 2-DG, and 2-DG-6-P with HKI
A 100-nanosecond (ns) molecular dynamics simulation was conducted to explore the movement and stability of G, G-6-P, 2-DG, and 2-DG-6-P within the catalytic pocket of HKI. The simulation results indicated that all ligands remained primarily confined within the catalytic pocket throughout the simulation period, with no instances of ligand egress observed (Supplementary Videos S1-S4;
Supplementary Figure S1. This confinement suggested that these molecules are likely to maintain long-term interactions with the enzyme in vivo, potentially exerting a sustained influence on enzymatic activity. Moreover, the restricted movement of the small molecules during the simulation may indicate multiple interaction points with amino acid residues within HKI's active pocket. Notably, the stability of 2-DG and 2-DG-6-P suggested that these molecules can effectively mimic the behavior of natural substrates. For 2-DG-6-P, its stable binding within the pocket was particularly significant, as a phosphorylated product of 2-DG, it is not further metabolized in vivo, which could lead to its accumulation.
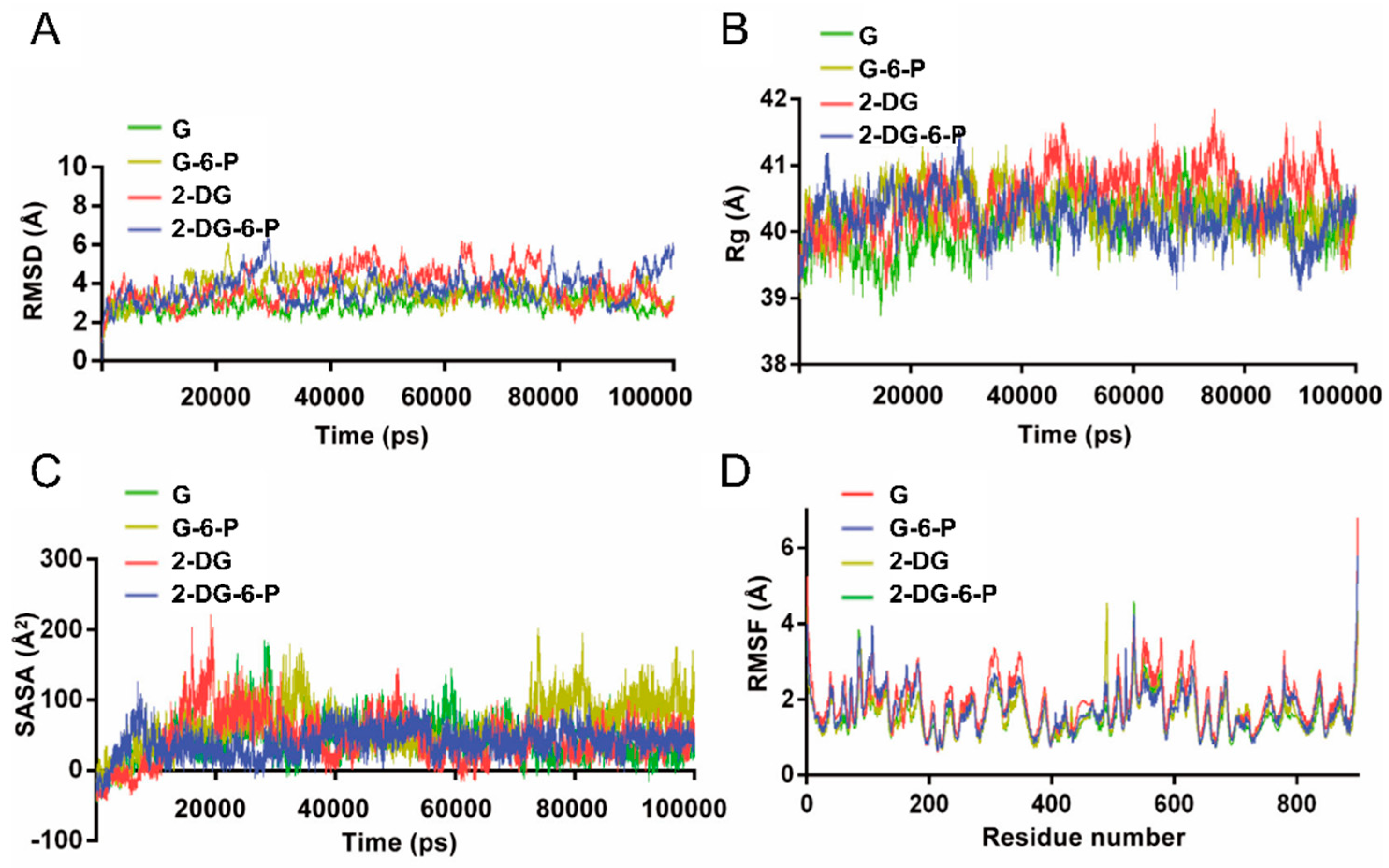
The root mean square deviation (RMSD) curves over time during the molecular dynamic simulations for the four sets of molecules were depicted (
Figure 2A,
Supplementary Figure S2). While fluctuations in RMSD indicate the dynamic nature of the structures, no large-scale conformational changes were observed in the protein-ligand complexes, suggesting that ligand bindings may have caused local rather than global structural adjustments. The RMSD values for the G with HKI complex were relatively stable throughout the simulation, mostly maintaining lower levels (approximately 2 to 3.5 angstroms). This smaller range of fluctuation indicates minimal overall conformational changes in the protein post-glucose binding, likely reflecting the high binding specificity and structural compatibility of glucose as the natural substrate for HKI. The low RMSD also implies that the protein-glucose complex maintained high structural stability during the simulation. In contrast, G-6-P, 2-DG, and 2-DG-6-P exhibited higher RMSD fluctuations, particularly at certain points, which might indicate that these small molecules induced more significant dynamic changes in the protein (
Figure 2A,
Supplementary Figure S2).
The radius of gyration (Rg) values for all complexes remained within a relatively narrow range of 39 to 42 angstroms throughout the simulation, showing no significant long-term trends, indicating that the overall conformation of the protein was maintained relatively stable (
Figure 2B). These findings were consistent with the RMSD results, further suggesting that the complexes did not undergo substantial structural transformations during the simulation, implying that the bindings of the ligands did not cause significant unfolding or tightening of the protein conformation. Specifically, no ligand exhibited a markedly different trend in Rg changes, suggesting that the various small molecule complexes with HKI exhibited similar stability in spatial conformation (
Figure 2B).
The Solvent Accessible Surface Area (SASA) is a parameter used to measure the degree of exposure of a protein or protein-ligand complex to the solvent, providing valuable insights into protein folding and conformational changes. The initial decrease in SASA values at the start of the simulation may indicate a transient closure of certain domains on the complex's surface due to the entry of ligands into the catalytic pocket. Subsequently, SASA values fluctuated for all complexes without showing a clear long-term increasing or decreasing trend, suggesting that ligand binding did not induce large-scale permanent structural unfolding of the complexes (
Figure 2C). The minimal SASA fluctuations observed with 2-DG-6-P throughout the simulation indicated a relatively stable structure of the complex with HKI, likely implying tight interactions with residues in the catalytic pocket that provide higher binding specificity and stable inhibitory effects (
Figure 2C). Moderate SASA fluctuations for G and 2-DG suggest that the complexes maintained balanced conformational dynamics, reflecting their adaptability as substrates or inhibitors in the protein structure (
Figure 2C). Larger SASA fluctuations observed with G-6-P suggest that it might induce relatively significant protein structural changes, potentially related to local conformational changes in the catalytic pocket (
Figure 2C).
Root Mean Square Fluctuation (RMSF) values reflect the mobility of individual amino acid residues during the simulation, with higher RMSF values indicating greater local flexibility or dynamic changes. G, G-6-P, and 2-DG-6-P exhibit smaller RMSF in the relevant residue areas of HKI catalytic pocket (approximately amino acids 500 to 850) (
Figure 2D). This suggests that their bindings to HKI stabilize the conformations of relevant residues, indicating robust interactions within the catalytic pocket, resulting in lower local flexibility and fewer dynamic changes. In contrast, 2-DG shows higher RMSF, particularly in the active pocket region, indicating that 2-DG binding might lead to greater conformational changes and increased dynamics in that region (
Figure 2D). This could be due to less stable interactions of 2-DG with HKI compared to the natural substrate glucose or other ligands, or because 2-DG induces different protein conformational changes than other substrates or ligands.
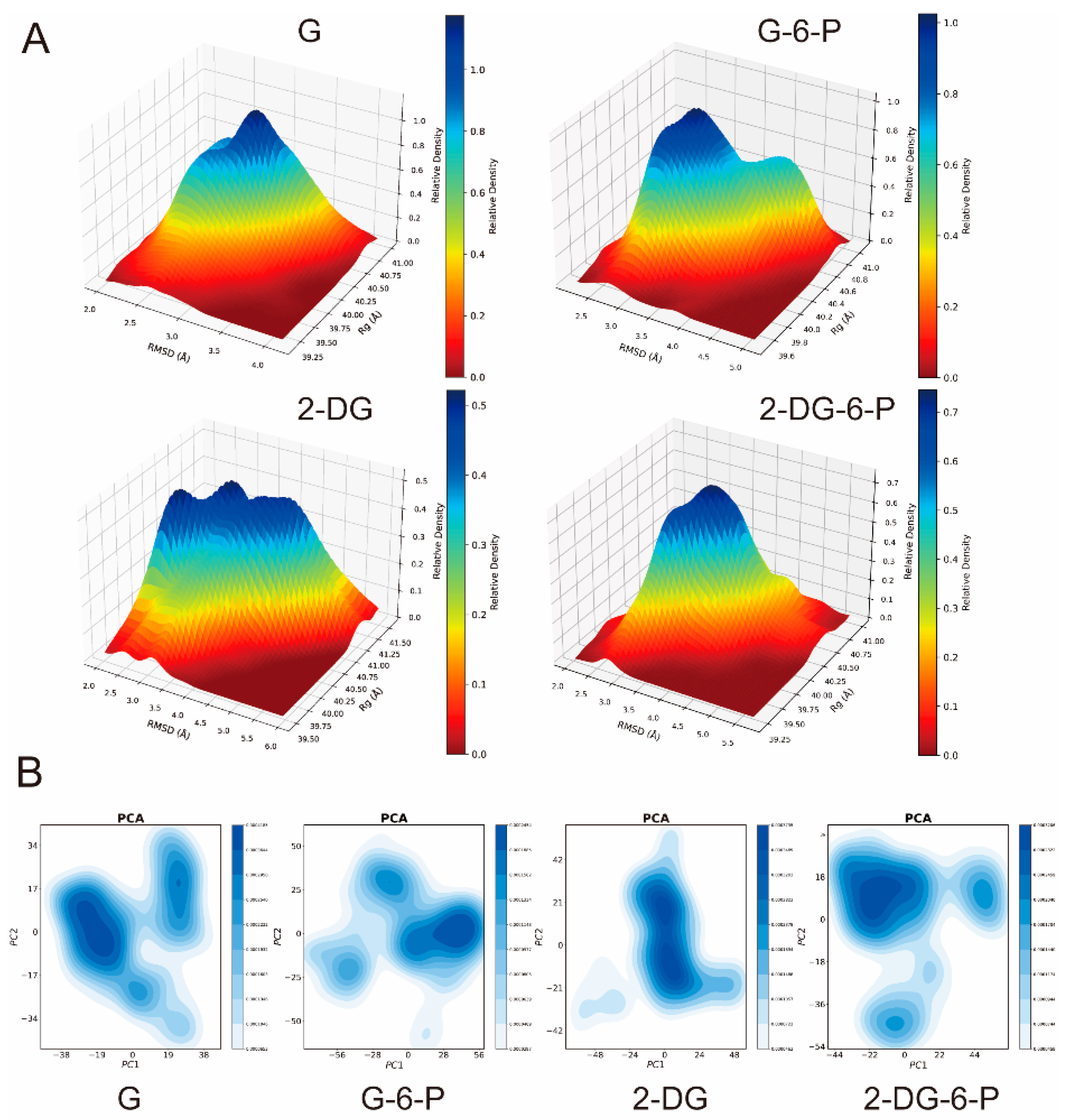
The free energy landscape, typically used to describe the relative probabilities of the system visiting various conformations during simulation, was constructed by combining the distributions of RMSD (reflecting the magnitude of conformational changes) and Rg (reflecting the compactness of the conformation). From the free energy landscape graph in
Supplementary Figure S3, complexes of G, G-6-P, and 2-DG-6-P with HKI tend to cluster in areas of lower RMSD and moderate Rg values, showing a clear high-probability "well," indicating that these ligands form relatively stable bindings with HKI, and the complexes undergo minimal conformational changes and are more compact. Particularly, G and 2-DG-6-P exhibit a narrow, deep free energy "well," further suggesting high structural stability and low conformational diversity upon binding to HKI. In contrast, 2-DG exhibits a broader area of high probability that includes higher RMSD and Rg values, suggesting that the 2-DG-HKI complex might dynamically alternate among various conformational states (
Supplementary Figure S3). This broader distribution of free energy could be due to higher conformational diversity following the binding of 2-DG to HKI, exhibiting greater flexibility and diverse conformational states.
The three-dimensional free energy landscape showed distinct peaks for G and 2-DG-6-P, indicating that during the simulation, these complexes mostly occupied specific, low-energy conformations. This narrow peak represented a stable conformational state, typically indicative of high binding affinity and specificity, suggesting that these ligands may form a series of strong interactions with the enzyme's active site (
Figure 3A). On the other hand, the free energy landscapes for G-6-P and 2-DG presented broader energy peaks, indicating that these ligands explored a wider conformational space during the simulation. Particularly, the broad peak for 2-DG suggested that it might have undergone multiple different conformational states during the simulation, possibly due to looser binding with HKI or inducing significant conformational adjustments in the protein (
Figure 3A).
The principal component analysis (PCA) displays the main motion modes of the protein-ligand complexes (
Figure 3B). Systems interacting with G, 2-DG-6-P, and G-6-P showed distinct compact and dispersed areas in the PCA plot, indicating that the complexes underwent flexible conformational changes when interacting with HKI, yet still maintained areas of high conformational stability. In contrast, the system interacting with 2-DG exhibited a wide range of stable distributions, which implied that HKI had more conformational freedom when bound to 2-DG (
Figure 3B). This increased range of motion could reflect the inherent flexibility and variety of conformational states accessible to HKI in the presence of 2-DG, highlighting the dynamic nature of enzyme-ligand interactions and the impact of different ligands on the enzyme's structural dynamics.
3.3. The End-Point Complex Structures and Interactions of G, G-6-P, 2-DG, and 2-DG-6-P with HKI
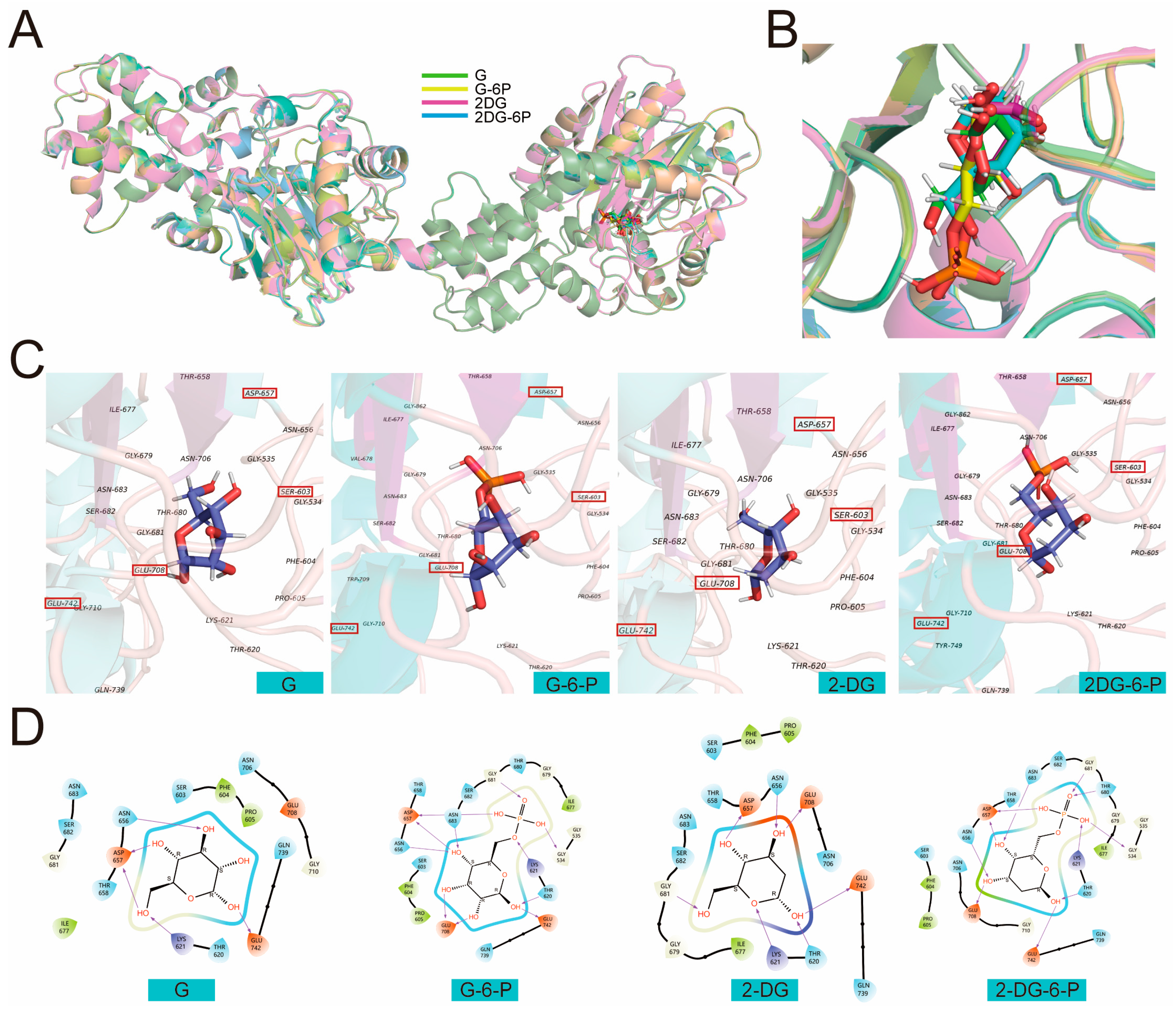
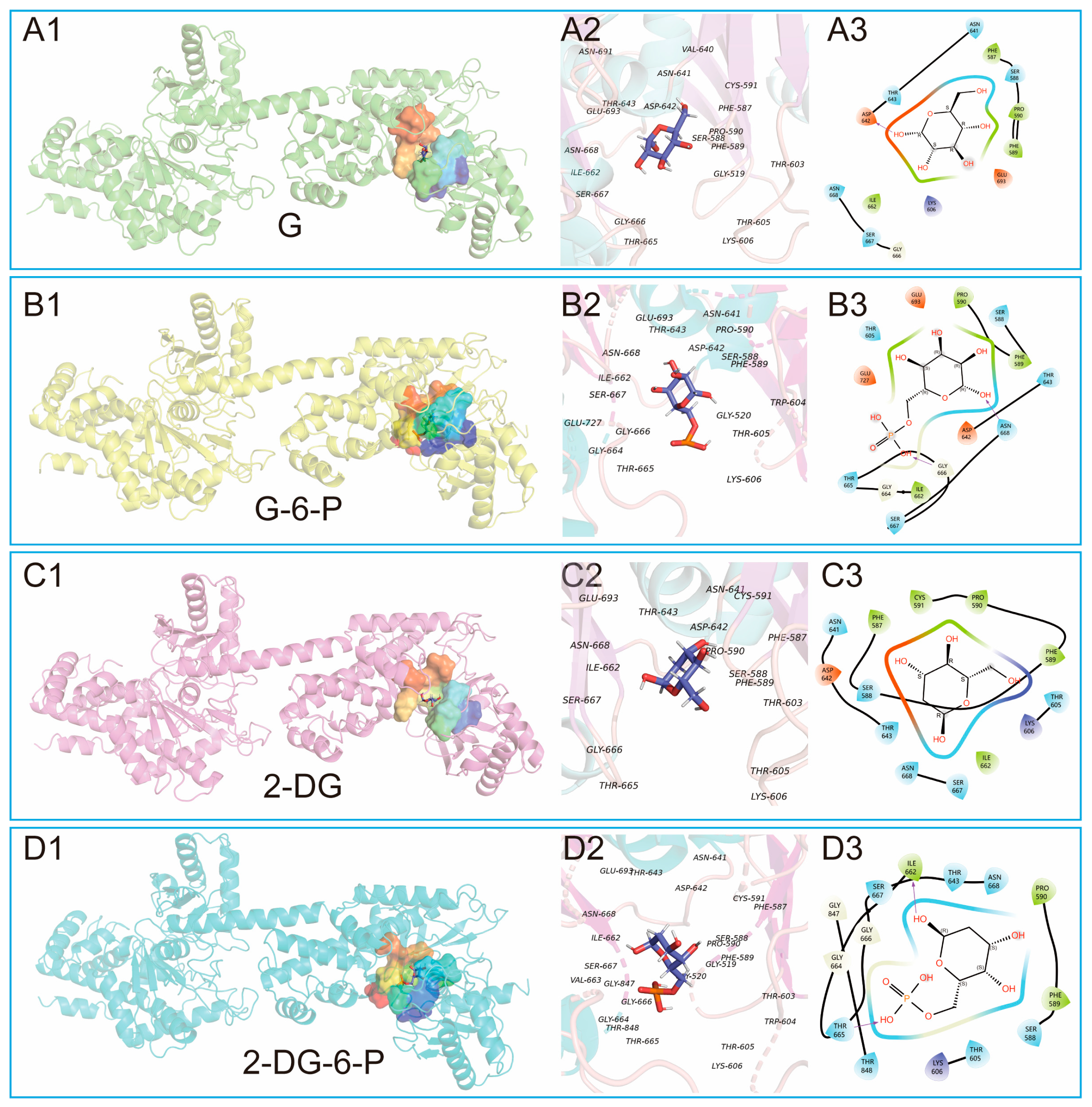
PyMOL was further used to visualize multiple facets of the optimal molecular simulation (MS) results depicting interactions between G, G-6-P, 2-DG, 2-DG-6-P, and HKI (
Figure 4). Each set's three-dimensional structures showed the ligands positioned within HKI’s catalytic pocket (
Figure 4A1, B1, C1, D1). The binding of 2-DG-6-P to HKI appears the tightest, characterized by the snug encapsulation of the small molecule within the binding pocket. Conversely, the binding of 2-DG is relatively loose, with a more open catalytic pocket, suggesting weaker interactions with HKI compared to other ligands. Zooming into the catalytic active site interactions (
Figure 4A2, B2, C2, D2), we observe direct interactions between each ligand and key residues, potentially mediated by hydrogen bonds (indicated by purple arrows), polar interactions, hydrophobic forces, and electrostatic interactions, each contributing to overall binding affinity and specificity. The two-dimensional diagrams simplify these complex interactions, offering an intuitive depiction of how each ligand may influence HKI's conformation and activity through different types of interactions (
Figure 4A3, B3, C3, D3).
The three-dimensional structure showed glucose positioned within HKI's catalytic pocket, tightly encased by specific amino acid residues, aiding in maintaining the correct orientation crucial for enzymatic catalysis (
Figure 4A1). Detailed interactions at the catalytic site involved residues within 5Å, including GLY 519, PHE 587, SER 588, and so on, contributing to a stable interaction (
Figure 4A2). The two-dimensional diagram simplified this view, showing potential polar interactions (e.g., ASN 641, THR 643), hydrophobic interactions (e.g., PHE 587, ILE 662), electrostatic interactions (e.g., LYS 606, ASP 642), and flexible glycine structures (GLY 666), along with possible hydrogen bonding (ASP 642) (
Figure 4A3).
The three-dimensional molecular simulation results depicted G-6-P within the catalytic pocket, interacting with residues that create a specific environment tailored to its phosphate group, causing shifts in interactions compared to glucose (
Figure 4B1). The two-dimensional diagram showed interactions including potential hydrogen bonds (ASN 668, LYS 606) and polar interactions with charged residues (LYS 606, GLU 727), which, along with the hydrophobic environment provided by residues like ILE 662, help stabilize G-6-P's position within the pocket (
Figure 4B2, B3).
The three-dimensional view of the molecular simulation of 2-DG with HKI showed 2-DG in a more open catalytic pocket, indicating weaker interactions with HKI (
Figure 4C1). This looser binding was reflected in the reduced number of interacting residues at the active site, potentially affecting its binding affinity and stability. The two-dimensional diagram highlighted reduced hydrogen bonding due to the absence of a hydroxyl group, with hydrophobic interactions still contributing to stabilizing its position (
Figure 4C3).
The three-dimensional structure of 2-DG-6-P with HKI showed a tight interaction within the catalytic pocket, indicating strong binding affinity (
Figure 4D1). 2-DG-6-P interacted with additional residues (e.g., PHE 587, VAL 663), enhancing its stability and inhibitory potential. The two-dimensional interaction diagram further illustrated how 2-DG-6-P's phosphate group might form additional hydrogen bonds (e.g., with THR 665), with its interactions mediated by polar, hydrophobic, and electrostatic forces, as well as flexible glycine structures (
Figure 4D3). Overall, these visual representations provide a detailed insight into how each ligand interacts within the catalytic pocket of HKI, highlighting the complex interplay of forces that govern binding specificity and enzyme modulation (
Figure 4).
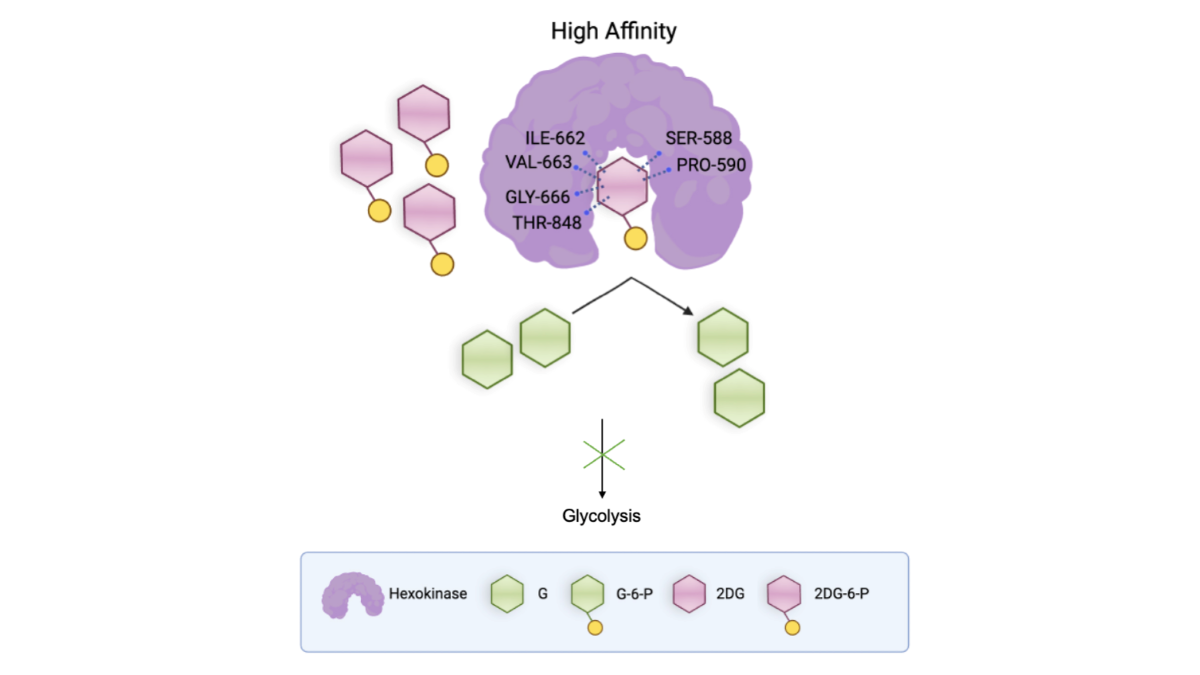
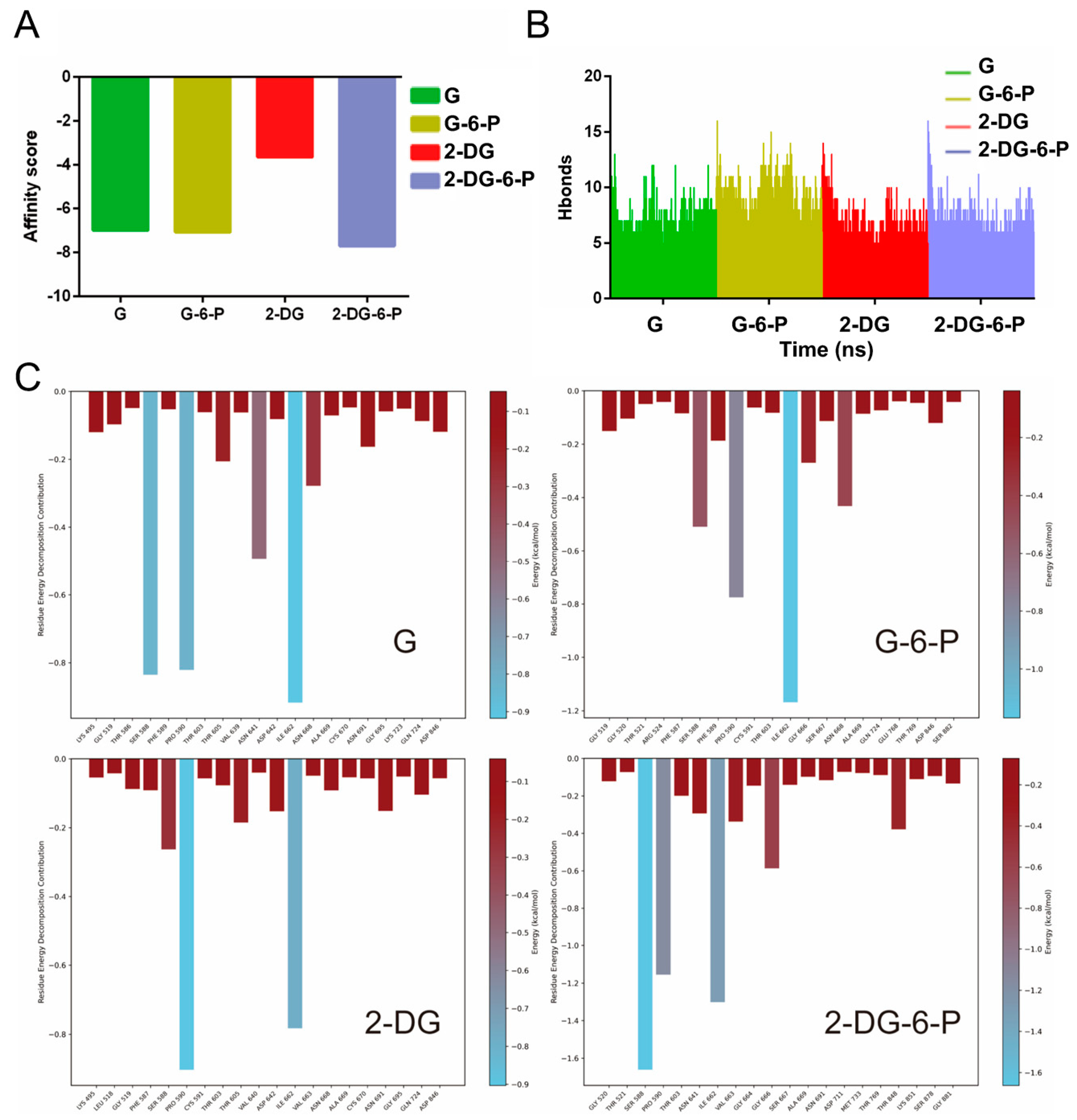
3.4. Binding Affinities of the End-Point Complexes of G, G-6-P, 2-DG, and 2-DG-6-P with HKI
The binding affinity scores for the complexes of each group provided insights into the interaction strengths between the ligands and HKI (
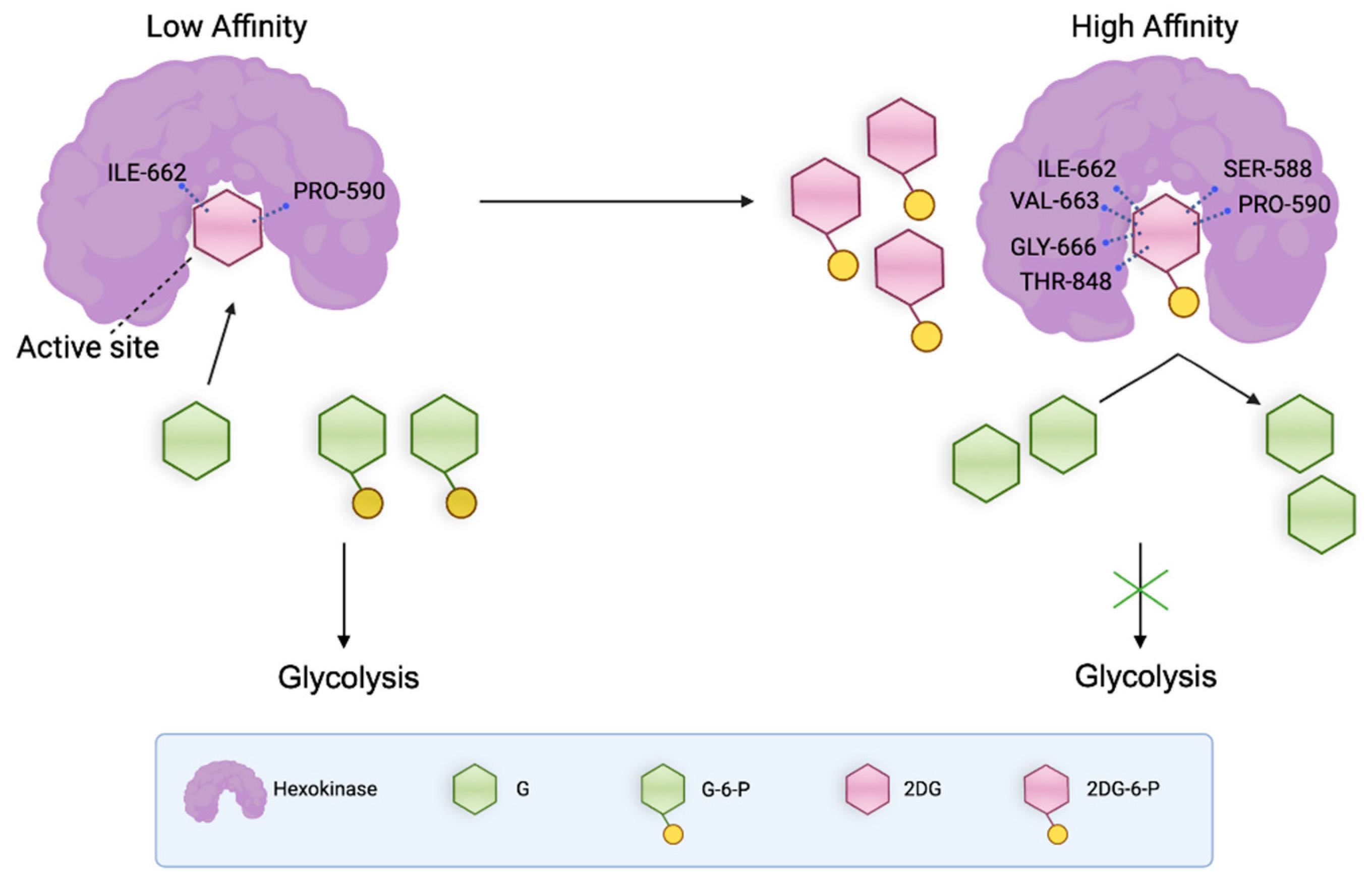
Figure 5A). The affinity score for G was -6.9873, indicating a strong binding affinity, which is expected for the natural substrate essential for catalytic activity. G-6-P displayed a similar affinity score of -7.0672, suggesting it also forms a stable complex with the enzyme. 2-DG showed a significantly higher affinity score of -3.6365 compared to both G and G-6-P, indicating the weakest binding affinity with HKI among the evaluated ligands. Such binding affinity suggested that 2-DG might not act as a strong competitive inhibitor on its own. 2-DG-6-P exhibited the lowest affinity score of -7.6934, indicating the tightest binding among the ligands studied (
Figure 5A). This tight binding could lead to the occlusion of the catalytic pocket, impeding the further transformation of glucose and thus potentially inhibiting the glycolytic pathway in cells.
The number of hydrogen bonds formed between different ligands (2-DG, 2-DG-6-P, G, G-6-P) and HKI over the course of the simulation was then illustrated (
Figure 5B and
Figure S4). Hydrogen bonds play a crucial role in stabilizing protein-ligand complexes. 2-DG, 2-DG-6-P, and G initially had a higher number of hydrogen bonds, which fluctuated significantly early in the simulation but then decreased and stabilized, suggesting that the complexes may reach a relatively stable conformation. Notably, 2-DG-6-P maintained a more stable and generally higher count of hydrogen bonds throughout the simulation, indicating a more constant and robust interaction. In contrast, G-6-P exhibited significant fluctuations in the number of hydrogen bonds during the simulation, suggesting multiple binding modes or dynamic changes in the binding conformation (
Figure 5B and
Figure S4).
The decomposition of total energy contributions from different amino acid residues within HKI when interacting with four ligands (G, G-6-P, 2-DG, and 2-DG-6-P) was shown in
Figure 5C. During the interaction with G, ILE 662 had a very significant negative energy contribution, indicating its crucial role in binding glucose through hydrophobic interactions with the non-polar parts of glucose (
Figure 5C1). SER 588 also provided a large contribution, likely through hydrogen bonding between its side chain hydroxyl group and the hydroxyl groups of glucose, helping to stabilize the ligand's position. PRO 590 contributed significantly, where its cyclic structure might provide a rigid framework for the correct positioning of glucose at HKI’s active site. ASN 641 showed a notable negative energy contribution, suggesting potential hydrogen bonding or dipole-dipole interactions, aiding in precise glucose positioning. THR 603 and THR 605 may also form hydrogen bonds with glucose’s hydroxyl groups, contributing to complex stability. GLY 519 and GLY 695 had smaller contributions, potentially due to their relative static nature during ligand binding or minor roles in complex stability (
Figure 5C1).
The energy contributions with G-6-P were: ILE 662 still showed the largest negative energy contribution, underscoring its core role in binding both G and G-6-P through hydrophobic interactions (
Figure 5C2). SER 588 and PRO 590 maintained significant energy contributions in the interaction with G-6-P, likely stabilizing the ligand through hydrogen and hydrophobic interactions. A notably increased contribution from ASN 668, whose energy contribution was more significant with G-6-P binding than with G, possibly due to the additional charge and spatial requirements introduced by G-6-P's phosphate group (
Figure 5C2). GLY 666 increased contribution to G-6P binding, possibly through the contacts with the phosphate group (
Figure 5C2).
For 2-DG, ILE 662 continued to show significant negative energy contribution, emphasizing its key role in maintaining ligand-enzyme interactions (
Figure 5C3). Its hydrophobic interactions were likely crucial for the stability of 2-DG. PRO 590 showed the largest contributions, highlighting the importance of this residue for the integrity and conformation of the enzyme's active pocket. A decrease in contributions from SER 588, THR 603, and THR 605 is likely due to reduced hydrogen bonding opportunities with 2-DG's structure (
Figure 5C3).
As for 2-DG-6-P, the energy contributions were as follows: ILE 662's contribution was the most significant in binding 2-DG-6-P, continuously highlighting its central role in binding phosphorylated ligands (
Figure 5C4). Its hydrophobic interactions were crucial for the stability of the phosphorylated ligand. SER 588 showed significant energy contributions in binding 2-DG-6-P, likely due to key hydrogen bonding in ligand recognition and binding. PRO 590 consistently showed high energy contributions across all ligands, suggesting its cyclic structure provides a stable environment for substrate binding. VAL 663 also contributed to the binding of 2-DG-6-P, indicating potential hydrophobic interactions with the phosphorylated ligand, enhancing ligand binding stability. An increase in energy contribution from GLY 666, possibly due to the small volume of GLY allowing necessary spatial flexibility for tighter contacts between the ligand and residues. THR 848 showed significant negative energy contributions, potentially because its side chain hydroxyl can form stable interactions with 2-DG-6-P's phosphate group (
Figure 5C4).
These observations indicate that certain amino acid residues like ILE 662, SER 588, and PRO 590 play key roles in maintaining the structural integrity and functionality of the protein-ligand complexes, critical for drug design to enhance the efficacy and specificity of new drug molecules. The unique interactions with 2-DG-6-P, particularly involving residues like VAL 663 and THR 848, reveal interaction patterns that may be key to its effective inhibitory effects (
Figure 5C).
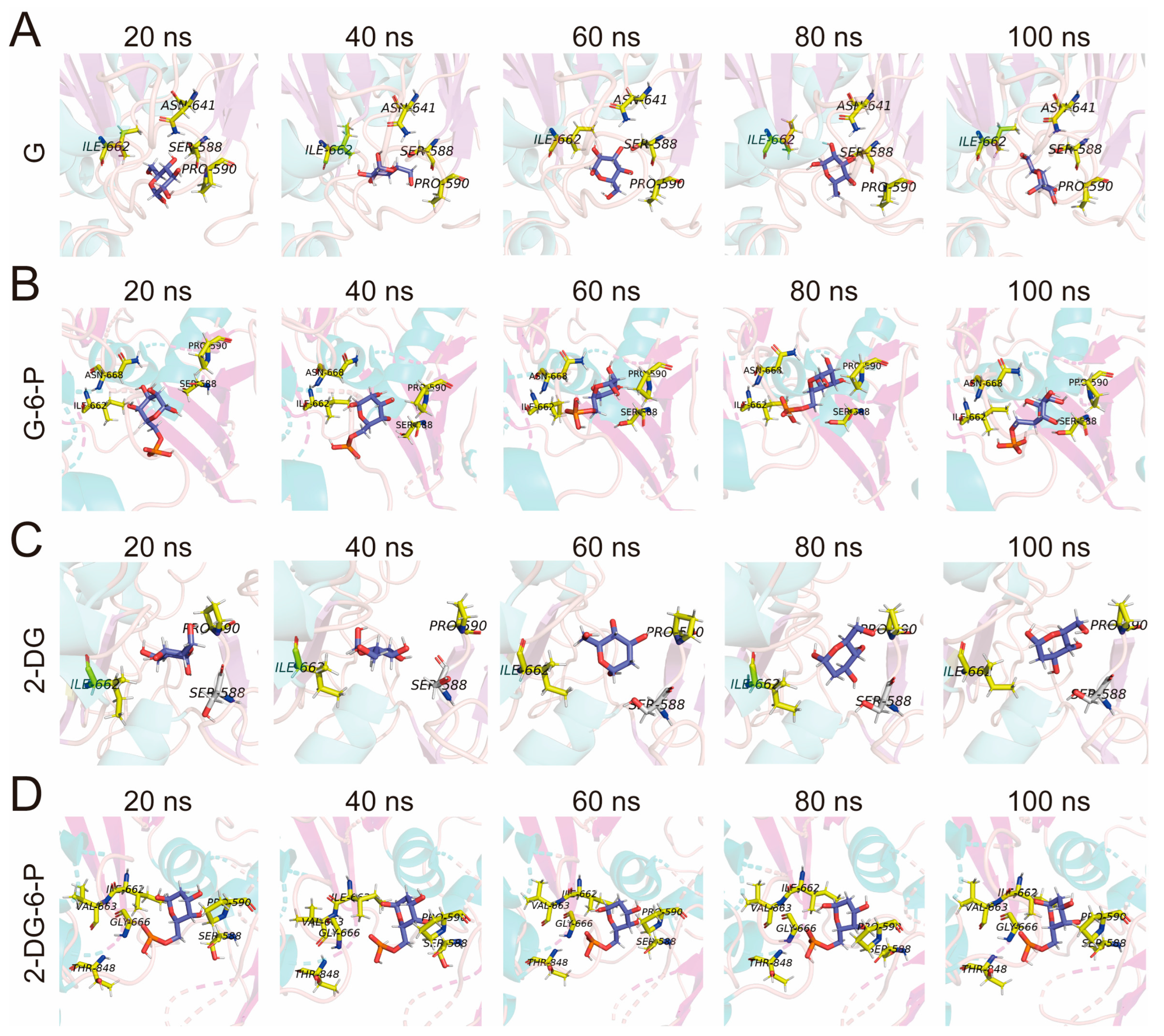
3.5. Interactions of G, G-6-P, 2-DG, and 2-DG-6-P with Key Binding Residues of HKI
The interactions of G with HKI over time at specific intervals (20 ns, 40 ns, 60 ns, 80 ns, and 100 ns) were illustrated (
Figure 6A). Key residues such as ILE 662, SER 588, PRO 590, and ASN 641 were highlighted for their roles in stabilizing the glucose within the active site of HKI. ILE 662 maintained a stable hydrophobic contact with glucose throughout the simulation, reinforcing its importance in stabilizing the ligand binding. SER 588, positioned near glucose, likely formed hydrogen bonds with glucose's hydroxyl groups, crucial for maintaining complex stability throughout the simulation. PRO 590 acted as an anchor around glucose, with its cyclic side chain potentially stabilizing the ligand's position and maintaining the overall shape of the active site. ASN 641's proximity to glucose remained relatively constant, suggesting its role in positioning glucose through dipole-dipole interactions or hydrogen bonds, adding further stability. Despite minor conformational changes observed between 60 ns and 80 ns, glucose remained within a pocket formed by these key residues, and the complex returned to a relatively stable state by 100 ns (
Figure 6A).
Interactions at similar time intervals for G-6-P with HKI were illustrated in
Figure 6B. ILE 662 continued to make significant hydrophobic contacts with G-6-P throughout the simulation, indicating its critical role irrespective of the ligand. SER 588, although slightly farther from the phosphate group of G-6-P, may still contribute to positioning and stabilizing G-6-P through hydrogen bonds with nearby water molecules or directly with the ligand. PRO 590 consistently supported G-6-P, suggesting that its proline ring structure helps maintain the ligand in the correct orientation for catalytic transformation. ASN 668, not ASN 641 as in the glucose interactions, played a significant role in stabilizing G-6-P, likely through hydrogen bonds with the phosphate group, which adds to the precision of ligand positioning (
Figure 6B). Despite minor positional and orientational changes of G-6-P, the binding environment remains consistent thanks to these residues.
The interactions of 2-DG with HKI were also examined. ILE 662 demonstrated significant energy contributions at every observed time point, indicating its essential role in the hydrophobic stabilization of 2-DG. PRO 590 also showed consistent contributions, suggesting its importance in maintaining the active site's conformation favorable for 2-DG binding. The interactions here were limited mainly to these two residues, as 2-DG lacked additional hydroxyl groups for more extensive hydrogen bonding, which might explain the larger conformational changes observed during the simulation (
Figure 6C).
The interactions of 2-DG-6-P with HKI were shown in
Figure 6D. ILE 662 is pivotal in stabilizing the phosphorylated ligand through significant hydrophobic interactions. SER 588's contribution remained stable, potentially interacting with the phosphate group of 2-DG-6-P or other hydrogen-bond-capable sites, aiding in ligand stabilization. PRO 590 contributed consistently across all time points, with its cyclic structure providing a stable scaffold. VAL 663 and GLY 666 showed particularly high energy contributions, likely enhancing the binding stability through additional hydrophobic forces and providing conformational flexibility. THR 848 significantly contributed by possibly forming hydrogen bonds with the phosphate group of 2-DG-6-P, further stabilizing the ligand (
Figure 6D).