1. Introduction
“Oral frailty” was first proposed by the Japanese Geriatrics Society in 2013, and is defined as an age-related decrease in oral function. Oral frailty is defined as “the overlap of minor declines in dental or oral functions that may increase the risk of adverse health outcomes ” [
1,
2].
This condition poses an increased risk of a further decline in oral function; however, it remains reversible if early and appropriate interventions are implemented. Older signs of oral frailty, including decreased tongue pressure, increased food spillage, slight chewing difficulties, and a dry mouth, are often subtle and easily overlooked. Recent studies have shown that oral frailty in older adults not only affects oral health but also has systemic implications, contributing to overall frailty and sarcopenia (age-related muscle loss) [
1,
3,
4].
In 2023, a new diagnostic criterion for oral frailty, known as the oral frailty five-item checklist (OF-5), was proposed [
1]. It comprises five items: fewer teeth, difficulty in chewing, difficulty in swallowing, dry mouth, and low articulatory oral motor skills. The OF-5 is designed to be implemented in various settings beyond dental care facilities, including non-dental healthcare facilities and community activities, and can be assessed by older individuals. The OF-5 has demonstrated robust predictive validity for physical frailty, physical impairment, and mortality among the older population in Japan [
1]. Despite these advancements, a longitudinal impact of oral frailty on the progression of physical frailty, as assessed by OF-5, remains poorly understood, particularly in rural populations.
On April 1, 2024, the Japanese Geriatrics Society, Japanese Geriatric Dentistry Society, and Japanese Society for Sarcopenia and Frailty introduced a joint statement on oral frailty diagnosed via the OF-5 [
5]. This tool allows non-dental professionals to assess oral frailty based on five key indicators: fewer teeth, difficulty in chewing, difficulty in swallowing, dry mouth, and low articulatory oral motor skills. The OF-5 facilitates early detection of oral frailty and promotes interdisciplinary collaboration in its management, particularly in the medical and dental fields.
In our epidemiological study conducted among community-dwelling older adults in Sasayama/Tamba, Hyogo Prefecture (the frail elderly in the Sasayama-Tamba Area [FESTA] study), we focused on the relationship between oral function and physical frailty. The rural environment of Sasayama/Tamba, which is relatively close to a metropolitan area and maintains a stable population without extreme depopulation or aging, offers a unique context. It features a modern, healthy, elderly population centered on suburban agriculture, with low population turnover. This setting provides an important backdrop for studying the interaction between oral and physical frailty given its distinctive demographic and health characteristics. In our previous study, we found a significant correlation between tongue pressure and cystatin C levels, an indicator of kidney function in the FESTA study. Our findings also revealed a correlation between tongue pressure, an indicator of oral function, and physical parameters, such as grip strength, walking speed, and muscle mass [
6].
The Oral Frailty Checklist/Oral Frailty Index-8 (OFI-8) was developed by the Japan Dental Association, [
7,
8] and consists of eight items: (1) difficulties in chewing; (2) difficulties in swallowing; (3) denture use; (4) dry mouth; (5) going out less frequently; (6) feasibility of chewing hard food; (7) brushing teeth at least twice a day; and (8) regular attendance at a dental clinic. Items (1) to (3) were scored as 2, whereas the other items were scored as 1. The maximum possible score was 11: low risk, 0–2 points; moderate risk, 3 points; and high risk, >4 points. Oral frailty, as assessed using the OFI-8, was independently associated with all-cause mortality, even after adjusting for physical and psychological frailty in older adults [
9].
On the other hand, there are many reports on the associations of cystatin C-related indices, including the creatinine to cystatin C ration (Cre/CysC ratio) and estimated glomerular filtration rate based on CysC (eGFRcys), with physical frailty and sarcopenia [
10,
11,
12,
13,
14,
15,
16,
17]. Our findings indicated that individuals at high risk for oral frailty, as assessed by the OFI-8, had lower levels of cystatin C-related indices, grip strength, hemoglobin, and albumin, with a higher prevalence of oral frailty observed in women [
18].
The OF-5 and OFI-8 share several common items, such as difficulties in chewing, difficulties in swallowing, and dry mouth. These three items are included in the Kihon checklist developed by the Japanese Ministry of Health, Labor, and Welfare, which consists of 25 questions in seven categories: physical strength, nutrition, eating, socialization, memory, mood, and lifestyle [
19,
20]. However, the OFI-8 differs in that the OF-5 includes objective evaluations, such as the remaining teeth count and articulatory oral motor skills assessed by a dental specialist. The relationship between oral frailty diagnosed using the OF-5, which includes objective measures based on dental examinations, grip strength, gait speed, and blood test indices, has not yet been examined. The comparative efficacy of the OF-5 in predicting physical frailty outcomes, especially in comparison to the OFI-8, has not been extensively explored, highlighting a vital area for investigation.
The longitudinal Kashiwa Study conducted by the University of Tokyo has also shown that oral frailty is a risk factor for physical frailty and is linked to life prognosis [
21]. In the present study, we examined whether oral frailty, as diagnosed by the OF-5, predicts worsening physical frailty according to the Japanese Cardiovascular Health Study (J-CHS) criteria. Oral frailty, as assessed using the OF-5, has also been shown to be related to the development of physical disabilities and frailty [
1].
This study aimed to assess, in a cross-sectional analysis, sex differences in physical and blood markers among individuals classified as having oral frailty by the OF-5. Additionally, using the OF-5, we aimed to investigate whether individuals classified as having suspected oral frailty (OF-5 score ≥2) exhibit differences in physical and biological markers, including height, weight, blood indices, and muscle strength, compared with those with lower OF-5 scores. We also explored whether these differences were associated with overall frailty. This study aimed to longitudinally assess the predictive value of the OF-5 checklist for physical frailty among older adults in Sasayama/Tamba by hypothesizing that higher OF-5 scores are significantly associated with an increased risk of physical frailty over time. Additionally, in a longitudinal analysis, we examined the association between OF-5 scores and the progression of physical frailty according to the J-CHS criteria. Finally, we evaluated the predictive value of OF-5 in comparison with other clinical markers over a follow-up period of 2-3 years.
2. Materials
2.1. Study Participants
This cross-sectional study in the FESTA study included individuals aged ≥65 years. Healthy community-dwelling older adults from the Sasayama-Tamba area, a rural region in Hyogo Prefecture, Japan, were recruited between 2017–2023. Body composition and blood sample analyses were performed as described previously [
14,
15]. Body composition was assessed using bioelectrical impedance analysis with an InBody 770 device (InBody Japan, Inc.). Skeletal muscle mass index (SMI) was calculated as skeletal muscle mass divided by height squared (kg/m²). Handgrip strength was measured according to previously established methods [
14,
15,
22].
This cross-sectional study included 313 men and 621 women (934 in total). For the longitudinal study, 105 men and 224 women (329 in total) from the first cross-sectional survey who had no missing data in the second survey conducted 2–3 years later were included.
All procedures performed in this study, which involving human participants, adhered to the ethical standards of the institutional and/or national research committee where the studies were conducted (IRB approval number Rinhi 0342 at Hyogo Medical University) and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
2.2. Evaluation of Physical Function
To assess gait speed, the participants were instructed to walk a 12-meter walkway at their usual pace, and the time taken to walk 10 m was recorded. Maximum grip strength was measured via a grip strength tester (GRIP-A; Takei Ltd., Niigata, Japan) [
23]. Knee extension strength (Nm) of the dominant leg was evaluated during isometric contraction of the knee extensor in a seated position, with the knee maintained at a 60°angle using a hand-held dynamometer (Sakai Medical Co., Ltd., Tokyo, Japan) [
24].
2.3. Diagnosis of Frailty
Frailty phenotypes were assessed based on the five clinical features defined in the Cardiovascular Health Study (CHS): slow gait speed, weakness, exhaustion, low physical activity, and weight loss [
25]. The frailty score was calculated using a modified version of the CHS (J-CHS) [
26]. The number of applicable frailty phenotypes of the five was used to determine the J-CHS score. A score of 0 was defined as robust, 1 or 2 as pre-frail, and ≥3 as frail.
In a longitudinal study, during the second survey conducted 2–3 years after the first survey, the participants were categorized based on changes in their J-CHS frailty scores. Seventy-four participants (27 men and 47 women) whose scores had increased were defined as "worsened," 167 (51 men, 116 women) whose scores remained unchanged were categorized as "unchanged," and 88 (27 men, 61 women) whose scores had decreased were classified as "improved." Changes in the J-CHS frailty scores were used to classify the participants as improved, unchanged, or worsened, and comparisons were made across groups in terms of physical indices, blood markers, OF-5 scores, and the number of positive subjects for each item at the time of the first survey. Logistic regression analysis, including other indices, was used to determine whether the J-CHS scores worsened during the second survey.
2.4. Evaluation of Oral Function
The participants were seated in reclinable nursing chairs and they underwent oral examinations. The number of remaining teeth, occlusal force, and tongue pressure were assessed. The occlusal force was measured by recording the maximum force of the first left and right molars via an occlusal force meter (Occlusal Force-Meter GM10; NAGANO KEIKI, Tokyo, Japan), and the sum of both measurements was evaluated. Tongue pressure was measured twice using a JMS Tongue Pressure Measuring Device (JMS Co., Ltd., Hiroshima, Japan), and the highest value was recorded [
27]. To evaluate tongue motor function (oral diadochokinesis [ODK]), we used an oral function measurement equipment (KENKOU-KUN Handy; Takei Scientific Instruments Co., Ltd., Niigata, Japan) to measure the articulatory velocity of /ta/ [
28].
2.5. Calculation of eGFR
We calculated creatine-based eGFR (eGFRcre) and eGFRcys using equations provided by the Japanese Society of Nephrology [
29,
30].
2.6. Statistical Analysis
The results are expressed as the means ± standard deviations or percentages. For intergroup comparisons, Student's t-test was used for data analysis. The Pearson’s correlation coefficient was used to assess the associations between tongue pressure and various factors, such as age, height, body weight, body mass index (BMI), renal function parameters, muscle volume and strength parameters, usual and maximal gait speed, number of teeth, and occlusal force.
Categorical variables are presented as absolute numbers (n) and relative frequencies (%), and were analyzed using the Fisher's exact test. Univariate and multivariate logistic regression analyses were performed to calculate the odds ratios and 95% confidence intervals. Data analysis was conducted using JMP version 17.1 software, with statistical significance set at p<05.
3. Results
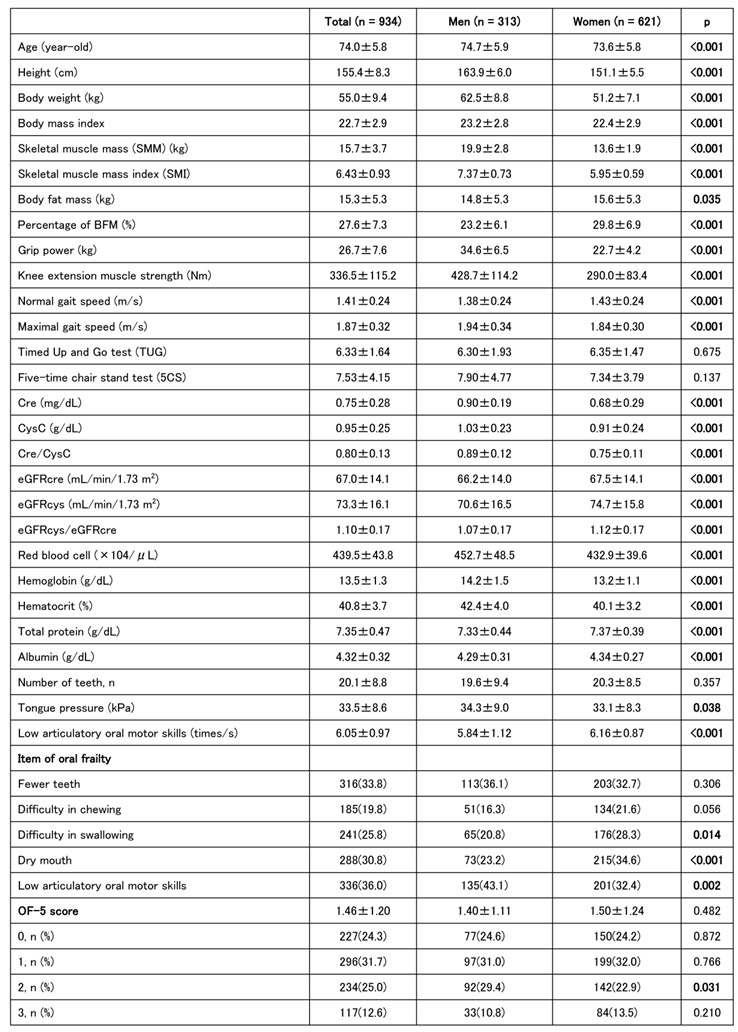
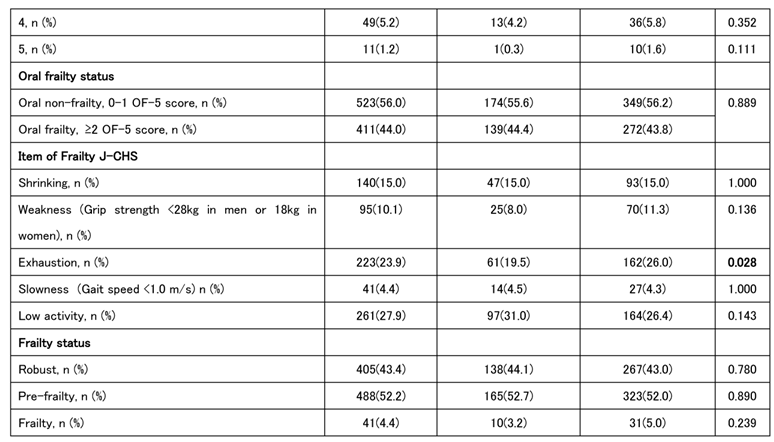
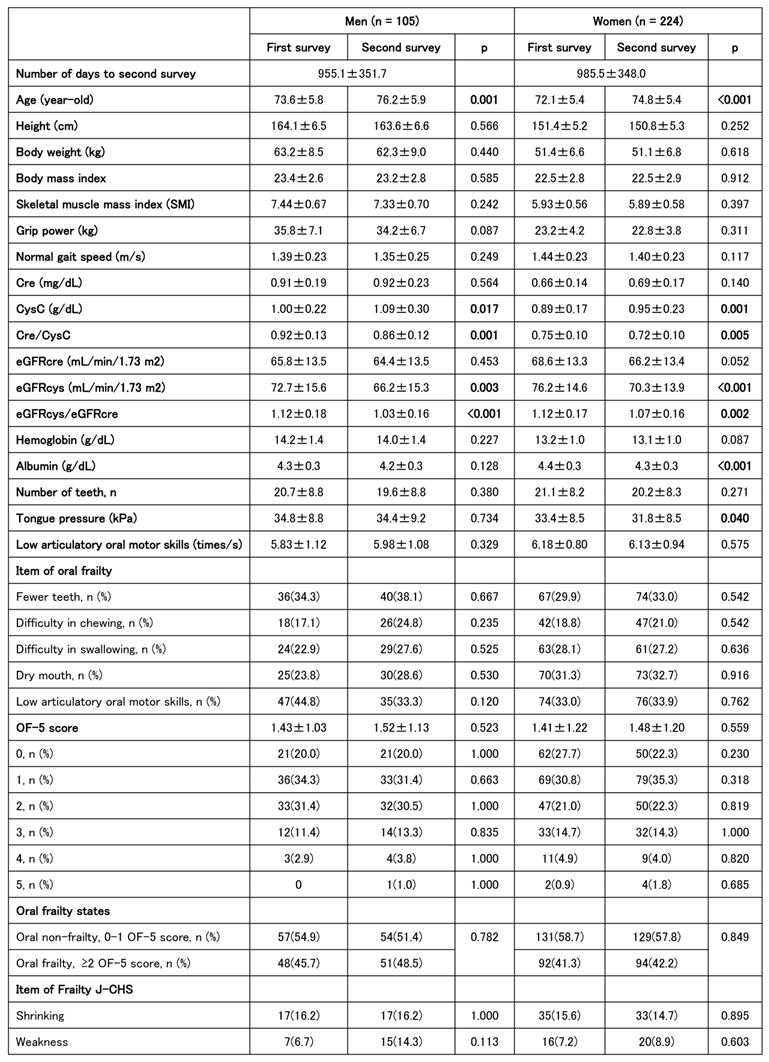
The characteristics of 313 men and 621 women (934 in total) are shown in
Table 1. The prevalence of oral frailty was slightly >40% in both sexes, with no significant difference between the sexes. According to the J-CHS frailty criteria by sex, exhaustion tended to be greater in women than that in men; however, there were no significant differences in the other four criteria. Muscle strength, muscle mass, and walking speed were generally greater in men than in women, although there were no sex differences in the timed up and go (TUG) test or the five-chair stand (5CS) test. Tongue pressure was greater in men, whereas ODK tended to be greater in women. However, no significant sex-related differences were observed in the number of teeth. Women also tended to be more prone to anemia, with higher total protein and albumin levels. Creatine, cystatin C, and Cre/CysC levels reflected muscle mass and tended to be higher in men than those in women, whereas eGFR tended to be higher in women than that in men. A score of ≥2 on the OF-5 indicates a diagnosis of oral frailty, which is associated with older age, shorter height, lower muscle mass and strength, and reduced physical functions, such as walking speed, TUG, and the 5CS test. Tongue pressure, number of teeth, and ODK were also reduced in individuals with oral frailty. (
Table 2)
Table 1.
Baseline Characteristics of Participants according to Sex.
Table 1.
Baseline Characteristics of Participants according to Sex.
Table 2.
Comparison of Physical and Oral Function according to Oral Frailty Status in Men and Women.
Table 2.
Comparison of Physical and Oral Function according to Oral Frailty Status in Men and Women.
Cystatin C-related indices, including the Cre/CysC ratio and eGFRcys, which we have previously reported, were lower in individuals of both sexes with oral frailty [
18]. Additionally, hemoglobin and albumin levels were lower in women with oral frailty. Both men and women with oral frailty were less robust and had more prefrailty; however, owing to the small number of frail individuals, the difference was not significant in the number of frailty.
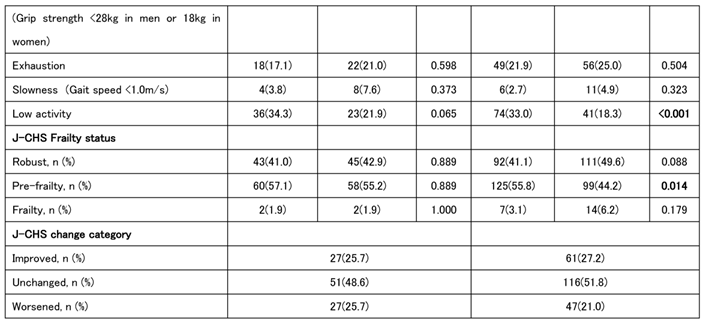
A longitudinal study involving 329 participants (105 men and 224 women) revealed changes in oral function between the first and second follow-up surveys. 2 to 3 years passed between the first and second follow-ups, during which no significant changes were observed in body size, grip strength, walking speed, SMI, or other parameters. However, cystatin C-related indices (Cre/CysC, eGFRcys, and eGFRcys/eGFRcre) were significantly lower at the second follow-up in both men and women. Albumin levels and tongue pressure were lower in women than those in men. The J-CHS frailty score showed a trend toward lower pre-frailty and greater robustness in women. Approximately half of the participants, both men and women, showed no changes in the relevant J-CHS items, whereas approximately a quarter showed either improvement or worsening (
Table 3).
Table 3.
Changes in Physical and Oral Function from Baseline to Follow-up according to Sex.
Table 3.
Changes in Physical and Oral Function from Baseline to Follow-up according to Sex.
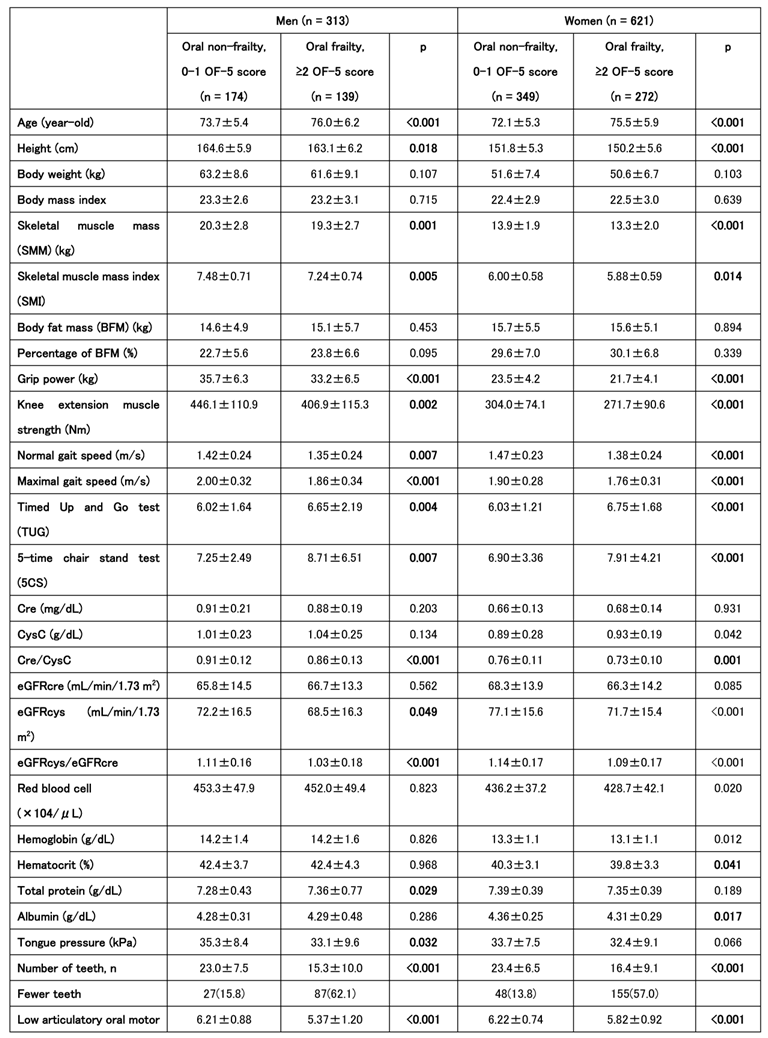
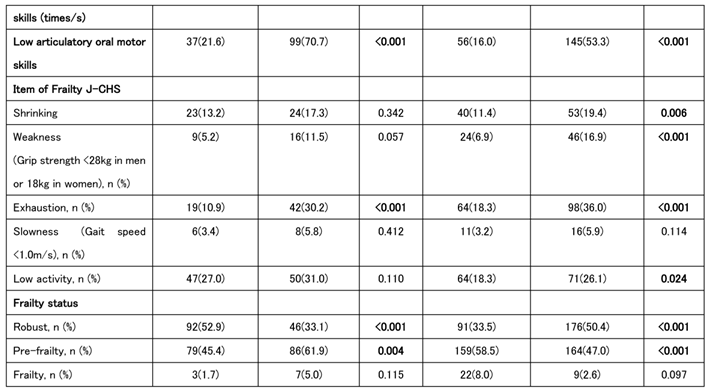
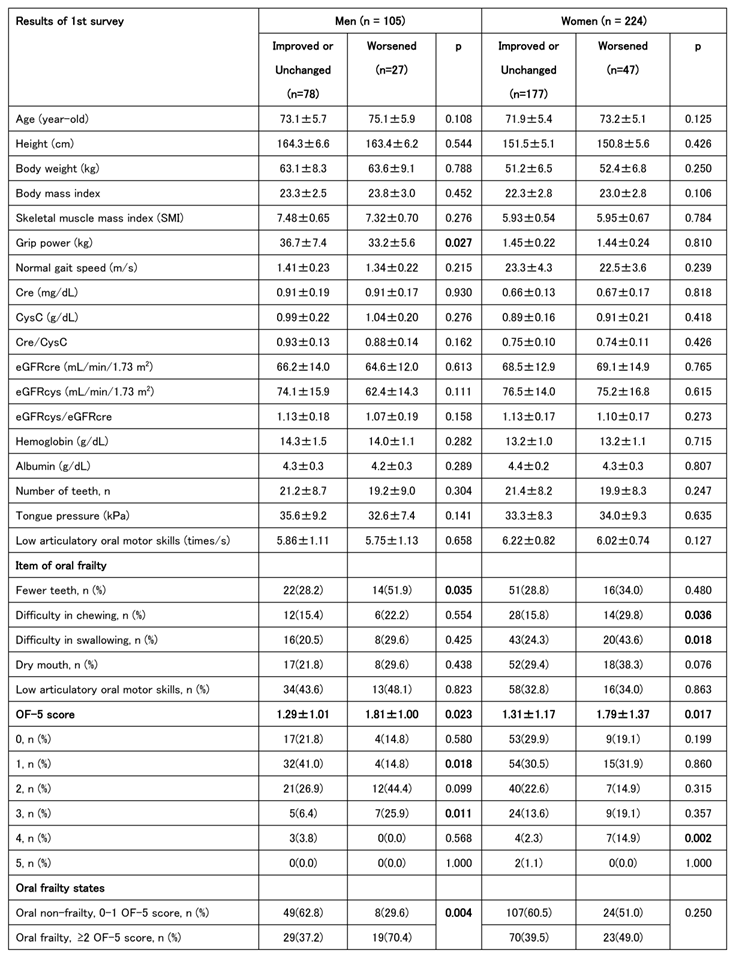
We also analyzed the baseline characteristics of the groups classified as improved/unchanged and worsened. In men, lower grip strength and fewer teeth at baseline were associated with disease worsening. In those who worsened, an OF-5 score of ≥2 at baseline was common, and many patients were assessed tas having oral frailty at the first time point. Among women, swallowing and chewing problems were more frequently reported at baseline in the worsened group, although the only significant sex difference was observed in the total OF-5 scores. In summary, individuals with higher baseline OF-5 scores were more likely to experience worsening J-CHS scores at the second time point (
Table 4).
Table 4.
Baseline Characteristics and Oral Frailty according to Frailty Progression Status in Men and Women.
Table 4.
Baseline Characteristics and Oral Frailty according to Frailty Progression Status in Men and Women.
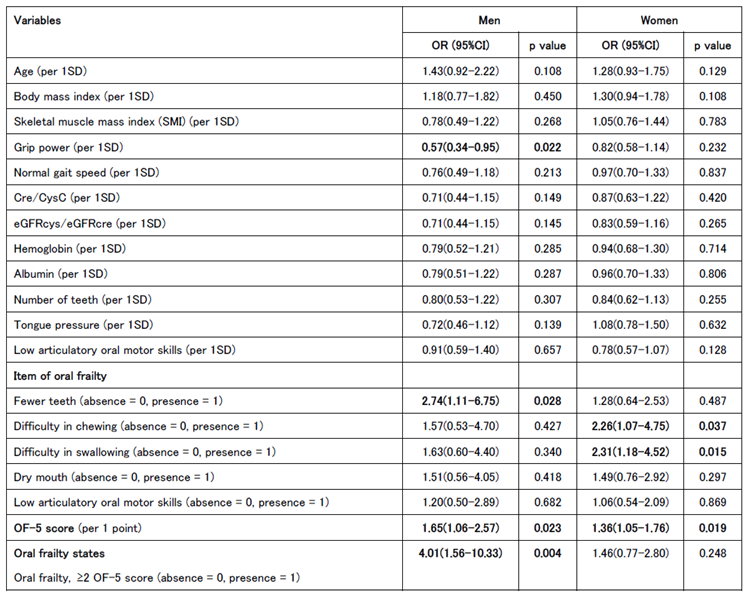
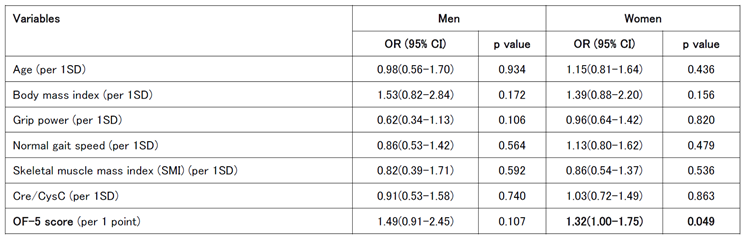
Univariate logistic regression analysis was conducted to calculate the odds ratios for each indicator at baseline in the worsening group in the second survey. For men, significant associations were found between reduced grip strength and tooth loss, whereas an OF-5 score of ≥2 and a diagnosis of oral frailty at the first visit were also significant worsening risk factors. Significant associations were observed between decreased chewing ability and swallowing function in the women. The OF-5 score was a significant worsening risk factor in both men and women; however, in women, there was no significant difference in those with an OF-5 score ≥2 (
Table 4A).
Table 5.
A. Univariate Logistic Regression Analysis for Baseline Factors Predicting Worsening Frailty according to Sex. B. Multivariate Logistic Regression Analysis for Baseline Factors Associated with Worsening of Frailty in Women. C. Multivariate Logistic Regression Analysis for Baseline Factors Associated with Worsening of Frailty in Men.
Table 5.
A. Univariate Logistic Regression Analysis for Baseline Factors Predicting Worsening Frailty according to Sex. B. Multivariate Logistic Regression Analysis for Baseline Factors Associated with Worsening of Frailty in Women. C. Multivariate Logistic Regression Analysis for Baseline Factors Associated with Worsening of Frailty in Men.
A
|
| SD, standard deviation; Cre, creatinine; CysC, cystatin C; eGFRcys, cystatin-based estimated glomerular filtration rate; eGFRcre, creatinine-based estimated glomerular filtration rate; OF-5, oral frailty five-item checklist. |
B
|
| OR, odds ratio; CI, confidence interval; SD, standard deviation; Cre, creatinine; CysC, cystatin C; OF-5, oral frailty five-item checklist. |
C
|
| OR, odds ratio; CI, confidence interval; SD, standard deviation; Cre, creatinine; CysC, cystatin C; OF-5, oral frailty five-item checklist. |
A multivariate logistic regression analysis was performed using age, BMI, grip strength, gait speed, SMI, Cre/CysC ratio, and OF-5 score, which are associated with frailty and sarcopenia, as explanatory variables. In men, grip strength was a significant univariate risk factor, whereas the OF-5 score did not differ significantly. However, in women, the OF-5 score remained a significant risk factor (
Table 4B).
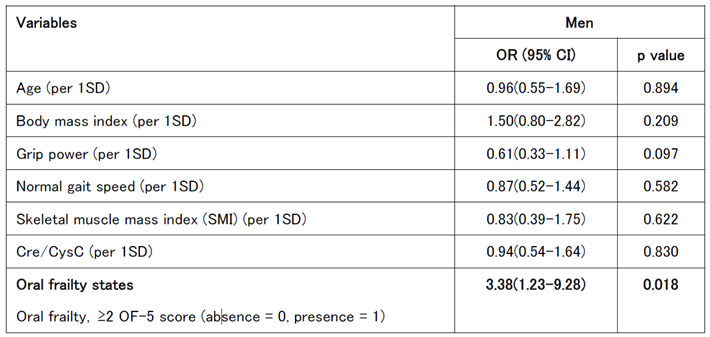
The same multivariate logistic regression analysis was repeated for men by adjusting the OF-5 score to ≥2, and a significant difference remained (
Table 4C).
4. Discussion
This study offers a comprehensive examination of sex-specific differences in oral frailty and related factors in a cohort of older adults, highlighting the important aspects of oral and physical function. Notably, the prevalence of oral frailty is similar between men and women, affecting >40% of the population. The 40% prevalence of oral frailty was in agreement with several previous reports [
1,
31,
32].
Women tended to report more fatigue and anemia, whereas men reported greater muscle strength, muscle mass, and tongue pressure. These findings underscore the need to consider sex-based physiological differences when evaluating frailty and sarcopenia, particularly oral health-related parameters.
One of the key results of this study was the significant association between oral frailty and reduced physical functions, such as walking speed, muscle mass, and tongue pressure, confirming an intricate link between systemic frailty and oral health. In both sexes, a higher OF-5 score of ≥2, which indicates a diagnosis of oral frailty, was correlated with diminished physical and oral functions, including grip strength and the number of teeth. These findings suggest that oral frailty can serve as a valuable early marker of declining physical capacity and could help identify individuals at risk of sarcopenia or broader systemic frailty.
The longitudinal component of this study provides further insights into the progression of oral frailty. Over a follow-up period of 2–3 years, significant declines in cystatin C-related indices (Cre/CysC and eGFRcys) and oral functions, including tongue pressure and albumin levels, were observed, particularly in women. These changes were not accompanied by significant alterations in muscle strength, walking speed, or other systemic parameters, indicating that oral frailty may progress rapidly or independently of systemic physical decline. This underlines the importance of targeted interventions focusing on oral health to mitigate the progression of frailty.
Importantly, the logistic regression analysis identified distinct risk factors for the worsening of oral frailty. In men, reduced grip strength and tooth loss were significant predictors, consistent with previous studies linking oral health to systemic physical capacity. In contrast, impaired chewing and swallowing functions were more prominent risk factors in women, underscoring the role of oral function in overall health deterioration in older women. Notably, although the OF-5 score was a significant risk factor for worsening frailty in both sexes, its effect was more pronounce in women, suggesting a potential sex difference in the relationship between oral health and progression of systemic frailty.
Despite these significant findings, it is important to acknowledge the limitations of this study. The sample size of frail individuals was relatively small, limiting the ability to detect subtle differences between sexes or within subgroups. Additionally, although longitudinal data were collected, the follow-up period may not have been sufficiently long to fully capture the trajectory of oral frailty in this population. Future studies with larger, more diverse cohorts and extended follow-up periods are needed to clarify the dynamics between oral and systemic frailty, and to identify effective interventions that target both domains. Moreover, the inclusion of healthy volunteers may have influenced the representativeness of the study population. Previous reports indicated that the prevalence of frailty, according to the J-CHS criteria, is approximately 10% among older adults in the Japanese populations [
33,
34,
35]. However, in this study, the prevalence of frailty was considerably lower at 3.2% for men and 5.0% for women.
During the 2-to 3-year observation period, no significant decline in grip strength, gait speed, or muscle mass was observed. However, even with a limited number of participants and short observation period, a diagnosis of oral frailty using the OF-5 was associated with an increase in J-CHS scores, suggesting that the OF-5 score is significantly linked to the worsening of long-term physical frailty. Oral frailty, as assessed using the OF-5, was also significantly associated with higher J-CHS scores after adjusting for age, BMI, grip strength, gait speed, SMI, and other frailty-related factors. These findings indicate that the OF-5 is a promising predictor of frailty onset. The novelty of this study lies in the significant relationship between OF-5 score and other frailty indices.
In the original article that introduced the OF-5 in 2023, difficulty in chewing, difficulty swallowing, and dry mouth were evaluated using subjective questionnaires, whereas objective data from dental examinations were used to assess the number of teeth and articulatory oral motor skills. Articulatory oral motor skills were also assessed. Similarly, the present study evaluated articulatory oral-motor skills via ODK, and a dentist assessed the number of teeth to objectively evaluate these aspects.
The results of the objective evaluation of ODK and subjective evaluation via questionnaires were in good agreement [
35]. In April 2024, a joint consensus statement on “Oral Frailty” in Japan suggested that the objective assessment of ODK is no longer necessary and can be replaced with the following question: “Have you had difficulty with clear pronunciation recently?” This statement also allows for a self-reported assessment of whether respondents had >20 teeth. Future studies should investigate whether oral frailty, as assessed by the OF-5 subjective questionnaire, is associated with lower muscle mass, slower gait speed, and reduced physical function in cross-sectional studies, and whether it significantly correlates with the progression of physical frailty in longitudinal studies.
In conclusion, this study provides valuable evidence on the relationships between oral frailty, systemic frailty, and risk factors in older adults. These findings emphasize the importance of integrating oral health assessments into frailty screening protocols, particularly for older women in whom oral dysfunction may serve as an early marker of systemic health decline. These insights have implications for the development of interventions aimed at preventing or mitigating frailty and its associated adverse outcomes in aging populations.