Introduction
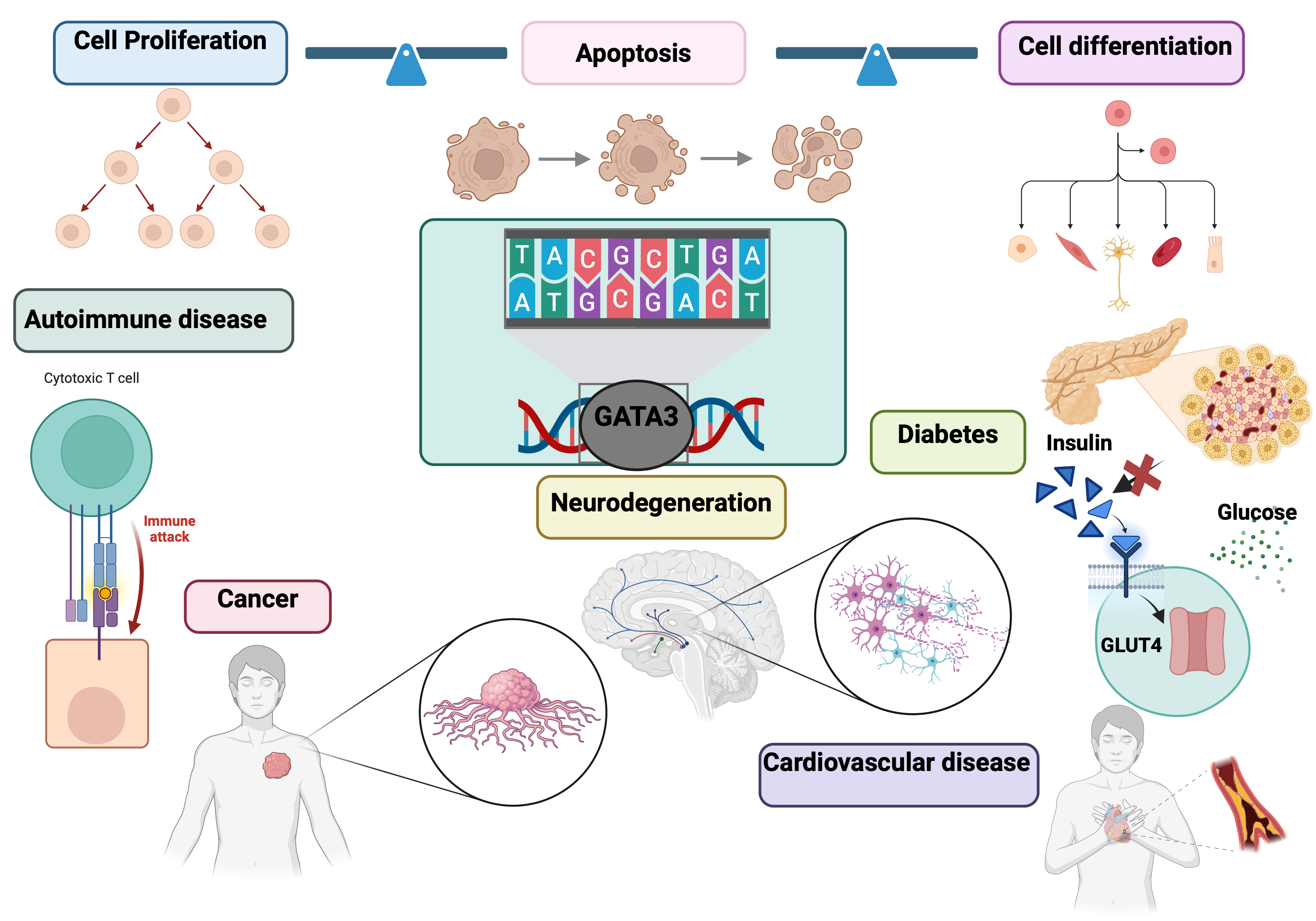
GATA3, a member of the GATA family of transcription factors, plays a vital role in regulating gene expression and key cellular processes, including differentiation, proliferation, and apoptosis. It is essential for the development and maintenance of various tissues and systems, including the immune, endocrine, and nervous systems. Dysregulation of GATA3 is implicated in several diseases, such as breast cancer (BC), haematological malignancies like T-cell lymphomas, autoimmune diseases, and neurodegenerative disorders. In cancer, GATA3 influences tumor development and progression by affecting cancer cell behaviour within the tumor microenvironment (TME). For instance, reduced GATA3 expression in breast cancer is linked to a poorer prognosis. In autoimmune diseases such as multiple sclerosis, abnormal GATA3 expression disrupts immune system balance, contributing to the autoimmune attack on the nervous system. In neurodegenerative disorders like Alzheimer’s disease, GATA3’s role in neuronal function and maintenance suggests that its dysregulation may accelerate disease progression.
GATA3’s involvement in these conditions highlights its potential as a biomarker for diagnosis, prognosis, and personalized treatment strategies. Its altered expression patterns provide insights into disease pathology and patient outcomes, making it a valuable tool in clinical practice. Furthermore, GATA3 analysis helps uncover the shared genetic architecture between conditions like cancer and autoimmune diseases, guiding therapeutic interventions and disease management. Overall, GATA3 stands out as a promising biomarker with significant implications for future research and medical applications.
1. GATA3 in Normal Physiology
1.1. Immune System Development
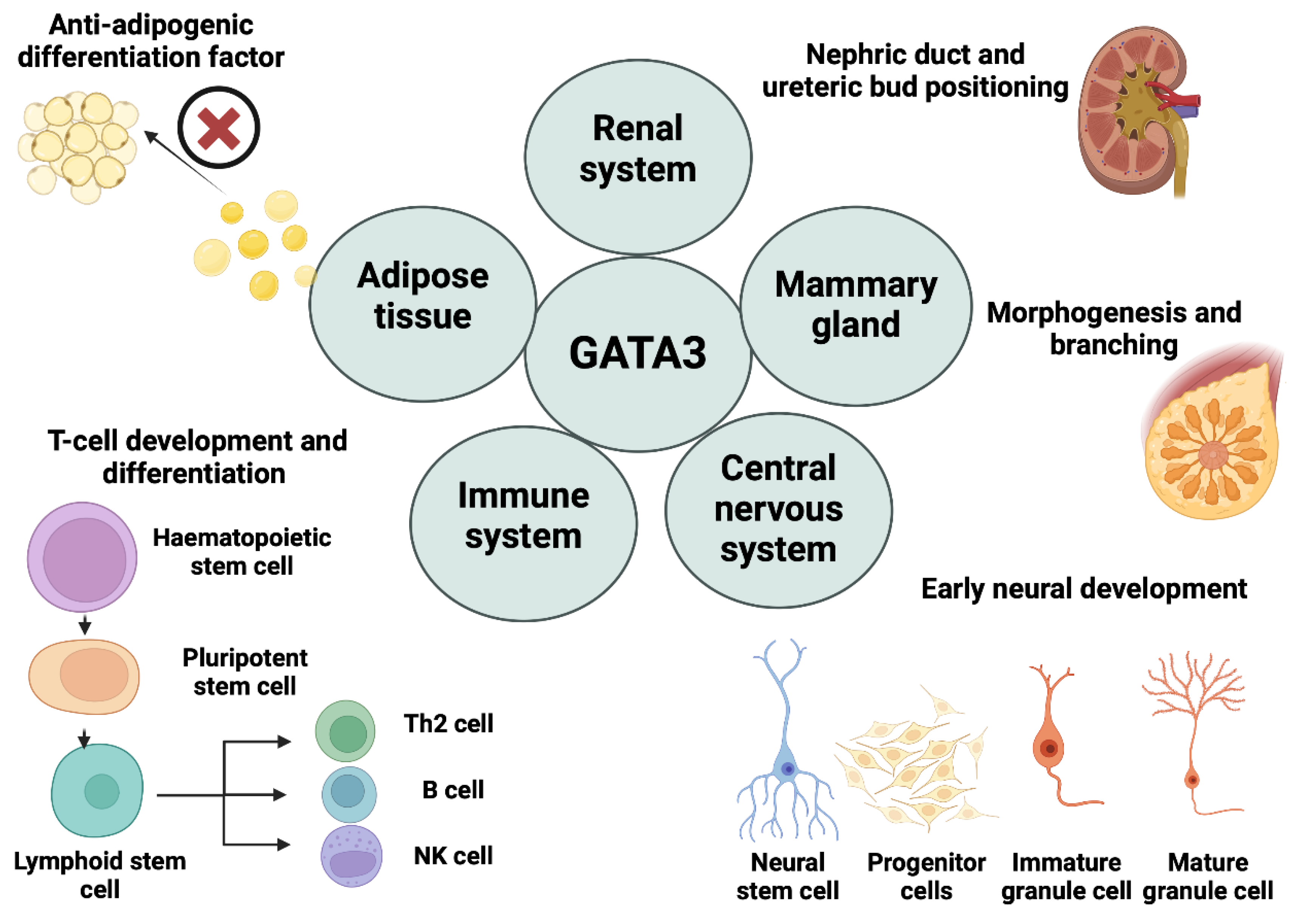
GATA3, a dual zinc finger transcription factor, is a critical modulator of the growth and differentiation of the immune cells, thereby influencing their function in the body's defence system[
2,
12]. Its significance is not restricted to specific cell types as it impacts the biological processes of various cell types of both innate and adaptive immunities. GATA3 is abundantly found in the hematopoietic compartment, where immune cells evolve and differentiate into multiple types with distinct roles[
2,
12]. While GATA3 is well-known as a key determinant of Th2 cells, via integrating different upstream signals to regulate target genes, which differentiate into the helper CD4+ T-cell subset, emerging evidence suggests that it also plays a role in maintaining the differentiation and function of other T-cell subsets, as well as B-cells and natural killer (NK) cells[
2,
12]. Several humoral mediators, particularly cytokines like interleukin-2 (IL-2) and interleukin -4 (IL-4), are crucial in the Wnt and Notch signalling pathways, which regulate GATA3 expression in Th2 cells
2. Moreover, GATA3 expression is upregulated in CD4 T-cells and innate lymphoid cells (ILCs) offering an additional role in immunoregulation via distinct genetic programs based on cellular and micro-environmental contexts[
2,
12]. Strikingly, GATA3 possesses its own activation machinery apart from cytokines-dependent stimulation enabling its stabilization in Th2 cells to maintain their function[
2,
13].
1.2. Mammary Gland Development
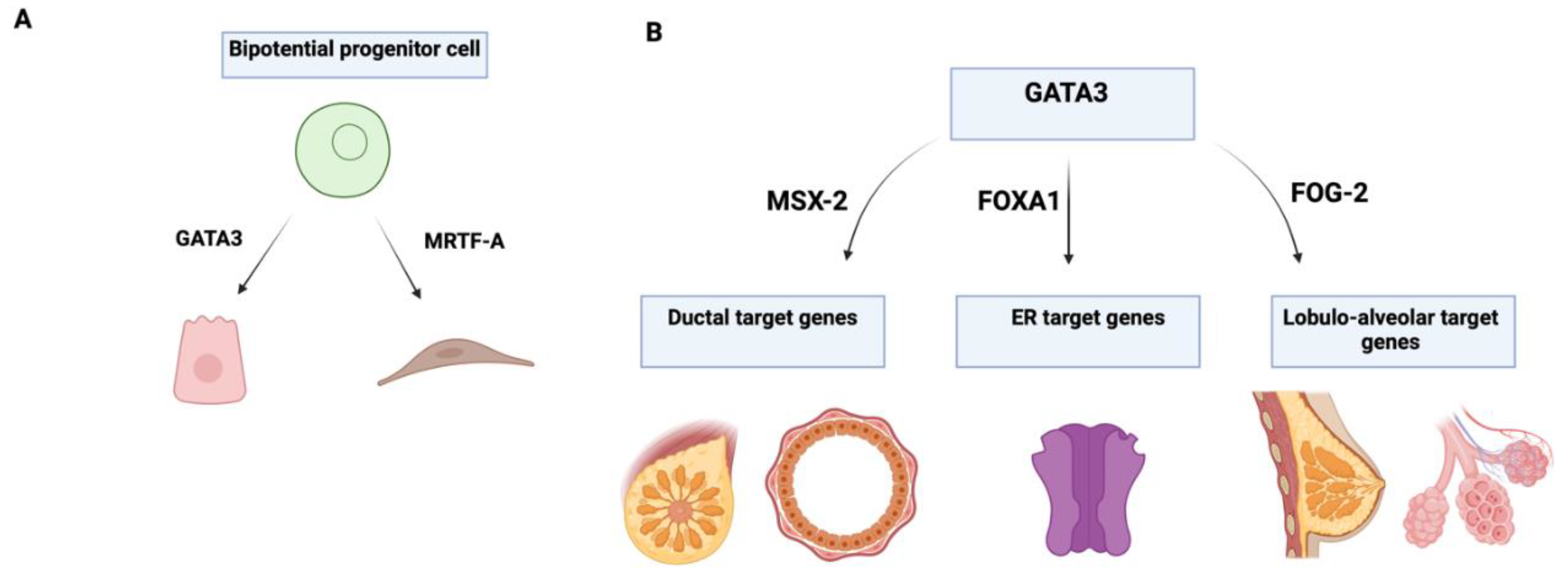
As GATA3 is a fundamental mediator for cell fate processes and mammary gland is a ductal epithelial organ comprising two main mature epithelial cell types (luminal epithelial and myoepithelial), thus, the role of GATA3 in the growth and differentiation of mammary glands has been the subject of speculation in various studies[
14,
15]. GATA3 regulates the morphogenesis, branching, and elongation of the mammary gland[
14]. Deleting GATA3 selectively in the mammary epithelium during puberty using MMTV-Cre92 failed in the development of terminal end buds (TEBs) and the formation of abnormal ductal structures[
1,
14,
15]. This suggests that GATA3 plays a role in tumorigenesis and breast cancer initiation by influencing the differentiation of both epithelial and non-epithelial tissues[
15].
1.3. Central Nervous System (CNS)
GATA3 is a key player in neural development and differentiation as its expression is linked to early motor neurons and interneuron precursors, underscoring its pivotal involvement in the early developmental stages of CNS[
16]. Mice embryos with GATA3 mutations were fatal, exhibiting symptoms like internal bleeding, growth delay, abnormalities in fetal liver haematopoiesis, brain deformities, and spinal cord damage [
17]. Moreover, blocking GATA3 activity depletes the ability of neural stem cells (NSCs) in the telencephalon of zebrafish to proliferate and generate neurons[
18]. After injury, GATA3 expression is reactivated in glial cells and newly formed neurons, demonstrating its involvement in reactive neurogenesis and the migration of neurons[
18].
1.4. Renal System
At renal developmental stages, GATA3 is predominantly found in the epithelial nephric duct of the renal primordium, where it regulates the positioning of the ureteric bud within the renal system[
19]. Grote et al.[
20] used a HoxB7-Cre transgenic line to specifically eliminate GATA3 from the nephric duct resulting in premature differentiation, and formation of misplaced metanephric kidney ducts. Renal dysplasia has been linked to GATA3 insufficiency as well, revealing the substantial role of GATA3 in renal cell development [
21]. Additionally, renin cells’ lineage identity, precursors in renal tissues, is strongly associated with GATA3 levels depending on previous research. Mice with conditional knockout of GATA3 (GATA3-cKO) displayed remarkably diminished GATA3 in juxtaglomerular, mesangial, and smooth muscle cells, implying massive elimination of GATA3 in renin lineage cells. These GATA3-cKO mice displayed a notable rise in blood urea nitrogen levels, suggesting hypovolemia and/or impaired renal function[
22].
1.5. Adipose Tissue:
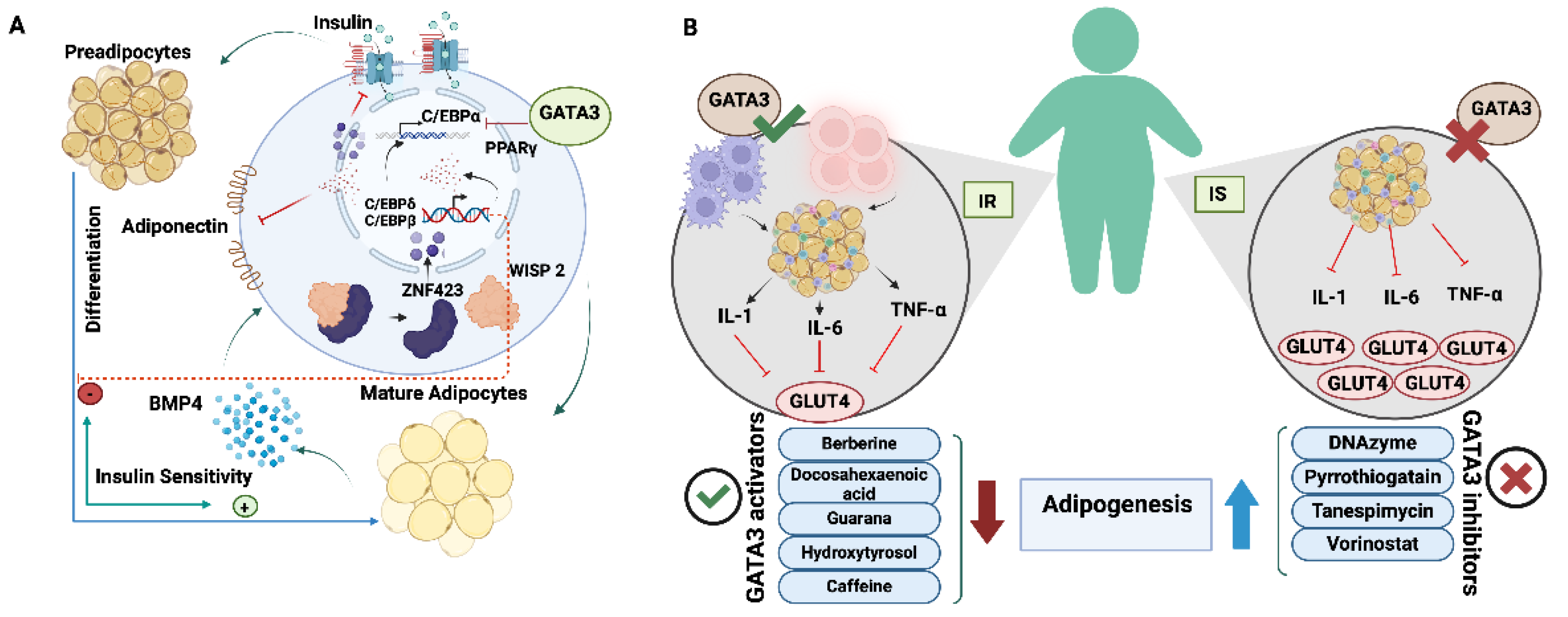
Remodelling the white adipose tissue (WAT) at early preadipocyte differentiation can hinder adipogenesis [
23]. Remodelling factors as GATA2 and GATA3, inhibit adipogenesis through direct binding to the Peroxisome proliferator-activated receptor γ (PPARγ) promoter, suppressing its activity, and antagonizing CCAAT/enhancer binding protein α and β (C/EBPs)[
24]. Continuous expression of GATA3 impedes adipogenesis by maintaining cells in the preadipocyte stage, with GATA3 levels gradually decreasing as adipocytes differentiate[
25]. Targeted therapies for obesity and insulin resistance (IR) have been speculated based on outcomes of silencing GATA3 via specific DNAzyme which induces adipogenesis in 3T3-L1 mouse pre-adipocytes[
26]. Therefore, GATA3 implementation enhances adipogenesis, modifies fat distribution, improves insulin sensitivity, and reduces obesity-related inflammation, supporting its therapeutic relevance in Type 2 Diabetes (T2D) pathology[
26,
27]. Overall, GATA3 acts as a vital mediator for maintaining the biological processes and homeostatic functions for optimal regulation of the dynamism and productivity of living cells (
Figure 1).
2. GATA3 in Pathological Conditions
2.1. Cancer
2.1.1. Breast Cancer (BC)
The eligibility of GATA3 as a key marker in BC diagnostics and prognostics is attributed to the close association with ER status (
Figure 2).
Luminal Differentiation and Tumour Progression:
GATA3 is essential for maintaining luminal cell differentiation in the mammary gland[
15], opposing the basal differentiation regulated by SOX10[
29]. GATA3 plays a key role in the morphogenesis and regulation of mammary epithelial differentiation, serving as an important tissue-specific marker for identifying whether tumours originate from epithelial or mesenchymal cells (
Figure 3).
In triple-negative breast cancer (TNBC), which is known for its aggressiveness and poor prognosis, the absence of GATA3 expression suggests that some TNBC subtypes may lose their specific markers as progenitor cells transition into tissues with distinct morphologies, thereby increasing tumor aggressiveness[
29]. While GATA3 is generally associated with luminal differentiation and better prognosis in breast cancer, some studies have reported conflicting results regarding its role in different subtypes. For instance, GATA3 expression is typically low in TNBC, which is associated with poor prognosis[
30]. However, some rare TNBC subtypes may still express GATA3, complicating the understanding of its role in these cancers[
29].
Tumour Microenvironment (TME) and Macrophage Infiltration (MI):
Previous studies have demonstrated that the tumor microenvironment (TME) has a significant impact on GATA3 expression[
31]. Elevated MI within the TME has been shown to reduce GATA3 levels, correlating with advanced tumor grade and poorly differentiated forms of BC[
31]. Tumor-associated macrophages (TAMs) of the M2 phenotype promote tumor progression, further implicating the role of GATA3 in tumor differentiation and aggressiveness[
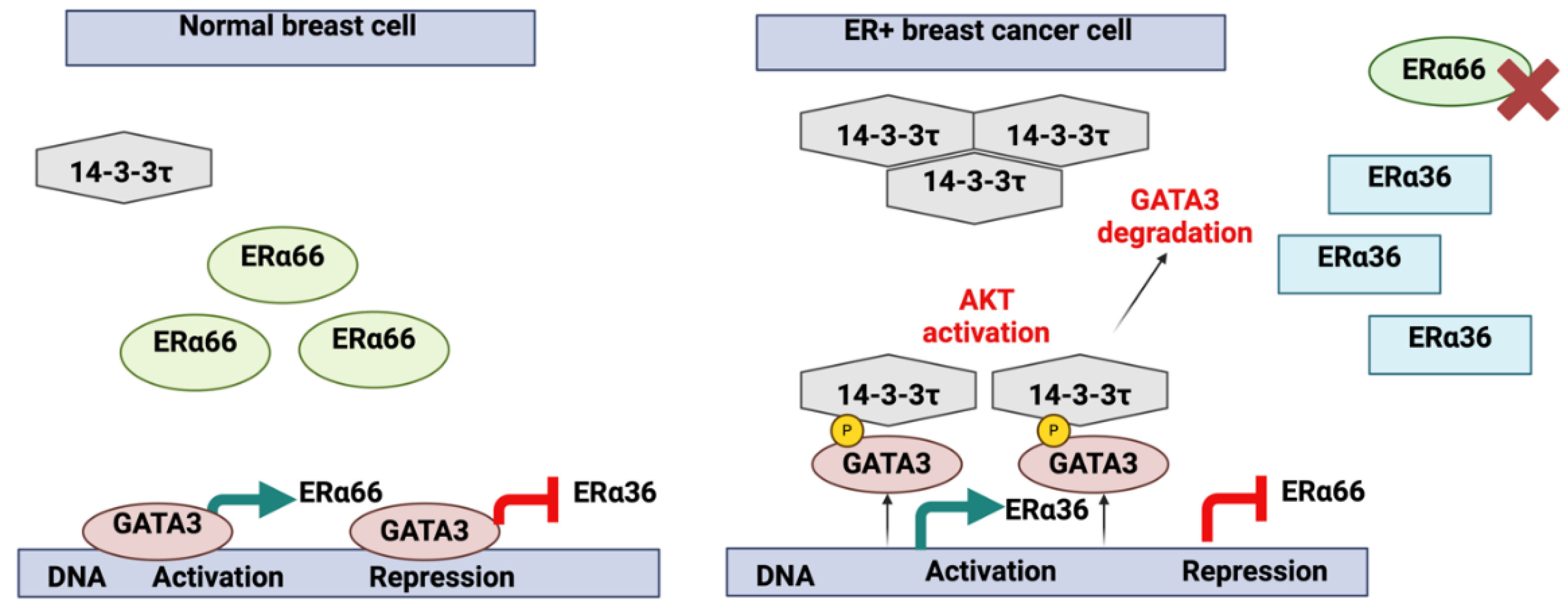
31]. This finding accentuates the importance of the TME in modulating GATA3 expression and its potential impact on tumor progression. GATA3 influences several molecular pathways in BC. It binds to ERα66 and ERα36 promoters, regulating their transcription. During BC progression, aberrant activation of protein kinase B (AKT) and high 14-3-3τ expression led to GATA3 phosphorylation, disrupting its transcriptional control, and promoting a basal-like cell phenotype(
Figure 2)[
28]. The potential of targeting GATA3 for therapeutic purposes is promising, but there are significant challenges. For example, the mechanisms underlying GATA3 downregulation in aggressive breast cancers are not fully understood, which complicates the development of targeted therapies. Moreover, the potential side effects and off-target impacts of modulating GATA3 expression need to be carefully evaluated in preclinical and clinical studies[
32].
GATA3 Is a Potential Diagnostic and Prognostic BC Marker:
GATA3 serves as a powerful diagnostic marker for BC, outperforming traditional markers like gross cystic disease fluid protein 15 (GCDFP-15) and mammaglobin due to its stronger positivity and consistency in both primary and metastatic tumors[
33,
34]. Its expression is positively linked to ER levels, indicating a better response to hormonal treatments in patients with high GATA3 expression[
35]. Although GATA3 is a powerful diagnostic marker for breast cancer, its utility may be limited by the heterogeneity of breast cancer subtypes. The variability in GATA3 expression across different subtypes and stages of breast cancer suggests that it may not be universally applicable as a prognostic marker[
36]. Additionally, the reliance on GATA3 expression alone may not provide a complete picture of the tumour’s behaviour and prognosis.
2.1.2. Leukemia and Lymphomas
Genetic Variants and Risk Associations:
The pathogenesis of hematopoietic malignancies, including leukemias is potentially linked to inherited germline or somatic genetic variations[
41]. While somatic genomic aberrations such as mutations, and rearrangements drive the progression of leukaemia, via maintaining the pro-survival of early-stage leukaemia precursor cells, the inherited risk variants, and their causative mechanisms in leukemogenesis remain poorly understood.
Upon ATAC-seq performed in a recent study, enriched GATA3 binding sites were in close approximate with the translocation breakpoints seen in Philadelphia chromosome-like acute lymphoblastic leukaemia (Ph-like ALL)[
41]. GATA3 overexpression was correlated potentially to the chromosomal instability and vulnerability to translocations that take place in earlier stages of cancer onset. Thus, GATA3 upregulation in leukemic patients might facilitate the enhancer seizing those results in closer rearrangement to the oncogenes, triggering oncogenesis without forming gene fusions.
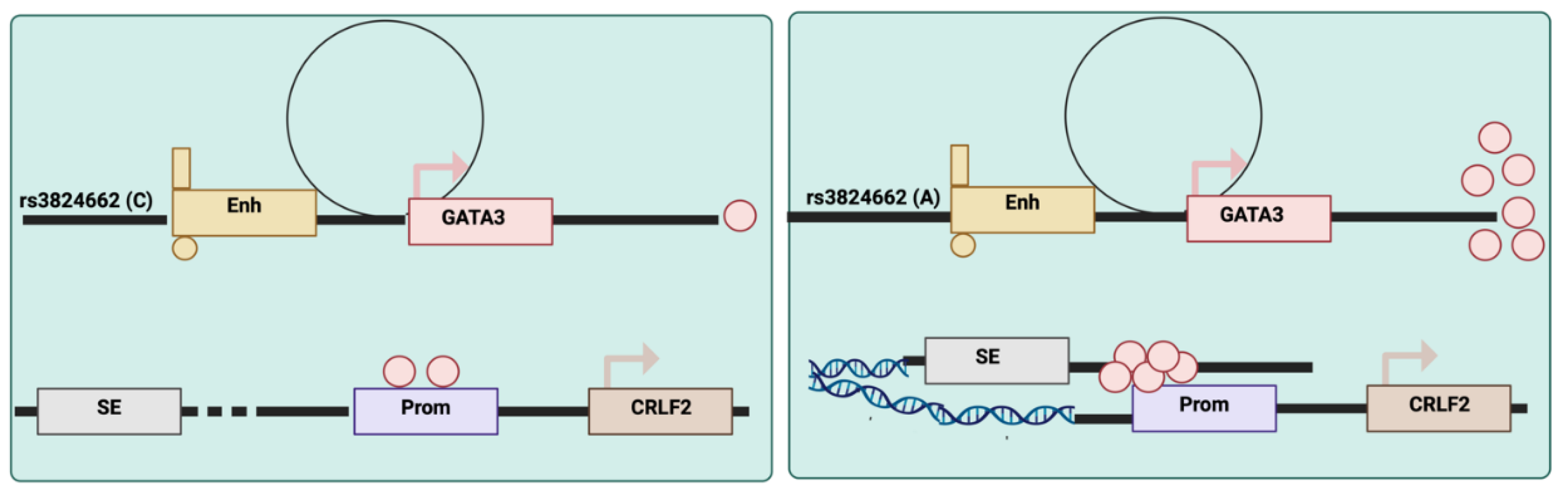
Recent research has identified noncoding genetic variations in GATA3 that increase the risk of acute lymphoblastic leukaemia (ALL). For example, the rs3824662 variant acts as a cis-acting enhancer that accelerates GATA3 transcriptional activity, reshaping global chromatin accessibility and increasing susceptibility to chromosomal rearrangements[
41], leading to increased incidence at regulatory sites and de novo occupancy in certain elements. Such a shift in the overall chromatin state plays a role in the development and progression of Ph-like ALL[
41]. This discovery provides insights into the genetic regulation of GATA3 and its role in leukemogenesis (
Figure 4).
Elevated GATA3 expressions had been detected in B cell malignancies including Hodgkin lymphoma as well. The constitutive induction of NFkB and Notch-1 led to an aberrant increase in GATA3 levels within Reed Sternberg cells accompanied by a massive amount of IL-13 ends up with typical signalling of Hodgkin lymphoma[
42]. GATA3 is not present in normal B cells, and mainly functions as a key regulator of lymphoid cell lineage commitment (B vs. T cells)[
43]. Collectively, it has been indicated that GATA3 is a major driver of epigenomic reprogramming that can directly influence the activity of oncogenes, suggesting a critical pathway through which inherited genetic variations might impact the risk of cancer[
41].
Oncogenic Role in T-cell Lymphoproliferative Neoplasms:
Interestingly, GATA3 activity is not limited to regulation and reprograming seen in genetically and clinically unique groups of T-cell lymphoproliferative neoplasms, as a recent work had provided the first direct evidence that GATA3 is, indeed, a bona fide proto-oncogene that contributes to the aggressiveness for these neoplasms[
44]. The oncogenic driving capacity of GATA3 had originated from induction of the transcriptional programs that promote cell growth and proliferation, hence divergent enhancer landscapes had been detected among GATA-3-associated T-cell neoplasms with different target genes[
44]. Most of these genes were linked to T-cell lymphomagenesis and chemotherapy resistance[
45], explaining the notable connection between GATA3 expression and adverse outcomes after receiving standard chemotherapy treatments[
44]. This finding stresses the potential of targeting GATA3 in therapeutic strategies for T-cell lymphomas.
2.1.3. Liver Carcinoma
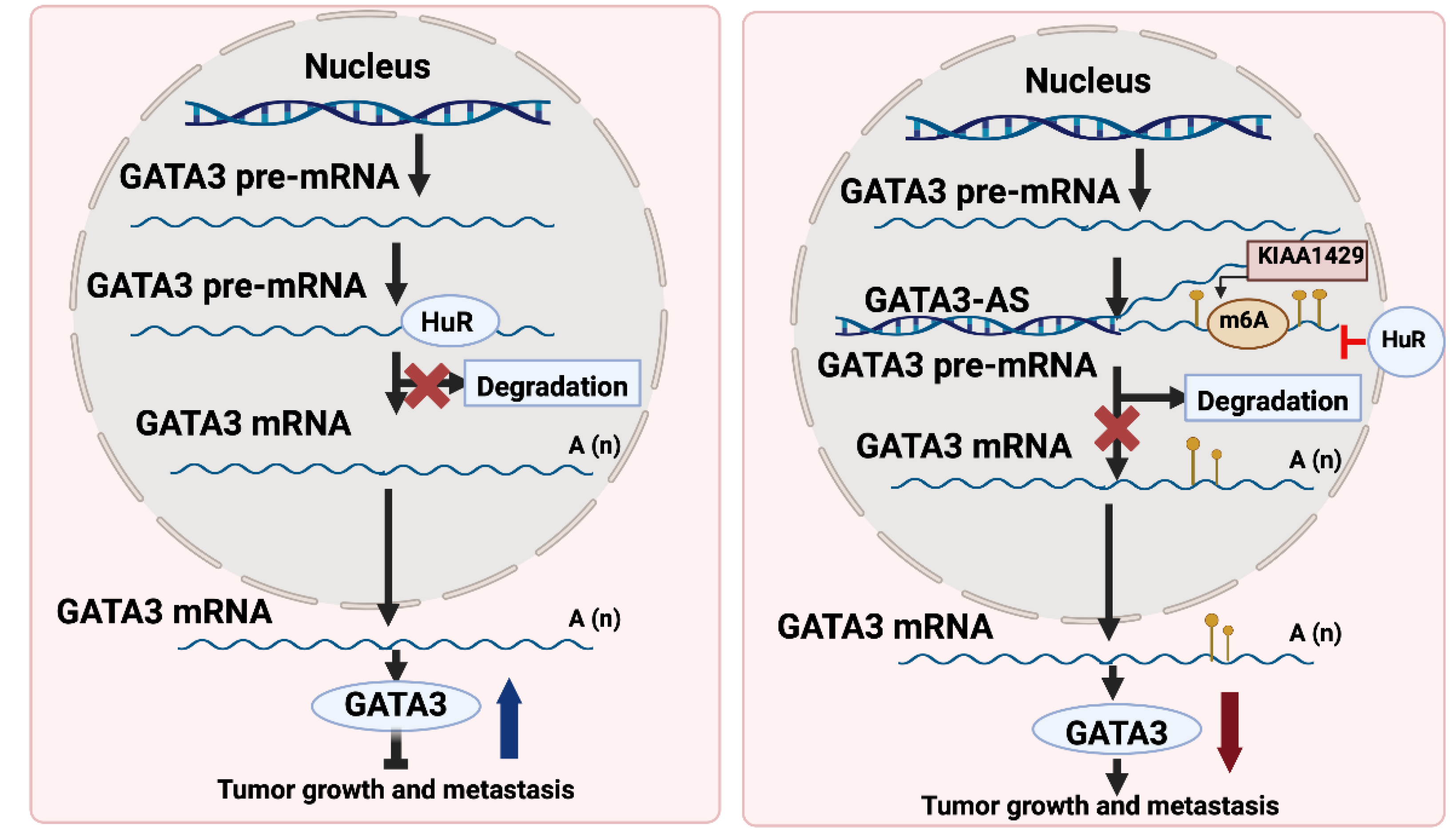
GATA3 functions as the main activator for tumor suppressor genes via binding to their promoters. Nonetheless, the mechanisms behind GATA3 downregulation led to switching off the tumor suppressor genes in most cancers remain elusive. One of the possible mechanisms of GATA3 depletion in hepatocellular carcinoma (HCC) is the post-transcriptional modification that disturbs its mRNA levels, thereby its protein levels are diminished[
46]. In a previous study, GATA3 downregulation was attributed in HCC to the formation of GATA3-antisense (GATA3-AS) that antagonizes GATA3, functions as a cis-regulatory element, thus, directing m6A modification by KIAA1429 on the GATA3 pre-mRNA[
46]. This post-transcriptional modification leads to the degradation of GATA3 mRNA, contributing to HCC progression[
46].On the contrary, GATA3-AS depletion was associated with suppressed malignant phenotypes in hepatoma cells that were rescued by GATA3 inhibition[
46]. Understanding this novel mechanism of GATA3 downregulation in HCC opens new avenues for therapeutic interventions targeting GATA3-AS and m6A modification. GATA3 mediates the metastasis driven by KIAA1429 or GATA3-AS as shown in
Figure 5.
Additionally, tumor suppressiveness of GATA3 was detected in HCC through its direct regulation of slug expression[
47]. Thereby, interfering with the EMT process, cell proliferation, invasion, and migration. Such finding represents GATA3 as a promising candidate for designing therapeutic strategies against HCC.
2.1.4. Prostate Cancer (PCa)
An antagonizing role of GATA3 in preventing the progression of prostate cancer has been determined in prior research[
48]. The enforced GATA3 expression in prostate tumors interferes with Akt signalling and maintains a differentiated prostatic duct phenotype that is predictive of low tumor recurrence[
48]. The latter work exhibited the potential of using GATA3 anti-oncogenic activity via regulating PI3K-Akt signalling as a prognostic biomarker for prostate cancer. Although GATA3 serves as a practical immunohistochemical marker for differentiating cancers with urothelial origins from prostate cancers, GATA3 positivity in metastatic prostate PCa may infrequently lead to misdiagnosis necessitating a panel of immunohistochemical markers, for both prostatic and urothelial cancers, primary and metastatic tumors[
49]
.
2.1.5. Bladder Cancer
Loss of GATA3 function was consistently seen in high-grade invasive bladder cancer suggesting the utility of GATA3 as a prognostic biomarker besides its utility in diagnostic procedures[
50]. In general, depleted level of GATA3 suggests a basal subtype of bladder cancer that is more sensitive to immune checkpoint blockade therapy (ICB) and neoadjuvant treatments. Conversely, upregulation of GATA3 refers to a luminal subtype of bladder cancer, exhibiting better response to targeted treatments, including a knockout of GATA3, β-catenin, and PPAR-γ pathways, as well as anti-angiogenic therapy. Therefore, the employment of GATA3 in the determination of TME phenotypes and bladder cancer subtypes had focused on GATA3 as a promising candidate for personalized medicine for better management of bladder cancer[
51]. GATA3 is a plausible diagnostic biomarker for urothelial carcinoma as it enables differentiation from other genitourinary malignancies[
52]. Tumor grade and invasion in biopsy material with poor morphological characteristics can be predicted as well. Thus, GATA3 represents an attractive sensitive, and specific marker for detection, staging, and therapeutic management in urothelial carcinoma. In invasive urothelial carcinomas, a notable difference in GATA3 expression between the subgroups with non-muscular invasion and those with muscular invasion had been recorded[
53]. Among the examined histological subtypes, the microcytic subtype had demonstrated the greatest GATA3 expression correlated with better prognosis and overall survival rates[
53].
2.2. Autoimmune Diseases
2.2.1. Type 1 Diabetes (T1D):
As indicated earlier, GATA3 acts as an essential TF in controlling T-cell lineage. Since autoimmunity alters the capabilities of T-cells and T1D is one of these diseases, then GATA3 is proposed to be involved in the underlying pathogenesis of T1D as it is examined in a recent study. Deficient levels of GATA3 and
forkhead box P3 (FoxP3) while elevated T-bet and RORγt mRNA levels in individuals with positive β-cell autoantibodies were associated with T1D pathology[
54]. So, considering the restoration of GATA3 levels in such T1D cases may exhibit beneficial effects for the treatment and management strategies of T1D.
2.2.2. Juvenile Idiopathic Arthritis (JIA)
A novel insight into the altered GATA3 activity that coincides with autoimmune arthritis had been unveiled in the first known case report of autoimmune arthritis[
55]. A dominant negative function of GATA3 transcriptional activity was determined due to the expression of mutant protein M401VfsX106 [
55]. It is well-established that autoimmune disorders are characterized by elevated T helper 1(Th1), and T helper 17 (Th17) cell activity, accompanied by increased Interferon-gamma (IFN-γ) release due to the epigenetic modifications; gain or loss of the histone modification and DNA methylation led to the production of Th0, Th1, and Th2 lineages[
56]. The enforcement of GATA3 expression concomitant with the reduction of Th17 cell differentiation led to a considerable reduction in joint-destructive inflammation in arthritic mice models indicating the protective role of GATA3 in alleviating the progression of autoimmune arthritis[
57]. This discovery provides a new understanding of GATA3's role in autoimmune diseases and potential therapeutic targets.
2.2.3. Multiple Sclerosis (MS)
MS is an autoimmune disorder described by altered Th-cell differentiation that is mainly driven by GATA3[
58,
59]. GATA3 is thought to decrease the onset and intensity of MS by directing the differentiation of Th cells towards Th2 and regulatory T cells (Tregs) while suppressing the differentiation of Th1 and Th17 cells. Dysfunctional Tregs, controlled by the
FoxP3 gene, contributed to the pathogenesis of MS[
58,
59]. Since the expression of
FoxP3 in Tregs is regulated by
GATA3, then in a retrospective case-control study, the connection among polymorphisms of both genes was evaluated and correlated to the development of MS[
58]. Upon comparison, there was no significant association between FoxP3 and GATA3 polymorphisms with MS susceptibility. Nevertheless, a bioinformatic analysis had identified genetic variants of GATA3 as risk factors for augmenting the incidence of relapsing-remitting multiple sclerosis (RRMS)[
59]. The latter is defined as episodes or attacks of MS with different signs, symptoms, and severities. Further work is required to elaborate on the implication of GATA3 in MS pathogenesis and the potential to design GATA3-mediated treatment strategies to manage MS.
2.3. Neurodegenerative Diseases
2.3.1. Alzheimer’s Disease (AD)
Late-onset Alzheimer's disease (LOAD) is characterized by systemic inflammation that occurs due to dysregulated T-cell activity[
60]. The cells are known for their rapid infiltration into the brain, hence aggravating the neurogenerative disease pathology. Since GATA3 represents an inevitable regulator for T-cell lineage determination, a connection between GATA3 activity and the pathogenesis of LOAD has been speculated. Network analysis detected a considerable decline in GATA3 mRNA levels in the LOAD patients’ group. Thus, molecular dysregulation in Th-related genes provided the potential for early diagnosis or targeted interventions of AD[
60]. Furthermore, genome-wide association studies (GWAS); functional analysis had detected LOAD-associated loci containing different genetic variants that are mostly present in non-coding regions in the human genome[
61]. GATA3 with other proteins were detected with their corresponding risk alleles for activating HLA-DRB1 in the brains of all patients suffering from AD[
61]. Prior transcriptomic analysis has shown that GATA2 and GATA3 were co-expressed with HLA-DQA1 in AD hippocampus[
62]. Taken together, GATA3 regulates AD pathology via transcriptional activation of HLA expression in the human brain that was found to be upregulated[
61]. Further AD studies are recommended to focus on interfering with GATA3 expression with related proteins that might relieve symptoms, decelerate neurodegeneration, and improve AD progression and clinical outcomes.
2.3.2. Parkinson’s Disease (PD)
GATA3 is a specific modulator for Th cells’ expression and activity in sympathetic nervous systems’ (SNS) neurons[
63]. T-cells’ levels are diminished due to PD pathophysiology, then, GATA3 was proposed to be involved in PD underlying mechanisms[
10]. GATA3 blood levels were depleted in PD patients who experienced sleep disturbances, suggesting that GATA3 could be potentially utilized as a novel non-invasive biomarker for early detection of PD as well as predicting individuals who are at risk of developing non-motor symptoms. In addition, the ectopic expression of GATA3 remarkably facilitated the neuronal cells’ proliferation and survival rate in PD models[
10]. This finding accentuates the importance of GATA3 in neurodegenerative disease research and its potential clinical applications. Hence, tailoring GATA3-mediated treatment and management plans for PD specifically, and neurodegenerative disease generally becomes plausible as it seems beneficial for patients to manage their symptoms and improve their outcomes.
2.4. Other Diseases
2.4.1. Coronavirus Disease 2019 (COVID-19)
The prevalence of COVID-19 is increasing worldwide and GATA3-mediated Th1 cells are the main fighters during the disease progression. Hence, GATA3 might be involved in COVID-19 dysregulated mechanisms due to the abnormal T-cell responses[
64]. The previous report reinforced the severity and fatal outcomes of COVID-19 to remarkable elevation in GATA3, RORγt, and T-bet while sharp reductions in FoxP3 expression levels were recorded[
64]. Further work is required to provide a deeper insight into the role of GATA3 in the pathophysiology of COVID-19 and how it may apply to improve therapeutic approaches.
2.4.2. Type 2 Diabetes (T2D)
Multiple earlier reports had pinpointed the role of GATA3 in obesity-related conditions that involve IR, whether left untreated or led to the incidence of T2D. Dysfunctional adipogenesis is a significant factor in the onset of IR linked to obesity and T2D[
65]. When GATA3 was silenced, adipocyte differentiation was improved, secretion of inflammatory cytokines was properly modulated, and insulin sensitivity was restored in IR cells[
65]. Consequently, GATA3 depletion represents a promising tool for alleviating the underlying pathological mechanisms associated with T2D. The beneficiary effect of inhibiting GATA3 to orchestrate the adipogenic differentiation, coordinate the release of inflammatory cytokines, and restore insulin sensitivity is summarized in
Figure 6.
2.4.3. Cardiovascular Diseases
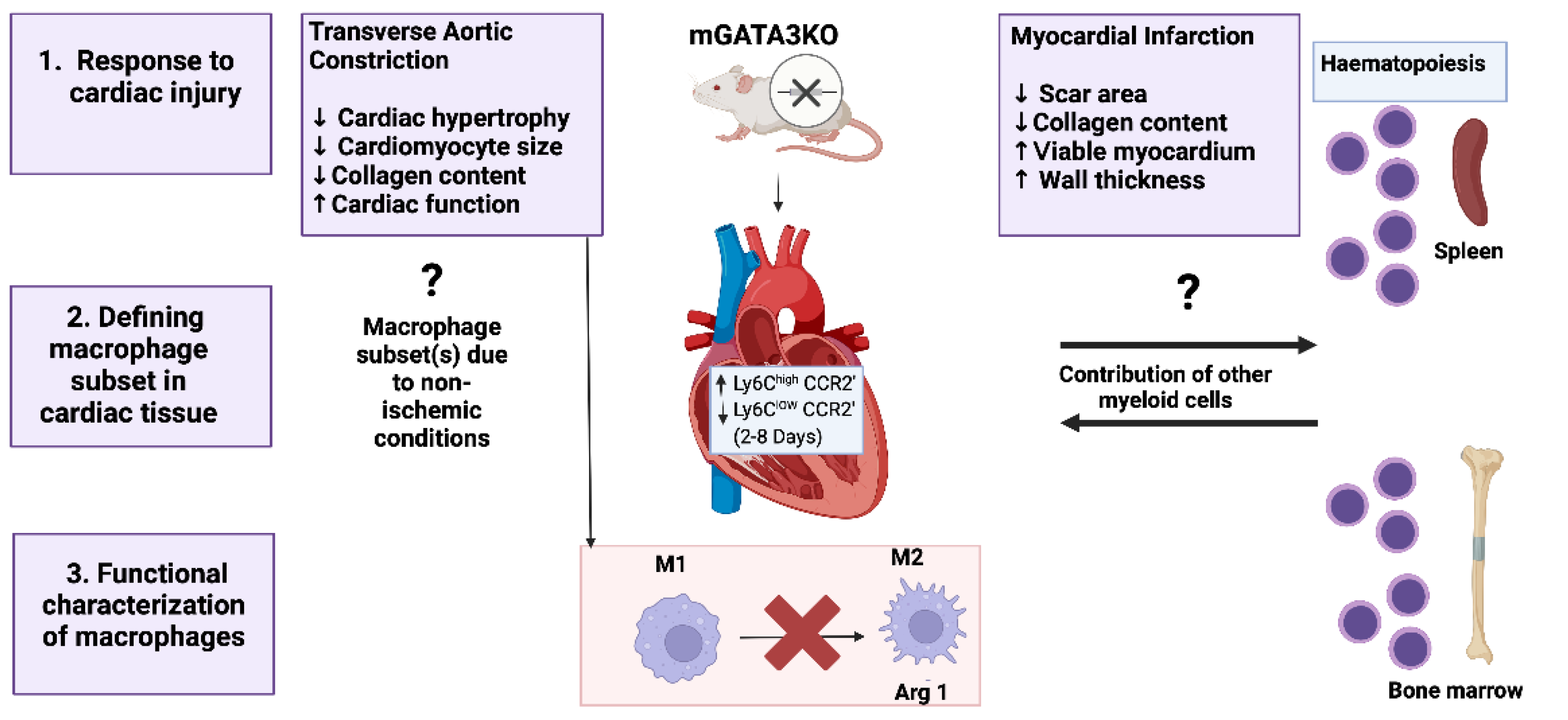
Despite the lack of research focusing on the role of GATA3 in the pathogenesis and complications of cardiovascular diseases, a prior study had shed light on the significance of dampening GATA3 in improving cardiac function following myocardial infarction (MI) and pressure overload hypertrophy[
66]. As GATA3 is not normally found in healthy cardiac tissues; it accumulates in the myocardium after MI injury exacerbating the pathology[
66]. Given that monocytes are the primary source of myocardial macrophage in acute MI, hence, built up of GATA3 most likely occurred in monocyte-derived macrophages. Dampening GATA3 levels within these subsets of macrophages were associated with enhanced cardiac function in knockdown mice models (
Figure 7)[
66,
67]. The latter finding provides insight into designing useful immunotherapeutic approaches involving GATA3 signalling in macrophages to treat cardiac diseases.
3. GATA3 Is a Potential Therapeutic Target
3.1. Molecular Mechanisms and Therapeutic Implications
3.1.1. GATA3 Pathways for Cancer Therapy
Designation of therapeutic targets for cancer treatment and disease management is challenging, due to the heterogeneity in tumor types, origin, epigenetic modifications, TME, and metastatic sites[
68]. Accordingly, GATA3 is differentially expressed in different tumor types and stages, thereby targeting GATA3 for cancer therapy is variable
1. Moreover, identifying resistance mechanisms that may develop in certain cancers is crucial, as these mechanisms may contribute to the limited or lack of response to certain treatment modalities, resulting in recurrent tumor growth[
69].
GATA3 expression had been linked to improved prognosis in BC. However, a study featured the alteration in gene signatures in BC patients led to a mutational shift in GATA3 causing the switch from anti-tumoral to pro-tumoral phenotype altering the overall survival rates in BC patients[
32]. Hence, mechanisms relying on GATA3 may require specific attention as the patient stratification based on GATA3 behaviour may add a significant layer in therapeutic considerations for accurate prognosis and treatment plans[
32]. Evidence indicated the association between GATA3 expression and the reversal of EMT that dampens tumor metastasis. The molecular mechanisms underlying the selection of GATA3 upregulation in well-differentiated epithelium-like BCs rather than invasive cancers have been elucidated so far[
70]. Therefore, GATA3 displays a non-invasive diagnostic and prognostic biomarker for BC. Noticeably, the necessity of determination of GATA3 expression in BC is not limited to tumor stratification and molecular subtyping as it is crucial for therapeutic designation and combination treatment plans. Prior research has revealed that BC tumours with high GATA3 levels will not respond to doxorubicin treatment as GATA3 contributes to resistance mechanisms[
71]. GATA3 promotes cell viability and survival by reducing the expression of the ferroptosis-associated gene
CYB5R2 which in turn maintains iron homeostasis. Therefore,
GATA3 and
CYB5R2 are directly associated with response to neoadjuvant chemotherapy (NAC) considered the primary preoperative therapy for BC[
71].
Emerging evidence has discussed the implication of genetic variants of GATA3 in the development and progression of leukaemia. Genetic polymorphism of GATA3 called rs3824662 risk allele was linked to a higher risk of relapse and inferior outcome in leukemic patients[
72,
73]. Through bioinformatics analyses and cellular experiment validations, potential GATA3-related genes, multiple signalling pathways (involved in T and B-cell leukemogenesis), and risk allele of
GATA3 SNP, had been unveiled that in turn increase the feasibility of targeting GATA3 for prognostic and management strategies in leukemia[
74]. The feasibility of targeting GATA3 for treatment strategies is completely reliable in comprehending the fundamental processes and clinical condition of each disease that alter the GATA3 expression levels and activity. Further investigations are required so far to provide a deeper insight into targeting GATA3 as novel therapeutic designations that might move from bench to bedside to benefit patients.
3.1.2. Modulating GATA3 for Therapeutic Interventions in Autoimmune Diseases
GATA3 serves as a key regulator of immune balance and T cell activity. Since that regulatory T cells (Tregs) that are governed by the Foxp3 gene, play a protective role against autoimmunity, then, a connection between GATA3 and the regulation of Treg activity is likely mediated through GATA3's modulation of Foxp3 expression
2. Notably, GATA3 exhibits a dual function in both the progression and control of autoimmune diseases. Reduced GATA3 levels, for instance, have been linked to the exacerbation of T1D pathology. Although lowered GATA3 levels were found to aggravate T1D pathology, a contradictive study revealed the detrimental consequences of elevated GATA3 expression on the progression of T1D[
75].The elevation of GATA3 altered the subpopulation of Tregs found in the islet cells, causing their dysfunction, compromising their function, and contributing to T1D onset[
75]. Furthermore, targeting GATA3 mutations associated with JIA is a potential strategy to alleviate symptoms and improve patients’ outcomes[
55]. As Th2 cells, stabilized by GATA3 activity, provide neuroprotection for MS disease, then GATA3 overexpression, stimulating a Th2 response, can reduce disease severity and delay the onset of experimental autoimmune encephalomyelitis (EAE), in MS animal model[
76]. These findings could pave the way for future translational studies in MS via exploring genetic biases towards different Th subsets influencing the clinical and histological patterns of demyelinating diseases.
3.1.3. Exploring GATA3 Regulation for Neurodegenerative Therapy
Bioinformatics analysis of transcriptomic data is widely used to identify molecular signatures and assign therapeutic targets for various conditions. GATA3 has been highlighted in the regulatory signature of vascular dementia (VaD) raising the potential of targeting GATA3 for therapeutic interventions assignment as neurodegenerative conditions. Considering the beneficial role of GATA3 in regulating AD pathology via transcriptional activation of HLA61, thereby GATA3 serves as a promising target for managing dementia-associated conditions. Since GATA3 was depleted in PD patients who experienced sleep disturbances, then, its ectopic expression remarkably facilitated the neuronal cells’ proliferation and survival via transcriptional inhibition of the transient receptor potential melastatin 2 (TRPM2)[
10,
78]. Consequently, the measurement of GATA3 expression in blood could be a potential novel biomarker for diagnosing idiopathic Parkinson’s disease (PD) and assessing the severity of the condition[
10].
3.1.4. GATA3 Role for the Management of Type 2 Diabetes
Given that, GATA3 silencing prevented fat accumulation, decreased inflammation, and restored insulin sensitivity, hence, targeting GATA3 demonstrates a promising tool for relieving T2D-associated symptoms[
65].
3.1.5. GATA3 Therapeutic Implications in Cardiovascular Disease
GATA3 is not normally expressed in healthy cardiac tissues but usually accumulates in monocyte-derived macrophages in response to myocardial infarction (MI) and pressure overload hypertrophy[
66]. Collectively, targeting GATA3 expresses an auspicious tool for recovery from cardiac injury.
3.2. Challenges and Limitations
While targeting GATA3 presents a promising therapeutic strategy for various diseases, several challenges and limitations must be addressed. The development of effective delivery systems, the specificity of gene silencing, and the potential side effects are critical factors that need careful consideration. Further research and clinical trials are essential to validate these approaches and translate them into effective treatments for patients.
3.3. Feasible Strategies for GATA3 Therapeutic Targeting
3.3.1.Delivery Mechanisms: siRNA and DNAzyme Delivery
Recent advancements have explored the use of small interfering RNAs (siRNAs) and DNAzymes to knock down GATA3 expression. For instance, pulmonary delivery of siRNAs using polyethylenimine (PEI)-based carriers has shown promise in targeting GATA3 in activated T cells for the treatment of allergic asthma. However, stability and targeting issues remain significant hurdles besides the complicated process of T-cells’ transfection[
79,
80].
3.3.2. Post-Translational Modifications: Acetylation and DNA Binding
GATA3's function is regulated by post-translational modifications, such as acetylation by the histone acetyltransferase p300. Acetylation at specific residues (e.g., K358) is required for optimal DNA binding. Targeting the GATA3/p300 complex or modulating GATA3's acetylation state presents a novel therapeutic approach, but further research is needed to develop effective inhibitors[
44,
81].
3.4. Potential Side Effects
3.4.1. Off-Target Effects: Specificity of Gene Silencing
The use of siRNAs and DNAzymes to silence GATA3 expression must be carefully designed to avoid off-target effects[
79,
80,
82]. Non-specific gene silencing can lead to unintended consequences, affecting other critical pathways and causing adverse effects[
83].
3.4.2. Immune Response: Immune Activation
Modulating GATA3 expression can impact the immune system, given its role in T-cell differentiation and function. For example, targeting GATA3 in T cells could potentially alter immune responses, leading to increased susceptibility to infections or autoimmune reactions[
2,
79,
84,
85].
3.5. Current Status of GATA3-Targeted Therapies
3.5.1. Preclinical Studies: siRNA and DNAzyme Delivery Systems
Preclinical studies have demonstrated the potential of siRNA and DNAzyme delivery systems in targeting GATA3. For instance, Tf-Mel-PEI polyplexes have shown effective GATA3 silencing in lung slices, indicating their feasibility for treating diseases like asthma. However, these approaches are still in the experimental stage and require further validation in clinical settings[
79,
80].
3.5.2. Clinical Trials
Currently, there is limited clinical data on GATA3-targeted therapies. Most research is still in the preclinical phase, and more studies are needed to evaluate the safety, efficacy, and long-term effects of these therapies in humans.
3.5.3. Combination Therapies
Combining GATA3-targeted therapies with other treatments, such as immune checkpoint inhibitors or chemotherapy, may enhance therapeutic outcomes. For example, targeting GATA3 in combination with other pathways involved in cancer progression could provide a more comprehensive approach to treatment [
32,
86].
4. Future Directions and Conclusions
4.1. Emerging Areas of GATA3 Research
Developing research on GATA3 is offering insights for novel GATA3 contributions beyond traditional immune system functions to unravel its implications in health and disease-related processes. Recent studies are exploring GATA3's expressions in cancer biology, where it is potentially used as a biomarker for diagnosis and prognosis in cancers like breast and prostate cancer. In neurobiology
, researchers are investigating GATA3’s importance for neuronal development, and survival, with potential applications in neurodegenerative diseases such as Parkinson’s disease[
10]. GATA3’s impact on metabolic disorders is being evaluated as well, particularly for adipogenesis and insulin sensitivity, which potentiates novel therapies for obesity and Type 2 Diabetes[
65]. Additionally, there is an increasing interest in the elucidation of the GATA3 regulatory mechanisms of stem cell biology and developmental processes, which includes its functions in tissue regeneration and embryonic development [
87,
88]. These new research areas suggest that GATA3 is a versatile transcription factor with broad implications for both basic research and clinical applications.
4.2. Implications of Novel Findings
4.2.1. Enhanced Diagnostic and Prognostic Tools
The identification of GATA3 as a sensitive diagnostic marker for various cancers, including breast cancer and urothelial carcinoma, enhances the accuracy of disease diagnosis and prognosis[
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53]. This can lead to better patient stratification and personalized treatment plans.
4.2.2. Targeted Therapeutic Strategies
Understanding the molecular mechanisms regulating GATA3 expression and activity opens new avenues for targeted therapies. For example, targeting the ZPO2/GATA3 signalling axis in breast cancer[
39] or modulating GATA3-AS in liver carcinoma[
46] could provide effective treatment options.
4.2.3. Biomarker Development
The potential use of GATA3 as a novel biomarker for early detection of diseases like Parkinson's disease[
10] and its role in predicting disease progression describes its importance in developing non-invasive diagnostic tools.
4.2.4. Research and Clinical Applications
These findings encourage further research into the regulatory mechanisms of GATA3 and its interactions with other molecular pathways. This can lead to the development of novel therapeutic interventions and improve clinical outcomes for patients with GATA3-related diseases.
References
- Chou J., Provot S, Werb Z. GATA3 in development and cancer differentiation: cells GATA have it!. J Cell Physiol. 2010;222(1):42-9. [CrossRef]
- Wan, YY. GATA3: a master of many trades in immune regulation. Trends Immunol. 2014 ;35(6):233-42. [CrossRef]
- Aguiari G, Crudele F, Taccioli C, Minotti L, Corrà F, Keillor JW, Grassilli S, Cervellati C, Volinia S, Bergamini CM, Bianchi N. Dysregulation of Transglutaminase type 2 through GATA3 defines aggressiveness and Doxorubicin sensitivity in breast cancer. Int J Biol Sci. 2022;18(1):1-14.
- Murga-Zamalloa C, Wilcox RA. GATA-3 in T-cell lymphoproliferative disorders. IUBMB Life. 2020;72(1):170-7. [CrossRef]
- Scazzone C, Agnello L, Lo Sasso B, Salemi G, Gambino CM, Ragonese P, Candore G, Ciaccio AM, Giglio RV, Bivona G, et al. FOXP3 and GATA3 Polymorphisms, Vitamin D3 and Multiple Sclerosis. Brain Sci. 2021;11(4):415. [CrossRef]
- Tsarovina K, Reiff T, Stubbusch J, Kurek D, Grosveld FG, Parlato R., Schütz G, Rohrer H. The Gata3 transcription factor is required for the survival of embryonic and adult sympathetic neurons. J Neurosci. 2010;30(32):10833-43. [CrossRef]
- Chen P, Wu Y, Zhuang J, Liu X, Luo Q, Wang Q, Jiang Z, He A, Chen S, Chen X, et al. Gata3 Silencing Is Involved in Neuronal Differentiation and Its Abnormal Expression Impedes Neural Activity in Adult Retinal Neurocytes. Int J Mol Sci. 2022;23(5):2495. [CrossRef]
- Querzoli P, Pedriali M, Rinaldi R, Secchiero P, Rossi PG, Kuhn E. GATA3 as an adjunct prognostic factor in breast cancer patients with less aggressive disease: a study with a review of the literature. Diagnostics (Basel). 2021;11(4):604. [CrossRef]
- Papoudou-Bai A, Koumpis E, Karpathiou G, Hatzimichael E, Kanavaros P. Expression Patterns of GATA3 in Classical Hodgkin Lymphoma: A Clinico-Pathological Study. Diseases. 2024;12(3):51. [CrossRef]
- Acharya S, Lumley AI, Zhang L, Vausort M, Devaux Y, On Behalf Of The NCER-PD Consortium. GATA3 as a Blood-Based RNA Biomarker for Idiopathic Parkinson's Disease. Int J Mol Sci. 2023;24(12):10040. [CrossRef]
- Yu X, Chen Y, Chen J, Fan Y., Lu H., Wu D., Xu Y. Shared genetic architecture between autoimmune disorders and B-cell acute lymphoblastic leukaemia: insights from large-scale genome-wide cross-trait analysis. BMC Med. 2024;22(1):161. [CrossRef]
- Wang Y, Misumi I, Gu AD, Curtis TA, Su L, Whitmire JK, Wan YY. GATA-3 controls the maintenance and proliferation of T cells downstream of TCR and cytokine signalling. Nat Immunol. 2013;14(7):714-722. [CrossRef]
- Ranganath S., Murphy KM. Structure and specificity of GATA proteins in Th2 development. Mol Cell Biol. 2001;21(8):2716-2725. [CrossRef]
- Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell. 2006;127(5):1041-1055. [CrossRef]
- Kouros-Mehr H, Kim J-W, Bechis SK, Werb Z. GATA-3 and the regulation of the mammary luminal cell fate. Curr Opin Cell Biol. 2008;20(2):164–70. [CrossRef]
- Nardelli J, Thiesson D, Fujiwara Y., Tsai F.-Y., Orkin SH. Expression and genetic interaction of transcription factors GATA-2 and GATA-3 during development of the mouse central nervous system. Dev Biol. 1999;210(2):305–21. [CrossRef]
- Pandolfi PP, Roth ME, Karis A, Leonard MW, Dzierzak E., Grosveld FG, et al. Targeted disruption of the GATA3 gene causes severe abnormalities in the nervous system and in fetal liver haematopoiesis. Nat Genet. 1995;11(1):40–4. [CrossRef]
- Celikkaya H, Cosacak MI, Papadimitriou C, Popova S, Bhattarai P., Biswas SN, et al. GATA3 promotes the neural progenitor state but not neurogenesis in 3D traumatic injury model of primary human cortical astrocytes. Front Cell Neurosci. 2019 ;13. [CrossRef]
- Grote D, Boualia SK, Souabni A, Merkel C, Chi X, Costantini F, et al. Gata3 Acts Downstream of β-Catenin Signaling to Prevent Ectopic Metanephric Kidney Induction. PLoS Genetics.2008;4(12). [CrossRef]
- Grote D, Souabni A, Busslinger M, Bouchard M. Pax2/8-regulated Gata3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development. 2006;133(1):53–61. [CrossRef]
- Zaidan N., Ottersbach K. The multi-faceted role of Gata3 in developmental haematopoiesis. Open Biol. 2018;8(11):180152. [CrossRef]
- Neyra JS, Medrano S, Goes Martini A, Sequeira-Lopez MLS, Gomez RA. The role of Gata3 in renin cell identity. Am J Physiol Renal Physiol. 2023;325(2). [CrossRef]
- Marchildon F, St-Louis C, Akter R, Roodman V., Wiper-Bergeron NL. Transcription factor Smad3 is required for the inhibition of adipogenesis by retinoic acid. J Biol Chem. 2010;285(17):13274–84. [CrossRef]
- Tong Q, Tsai J, Tan G, Dalgin G., Hotamisligil GS. Interaction between GATA and the C/EBP family of transcription factors is critical in GATA-mediated suppression of adipocyte differentiation. Mol Cell Biol. 2005;25(2):706–15. [CrossRef]
- Tong, Q. Function of GATA Transcription Factors in Preadipocyte-Adipocyte Transition. Science. 2000 Oct 6;290(5489):134–8. [CrossRef]
- Al-Mansoori L, Al-Jaber H, Madani AY, Mazloum NA, Agouni A, Ramanjaneya M, et al. Suppression of GATA-3 increases adipogenesis, reduces inflammation and improves insulin sensitivity in 3T3L-1 preadipocytes. Cellular Signalling. 2020;75:109735. [CrossRef]
- Al-Jaber H, Mohamed NA, Govindharajan VK, Taha S, John J, Halim S, et al. In vitro and in vivo validation of GATA-3 suppression for induction of adipogenesis and improving insulin sensitivity. Int J Mol Sci. 2022;23(19):11142. [CrossRef]
- Garan LAW, Xiao Y, Lin W-C. 14-3-3τ drives estrogen receptor loss via ERα36 induction and GATA3 inhibition in breast cancer. Proc Natl Acad Sci USA.2022;119(43). [CrossRef]
- Yoon EC, Wang G, Parkinson B, Huo L, Peng Y., Wang J, et al. TRPS1, GATA3, and SOX10 expression in triple-negative breast carcinoma. Hum Pathol. 2022;125:97–107. [CrossRef]
- Yang Y, Lu S, Zeng W, Xie S, Xiao S. GATA3 expression in clinically useful groups of breast carcinoma: a comparison with GCDFP15 and mammaglobin for identifying paired primary and metastatic tumours. Ann Diagn Pathol. 2017;26:1-5. [CrossRef]
- Oda H, Hedayati E, Lindström A, Shabo I. GATA-3 expression in breast cancer is related to intratumoral M2 macrophage infiltration and tumour differentiation.PLoSOne.2023 ;18(3). [CrossRef]
- Cohen H, Ben-Hamo R, Gidoni M, Yitzhaki I, Kozol R, Zilberberg A., et al. Shift in GATA3 functions, and GATA3 mutations, control progression and clinical presentation in breast cancer. Breast Cancer Res. 2014;16(6). [CrossRef]
- Sangoi AR, Shrestha B, Yang G, Mego O, Beck AH. The novel marker GATA3 is significantly more sensitive than traditional markers mammaglobin and GCDFP15 for identifying breast cancer in surgical and cytology specimens of metastatic and matched primary tumours. Appl Immunohistochem Mol Morphol. 2016;24(4):229–37. [CrossRef]
- Ahadi M, Moradi A, Rabiee E, Pourmotahari F. Evaluation of GATA3 and GCDFP15 Expression in Triple Negative Breast Cancers. Iran J Pathol. 2023;18(1):90-95. [CrossRef]
- de Medeiros Souza P, Carvalho FM, Aguiar FN, Gagliato D., de Barros ACSD. Association between GATA3 and histopathological and immunohistochemical parameters in early-infiltrating breast carcinomas.Eur J Breast Health. 2022;18(3):229-234. 2022. [CrossRef]
- Ricks-Santi LJ, Fredenburg K., Rajaei M., et al. Characterization of GATA3 and Mammaglobin in breast tumours from African American women. Res Sq. 2023. [CrossRef]
- Asselin-Labat M-L, Sutherland KD, Vaillant F, Gyorki DE, Wu D, Holroyd S, et al. Gata-3 negatively regulates the tumor-initiating capacity of mammary luminal progenitor cells and targets the putative tumor suppressor caspase-14. Mol Cell Biol. 2011;31(22):4609–22. [CrossRef]
- Liu X, Bai F, Wang Y, Wang C, Chan HL, Zheng C, et al. Loss of function of GATA3 regulates FRA1 and c-FOS to activate EMT and promote mammary tumorigenesis and metastasis. Cell Death Dis. 2023;14(6). [CrossRef]
- Shahi P, Wang C-Y, Lawson DA, Slorach EM, Lu A, Yu Y, et al. ZNF50 3/Zpo2 drives aggressive breast cancer progression by down-regulation of GATA3 expression. Proc Natl Acad Sci USA. 2017;114(12):3169–74. [CrossRef]
- Lin H-Y, Zeng D, Liang Y-K, Wei X-L, Chen C-F. GATA3 and TRPS1 are distinct biomarkers and prognostic factors in breast cancer: database mining for GATA family members in malignancies. Oncotarget. 2017;8(21):34750–61. [CrossRef]
- Yang H, Zhang H, Luan Y, Liu T, Yang W, Roberts KG, et al. Noncoding genetic variation in GATA3 increases acute lymphoblastic leukaemia risk through local and global changes in chromatin conformation. Nat Genet. 2022;54(2):170–9. [CrossRef]
- Stanelle J, Döring C, Hansmann M.-L., Küppers R. Mechanisms of aberrant GATA3 expression in classical Hodgkin lymphoma and its consequences for the cytokine profile of Hodgkin and Reed/Sternberg cells. Blood. 2010;116(20):4202–11. [CrossRef]
- Banerjee A, Northrup D, Boukarabila H, Jacobsen SEW, Allman D. Transcriptional repression of Gata3 is essential for early B cell commitment. Immunity. 2013;38(5):930–42. [CrossRef]
- Geng X, Wang C, Gao X, Chowdhury P, Weiss J, Villegas JA, et al. GATA-3 is a proto-oncogene in T-cell lymphoproliferative neoplasms. Blood Cancer J. 2022;12(11). [CrossRef]
- Fiore D, Cappelli LV, Broccoli A, Zinzani PL, Chan WC, Inghirami G. Peripheral T cell lymphomas: from the bench to the clinic. Nat Rev Cancer. 2020;20(6):323–42. [CrossRef]
- Lan T, Li H, Zhang D, Xu L, Liu H, Hao X, et al. KIAA1429 contributes to liver cancer progression through N6-methyladenosine-dependent post-transcriptional modification of GATA3. Mol Cancer. 2019;18(1). [CrossRef]
- Zhang Z, Fang X, Xie G, Zhu J. GATA3 is downregulated in HCC and accelerates HCC aggressiveness by transcriptionally inhibiting slug expression. Oncol Lett. 2021;21(3). [CrossRef]
- Nguyen AHT, Tremblay M, Haigh K, Koumakpayi IH, Paquet M., Pandolfi PP, et al. Gata3 antagonizes cancer progression in Pten-deficient prostates. Hum Mol Genet. 2013;22(12):2400–10. [CrossRef]
- Lobo J, Tenace NP, Cañete-Portillo S., et al. Aberrant expression of GATA3 in metastatic adenocarcinoma of the prostate: an important pitfall. Histopathology. 2024;84(3):507-514. [CrossRef]
- Li Y, Ishiguro H, Kawahara T, Kashiwagi E, Izumi K., Miyamoto H. Loss of GATA3 in bladder cancer promotes cell migration and invasion. Cancer Biol Ther. 2014;15(4):428–35. [CrossRef]
- Zhang Q, Qi T, Long Y, Li X, Yao Y, Wu Q, et al. GATA3 predicts the tumor microenvironment phenotypes and molecular subtypes for bladder carcinoma. Front Surg. 2022;9. [CrossRef]
- Rana C, Agarwal H, Babu S, Kumar M, Singhai A, Shankhwar S., et al. Diagnostic utility of GATA3 immunohistochemical expression in urothelial carcinoma. Indian J Pathol Microbiol. 2019;62(2):244-250. [CrossRef]
- Yoo D, Min KW, Pyo JS, Kim NY. Diagnostic and prognostic roles of GATA3 immunohistochemistry in urothelial carcinoma. Medicina (Kaunas). 2023;59(8):1452. [CrossRef]
- Ozgur BA, Cinar SA, Coskunpinar E, Yilmaz A, Altunkanat D., Deniz G., et al. The role of cytokines and T-bet, GATA3, ROR-γt, and FOXP3 transcription factors of T cell subsets in the natural clinical progression of Type 1 Diabetes. Immunol Res. 2023;71(3):451–62. [CrossRef]
- Patrick AE, Wang W, Brokamp E, Graham TB, Aune TM, Duis JB. Juvenile idiopathic arthritis associated with a mutation in GATA3. Arthritis Res Ther. 2019;21(1). [CrossRef]
- Aune TM, Collins PL, Collier SP, Henderson MA, Chang S. Epigenetic activation and silencing of the gene that encodes IFN-γ. Front Immunol. 2013;4:112. [CrossRef]
- van Hamburg JP, Mus A-M, de Bruijn MJW, de Vogel L, Boon L, Cornelissen F, et al. GATA-3 protects against severe joint inflammation and bone erosion and reduces differentiation of Th17 cells during experimental arthritis. Arthritis Rheum. 2009;60(3):750–9. [CrossRef]
- Scazzone C, Agnello L, Lo Sasso B, Salemi G, Gambino CM, Ragonese P, et al. FOXP3 and GATA3 polymorphisms,vitamin D3 and Multiple Sclerosis. Brain Sci.2021;11(4):415. [CrossRef]
- Anarlouei S, Roohy F, Mohamadynejad P. Effect of rs1058240 polymorphism in 3′-UTR of GATA3 gene on potential binding of miRNAs and its association with RRMS risk: bioinformatics analysis and case-control study. Int J Neurosci. 2023;1–6. [CrossRef]
- Neshan M, Malakouti SK, Kamalzadeh L, Makvand M, Campbell A., Ahangari G. Alterations in T-cell transcription factors and cytokine gene expression in late-onset Alzheimer’s disease. J Alzheimers Dis. 2022;85(2):645–65. [CrossRef]
- Zhang X, Zou M, Wu Y, Jiang D, Wu T, Zhao Y, et al. Regulation of the late onset alzheimer’s disease associated HLA-DQA1/DRB1 expression. Am J Alzheimers Dis Other Demen. 2022;37:153331752210850. [CrossRef]
- Dharshini SAP, Taguchi Y.-H., Gromiha MM. Investigating the energy crisis in Alzheimer disease using transcriptome study. Sci Rep. 2019;9(1). [CrossRef]
- Hong SJ, Huh Y, Chae H, Hong S, Lardaro T, Kim K-S. GATA-3 regulates the transcriptional activity of tyrosine hydroxylase by interacting with CREB. J Neurochem. 2006;98(3):773–81. [CrossRef]
- Abbasi-Dokht T, Vafaeinezhad A, Khalesi N, Malek F, Haghmorad D., Baharlou R. T-cell immune responses and immunological factors associated with Coronavirus disease 2019 progression as predictors for the severity of the disease in hospitalized patients. Int Arch Allergy Immunol. 2023;184(6):557–66. [CrossRef]
- Al-Jaber H, Al-Mansoori L, Elrayess MA. GATA-3 as a potential therapeutic target for insulin resistance and type 2 diabetes mellitus. Curr Diabetes Rev. 2021;17(2):169–79. [CrossRef]
- Yang M, Song L, Wang L, Yukht A, Ruther H, Li F, et al. Deficiency of GATA3-positive macrophages improves cardiac function following myocardial infarction or pressure overload hypertrophy. J Am Coll Cardiol. 2018;72(8):885–904. [CrossRef]
- Giannarelli, C. , Fernandez DM. Manipulating macrophage polarization to fix the broken heart. J Am Coll Cardiol. 2018;72(8):905–7. [CrossRef]
- Xulu KR, Nweke EE, Augustine TN. Delineating intra-tumoral heterogeneity and tumor evolution in breast cancer using precision-based approaches. Front Genet. 2023;14:1087432. [CrossRef]
- Lei ZN, Tian Q., Teng QX, et al. Understanding and targeting resistance mechanisms in cancer. MedComm (2020). 2023;4(3). [CrossRef]
- Yan W, Cao QJ, Arenas RB, Bentley B, Shao R. GATA3 inhibits breast cancer metastasis through the reversal of epithelial-mesenchymal transition. J Biol Chem. 2010;285(18):14042–51. [CrossRef]
- Zhu Z, Shen H, Xu J, Fang Z., Wo G, Ma Y, et al. GATA3 mediates doxorubicin resistance by inhibiting CYB5R2-catalyzed iron reduction in breast cancer cells. Drug Resist Updat. 2023;69(100974):100974. [CrossRef]
- Zhang H, Liu AP-Y, Devidas M, Lee SHR, Cao X., Pei D., et al. Association ofGATA3polymorphisms with minimal residual disease and relapse risk in childhood acute lymphoblastic leukaemia. J Natl Cancer Inst. 2021;113(4):408–17. [CrossRef]
- Perez-Andreu V, Roberts KG, Harvey RC, Yang W, Cheng C, Pei D, et al. Inherited GATA3 variants are associated with Ph-like childhood acute lymphoblastic leukaemia and risk of relapse. Nat Genet. 2013;45(12):1494–8. [CrossRef]
- Hou Q, Liao F, Zhang S, Zhang D, Zhang Y, Zhou X, et al. Regulatory network of GATA3 in pediatric acute lymphoblastic leukaemia. Oncotarget. 2017;8(22):36040–53. [CrossRef]
- Kiaf B, Bode K, Schuster C, Kissler S. Gata3 is detrimental to regulatory T cell function in autoimmune diabetes. 2023. [CrossRef]
- Fernando V, Omura S, Sato F, Kawai E, Martinez NE, Elliott SF, Yoh K, Takahashi S, Tsunoda I. Regulation of an autoimmune model for multiple sclerosis in Th2-biased GATA3 transgenic mice. Int J Mol Sci. 2014;15(2):1700-1718. [CrossRef]
- Shu J., Wei W., Zhang L. Identification of molecular signatures and candidate drugs in vascular dementia by bioinformatics analyses. Front Mol Neurosci. 2022;15:751044. [CrossRef]
- Zhou Y, Han D. GATA3 modulates neuronal survival through regulating TRPM2 in Parkinson’s disease. Neurosci Lett. 2017;655:51-58. [CrossRef]
- Kandil R, Baldassi D, Böhlen S, Müller JT, Jürgens DC, Bargmann T, Dehmel S, Xie Y, Mehta A, Sewald K, Merkel OM. Targeted GATA3 knockdown in activated T cells via pulmonary siRNA delivery as novel therapy for allergic asthma. J Control Release. 2023;354:305-315. [CrossRef]
- Gavitt TD, Hartmann AK, Sawant SS, et al. A GATA3 targeting nucleic acid nanocapsule for in vivo gene regulation in asthma. ACS Nano. 2020;14(12):16302-16317. [CrossRef]
- Hosokawa H, Tanaka T, Suzuki Y, Iwamura C, Ohkubo S, Endoh K, Kato M, Endo Y, Onodera A, Tumes DJ, Kanai A, Sugano S, Nakayama T. Functionally distinct Gata3/Chd4 complexes coordinately establish T helper 2 (Th2) cell identity. Proc Natl Acad Sci U S A. 2013 ;110(12):4691-6. [CrossRef]
- Hannus M, Beitzinger M, Engelmann JC, Weickert MT, Spang R, Hannus S, Meister G. siPools: highly complex but accurately defined siRNA pools eliminate off-target effects. Nucleic Acids Res. 2014;42(12):8049-61. [CrossRef]
- Olejniczak M, Galka P, Krzyzosiak WJ. Sequence-non-specific effects of RNA interference triggers and microRNA regulators. Nucleic Acids Res. 2010 ;38(1):1-16. [CrossRef]
- Ku CJ, Hosoya T, Maillard I, Engel JD. GATA-3 regulates hematopoietic stem cell maintenance and cell-cycle entry. Blood. 2012 ;119(10):2242-51. [CrossRef]
- Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, Voehringer D, Busslinger M, Diefenbach A. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012 ;37(4):634-48. [CrossRef]
- Bai R, Jia L, Gao Y, Sun X, Chen N., Lv Z., Cui J. Targeted therapy combined with immunotherapy in patients with breast infiltrating ductal carcinoma with axillary lymph node metastasis of metaplastic SCC. Thorac Cancer. 2022 ;13(19):2799-2807. [CrossRef]
- Blinkiewicz PV, Long MR, Stoner ZA, Ketchum EM, Sheltz-Kempf SN, Duncan JS. Gata3 is required in late proneurosensory development for proper sensory cell formation and organization. Research Square. 2023. [CrossRef]
- Lentjes MH, Niessen HE, Akiyama Y, de Bruïne AP, Melotte V., van Engeland M. The emerging role of GATA transcription factors in development and disease. Expert Rev Mol Med. 2016;18. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).