1. Introduction
Ferritin is a ubiquitously expressed heteropolymer composed of 24 polypeptide chains assembled into a shell-like structure delimiting a cavity where iron atoms are stored in non-toxic ferric form. Here, the discoveries which have led to the current understanding of the biochemical properties of ferritin and to the appreciation of its role in iron metabolism are summarized from a historical viewpoint. Ferritin is indeed a remarkable and fascinating molecule with a rich history in biochemical research. It is one of the first proteins to be identified and purified and its stability, distinctive color, and ability to crystallize made it a model for early protein studies. Ferritin plays a crucial role in iron metabolism, a vital metal for nearly all living organisms, underscoring its biological importance across species.
Its tight regulation by body iron levels makes ferritin an attractive subject for gene expression studies, and its polymeric and highly stable structure, which exhibits remarkable protein self-assembly, has fascinated researchers for decades. In addition, ferritin stimulates a strong immune response to produce high affinity antibodies that can be useful for immunological studies. In the eight decades after its discovery major progress has been made in understanding the role of ferritin in iron oxidation, storage, and release, but some of the intricate biochemical mechanisms of these processes remain unresolved. An interesting characteristic of ferritin’s history is the variety of biological functions attributed to this protein over the years. The major one is its role in iron storage and regulation, but it has been reported to have roles in regulation of cellular defense and proliferation, oxidative stress, and even disease progression. This 80-year journey of discovery mirrors the evolution of biochemical techniques, making ferritin a testament to the growing sophistication of chemical and physical tools used in modern science.
2. The Origin
The history of ferritin began with a discovery by the Czech scientist Vilem Laufberger who in 1937 named "ferritin" an iron-rich compound obtained by crystallization of horse spleen extract. The groundwork for ferritin discovery was done by earlier scientists who identified iron deposits in tissues like the liver and spleen. In the late 19th century, scientists such as Perls (1867) discovered iron-containing granules, visible as positive Prussian blue stains, in these tissues. These granules, which contained iron oxide and phosphate, varied in color from pale yellow to brown and were particularly abundant in the spleens of horses, accounting for up to 5% of the organ's dry weight. Further research revealed that these iron deposits appeared also in bone marrow cells following hemoglobin degradation and were later termed hemosiderin. Around the same time, researchers began investigating more diffuse forms of iron in the body. Schmiedeberg in 1894 boiled pig liver homogenates and precipitated the resulting compounds with tartaric acid, isolating a substance containing around 6% iron, which he named "ferratin.". This preparation was initially thought to contain nucleic acids or lipids.
2.1. Vilém Laufberger
Crystallization was one of the few methods available at that time to purify proteins, and was used to identify hemoglobin in 1840, followed by few other proteins that included globulins, albumin, concanavalin A, urease, trypsin and chymotrypsin. Laufberger isolated from horse spleen a highly stable compound containing over 20% iron. His preparation was easily crystallizable using cadmium sulfate and could endure heating at 80°C, marking a significant discovery [
1]. The name "ferritin" was chosen in honor of Schmiedeberg's earlier work on "ferratin." These results were confirmed by other groups who identified phosphorus in the ferritin preparation and speculated that it might be a nucleoprotein. Vilém Laufberger, a versatile scientist from Czechoslovakia, initially worked on animal metamorphosis before focusing on insulin purification and ultimately made the groundbreaking discovery of ferritin. His work received some international attention, with presentations in Moscow, Leningrad, and Zurich, leading to a publication in 1937. Later, Laufberger shifted his focus to neurology, publishing a book on the "Theory of Excitement" and pioneering spaciocardiography for heart disease diagnosis. He lived a long and influential life, passing away at age 96.
2.2. Michaelis and Granick
Leonor Michaelis, a renowned German scientist celebrated for his work on enzyme kinetics, turned his attention to ferritin later in his career. Although famous for the Michaelis-Menten equation, developed in 1913 with Canadian scientist Maud Leonora Menten, which was foundational for understanding enzyme-substrate interactions, Michaelis faced challenges in establishing a stable career in Germany. He spent three years in Japan before ultimately moving to the United States, where he joined the Rockefeller Institute and focused on oxidation-reduction processes and free radical formation. His interest in ferritin developed during this period, and in 1942 [
2], he demonstrated that ferritin’s iron could be readily removed by reduction with sodium dithionite, introducing the concept of apoferritin, ferritin’s iron-free form. Michaelis encouraged his colleague Sam Granick to study ferritin further, sparking a series of groundbreaking studies from 1942 to 1947.
The research by Granick and Michaelis on ferritin established the foundation for modern iron metabolism studies. Key reviews in 1946 and 1951 [
3,
4] summarized their findings, highlighting ferritin’s unique ability to crystallize in tissues upon the addition of cadmium sulfate, allowing visualization and quantification of crystals under a microscope. Their work demonstrated that ferritin could be crystallized from the organs of various species, including horses, humans, dogs, guinea pigs, rats, pigs, cats, and rabbits, though it did not readily crystallize from cows, sheep, deer, fowl, fish, and bullfrogs. They also detected ferritin in tissues like the spleen, liver, bone marrow, kidneys, and testes, where it appeared pale or colorless. A significant finding was the increase in ferritin levels in the duodenum of guinea pigs following oral ferrous sulfate administration, indicating that mucosal ferritin may regulate iron absorption, aligning with the "mucosal block" mechanism described by the Whipple group [
5], which involves reduced iron absorption after an oral iron dose. Additionally, purified ferritin enabled antibody production for precipitin reactions that detected ferritin across tissues, though not in blood, muscle, or the pituitary. Interestingly, horse ferritin antibodies crossreacted with dog ferritin but not with human apoferritin.
Further research demonstrated that when radioactive iron from hemoglobin or ferric ammonium citrate was injected into animals, it later appeared in liver ferritin, thus showing that liver ferritin stored iron; conversely, when the body's demand for iron increased, as seen in horses that had undergone extensive bloodletting, ferritin levels in the liver decreased. This indicated that ferritin breakdown occurred in the liver to meet the body's iron needs, although the exact mechanism remained unclear. Granick and Michaelis also proposed that ferritin might be involved in transporting iron to the fetus via the placenta. Their findings confirmed that ferritin acted primarily as an iron storage molecule, except in the testes, where only apoferritin was found. This extensive work established ferritin’s biochemical and physiological roles, particularly in iron storage and regulation. Their work was followed by various groups, notably Tecce in 1952 [
6], who first observed ferritin crystals in elasmobranch fishes, broadening ferritin studies beyond mammals.
After making significant contributions to ferritin research, Granick shifted his focus to the biosynthesis of heme and chlorophyll, where he achieved notable recognition.
2.3. Mazur and Shorr
Following Sam Granick's pioneering research on ferritin, a new line of inquiry emerged in the late 1940s, led by Shorr and Mazur, who explored ferritin from an entirely different perspective. Their work investigated ferritin’s potential role in regulating blood pressure, particularly its "vaso-depressor" activity.
The research was prompted by observations in the later stages of shock, where capillary dilation causes a significant drop in blood pressure. Shorr and Mazur hypothesized the presence of a "Vaso-Depressor Material" (VDM) responsible for this effect in animals undergoing shock. To test this, the authors used the rat mesoappendix to assess the sensitivity of precapillary sphincters to adrenaline (epinephrine). In their assay, they injected the test substance into the rat's bloodstream and then applied a fixed amount of adrenaline topically to the mesoappendix to measure vasoconstriction [
7]. Their experiments revealed that saline washes of liver and skeletal muscle from animals subjected to shock contained VDM. Notably, anaerobic treatment of the liver was necessary to release VDM. They also discovered that crystalline liver ferritin, but not denatured ferritin, inhibited the epinephrine response in the mesoappendix test. This inhibition was blocked when ferritin was pre-treated with anti-ferritin antibodies, suggesting a role for ferritin in the vasodepressor effect [
8]. These findings spurred further research into the chemical properties of ferritin. They found that its vasodepressor activity was linked to the presence of SH (sulfhydryl) groups. Moreover, they observed that cysteine and various forms of iron also exhibited vasodepressor activity, which was similarly inhibited by anti-ferritin antibodies, raising questions about ferritin's functions beyond iron storage. Further studies revealed that ferritin could oxidize and deactivate adrenaline in vitro, especially in the presence of hydrogen peroxide [
9], providing a partial explanation for its vasodepressor activity and linking ferritin's ability to release iron with its impact on adrenaline metabolism. Later, the researchers identified products of uric acid metabolism and the enzyme xanthine oxidase as agents that could facilitate iron release from ferritin [
10].
Although the theory of ferritin acting as a VDM was not confirmed, the research by Shorr and Mazur made significant contributions to the chemical and physical characterization of ferritin. Importantly, they suggested that ferritin may be present in circulation and may have physiological roles beyond iron storage. This hypothesis sparked subsequent studies exploring its presence in blood circulation and its various biochemical functions.
2.4. Electron Microscopy
In the mid-1940s, electron microscopy (EM) began to be applied to biological specimens, and by 1954, Farrant had applied it to study ferritin [
11]. This groundbreaking work provided new insights into ferritin's structure, revealing that its iron core resided within a nearly spherical protein shell approximately 93 Å in diameter. The electron dense iron core, up to 55 Å across, was clearly visible under EM, greatly facilitating the identification of ferritin in tissues. This discovery prompted new research directions, including investigations into ferritin's relationship with hemosiderin and its potential as a biological tracer.
One notable area of research focused on distinguishing ferritin from hemosiderin, the two main iron storage forms in the body. In 1953, Gabrio, Shoden, and Finch [
12] identified differences in the size and morphology of the opaque hemosiderin iron granules. This work was extended by Richter in 1957 [
13], who used EM to examine ferritin and hemosiderin in patients with hemosiderosis. Other studies highlighted the identification of ferritin and crystalline lattices within hemosiderin and demonstrated the conversion of colloidal iron injected into animals into both ferritin and hemosiderin. By the mid-1960s, Drysdale and colleagues further advanced the understanding of the interconnected roles of ferritin and hemosiderin in iron storage [
14]. Their research demonstrated that ferritin is the primary site for iron accumulation, but its storage capacity is limited. Once tissue iron levels exceed ferritin's storage limit, excess iron is deposited in hemosiderin. Despite their structural differences, ferritin and hemosiderin were shown to be functionally inseparable, with both releasing stored iron as needed. In fact, ferritin molecules were often found within hemosiderin granules, further highlighting their close association.
The easy recognition of ferritin in EM led to its use as a biological tracer. The iron-rich ferritin from horse spleen, which was relatively simple to purify and easily available, became a valuable tool for studying the protein diffusion in tissues and cells, appearing in numerous publications. For example, Nobel laureate George Palade used it to investigate the transfer across capillary wall [
15]. In addition, ferritin was readily bound to antibodies that were widely used in immunohistochemistry to visualize specific antigens in tissues. This technique, known as immunoelectron microscopy, helped advance the fields of cell biology and molecular medicine, allowing scientists to label and track various biomolecules in precise locations within cells. In this way, EM played a crucial role in studying ferritin’s structure and tracing its interactions in the body, with numerous applications in cell biology and immunology.
EM also enabled researchers to identify ferritin iron cores in various organisms beyond mammals. These studies showed that ferritin is present across the biological world. It was detected in the eggs and embryos of
Rana pipiens [
16], in various mollusks [
17], including
Corbicula sandai [
18], in the dental cells of urodeles [
19], in snails [
20], in fungi [
21], in the octopus and tuna fish [
22], and in the plasma of birds [
23]. Further research revealed the presence of ferritin in plants, where it was referred to as "phytoferritin." Unlike in animals, where ferritin is found in the cytosol, plant ferritin was localized in plastids, a type of organelle [
24]. Ferritin was also detected in the plant roots [
25] and in the root tips of beans [
26]. It was found in various other plants, including the leaves of
Xanthium [
27]. Craig and William explored the connection between phytoferritin and viral infections, suggesting a potential role in plant immune responses [
28]. This series of EM-based studies confirmed the widespread presence of ferritin across various species, highlighting its fundamental role in essential biological processes such as iron storage, detoxification, and regulation across all eukaryotic life forms.
2.5. Early Studies of Ferritin Biosynthesis and Structure
Sam Granick's pioneering work revealed that ferritin accumulation is rapidly induced by iron in animals, making ferritin an excellent model for studying the regulation of gene expression and protein synthesis. As a result, various laboratories began investigating ferritin biosynthesis. In 1955, Fineberg and Greenberg [
29] conducted research on guinea pigs, showing that ferritin biosynthesis is accelerated by iron, with apoferritin (the protein shell without iron) being produced before ferritin itself [
30]. Later, Richter explored ferritin biosynthesis in liver cells and HeLa cells [
31], followed by studies from Shoden & Sturgeon [
32] and Friedberg et al. in guinea pigs [
33]. A significant breakthrough occurred in 1965 when Drysdale and Munro demonstrated that actinomycin D, a transcription inhibitor, failed to inhibit iron-induced ferritin synthesis in rats, proving that ferritin synthesis is mainly regulated at a post-transcriptional level [
34].
In 1957, Loewus and Fineberg [
35] discovered the conditions to incorporate iron into apoferritin, thereby reconstituting ferritin in vitro. During the 1960s, the development of new biochemical and biophysical techniques facilitated a deeper understanding of ferritin structure and function. X-ray diffraction studies by Kleinwachter [
36] and Harrison [
37] provided insights into ferritin’s symmetry, size, and shape. Concurrently, Crichton and colleagues focused on the biochemical characterization of ferritin, employing proteolytic enzymes, circular dichroism and other techniques[
38]. The Radola applied gel filtration analysis for molecular weight determination [
39], while starch electrophoresis revealed that liver ferritin could be separated into two bands, potentially representing ferritin monomers and oligomers [
40]. The introduction of polyacrylamide gel electrophoresis (PAGE), first described in 1959 by Raymond & Weintraub [
41], offered greater resolution in protein separation. In 1965 its use by Richter revealed differences in the ferritins from normal versus neoplastic cells [
42]. Subsequent studies confirmed that ferritin from different tissues or species exhibited variations in electrophoretic mobility, suggesting the potential existence of tissue-specific forms of ferritin. Initially, these electrophoretic techniques were cumbersome, as the runs were conducted in tubes, making band alignment very challenging. This issue was resolved with the development of slab gel electrophoresis, which greatly improved band visualization. Another significant advancement was the introduction of isoelectric focusing in 1971 by Urishizaki et al. [
43]. This technique demonstrated that even crystallized horse spleen ferritin could be separated into several bands with different electric charges. When applied to human specimens, the most acidic ferritin bands were found in tumor tissues, giving rise to the concept of "carcino-fetal isoferritins"[
44]. This finding sparked further interest due to its parallel with the recently discovered carcinoembryonic antigen (CEA) and alpha-fetoprotein.
A major advancement in electrophoresis came with the development of SDS-PAGE in 1970 by Laemmli [
45], which allowed for the analysis of denatured proteins and their subunits. This technique was used to determine whether ferritin consisted of one or multiple subunit types, initially yielding conflicting results. However, it was eventually demonstrated that human ferritin is composed of two distinct subunit types: H (heavy, heart, MW 21,000) and L (light, liver, MW 19,000), with the H subunit being more prevalent in the heart and the L subunit in the liver. These subunits were later found to have different amino acid compositions and to be encoded by separate mRNAs. This clarified that the isoferritins observed in different tissues were due to varying proportions of the H and L subunits, with more acidic ferritins being richer in the H subunit [
46]. These discoveries marked a significant leap in understanding ferritin’s complex structure, regulation, and functional diversity across different tissues and species.
2.6. Radioimmunoassays and serum ferritin
The studies of Mazur and Shorr suggested that ferritin might circulate in the blood and have additional functions. Some research groups used antibodies to detect ferritin in blood, but the low sensitivity of available techniques at the time only allowed its detection in the serum of animals experiencing experimental shock [
47], but not in healthy individuals. It was not until the development of sensitive radioimmunoassays that ferritin was reliably detected in the serum of all individuals, including healthy subjects. In 1972 Addison et al. demonstrated that serum ferritin concentrations were higher in individuals with iron-overload and lower in those with iron deficiency, establishing ferritin as a useful marker for assessing body iron status [
48]. This discovery led to the widespread use of serum ferritin measurements as a clinically important diagnostic tool for anemia, iron-related diseases and siderosis [
49]. Initially, competitive assays using radiolabeled human ferritin and antibodies for horse spleen ferritin were employed. Later, the symmetrical structure of ferritin made it an ideal model for developing more advanced two-site (sandwich) immunoassays. These sensitive assays also revealed that ferritin is present in various body fluids, including cerebrospinal fluid (CSF), urine, milk, and colostrum, although extracellular ferritin levels are much lower than intracellular concentrations.
While serum ferritin became—and continues to be—a key clinical indicator for diagnosing iron deficiency (or overload) and other diseases, only a limited number of studies focused on its specific characteristics, showing that it is either iron-free or iron-poor and shares structural similarities with liver apoferritin [
50]. It was found to predominantly consist of the L-subunit, which is partially glycosylated and recognized by the lectin concanavalin A [
51,
52]. However, open questions remained about its origin and the mechanisms by which it enters the bloodstream. Konijn et al. showed that ferritin synthesis occurs on both free and membrane-bound polyribosomes, suggesting the possibility of active secretion [
53]. Moreover, the presence of ferritin mRNAs on bound polysomes was found to be elevated in rat liver during experimental inflammation [
54] and in experimental tumors [
55]. However, in vitro translation experiments did not reveal post-translational modifications typical of secreted proteins [
56] and ferritin mRNA injection into Xenopus oocytes did not result in ferritin secretion in the medium [
55].
The discovery that ferritin exists in extracellular spaces spurred investigations into potential non-iron-related functions. Research by Matzner et al. [
57] found that ferritin had a suppressive effect on lymphocyte function, indicating it might have immunomodulatory roles, in addition to its well-known function as an iron storage protein. The dual roles of ferritin, both as an intracellular iron storage protein and a potential extracellular signaling molecule, continue to intrigue researchers, with many studies focused on unraveling the complexities of its secretion, circulation, and broader physiological functions. Building on the groundbreaking work on monoclonal antibodies by Köhler and Milstein of 1975 [
58], the production and characterization of the first monoclonal antibodies for heart ferritin [
59] marked a significant advancement. Subsequently, monoclonal antibodies targeting both ferritin subunits (H and L) were developed [
60], offering valuable tools for further ferritin studies.
2.7. Ferritin Structure
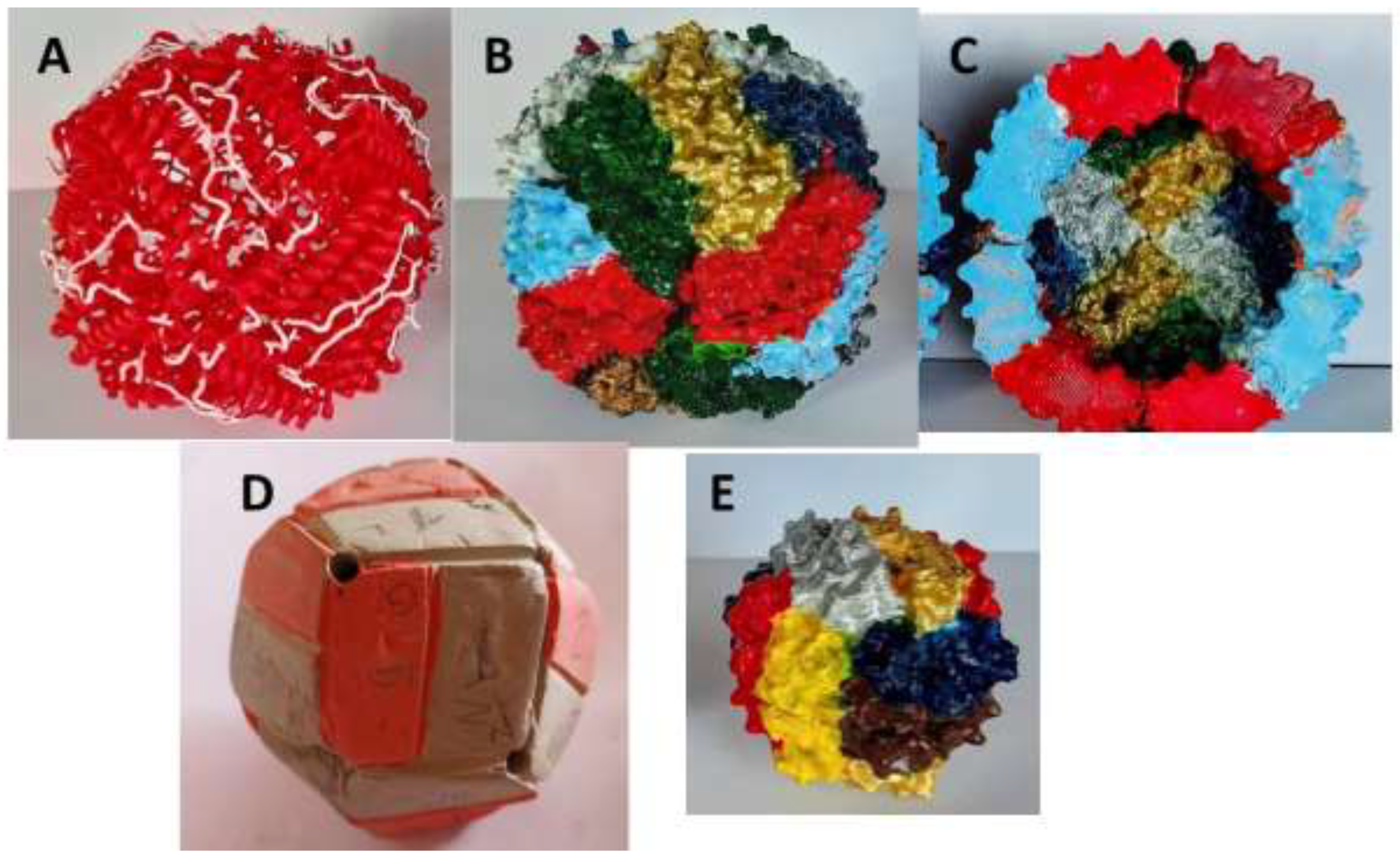
A major milestone in ferritin research was the publication of the crystallographic structure of horse spleen ferritin in 1984 by Ford et al., which revealed that its subunits are composed of a bundle of four alpha helices (
Figure 1). These 24 subunits come together to form an almost spherical molecule with 2-, 3-, and 4-fold symmetries [
61]. The structure includes pores along the 3- and 4-fold symmetry axes, which were proposed as potential routes for iron entry and exit. The resolution of this structure was complex and painstaking, involving the use of heavy atom derivatives, but it greatly simplified the subsequent determination of ferritin structures from many different origins. Moreover, it was critical for understanding the mechanism of iron incorporation into the protein cavity, which was suggested to occur through the hydrophilic 3-fold channels rather than the hydrophobic 4-fold channels.
The 1980s witnessed rapid advancements in molecular biology, highlighted by the first publication of “Molecular Cloning: A Laboratory Manual” by Tom Maniatis et al. [
62]. This influential work became an essential resource for molecular biology research, supporting numerous laboratories in the identification and cloning of genes. The cDNAs for the ferritin chains were cloned by various groups: initially in 1983 the cDNA for the rat L subunit by Brown et al. [
63], followed by the human L subunit cDNA by Costanzo et al. [
64], and shortly thereafter the cDNA for the H-chain was cloned by Boyd et al. [
65]. However, identifying the functional ferritin genes was complicated by the discovery of multiple bands in Southern blots, which were later attributed to inactive retro-pseudogenes [
66]. The functional human L-ferritin gene was mapped in 1983 to chromosome 19 by Caskey et al.), [
67] and the H-chain gene was localized to chromosome 11 by Worwood et al. [
68]. Subsequently in 1987, the cDNAs for both human H and L chains were cloned into prokaryotic expression vectors, and the corresponding ferritins were expressed in
Escherichia coli as recombinant proteins [
69,
70]. This work demonstrated that the H-chain possesses ferroxidase activity, which was absent in the L-chain [
71]. Recombinant H-ferritin was then crystallized for 3D structural determination, identifying the ferroxidase site within the 4-helix bundles of the subunit, composed of two iron-binding sites [
72]. Site-directed mutagenesis on recombinant ferritins was employed to explore the residues involved in iron uptake and the stability of the protein. The ferroxidase site was identified as essential for iron metabolism [
73]. This breakthrough paved the way for further research into ferritin's function, particularly the detailed mechanisms of iron oxidation and storage within the ferritin molecule.
2.8. Regulation of Ferritin Expression
Cloning the ferritin cDNAs was also fundamental for investigating the control of ferritin expression. In particular, significant advances were made in the area of the iron-dependent post-transcriptional regulation cited above. Early studies by Aziz & Munro revealed that both ferritin subunits (H and L) are similarly stimulated in response to increased iron levels in mammals [
74]. Confirming the remarkable foresight of the post-transcriptional model proposed by Munro and colleagues [
75], this regulation was traced to a conserved sequence located in the 5' untranslated region (UTR) of the ferritin mRNA, termed the Iron Responsive Element (IRE) [
76]. The discovery of the IRE was crucial in understanding how cells regulate ferritin synthesis in response to iron availability. The proteins responsible for binding to the IRE and regulating ferritin translation, known as Iron Regulatory Proteins (IRP1 and IRP2), were identified the following year. This discovery was made by comparing the translation efficiency of mammalian (reticulocyte lysate) and plant (wheat germ extract not containing IRPs) in vitro systems [
77].
In the same year a similar binding activity was documented in rat liver [
78]. Importantly, it was shown that the IRPs binding activity could be measured by electro mobility shift assay (EMSA) using radioactive IRE probes, a highly sensitive method that was employed in many studies on cellular iron homeostasis. The IRPs regulate ferritin production by binding to the IRE of both H and L mRNAs under low iron conditions, blocking translation and thus reducing ferritin synthesis. Conversely, when iron levels are sufficient, IRP1 assembles an iron sulfur cluster, becoming inactive, while IRP2 undergoes degradation, allowing ferritin synthesis to proceed. This regulatory mechanism not only clarified how ferritin expression is finely tuned in response to iron availability, but also underscored the broader importance of post-transcriptional regulation in controlling protein expression. As IRE were later discovered in the mRNAs of other key proteins of iron metabolism (see below), these findings paved the way for further exploration of iron metabolism and related disorders.
2.9. Novel Ferritin Functions
Once the fundamental aspects of ferritin were understood and the proper experimental tools became available, research on ferritin diversified into several important directions. One of the notable findings came from Konijn et al. who demonstrated that inflammation affects ferritin synthesis, suggesting a link between ferritin and the body’s response to inflammatory processes [
79]. In 1980, Dorner et al. published a key study showing that ferritin synthesis occurs in T-lymphocytes [
80], expanding the understanding of ferritin’s role in immune function. A particularly important discovery was made by Broxmeyer, who found that acidic ferritins exhibit an inhibitory effect on granulocyte-macrophage progenitor cells [
81] . The same group later showed that the myelosuppressive activity of ferritin was related to the H-chain's ferroxidase activity [
82]. These findings highlighted a functional connection between ferritin’s role in iron metabolism and immune regulation, especially in hematopoietic processes. Other important discoveries during this period included the observation by Konijn et al. that ferritin is taken up by erythroid precursor cells for heme synthesis [
83], underscoring ferritin’s contribution to iron utilization in red blood cell development. Furthermore, Pollack and Campana discovered ferritin receptors on immature red blood cells [
84], contributing to the understanding of how ferritin mediates iron transport and uptake in developing erythroid cells. Moreover, in 1989, Fracanzani et al. made a key observation regarding hemochromatosis, finding a lack of ferritin in the duodenal absorptive epithelial cells of patients [
85], which was likely due to increased IRP binding activity [
86]. This was in line with the previously suggested role of ferritin [
3,
4] in the hypothesis of a "mucosal block" in iron absorption regulation. Collectively, these findings expanded the scope of ferritin research, establishing its role beyond simple iron storage and introducing its involvement in immune regulation, cancer treatment, and iron homeostasis in various diseases (
Table 1). Additionally, studies by Leichner et al. using radiolabeled anti-ferritin antibodies to target primary liver cancer [
87] were followed by various other tumor-targeting studies using radio-iodinated anti-hepatocellular carcinoma ferritin [
88]. These efforts highlighted the potential of ferritin-based therapies for cancer diagnosis and treatment.
2.10. Ferritins in Animals, Plants And Bacteria
Although the primary focus of ferritin research remained on human and mammalian ferritins, several laboratories explored ferritins from other species and phyla, providing valuable comparative insights. For example, Bottke [
89] discovered a secreted ferritin in snails, marking an early observation of ferritin functioning beyond intracellular iron storage. In plants, ferritin was characterized by the presence of an N-terminal transit peptide, a feature enabling its transport into plastids [
90], emphasizing a specialized role for plant ferritins in different cellular compartments. Bacterial ferritins also became a major area of interest. Research identified three distinct types of bacterial ferritins. One type, structurally similar to animal ferritins (FTN) was first identified in
E. coli [
91]. Another type, termed bacterioferritin (BFR), was found to contain 24 heme groups, highlighting its unique iron-storage and oxidative properties [
92]. A third type, initially identified in
Listeria innocua by Bozzi et al. [
93], was composed of 12 subunits and was previously referred to as DPS (DNA-binding proteins from starved cells). This ferritin variant garnered attention because its crystallographic structure revealed that its subunits also formed 4-helical bundles, resembling those found in other ferritins [
94]. The DPS ferritin was shown to possess ferroxidase activity, with the active site localized at the interface between the subunits [
95]. Further studies on ferritins from various organisms, including
E. coli,
Azotobacter and pea seeds [
96] contributed to a broader understanding of the structural diversity and functional specialization of ferritins across different species. These studies highlighted the evolutionary conservation of ferritin’s core structure while revealing distinct adaptations in specific species, thus enriching the overall knowledge of iron metabolism across biological systems.
Table 1.
Some functional roles attributed to ferritin.
Table 1.
Some functional roles attributed to ferritin.
| Function |
Experimental model |
Reference |
| Iron storage |
Guinea pigs and other animals |
Granick, 1951 [3] |
| Vaso depressor material |
Rat |
Mazur Shorr, 1948 [97] |
| Antidiuretic |
Rat |
Mazur, [98] |
| Serum ferritin, marker of iron status |
Humans and other mammals |
Addison, 1972 [48] |
| Inhibitor of granulocytes-macrophage progenitors |
Cultured human cells |
Broxmeyer, 1982 [81] |
| Radiolabeled antiferritin antibody targets liver cancer |
Human patients |
Leichner, 1984 [87] |
| Source of iron for ROS formation |
Cellular models |
Reif, 1992 [99] |
| Cytoprotective antioxidant |
Cellular models |
Balla, 1992 [100] |
| Delivery of iron to erythroid precursor cells |
Cultured erythroid cells |
Konijn, 1994 [83] |
| Regulated in oncogenesis |
Cellular models |
Bevilacqua, 1997 [101] Wu, 1999 [102] |
| Binds kininogen |
Cellular models |
Torti, 1998 [103] |
| Binds microtubules |
Cellular models |
Hasan, 2006 [104] |
| Inhibitor of calcification and osteogenesis |
Cellular models |
Zarjou, 2010 [105] |
| Stabilizes HIF1a |
Cellular models |
Siegert, 2015 [106] |
| Regulates ferroptosis |
Cellular models |
Park & Chung, 2019 [107] |
| Stimulates inflammasome |
Cellular models |
Fernandez-Rojo 2024 [108] |
2.11. Ferritin Stability and Function
Ferritin was among the first multimeric proteins to be expressed recombinantly in
E. coli. It was produced in a soluble and stable form with good yields and was subsequently modified through site-directed mutagenesis to investigate its structural stability and its interaction with iron. Alterations in the 2-fold symmetry axis inhibited ferritin assembly, while changes at the 3- and 4-fold axes mainly reduced stability without entirely preventing function [
109]. Crucially, it was shown that iron enters and exits through the hydrophilic 3-fold channels, while the 4-fold channels are thought to be permeable only to protons. Efficient and rapid iron incorporation required both the ferroxidase activity of the H-chain and the nucleation center of the L-chain inside the ferritin cavity [
110]. Research also demonstrated the formation of a transient blue Fe(III)-tyrosinate complex during the early steps of ferroxidase activity, as reported by Waldo et al. [
111]. Efforts to introduce a functional ferroxidase center into the L-chain were initially hampered by mutations that disrupted subunit folding. However, this issue was resolved by co-assembling mutant L-chains with wild-type L-chains, yielding functional molecules with ferroxidase activity [
112]. Takagi et al. [
113] further suggested that the 3-fold channel could undergo partial unfolding under mild conditions, facilitating iron exchange between the ferritin core and its external environment.
A significant breakthrough occurred with the successful modification of the iron core chemistry, transforming it from ferrihydrite into iron sulfide. This advancement paved the way for the use of ferritin as a cargo carrier for various mineral compounds. This was exemplified by a bioinorganic composite created within ferritin by Douglas et al. [
114], highlighting ferritin's potential in nanotechnology and materials science.
In addition to these structural insights, a key milestone enabled by the availability of recombinant ferritins was the establishment of an international standard for serum ferritin based on recombinant human L-ferritin [
115]. This marked the first standardized measurement of serum ferritin levels, with significant clinical implications for diagnosing iron-related disorders.
2.12. The Importance of the IRE/IRPs Machinery
The IRP/IRE machinery became a fascinating example of post-transcriptional regulation, particularly after it was discovered that this regulatory system extends beyond ferritins and iron storage. It also controls other proteins involved in iron uptake (transferrin receptor, TfR1), export (ferroportin) and utilization (erythroid 5-aminolevulinic acid synthase (eALAS, the key enzyme in heme synthesis) [
116]. Gray and Hentze [
117] first highlighted this broader regulatory role which extends to other proteins related to iron metabolism indicated above. Detailed studies showed that IRP1 inhibited the binding of eIF4F to the 5’ untranslated region of ferritin transcripts, thereby blocking translation initiation [
118]. Interest in the IRP/IRE system grew significantly with the discovery of its involvement in a genetic disorder called Hereditary Hyperferritinemia Cataract Syndrome (HHCS). This autosomal dominant condition results from mutations in the IRE of the L-ferritin mRNA, which diminish the binding affinity of IRPs, leading to unregulated overproduction of L-ferritin. Elevated serum ferritin levels in these patients are accompanied by early-onset bilateral cataracts. The genetic basis of HHCS was elucidated almost simultaneously in 1995 by two groups in France and Italy [
119,
120]. The close relationship between HHCS-dependent hyperferritinemia and L subunit ferritin content in blood mononuclear cells strongly suggested that the latter cells are the source of serum ferritin [
121], an indication that was confirmed many years later (more below).
2.13. Other Ferritin Functions
Several additional studies have expanded our understanding of ferritin's functions (
Table 1). Cai et al. reported the presence of ferritin in the nuclei of mammalian cells, specifically in corneal epithelial cells [
123], while Pountney et al. found nuclear ferritin in human K562 cells, suggesting new roles for ferritin beyond iron storage [
124]. Torti and Torti discovered that ferritin could bind to kininogen, a multifunctional protein [
103], while Hulet et al. identified ferritin binding sites in the mouse brain [
125], which might be involved in iron transfer to brain cells. Notably, further studies on ferritin regulation revealed an association between ferritin and oncogenic pathways; in fact, the gene product of E1A, a viral oncogene [
101], and the proto-oncogene c-MYC [
102] were shown to repress ferritin transcription. Another discovery was that ferritin forms dynamic oligomers that associate with microtubules, offering insights into its structural interactions within cells Hasan et al. [
104]. These findings indicated novel regulatory and functional roles for ferritins in cellular processes beyond simple iron storage, broadening the scope of research in this area.
2.14. The New Century, 2000: KO Mice, Mitochondrial Ferritin and Neuroferritinopathy
The early 2000s brought significant advancements in the understanding of ferritin functions, also thanks to the development of a knockout (KO) mouse model lacking the H-ferritin subunit. This study by Ferreira et al. [
126] revealed that H-ferritin deletion results in embryonic lethality, highlighting the essential role of ferritin’s ferroxidase activity in development and reinforcing the idea that ferritin is crucial across almost all living organisms. A second landmark discovery of 2001 was the identification of neuroferritinopathy, a rare genetic disorder first described in England that affects the L-ferritin subunit [
127]. This neurological disorder is a form of late-onset parkinsonism with dominant transmission. It is caused by nucleotide insertions or duplications at the 3’ end of the L-ferritin coding sequence that alter the C-terminus of the protein and lead to the accumulation of iron precipitates in tissues, especially in the brain. A third major milestone of 2001 was the discovery of mitochondrial ferritin (MtF) by Levi et al. [
128], which is encoded by an intronless gene. The gene product contains a long N-terminal sequence for mitochondrial targeting, which is cleaved to form a mature protein similar in size and function to the H-ferritin subunit, as it is endowed with ferroxidase activity. This discovery was considered important because mitochondria, being involved in both heme synthesis and Fe-S cluster formation, are critical for intracellular iron processing and are a major source of reactive oxygen species (ROS). MtF gained further attention because of its overexpression in the red blood cells of patients with sideroblastic anemia [
129]. More detailed analysis in mice showed its presence primarily in cells with high metabolic activity, such as those in the heart, spermatozoa, and kidneys, but not in other cells [
130]. Interestingly, knockout mice lacking MtF did not display obvious phenotypes, except for increased sensitivity to the cardiotoxic effects of doxorubicin, a chemotherapeutic drug that mainly targets mitochondria, and reduced male fertility [
131]. This suggests a specific role for mitochondrial ferritin in maintaining cellular homeostasis in energy-intensive tissues. MtF was later found in plants and Drosophila [
132,
133].
2.15. Ferritin and Oxidative Stress
During this period, several intriguing findings expanded the understanding of ferritin beyond its traditional role in iron storage. While ferritin may act as a chemical source of iron contributing to oxidative damage [
99], H-ferritin was shown to reduce lipid peroxidation in vitro [
122] and to function as a cytoprotective antioxidant, particularly in endothelial cells, by mitigating oxidative stress Balla et al. [
100]. Oxidative stress was shown to induce coordinated transcriptional and translational regulation of ferritin, as initially demonstrated in vivo by Cairo et al. [
134] and later confirmed by Tsuji et al. [
135], who precisely characterized an antioxidant response element in the regulatory region of the H subunit, highlighting the protein's role in the cellular response to stress.
2.16. Ferritin Receptors
Given the existence of circulating ferritin and its role in hematopoiesis, as demonstrated by Broxmeyer’s studies, the search for ferritin receptors also progressed. Various earlier reports suggested the presence of ferritin binding sites on the cell membrane of mammalian cells, but their precise identity remained elusive. In 2005, Chen et al. identified TIM-2 [
136] as a receptor for H-ferritin endocytosis in mouse B cells and in the liver and kidney. However, TIM-2 lacks a human homolog, leaving the human H-ferritin receptor unknown until Li et al. [
137] discovered that TfR1 binds and internalizes H-ferritin in human cells. Further progress in 2014 identified SCARA5 as the receptor for L-ferritin, with potential implications in retinopathy [
138].
2.17. Novel Ferritin Structures
In parallel, structural studies continued to shed light on ferritin's diversity. One significant finding was the serendipitous crystallization in 2005 of a secreted ferritin from an insect species, which showed to be a heteropolymer composed of two distinct subunit types forming heterodimers. This marked the first 3D structure of a ferritin heteropolymer, achieved by Hamburger et al. [
139]. Additional crystallographic studies unveiled the structure of tetrahedral open-pore ferritin from the hyperthermophilic archaeon
Archaeoglobus fulgidus [
140]. Another milestone was the structural elucidation of IRP1 complexed with the IRE motif in ferritin mRNA [
141], providing deeper insights into the post-transcriptional regulation of iron metabolism. More recently, a system was engineered that permitted the synthesis of human heteropolymeric ferritins with different H to L subunit ratio [
142], thus allowing to study the 3D structured of the isoferritins with different H and L chain composition [
143].
Overall, these discoveries helped connect ferritin’s functions to a broader array of biological processes, and highlighted its importance in cellular homeostasis, oxidative stress response, and iron regulation across different organisms and tissues.
2.18. Iron Trafficking To And From Ferritin, Chaperones And Ferritinophagy”
The process of iron incorporation by ferritin has been a subject of extensive research, with early studies showing that ferritin readily takes up iron in vitro. However, the in vivo mechanism remained elusive until in 2008 an ingenious system in yeast identified a cytosolic iron chaperone named PCBP1 that delivered iron to ferritin and other iron-dependent proteins [
144]. This discovery revealed a regulated and controlled iron delivery system to ferritin, challenging earlier assumptions of passive iron incorporation.
The reverse process—how iron stored in ferritin is made available to cells—was an ancient problem, originally raised by Granick, that presented a significant challenge. Direct solubilization of the iron core was thought to involve reduction reactions and a ferritin reductase was described in early times by Zaman & Verwilghen [
145], but never confirmed. Since the released divalent iron could produce harmful ROS, releasing iron from ferritin in a safe manner is crucial for cellular health. Early studies suggested that ferritin was degraded in the lysosome to release iron, with a mechanism differing in iron-depleted and iron-replete cells [
146]. However, the breakthrough came with the discovery of ferritinophagy by Mancias et al., a specific autophagic process. In this pathway, the protein NCOA4 binds the H-chain of ferritin and transports it to lysosomes, where ferritin is degraded and the stored iron is solubilized and recycled back into the cytosol [
147]. This iron-regulated mechanism offered a clearer understanding of how ferritin participates in iron recycling and contributes to cellular iron homeostasis. Interestingly, similar mechanisms seem to be involved in ferritin export. Macrophages, which were identified as the primary source of extracellular ferritin [
148], secrete ferritin through a NCOA4-independent lysosomal pathway involving secretory autophagosomes [
149] as well as via the multivesicular body–exosome pathway [
150].
2.19. Discovery of Ferroptosis
Ferritin’s role in cellular protection includes apoptosis, where it has been shown to inhibit cell death by suppressing ROS production, another evidence of the antioxidant role of ferritin [
151]. However, a more critical connection between ferritin and programmed cell death emerged with the description of ferroptosis in 2012 [
152]. Ferroptosis is a distinct form of programmed cell death caused by lipid peroxidation, facilitated by iron. This discovery has sparked large interest, as ferroptosis has been linked to various diseases, including cancer and neurodegeneration [
153]. In this context, the iron sequestering capacity of ferritin might be key in regulating ferroptosis, as shown by the ferroptosis-mediated cardiomiopathy triggered by H-ferritin gene deletion [
154]. The role of iron and iron-related proteins, including ferritin, in regulating this pathway has generated extensive research into the mechanisms of ferroptosis, while also driving efforts to identify agents that can either promote or inhibit this process. The uncovering of ferritinophagy and ferroptosis has expanded the understanding of ferritin’s biological roles, showing that it is not only an iron storage molecule, but also a dynamic regulator of cellular iron homeostasis, and a key player in cell survival and death mechanisms.
2.20. Novel Ferritin Functions
Studies on conditional H-ferritin KO mice, produced by Lukas Kuhn's lab revealed novel tissue-specific roles of ferritin. These authors in 2010 showed that intestinal H-ferritin is necessary for regulating iron absorption, aligning with the "mucosal block" theory [
155]. Additionally, deleting H-ferritin in mouse bone marrow was shown to reduce B and T lymphocyte populations, supporting its role in immune function [
156]. Interestingly, KO mice for L-ferritin exhibited no significant phenotype, consistent with clinical data showing that mutations affecting L-ferritin’s start codon produce no hematological or neurological symptoms [
157].
Ferritin’s ferroxidase activity and its role in iron storage are involved in a wide range of biological processes (
Table 1). In human and animal models H-ferritin showed inhibitory role in osteogenesis [
158] and is involved in a chemokine receptor CXCR4-dependent dysfunction in neurons due to opiates [
159]. Furthermore, it stabilizes hypoxia-inducible factor-1α (HIF1α) under lipopolysaccharide activation in oxygen-rich environments [
106], showcasing its regulatory role in cellular responses to inflammation and oxidative stress. Moreover, upon endocytosis, ferritin H subunits stimulate the formation of the NLRP3 inflammasomes in liver stellate cells to drive hepatic inflammation [
108].
Studies of ferritin across different species revealed some surprising findings. In marine diatoms, for instance, ferritin plays a crucial role in providing iron, which contributes to their ability to form blooms [
160]. In bay scallops, ferritin is involved in immune defense mechanisms, while in marine phytoplankton, it regulates iron homeostasis [
161]. Interestingly, ferritin has also been linked to light production in the marine worm
haetopterus [
162], and in oysters ferritin regulates mitophagy [
163], highlighting its diverse roles across different marine species.
2.21. Recent Biotechnological and Clinical Applications
The unique structural properties of ferritin have been leveraged in various innovative approaches and for different applications. Cohen et al. [
164] demonstrated that ferritin overexpression in cells could be monitored using magnetic resonance imaging (MRI), as the accumulation of iron lowers T1 and T2 signals, showcasing the utility of the iron core's magnetic properties for tissue identification. This characteristic has allowed ferritin to be used as an endogenous MRI reporter for noninvasive imaging of gene expression in several studies. One notable example is the use of MRI to quantify signals in transgenic grafts overexpressing ferritin in murine myocardial infarcts [
165]. Beyond imaging, the magnetic and temperature-sensitive properties of ferritin's iron core have been exploited in more advanced applications, such as the magnetogenetic manipulation of proteins and organelles within living cells, facilitated by engineered ferritin [
166]. On the technological front, ferritin nanoparticles have been incorporated into organic field-effect transistor memory devices [
167]. Moreover, ferritin remains a model system for protein crystallization studies and advancements in cryo-electron microscopy (Cryo-EM) [
168].
Furthermore, the ferritin nanocage structure possesses remarkable properties, including high stability and the ability to encapsulate drugs or chemical agents within its cavity. Such properties are currently being exploited in the medical field for theranostic applications. As a drug delivery system, drug-loaded ferritin nanoparticles have shown higher efficacy in targeting cancer cells compared to free drugs [
169], especially since TfR1, which binds H-ferritin, is highly expressed on the surface of many cancer cells. Additionally, ferritin is being explored as a platform for vaccine development, with epitopes genetically fused or chemically attached to ferritin molecules to boost immunogenicity [
170]. Ferritin’s clinical relevance surged during the COVID-19 pandemic, as serum ferritin levels became a key prognostic marker for disease severity [
171], reflecting its central role in inflammatory and other iron-related pathways. Overall, ferritin’s unique properties have made it an indispensable tool across various scientific disciplines, from bioengineering and drug delivery to immune research and materials science.
Table 2.
Ferritin milestones.
Table 2.
Ferritin milestones.
| Year |
Milestone |
Author |
| 1937 |
Ferritin crystallization |
Laufberger [1] |
| 1942 |
Apoferritin |
Michaelis [2] |
| 1951 |
Ferritin iron storage |
Granick [3] |
| 1948? |
Vaso Depressive Material |
Mazur-Shorr [7] |
| 1954 |
Electron Microscopy |
Farrant et al. [11] |
| 1971 |
Isoferritins |
Urushizaki et al. [43] |
| 1972 |
Serum ferritin |
Addison et al. [48] |
| 1978 |
H-L subunits |
Arosio et al. [46] |
| 1983 |
Monoclonal antibodies |
Cavanna et al. [59] |
| 1983 |
Cloning of ferritin subunits |
Brown et al. [63] |
| 1984 |
Solving the crystallographic structure of ferritin |
Ford et al. [61] |
| 1987 |
Production of recombinant ferritin |
Levi et al. [69] |
| 1987 |
IRE identification |
Hentze et al. [76] |
| 1988 |
IRPs purification |
Walden [ et al. 77] |
| 1988 |
Ferroxidase activity in H-chain |
Levi et al. [71] |
| 1990 |
Bacterial Heme ferritin BFR |
Kadir & Moore [92] |
| 1991 |
Identification of ferroxidase center |
Lawson et al. [73] |
| 1997 |
Dodecameric DPS |
Bozzi et al. [93] |
| 2000 |
H Ferritin KO mice |
Ferreira et al. [126] |
| 2001 |
Neuroferritinopathy |
Curtis et al. [127] |
| 2001 |
Mitochondrial ferritin |
Levi et al. [128] |
| 2008 |
Iron chaperone to ferritin |
Shi et al. [144] |
| 2012 |
Ferroptosis |
Dixon et al. [152] |
| 2014 |
Ferritinophagy |
Mancias et al. [147] |
| 2021 |
Recombinant human isoferritins |
Srivastava et al. [142] |
| 2024 |
Structure of isoferritins |
Bou-Abdallah et al [143]. |
3. Conclusions
Over the past 80 years, with more than 40,000 original papers and hundreds of review articles our understanding of the ferritin molecule has advanced significantly, as indicated by the milestones summarized in Table II. We now have a comprehensive understanding of its intricate structure, its interactions with iron, and its essential role in regulating cellular iron availability and preventing toxicity. Ferritin’s presence in nearly all living organisms, likely since the early stages of life, underscores its evolutionary significance. Despite variations in sequence across different species, the fundamental structure—a highly conserved alpha-helix bundle forming a spherical cage—remains unchanged. This structural conservation has enabled ferritin to take on various specialized roles depending on the organism and cellular context.
Its primary and conserved function is to safely store excess iron, thereby protecting cells from ROS, but also providing a reservoir that can be easily accessed when needed. In certain organisms like seashells, ferritin has assumed a prominent role in immune defense, while in mammals, it plays a crucial part in regulating programmed cell death mechanisms such as apoptosis and ferroptosis. Ferritin may also influence calcification processes [
105] further expanding its biological significance.
While the intracellular roles of ferritin are well established, its extracellular functions, including its presence in serum, are not yet fully understood. Despite ongoing research, the origins and specific functions of serum ferritin remain inadequately characterized.
Over the years, interest in ferritin has surged, driven by its expanding roles in a wide range of biological processes, from iron metabolism to immune defense, disease mechanisms, and groundbreaking applications in biotechnology and nanomedicine [
172,
173] .[
174] and it has also been used for iron supplement and treatment of anemias. Ferritin’s versatility, both structurally and functionally, ensures that it will continue to be a focal point of research for years to come
Author Contributions
writing—original draft preparation, P.A.; writing—review and editing, G.C. and F.B-A. All authors have read and agreed to the published version of the manuscript.”.
Funding
This research received no external funding.
References
- Laufberger V, Sur Cristal. Ferritine 1937, 19, 1575–1582.
- S. Granick e L. Michaelis. FERRITIN AND APOFERRITIN. Science 1942, 95, 439–440. [CrossRef] [PubMed]
- S. Granick. Structure and physiological functions of ferritin. Physiol. Rev. 1951, 31, 489–511. [CrossRef]
- S. GRANICK. Ferritin; its properties and significance for iron metabolism. Chem. Rev. 1946, 38, 379–403. [CrossRef]
- W. M. Balfour, P. F. Hahn, W. F. Bale, W. T. Pommerenke, e G. H. Whipple. RADIOACTIVE IRON ABSORPTION IN CLINICAL CONDITIONS: NORMAL, PREGNANCY, ANEMIA, AND HEMOCHROMATOSIS. J. Exp. Med. 1942, 76, 15–30. [CrossRef]
- G. TECCE. Presence of ferritin in a marine elasmobranch. Scyllium canicula. Nature 1952, 170, 75. [CrossRef]
- MAZUR e, E. SHORR. Hepatorenal factors in circulatory homeostasis; the identification of the hepatic vasodepressor substance, VDM, with ferritin. J. Biol. Chem. 1948, 176, 771–787. [Google Scholar]
- MAZUR, I. LITT, e E. SHORR. The relation of sulfhydryl groups in ferritin to its vasodepressor activity. J. Biol. Chem. 1950, 187, 485–495. [Google Scholar] [CrossRef]
- MAZUR, S. GREEN, e E. SHORR. The oxidation of adrenaline by ferritin iron and hydrogen peroxide. J. Biol. Chem. 1956, 220, 227–235. [Google Scholar] [CrossRef]
- S. GREEN e A. MAZUR. Relation of uric acid metabolism to release of iron from hepatic ferritin. J. Biol. Chem. 1957, 227, 652–668.
- J. L. Farrant. An electron microscopic study of ferritin», Biochim. Biophys. Acta 1954, 13, 569–576.
- W. GABRIO, A. SHODEN, e C. A. FINCH. Quantitative fractionation of tissue ferritin and hemosiderin. J. Biol. Chem. 1953, 204, 815–821. [CrossRef]
- G. W. RICHTER. A study of hemosiderosis with the aid of electron microscopy; with observations on the relationship between hemosiderin and ferritin. J. Exp. Med. 1957, 106, 203–218. [CrossRef] [PubMed]
- J. W. DRYSDALE e W. N. RAMSAY. THE SEPARATION OF FERRITIN AND HAEMOSIDERIN FOR STUDIES IN THE METABOLISM OF IRON. Biochem. J. 1965, 95, 282–288. [CrossRef] [PubMed]
- M. G. FARQUHAR, S. L. WISSIG, e G. E. PALADE. Glomerular permeability. I. Ferritin transfer across the normal glomerular capillary wall. J. Exp. Med. 1961, 113, 47–66. [CrossRef]
- D. BROWN e J. D. CASTON. Biochemistry of amphibian development. III. Identification of ferritin in the egg and early embryos of Rana pipiens. Dev. Biol. 1962, 5, 445–451. [CrossRef]
- K. M. TOWE, H. A. LOWENSTAM, e M. H. NESSON. INVERTEBRATE FERRITIN: OCCURRENCE IN MOLLUSCA. Science 1963, 142, 63–64. [CrossRef]
- Baba. Crystallization of ferritin from coelomic fluid of Corbicula sandai with ammonium sulfate. J. Biochem. (Tokyo) 1969, 65, 915–923. [CrossRef]
- M. Randall. Electron microscopical demonstration of ferritin in the dental epithelial cells of urodeles. Nature 1966, 210, 1325–1326. [CrossRef]
- F. Heneine, G. Gazzinelli, e W. L. Tafuri. Iron metabolism in the snail Biomphalaria glabrata: uptake, storage and transfer. Comp. Biochem. Physiol. 1969, 28, 391–399. [CrossRef]
- N. David e K. Easterbrook. Ferritin in the fungus Phycomyces. J. Cell Biol. 1971, 48, 15–28. [CrossRef] [PubMed]
- T. Kato, S. Shinjo, e T. Shimada. Isolation and properties of ferritin from tuna fish (Thunnus obesus) spleen. J. Biochem. (Tokyo) 1968, 63, 170–175. [CrossRef] [PubMed]
- le, Q. Darcel e M. J. Merriman. Ferritin in the plasma of birds with erythroblastosis. Poult. Sci. 1971, 50, 1029–1035. [Google Scholar] [CrossRef]
- W. Robards e P. G. Humpherson. Phytoferritin in plastids of the cambial zone of willow. Planta 1967, 76, 169–178. [CrossRef]
- B. HYDE, A. J. HODGE, A. KAHN, e M. L. BIRNSTIEL. STUDIES ON PHYTOFERRITIN. I. IDENTIFICATION AND LOCALIZATION. J. Ultrastruct. Res. 1963, 59, 248–258. [CrossRef]
- H. Newcomb. Fine structure of protein-storing plastids in bean root tips. J. Cell Biol. 1967, 33, 143–163. [CrossRef]
- Seekbach. Iron content and ferritin in leaves of iron treated Xanthium pensylvanicum plants. Plant Physiol. 1969, 44, 816–820. [CrossRef]
- S. Craig e K. I. William. Phytoferritin and viral infection. Virology 1969, 39, 616–617. [CrossRef]
- R. A. FINEBERG e D. M. GREENBERG. Ferritin biosynthesis. II. Acceleration of synthesis by the administration of iron. J. Biol. Chem. 1955, 214, 97–106.
- R. A. FINEBERG e D. M. GREENBERG. Ferritin biosynthesis. III. Apoferritin, the initial product. J. Biol. Chem. 1955, 214, 107–113.
- W. RICHTER. Activation of ferritin synthesis and induction of changes in fine structure in HeLa cells in vitro: implications for protein synthesis. Nature 1961, 190, 413–415. [CrossRef] [PubMed]
- SHODEN e, P. STURGEON. Iron storage. III. The influence of rates of administration of iron on its distribution between ferritin and hemosiderin. Acta Haematol. 1962, 27, 33–46. [Google Scholar] [CrossRef]
- FRIEDBERG. Some properties of apoferritin isolated from guinea pig liver. Can. J. Biochem. Physiol. 1962, 40, 983–987. [CrossRef]
- W. DRYSDALE e H. N. MUNRO. FAILURE OF ACTINOMYCIN D TO PREVENT INDUCTION OF LIVER APOFERRITIN AFTER IRON ADMINISTRATION. Biochim. Biophys. Acta 1965, 103, 185–188. [CrossRef] [PubMed]
- W. LOEWUS e R. A. FINEBERG. The incorporation of iron by apoferritin. Biochim. Biophys. Acta 1957, 26, 441–443. [CrossRef]
- V. KLEINWACHTER. X-ray diffraction study of denatured ferritin and apoferritin. Nature 1960, 186, 313–314. [CrossRef]
- P. M. HARRISON. The structure of apoferritin: molecular size, shape and symmetry from x-ray data. J. Mol. Biol. 1963, 6, 404–422. [CrossRef]
- R. R. Crichton. Ferritin: structure, synthesis and function. N. Engl. J. Med. 1971, 284, 1413–1422. [CrossRef]
- J. Radola, G. Kellner, e J. S. Frimmel. Gel-filtration of isotopically labelled ferritin from HeLa cells. Nature 1965, 207, 206–208. [CrossRef]
- J. J. THERON, A. O. HAWTREY, e V. SCHIRREN. Characterization of human liver ferritin by starch-gel electrophoresis. Clin. Chim. Acta Int. J. Clin. Chem. 1963, 8, 165–167. [CrossRef]
- S. RAYMOND e L. WEINTRAUB. Acrylamide gel as a supporting medium for zone electrophoresis. Science 1959, 130, 711. [CrossRef] [PubMed]
- W. Richter. Comparison of ferritins from neoplastic and non-neoplastic human cells. Nature 1965, 207, 616–618. [CrossRef] [PubMed]
- Urushizaki, Y. Niitsu, K. Ishitani, M. Matsuda, e M. Fukuda. Microheterogeneity of horse spleen ferritin and apoferritin. Biochim. Biophys. Acta 1971, 243, 187–192. [Google Scholar] [CrossRef] [PubMed]
- W. Drysdale e E. Alpert. Carcinofetal human isoferritins. Ann. N. Y. Acad. Sci. 1975, 259, 427–434. [CrossRef] [PubMed]
- U. K. Laemmli. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [CrossRef]
- P. Arosio, T. G. Adelman, e J. W. Drysdale. On ferritin heterogeneity. Further evidence for heteropolymers. J. Biol. Chem. 1978, 253, 4451–4458. [CrossRef]
- JANOFF, B. W. ZWEIFACH, A. L. NAGLER, e Z. OVARY. Detection of ferritin in the plasma of guinea pigs in experimental shock. Circ. Res. 1961, 9, 407–412. [Google Scholar] [CrossRef]
- G. M. Addison, M. R. Beamish, C. N. Hales, M. Hodgkins, A. Jacobs, e P. Llewellin. An immunoradiometric assay for ferritin in the serum of normal subjects and patients with iron deficiency and iron overload. J. Clin. Pathol. 1972, 25, 326–329. [CrossRef]
- Jacobs e, M. Worwood. Ferritin in serum. Clinical and biochemical implications. N. Engl. J. Med. 1975, 292, 951–956. [Google Scholar] [CrossRef]
- P. Arosio, M. Yokota, e J. W. Drysdale. Characterization of serum ferritin in iron overload: possible identity to natural apoferritin. Br. J. Haematol. 1977, 36, 199–207. [CrossRef]
- S. J. Cragg, M. Wagstaff, e M. Worwood. Detection of a glycosylated subunit in human serum ferritin. Biochem. J. 1981, 199, 565–571. [CrossRef] [PubMed]
- P. Santambrogio, A. Cozzi, S. Levi, e P. Arosio. Human serum ferritin G-peptide is recognized by anti-L ferritin subunit antibodies and concanavalin-A. Br. J. Haematol. 1987, 65, 235–237. [CrossRef]
- M. Konijn, B. S. Baliga, e H. N. Munro. Synthesis of liver ferritin on free and membrane-bound polyribosomes of different sizes. FEBS Lett. 1973, 37, 249–252. [CrossRef] [PubMed]
- Schiaffonati; et al. Mechanisms of regulation of ferritin synthesis in rat liver during experimental inflammation. Exp. Mol. Pathol. 1988, 48, 174–181. [Google Scholar] [CrossRef]
- C. Linder et al. Ferritin synthesis on polyribosomes attached to the endoplasmic reticulum. J. Inorg. Biochem. 1992, 47, 229–240. [CrossRef]
- Tacchini, E. Rappocciolo, M. Ferrero, L. Schiaffonati, e G. Cairo. Ferritin mRNAs on rat liver membrane-bound polysomes synthesize ferritin that does not translocate across membranes. Biochim. Biophys. Acta 1992, 1131, 133–138. [Google Scholar] [CrossRef]
- Y. Matzner, C. Hershko, A. Polliack, A. M. Konijn, e G. Izak. Suppressive effect of ferritin on in vitro lymphocyte function. Br. J. Haematol. 1979, 42, 345–353. [CrossRef]
- G. Köhler e C. Milstein. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [CrossRef]
- F. Cavanna et al. Development of a monoclonal antibody against human heart ferritin and its application in an immunoradiometric assay. Clin. Chim. Acta Int. J. Clin. Chem. 1983, 134, 347–356. [CrossRef]
- Luzzago; et al. Immunochemical characterization of human liver and heart ferritins with monoclonal antibodies. Biochim. Biophys. Acta 1986, 872, 61–71. [Google Scholar] [CrossRef]
- G. C. Ford et al. Ferritin: design and formation of an iron-storage molecule», Philos. Trans. R. Soc. Lond. B Biol. Sci. 1984, 304, 551–565.
- J. Sambrook. Molecular cloning: a laboratory manual», Cold Spring Habor Lab., 1989.
- J. Brown, E. A. Leibold, e H. N. Munro. Isolation of cDNA clones for the light subunit of rat liver ferritin: evidence that the light subunit is encoded by a multigene family. Proc. Natl. Acad. Sci. U. S. A. 1983, 80, 1265–1269. [CrossRef] [PubMed]
- F. Costanzo et al. Cloning and sequencing of a full length cDNA coding for a human apoferritin H chain: evidence for a multigene family. EMBO J. 1984, 3, 23–27. [CrossRef]
- Boyd, S. K. Jain, J. Crampton, K. J. Barrett, e J. Drysdale. Isolation and characterization of a cDNA clone for human ferritin heavy chain. Proc. Natl. Acad. Sci. U. S. A. 1984, 81, 4751–4755. [Google Scholar] [CrossRef]
- S. K. Jain, K. J. Barrett, D. Boyd, M. F. Favreau, J. Crampton, e J. W. Drysdale. Ferritin H and L chains are derived from different multigene families. J. Biol. Chem. 1985, 260, 11762–11768. [CrossRef]
- J. H. Caskey, C. Jones, Y. E. Miller, e P. A. Seligman. Human ferritin gene is assigned to chromosome 19. Proc. Natl. Acad. Sci. U. S. A. 1983, 80, 482–486. [CrossRef]
- Worwood; et al. Assignment of human ferritin genes to chromosomes 11 and 19q13.3----19qter. Hum. Genet. 1985, 69, 371–374. [Google Scholar] [CrossRef]
- S. Levi et al. Characterization of human ferritin H chain synthetized in Escherichia coli. Gene 51, fasc. 2– 1987, 3, 269–274. [CrossRef]
- S. Levi, J. Salfeld, F. Franceschinelli, A. Cozzi, M. H. Dorner, e P. Arosio. Expression and structural and functional properties of human ferritin L-chain from Escherichia coli. Biochemistry 1989, 28, 5179–5184. [CrossRef]
- S. Levi et al. Mechanism of ferritin iron uptake: activity of the H-chain and deletion mapping of the ferro-oxidase site. A study of iron uptake and ferro-oxidase activity of human liver, recombinant H-chain ferritins, and of two H-chain deletion mutants. J. Biol. Chem. 1988, 263, 18086–18092. [CrossRef]
- M. Lawson et al. Identification of the ferroxidase centre in ferritin. FEBS Lett. 254, fasc. 1– 1989, 2, 207–210. [CrossRef]
- M. Lawson et al. Solving the structure of human H ferritin by genetically engineering intermolecular crystal contacts. Nature 1991, 349, 541–544. [CrossRef] [PubMed]
- Aziz e H., N. Munro. Both subunits of rat liver ferritin are regulated at a translational level by iron induction. Nucleic Acids Res. 1986, 14, 915–927. [Google Scholar] [CrossRef] [PubMed]
- J. Zähringer, B. S. Baliga, e H. N. Munro. Novel mechanism for translational control in regulation of ferritin synthesis by iron. Proc. Natl. Acad. Sci. U. S. A. 1976, 73, 857–861. [CrossRef] [PubMed]
- W. Hentze et al. Identification of the iron-responsive element for the translational regulation of human ferritin mRNA. Science 1987, 238, 1570–1573. [CrossRef]
- W. E. Walden et al. Translational repression in eukaryotes: partial purification and characterization of a repressor of ferritin mRNA translation. Proc. Natl. Acad. Sci. U. S. A. 1988, 85, 9503–9507. [CrossRef]
- A. Leibold e H. N. Munro. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5’ untranslated region of ferritin heavy- and light-subunit mRNAs. Proc. Natl. Acad. Sci. U. S. A. 1988, 85, 2171–2175. [CrossRef]
- M. Konijn, N. Carmel, R. Levy, e C. Hershko. Ferritin synthesis in inflammation. II. Mechanism of increased ferritin synthesis. Br. J. Haematol. 1981, 49, 361–370. [CrossRef]
- H. Dörner, A. Silverstone, K. Nishiya, A. de Sostoa, G. Munn, e M. de Sousa. Ferritin synthesis by human T lymphocytes. Science 1980, 209, 1019–1021. [CrossRef]
- E. Broxmeyer, J. Bognacki, P. Ralph, M. H. Dörner, L. Lu, e H. Castro-Malaspina. Monocyte-macrophage-derived acidic isoferritins: normal feedback regulators of granulocyte-macrophage progenitor cells in vitro. Blood 1982, 60, 595–607. [CrossRef]
- E. Broxmeyer et al. Suppressive effects in vivo of purified recombinant human H-subunit (acidic) ferritin on murine myelopoiesis. Blood 1989, 73, 74–79. [CrossRef]
- M. Konijn, E. G. Meyron-Holtz, E. Fibach, e D. Gelvan. Cellular ferritin uptake: a highly regulated pathway for iron assimilation in human erythroid precursor cells. Adv. Exp. Med. Biol. 1994, 356, 189–197. [CrossRef]
- S. Pollack e T. Campana. Immature red cells have ferritin receptors. Biochem. Biophys. Res. Commun. 1981, 100, 1667–1672. [CrossRef] [PubMed]
- L. Francanzani et al. Immunohistochemical evidence for a lack of ferritin in duodenal absorptive epithelial cells in idiopathic hemochromatosis. Gastroenterology 1989, 96, 1071–1078. [CrossRef]
- Pietrangelo; et al. Duodenal ferritin synthesis in genetic hemochromatosis. Gastroenterology 1995, 108, 208–217. [Google Scholar] [CrossRef]
- K. Leichner, J. L. Klein, E. K. Fishman, S. S. Siegelman, D. S. Ettinger, e S. E. Order. Comparative tumor dose from 131I-labeled polyclonal anti-ferritin, anti-AFP, and anti-CEA in primary liver cancers. Cancer Drug Deliv. 1984, 1, 321–328. [CrossRef]
- M. Vriesendorp et al. Phase I-II studies of yttrium-labeled antiferritin treatment for end-stage Hodgkin’s disease, including Radiation Therapy Oncology Group 87-01. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1991, 9, 918–928. [CrossRef]
- W. Bottke, M. Burschyk, e J. Volmer. On the origin of the yolk protein ferritin in snails. Rouxs Arch. Dev. Biol. Off. Organ EDBO 1988, 197, 377–382. [CrossRef]
- M. Ragland, J. F. Briat, J. Gagnon, J. P. Laulhere, O. Massenet, e E. C. Theil. Evidence for conservation of ferritin sequences among plants and animals and for a transit peptide in soybean. J. Biol. Chem. 1990, 265, 18339–18344. [CrossRef]
- S. C. Andrews et al. Structure, function, and evolution of ferritins. J. Inorg. Biochem. 1992, 47, 161–174. [CrossRef]
- F. H. Kadir e G. R. Moore. Bacterial ferritin contains 24 haem groups. FEBS Lett. 1990, 271, 141–143. [CrossRef] [PubMed]
- M. Bozzi et al. A novel non-heme iron-binding ferritin related to the DNA-binding proteins of the Dps family in Listeria innocua. J. Biol. Chem. 1997, 272, 3259–3265. [CrossRef] [PubMed]
- R. A. Grant, D. J. Filman, S. E. Finkel, R. Kolter, e J. M. Hogle. The crystal structure of Dps, a ferritin homolog that binds and protects DNA. Nat. Struct. Biol. 1998, 5, 294–303. [CrossRef] [PubMed]
- Ilari, S. Stefanini, E. Chiancone, e D. Tsernoglou. The dodecameric ferritin from Listeria innocua contains a novel intersubunit iron-binding site. Nat. Struct. Biol. 2000, 7, 38–43. [Google Scholar] [CrossRef]
- M. J. Grossman, S. M. Hinton, V. Minak-Bernero, C. Slaughter, e E. I. Stiefel. Unification of the ferritin family of proteins. Proc. Natl. Acad. Sci. U. S. A. 1992, 89, 2419–2423. [CrossRef]
- MAZUR, I. LITT, e E. SHORR. Chemical properties of ferritin and their relation to its vasodepressor activity. J. Biol. Chem. 1950, 187, 473–484. [Google Scholar] [CrossRef]
- E. SHORR, S. BAEZ, B. W. ZWEIFACH, M. A. PAYNE, A. MAZUR, e D. B. METZ. The antidiuretic action of the hepatic vasodepressor ferritin (VDM) and its occurrence in conditions associated with antidiuresis in man. Trans. Assoc. Am. Physicians 1950, 63, 39–50.
- W. Reif. Ferritin as a source of iron for oxidative damage. Free Radic. Biol. Med. 1992, 12, 417–427. [CrossRef]
- G. Balla et al. Ferritin: a cytoprotective antioxidant strategem of endothelium. J. Biol. Chem. 1992, 267, 18148–18153. [CrossRef]
- M. A. Bevilacqua et al. A common mechanism underlying the E1A repression and the cAMP stimulation of the H ferritin transcription. J. Biol. Chem. 1997, 272, 20736–20741. [CrossRef]
- J. Wu, A. Polack, e R. Dalla-Favera. Coordinated regulation of iron-controlling genes, H-ferritin and IRP2, by c-MYC. Science 1999, 283, 676–679. [CrossRef] [PubMed]
- S. V. Torti e F. M. Torti. Human H-kininogen is a ferritin-binding protein. J. Biol. Chem. 1998, 273, 13630–13635. [CrossRef] [PubMed]
- M. R. Hasan, S. Koikawa, S. Kotani, S. Miyamoto, e H. Nakagawa. Ferritin forms dynamic oligomers to associate with microtubules in vivo: implication for the role of microtubules in iron metabolism. Exp. Cell Res. 2006, 312, 1950–1960. [CrossRef] [PubMed]
- Zarjou; et al. Ferritin prevents calcification and osteoblastic differentiation of vascular smooth muscle cells. J. Am. Soc. Nephrol. JASN 2009, 20, 1254–1263. [Google Scholar] [CrossRef]
- Siegert; et al. Ferritin-Mediated Iron Sequestration Stabilizes Hypoxia-Inducible Factor-1α upon LPS Activation in the Presence of Ample Oxygen. Cell Rep. 2015, 13, 2048–2055. [Google Scholar] [CrossRef]
- Park e S., W. Chung. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019, 10, 822. [Google Scholar] [CrossRef]
- M. A. Fernandez-Rojo et al. The heavy subunit of ferritin stimulates NLRP3 inflammasomes in hepatic stellate cells through ICAM-1 to drive hepatic inflammation. Sci. Signal. 2024, 17, eade4335. [CrossRef]
- Santambrogio; et al. Effects of modifications near the 2-, 3- and 4-fold symmetry axes on human ferritin renaturation. Biochem. J. 1997, 322, 461–468. [Google Scholar] [CrossRef]
- Santambrogio, S. Levi, A. Cozzi, B. Corsi, e P. Arosio. Evidence that the specificity of iron incorporation into homopolymers of human ferritin L- and H-chains is conferred by the nucleation and ferroxidase centres. Biochem. J. 1996, 314, 139–144. [Google Scholar] [CrossRef]
- G. S. Waldo, J. Ling, J. Sanders-Loehr, e E. C. Theil. Formation of an Fe(III)-tyrosinate complex during biomineralization of H-subunit ferritin. Science 1993, 259, 796–798. [CrossRef]
- Levi; et al. The role of the L-chain in ferritin iron incorporation. Studies of homo and heteropolymers. J. Mol. Biol. 1994, 238, 649–654. [Google Scholar] [CrossRef] [PubMed]
- H. Takagi, D. Shi, Y. Ha, N. M. Allewell, e E. C. Theil. Localized unfolding at the junction of three ferritin subunits. A mechanism for iron release?», J. Biol. Chem. 1998, 273, 18685–18688. [CrossRef]
- Douglas, D. P. Dickson, S. Betteridge, J. Charnock, C. D. Garner, e S. Mann. Synthesis and Structure of an Iron(III) Sulfide-Ferritin Bioinorganic Nanocomposite. Science 1995, 269, 54–57. [Google Scholar] [CrossRef] [PubMed]
- J. Thorpe, D. Walker, P. Arosio, A. Heath, J. D. Cook, e M. Worwood. International collaborative study to evaluate a recombinant L ferritin preparation as an International Standard. Clin. Chem. 1997, 43, 1582–1587. [CrossRef]
- M. W. Hentze e L. C. Kühn. Molecular control of vertebrate iron metabolism: mRNA-based regulatory circuits operated by iron, nitric oxide, and oxidative stress. Proc. Natl. Acad. Sci. U. S. A. 1996, 93, 8175–8182. [CrossRef]
- N. K. Gray e M. W. Hentze. Iron regulatory protein prevents binding of the 43S translation pre-initiation complex to ferritin and eALAS mRNAs. EMBO J. 1994, 13, 3882–3891. [CrossRef]
- M. Muckenthaler, N. K. Gray, e M. W. Hentze. IRP-1 binding to ferritin mRNA prevents the recruitment of the small ribosomal subunit by the cap-binding complex eIF4F. Mol. Cell 1998, 2, 383–388. [CrossRef]
- Girelli; et al. Molecular basis for the recently described hereditary hyperferritinemia-cataract syndrome: a mutation in the iron-responsive element of ferritin L-subunit gene (the “Verona mutation”). Blood 1995, 86, 4050–4053. [Google Scholar] [CrossRef]
- Beaumont; et al. Mutation in the iron responsive element of the L ferritin mRNA in a family with dominant hyperferritinaemia and cataract. Nat. Genet. 1995, 11, 444–446. [Google Scholar] [CrossRef]
- M. Cazzola et al. Hereditary hyperferritinemia-cataract syndrome: relationship between phenotypes and specific mutations in the iron-responsive element of ferritin light-chain mRNA. Blood 1997, 90, 814–821. [CrossRef]
- Cozzi, P. Santambrogio, S. Levi, e P. Arosio. Iron detoxifying activity of ferritin. Effects of H and L human apoferritins on lipid peroxidation in vitro. FEBS Lett. 1990, 277, 119–122. [Google Scholar] [CrossRef] [PubMed]
- X. Cai, D. E. Birk, e T. F. Linsenmayer. Ferritin is a developmentally regulated nuclear protein of avian corneal epithelial cells. J. Biol. Chem. 1997, 272, 12831–12839. [CrossRef] [PubMed]
- Pountney, G. Trugnan, M. Bourgeois, e C. Beaumont. The identification of ferritin in the nucleus of K562 cells, and investigation of a possible role in the transcriptional regulation of adult beta-globin gene expression. J. Cell Sci. 1999, 112, 825–831. [Google Scholar] [CrossRef] [PubMed]
- S. W. Hulet et al. Characterization and distribution of ferritin binding sites in the adult mouse brain. J. Neurochem. 1999, 72, 868–874. [CrossRef] [PubMed]
- Ferreira; et al. Early embryonic lethality of H ferritin gene deletion in mice. J. Biol. Chem. 2000, 275, 3021–3024. [Google Scholar] [CrossRef]
- R. Curtis et al. Mutation in the gene encoding ferritin light polypeptide causes dominant adult-onset basal ganglia disease. Nat. Genet. 2001, 28, 350–354. [CrossRef]
- S. Levi et al. A human mitochondrial ferritin encoded by an intronless gene», J. Biol. Chem. 2001, 276, 24437–24440.
- M. Cazzola et al. Mitochondrial ferritin expression in erythroid cells from patients with sideroblastic anemia. Blood 2003, 101, 1996–2000. [CrossRef]
- Santambrogio, G. Biasiotto, F. Sanvito, S. Olivieri, P. Arosio, e S. Levi. Mitochondrial ferritin expression in adult mouse tissues. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2007, 55, 1129–1137. [Google Scholar] [CrossRef]
- Maccarinelli; et al. Mice lacking mitochondrial ferritin are more sensitive to doxorubicin-mediated cardiotoxicity. J. Mol. Med. Berl. Ger. 2014, 92, 859–869. [Google Scholar] [CrossRef]
- M. Zancani et al. Evidence for the presence of ferritin in plant mitochondria. Eur. J. Biochem. 2004, 271, 3657–3664. [CrossRef] [PubMed]
- Missirlis, S. Holmberg, T. Georgieva, B. C. Dunkov, T. A. Rouault, e J. H. Law. Characterization of mitochondrial ferritin in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 5893–5898. [Google Scholar] [CrossRef] [PubMed]
- Cairo, L. Tacchini, G. Pogliaghi, E. Anzon, A. Tomasi, e A. Bernelli-Zazzera. Induction of ferritin synthesis by oxidative stress. Transcriptional and post-transcriptional regulation by expansion of the “free” iron pool. J. Biol. Chem. 1995, 270, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Y. Tsuji, H. Ayaki, S. P. Whitman, C. S. Morrow, S. V. Torti, e F. M. Torti. Coordinate transcriptional and translational regulation of ferritin in response to oxidative stress. Mol. Cell. Biol. 2000, 20, 5818–5827. [CrossRef] [PubMed]
- T. Chen et al. TIM-2 is expressed on B cells and in liver and kidney and is a receptor for H-ferritin endocytosis. J. Exp. Med. 2005, 202, 955–965. [CrossRef]
- Li; et al. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 3505–3510. [Google Scholar] [CrossRef]
- Mendes-Jorge; et al. L-ferritin binding to scara5: a new iron traffic pathway potentially implicated in retinopathy. PloS One 2014, 9, e106974. [Google Scholar] [CrossRef]
- E. Hamburger, A. P. J. West, Z. A. Hamburger, P. Hamburger, e P. J. Bjorkman. Crystal structure of a secreted insect ferritin reveals a symmetrical arrangement of heavy and light chains. J. Mol. Biol. 2005, 349, 558–569. [CrossRef]
- Johnson, D. Cascio, M. R. Sawaya, M. Gingery, e I. Schröder. Crystal structures of a tetrahedral open pore ferritin from the hyperthermophilic archaeon Archaeoglobus fulgidus. Struct. Lond. Engl. 2005, 13, 637–648. [Google Scholar] [CrossRef]
- E. Walden et al. Structure of dual function iron regulatory protein 1 complexed with ferritin IRE-RNA. Science 2006, 314, 1903–1908. [CrossRef]
- K. Srivastava, P. Arosio, M. Poli, e F. Bou-Abdallah. A Novel Approach for the Synthesis of Human Heteropolymer Ferritins of Different H to L Subunit Ratios. J. Mol. Biol. 2021, 433, 167198. [CrossRef]
- Bou-Abdallah, J. Fish, G. Terashi, Y. Zhang, D. Kihara, e P. Arosio. Unveiling the stochastic nature of human heteropolymer ferritin self-assembly mechanism. Protein Sci. Publ. Protein Soc. 2024, 33, e5104. [Google Scholar] [CrossRef] [PubMed]
- Shi, K. Z. Bencze, T. L. Stemmler, e C. C. Philpott. A cytosolic iron chaperone that delivers iron to ferritin. Science 2008, 320, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Z. Zaman e R. L. Verwilghen. Some properties of the purified ferritin reductase from the rat liver microsomes [proceedings]. Biochem. Soc. Trans. 1979, 7, 201–202. [CrossRef] [PubMed]
- T. Asano, M. Komatsu, Y. Yamaguchi-Iwai, F. Ishikawa, N. Mizushima, e K. Iwai. Distinct mechanisms of ferritin delivery to lysosomes in iron-depleted and iron-replete cells. Mol. Cell. Biol. 2011, 31, 2040–2052. [CrossRef]
- D. Mancias, X. Wang, S. P. Gygi, J. W. Harper, e A. C. Kimmelman. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 2014, 509, 105–109. [CrossRef]
- A. Cohen et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood 2010, 116, 1574–1584. [CrossRef]
- T. Kimura et al. Dedicated SNAREs and specialized TRIM cargo receptors mediate secretory autophagy. EMBO J. 2017, 36, 42–60. [CrossRef]
- Truman-Rosentsvit; et al. Ferritin is secreted via 2 distinct nonclassical vesicular pathways. Blood 2018, 131, 342–352. [Google Scholar] [CrossRef]
- G. Pham et al. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell 2004, 119, 529–542. [CrossRef]
- S. J. Dixon et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [CrossRef] [PubMed]
- R. Stockwell. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [CrossRef] [PubMed]
- Fang; et al. Loss of Cardiac Ferritin H Facilitates Cardiomyopathy via Slc7a11-Mediated Ferroptosis. Circ. Res. 2020, 127, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Vanoaica, D. Darshan, L. Richman, K. Schümann, e L. C. Kühn. Intestinal ferritin H is required for an accurate control of iron absorption. Cell Metab. 2010, 12, 273–282. [Google Scholar] [CrossRef]
- Vanoaica, L. Richman, M. Jaworski, D. Darshan, S. A. Luther, e L. C. Kühn. Conditional deletion of ferritin h in mice reduces B and T lymphocyte populations. PloS One, 2014. [Google Scholar] [CrossRef]
- L. Cremonesi et al. Case report: a subject with a mutation in the ATG start codon of L-ferritin has no haematological or neurological symptoms. J. Med. Genet. 2004, 41, e81. [CrossRef]
- Zarjou; et al. Ferritin ferroxidase activity: a potent inhibitor of osteogenesis. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2010, 25, 164–172. [Google Scholar] [CrossRef]
- C. Abt e O. Meucci. Regulation of neuronal ferritin heavy chain, a new player in opiate-induced chemokine dysfunction. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2011, 6, 466–476. [CrossRef]
- Marchetti; et al. Ferritin is used for iron storage in bloom-forming marine pennate diatoms. Nature 2009, 457, 467–470. [Google Scholar] [CrossRef]
- H. Botebol et al. Central role for ferritin in the day/night regulation of iron homeostasis in marine phytoplankton. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 14652–14657. [CrossRef]
- Rawat e D., D. Deheyn. Evidence that ferritin is associated with light production in the mucus of the marine worm Chaetopterus. Sci. Rep. 2016, 6, 36854. [Google Scholar] [CrossRef]
- H. Li, X. Xia, S. Cheng, J. Zang, Z. Wang, e M. Du. Oyster (Crassostrea gigas) ferritin relieves lead-induced liver oxidative damage via regulating the mitophagy. Int. J. Biol. Macromol. 2023, 253, 126965. [CrossRef]
- Cohen, H. Dafni, G. Meir, A. Harmelin, e M. Neeman. Ferritin as an endogenous MRI reporter for noninvasive imaging of gene expression in C6 glioma tumors. Neoplasia N. Y. N 2005, 7, 109–117. [Google Scholar] [CrossRef] [PubMed]
- V. Naumova, V. L. Yarnykh, N. Balu, H. Reinecke, C. E. Murry, e C. Yuan. Quantification of MRI signal of transgenic grafts overexpressing ferritin in murine myocardial infarcts. NMR Biomed. 2012, 25, 1187–1195. [CrossRef]
- Liße; et al. Engineered Ferritin for Magnetogenetic Manipulation of Proteins and Organelles Inside Living Cells. Adv. Mater. Deerfield Beach Fla 2017, 29, 42. [Google Scholar] [CrossRef]
- J. Kim, Y. Ko, J. H. Cho, e J. Cho. Organic field-effect transistor memory devices using discrete ferritin nanoparticle-based gate dielectrics. Small Weinh. Bergstr. Ger. 2013, 9, 3784–3791. [CrossRef]
- J. Russo e L. A. Passmore. Electron microscopy: Ultrastable gold substrates for electron cryomicroscopy. Science 2014, 346, 1377–1380. [CrossRef]
- Wang, Q. Liu, X. Huang, e J. Zhuang. Ferritin nanocages: a versatile platform for nanozyme design. J. Mater. Chem. B 2023, 11, 4153–4170. [Google Scholar] [CrossRef]
- A. Reutovich, A. K. Srivastava, P. Arosio, e F. Bou-Abdallah. Ferritin nanocages as efficient nanocarriers and promising platforms for COVID-19 and other vaccines development. Biochim. Biophys. Acta Gen. Subj. 2023, 1867, 130288. [CrossRef]
- Girelli, G. Marchi, F. Busti, e A. Vianello. Iron metabolism in infections: Focus on COVID-19. Semin. Hematol. 2021, 58, 182–187. [Google Scholar] [CrossRef]
- Mohanty, A. Parida, R. K. Raut, e R. K. Behera. Ferritin: A Promising Nanoreactor and Nanocarrier for Bionanotechnology. ACS Bio Med Chem Au 2022, 2, 258–281. [Google Scholar] [CrossRef]
- Zhu; et al. Ferritin-based nanomedicine for disease treatment. Med. Rev. 2023, 3, 49–74. [Google Scholar] [CrossRef]
- V. V. Sudarev et al. Ferritin self-assembly, structure, function, and biotechnological applications. Int. J. Biol. Macromol. 2023, 224, 319–343. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).