Submitted:
01 April 2024
Posted:
01 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. What Can (and Cannot) Be Expected from this Reading?
1.2. The Cryptic 'Poisonous' agent Causing Tobacco Mosaic Disease: Early 'Pre-Purification' Experiments towards Virus Separation
1.3. Tobamovirus Purification Over Time: How and Why
1.3.1. From Crystal-Like Needles to 'Ribonucleoprotein' Helices
1.3.2. Manifold Routes of Research and Technical Progress Thereafter
1.3.3. Novel Applications: Increasing Demand: Tobamovirus Particles as Tools
1.4. Tobamovirus Properties and Variability - Essential Knowledge
2. Techniques for Virion Enrichment and Storage, One by One
2.1. Grinding, Blending, and More: Plant Disruption
2.2. Getting Rid of Plant Stuff: Clarification
2.3. Precipitation of Virus Particles
2.4. Centrifugation
2.4.1. Differential Sedimentation
2.4.2. Continuous Ultracentrifugation
2.4.3. Density Gradient Centrifugation
2.4.4. Solubility Gradient Centrifugation
2.5. Chromatography
2.5.1. Flow-through Chromatography
2.5.2. Bind/Elute Chromatography
2.6. Finishing
2.6.1. Ultrafiltration
2.6.2. Lyophilization and Storage of Virus Preparations
3. Combined Recipes: Stirred, Shaken and Poured
3.1. Adaptating Purification Strategies to Specific Needs: Combined Protocols and a Few Pitfalls to Keep in Mind
3.2. Methods Worth Being Explored in Future Work
4. The Proven and the New - Back to the Future? A Case Study
4.1. Motivation and Challenge
4.2. Strategy
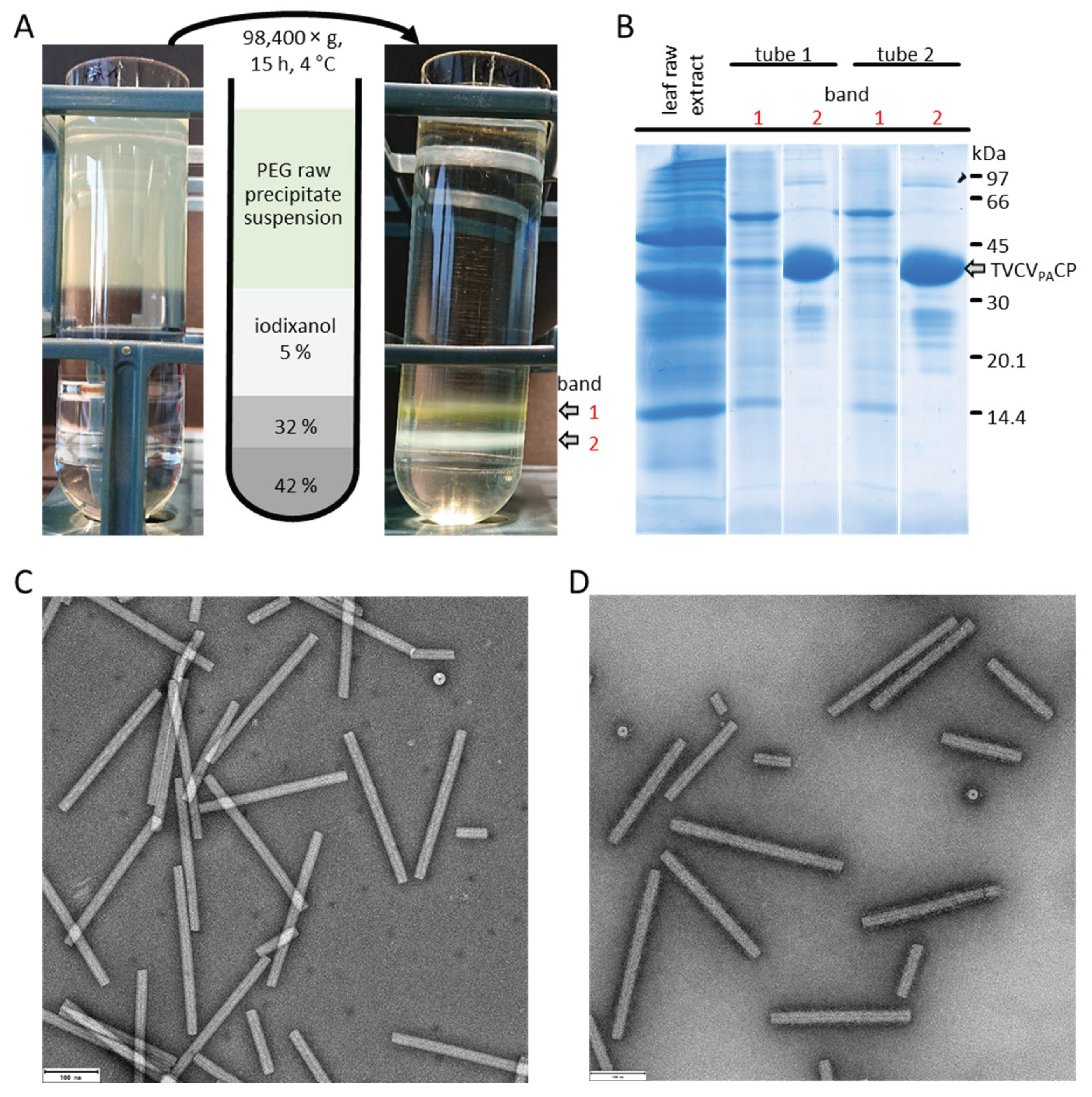
4.3. Results
4.4. Perspectives for the Novel Method
5. An Attempt to a Conclusion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
References
- Clark, F. Polyethylene glycol solubility gradients, a new and rapid technique for investigations of plant viruses. Virology 1970, 42, 246–247. [Google Scholar] [CrossRef]
- Clark, M.F.; Lister, R.M. The application of polyethylene glycol solubility-concentration gradients in plant virus research. Virology 1971, 43, 338–351. [Google Scholar] [CrossRef]
- Wendlandt, T.; Koch, C.; Britz, B.; Liedek, A.; Schmidt, N.; Werner, S.; Gleba, Y.; Vahidpour, F.; Welden, M.; Poghossian, A.; et al. Facile purification and use of tobamoviral nanocarriers for antibody-mediated display of a two-enzyme system. Viruses 2023, 15. [Google Scholar] [CrossRef]
- Altintoprak, K.; Seidenstücker, A.; Krolla-Sidenstein, P.; Plettl, A.; Jeske, H.; Gliemann, H.; Wege, C. RNA-stabilized protein nanorings: high-precision adapters for biohybrid design. Bioinspired, Biomimetic Nanobiomater. 2017, 6, 208–223. [Google Scholar] [CrossRef]
- Schenk, A.; Eiben, S.; Goll, M.; Reith, L.; Kulak, A.N.; Meldrum, F.; Jeske, H.; Wege, C.; Ludwigs, S. Virus-directed formation of electrocatalytically active nanoparticle-based Co3O4 tubes. Nanoscale 2017, 9, 6334–6345. [Google Scholar] [CrossRef]
- Schneider, A.; Eber, F.J.; Wenz, N.L.; Altintoprak, K.; Jeske, H.; Eiben, S.; Wege, C. Dynamic DNA-controlled "stop-and-go" assembly of well-defined protein domains on RNA-scaffolded TMV-like nanotubes. Nanoscale 2016, 8, 19853–19866. [Google Scholar] [CrossRef]
- Wenz, N.; Piasecka, S.; Kalinowski, M.; Schneider, A.; Richert, C.; Wege, C. Building expanded structures from tetrahedral DNA branching elements, RNA and TMV protein. Nanoscale 2018, 10, 6496–6510. [Google Scholar] [CrossRef]
- Eber, F.J.; Eiben, S.; Jeske, H.; Wege, C. RNA-controlled assembly of tobacco mosaic virus-derived complex structures: from nanoboomerangs to tetrapods. Nanoscale 2015, 7, 344–355. [Google Scholar] [CrossRef]
- Eber, F.J.; Eiben, S.; Jeske, H.; Wege, C. Bottom-up-assembled nanostar colloids of gold cores and tubes derived from tobacco mosaic virus. Angew. Chem. Int. Ed. Engl. 2013, 52, 7203–7207. [Google Scholar] [CrossRef]
- Eiben, S.; Stitz, N.; Eber, F.; Wagner, J.; Atanasova, P.; Bill, J.; Wege, C.; Jeske, H. Tailoring the surface properties of tobacco mosaic virions by the integration of bacterially expressed mutant coat protein. Virus Res. 2014, 180, 92–96. [Google Scholar] [CrossRef]
- Koch, C.; Eber, F.J.; Azucena, C.; Foerste, A.; Walheim, S.; Schimmel, T.; Bittner, A.; Jeske, H.; Gliemann, H.; Eiben, S.; et al. Novel roles for well-known players: from tobacco mosaic virus pests to enzymatically active assemblies. Beilstein J. Nanotechnol. 2016, 7, 613–629. [Google Scholar] [CrossRef]
- Altintoprak, K.; Seidenstücker, A.; Welle, A.; Eiben, S.; Atanasova, P.; Stitz, N.; Plettl, A.; Bill, J.; Gliemann, H.; Jeske, H.; et al. Peptide-equipped tobacco mosaic virus templates for selective and controllable biomineral deposition Beilstein J. Nanotechnol. 2015, 6, 1399–1412. [Google Scholar] [CrossRef]
- Geiger, F.C.; Eber, F.J.; Eiben, S.; Mueller, A.; Jeske, H.; Spatz, J.P.; Wege, C. TMV nanorods with programmed longitudinal domains of differently addressable coat proteins. Nanoscale 2013, 5, 3808–3816. [Google Scholar] [CrossRef]
- Atanasova, P.; Rothenstein, D.; Schneider, J.J.; Hoffmann, R.C.; Dilfer, S.; Eiben, S.; Wege, C.; Jeske, H.; Bill, J. Virus-templated synthesis of ZnO nanostructures and formation of field-effect transistors. Adv. Mater. 2011, 23, 4918–4922. [Google Scholar] [CrossRef]
- Mueller, A.; Eber, F.J.; Azucena, C.; Petershans, A.; Bittner, A.M.; Gliemann, H.; Jeske, H.; Wege, C. Inducible site-selective bottom-up assembly of virus-derived nanotube arrays on RNA-equipped wafers. ACS Nano 2011, 5, 4512–4520. [Google Scholar] [CrossRef]
- Kadri, A.; Maiss, E.; Amsharov, N.; Bittner, A.M.; Balci, S.; Kern, K.; Jeske, H.; Wege, C. Engineered Tobacco mosaic virus mutants with distinct physical characteristics in planta and enhanced metallization properties. Virus Res. 2011, 157, 35–46. [Google Scholar] [CrossRef]
- Mueller, A.; Kadri, A.; Jeske, H.; Wege, C. In vitro assembly of Tobacco mosaic virus coat protein variants derived from fission yeast expression clones or plants. J. Virol. Methods 2010, 166, 77–85. [Google Scholar] [CrossRef]
- Balci, S.; Bittner, A.M.; Schirra, M.; Thonke, K.; Sauer, R.; Hahn, K.; Kadri, A.; Wege, C.; Jeske, H.; Kern, K. Catalytic coating of virus particles with zinc oxide. Electrochimica Acta 2009, 54, 5149–5154. [Google Scholar] [CrossRef]
- Balci, S.; Leinberger, D.M.; Knez, M.; Bittner, A.M.; Boes, F.; Kadri, A.; Wege, C.; Jeske, H.; Kern, K. Printing and aligning mesoscale patterns of tobacco mosaic viruses on surfaces. Adv. Mater. 2008, 20, 2195–2200. [Google Scholar] [CrossRef]
- Balci, S.; Noda, K.; Bittner, A.M.; Kadri, A.; Wege, C.; Jeske, H.; Kern, K. Self-assembly of metal-virus nanodumbbells. Angew. Chem. Int. Ed. Engl. 2007, 46, 3149–3151. [Google Scholar] [CrossRef]
- Knez, M.; Bittner, A.M.; Boes, F.; Wege, C.; Jeske, H.; Maiss, E.; Kern, K. Biotemplate synthesis of 3-nm nickel and cobalt nanowires. Nano Lett. 2003, 3, 1079–1082. [Google Scholar]
- Knez, M.; Sumser, M.; Bittner, A.M.; Wege, C.; Jeske, H.; Kooi, S.; Burghard, M.; Kern, K. Electrochemical modification of individual nano-objects. Journal of Electroanalytical Chemistry 2002, 522, 70–74. [Google Scholar] [CrossRef]
- Steere, R.L. The purification of plant viruses. In Adv. Virus Res., Smith, K.M., Lauffer, M.A., Eds.; Academic Press: 1959; Volume 6, pp. 1–73.
- Stanley, W.M. Chemical studies on the virus of tobacco mosaic: VIII. The isolation of a crystalline protein possessing the properties of aucuba mosaic virus. J. Biol. Chem. 1937, 117, 325–340. [Google Scholar]
- Kreibig, U.; Wetter, C. Light diffraction of in vitro crystals of six tobacco mosaic viruses. Z. Naturforsch. Sect. C 1980, 35, 750–762. [Google Scholar] [CrossRef]
- Bos, L. Beijerinck's work on tobacco mosaic virus: historical context and legacy. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 1999, 354, 675–685. [Google Scholar] [CrossRef]
- Denyer, S.P.; Hodges, N.A. 12.4 Filtration sterilization. Russell, Hugo & Ayliffe's Principles and Practice of Disinfection, Preservation and Sterilization.
- Beijerinck, M.W. Über ein contagium vivum fluidum als Ursache der Fleckenkrankheit der Tabaksblätter. Verhandel. Koninkl. Akad. van Wettenschappen te Amsterdam 1898, 5, 3–21. [Google Scholar]
- Ivanowski, D. Ueber die Mosaikkrankheit der Tabakpflanze / Concerning the mosaic disease of the tobacco plant (translation). Bulletin de l’Académie Impériale des Sciences de Saint-Pétersbourg/ translated in: Phytopathological Classics, 1892; 35. [Google Scholar]
- Lecoq, H. Découverte du premier virus, le virus de la mosaïque du tabac : 1892 ou 1898 ? Comptes Rendus de l'Académie des Sciences - Series III - Sciences de la Vie 2001, 324, 929–933. [Google Scholar]
- Lustig, A.; Levine, A.J. One hundred years of virology. J. Virol. 1992, 66, 4629–4631. [Google Scholar] [CrossRef]
- Scholthof, K.B. Tobacco mosaic virus: a model system for plant biology. Annu. Rev. Phytopathol. 2004, 42, 13–34. [Google Scholar] [CrossRef]
- Creager, A.N.H. Tobacco Mosaic Virus and the History of Molecular Biology. Annual Review of Virology 2022, 9, 39–55. [Google Scholar] [CrossRef]
- Scholthof, K.-B.G.; Shaw, J.G.; Zaitlin, M. , (Eds.) Tobacco mosaic virus: One hundred years of contributions to virology. APS Press, The American Phytopathological Society: St Paul, Minnesota, USA, 1999.
- Bos, L. 100 years of virology: from vitalism via molecular biology to genetic engineering. Trends Microbiol. 2000, 8, 82–87. [Google Scholar] [CrossRef]
- van der Want, J.P.H.; Dijkstra, J. A history of plant virology. Arch. Virol. 2006, 151, 1467–1498. [Google Scholar] [CrossRef]
- Zerbini, F.M.; Kitajima, E.W. From Contagium vivum fluidum to Riboviria: A tobacco mosaic virus-centric history of virus taxonomy. Biomolecules 2022, 12, 1363. [Google Scholar] [CrossRef]
- Baur, E. Über die infektiöse Chlorose der Malvaceen. Kgl. Preuss. Akad. Wiss. 1906, 11–29. [Google Scholar]
- Wege, C.; Gotthardt, R.D.; Frischmuth, T.; Jeske, H. Fulfilling Koch's postulates for Abutilon mosaic virus. Arch. Virol. 2000, 145, 2217–2225. [Google Scholar] [CrossRef]
- Smith, E.F. Ueber die Mosaikkrankheit des Tabaks by Adolf Mayer. The Journal of Mycology 1894, 7, 382–385. [Google Scholar] [CrossRef]
- Koning, C.J. Die Flecken- oder Mosaikkrankheit des holländischen Tabaks. Zeitschrift für Pflanzenkrankheiten 1899, 9, 65–80. [Google Scholar]
- Bechhold, H. Die Gallertfiltration (Ultrafiltration.). Zeitschrift für Chemie und Industrie der Kolloide 1907, 2, 33–41. [Google Scholar]
- Holmes, F.O. Local lesions in tobacco mosaic. Bot. Gaz. 1929, 87, 39–55. [Google Scholar] [CrossRef]
- Purdy, H.A. Immunologic reactions with tobacco mosaic virus. J. Exp. Med. 1929, 49, 919–935. [Google Scholar] [CrossRef]
- Zaitlin, M.; Palukaitis, P. Advances in understanding plant viruses and virus diseases. Annu. Rev. Phytopathol. 2000, 38, 117–143. [Google Scholar] [CrossRef]
- Vinson, C. Possible chemical nature of tobacco mosaic virus. Science 1934, 79, 548–549. [Google Scholar] [CrossRef]
- Stanley, W.M.; Loring, H.S. The isolation of crystalline tobacco mosaic virus protein from diseased tomato plants. Science 1936, 83, 85. [Google Scholar] [CrossRef]
- Stanley, W.M. Isolation of a crystalline protein possessing the properties of tobacco-mosaic virus. Science 1935, 81, 644–645. [Google Scholar] [CrossRef]
- Stanley, W. Isolation and properties of tobacco mosaic and other virus proteins: harvey lecture, March 17, 1938. Bulletin of the New York Academy of Medicine 1938, 14, 398. [Google Scholar]
- Svedberg, T.; Rinde, H. The ultra-centrifuge, a new instrument for the determination of size and distribution of size of particle in amicroscopic colloids. J. Am. Chem. Soc. 1924, 46, 2677–2693. [Google Scholar]
- Biscoe, J.; Pickels, E.G.; Wyckoff, R.W. An air-driven ultracentrifuge for molecular sedimentation. J. Exp. Med. 1936, 64, 39–45. [Google Scholar] [CrossRef]
- Eriksson-Quensel, I.-B.; Svedberg, T. Sedimentation and electrophoresis of the tobacco mosaic virus protein. J. Am. Chem. Soc. 1936, 58, 1863–1867. [Google Scholar] [CrossRef]
- Wyckoff, R.W.G. The ultracentrifugal study of virus proteins. Proceedings of the American Philosophical Society 1937, 77, 455–462. [Google Scholar]
- Bawden, F.C.; Pirie, N.W.; Bernal, J.D.; Fankuchen, I. Liquid crystalline substances from virus infected plants. Nature 1936, 138, 1051–1052. [Google Scholar]
- Bawden, F.C.; Pirie, N.W. The isolation and some properties of liquid crystalline substances from solanaceous plants infected with three strains of tobacco mosaic virus. Proceedings of the Royal Society of London. Series B, Biological sciences 1937, 123, 274–320. [Google Scholar] [CrossRef]
- Pennazio, S.; Roggero, P. The discovery of the chemical nature of tobacco mosaic virus. Riv Biol 2000, 93, 253–281. [Google Scholar]
- Bernal, J.D.; Fankuchen, I. Structure types of protein 'crystals' from virus infected plants. Nature 1937, 139, 923–924. [Google Scholar]
- Kausche, G.; Guggisberg, H.; Wissler, A. Quantitative Untersuchung der Strömungsdoppel-brechung von Tabakmosaik-und Kartoffel-X-Virus. Naturwissenschaften 1939, 27, 303–304. [Google Scholar]
- Lauffer, M.A. The electro-optical effect in certain viruses. J. Am. Chem. Soc. 1939, 61, 2412–2416. [Google Scholar]
- Robinson, J. Shape of tobacco mosaic virus particles in solution. Nature 1939, 143, 923–926. [Google Scholar] [CrossRef]
- Robinson, J. Studies in the viscosity of colloids. I. The anomalous viscosity of dilute suspensions of rigid anisometric particles. Proceedings of the Royal Society of London. Series A. Mathematical and Physical Sciences 1939, 170, 519–550. [Google Scholar]
- Bernal, J.D.; Fankuchen, I. X-Ray and crystallographic studies of plant virus preparations : I. Introduction and preparation of specimens ii. Modes of aggregation of the virus particles. The Journal of General Physiology 1941, 25, 111–146. [Google Scholar] [CrossRef]
- Kausche, G. Über die Charakterisierung von pflanzlichen Virussolen mit kolloidem Gold. Naturwissenschaften 1938, 26, 445–445. [Google Scholar]
- Adams, M.; Fraden, S. Phase behavior of mixtures of rods (tobacco mosaic virus) and spheres (polyethylene oxide, bovine serum albumin). Biophys. J. 1998, 74, 669–677. [Google Scholar] [CrossRef]
- Liljeström, V.; Ora, A.; Hassinen, J.; Rekola, H.T.; Nonappa; Heilala, M. ; Hynninen, V.; Joensuu, J.J.; Ras, R.H.A.; Törmä, P.; et al. Cooperative colloidal self-assembly of metal-protein superlattice wires. Nature Communications 2017, 8, 671. [Google Scholar] [CrossRef]
- Wu, Z.; Mueller, A.; Degenhard, S.; Ruff, S.E.; Geiger, F.; Bittner, A.; Wege, C.; Krill III, C. Enhancing the magnetoviscosity of ferrofluids by the addition of biological nanotubes. ACS Nano 2010, 4, 4531–4538. [Google Scholar] [CrossRef]
- Khan, A.A.; Fox, E.K.; Gorzny, M.L.; Nikulina, E.; Brougham, D.F.; Wege, C.; Bittner, A.M. pH Control of the Electrostatic Binding of Gold and Iron Oxide Nanoparticles to Tobacco Mosaic Virus. Langmuir 2013, 29, 2094–2098. [Google Scholar] [CrossRef]
- Bawden, F.C.; Pirie, N.W. Methods for the purification of tomato bushy stunt and tobacco mosaic viruses. Biochem. J. 1943, 37, 66–70. [Google Scholar] [CrossRef]
- Pfankuch, E.; Hagenguth, K. Isolierung einiger pflanzlicher Viren. Naturwissenschaften 1943, 31, 370–371. [Google Scholar]
- Lauffer, M.A. The size and shape of tobacco mosaic virus particles1. J. Am. Chem. Soc. 1944, 66, 1188–1194. [Google Scholar]
- Gelderblom, H.R.; Krüger, D.H. Helmut Ruska (1908–1973): his role in the evolution of electron microscopy in the life sciences, and especially virology. In Advances in imaging and electron physics, Hawkes, P.W., Ed.; Elsevier: 2014; Volume Volume 182, pp. 1–94.
- Kausche, G.A.; Pfankuch, E.; Ruska, H. Die Sichtbarmachung von pflanzlichem Virus im Übermikroskop. Naturwissenschaften 1939, 27, 292–299. [Google Scholar]
- Creager, A.N. The life of a virus: Tobacco mosaic virus as an experimental model, 1930-1965; University of Chicago Press: 2002.
- Creager, A.N.; Morgan, G.J. After the double helix: Rosalind Franklin's research on Tobacco mosaic virus. Isis 2008, 99, 239–272. [Google Scholar] [CrossRef]
- Franklin, R.E.; Klug, A.; Holmes, K.C. X-ray diffraction studies of the structure and morphology of tobacco mosaic virus. In Proceedings of the Ciba Foundation Symposium on the Nature of Viruses, London; 1957; pp. 39–55. [Google Scholar]
- Klein, J. Max-Planck-Institut für Biologie, Tübingen: Die Geschichte des Instituts von 1912 bis 1983; Tübingen, 1983; p. 71.
- Schramm, G. Zur Chemie des Mutationsvorganges beim Tabakmosaik-Virus. Zeitschrift für Naturforschung B 1948, 3, 320–327. [Google Scholar]
- Lewis, J. From virus research to molecular biology: Tobacco mosaic virus in Germany, 1936-1956. Journal of the History of Biology 2004, 37, 259–301. [Google Scholar] [CrossRef]
- Fraenkel-Conrat, H.; Singer, B.; Williams, R. Infectivity of viral nucleic acid. Biochim. Biophys. Acta 1957, 25, 87–96. [Google Scholar] [CrossRef]
- Watanabe, I.; Kawade, Y. Purification and characterization of tobacco mosaic virus. Bulletin of the Chemical Society of Japan, 2006; 26. [Google Scholar] [CrossRef]
- Fukushi, T.; Shikata, E. Electron microscope studies on plant viruses I. Journal of the Faculty of Agriculture, Hokkaido University 1955, 50, 74–94. [Google Scholar]
- Ryjkoff, V.; Smirnova, M.V. Liquid crystals of the virus of the tobacco mosaic (Nicotiana virus 1 Allard). Doklady Akademii nauk SSSR 1941, 31, 930–932. [Google Scholar]
- Ryzkov, V.; Tarasevic, L.; Loïdina, G. The effect of strong solutions of sodium nucleinate on the virus of the mosaic disease of tobacco (VTM) and on albumen. Doklady Akademii nauk SSSR 1950, 74, 1023–1024. [Google Scholar]
- Carr, J.P. Tobacco mosaic virus. In Plant-Pathogen Interactions, Talbot, N.J., Ed.; Annual Plant Reviews; Blackwell Publishers: Oxford, U.K, 2004; pp. 25–67. [Google Scholar]
- Hamilton, R.I.; Edwardson, J.R.; Francki, R.I.B.; Hsu, H.T.; Hull, R.; Koenig, R.; Milne, R.G. Guidelines for the identification and characterization of plant viruses. J. Gen. Virol. 1981, 54, 223–241. [Google Scholar] [CrossRef]
- Van Regenmortel, M.H.V. Antigenic relationships between strains of tobacco mosaic virus. Virology 1975, 64, 415–420. [Google Scholar] [CrossRef]
- Van Regenmortel, M.H.V. Tobacco mosaic virus antigenic structure. In The plant viruses: The rod-shaped plant viruses, Van Regenmortel, M.H.V., Fraenkel-Conrat, H., Eds.; Springer US: Boston, MA, 1986; pp. 79–104. [Google Scholar]
- Smith, K.M. On the composite nature of certain potato virus diseases of the mosaic group as revealed by the use of plant indicators and selective methods of transmission. Proceedings of the Royal Society of London. Series B, Containing Papers of a Biological Character 1931, 109, 251–267. [Google Scholar] [CrossRef]
- Mehetre, G.T.; Leo, V.V.; Singh, G.; Sorokan, A.; Maksimov, I.; Yadav, M.K.; Upadhyaya, K.; Hashem, A.; Alsaleh, A.N.; Dawoud, T.M.; et al. Current developments and challenges in plant viral diagnostics: A systematic review. Viruses 2021, 13, 412. [Google Scholar] [CrossRef]
- Bernabé-Orts, J.M.; Torre, C.; Méndez-López, E.; Hernando, Y.; Aranda, M.A. New resources for the specific and sensitive detection of the emerging tomato brown rugose fruit virus. Viruses 2021, 13, 1680. [Google Scholar] [CrossRef]
- Mandal, S.; Mandal, B.; Haq, Q.; Varma, A. Properties, diagnosis and management of Cucumber green mottle mosaic virus. Plant viruses 2008, 2, 25–34. [Google Scholar]
- Wilkins, M.; Stokes, A.; Seeds, W.; Oster, G. Tobacco mosaic virus crystals and three-dimensional microscopic vision. Nature 1950, 166, 127–129. [Google Scholar] [CrossRef]
- Klug, A. The tobacco mosaic virus particle: structure and assembly. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 1999, 354, 531–535. [Google Scholar] [CrossRef]
- Boedtker, H.; Simmons, N.S. The preparation and characterization of essentially uniform tobacco mosaic virus particles. J. Am. Chem. Soc. 1958, 80, 2550–2556. [Google Scholar] [CrossRef]
- Kendall, A.; Stubbs, G. Fibre diffraction in the analysis of filamentous virus structure. Crystallogr. Rev. 2016, 22, 84–101. [Google Scholar] [CrossRef]
- Gebhardt, R.; Teulon, J.-M.; Pellequer, J.-L.; Burghammer, M.; Colletier, J.-P.; Riekel, C. Virus particle assembly into crystalline domains enabled by the coffee ring effect. Soft Matter 2014, 10, 5458–5462. [Google Scholar] [CrossRef]
- Wang, H.; Planchart, A.; Allen, D.; Pattanayek, R.; Stubbs, G. Preliminary X-ray diffraction studies of ribgrass mosaic virus. J. Mol. Biol. 1993, 234, 902–904. [Google Scholar] [CrossRef]
- Fromm, S.A.; Bharat, T.A.; Jakobi, A.J.; Hagen, W.J.; Sachse, C. Seeing tobacco mosaic virus through direct electron detectors. Journal of Structural Biology 2015, 189, 87–97. [Google Scholar] [CrossRef]
- Song, B.; Lenhart, J.; Flegler, V.J.; Makbul, C.; Rasmussen, T.; Böttcher, B. Capabilities of the Falcon III detector for single-particle structure determination. Ultramicroscopy 2019, 203, 145–154. [Google Scholar]
- Lewis, J.K.; Bendahmane, M.; Smith, T.J.; Beachy, R.N.; Siuzdak, G. Identification of viral mutants by mass spectrometry. Proc. Natl. Acad. Sci. USA 1998, 95, 8596–8601. [Google Scholar] [CrossRef]
- Lumata, J.L.; Ball, D.; Shahrivarkevishahi, A.; Luzuriaga, M.A.; Herbert, F.C.; Brohlin, O.; Lee, H.; Hagge, L.M.; D'Arcy, S.; Gassensmith, J.J. Identification and physical characterization of a spontaneous mutation of the tobacco mosaic virus in the laboratory environment. Sci. Rep. 2021, 11, 15109. [Google Scholar] [CrossRef]
- Gooding, G.V., Jr.; Hebert, T.T. A simple technique for purification of tobacco mosaic virus in large quantities. Phytopathology 1967, 57, 1285. [Google Scholar]
- Hebert, T.T. Precipitation of plant viruses by polyethylene glycol. Phytopathology 1963, 53, 362. [Google Scholar]
- Fraile, A.; Escriu, F.; Aranda, M.A.; Malpica, J.M.; Gibbs, A.J.; García-Arenal, F. A century of tobamovirus evolution in an Australian population of Nicotiana glauca. J. Virol. 1997, 71, 8316–8320. [Google Scholar] [CrossRef]
- Gibbs, A. Evolution and origins of tobamoviruses. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 1999, 354, 593–602. [Google Scholar] [CrossRef]
- Gibbs, A.J.; Wood, J.; Garcia-Arenal, F.; Ohshima, K.; Armstrong, J.S. Tobamoviruses have probably co-diverged with their eudicotyledonous hosts for at least 110 million years. Virus Evol. 2015, 1, vev019. [Google Scholar] [CrossRef]
- Wilson, T.M.A. Plant viruses: A tool-box for genetic engineering and crop protection. Bioessays 1989, 10, 179–186. [Google Scholar] [CrossRef]
- Haynes, J.R.; Cunningham, J.; von Seefried, A.; Lennick, M.; Garvin, R.T.; Shen, S.-H. Development of a genetically–engineered, candidate polio vaccine employing the self–assembling properties of the tobacco mosaic virus coat protein. Bio/Technology 1986, 4, 637. [Google Scholar] [CrossRef]
- Lomonossoff, G.P.; Wege, C. Chapter Six - TMV particles: the journey from fundamental studies to bionanotechnology applications. In Adv. Virus Res., Palukaitis, P., Roossinck, M.J., Eds.; Academic Press: 2018; Volume 102, pp. 149–176.
- Wen, A.M.; Lee, K.L.; Steinmetz, N.F. Plant Virus-Based Nanotechnologies. In Women in Nanotechnology: Contributions from the Atomic Level and Up, Norris, P.M., Friedersdorf, L.E., Eds.; Springer International Publishing: Cham, 2020; pp. 57–69. [Google Scholar]
- Zhang, J.; He, H.; Zeng, F.; Du, M.; Huang, D.; Chen, G.; Wang, Q. Advances of structural design and biomedical applications of tobacco mosaic virus coat protein. Adv. Nanobiomed Res. 2024, n/a, 2300135. [Google Scholar] [CrossRef]
- Fan, X.Z.; Pomerantseva, E.; Gnerlich, M.; Brown, A.; Gerasopoulos, K.; McCarthy, M.; Culver, J.; Ghodssi, R. Tobacco mosaic virus: A biological building block for micro/nano/biosystems. J. Vac. Sci. Technol., A 2013, 31, 050815. [Google Scholar] [CrossRef]
- Culver, J.N.; Brown, A.D.; Zang, F.; Gnerlich, M.; Gerasopoulos, K.; Ghodssi, R. Plant virus directed fabrication of nanoscale materials and devices. Virology 2015, 479–480, 200–212. [Google Scholar] [CrossRef]
- Wege, C.; Koch, C. From stars to stripes: RNA-directed shaping of plant viral protein templates—structural synthetic virology for smart biohybrid nanostructures. WIREs Nanomedicine and Nanobiotechnology 2020, 12, e1591. [Google Scholar] [CrossRef]
- Dragnea, B. Virus-based devices: prospects for allopoiesis. ACS Nano 2017, 11, 3433–3437. [Google Scholar] [CrossRef]
- Steele, J.F.C.; Peyret, H.; Saunders, K.; Castells-Graells, R.; Marsian, J.; Meshcheriakova, Y.; Lomonossoff, G.P. Synthetic plant virology for nanobiotechnology and nanomedicine. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology 2017, 9, e1447. [Google Scholar] [CrossRef]
- Lin, B.; Ratna, B. Virus hybrids as nanomaterials : methods and protocols; Humana Press - Springer: New York, Heidelberg, Dordrecht, London, 2014. [Google Scholar]
- Nkanga, C.I.; Steinmetz, N.F. The pharmacology of plant virus nanoparticles. Virology 2021, 556, 39–61. [Google Scholar]
- Czapar, A.E.; Steinmetz, N.F. Plant viruses and bacteriophages for drug delivery in medicine and biotechnology. Curr. Opin. Chem. Biol. 2017, 38, 108–116. [Google Scholar] [CrossRef]
- Khudyakov, Y.; Pumpens, P. Viral Nanotechnology; CRC Press, Taylor & Francis: Boca Raton, London, New York, 2016. [Google Scholar]
- Arul, S.S.; Balakrishnan, B.; Handanahal, S.S.; Venkataraman, S. Viral nanoparticles: Current advances in design and development. Biochimie 2024, 219, 33–50. [Google Scholar]
- Eiben, S.; Koch, C.; Altintoprak, K.; Southan, A.; Tovar, G.; Laschat, S.; Weiss, I.; Wege, C. Plant virus-based materials for biomedical applications: trends and prospects. Adv. Drug Del. Rev. 2019, 145, 96–118. [Google Scholar] [CrossRef]
- Chariou, P.L.; Ortega-Rivera, O.A.; Steinmetz, N.F. Nanocarriers for the Delivery of Medical, Veterinary, and Agricultural Active Ingredients. ACS Nano 2020, 14, 2678–2701. [Google Scholar] [CrossRef]
- Takeyama, N.; Kiyono, H.; Yuki, Y. Plant-based vaccines for animals and humans: recent advances in technology and clinical trials. Therapeutic Advances in Vaccines 2015, 3, 139–154. [Google Scholar] [CrossRef]
- Kolotilin, I.; Topp, E.; Cox, E.; Devriendt, B.; Conrad, U.; Joensuu, J.; Stoger, E.; Warzecha, H.; McAllister, T.; Potter, A.; et al. Plant-based solutions for veterinary immunotherapeutics and prophylactics. Vet. Res. 2014, 45, 117. [Google Scholar] [CrossRef]
- Chung, Y.H.; Church, D.; Koellhoffer, E.C.; Osota, E.; Shukla, S.; Rybicki, E.P.; Pokorski, J.K.; Steinmetz, N.F. Integrating plant molecular farming and materials research for next-generation vaccines. Nature Reviews Materials 2021, 1–17. [Google Scholar] [CrossRef]
- Crisci, E.; Bárcena, J.; Montoya, M. Virus-like particles: The new frontier of vaccines for animal viral infections. Vet. Immunol. Immunopathol. 2012, 148, 211–225. [Google Scholar] [CrossRef]
- Nikitin, N.; Vasiliev, Y.; Kovalenko, A.; Ryabchevskaya, E.; Kondakova, O.; Evtushenko, E.; Karpova, O. Plant Viruses as Adjuvants for Next-Generation Vaccines and Immunotherapy. Vaccines 2023, 11, 1372. [Google Scholar] [CrossRef]
- Williams, L.; Jurado, S.; Llorente, F.; Romualdo, A.; González, S.; Saconne, A.; Bronchalo, I.; Martínez-Cortes, M.; Pérez-Gómez, B.; Ponz, F.; et al. The C-Terminal Half of SARS-CoV-2 Nucleocapsid Protein, Industrially Produced in Plants, Is Valid as Antigen in COVID-19 Serological Tests. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef]
- Azizi, M.; Shahgolzari, M.; Fathi-Karkan, S.; Ghasemi, M.; Samadian, H. Multifunctional plant virus nanoparticles: An emerging strategy for therapy of cancer. WIREs Nanomedicine and Nanobiotechnology 2023, 15, e1872. [Google Scholar]
- Venkataraman, S.; Hefferon, K. Application of plant viruses in biotechnology, medicine, and human health. Viruses 2021, 13. [Google Scholar] [CrossRef]
- Chariou, P.L.; Ma, Y.; Hensley, M.; Rosskopf, E.N.; Hong, J.C.; Charudattan, R.; Steinmetz, N.F. Inactivated plant viruses as an agrochemical delivery platform. ACS Agricultural Science & Technology 2021, 1, 124–130. [Google Scholar] [CrossRef]
- Charudattan, R. Use of plant viruses as bioherbicides: the first virus-based bioherbicide and future opportunities. Pest Manage. Sci. 2024, 80, 103–114. [Google Scholar]
- Schlick, T.L.; Ding, Z.B.; Kovacs, E.W.; Francis, M.B. Dual-surface modification of the tobacco mosaic virus. J. Am. Chem. Soc. 2005, 127, 3718–3723. [Google Scholar] [CrossRef]
- González-Gamboa, I.; Caparco, A.A.; McCaskill, J.; Fuenlabrada-Velázquez, P.; Hays, S.S.; Jin, Z.; Jokerst, J.V.; Pokorski, J.K.; Steinmetz, N.F. Inter-coat protein loading of active ingredients into Tobacco mild green mosaic virus through partial dissociation and reassembly of the virion. Sci. Rep. 2024, 14, 7168. [Google Scholar] [CrossRef]
- Gulati, N.M.; Pitek, A.S.; Steinmetz, N.F.; Stewart, P.L. Cryo-electron tomography investigation of serum albumin-camouflaged tobacco mosaic virus nanoparticles. Nanoscale 2017, 9, 3408–3415. [Google Scholar] [CrossRef]
- Dickmeis, C.; Kauth, L.; Commandeur, U. From infection to healing: The use of plant viruses in bioactive hydrogels. WIREs Nanomed Nanobiotechnol. 2021, 13, e1662. [Google Scholar] [CrossRef]
- Maturavongsadit, P.; Luckanagul, J.A.; Metavarayuth, K.; Zhao, X.; Chen, L.M.; Lin, Y.; Wang, Q. Promotion of in vitro chondrogenesis of mesenchymal stem cells using in situ hyaluronic hydrogel functionalized with rod-like viral nanoparticles. Biomacromolecules 2016, 17, 1930–1938. [Google Scholar] [CrossRef]
- Schuphan, J.; Stojanović, N.; Lin, Y.-Y.; Buhl, E.M.; Aveic, S.; Commandeur, U.; Schillberg, S.; Fischer, H. A combination of flexible modified plant virus nanoparticles enables additive effects resulting in improved osteogenesis. Adv. Healthc. Mater. 2024, n/a, 2304243. [Google Scholar]
- Alonso, J.M.; Górzny, M.Ł.; Bittner, A.M. The physics of tobacco mosaic virus and virus-based devices in biotechnology. Trends Biotechnol. 2013, 31, 530–538. [Google Scholar] [CrossRef]
- Riekel, C.; Burghammer, M.; Snigirev, I.; Rosenthal, M. Microstructural metrology of tobacco mosaic virus nanorods during radial compression and heating. Soft Matter 2018, 14, 194–204. [Google Scholar] [CrossRef]
- Wu, L.Y.; Zang, J.F.; Lee, L.A.; Niu, Z.W.; Horvatha, G.C.; Braxtona, V.; Wibowo, A.C.; Bruckman, M.A.; Ghoshroy, S.; zur Loye, H.C.; et al. Electrospinning fabrication, structural and mechanical characterization of rod-like virus-based composite nanofibers. J Mater Chem 2011, 21, 8550–8557. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ölçeroğlu, E.; McCarthy, M. Biotemplates: Scalable nanomanufacturing of virus-templated coatings for enhanced boiling. Adv. Mater. Interfaces 2014, 1. [Google Scholar] [CrossRef]
- Poghossian, A.; Jablonski, M.; Koch, C.; Bronder, T.S.; Rolka, D.; Wege, C.; Schöning, M.J. Field-effect biosensor using virus particles as scaffolds for enzyme immobilization. Biosensors Bioelectron. 2018, 110, 168–174. [Google Scholar] [CrossRef]
- Koch, C.; Poghossian, A.; Schoening, M.J.; Wege, C. Penicillin detection by tobacco mosaic virus-assisted colorimetric biosensors. Nanotheranostics 2018, 2, 184–196. [Google Scholar] [CrossRef]
- Poghossian, A.; Jablonski, M.; Molinnus, D.; Wege, C.; Schoning, M.J. Field-effect sensors for virus detection: from Ebola to SARS-CoV-2 and plant viral enhancers. Front. Plant Sci. 2020, 11, 598103. [Google Scholar]
- Douglas, T.; Young, M. Virus particles as templates for materials synthesis. Adv. Mater. 1999, 11, 679–681. [Google Scholar] [CrossRef]
- Shenton, W.; Douglas, T.; Young, M.; Stubbs, G.; Mann, S. Inorganic-organic nanotube composites from template mineralization of tobacco mosaic virus. Adv. Mater. 1999, 11, 253–256. [Google Scholar] [CrossRef]
- Young, M.; Willits, D.; Uchida, M.; Douglas, T. Plant viruses as biotemplates for materials and their use in nanotechnology. Annu. Rev. Phytopathol. 2008, 46, 361–384. [Google Scholar] [CrossRef]
- Bittner, A.M.; Alonso, J.M.; Górzny, M.L.; Wege, C. Nanoscale science and technology with plant viruses and bacteriophages. In Structure and physics of viruses: an integrated textbook, Mateu, M.G., Ed.Harris, J.R., Ed.; Subcellular Biochemistry (); Springer Science+Business Media: Dordrecht, 2013; Volume 68, pp. 667–702. ISSN 0306-0225. [Google Scholar]
- Fan, X.Z.; Naves, L.; Siwak, N.P.; Brown, A.; Culver, J.; Ghodssi, R. Integration of genetically modified virus-like-particles with an optical resonator for selective bio-detection. Nanotechnology 2015, 26, 205501. [Google Scholar] [CrossRef]
- Soto, C.M.; Ratna, B.R. Virus hybrids as nanomaterials for biotechnology. Curr. Opin. Biotechnol. 2010, 21, 426–438. [Google Scholar] [CrossRef]
- Chen, Z.; Li, N.; Li, S.; Dharmarwardana, M.; Schlimme, A.; Gassensmith, J.J. Viral chemistry: the chemical functionalization of viral architectures to create new technology. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology 2016, 8, 512–534. [Google Scholar] [CrossRef]
- Chu, S.; Brown, A.D.; Culver, J.N.; Ghodssi, R. Tobacco mosaic virus as a versatile platform for molecular assembly and device fabrication. Biotechnol. J. 2018, 13, 1800147. [Google Scholar] [CrossRef]
- Balci, S.; Hahn, K.; Kopold, P.; Kadri, A.; Wege, C.; Kern, K.; Bittner, A.M. Electroless synthesis of 3 nm wide alloy nanowires inside Tobacco mosaic virus. Nanotechnology 2012, 23. [Google Scholar] [CrossRef]
- Bittner, A.M. TMV-Templated Formation of Metal and Polymer Nanotubes. In Virus-Derived Nanoparticles for Advanced Technologies: Methods and Protocols, Wege, C., Lomonossoff, G.P., Eds.; Springer New York: New York, NY, 2018; pp. 383–392. [Google Scholar]
- Shah, S.N.; Heddle, J.G.; Evans, D.J.; Lomonossoff, G.P. Production of Metallic Alloy Nanowires and Particles Templated Using Tomato Mosaic Virus (ToMV). Nanomaterials 2023, 13, 2705. [Google Scholar]
- Miller, R.A.; Stephanopoulos, N.; McFarland, J.M.; Rosko, A.S.; Geissler, P.L.; Francis, M.B. Impact of assembly state on the defect tolerance of TMV-based light harvesting arrays. J. Am. Chem. Soc. 2010, 132, 6068–6074. [Google Scholar] [CrossRef]
- Rong, J.H.; Oberbeck, F.; Wang, X.N.; Li, X.D.; Oxsher, J.; Niu, Z.W.; Wang, Q. Tobacco mosaic virus templated synthesis of one dimensional inorganic-polymer hybrid fibres. J Mater Chem 2009, 19, 2841–2845. [Google Scholar] [CrossRef]
- Dönmez Güngüneş, Ç.; Başçeken, S.; Elçin, A.E.; Elçin, Y.M. Fabrication and molecular modeling of navette-shaped fullerene nanorods using tobacco mosaic virus as a nanotemplate. Mol. Biotechnol. 2022, 64, 681–692. [Google Scholar]
- Riccò, R.; Liang, W.; Li, S.; Gassensmith, J.J.; Caruso, F.; Doonan, C.; Falcaro, P. Metal–organic frameworks for cell and virus biology: a perspective. ACS Nano 2018, 12, 13–23. [Google Scholar] [CrossRef]
- Moreira Da Silva, C.; Ortiz-Peña, N.; Boubekeur-Lecaque, L.; Dušek, J.; Moravec, T.; Alloyeau, D.; Ha-Duong, N.-T. In situ insights into the nucleation and growth mechanisms of gold nanoparticles on tobacco mosaic virus. Nano Lett. 2023, 23, 5281–5287. [Google Scholar] [CrossRef]
- Gerasopoulos, K.; McCarthy, M.; Royston, E.; Culver, J.N.; Ghodssi, R. Nanostructured nickel electrodes using the Tobacco mosaic virus for microbattery applications. J Micromech Microeng 2008, 18. [Google Scholar] [CrossRef]
- Royston, E.; Ghosh, A.; Kofinas, P.; Harris, M.T.; Culver, J.N. Self-assembly of virus-structured high surface area nanomaterials and their application as battery electrodes. Langmuir 2008, 24, 906–912. [Google Scholar] [CrossRef]
- Tseng, R.J.; Tsai, C.; Ma, L.P.; Ouyang, J.; Ozkan, C.S.; Yang, Y. Digital memory device based on tobacco mosaic virus conjugated with nanoparticles. Nature nanotechnology 2006, 1, 72–77. [Google Scholar] [CrossRef]
- Yang, C.X.; Choi, C.H.; Lee, C.S.; Yi, H.M. A facile synthesis-fabrication strategy for integration of catalytically active viral-palladium nanostructures into polymeric hydrogel microparticles via replica molding. ACS Nano 2013, 7, 5032–5044. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Epstein, J.; Brown, A.; Munday, J.N.; Culver, J.N.; Ehrman, S. Biological templates for antireflective current collectors for photoelectrochemical cell applications. Nano Lett. 2012, 12, 6005–6011. [Google Scholar] [CrossRef]
- Lee, K.Z.; Basnayake Pussepitiyalage, V.; Lee, Y.-H.; Loesch-Fries, L.S.; Harris, M.T.; Hemmati, S.; Solomon, K.V. Engineering tobacco mosaic virus and its virus-like-particles for synthesis of biotemplated nanomaterials. Biotechnol. J. 2021, 16, 2000311. [Google Scholar]
- Zhou, Q.; Liu, X.; Tian, Y.; Wu, M.; Niu, Z. Mussel-inspired polydopamine coating on tobacco mosaic virus: one-dimensional hybrid nanofibers for gold nanoparticle growth. Langmuir 2017, 33, 9866–9872. [Google Scholar] [CrossRef]
- Love, A.J.; Makarov, V.; Yaminsky, I.; Kalinina, N.O.; Taliansky, M.E. The use of tobacco mosaic virus and cowpea mosaic virus for the production of novel metal nanomaterials. Virology 2014, 449, 133–139. [Google Scholar] [CrossRef]
- Hou, C.; Xu, H.; Jiang, X.; Li, Y.; Deng, S.; Zang, M.; Xu, J.; Liu, J. Virus-based supramolecular structure and materials: concept and prospects. ACS Appl.. Bio Mater. 2021, 4, 5961–5974. [Google Scholar] [CrossRef]
- Love, A.J.; Makarov, V.V.; Sinitsyna, O.V.; Shaw, J.; Yaminsky, I.V.; Kalinina, N.O.; Taliansky, M. A genetically modified tobacco mosaic virus that can produce gold nanoparticles from a metal salt precursor. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Zakeri, B.; Fierer, J.O.; Celik, E.; Chittock, E.C.; Schwarz-Linek, U.; Moy, V.T.; Howarth, M. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proceedings of the National Academy of Sciences 2012, 109, E690–E697. [Google Scholar] [CrossRef]
- Schuphan, J.; Commandeur, U. Analysis of engineered tobacco mosaic virus and potato virus X nanoparticles as carriers for biocatalysts. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef]
- Lomonossoff, G.P.; Wege, C. Chapter Six - TMV Particles: The Journey From Fundamental Studies to Bionanotechnology Applications. In Advances in Virus Research, Vol. 102, Palukaitis, P., Roossinck, M.J., Eds.; Academic Press: 2018; Volume 102, pp. 149–176.
- Röder, J.; Fischer, R.; Commandeur, U. Adoption of the 2A ribosomal skip principle to tobacco mosaic virus for peptide display. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Frolova, O.Y.; Petrunia, I.V.; Komarova, T.V.; Kosorukov, V.S.; Sheval, E.V.; Gleba, Y.Y.; Dorokhov, Y.L. Trastuzumab-binding peptide display by Tobacco mosaic virus. Virology 2010, 407, 7–13. [Google Scholar] [CrossRef]
- Werner, S.; Marillonnet, S.; Hause, G.; Klimyuk, V.; Gleba, Y. Immunoabsorbent nanoparticles based on a tobamovirus displaying protein A. Proceedings of the National Academy of Sciences USA 2006, 103, 17678–17683. [Google Scholar] [CrossRef]
- Bäcker, M.; Koch, C.; Eiben, S.; Geiger, F.; Eber, F.J.; Gliemann, H.; Poghossian, A.; Wege, C.; Schöning, M.J. Tobacco mosaic virus as enzyme nanocarrier for electrochemical biosensors. Sens. Actuators, B 2017, 238, 716–722. [Google Scholar] [CrossRef]
- Koch, C.; Wabbel, K.; Eber, F.J.; Krolla-Sidenstein, P.; Azucena, C.; Gliemann, H.; Eiben, S.; Geiger, F.; Wege, C. Modified TMV particles as beneficial scaffolds to present sensor enzymes. Frontiers in Plant Science 2015, 6, 1137. [Google Scholar] [CrossRef]
- Welden, M.; Poghossian, A.; Vahidpour, F.; Wendlandt, T.; Keusgen, M.; Christina, W.; Schöning, M.J. Capacitive field-effect biosensor modified with a stacked bilayer of weak polyelectrolyte and plant virus particles as enzyme nanocarriers. Bioelectrochemistry 2023, 151, 108397. [Google Scholar]
- Welden, M.; Poghossian, A.; Vahidpour, F.; Wendlandt, T.; Keusgen, M.; Wege, C.; Schöning, M.J. Towards multi-analyte detection with field-effect capacitors modified with tobacco mosaic virus bioparticles as enzyme nanocarriers. Biosensors 2022, 12, 43. [Google Scholar] [CrossRef]
- Welden, M.; Severins, R.; Poghossian, A.; Wege, C.; Bongaerts, J.; Siegert, P.; Keusgen, M.; Schoning, M.J. Detection of acetoin and diacetyl by a tobacco mosaic virus-assisted field-effect biosensor. Chemosensors 2022, 10, 218. [Google Scholar]
- Zang, F.; Gerasopoulos, K.; Fan, X.Z.; Brown, A.D.; Culver, J.N.; Ghodssi, R. Real-time monitoring of macromolecular biosensing probe self-assembly and on-chip ELISA using impedimetric microsensors. Biosensors Bioelectron. 2016, 81, 401–407. [Google Scholar]
- Zang, F.; Gerasopoulos, K.; Brown, A.D.; Culver, J.N.; Ghodssi, R. Capillary microfluidics-assembled virus-like particle bionanoreceptor interfaces for label-free biosensing. ACS Appl. Mater. Interfaces 2017, 9, 8471–8479. [Google Scholar] [CrossRef]
- Grübel, J.; Wendlandt, T.; Urban, D.; Jauch, C.O.; Wege, C.; Tovar, G.E.M.; Southan, A. Soft sub-structured multi-material biosensor hydrogels with enzymes retained by plant viral scaffolds. Macromol Biosci 2023, e2300311. [Google Scholar] [CrossRef]
- Wege, C.; Koch, C. From stars to stripes: RNA-directed shaping of plant viral protein templates—structural synthetic virology for smart biohybrid nanostructures. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology 2020, 12, e1591. [Google Scholar]
- Paiva, T.O.; Schneider, A.; Bataille, L.; Chovin, A.; Anne, A.; Michon, T.; Wege, C.; Demaille, C. Enzymatic activity of individual bioelectrocatalytic viral nanoparticles: dependence of catalysis on the viral scaffold and its length. Nanoscale 2022, 14, 875–889. [Google Scholar]
- Yi, H.; Rubloff, G.W.; Culver, J.N. TMV microarrays: Hybridization-based assembly of DNA-programmed viral nanotemplates. Langmuir 2007, 23, 2663–2667. [Google Scholar] [CrossRef]
- Bruckman, M.A.; Steinmetz, N.F. Chemical Modification of the Inner and Outer Surfaces of Tobacco Mosaic Virus (TMV). In Virus Hybrids as Nanomaterials: Methods and Protocols, Lin, B., Ratna, B., Eds.; Humana Press: Totowa, NJ, 2014; pp. 173–185. [Google Scholar]
- Niu, Z.; Bruckman, M.A.; Li, S.; Lee, L.A.; Lee, B.; Pingali, S.V.; Thiyagarajan, P.; Wang, Q. Assembly of tobacco mosaic virus into fibrous and macroscopic bundled arrays mediated by surface aniline polymerization. Langmuir 2007, 23, 6719–6724. [Google Scholar] [CrossRef]
- Mukerrem, D.; Michael, H.B.S. A chemoselective biomolecular template for assembling diverse nanotubular materials. Nanotechnology 2002, 13, 541. [Google Scholar] [CrossRef]
- Dickmeis, C.; Altintoprak, K.; van Rijn, P.; Wege, C.; Commandeur, U. Bioinspired silica mineralization on viral templates. In Virus-Derived Nanoparticles for Advanced Technologies, Wege, C., Lomonossoff, G.P., Eds.Walker, J.M., Ed.; Methods in Molecular Biology; Humana Press, Springer Science+Business Media: Heidelberg, London, New York, 2018; Volume 1776, pp. 337–362. [Google Scholar]
- Sacco, A.; Barzan, G.; Matić, S.; Giovannozzi, A.M.; Rossi, A.M.; D’Errico, C.; Vallino, M.; Ciuffo, M.; Noris, E.; Portesi, C. Raman-dielectrophoresis goes viral: towards a rapid and label-free platform for plant virus characterization. Front. Microbiol. 2023, 14. [Google Scholar] [CrossRef]
- Saunders, K.; Thuenemann, E.C.; Shah, S.N.; Peyret, H.; Kristianingsih, R.; Lopez, S.G.; Richardson, J.; Lomonossoff, G.P. The use of a replicating virus vector for in planta generation of tobacco mosaic virus nanorods suitable for metallization. Frontiers in Bioengineering and Biotechnology 2022, 10, 877361. [Google Scholar] [CrossRef]
- Negrouk, V.; Eisner, G.; Midha, S.; Lee, H.-i.; Bascomb, N.; Gleba, Y. Affinity purification of streptavidin using tobacco mosaic virus particles as purification tags. Anal. Biochem. 2004, 333, 230–235. [Google Scholar]
- Dobrov, E.N.; Nikitin, N.A.; Trifonova, E.A.; Parshina, E.Y.; Makarov, V.V.; Maksimov, G.V.; Karpova, O.V.; Atabekov, J.G. β-structure of the coat protein subunits in spherical particles generated by tobacco mosaic virus thermal denaturation. J. Biomol. Struct. Dyn. 2014, 32, 701–708. [Google Scholar] [CrossRef]
- Atabekov, J.; Nikitin, N.; Arkhipenko, M.; Chirkov, S.; Karpova, O. Thermal transition of native tobacco mosaic virus and RNA-free viral proteins into spherical nanoparticles. J. Gen. Virol. 2011, 92, 453–456. [Google Scholar]
- Ambrico, M.; Ambrico, P.F.; Minafra, A.; De Stradis, A.; Vona, D.; Cicco, S.R.; Palumbo, F.; Favia, P.; Ligonzo, T. Highly sensitive and practical detection of plant viruses via electrical impedance of droplets on textured silicon-based devices. Sensors 2016, 16, 1946. [Google Scholar]
- Sachse, C.; Chen, J.Z.; Coureux, P.D.; Stroupe, M.E.; Fandrich, M.; Grigorieff, N. High-resolution electron microscopy of helical specimens: a fresh look at tobacco mosaic virus. J. Mol. Biol. 2007, 371, 812–835. [Google Scholar]
- Gregory, J.; Holmes, K.C. Methods of preparing orientated tobacco mosaic virus sols for X-ray diffraction. J. Mol. Biol. 1965, 13, 796–IN715. [Google Scholar] [CrossRef]
- Weis, F.; Beckers, M.; von der Hocht, I.; Sachse, C. Elucidation of the viral disassembly switch of tobacco mosaic virus. EMBO reports 2019, 20, e48451. [Google Scholar]
- Chapman, S.N. Tobamovirus Isolation and RNA Extraction. In Plant Virology Protocols: From Virus Isolation to Transgenic Resistance, Foster, G.D., Taylor, S.C., Eds.; Humana Press: Totowa, NJ, 1998; pp. 123–129. [Google Scholar]
- Martelli, G.P.; Russo, M. Plant virus inclusion bodies. In Adv. Virus Res., Lauffer, M.A., Bang, F.B., Maramorosch, K., Smith, K.M., Eds.; Academic Press: 1977; Volume 21, pp. 175–266.
- Dierking, I.; Al-Zangana, S. Lyotropic Liquid Crystal Phases from Anisotropic Nanomaterials. Nanomaterials 2017, 7, 305. [Google Scholar] [CrossRef]
- Bawden, F.C.; Sheffield, F.M.L. The intracellular inclusions of some plant virus diseases. Ann. Appl. Biol. 1939, 26, 102–115. [Google Scholar] [CrossRef]
- Wehrmeyer, W. Darstellung und Strukturordnung eines Tabakmosalkvirus-Einschlußkörpers in der Zelle. Naturwissenschaften 1957, 44, 519–520. [Google Scholar]
- Adams, M.J.; Adkins, S.; Bragard, C.; Gilmer, D.; Li, D.; MacFarlane, S.A.; Wong, S.-M.; Melcher, U.; Ratti, C.; Ryu, K.H.; Consortium, I.R. ICTV virus taxonomy profile: Virgaviridae. J. Gen. Virol. 2017, 98, 1999–2000. [Google Scholar] [CrossRef]
- Gibbs, A.J. Tobamovirus group CMI/AAB descriptions of plant viruses. Association of Applied Biologicts 1977, 184. [Google Scholar]
- Van Regenmortel, M.H.; Fraenkel-Conrat, H. The Plant Viruses: The rod-shaped plant viruses; Van Regenmortel, M.H., Fraenkel-Conrat, H., Eds.; New York Plenum Press: 1986.
- Melcher, U.; Lewandowski, D.J.; Dawson, W.O. Tobamoviruses (Virgaviridae). In Encyclopedia of Virology (Fourth Edition), Bamford, D.H., Zuckerman, M., Eds.; Academic Press: Oxford, 2021; pp. 734–742. [Google Scholar]
- Higgins, T.J.; Goodwin, P.B.; Whitfeld, P.R. Occurrence of short particles in beans infected with the cowpea strain of TMV. II. Evidence that short particles contain the cistron for coat-protein. Virology 1976, 71, 486–497. [Google Scholar] [CrossRef]
- Beachy, R.N.; Zaitlin, M. Characterization and in vitro translation of the RNAs from less-than-full-length, virus-related, nucleoprotein rods present in tobacco mosaic virus preparations. Virology 1977, 81, 160–169. [Google Scholar] [CrossRef]
- Fukuda, M.; Meshi, T.; Okada, Y.; Otsuki, Y.; Takebe, I. Correlation between particle multiplicity and location on virion RNA of the assembly initiation site for viruses of the tobacco mosaic virus group. Proc. Natl. Acad. Sci. USA 1981, 78, 4231–4235. [Google Scholar] [CrossRef]
- Kim, S.-M.; Lee, J.-M.; Yim, K.-O.; Oh, M.-H.; Park, J.-W.; Kim, K.-H. Nucleotide sequences of two korean isolates of cucumber green mottle mosaic virus. Molecules and Cells 2003, 16, 407–412. [Google Scholar] [CrossRef]
- Finch, J.T. The hand of the helix of tobacco mosaic virus. J. Mol. Biol. 1972, 66, 291–294. [Google Scholar] [CrossRef]
- Namba, K.; Pattanayek, R.; Stubbs, G. Visualization of protein-nucleic acid interactions in a virus. Refined structure of intact tobacco mosaic virus at 2.9 A resolution by X-ray fiber diffraction. J. Mol. Biol. 1989, 208, 307–325. [Google Scholar]
- Ilyas, R.; Rohde, M.J.; Richert-Pöggeler, K.R.; Ziebell, H. To be seen or not to be seen: Latent infection by tobamoviruses. Plants 2022, 11, 2166. [Google Scholar] [CrossRef]
- Stubbs, G. Tobacco mosaic virus particle structure and the initiation of disassembly. Phil. Trans. R. Soc. Lond. B 1999, 354, 551–557. [Google Scholar]
- Culver, J.N. Tobacco mosaic virus assembly and disassembly: Determinants in pathogenicity and resistance. Annu. Rev. Phytopathol. 2002, 40, 287–308. [Google Scholar]
- Wilson, T.M.A.; Perham, R.N.; Finch, J.T.; Butler, P.J.G. Polarity of the RNA in the tobacco mosaic virus particle and the direction of protein stripping in sodium dodecyl sulphate. FEBS Lett. 1976, 64, 285–289. [Google Scholar] [CrossRef]
- Goelet, P.; Lomonossoff, G.P.; Butler, P.J.; Akam, M.E.; Gait, M.J.; Karn, J. Nucleotide sequence of tobacco mosaic virus RNA. Proceedings of the National Academy of Sciences of the United States of America 1982, 79, 5818–5822. [Google Scholar]
- Wetter, C.; Conti, M.; Altschuh, D.; Tabillion, R.; Van Regenmortel, M. Pepper mild mottle virus, a tobamovirus infecting pepper cultivars in Sicily. Phytopathology 1984, 74, 405–410. [Google Scholar] [CrossRef]
- Silber, G.; Burk, L.G. Infectivity of tobacco mosaic virus stored for fifty years in extracted, ‘unpreserved’ plant juice. Nature 1965, 206, 740–741. [Google Scholar] [CrossRef]
- Fraile, A.; García-Arenal, F. Chapter four - Tobamoviruses as models for the study of virus evolution. In Adv. Virus Res., Palukaitis, P., Roossinck, M.J., Eds.; Academic Press: 2018; Volume 102, pp. 89–117.
- Lartey, R.T.; Voss, T.C.; Melcher, U. Tobamovirus evolution: gene overlaps, recombination, and taxonomic implications. Mol. Biol. Evol. 1996, 13, 1327–1338. [Google Scholar] [CrossRef]
- Min, B.E.; Chung, B.N.; Kim, M.J.; Ha, J.H.; Lee, B.Y.; Ryu, K.H. Cactus mild mottle virus is a new cactus-infecting tobamovirus. Arch. Virol. 2006, 151, 13–21. [Google Scholar] [CrossRef]
- Dombrovsky, A.; Smith, E. Seed transmission of Tobamoviruses: Aspects of global disease distribution; 2017; Volume 12, pp. 233–260.
- Dombrovsky, A.; Tran-Nguyen, L.T.T.; Jones, R.A.C. Cucumber green mottle mosaic virus: Rapidly increasing global distribution, etiology, epidemiology, and management. Annu. Rev. Phytopathol. 2017, 55, 231–256. [Google Scholar] [CrossRef]
- Ishibashi, K.; Kubota, K.; Kano, A.; Ishikawa, M. Tobamoviruses: old and new threats to tomato cultivation. J. Gen. Plant Pathol. 2023, 89, 305–321. [Google Scholar] [CrossRef]
- 231. Anses. Expert Committee on "Biological risks for plant health" ToBRFV Working Group Request No. 2019-SA-0080 ToBRFV Express pest risk analysis of tomato brown rugose fruit virus for France. Opinion Collective Expert Appraisal Report.
- Zhang, S.; Griffiths, J.S.; Marchand, G.; Bernards, M.A.; Wang, A. Tomato brown rugose fruit virus: An emerging and rapidly spreading plant RNA virus that threatens tomato production worldwide. Mol. Plant Pathol. 2022, 23, 1262–1277. [Google Scholar] [CrossRef]
- Salem, N.M.; Jewehan, A.; Aranda, M.A.; Fox, A. Tomato brown rugose fruit virus pandemic. Annu. Rev. Phytopathol. 2023, 61, 137–164. [Google Scholar] [CrossRef]
- Matthews, R.E. Effects of some purine analogues on tobacco mosaic virus. Journal of General Microbiology 1954, 10, 521–532. [Google Scholar] [CrossRef]
- Commoner, B.; Shearer, G.B.; Yamada, M. Linear biosynthesis of tobacco mosaic virus: changes in rod length during the course of infection. Proc. Natl. Acad. Sci. USA 1962, 48, 1788–1795. [Google Scholar] [CrossRef]
- Kaper, J.M.; Siberg, R.A. The effect of freezing on the structure of turnip yellow mosaic virus and a number of other simple plant viruses: An ultracentrifugal analysis. Cryobiology 1969, 5, 366–374. [Google Scholar] [CrossRef]
- Mundry, K.-W. Die Abhängigkeit des Auftretens neuer Virusstämme von der Kulturtemperatur der Wirtspflanzen. Zeitschrift für Induktive Abstammungs- und Vererbungslehre 1957, 88, 407–426. [Google Scholar] [CrossRef]
- Wetter, C. Die Flüssigkristalle des Tabakmosaikvirus. Biologie in unserer Zeit 1985, 15, 81–89. [Google Scholar] [CrossRef]
- Zaitlin, M. Tobacco mosaic virus CMI/AAB descriptions of plant viruses. Association of Applied Biologicts 2000, 370. [Google Scholar]
- Bawden, F.; Pirie, N. The separation and properties of tobacco mosaic virus in different states of aggregation. B.r J. Exp. Pathol. 1945, 26, 294. [Google Scholar]
- McNulty, M.J.; Schwartz, A.; Delzio, J.; Karuppanan, K.; Jacobson, A.; Hart, O.; Dandekar, A.; Giritch, A.; Nandi, S.; Gleba, Y.; McDonald, K.A. Affinity sedimentation and magnetic separation with plant-made immunosorbent nanoparticles for therapeutic protein purification. Frontiers in Bioengineering and Biotechnology 2022, 10, 865481. [Google Scholar] [CrossRef]
- Devash, Y.; Hauschner, A.; Sela, I.; Chakraburtty, K. The antiviral factor (AVF) from virus-infected plants induces discharge of histidinyl-TMV-RNA. Virology 1981, 111, 103–112. [Google Scholar] [CrossRef]
- Zaitlin, M.; Israel, H.W. Tobacco mosaic virus (type strain). AAB Descriptions of Plant Viruses.
- Stanley, W.M. Chemical studies on the virus of tobacco mosaic. VI. The isolation from diseased Turkish tobacco plants of a crystalline protein possessing the properties of tobacco mosaic virus. Phytopathology 1936, 26, 305–320. [Google Scholar]
- Leberman, R. The isolation of plant viruses by means of “simple” coacervates. Virology 1966, 30, 341–347. [Google Scholar]
- Wyckoff, R.W.G.; Biscoe, J.; Stanley, W.M. An ultracentrifugal analysis of the crystalline virus proteins isolated from plants diseased with different strains of tobacco mosaic virus. J. Biol. Chem. 1937, 117, 57–71. [Google Scholar] [CrossRef]
- Mächtle, W.; Börger, L. Analytical ultracentrifugation of polymers and nanoparticles; Springer laboratory: Springer, Berlin, New York, 2006. [Google Scholar]
- Wyckoff, R.W.G.; Corey, R.B. The ultracentrifugal crystallization of tobacco mosaic virus protein. Science 1936, 84, 513–513. [Google Scholar] [CrossRef]
- Stanley, W.M. Chemical studies on the virus of tobacco mosaic: X. The activity and yield of virus protein from plants diseased for different periods of time. J. Biol. Chem. 1937, 121, 205–217. [Google Scholar] [CrossRef]
- Stanley, W.M. Chemical studies on the virus of tobacco mosaic: IX. Correlation of virus activity and protein on centrifugation of protein from solution under various conditions. J. Biol. Chem. 1937, 117, 755–770. [Google Scholar] [CrossRef]
- Stanley, W.M.; Wyckoff, R.W.G. The isolation of tobacco ring spot and other virus proteins by ultracentrifugation. Science 1937, 85, 181–183. [Google Scholar] [CrossRef]
- Wyckoff, R.W.G. An ultracentrifugal study of the pH stability of tobacco mosaic virus protein. J. Biol. Chem. 1937, 122, 239–247. [Google Scholar] [CrossRef]
- Lauffer, M.A. Ultracentrifugation studies on tobacco mosaic and bushy stunt viruses. The Journal of Physical Chemistry 1940, 44, 1137–1146. [Google Scholar] [CrossRef]
- Schachman, H.K.; Kauzmann, W.J. Viscosity and sedimentation studies on tobacco mosaic virus. The Journal of Physical and Colloid Chemistry 1949, 53, 150–161. [Google Scholar] [CrossRef]
- Williams, R.C.; Steere, R.L. Electron microscopic observations on the unit of length of the particles of tobacco mosaic virus1. J. Am. Chem. Soc. 1951, 73, 2057–2061. [Google Scholar] [CrossRef]
- Francki, R.; McLean, G. Purification of potato virus X and preparation of infectious ribonucleic acid by degradation with lithium chloride. Australian J. biol. Sci. 1968, 21, 1311–1318. [Google Scholar] [CrossRef]
- Bruening, G.; Beachy, R.N.; Scalla, R.; Zaitlin, M. In vitro and in vivo translation of the ribonucleic acids of a cowpea strain of tobacco mosaic virus. Virology 1976, 71, 498–517. [Google Scholar] [CrossRef]
- Gardner, R.C.; Shepherd, R.J. A procedure for rapid isolation and analysis of cauliflower mosaic virus DNA. Virology 1980, 106, 159–161. [Google Scholar] [CrossRef]
- Jensen, S.M.; Nguyen, C.T.; Jewett, J.C. A gradient-free method for the purification of infective dengue virus for protein-level investigations. J. Virol. Methods 2016, 235, 125–130. [Google Scholar] [CrossRef]
- Stanley, W.M. The concentration and purification of tobacco mosaic virus by means of the sharples super-centrifuge. J. Am. Chem. Soc. 1942, 64, 1804–1806. [Google Scholar] [CrossRef]
- McAleer, W.J.; Hurni, W.; Wasmuth, E.; Hilleman, M.R. High-resolution flow-zonal centrifuge system. Biotechnol. Bioeng. 1979, 21, 317–321. [Google Scholar] [CrossRef]
- Brakke, M.K. Density gradient centrifugation and its application to plant viruses. In Adv. Virus Res.; Advances in Virus Research; Elsevier: 1961; Volume 7, pp. 193–224.
- Brakke, M.K. Density gradient centrifugation: a new separation technique1. J. Am. Chem. Soc. 1951, 73, 1847–1848. [Google Scholar] [CrossRef]
- Hills, G.J. Partial crystallization of tobacco mosaic virus. Virology 1959, 7, 239–240. [Google Scholar] [CrossRef]
- Brakke, M.K. Nonideal sedimentation and the capacity of sucrose gradient columns for virus in density-gradient centrifugation. Archives of Biochemistry and Biophysics 1964, 107, 388–403. [Google Scholar] [CrossRef]
- Brakke, M.K.; Van Pelt, N. Linear-log sucrose gradients for estimating sedimentation coefficients of plant viruses and nucleic acids. Anal. Biochem. 1970, 38, 56–64. [Google Scholar] [CrossRef]
- Saunders, K.; Thuenemann, E.C.; Peyret, H.; Lomonossoff, G.P. The tobacco mosaic virus origin of assembly sequence is dispensable for specific viral rna encapsidation but necessary for initiating assembly at a single site. J. Mol. Biol. 2022, 434, 167873. [Google Scholar] [CrossRef]
- Liu, X.; Wu, F.; Tian, Y.; Wu, M.; Zhou, Q.; Jiang, S.; Niu, Z. Size Dependent Cellular Uptake of Rod-like Bionanoparticles with Different Aspect Ratios. Sci. Rep. 2016, 6, 24567. [Google Scholar] [CrossRef]
- Perham, R.N. Sucrose density-gradient analysis of the alkaline degradation of tobacco mosaic virus. J. Mol. Biol. 1969, 45, 439–441. [Google Scholar] [CrossRef]
- Siegel, A.; Hudson, W. Equilibrium centrifugation of two strains of tobacco mosaic virus in density gradients. Biochim. Biophys. Acta 1959, 34, 254–255. [Google Scholar] [CrossRef]
- Gugerli, P. Isopycnic centrifugation of plant viruses in Nycodenz® density gradients. J. Virol. Methods 1984, 9, 249–258. [Google Scholar] [CrossRef]
- Skotnicki, A.; Scotti, P.D.; Gibbs, A. On the nature of the difference in the densities of the particles of two tobamoviruses. Intervirology 1976, 7, 292–302. [Google Scholar] [CrossRef]
- Rickwood, D.; Ford, T.; Graham, J. Nycodenz: A new nonionic iodinated gradient medium. Anal. Biochem. 1982, 123, 23–31. [Google Scholar] [CrossRef]
- Rickwood, D.; Birnie, G. Metrizamide, a new density-gradient medium. FEBS Lett. 1975, 50, 102–110. [Google Scholar]
- Pertoft, H.; Philipson, L.; Oxelfelt, P.; Höglund, S. Gradient centrifugation of viruses in colloidal silica. Virology 1967, 33, 185–196. [Google Scholar] [CrossRef]
- Pertoft, H. Fractionation of cells and subcellular particles with Percoll. J. Biochem. Biophys. Methods 2000, 44, 1–30. [Google Scholar] [CrossRef]
- Prucha, M.J.; Tanner, F.W. TIME SAVING BACTERIOLOGICAL APPARATUS. J. Bacteriol. 1920, 5, 559–563. [Google Scholar] [CrossRef]
- Arrua, R.D.; Strumia, M.C.; Alvarez Igarzabal, C.I. Macroporous Monolithic Polymers: Preparation and Applications. Materials 2009, 2, 2429–2466. [Google Scholar]
- Turpeinen, D.G.; Joshi, P.U.; Kriz, S.A.; Kaur, S.; Nold, N.M.; O'Hagan, D.; Nikam, S.; Masoud, H.; Heldt, C.L. Continuous purification of an enveloped and non-enveloped viral particle using an aqueous two-phase system. Separation and Purification Technology 2021, 269, 118753. [Google Scholar] [CrossRef]
- McLean, G.D.; Francki, R.I.B. Purification of lettuce necrotic yellows virus by column chromatography on calcium phosphate gel. Virology 1967, 31, 585–591. [Google Scholar] [CrossRef]
- Mahy, B.W.J. Virology: A practical approach, Reprint. ed, ed.; IRL Press: Oxford, 1985. [Google Scholar]
- Francki, R.; Zaitlin, M.; Grivell, C. An unusual strain of tobacco mosaic virus from Plumeria acutifolia. Australian J. biol. Sci. 1971, 24, 811–814. [Google Scholar] [CrossRef]
- Cochran, G. A chromatographic method for the detection of tobacco-mosaic virus in juice from diseased Turkish tobacco plants. Phytopathology 1947, 37, 850–851. [Google Scholar]
- Gray, R.A. The electrophoresis and chromatography of plant viruses on filter paper. Arch. Biochem. Biophys. 1952, 38, 305–316. [Google Scholar] [CrossRef]
- Barton, R.J. An examination of permeation chromatography on columns of controlled pore glass for routine purification of plant viruses. J. Gen. Virol. 1977, 35, 77–87. [Google Scholar] [CrossRef]
- Čech, M.; Jelinkova, M.; Čoupek, J. High-pressure chromatography of tobacco mosaic virus on Spheron gels. J. Chromatogr. 1977, 135, 435–440. [Google Scholar] [CrossRef]
- Brunt, A.; Phillips, S.; Jones, R.; Kenten, R. Viruses detected in Ullucus tuberosus (Basellaceae) from Peru and Bolivia. Ann. Appl. Biol. 1982, 101, 65–71. [Google Scholar] [CrossRef]
- Orita, H.; Sakai, J.-I.; Kubota, K.; Okuda, M.; Tanaka, Y.; Hanada, K.; Imamura, Y.; Nishiguchi, M.; Karasev, A.V.; Miyata, S.-I. Molecular and serological characterization of cucumber mottle virus, a new cucurbit-infecting tobamo-like virus. Plant Dis. 2007, 91, 1574–1578. [Google Scholar] [CrossRef]
- Cochran, G.W.; Chidester, J.L.; Stocks, D.L. Chromatography of tobacco mosaic virus on a cellulose cation exchange adsorbent. Nature 1957, 180, 1281–1282. [Google Scholar] [CrossRef]
- Commoner, B.; Lippincott, J.A.; Shearer, G.B.; Richman, E.E.; Wu, J.-H. Reconstitution of tobacco mosaic virus components. Nature 1956, 178, 767–771. [Google Scholar] [CrossRef]
- Levin, Ö. Chromatography of tobacco mosaic virus and potato virus X. Arch. Biochem. Biophys. 1958, 78, 33–45. [Google Scholar] [CrossRef]
- Ruščić, J.; Gutiérrez-Aguirre, I.; Tušek Žnidarič, M.; Kolundžija, S.; Slana, A.; Barut, M.; Ravnikar, M.; Krajačić, M. A new application of monolithic supports: The separation of viruses from one another. J. Chromatogr. 2015, 1388, 69–78. [Google Scholar] [CrossRef]
- Krajacic, M.; Ravnikar, M.; Štrancar, A.; Gutiérrez-Aguirre, I. Application of monolithic chromatographic supports in virus research. Electrophoresis 2017, 38, 2827–2836. [Google Scholar] [CrossRef]
- Kramberger, P.; Petrovič, N.; Štrancar, A.; Ravnikar, M. Concentration of plant viruses using monolithic chromatographic supports. J. Virol. Methods 2004, 120, 51–57. [Google Scholar] [CrossRef]
- Kramberger, P.; Peterka, M.; Boben, J.; Ravnikar, M.; Štrancar, A. Short monolithic columns-A breakthrough in purification and fast quantification of tomato mosaic virus. J. Chromatogr. 2007, 1144, 143–149. [Google Scholar] [CrossRef]
- Fraser, D.; Johnson, F. The influence of buffer composition, pH and aggregation on the thermal denaturation of tobacco mosaic virus. Arch. Biochem. 1949, 24, 338–349. [Google Scholar]
- Desjardins, P.R.; French, J.V. Storage of purified plant viruses in the unfrozen state free of microbial contamination. Experientia 1969, 25, 444–446. [Google Scholar] [CrossRef]
- Best, R.J. Longevity of tobacco mosaic virus: Part I. in vitro life of the pure virus in buffer solution at pH 4. Aust. J. Exp. Bio.l Med. Sci. 1948, 26, 163–170. [Google Scholar] [CrossRef]
- Byrne, N.; Rodoni, B.; Constable, F.; Varghese, S.; Davis, J.H. Enhanced stabilization of the tobacco mosaic virus using protic ionic liquids. Physical Chemistry Chemical Physics 2012, 14, 10119–10121. [Google Scholar] [CrossRef]
- Rice, R.V.; Kaesberg, P.; Stahmann, M.A. Further studies concerning the breaking of tobacco mosaic virus. Biochim. Biophys. Acta 1956, 20, 488–496. [Google Scholar] [CrossRef]
- Donev, T.; Yordanova, A.; Stoimenova, E.; Damjanova, S. Determination of parameters for tobacco mosaic virus cryogenic treatment and freeze-drying. Biotechnol. Tech. 1996, 10, 971–976. [Google Scholar] [CrossRef]
- Wacker, W.E.; Gordon, M.P.; Huff, J.W. Metal content of tobacco mosaic virus and tobacco mosaic virus RNA. Biochemistry 1963, 2, 716–719. [Google Scholar] [CrossRef]
- Hulett, H.R.; Loring, H.S. Effect of particle length distribution on infectivity of tobacco mosaic virus. Virology 1965, 25, 418–430. [Google Scholar] [CrossRef]
- Loring, H.S.; Fujimoto, Y.; Tu, A.T. Tobacco mosaic virus—A calcium-magnesium coordination complex. Virology 1962, 16, 30–40. [Google Scholar] [CrossRef]
- Ladipo, J.L.; Koenig, R.; Lesemann, D.E. Nigerian tobacco latent virus: a new tobamovirus from tobacco in Nigeria. Eur. J. Plant Pathol. 2003, 109, 373–379. [Google Scholar] [CrossRef]
- Whitfeld, P.R.; Higgins, T.J. Occurrence of short particles in beans infected with the cowpea strain of TMV. I. Purification and characterization of short particles. Virology 1976, 71, 471–485. [Google Scholar] [CrossRef]
- Eskelin, K.; Poranen, M.M.; Oksanen, H.M. Asymmetrical flow field-flow fractionation on virus and virus-like particle applications. Microorganisms 2019, 7, 555. [Google Scholar] [CrossRef]
- Lampi, M.; Oksanen, H.M.; Meier, F.; Moldenhauer, E.; Poranen, M.M.; Bamford, D.H.; Eskelin, K. Asymmetrical flow field-flow fractionation in purification of an enveloped bacteriophage ϕ6. Journal of Chromatography B 2018, 1095, 251–257. [Google Scholar] [CrossRef]
- Albertsson, P.A.; Frick, G. Partition of virus particles in a liquid two-phase system. Biochimica et Biophysica Acta 1960, 37, 230–237. [Google Scholar] [CrossRef]
- Teepakorn, C.; Fiaty, K.; Charcosset, C. Comparison of Membrane Chromatography and Monolith Chromatography for Lactoferrin and Bovine Serum Albumin Separation. Processes 2016, 4, 31. [Google Scholar]
- Yang, Z.; Xu, X.; Silva, C.A.T.; Farnos, O.; Venereo-Sanchez, A.; Toussaint, C.; Dash, S.; González-Domínguez, I.; Bernier, A.; Henry, O.; Kamen, A. Membrane chromatography-based downstream processing for cell-culture produced influenza vaccines. Vaccines 2022, 10, 1310. [Google Scholar] [CrossRef]
- Chen, J.; Yu, B.; Cong, H.; Shen, Y. Recent development and application of membrane chromatography. Anal. Bioanal. Chem. 2023, 415, 45–65. [Google Scholar] [CrossRef]
- 313. Sartorius. Resins, monoliths, or membranes. Which chromatographic method should you use? Sartorius AG Science-Snippets.
- Thuenemann, E.C.; Le, D.H.T.; Lomonossoff, G.P.; Steinmetz, N.F. Bluetongue virus particles as nanoreactors for enzyme delivery and cancer therapy. Mol. Pharmacol. 2021, 18, 1150–1156. [Google Scholar] [CrossRef]
- Thuenemann, E.C.; Meyers, A.E.; Verwey, J.; Rybicki, E.P.; Lomonossoff, G.P. A method for rapid production of heteromultimeric protein complexes in plants: assembly of protective bluetongue virus-like particles. Plant Biotechnology Journal 2013, 11, 839–846. [Google Scholar] [CrossRef]
- Dennis, S.J.; Meyers, A.E.; Guthrie, A.J.; Hitzeroth, II; Rybicki, E. P. Immunogenicity of plant-produced African horse sickness virus-like particles: implications for a novel vaccine. Plant Biotechnol. J. 2018, 16, 442–450. [Google Scholar] [CrossRef]
- Ford, T.; Graham, J.; Rickwood, D. Iodixanol: a nonionic iso-osmotic centrifugation medium for the formation of self-generated gradients. Analytical Biochemistry 1994, 220, 360–366. [Google Scholar] [CrossRef]
- Rybicki, E.P. Plant molecular farming of virus-like nanoparticles as vaccines and reagents. WIREs Nanomedicine and Nanobiotechnology 2020, 12, e1587. [Google Scholar] [CrossRef]
- Marsian, J.; Lomonossoff, G.P. Molecular pharming — VLPs made in plants. Curr. Opin. Biotechnol. 2016, 37, 201–206. [Google Scholar] [CrossRef]
- Zahmanova, G.; Aljabali, A.A.; Takova, K.; Toneva, V.; Tambuwala, M.M.; Andonov, A.P.; Lukov, G.L.; Minkov, I. The Plant Viruses and Molecular Farming: How Beneficial They Might Be for Human and Animal Health? International Journal of Molecular Sciences 2023, 24, 1533. [Google Scholar]
- Margolin, E.; Allen, J.D.; Verbeek, M.; Van Diepen, M.; Ximba, P.; Chapman, R.; Meyers, A.; Williamson, A.-L.; Crispin, M.; Rybicki, E. Site-specific glycosylation of recombinant viral glycoproteins produced in Nicotiana benthamiana. Front. Plant Sci. 2021, 12, 709344. [Google Scholar]
| Type | Stationary phase | Mobile Phase | Comment | Citation |
|---|---|---|---|---|
| Cation exchange | Carboxymethyl-cellulose | 5 mM Citric acid pH 3 50 mM Na2HPO4 /0.1 M NaCl pH 9.05 |
-- | (Cochran et al., 1957) |
| Cation exchange/ Ca2+ affinity | Hydroxyapatite | 0.001 M Phosphate pH 6.8 0.3 M Phosphate pH 6.8 |
Used for separation of partially and completely in vitro reconstituted particles | (Guilley et al., 1972) |
| Anion exchange | Cellulose modified with epichlorhydrin and triethanolamine | 0.01 M Phosphate pH 7 0.01 M Phosphate / 1 M NaCl pH 7 |
Used for separation of in vitro reconstituted particles | (Commoner et al., 1956) |
| Anion exchange | DEAE-cellulose | 0.01 M Tris-HCl pH 7.3 0.8 M Tris-HCl pH 7.3 |
-- | (Levin, 1958) |
| Anion exchange | CIM - QA | 1.20 mM NaOAc pH 5.5 2.20 mM NaOAc / 1.5 M NaCl pH 5.5 |
Yields 70-90 % Tomato mosaic virus only |
(Kramberger et al., 2004) |
| Anion exchange | CIM - QA + CIM - DEAE | Various; best performing with CIM QA: 1. 20 mM NaOAc pH 5.5 2. 20 mM NaOAc / 0.45 M NaCl pH 5.5 3. 20 mM NaOAc / 1.5 M NaCl pH 5.5 |
Highest yield with CIM – QA Up to 98 % yield, on average 75 % Allows purification from clarified extract within one step; tomato mosaic virus only |
(Kramberger et al., 2007) |
| Anion exchange | CIM - QA + CIM - DEAE | 20 mM NaOAc pH 5.5 20 mM NaOAc / 1 M NaCl pH 5.5 |
Separation of different viruses from their mixtures | (Ruščić et al., 2015) |
| various | Chitin | 1. 0.01 M Tris-HCl pH 6.8 2. Water 0.5 M K2HPO4 |
-- | (Townsley, 1961) |
| various | Methylated albumin on kieselgur (MAK) | 0.05 M Phosphate / 0.2 M NaCl 0.05 M Phosphate / 1 M NaCl |
Separation RNA, TMV CP and TMV particles | (Kubo et al., 1965) |
| Size exclusion | Spheron (Hydroxyalkyl methacrylate gel) | 0.05 M Tris-HCl pH 7.5 | Various particle sizes and exclusion limits tested Yield about 90% |
(Čech et al., 1977) |
| Size exclusion | Controlled pore glass | 0.05 M phosphate pH 7 | Recovery close to 100 % Comparison with Sucrose gradient centrifugation; Yield of centrifugation: 80% Comparison of different viruses |
(Barton, 1977) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





