Submitted:
16 June 2025
Posted:
17 June 2025
Read the latest preprint version here
Abstract
Keywords:
Introduction
1. Foundational Analysis
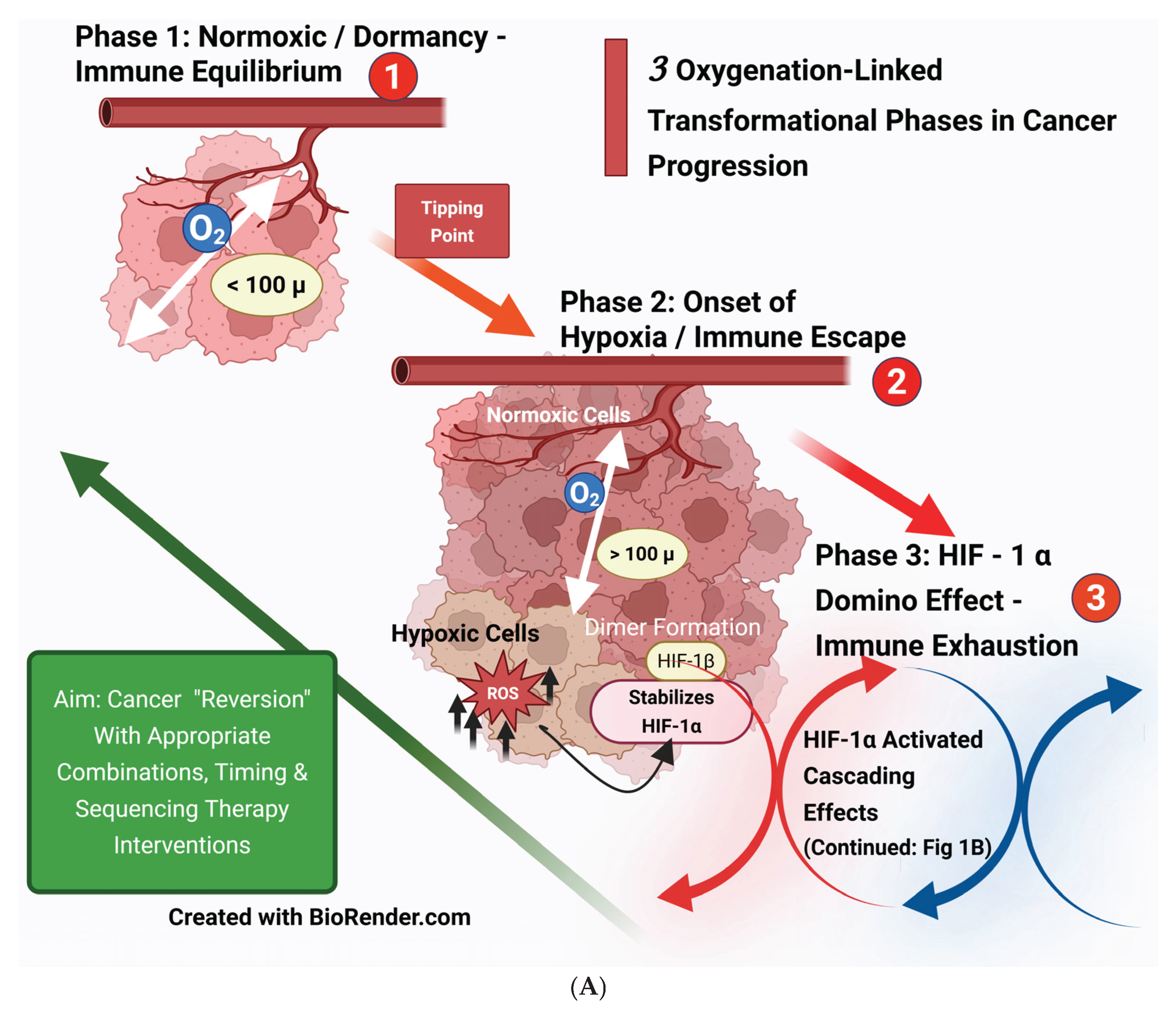
1.1. Foundational Factor 1: Hypoxia, HIF1-α, and the Domino Effects
1.1.1. 100-micron Factor—The Genesis
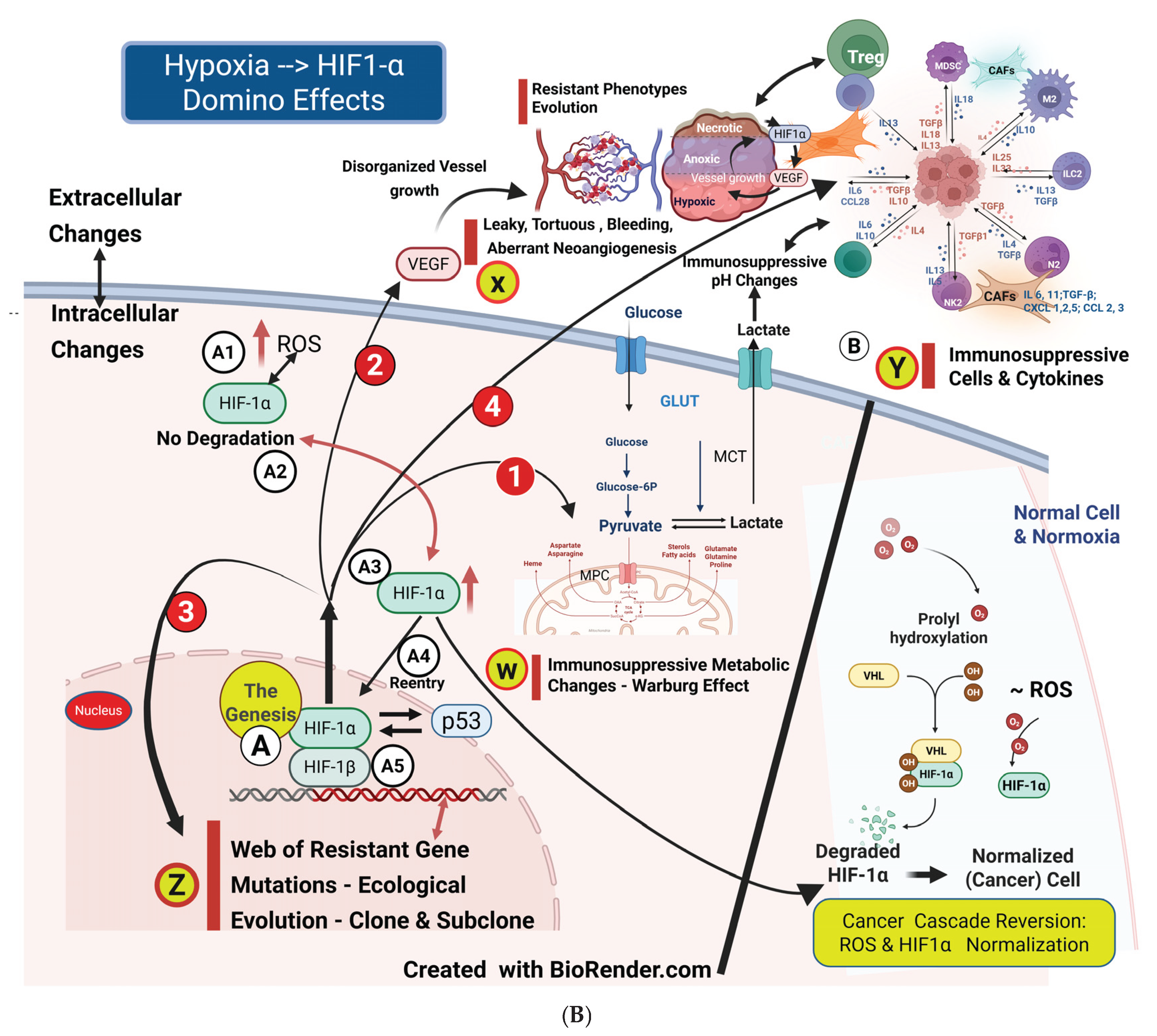
1.1.2. HIF-1α Cascading Effects
1.2. Foundational Factor 2: Vascular Changes
1.2.1. Angiogenesis in Healing vs. Tumor Angiogenesis (Neoangiogenesis)
1.2.2. Process and Types of Tumor Angiogenesis
- Sprouting and Splitting Angiogenesis: In sprouting angiogenesis, the formation of “tip cells” is supported by the growth of “stalk cells,” which help elongate from the side of the blood vessel through the activation of endothelial cells. Intussusceptive angiogenesis (or splitting angiogenesis) involves dividing an existing blood vessel into two. Splitting/intussusceptive angiogenesis, which does not require endothelial proliferation, has a lower metabolic demand. Therefore, relapse after post-tyrosine kinase inhibitor (TKI) therapy may occur due to extensive splitting angiogenesis. [32] [Ribatti D et al.]
- Vasculogenesis: This is the type of angiogenesis through which precursor cells differentiate into endothelial cells. The term neo-vascularization encompasses both neoangiogenesis and vasculogenesis. [24] [Al-Ostoot FH et al.]. Contribution to vasculogenesis can arise from differentiating hematopoietic (stem) cells or cancer stem cells through direct endothelial differentiation. This vasculogenesis operates as a VEGF-independent mechanism and remains unaffected by anti-VEGF bevacizumab. However, it could be inhibited by the tyrosine kinase inhibitor sunitinib or the anti-VEGF-receptor-2 neutralizing antibody. [33] [Brossa A]
- Vascular cooption: The immediate adoption of preexisting vasculature is known as vascular cooption. This process increases metastatic potential and the Ang2-mediated apoptotic cascade, which worsens neoangiogenesis. It is resistant to AAGs because it is independent of angiogenic switches. [29] [Caporarello N]. [34] [Haibe Y et al.]. [32] [Ribatti D et al.].
- Vascular mimicry: This occurs when cancer cells directly form vascular-like structures. Research has shown that this process is upregulated after treatment with bevacizumab or during the induction of hypoxia in the resistance phase. [34] [Haibe Y]. It is proposed that vasculogenic mimicry might depend on cancer stem cells, as classical angiogenics do not play a role. [32] [Ribatti D].
1.2.3. Neoangiogenesis, Hypoxia, and Treatment Resistance
1.2.4. The Changing Paradigm of the Antiangiogenesis Approach (From Vascular Disruption to Normalization)
Vascular Disruption Approach
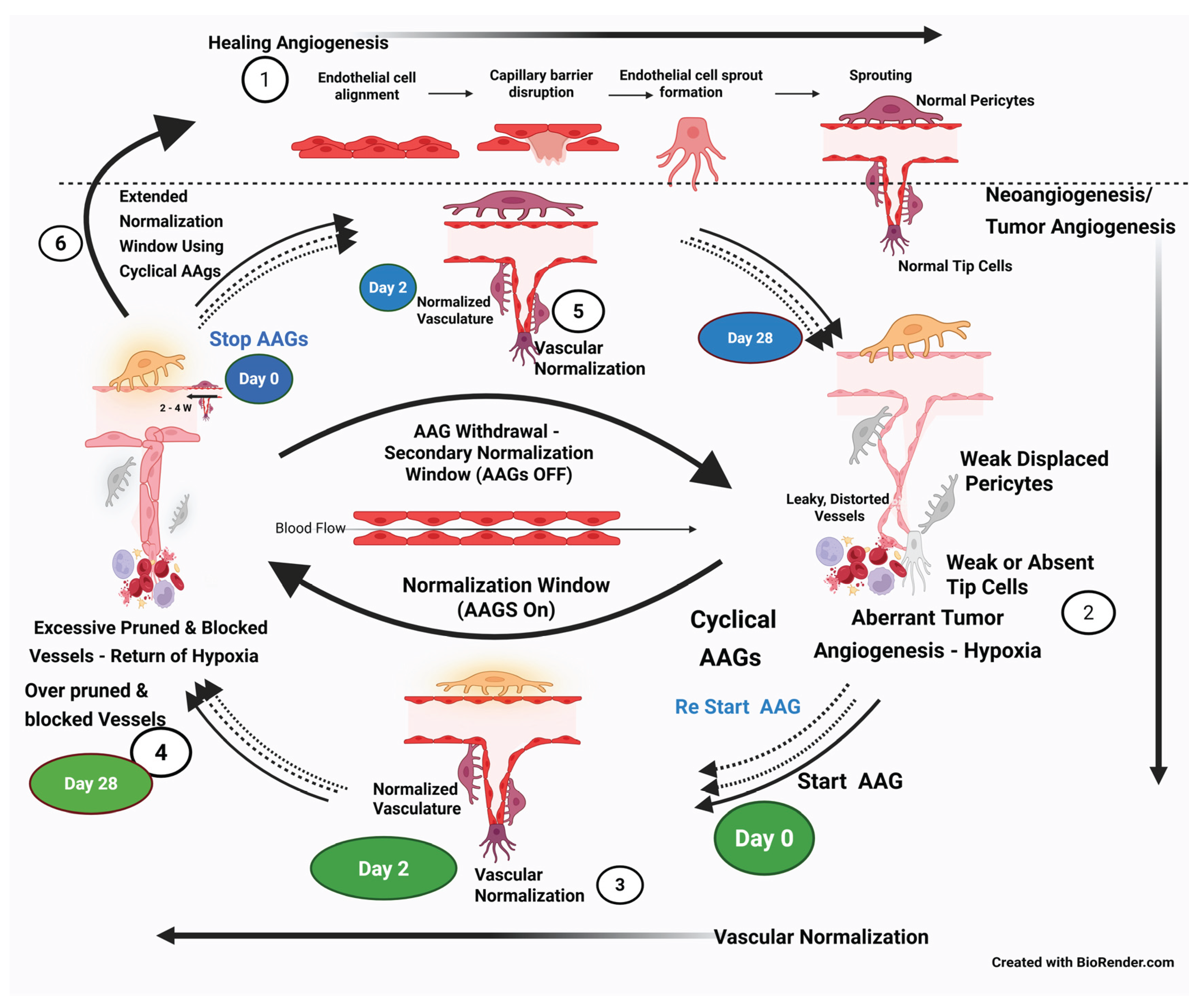
- Vascular Normalization, Normalization Window Approach (first switch): An alternative concept emerged that favors vascular normalization over shutting it off [43] [Jain RK]. RK Jain et al. (2005) showed specific antiangiogenics transiently “normalized” tumor vasculature, alleviating hypoxia and improving oxygen/drug delivery, thereby enhancing conventional therapies [43] [Jain RK]. Cediranib resulted in a consistent and dramatic reduction in tumor enhancement within 24 hours of therapy and a decrease in vascular permeability, indicating the onset of normalization. This aligns with the onset of normalization following blockade of the VEGF/VEGFR2 pathway in preclinical studies. Upregulation of tumor Ang-1 gene expression, typically produced by perivascular cells (PVCs), activates the Tie-2 receptor on ECs. The reorganization of the basement membrane of vessels, which is usually haphazard and thick in cancer, is restored to a thinner and more closely associated structure, beginning in 48–72 hours [35] [Goel S]. Tumor vascularization and oxygenation typically improve from day 1 of AAG administration. However, by Day 28, continued AAG administration leads to excessive pruning/regression of blood vessels due to anti-VEGF overaction, and the features of the aberrant vasculature, anoxia, and reproliferation of cancer cells return. This interval between the onset of normalization (around Day 2) and the excessive pruning of vessels with continued AAGs (around Day 28) is defined as a normalization time window. The time window opens up a period of enhanced delivery of cytotoxic drugs into the CCME. Additionally, during this normalization window, the effectiveness of combinatorial radiation therapy and chemotherapy/immunotherapy is enhanced due to improved drug delivery and oxygenation. [43] [RK Jain ] [35] [Goel S].
- Reversibility of Vascular Pruning/regression During the Therapy Gap of AAGs (second switch): This is another critical finding after a long course of AAGs, when the hypoxia resets due to excessive pruning of the vasculature at the end of the normalization window (around day 28). This was initially demonstrated in patients who required “drug holidays” due to toxicity, with the reversion to the normalization phenotype observed on MRa (second “normalization window”). Stopping AAGs at this stage (Day 28) reverses the excessive regression of long-term AAGs and restores vascular normalization. [35] [Goel S].
- Restarting AAGs and Extending the Normalization Window (third switch): Later, when aberrant angiogenesis sets in again (at the end of the drug holiday gap), restarting the AAGs restores normalization once more. [35] [Goel S].
- Stages of Normalization and Cyclical AAG Therapy Opportunity: Studying the initiation process of the second and potentially additional normalization windows may be foundational to adopting the “cyclical” AAG treatment concept. Theoretically, extending the normalization window reasonably indefinitely while dynamically mapping tumor vasculature during therapy is feasible and can guide the on-and-off scheduling of AAGs. [32] [Ribatti D].
- Other Methods of Extending the Normalization Window: Hypoxia-driven fibroblast growth factor (bFGF) may be responsible for escaping the normalization window, and bFGF levels increased alongside microvessel density and tumor cell proliferation after 7 weeks of ongoing VEGF blockade. [38] [Zahra].. Temporal changes in circulating fibroblast growth factor 2 (FGF2) levels due to the inhibition of the VEGF axis are associated with the disease progression observed in glioblastoma. [28] [Clarke J M].
Mapping the Tumor Angiogenesis During Anticancer Therapy—Vascular Guided Therapy
1.3. Foundational Factor 3: Metabolic Aspects—Hypoxia, Acidic pH, Differential glycolysis TILs and Tumor Cells
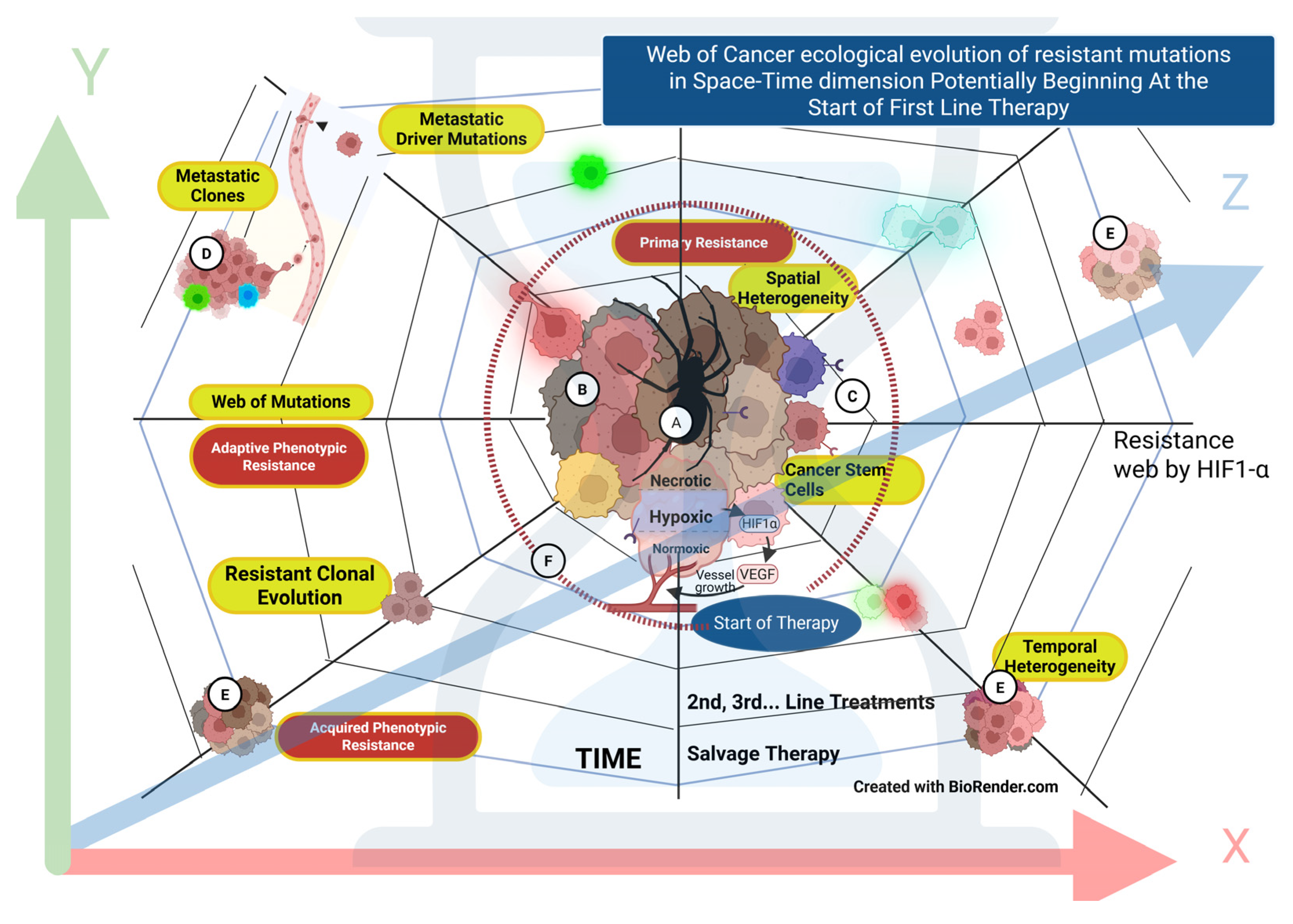
1.4. Foundational Factor 4: Genomic–Phenotypic Alterations and Cancer Cell Heterogeneity
1.4.1. Clonal–Sub-Clonal Evolution
1.5. Foundational Factor 5: Changes in CCME Within TME and Optimizing Immunological Cross-Talk
1.5.1. Normal Vascular-Immune Crosstalk
1.5.2. Endothelial Cell–Immune Crosstalk During Vascular Normalization
1.5.3. Pericyte–Immune Crosstalk
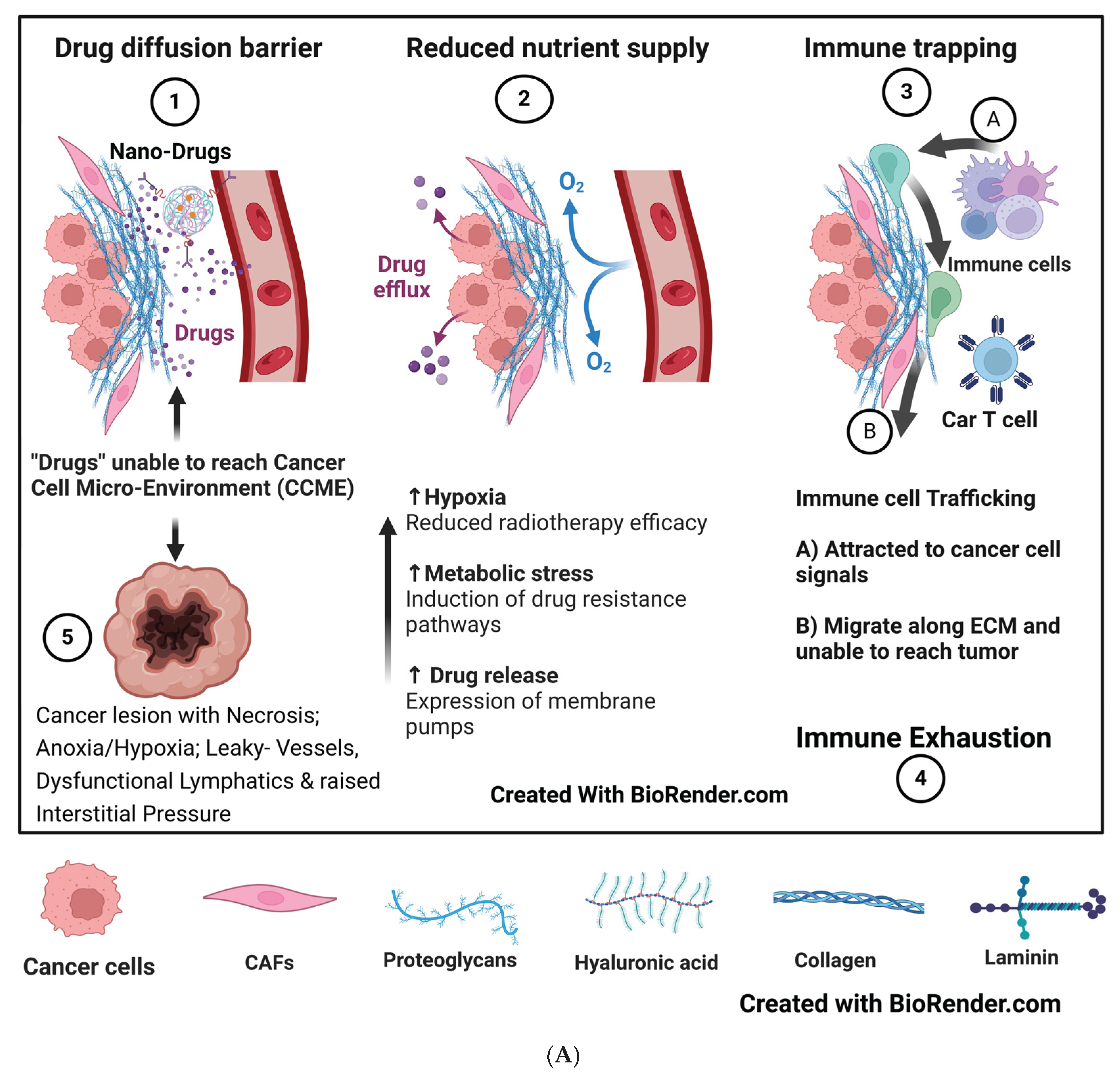
1.6. Foundational Factor 6: Multi-Dimensional, Multi-Layered Cancer Cell Protective Shields and Cancer Cell Sequestration Within CCME
1.6.1. TME/CCME Mechanobiological Forces
1.6.2. Acidic pH—Metabolic Shield
1.6.3. Fibroblasts and Rigid ECM as Physical Shields
1.6.4. Cancer Cell Lysis Products and ISP Shield
2. Actionable Analytics
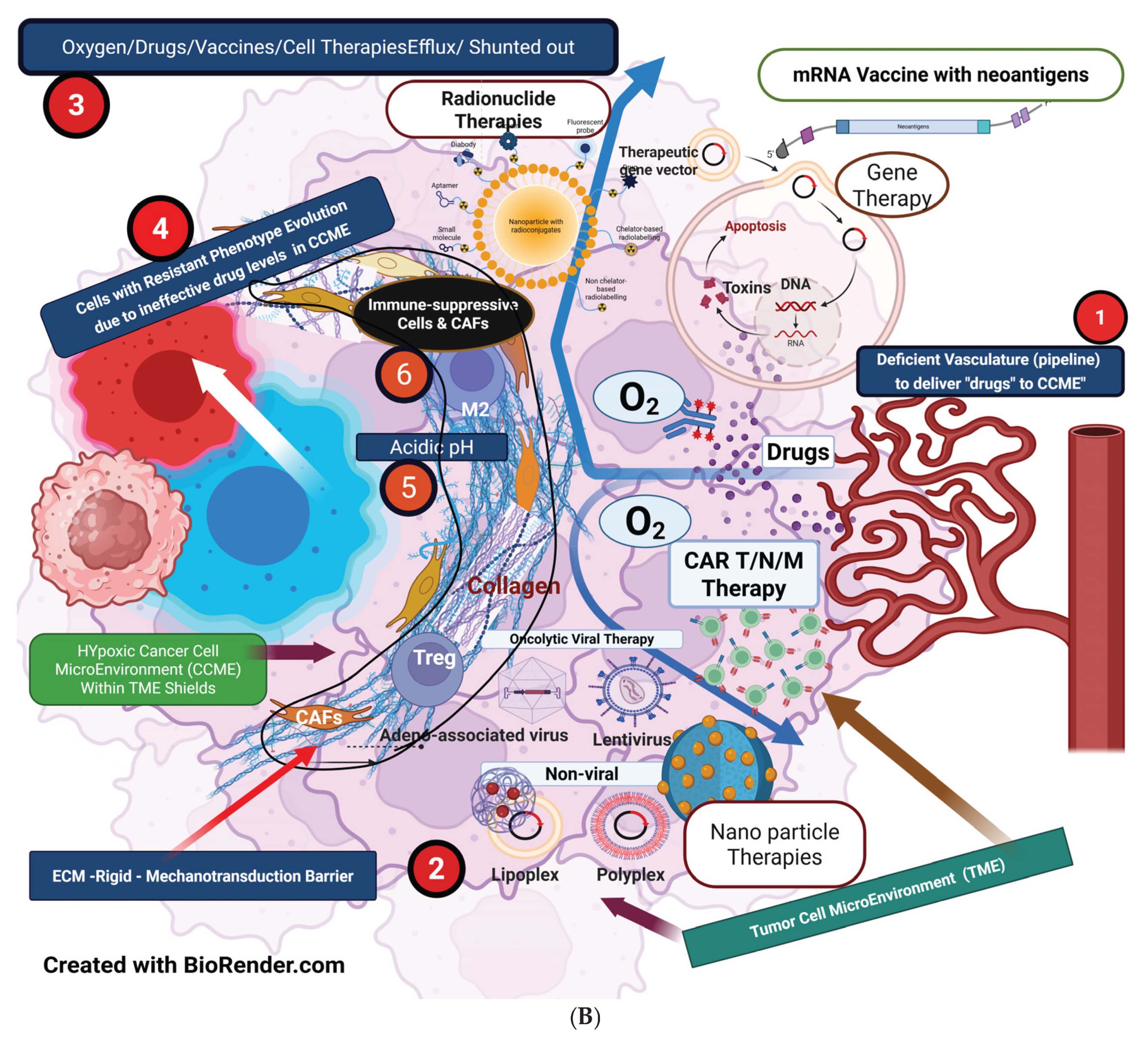
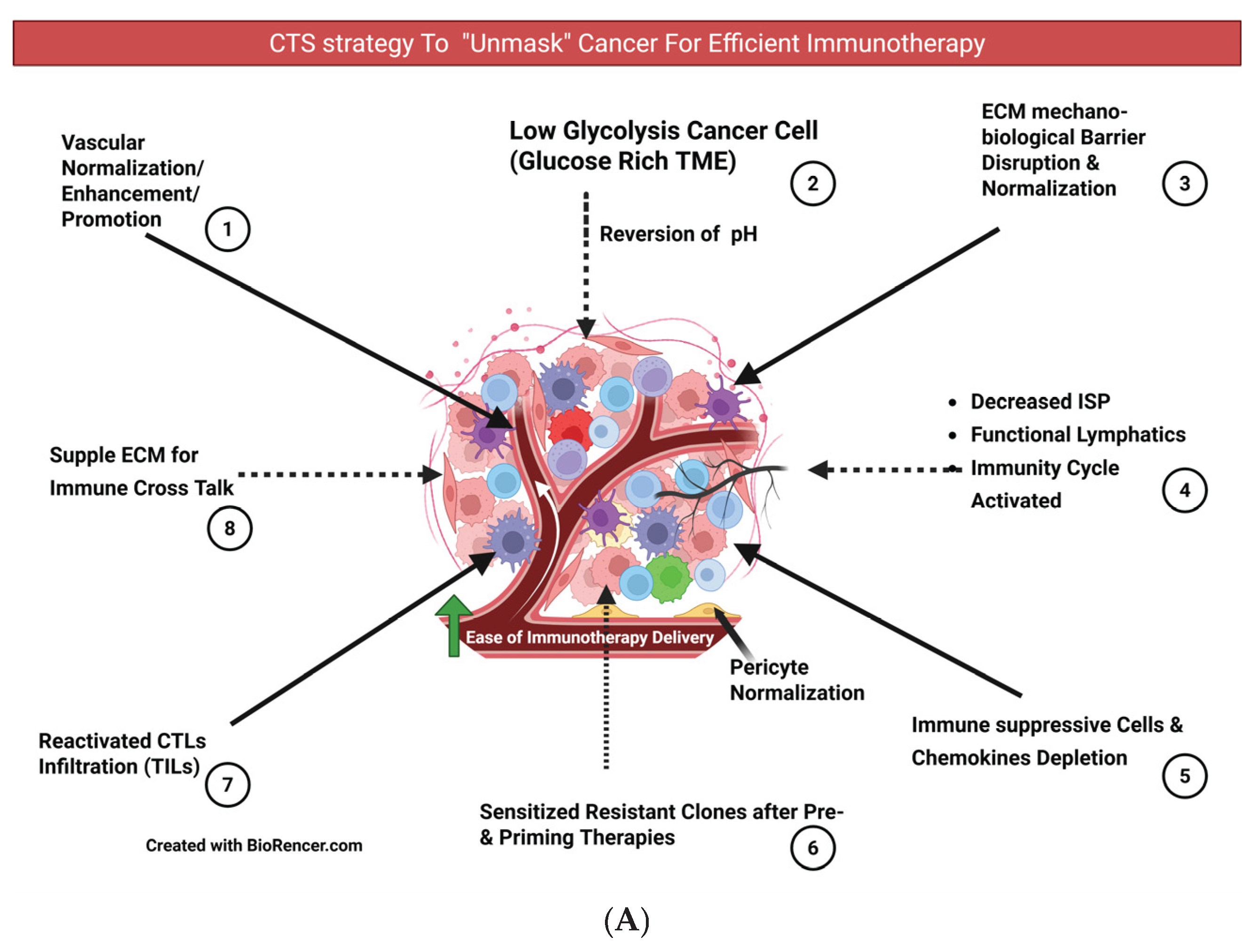
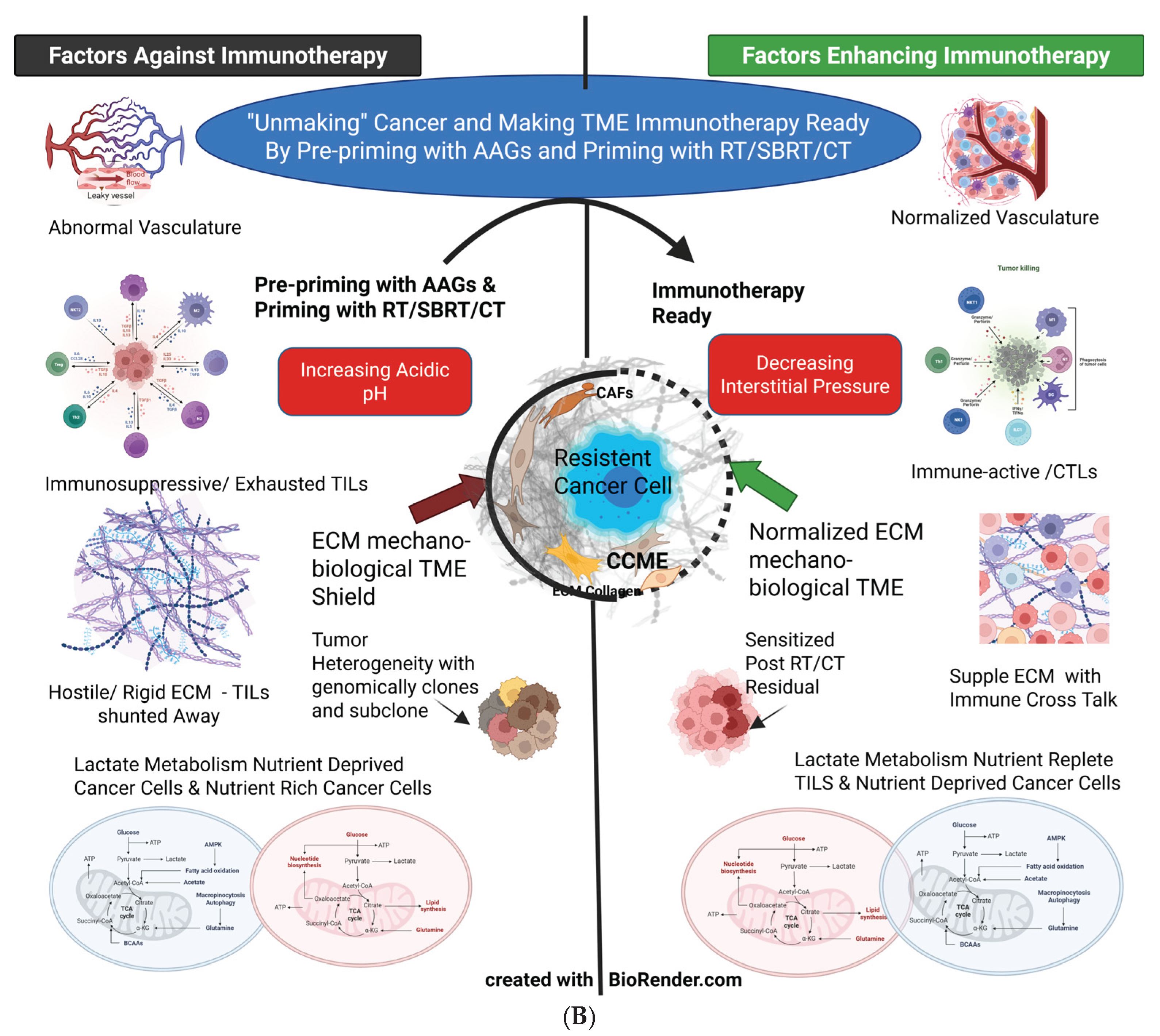
2.1. “Unmasking” the Tumor and Preparing CCME for Enhanced Immunotherapy Response
2.1.1. Rationale and Importance of Developing a Planned, Verifiable Combination, Timing, and Sequencing (CTS) Strategy
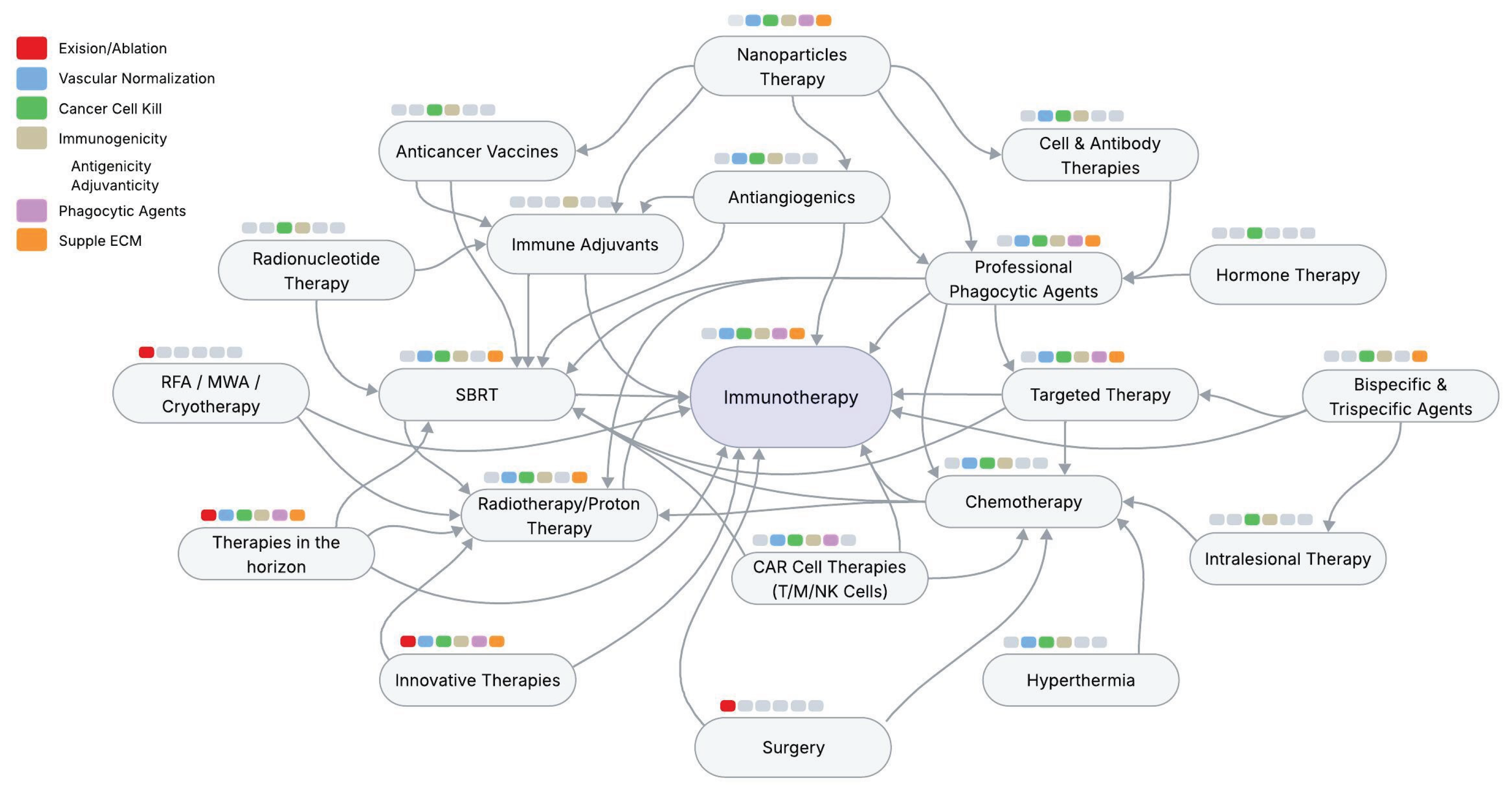
2.1.2. Classification of Treatments, Combinations, and Slotting (BOX 1)
- Vascular Normalization: Antiangiogenics, genetic modulation, newer approaches.
- Cancer Cell Kill / Vascular Enhancement or Promotion: Radiotherapy/SBRT/Chemotherapy/Targeted therapies/Radionuclide therapies
- Immune Therapy / Immune Promotion: Immunotherapies/Cell-CAR & Antibody Therapies
- Consolidation therapies
- Cancer Reversion: Maintenance/metronomic therapies, ROS targeting, repurposing drugs, senolytics, Lifestyle modifications
- *
- ECM Normalization agents, eg, TGF-β blockers: Applicable from Phase I to Phase V
- *
- Phagocytic Agents – Applicable from Phase II to Phase V
- *
- Immune Adjuvants - Applicable from Phase II to Phase V
- *
- Nanotherapies - Applicable from Phase I to Phase V
2.1.3. Mitigating Toxicities Through CTS Strategy
2.1.4. Fundamental Conditions for Effective Combinations, Timing, and Sequencing (CTS Strategy)
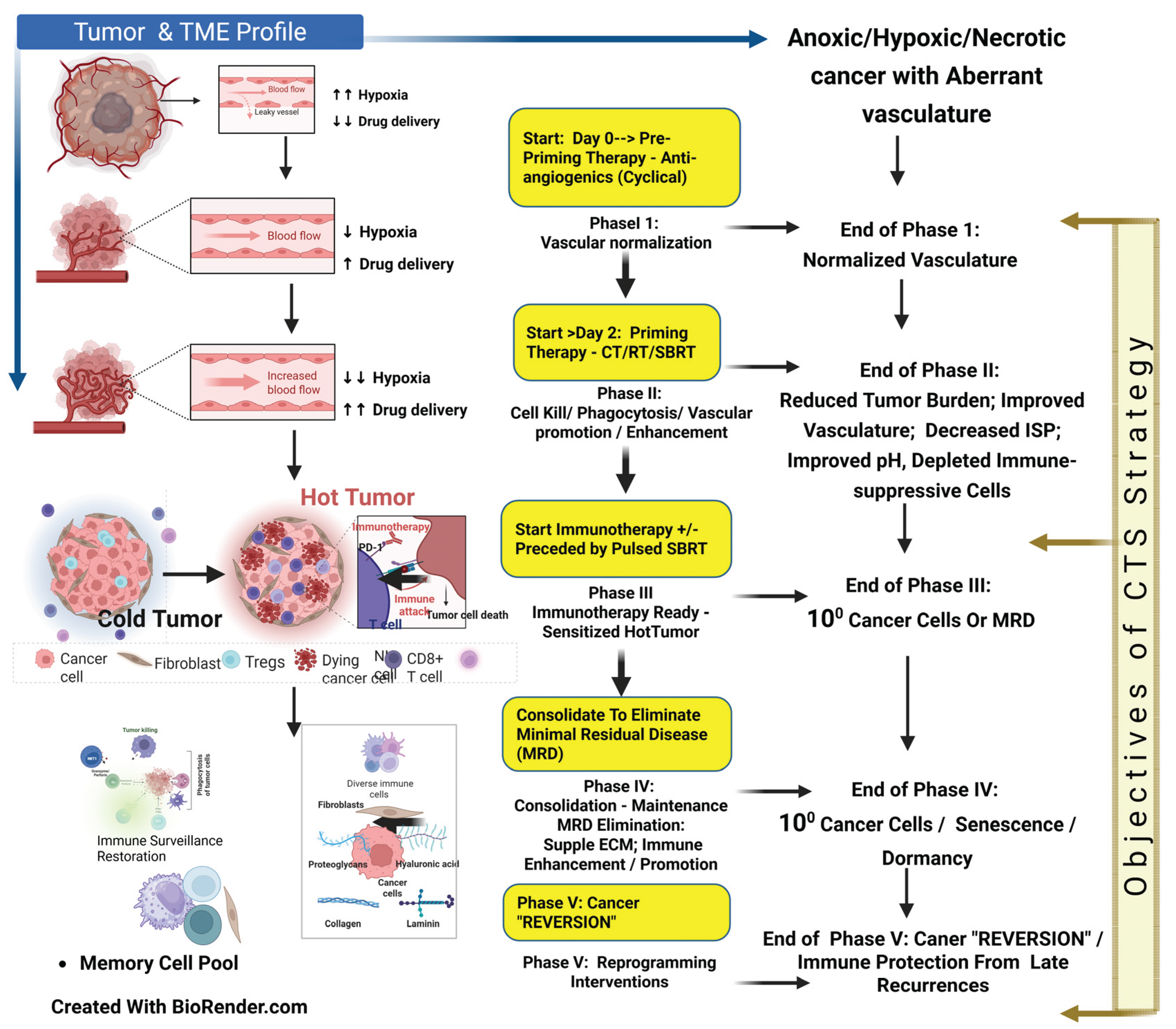
2.2. CTS Therapy Strategy Phases
2.2.1. CTS Therapy Phase I: Normalization of vasculature and Targeting Hypoxia
- Strategy:
- Prepriming with AAGs to initiate vascular normalization & hypoxia targeting.
- Time of Start:
- Day 0, Day 2: Improves the vasculature.
- Objective 1:
- Normalization of Vasculature for effective subsequent chemo-immunotherapy delivery and improves sensitivity to RT/SBRT.
- Objective 2:
- Improve by adding newer strategies/drugs for vascular normalization.
- Targeting HIF-1α, the root: Efforts have been made to develop HIF inhibitors, such as dissociating HIF-1α and HIF-1β dimers, which inhibit the transcriptional activation of HIF-2. This treatment has been tested in patients with multi-treated renal cell carcinoma and can be combined with classical antiangiogenic drugs or immunotherapies. [27] [Montemagno C]. Targeting HIF-1α is impractical due to a wide range of gene mutations and its normal presence in the homeostatic environment, which contributes to toxicity [22] [Jun JC, ] [23] [Yong L]. Therefore, hypothetically, the optimal approach to targeting HIF-1α involves normalizing oxygenation, restoring normal homeostasis, and reducing cancer-induced inflammation to decrease ROS. For this reason, vascular normalization is essential for restoring normal homeostasis and “reversion/reprogramming” of the cancer process. [95] [Swamy K].
- Vascular normalization and its window: The most accessible vascular normalization agents include antiangiogenics and several small-molecule TKIs, which enhance the effects of subsequent RT/SBRT/CT and immunotherapy during their window period. [35] [Goel S] (Figure 6). Initially, VEGF was the preferred target for normalization, as it was deemed the primary driver. Soon, it became clear that the initial response was followed by re-growth due to the emergence of multiple complementary VEGF and non-VEGF angiogenic pathways [28] [Clarke J M]. Consequently, to inhibit the parallel vertical and horizontal paths, combinations of anti-angiogenics were introduced. However, toxicity and the subsequent development of resistance emerged as significant issues [28] [Clarke J M]. In clinical settings (unlike animal studies), bevacizumab improved survival only when administered with chemotherapy. [32] [Ribatti D].
- Extending the Normalization Window—Temporal Kinetics—Cyclic antiangiogenic schedules: Stopping AAGs during the excessive regression of vessels (around day 28) reverses normalization phenotype, reducing the vascular obstruction and reestablishes normalization due to the withdrawal effect of anti-VEGF. This was observed clinically (MRI) during toxicity-mandated drug holidays. After this drug holiday, restarting AAGs reinitiates the normalization process. [35] (Figure 2) [Goel S]. This finding theoretically enables continuous vascular normalization through cyclical three-to-four-week on/off AAG administration. Several other approaches have been tried to extend the normalization window by targeting bFGF [38] [Zahra]; PDGFR-β [44] [ Hu, X]; soluble Tie-2, Ang-2 neutralization antibodies [45] [Xiaolan Yu et al.]; combined VEGFRs and ANG-2 dual blockade [46] [Li S]; as discussed previously.
- Monitoring normalization window: The normalization window has been identified using functional magnetic resonance imaging (fMRI) 2–4 days after starting sunitinib, demonstrating improved perfusion, reduced hypoxia, and metabolic shifts. FDG-PET measurements of reduced glucose uptake indicate vessel normalization. [76] [Liu, Y]. Therefore, the cyclical AAGs or other approaches discussed above can be personalized for individual patients in clinical trials (“Vascular-guided therapy”) using modern imaging techniques and specific systemic biomarkers like VEGF and miRNA. [37] [Ayoub NM].
- Timing Ang2 & Ang-1: An alternative strategy is to target Ang-1 and Ang-2. A sufficient presence of Ang-1 (with restricted Ang-2) at the maturation stage results in vessels with robust “tip cells” and a thinner vasculature penetrating deeper into the tumor’s’ hypovascular/avascular part. Theoretically, alongside the restriction of Ang-2 at the maturation stage of the vasculature, Ang-1 would induce normalization, overcoming the “trimming effect” of long-term AAGs. [96] [Biel NM].
- Moderate/Lower-Dose Antiangiogenic Therapy: Using low to moderate doses of AAGs effectively normalizes aberrant tumor microvessels and optimizes blood perfusion. [97] [Chatterjee S]. The normalization window is dose- and time-dependent, with higher doses causing excessive vascular pruning earlier. [98] [Huang Y]. High-dose AAG leads to exaggerated hypoxia, shortens the normalization window, and promotes post-window immunosuppressive cell infiltration. [46] [Li S]. Studies showed that short-term high-dose sunitinib (120 mg/kg/day) increased tumor growth, while low doses (30–60 mg/kg/day) did not stimulate metastasis in mice. [34] [Haibe Y]. Low-dose bevacizumab yielded better results in glioblastoma. [46] [Li S]. VEGFR-2 blockade doses increased perfusion and promoted M1-type TAMs and CD8+ T-cell infiltration, while higher doses expanded immunosuppressive Tregs in breast cancer. [47] [Ramjiawan RR]. While optimal normalization dosing remains unclear, lower bevacizumab doses (5 mg/kg for colorectal cancer, 15 mg/kg for lung cancer) warrant further investigation. [56] [Lee W S].
2.2.2. CTS Therapy Phase II: Normalization of Cancer Cell Sequestration, Cancer Cell Lysis, Vascular Enhancement, and Immune-Suppressive Cells Depletion—Making the TME Immunotherapy Ready
- Objective 1:
- Cancer cell lysis (preferably by ICD); waves of neoantigen generation; Immune suppressive/exhausted cell depletion in TME; decreased ISP; enhanced vascularity (vascular promotion), improved lymphatic drainage for neoantigen presentation in the lymph nodes, and reinvigorating the Immunity cycle; enhanced fresh TME CTLs infiltration.
- Objective 2:
- Endothelial integrity eg. Gene editing (moding ref); improving lymphatic functions, eg, TGF-β blockers; Improving antigenicity and adjuvanticity by immune adjuvants/ nanoedjuvants [4] [Appleton E], professional phagocytic agents [75] [Li SY]; metabolic & pH modulators; Surgery/SBRT for residual resistant mass.
- Effective Cancer Cell-Killing/Lysis and Immune-Suppressive Cell Population Depletion: Routinely used radiotherapy, SBRT, and chemotherapy enhance the normalization window discussed in Phase 1, [35] [Goel S] [76] [Liu, Y.] for subsequent immunotherapy. This ensures more efficient drug distribution due to the space created by cancer cell lysis, normalization of ECM, depletion of immune-suppressive (and immune-exhausted) cells, and reduction of CAF populations. Depleting cancer cells decreases ISP, leading to lower oxygen demand and increased vascularization. Professional phagocytic agents can further aid in decreasing ISP. [75] [Li SY]. TGF-β blockers can also mitigate ISP by reducing inflammation in the ECM and improving the function of uncollapsed lymphatics for presenting neoantigens by APCs at lymph nodes. [99] [Ariffin AB]. An activated immunity cycle and reduced ISP promote the fresh infiltration of antigen-specific CTLs/TILs [100] [Mellman I] [48] [Yang T]. SBRT, at a dose range of 6 Gy to 10 Gy per fraction, induces ICD and vascularization without disrupting endothelial function or stimulating immunosuppressive pathways, thereby preserving the flexible ECM. [71] [Martinez-Zubiaurre I]. Theoretically, SBRT within a non-disruptive vascular range (6 to 10 Gy per fraction) can be scheduled during the extended normalization window of AAG administration as a booster dose against residual disease in advanced malignancies and oligometastases. [101] [Swamy K].
- Fortification of the Vascular Normalization Window—Beyond Antiangiogenics: Several targets can be utilized to normalize the vasculature. Angiostatic factors like TNFα, TSP-1, and endostatin enhance vascular perfusion. Injection of TILs and TNFα intratumorally improves in vivo vaccination effects. The use of the Herpes virus entry mediator Ligand (HVEM-L) repairs abnormal tumor vasculature by activating various intermediaries. Incorporating a modified type 1 repeat peptide of thrombospondin (ABT-510), a TSP-1 mimic that promotes normalization and immune modulation without reducing vascular density. [48] [Yang T]. miRNAs can restore vascular integrity, as with miR-20b inhibiting the nuclear aggregation of HIF-1α and Signal Transducer and Activator of Transcription 3 (STAT3) activation. [102] [Cascio S]. Research has evaluated miRNA-153 suppression of HIF-1α and Ang-1 targeting in breast cancer. [37] [Ayoub NM]. Studies show that miRNA-140-5p silences VEGF-A; miRNA-29b inhibits angiogenesis by downregulating VEGF. Cellular myelocytomatosis oncogene (c-Myc) and miRNA-497 overexpression decrease VEGF and HIF-1α. [48] [Yang T].
- Exploiting Ang2–Ang1 interplay: Combining AAGs with properly timed Ang-1 can produce more viable vasculature during the normalization window period. Even with prolonged treatment of pancreatic tumors in transgenic mouse models using anti-VEGFR2 antibody, a delay in growth and modest survival benefit was noted due to increased expression of the pro-angiogenic Ang-1 and associated growth factors. The Ang–1–Tie pathway leads to the maturation or stabilization of blood vessels when accumulated Ang-2 is blocked. [34] [Haibe Y].
- Genetic modification: Targeting G-protein signaling 5 (Rgs5) can enhance TILs in the tumor parenchyma and the survival of tumor-bearing mice through an unknown mechanism. [46] [Li S]. Another approach is to utilize Dual Recombinase Technology to preferentially radiosensitize tumor cells while protecting the endothelium, particularly during SBRT. [42] [Moding EJ].
- Enhancing primary therapies (chemo-radio-immunotherapy)—antigenicity and adjuvanticity strategies: Localized/intralesional therapies, including oncolytic viruses, oncolytic peptides, STING, and Toll-like receptors (TLR) agonists, can activate immunogenic cold tumors through adjuvanticity and antigenicity [4] [Appleton]. Immunoadjuvants, hyperthermia, and novel nanotechnology-based targeting of the cancer cell cycle enhance the efficacy of chemo-radiotherapy. [103] [Vlastou, E]. The vascular non-disruptive, immunogenic pulsed-SBRT schedule generates an in situ/in vivo therapeutic vaccine effect. This strategy amplifies immune activation and drug penetration within the CCME [81] [Ferretti S] [104] [Liu Z] [105] [Magnussen AL]. Facilitate the neoantigen–APC flow to the lymph nodes by restoring lymphatic functions after opening the lymphatic lumens with reduced interstitial pressure [106] [Padera TP] [107] [Liao S] [108] [Avraham T]. Nanodynamic therapies are open to exploration and can be incorporated for their ability to modify pH and improve oxygen diffusion [109] [Zhang, B].
2.2.3. CTS Therapy Phase III: Initiation of Immunotherapy, Immune Promotion/Enhancement, and Memory Cell Pool Expansion
- Objective 1:
- Optimize the immunotherapy schedule by starting immunotherapy when cancer cells are unmasked and CCME modulated for maximum response & least toxicity.
- Objective 2:
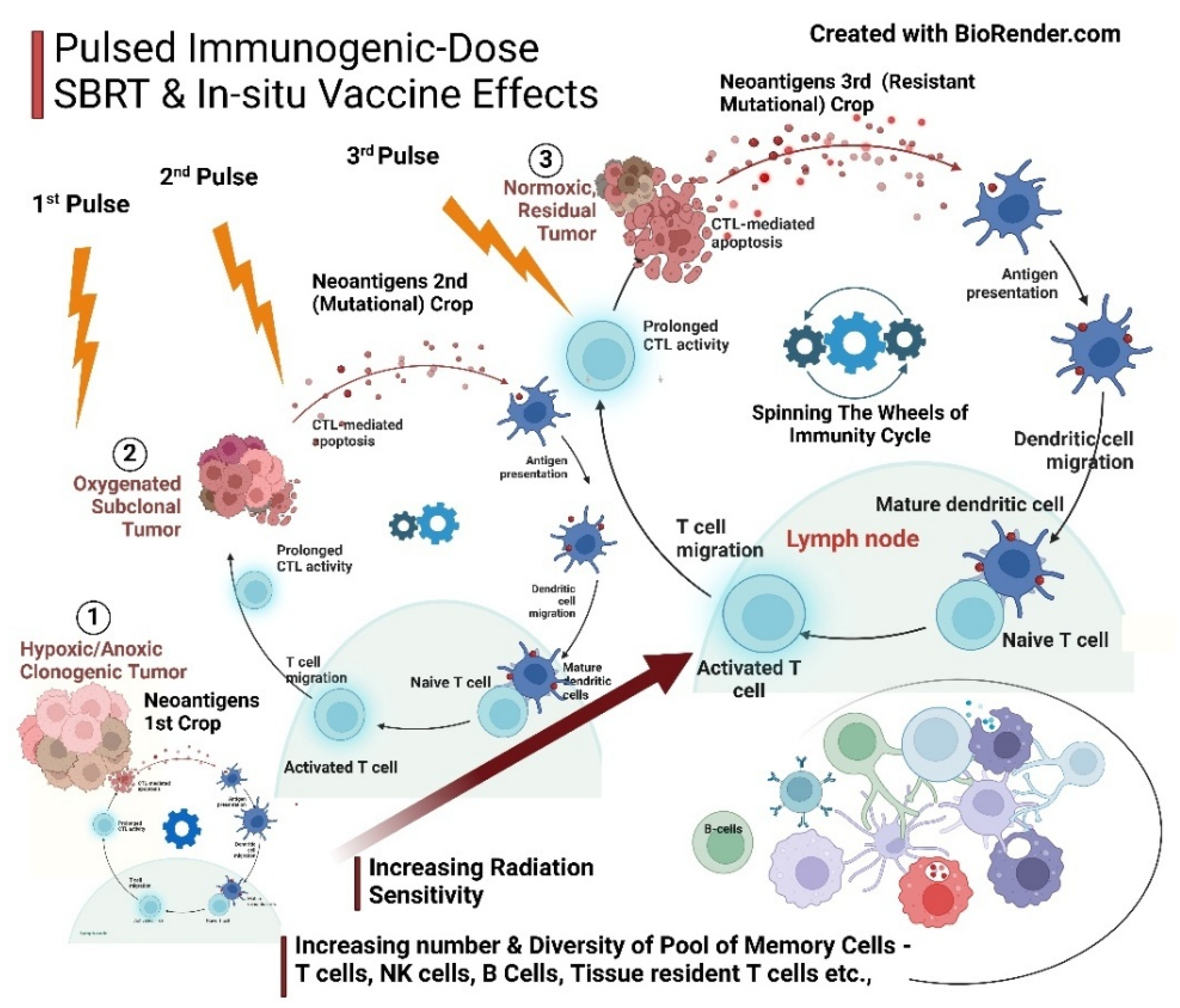
- Integrated Boost/Pulsed SBRT for dynamic generation of contemporary neoantigen for in-situ vaccination effect and to improve memory cell pool.
- Basis for unmasking for immunotherapy as a priming strategy: Vascular normalization and modulation are gaining traction, enhancing the efficacy of immunotherapies such as PD-1/PD-L1 and CTLA-4 antibodies, CAR T cells, and cancer vaccines. Before initiating the immunotherapy strategy of establishing vascular normalization, promotion, or modulation, increases in cancer cell sensitivity, oxygen diffusion, effective drug delivery, and TILs. Chemoradiotherapy depletes TME immune-suppressive elements, particularly Tregs, sensitizing cancer cells and priming the immunity cycle [17] [Zhao Y] [110] [Newport EL]. Infiltrating immune-suppressive cells, stromal cells, and abnormal vascular and lymphatic vessels—key components of the TME—are initially immunologically compromised due to hypoxia, characterized by high interstitial pressure and low pH [46] [Li S]. By the end of Phase II, following chemotherapy or radiotherapy (SBRT) or both, vascular promotion and remodeling facilitate optimal delivery of immunotherapies, immunoadjuvants, newer nanotechnological targets, and oxygen perfusion to the CCME. [17] [Zhao Y] [85] [Shibamoto Y] [35] [Goel S] [111] [Potiron V]. This vascular reset and remodeling, alongside normalized angiogenesis after RT/SBRT, form the foundation for subsequent treatment interventions of other types. [105] [Magnussen AL]. Cancer cell lysis by CTLs initiates the immunity cycle, leading to antigen uptake and presentation by dendritic cells for subsequent iterations of the immunity cycle, adapting to tumor evolution. This creates a virtuous cycle that sustains active immunity. A better understanding of the checkpoint mechanism and the maintenance of anti-tumor immunity by dendritic cells becomes relevant. Tumors exhibit three classical “immunotypes” (immunological phenotypes): immune inflamed (hot), immune excluded (cold), and immune desert. This simplification of tumors’ immunological classification should be considered in the context of the heterogeneity of all three within each particular immunotype. The proposed CTS strategy in the present article is relevant because even next-generation checkpoint inhibitors are unlikely to overcome immune-excluded and immune-desert barriers. Overall, 60%–70% of all cancers exhibit features demonstrating an immune-restrictive phenotype, with shared immune escape essentials refractory to immunotherapy. Amid all the complexities, the goal of a therapeutic strategy should be to ensure the wheel of the cancer immunity cycle keeps turning continuously. [100] [Mellman I] [48] [Yang T].
- Synergism of Immunotherapy with AAGs: Immunotherapy agents also enhance vascular enhancement by improving the immune-vascular and ECM-immune cross-talk. [17] [Zhao Y]. The vascular promotion/ remodelling induced by immunotherapy forms an “enhanced loop” by improving TILs, which improves the response to further immunotherapy doses. [48] [Yang T]. Additionally, resistant phenotypes become sensitive as “immunotypes.” [100] [Mellman I]. For example, PDL-1 expression was upregulated in tumor endothelial cells after AAG treatment, with increased infiltration of CD4+ and CD8+ T cells resulting in improved tumor control following immunotherapy. Also, VEGFA and Ang-2 blockade with the added bispecific antibody Ang-2-VEGF-A CrossMab (A2 V) resulted in enhanced infiltration of CD8+ T cells (leading to increased tumor antigen presentation) and promoted perivascular T cell accumulation. [46] [Li S]. Combining AAG and immunotherapy agents such as bevacizumab, atezolizumab, and apatinib with an anti-PD-L1 antibody could normalize the TME. [38] [Zahra, F.T] [48] [Yang T]. IFN-ϒ secreted by Th1 cells is positively associated with vessel normalization [76] [Liu, Y]. When PD-1/PD-L1 antibody is combined with an anti-VEGF, it has positive results in several phase III studies. This combination overcomes the inhibition of dendritic cell (DC) maturation, which facilitates the recruitment of T cells to the TME with improved perfusion [112] [Hack SP].
- Pulsed Immunogenic Dose SBRT and In situ Vaccination Effects Complementing Immunotherapy in Phase III: This technique has dual advantages. One is preparing the CCME for better penetration of TILs and immunotherapy drugs due to normalization of vessels and perfusion. [85] [Shibamoto Y]. Second, He K et al. (2021), based on indirect clinical evidence and preclinical studies, propose that repeated “pulsed-RT” resulted in the release of tumor antigens, expanded the tumor-specific T-cell receptor repertoire, high-affinity antibodies against the tumor, and memory cells with an in situ vaccination effect. [113] [Sezen D] [114] [Moore C] [115] [He K]. ECM suppleness is paramount for improving immune cells’ memory pool and cross-talk, highlighting all combination therapies’ appropriate dose, timing, and sequence. [17] [Zhao Y].
- Antigenicity and Adjuvanticity acceleration: In vitro-designed vaccines and cell therapies will be adjuncts at the immune-promotion level. Immune adjuvants enhance immune promotion via immune antigenicity–adjuvanticity actions, especially in cancer vaccines. [116] [Baxevanis, C.N]. Intralesional therapies add value to immunological promotion methods through adjuvanticity and antigenicity [117] [DePalo][4] [Appleton E]. Surgical excision after neoadjuvant therapy to downstage the tumor is prevalent in resectable/unresectable locally advanced head and neck cancer. [118] [Chen Y]. Additionally, in neoadjuvant therapy, an optimum immunological cascade may set in before surgery in the presence of intact drainage lymph nodes. [119] [Baniel CC]. The infrequent possibility of progression during neoadjuvant therapy can be minimized by evaluating for response to treatment often [120] [Caudle AS], and the results of several ongoing clinical trials are pending [121] [Rao, Y.J].
2.2.4. CTS Therapy Phase IV: Therapy Consolidation and Cancer Reversion/Reprogramming
- Strategy:
- Consolidation of Immunotherapy Effects, Normalization of CCME, Enhanced Long-term Control and Cancer reversion/reprogramming
- Objective 1:
- Design the maintenance therapy with the least long-term side effects.
- Objective 2:
- Develop anticancer drugs suitable for long-term medications to prevent the recurrence/ eliminate dormant cells, like any other chronic disease.
- The maintenance treatments for various malignancies and their duration are still in the early stages of development. A critical issue is creating methods to monitor the quantification of microscopic, quiescent, or dormant cancer cells. However, this represents a crucial step toward evolving therapies for “reversion” and a cure. The presence of dying or apoptotic cells also contributes to the measured cfDNA levels, limiting their usefulness as indirect information about living cancer cells. [122] [Heitzer E]. The marker for living cancer cell activity, which is reasonably stable, is microRNA (miRNA). [123] [Ruksha TG] Even though specific cirRNA has not made its foray into monitoring dormancy, due to its abundance, high stability, functional diversity, ability to act as a protein sponge, and evolutionary conservation, cirRNA is the dormancy biomarker of the future. [124] [Bach DH]. CirRNA may finally turn out to be the choice for cancer elimination monitoring. Hence, it is worth exploring how to extend the cancer therapy intervention period beyond the current strategy of merely achieving remission and “leaving it” halfway, much before the first step in cancer elimination is completed.
- <105 Cells and Dormancy Factor: The stage is now set for “reversion/reprogramming” of cancer by reestablishing normalized phenotypes and the TME, potentially overriding persistent genetic mutations. The adoption of the embryonic microenvironment within the TME can further support this. [6] [Pensotti A].
- Consolidation therapy / Cancer Cell “Reversion” monitoring: Using liquid biopsy to detect minimal residual disease (MRD) by adopting circulating tumor cells (CTC) and cell-free DNA (cfDNA) for establishing the presence of residual cells is gaining acceptance over imaging in follow-up for solid tumors. This is particularly useful for guiding maintenance treatment. [125] [Ma Y] (Figure 1B).
2.2.5. CTS Therapy Phase V: Prevention of late recurrences and Cancer Reversion/Reprogramming
- Strategy:
- To prevent late recurrences
- Objective 1:
- To keep the ECM supple. Secondly, to target HIF1-α and ROS to flip towards normalization of CCME and elimination of dormant cells.
- Objective 2:
- Reducing inflammation by maintenance therapy, senolytics, and lifestyle modifications or combinations thereof.
- Steps to Prevent Late Recurrences: The treatment mandate at this stage is to preserve a supple ECM following all combination therapies to prevent late recurrences while minimizing long-term side effects. [126] [Abyaneh HS]. Therefore, planning for this step begins with Actionable Phase I. Two factors meet this criterion: ensuring vascular endothelial integrity (especially of the endothelial stem cells) [42] [Moding EJ] and preventing fibrosis in the ECM. [17] [Zhao Y]. When SBRT is part of treatment, the dose per fraction (total dose in regular radiotherapy) and the complex role of TGF-β on ECM are crucial for ECM suppleness. TGF-β plays a significant role in normal homeostasis and can contribute to post-therapy tissue inflammation and fibrosis. TGF-β blockers help keep the ECM supple, maintaining immunological cross-talk and preventing breakthroughs in cancer cells dormancy [71] [ Martinez-Zubiaurre I ] [17] [Zhao Y]. The elements of the consolidation phase include metronomic chemotherapy. [71] [Martinez-Zubiaurre I]. repurposing drugs [94] [Xia, Y], senolytics [127] [Wyld, L] [128] [Kirkland, J.L], lifestyle modifications [129] [Berrino F], or a combination of these for the cancer reversion/reprogramming process. The future should focus on developing targets that emphasize the fundamental processes involved in the initiation and progression of cancer. This includes targeting increased ROS, persistent HIF-1α in the cytosol, and the HIF-1α-HIF-1β dimer within the cell nucleus. [130] [Ziello JE].
- Immune Protection—Expanding the Memory Cell Pool “Repertoire”: The background objective of Phases I through IV of the CTS strategy is to establish a memory cell pool that provides long-term immunological protection while targeting cancer cells. Although CT has traditionally been viewed as harmful to the immune system, a growing body of evidence suggests its capacity to engage innate and adaptive immunity. Studies have demonstrated a CD44hi memory T cell response associated with specific CT low-dose schedules and an enhanced peptide-specific CD8+ effector T cell response to DTIC. [131] [Emens LA]. After CT, resilient effector memory CD8+ T cells, which inherently possess strong resistance, are the primary contributors to the re-expansion of the memory pool. Reforming the T cell memory subpopulation is supported by an in vitro study showing strong proliferation of these subsets after IL-7/IL-15 supplementation. Eventually, there will be a shift from terminal effector phenotype cells to effector memory cells following the recovery period, alongside reduced MDSCs and Tregs. Importantly, subsequent adoptive cell therapy regimens may lead to immune memory in solid tumors. [132] [Truong, N]. Pretreatment with CT can activate virtual memory (VM) CD8+ T cells in an antigen-independent manner, mediating cancer cell cytotoxicity. [133] [Schmiechen ZC].
2.3. Methodology for Adopting the CTS Strategy
- Regardless of how innovative the treatment may be, a singular or limited approach cannot sufficiently address the complex and evolved architecture of cancer lesions in today’s context. Even in lung cancer, where immunotherapy, targeted drug therapy, and conventional chemotherapy have advanced significantly over the decades, drug resistance remains a major challenge. Tumor heterogeneity, physical barriers, adaptive dynamic mutations, and dormancy contribute to treatment failures. Understanding and targeting multiple pathways is essential for progressing toward more reliable therapies. [78] [Kang, D.H].
- The CTS strategy outlined above could be crucial for addressing the complexities of cancer. Efforts to explore the right combination of radiotherapy, chemotherapy, and immunotherapy have demonstrated benefits in only a few trials. Insights from spatial transcriptomics and single-cell sequencing may aid individual CCME in determining optimal scheduling. [79] [S.Sordo-Bahamonde].
- During trials, an adaptive multi-arm, multi-stage (MAMS) methodology is used by adding or removing therapies from a combination. This can be achieved through either the drop-the-losers design or a Bayesian design by selecting all promising treatments in the order of their treatment effects based on their mechanisms of action, as proposed in the classification above. Designing the testing models with artificial intelligence and machine learning can be accomplished using the analytical approach discussed. [138] [Wason J] [139] [Serra A].
- An earlier version of this work is available on Preprint.org [140] [Swamy, K.]. A detailed exploration of Pulsed SBRT as a practical, precisely timed in situ/in vivo vaccine modulator is discussed elsewhere. These findings highlight the potential of incorporating advanced radiotherapy techniques like SBRT into evolving therapeutic strategies. [141] [Swamy, K].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Grammar Check
References
- Passariello, M., Manna, L., Rapuano Lembo, R. et al. Tri-specific tribodies targeting 5T4, CD3, and immune checkpoint drive stronger functional T-cell responses than combinations of antibody therapeutics. Cell Death Discov. 11, 58 (2025). [CrossRef]
- Pascual J, Lim JSJ, Macpherson IR, et al., Triplet Therapy with Palbociclib, Taselisib, and Fulvestrant in PIK3CA-Mutant Breast Cancer and Doublet Palbociclib and Taselisib in Pathway-Mutant Solid Cancers. Cancer Discov. 2021 Jan;11(1):92-107. [CrossRef]
- Zhi Li, Peihao Yin, Tumor microenvironment diversity and plasticity in cancer multidrug resistance, Biochimica et Biophysica Acta (BBA)—Reviews on Cancer, Volume 1878, Issue 6, 2023, 188997. [CrossRef]
- Appleton E, Hassan J, Chan Wah Hak C, Sivamanoharan N, Wilkins A, Samson A, Ono M, Harrington KJ, Melcher A, Wennerberg E. Kickstarting Immunity in Cold Tumours: Localised Tumour Therapy Combinations With Immune Checkpoint Blockade. Front Immunol. 2021 Oct 18;12:754436. [CrossRef]
- Putzu C, Canova S, Paliogiannis P, Lobrano R, Sala L, Cortinovis DL, Colonese F. Duration of Immunotherapy in Non-Small Cell Lung Cancer Survivors: A Lifelong Commitment? Cancers (Basel). 2023 Jan 22;15(3):689. [CrossRef]
- Pensotti A, Bizzarri M, Bertolaso M. The phenotypic reversion of cancer: Experimental evidence on cancer reversibility through epigenetic mechanisms (Review). Oncol Rep. 2024 Mar;51(3):48 . https://pmc.ncbi.nlm.nih.gov/articles/PMC10835663. [CrossRef]
- Tuynder M, Susini L, Prieur S, Besse S, Fiucci G, Amson R, Telerman A. Biological models and genes of tumor reversion: cellular reprogramming through tpt1/TCTP and SIAH-1. Proc Natl Acad Sci U S A. 2002 Nov 12;99(23):14976-81. [CrossRef]
- Shin D, Cho KH. Critical transition and reversion of tumorigenesis. Exp Mol Med. 2023 Apr;55(4):692-705. [CrossRef]
- Garg, P.; Malhotra, J.; Kulkarni, P.; Horne, D.; Salgia, R.; Singhal, S.S. Emerging Therapeutic Strategies to Overcome Drug Resistance in Cancer Cells. Cancers 2024, 16, 2478. [CrossRef]
- Lei ZN, Tian Q, Teng QX, Wurpel JND, Zeng L, Pan Y, Chen ZS. Understanding and targeting resistance mechanisms in cancer. MedComm (2020). 2023 May 22;4(3):e265. [CrossRef]
- Škarková A, Bizzarri M, Janoštiak R, Mašek J, Rosel D, Brábek J. Educate, not kill: treating cancer without triggering its defenses. Trends Mol Med. 2024 Jul;30(7):673-685. [CrossRef]
- Ingber DE. Can cancer be reversed by engineering the tumor microenvironment? Semin Cancer Biol. 2008 Oct;18(5):356-64. [CrossRef]
- Jacquemin, V.; Antoine, M.; Dom, G.; Detours, V.; Maenhaut, C.; Dumont, J.E. Dynamic Cancer Cell Heterogeneity: Diagnostic and Therapeutic Implications. Cancers 2022, 14, 280. [CrossRef]
- Newton JM, Hanoteau A, Liu HC, Gaspero A, Parikh F, Gartrell-Corrado RD, Hart TD, Laoui D, Van Ginderachter JA, Dharmaraj N, Spanos WC, Saenger Y, Young S, Sikora AG. Immune microenvironment modulation unmasks therapeutic benefit of radiotherapy and checkpoint inhibition. J Immunother Cancer. 2019 Aug 13;7(1):216. [CrossRef]
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004 Aug;21(2):137-48. PMID: 15308095. [CrossRef]
- Battaglia S, Muhitch JB. Unmasking targets of antitumor immunity via high-throughput antigen profiling. Curr Opin Biotechnol. 2016 Dec;42:92-97. [CrossRef]
- Zhao Y, Yu X, Li J. Manipulation of immune‒vascular cross-talk: new strategies towards cancer treatment. Acta Pharm Sin B. 2020 Nov;10(11):2018-2036. [CrossRef]
- Schmitt MW, Loeb LA, Salk JJ. The influence of subclonal resistance mutations on targeted cancer therapy. Nat Rev Clin Oncol. 2016 Jun;13(6):335-47. [CrossRef]
- Gatenby RA, Brown JS. The Evolution and Ecology of Resistance in Cancer Therapy. Cold Spring Harb Perspect Med. 2020 Nov 2;10(11):a040972. https://pmc.ncbi.nlm.nih.gov/articles/PMC7605238/. [CrossRef]
- Groebe K, Vaupel P. Evaluation of oxygen diffusion distances in human breast cancer xenografts using tumour-specific in vivo data: role of various mechanisms in the development of tumour hypoxia. Int J Radiat Oncol Biol Phys. 1988 Sep;15(3):691-7. [CrossRef]
- MOORE GE, SANDBERG AA, WATNE AL. The comparative size and structure of tumour cells and clumps in the blood, bone marrow, and tumour imprints. Cancer. 1960 Jan-Feb;13:111-7. [CrossRef]
- Jun JC, Rathore A, Younas H, Gilkes D, Polotsky VY. Hypoxia-Inducible Factors and Cancer. Curr Sleep Med Rep. 2017 Mar;3(1):1-10. [CrossRef]
- Yong L, Tang S, Yu H, Zhang H, Zhang Y, Wan Y, Cai F. The role of hypoxia-inducible factor-1 alpha in multidrug-resistant breast cancer. [CrossRef]
- Al-Ostoot FH, Salah S, Khamees HA, Khanum SA. Tumor angiogenesis: Current challenges and therapeutic opportunities. Cancer Treat Res Commun. 2021;28:100422. [CrossRef]
- Multhoff, G., Radons, J., & Vaupel, P. (2014). Critical Role of Aberrant Angiogenesis in Developing Tumor Hypoxia and Associated Radioresistance. Cancers, 2014, 6(2), 813-828. [CrossRef]
- Wegiel B, Vuerich M, Daneshmandi Sand Seth P (2018) Metabolic Switch in the Tumor Microenvironment Determi[nes Immune Responses to Anticancer Therapy. Front. Oncol. (2018) 8:284. [CrossRef]
- Montemagno C and Pagès G (2020) Resistance to Antiangiogenic Therapies: A Mechanism Depending on the Time of Exposure to the Drugs. Front. Cell Dev. Biol. 8:584. 2020. [CrossRef]
- Clarke J M, Hurwitz H I. Understanding and targeting resistance to antiangiogenic therapies. J Gastrointest Oncol 2013;4(3):253-263. [CrossRef]
- Caporarello N, Lupo G, Olivieri M et al. Classical VEGF, Notch, and Ang signalling in cancer angiogenesis, alternative approaches and future directions (Review). Mol Med Rep 16: 4393-4402, 2017. PMCID: https://pubmed.ncbi.nlm.nih.gov/28791360/. [CrossRef]
- Zahra, F.T.; Sajib, M. S.; Mikelis, C.M. Role of bFGF in Acquired Resistance upon Anti-VEGF Therapy in Cancer. Cancers 2021, 13, 1422. [CrossRef]
- Jiang Z, Zhou J, Li L, Liao S, He J, Zhou S, Zhou Y. Pericytes in the tumor microenvironment. Cancer Lett. 2023 Mar 1;556:216074. [CrossRef]
- Ribatti D. Tumor refractoriness to anti-VEGF therapy. Oncotarget. 2016 Jul 19;7(29):46668-46677. [CrossRef]
- Brossa A, Grange C, Mancuso L, Annaratone L, Satolli MA, Mazzone M, Camussi G, Bussolati B. Sunitinib but not VEGF blockade inhibits cancer stem cell endothelial differentiation. Oncotarget. 2015 May 10;6(13):11295-309. [CrossRef]
- Haibe Y, Kreidieh M, El Hajj H et al. Resistance Mechanisms to Antiangiogenic Therapies in Cancer. Front. Oncol. (2020) 10:221. [CrossRef]
- Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. Normalisation of the vasculature is needed to treat cancer and other diseases. Physiol Rev. 2011 Jul;91(3):1071-121. [CrossRef]
- Fong SS, Nanchen A, Palsson BO, Sauer U. Latent pathway activation and increased pathway capacity enable Escherichia coli adaptation to loss of key metabolic enzymes. J Biol Chem. 2006 Mar 24;281(12):8024-33. [CrossRef]
- Ayoub NM, Jaradat SK, Al-Shami KM, Alkhalifa AE. Targeting Angiogenesis in Breast Cancer: Current Evidence and Future Perspectives of Novel Antiangiogenic Approaches. Front Pharmacol. 2022 Feb 25;13:838133. [CrossRef]
- Zahra, F.T.; Sajib, M. S.; Mikelis, C.M. Role of bFGF in Acquired Resistance upon Anti-VEGF Therapy in Cancer. Cancers 2021, 13, 1422. [CrossRef]
- Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E, Munn LL, Jain RK. Kinetics of vascular normalisation by VEGFR2 blockade governs brain tumour response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004 Dec;6(6):553-63. [CrossRef]
- Chae SS, Kamoun WS, Farrar CT, Kirkpatrick ND, Niemeyer E, de Graaf AM, Sorensen AG, Munn LL, Jain RK, Fukumura D. Angiopoietin-2 interferes with anti-VEGFR2-induced vessel normalisation and survival benefit in mice bearing gliomas. Clin Cancer Res. 2010 Jul 15;16(14):3618-27. [CrossRef]
- Thomas Nielsen; Lise Bentzen; Michael Pedersen; Trine Tramm; Paul F.J.W. Rijken; Johan Bussink; Michael R. Horsman; Leif Østergaard. Combretastatin A-4 Phosphate Affects Tumor Vessel Volume and Size Distribution as Assessed Using MRI-Based Vessel Size Imaging. Clin Cancer Res (2012) 18 (23): 6469–6477. [CrossRef]
- Moding EJ, Castle KD, Perez BA, Oh P, Min HD, Norris H, et al. Tumor cells, but not endothelial cells, mediate the eradication of primary sarcomas by stereotactic body radiation therapy. Sci Trans Med (2015) 7(278):278ra34– 278ra34. [CrossRef]
- Jain RK. Normalisation of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005 Jan 7;307(5706):58-62. [CrossRef]
- Hu, X., Ye, K., Bo, S. et al. Monitoring imatinib decreasing pericyte coverage and HIF-1α level in a colorectal cancer model by an ultrahigh-field multiparametric MRI approach. J Transl Med 22, 712 (2024). [CrossRef]
- Xiaolan Yu and Fengchun Ye. Role of Angiopoietins in Development of Cancer and Neoplasia Associated with Viral Infection. Cells 2020, 9, 457. [CrossRef]
- Li S, Zhang Q, Hong Y. Tumor Vessel Normalization: A Window to Enhancing Cancer Immunotherapy. Technol Cancer Res Treat. 2020 Jan-Dec;19:1533033820980116. [CrossRef]
- Ramjiawan RR, Griffioen AW, Duda DG. Antiangiogenesis for Cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis. 2017 May;20(2):185-204. [CrossRef]
- Yang T, Xiao H, Liu X, Wang Z, Zhang Q, Wei N, Guo X. Vascular Normalization: A New Window Opened for Cancer Therapies. Front Oncol. 2021 Aug 12;11:719836. [CrossRef]
- Ganapathy-Kanniappan, S., Geschwind, JF.H. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer 12, 152 (2013). [CrossRef]
- Weihua Wu, Shimin Zhao, Metabolic changes in Cancer: beyond the Warburg effect, Acta Biochimica et Biophysica Sinica, Volume 45, Issue 1, January 2013, Pages 18–26. [CrossRef]
- Liberti MV, Locasale JW. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci. 2016 Mar;41(3):211-218. Erratum in: Trends Biochem Sci. 2016 Mar;41(3):287. Erratum in: Trends Biochem Sci. 2016 Mar;41(3):287. https://doi.org/10.1016/j.tibs.2016.01.004. [CrossRef]
- Schreier A, Zappasodi R, Serganova I, Brown KA, Demaria S and Andreopoulou E (2023) Facts and Perspectives: Implications of tumor glycolysis on immunotherapy response in triple negative breast cancer. Front. Oncol. 12:1061789. [CrossRef]
- James Ralston Kennedy Paterson. The Treatment of Malignant Disease by Radiotherapy. Williams & Wilkins Company, 1963. Page 45.
- Lipinski KA, Barber LJ, Davies MN, Ashenden M, Sottoriva A, Gerlinger M. Cancer Evolution and the Limits of Predictability in Precision Cancer Medicine. Trends Cancer. 2016 Jan;2(1):49-63. [CrossRef]
- Brady SW, McQuerry JA, Qiao Y, et al. Combating subclonal evolution of resistant cancer phenotypes. Nat Commun. 2017 Nov 1;8(1):1231. Erratum in: Nat Commun. 2018 Feb 5;9(1):572. [CrossRef]
- Lee W S, Yang H, Chon H J and Kim C. Combination of antiangiogenic therapy and immune checkpoint blockade normalise vascular immune cross-talk to potentiate cancer immunity. Experimental & Molecular Medicine (2020) 52:1475–1485. [CrossRef]
- Amersfoort J, Eelen G, and Carmeliet P. Immunomodulation by endothelial cells — partnering up with the immune system? PERSPECTIVES Nature Reviews. Nat Rev Immunol 2022 Mar 14;1-13. [CrossRef]
- Finger AM, Hendley AM, Figueroa D, Gonzalez H, Weaver VM. Tissue mechanics in tumor heterogeneity and aggression. Trends Cancer. 2025 Apr 29:S2405-8033(25)00096-2. [CrossRef]
- Jain RK, Martin JD, Stylianopoulos T. The role of mechanical forces in tumour growth and therapy. Annu Rev Biomed Eng. 2014 Jul 11;16:321-46. [CrossRef]
- Karin E. de Visser, Johanna A. Joyce, The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth, Cancer Cell, Volume 41, Issue 3, 2023, Pages 374-403. [CrossRef]
- Zhou H, Wang M, Zhang Y, Su Q, Xie Z, Chen X, Yan R, Li P, Li T, Qin X, Yang H, Wu C, You F, Li S, Liu Y. Functions and Clinical significance of mechanical tumour microenvironment: cancer cell sensing, mechanobiology and metastasis. Cancer Commun (Lond). 2022 May;42(5):374-400. [CrossRef]
- Chung, S.W., Xie, Y. & Suk, J.S. Overcoming physical stromal barriers to cancer immunotherapy. Drug Deliv. and Transl. Res. 11, 2430–2447 (2021). [CrossRef]
- Kim G B, Riley, J L, Levine L B. Engineering T cells to survive and thrive in the hostile tumor microenvironment, Current Opinion in Biomedical Engineering, Volume 21, 2022, 100360. [CrossRef]
- Huber V, Camisaschi C, Berzi A, Ferro S, Lugini L, Triulzi T, Tuccitto A, et al. Cancer acidity: An ultimate frontier of tumour immune escape and a novel target of immunomodulation. Semin Cancer Biol. 2017 Apr;43:74-89. [CrossRef]
- Liu, S., Wang, W., Hu, S. et al. Radiotherapy remodels the tumour microenvironment to enhance immunotherapeutic sensitivity. Cell Death Dis 14, 679 (2023). [CrossRef]
- Bogdanov A, Bogdanov A, Chubenko V, Volkov N, Moiseenko F, Moiseyenko V. Tumor acidity: From hallmark of cancer to target of treatment. Front Oncol. 2022 Aug 29;12:979154. [CrossRef]
- Druzhkova I, Lukina M, Dudenkova V, Ignatova N, Snopova L, Gavrina A, Shimolina L, Belousov V, Zagaynova E, Shirmanova M. Tracing of intracellular pH in cancer cells in response to Taxol treatment. Cell Cycle. 2021 Aug;20(16):1540-1551. [CrossRef]
- Shirmanova MV, Druzhkova IN, Lukina MM, Dudenkova VV, Ignatova NI, Snopova LB, Shcheslavskiy VI, Belousov VV, Zagaynova EV. Chemotherapy with cisplatin: insights into intracellular pH and metabolic landscape of cancer cells in vitro and in vivo. Sci Rep. 2017 Aug 21;7(1):8911. [CrossRef]
- Matthew D. Galsky, Xiangnan Guan, et al. Immunomodulatory effects and improved outcomes with cisplatin- versus carboplatin-based chemotherapy plus atezolizumab in urothelial cancer, Cell Reports Medicine, Volume 5, Issue 2, 2024, 101393. [CrossRef]
- Piersma B, Hayward MK, Weaver VM. Fibrosis and cancer: A strained relationship. Biochim Biophys Acta Rev Cancer. 2020 Apr;1873(2):188356. [CrossRef]
- Martinez-Zubiaurre I, Chalmers AJ, Hellevik T. Radiation-induced transformation of immunoregulatory networks in the tumour stroma. Front Immunol (2018) 9:167. [CrossRef]
- Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated But Not Single-Dose Radiotherapy Induces an ImmuneMediated Abscopal Effect When Combined With Anti-CTLA–4 Antibody. Clin Cancer Res (2009) 15(17):5379–88. [CrossRef]
- Nguyen DT, Ogando-Rivas E, Liu R, Wang T, Rubin J, Jin L, Tao H, Sawyer WW, Mendez-Gomez HR, Cascio M, Mitchell DA, Huang J, Sawyer WG, Sayour EJ, Castillo P. CAR T Cell Locomotion in Solid Tumor Microenvironment. Cells. 2022 Jun 20;11(12):1974. [CrossRef]
- Miao L, Huang L. Exploring the tumour microenvironment with nanoparticles. Cancer Treat Res. 2015;166:193-226. PMID: 25895870; PMCID: PMC5010228. [CrossRef]
- Li SY, Guo YL, Tian JW, Zhang HJ, Li RF, Gong P, Yu ZL. Antitumor Strategies by Harnessing the Phagocytosis of Macrophages. Cancers (Basel). 2023 May 11;15(10):2717. [CrossRef]
- Liu, Y., Wang, Y., Yang, Y. et al. Emerging phagocytosis checkpoints in cancer immunotherapy. Sig Transduct Target Ther 8, 104 (2023). [CrossRef]
- Brunner TB. The rationale of combined radiotherapy and chemotherapy—Joint action of Castor and Pollux. Best Pract Res Clin Gastroenterol. 2016 Aug;30(4):515-28. [CrossRef]
- Kang, D.H.; Lee, J.; Im, S.; Chung, C. Navigating the Complexity of Resistance in Lung Cancer Therapy: Mechanisms, Organoid Models, and Strategies for Overcoming Treatment Failure. Cancers 2024, 16, 3996. [CrossRef]
- Sordo-Bahamonde, C.; Lorenzo-Herrero, S.; Gonzalez-Rodriguez, A.P.; Martínez-Pérez, A.; Rodrigo, J.P.; García-Pedrero, J.M.; Gonzalez, S. Chemo-Immunotherapy: A New Trend in Cancer Treatment. Cancers 2023, 15, 2912. [CrossRef]
- Sun, X.X.; Nosrati, Z.; Ko, J.; Lee, C.-M.; Bennewith, K.L.; Bally, M.B. Induced Vascular Normalization—Can One Force Tumors to Surrender to a Better Microenvironment? Pharmaceutics 2023, 15, 2022. [CrossRef]
- Ferretti S, Allegrini PR, Becquet MM, McSheehy PM. Tumor interstitial fluid pressure as an early-response marker for anticancer therapeutics. Neoplasia. 2009 Sep;11(9):874-81. [CrossRef]
- K.E. Hoffman ∙ R. Abi-Raad ∙ M. Ancukiewicz ∙ E. Yeh ∙ P. Ryan ∙ L. Schapira ∙ J. Younger ∙ B.L. Smith ∙ I. Kuter ∙ A.G. Taghian. Impact of Interstitial Fluid Pressure, Tumor Oxygenation, and Chemotherapy Drug Sequencing on Response to Neoadjuvant Chemotherapy and Long-Term Local Control in Women Treated for Locally Advanced Breast Cancer. International Journal of Radiation Oncology, Biology, Physics, Volume 69, Issue 3, Supplement S61-S62November 01, 2007.
- 83. [CrossRef]
- Taghian AG, Abi-Raad R, Assaad SI, Casty A, Ancukiewicz M, Yeh E, Molokhia P, Attia K, Sullivan T, Kuter I, Boucher Y, Powell SN. Paclitaxel decreases the interstitial fluid pressure and improves oxygenation in breast cancers in patients treated with neoadjuvant chemotherapy: clinical implications. J Clin Oncol. 2005 Mar 20;23(9):1951-61. [CrossRef]
- Barsoumian HB, Sheth RA, Ramapriyan R, Hsu E, Gagea M, Crowley K, Sezen D, Williams M, Welsh JW. Radiation Therapy Modulates Tumor Physical Characteristics to Reduce Intratumoral Pressure and Enhance Intratumoral Drug Delivery and Retention. Adv Radiat Oncol. 2022 Dec 5;8(2):101137. [CrossRef]
- Shibamoto Y, Miyakawa A, Otsuka S, Iwata H. Radiobiology of hypofractionated stereotactic radiotherapy: what are the optimal fractionation schedules? J Radiat Res. 2016 Aug;57 Suppl 1(Suppl 1):i76-i82. [CrossRef]
- Sophie A. Lelièvre, Kurt B. Hodges, Pierre-Alexandre Vidi. Chapter 26—Application of Theranostics to Measure and Treat Cell Heterogeneity in Cancer. Editor(s): Xiaoyuan Chen, Stephen Wong, Cancer Theranostics, Academic Press, 2014, Pages 493-516. [CrossRef]
- Li Q, Lei X, Zhu J, Zhong Y, Yang J, Wang J, Tan H. Radiotherapy/Chemotherapy-Immunotherapy for Cancer Management: From Mechanisms to Clinical Implications. Oxid Med Cell Longev. 2023 Feb 2;2023:7530794. [CrossRef]
- Ling, S.P.; Ming, L.C.; Dhaliwal, J.S.; Gupta, M.; Ardianto, C.; Goh, K.W.; Hussain, Z.; Shafqat, N. Role of Immunotherapy in the Treatment of Cancer: A Systematic Review. Cancers 2022, 14, 5205. [CrossRef]
- Tie, Y., Tang, F., Wei, Yq. et al. Immunosuppressive cells in cancer: mechanisms and potential therapeutic targets. J Hematol Oncol 15, 61 (2022). [CrossRef]
- Liu, N., Wang, X., Wang, Z. et al. Nanomaterials-driven in situ vaccination: a novel frontier in tumor immunotherapy. J Hematol Oncol 18, 45 (2025). [CrossRef]
- Mortaja M, Adams SR, McKay RR, Gutkind JS, Advani SJ. Spatially precise chemo-radio-immunotherapy by antibody drug conjugate directed tumor radiosensitization to potentiate immunotherapies. NPJ Precis Oncol. 2025 Apr 4;9(1):97. [CrossRef]
- Chmura S, Winter KA, Robinson Cet al., Evaluation of Safety of Stereotactic Body Radiotherapy for the Treatment of Patients With Multiple Metastases: Findings From the NRG-BR001 Phase 1 Trial. JAMA Oncol. 2021 Jun 1;7(6):845-852. [CrossRef]
- Wang, M., Yu, F. & Zhang, Y. Present and future of cancer nano-immunotherapy: opportunities, obstacles and challenges. Mol Cancer 24, 26 (2025). [CrossRef]
- Xia, Y., Sun, M., Huang, H. et al. Drug repurposing for cancer therapy. Sig Transduct Target Ther 9, 92 (2024). [CrossRef]
- Swamy K. Vascular-Immuno-Phenotypic (VIP) Model for Locally Advanced and Oligo-Metastatic Cancer: A Hypothesis. Med Hypotheses (2021) 152:110618. [CrossRef]
- Biel NM, Siemann DW. Targeting the Angiopoietin-2/Tie-2 axis in conjunction with VEGF signal interference. Cancer Lett. 2016 Oct 1;380(2):525-533. [CrossRef]
- Chatterjee S, Wieczorek C, Schöttle J, Siobal M, Hinze Y, Franz T, Florin A, Adamczak J, Heukamp LC, Neumaier B, Ullrich RT. Transient antiangiogenic treatment improves the delivery of cytotoxic compounds and therapeutic outcomes in lung cancer. Cancer Res. 2014 May 15;74(10):2816-24. [CrossRef]
- Huang Y, Stylianopoulos T, Duda DG, Fukumura D, Jain RK. The benefits of vascular normalisation are dose and time-dependent--letter. Cancer Res. 2013 Dec 1;73(23):7144-6. [CrossRef]
- Ariffin AB, Forde PF, Jahangeer S, Soden DM, Hinchion J. Releasing pressure in tumours: what do we know so far and where do we go from here? A review. Cancer Res. 2014 May 15;74(10):2655-62. [CrossRef]
- Mellman I, Chen DS, Powles T, Turley SJ. The cancer-immunity cycle: Indication, genotype, and immunotype. Immunity. 2023 Oct 10;56(10):2188-2205. [CrossRef]
- Swamy K. Stereotactic Body Radiotherapy Immunological Planning-A Review With a Proposed Theoretical Model. Front Oncol. 2022 Jan 26;12:729250. [CrossRef]
- Cascio S, D’Andrea A, Ferla R, Surmacz E, Gulotta E, Amodeo V, Bazan V, Gebbia N, Russo A. miR-20b modulates VEGF expression by targeting HIF-1 alpha and STAT3 in MCF-7 breast cancer cells. J Cell Physiol. 2010 Jul;224(1):242-9. [CrossRef]
- Vlastou, E.; Kougioumtzopoulou, A.; Platoni, K.; Georgakopoulos, I.; Lagopati, N.; Kouloulias, V.; Zygogianni, A. The Emerging Role of Nanoparticles Combined with Either Radiotherapy or Hyperthermia in Head and Neck Cancer: A Current Review. Cancers 2025, 17, 899. [CrossRef]
- Liu Z, Zhao Q, Zheng Z, Liu S, Meng L, Dong L, Jiang X. Vascular normalisation in immunotherapy: A promising mechanisms combined with radiotherapy. Biomed Pharmacother. 2021 Jul;139:111607. [CrossRef]
- Magnussen AL, Mills IG. Vascular normalisation as the stepping stone into tumour microenvironment transformation. Br J Cancer. 2021 Aug;125(3):324-336. [CrossRef]
- Padera TP, Stoll BR, Tooredman JB, Capen D, di Tomaso E, Jain RK. Pathology: cancer cells compress intratumor vessels. Nature. 2004 Feb 19;427(6976):695. [CrossRef]
- Liao S, Liu J, Lin P, Shi T, Jain RK, Xu L. TGF-beta blockade controls ascites by preventing abnormalization of lymphatic vessels in orthotopic human ovarian carcinoma models. Clin Cancer Res. 2011 Mar 15;17(6):1415-24. [CrossRef]
- Avraham T, Daluvoy S, Zampell J, Yan A, Haviv YS, Rockson SG, Mehrara BJ. Blockade of transforming growth factor-beta1 accelerates lymphatic regeneration during wound repair. Am J Pathol. 2010 Dec;177(6):3202-14. [CrossRef]
- Zhang, B.; Huang, Y.; Huang, Y. Advances in Nanodynamic Therapy for Cancer Treatment. Nanomaterials 2024, 14, 648. [CrossRef]
- Newport EL, Pedrosa AR, Njegic A, Hodivala-Dilke KM, Muñoz-Félix JM. Improved Immunotherapy Efficacy by Vascular Modulation. Cancers (Basel). 2021 Oct 17;13(20):5207. [CrossRef]
- Potiron V, Clément-Colmou K, Jouglar E, Pietri M, Chiavassa S, Delpon G, Paris F, Supiot S. Tumor vasculature remodeling by radiation therapy increases doxorubicin distribution and efficacy. Cancer Lett. 2019 Aug 10;457:1-9. [CrossRef]
- Hack SP, Zhu AX and Wang Y (2020) Augmenting Anticancer Immunity Through Combined Targeting of Angiogenic and PD-1/PD-L1 Pathways: Challenges and Opportunities. Front. Immunol. 11:598877. [CrossRef]
- Sezen D, Barsoumian HB, He K, Y, Wang Q, Abana CO, Puebla-Osorio N, Hsu EY, high tumour burden and generate immune memory. Front Immunol. 2022 Oct 6;13:984318. [CrossRef]
- Moore C, Hsu CC, Chen WM, Chen BPC, Han C, Story M, Aguilera T, Pop LM, Hannan R, Fu YX, Saha D, Timmerman R. Personalized Ultrafractionated Stereotactic Adaptive Radiotherapy (PULSAR) in Preclinical Models Enhances Single-Agent Immune Checkpoint Blockade. Int J Radiat Oncol Biol Phys. 2021 Aug 1;110(5):1306-1316. [CrossRef]
- He K, Barsoumian HB, Sezen D, Puebla-Osorio N, Hsu EY, Verma V, Abana CO, Chen D, Patel RR, Gu M, Cortez MA, Welsh JW. Pulsed Radiation Therapy to Improve Systemic Control of Metastatic Cancer. Front Oncol. 2021 Aug 23;11:737425. [CrossRef]
- Baxevanis, C.N.; Tsitsilonis, O.E.; Goulielmaki, M.; Tsakirakis, N.; Gritzapis, A.D. The Role of Therapeutic Vaccines in Cancer Immunotherapy. Onco 2025, 5, 11. [CrossRef]
- DePalo, D.K.; Zager, J.S. Advances in Intralesional Therapy for Locoregionally Advanced and Metastatic Melanoma: Five Years of Progress. Cancers 2023, 15, 1404. [CrossRef]
- Chen Y, Zhong NN, Cao LM, Liu B, Bu LL. Surgical margins in head and neck squamous cell carcinoma: A narrative review. Int J Surg. 2024 Jun 1;110(6):3680-3700. [CrossRef]
- Baniel CC, Heinze CM, Hoefges A, Sumiec EG, Hank JA, Carlson PM, Jin WJ, Patel RB, Sriramaneni RN, Gillies SD, Erbe AK, Schwarz CN, Pieper AA, Rakhmilevich AL, Sondel PM, Morris ZS. In situ, Vaccine Plus Checkpoint Blockade Induces Memory Humoral Response. Front Immunol. 2020 Jul 24;11:1610. [CrossRef]
- Caudle AS, Gonzalez-Angulo AM, Hunt KK, Pusztai L, Kuerer HM, Mittendorf EA, Hortobagyi GN, Meric-Bernstam F. Impact of progression during neoadjuvant chemotherapy on surgical management of breast cancer. Ann Surg Oncol. 2011 Apr;18(4):932-8. [CrossRef]
- Rao, Y.J.; Goodman, J.F.; Haroun, F.; Bauman, J.E. Integrating Immunotherapy into Multimodal Treatment of Head and Neck Cancer. Cancers 2023, 15, 672. [CrossRef]
- Ellen Heitzer, Lisa Auinger, Michael R. Speicher, Cell-Free DNA and Apoptosis: How Dead Cells Inform About the Living, Trends in Molecular Medicine, Volume 26, Issue 5, 2020, Pages 519-528. [CrossRef]
- Ruksha TG. MicroRNAs’ control of cancer cell dormancy. Cell Div. 2019 Oct 10;14:11. [CrossRef]
- Bach DH, Lee SK, Sood AK. Circular RNAs in Cancer. Mol Ther Nucleic Acids. 2019 Jun 7;16:118-129. [CrossRef]
- Ma Y, Gan J, Bai Y, Cao D, Jiao Y. Minimal residual disease in solid tumors: an overview. Front Med. 2023 Aug;17(4):649-674. [CrossRef]
- Abyaneh HS, Regenold M, McKee TD, Allen C, Gauthier MA. Towards extracellular matrix nor-malization for improved treatment of solid tumors. Theranostics. 2020 Jan 12;10(4):1960-1980. [CrossRef]
- Wyld, L., Bellantuono, I., Tchkonia, T., Morgan, J., Turner, O., Foss, F., George, J., Danson, S., & Kirkland, J. L. (2020). Senescence and Cancer: A Review of Clinical Implications of Senescence and Senotherapies. Cancers, 12(8), 2134. [CrossRef]
- Kirkland, J.L. Tumor dormancy and disease recurrence. Cancer Metastasis Rev 42, 9–12 (2023). [CrossRef]
- Berrino F. Lifestyle prevention of cancer recurrence: the yin and the yang. Cancer Treat Res. 2014;159:341-51. [CrossRef]
- Ziello JE, Jovin IS, Huang Y. Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J Biol Med. 2007 Jun;80(2):51- 60. PMID: 18160990; PMCID: PMC2140184.
- Emens LA. Chemoimmunotherapy. Cancer J. 2010 Jul-Aug;16(4):295-303. [CrossRef]
- Truong, N.T.H.; Gargett, T.; Brown, M.P.; Ebert, L.M. Effects of Chemotherapy Agents on Circulating Leukocyte Populations: Potential Implications for the Success of CAR-T Cell Therapies. Cancers 2021, 13, 2225. [CrossRef]
- Schmiechen ZC, Burrack AL, Stromnes IM. Chemotherapy brings virtual memory T cells into reality for cancer therapy. Cell Mol Immunol. 2021 May;18(5):1339-1340. [CrossRef]
- Huang, J., Michaud, E., Shinde-Jadhav, S. et al. (2024). Effects of combined radiotherapy with immune checkpoint blockade on immunological memory in luminal-like subtype murine bladder cancer model. Cancer Biology & Therapy, 25(1). [CrossRef]
- Breen WG, Leventakos K, Dong H, Merrell KW. Radiation and immunotherapy: emerging mechanisms of synergy. J Thorac Dis. 2020 Nov;12(11):7011-7023. [CrossRef]
- N.E. Donlon, R. Power, C. Hayes, J.V. Reynolds, J. Lysaght. Radiotherapy, immunotherapy, and the tumour microenvironment: Turning an immunosuppressive milieu into a therapeutic opportunity. Cancer Letters, Volume 502, 2021, Pages 84-96. [CrossRef]
- Zhai D, An D, Wan C, Yang K. Radiotherapy: Brightness and darkness in the era of immunotherapy. Transl Oncol. 2022 May;19:101366. [CrossRef]
- Wason J, Stallard N, Bowden J, Jennison C. A multi-stage drop-the-losers design for multi-arm clinical trials. Stat Methods Med Res. 2017 Feb;26(1):508-524. [CrossRef]
- Serra A, Mozgunov P, Jaki T. A Bayesian multi-arm multi-stage clinical trial design incorporating information about treatment order. Stat Med. 2023 Jul 20;42(16):2841-2854. [CrossRef]
- Swamy, K.; Basavalingaiah S, A. Bridging the Foundational Versus Actionable Cycle in Cancer: Hypoxia Domino Effect and Cancer Cell Microenvironment (CCME) “Drug” Delivery. Preprints 2024, 2024111830. [CrossRef]
- Swamy, K. (2023). Therapeutic In Situ Cancer Vaccine Using Pulsed Stereotactic Body Radiotherapy—A Translational Model. Vaccines, 12(1), 7. [CrossRef]
- Bae, T., Hallis, S.P. & Kwak, MK. Hypoxia, oxidative stress, and the interplay of HIFs and NRF2 signaling in cancer. Exp Mol Med 56, 501–514 (2024). [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




