1. Introduction
A practical approach to protect metals is by creating physical barriers to prevent exposure to elements like water, oxygen, and hydrogen. Metallic coatings and other types of protective layers are regarded as highly effective in this role. Among the various methods available, thermal spray processes—including detonation-gun (DG), plasma, high-velocity air-fuel (HVAF), and high-velocity oxy-fuel (HVOF) spraying—are commonly used to produce cermet coatings [
1]. In the thermal spraying of fine Cr
3C
2–NiCr powders, chemical degradation of chromium carbides within the feedstock powder can occur, along with the reprecipitation and dissolution of carbide phases into the NiCr matrix, forming phases such as Cr
7C
3 or Cr
24C
6 [
2]. Consequently, using the cold spray method for Cr
3C
2–NiCr coatings can significantly minimize thermally induced phase reactions and the decomposition effects often observed in fine Cr
3C
2–NiCr powders.
Cold spray (CS) is an emerging technology primarily used to produce and repair metal coatings, enhancing mechanical properties and improving corrosion resistance in various metal components. During the cold spraying process, metallic particles (ranging from 5 to 50 µm in diameter) are propelled at supersonic speeds through an inert gas flow in a de Laval nozzle, impacting the substrate to form metallic coatings. These particles collide with the substrate at velocities between 300 and 1200 m/s. As a result, the temperature of the process gas remains sufficiently low to prevent melting of the spray material [
3,
4,
5]. If the velocity of the metal particles falls below a critical threshold, the substrate may experience damage due to abrasion. Notably, increasing the powder feed rate within the nozzle decreases the velocity of the deposited particles. This reduction occurs due to interactions between the gas and particles as they exit the nozzle [
6,
7,
8].
Aluminum-zinc-magnesium (Al7075) alloys exhibit a stronger response to heat treatment compared to binary aluminum-zinc alloys, resulting in higher strength. However, the addition of zinc and magnesium reduces corrosion resistance, making it necessary to protect these alloys (such as Al7075) with corrosion-resistant metallic coatings. Titanium or nickel coatings are commonly applied for this purpose [
9,
10]. Traditional Cr
3C
2–NiCr coatings are widely used to add carbide cermet layers to industrial equipment, providing excellent resistance to wear, erosion, thermal shock, and stability at high temperatures in thermal spray applications. The effectiveness of these coatings relies heavily on factors such as deposition methods (parameters) and the coatings' microstructure [
11,
12]. Moreover, chromium carbide particles in these coatings are dispersed in a nickel-chromium alloy matrix, with the Cr
3C
2–NiCr system specifically designed for applications requiring resistance to corrosion and wear.
The combination of ceramic and metal phases in Cr
3C
2–NiCr coatings contributes to achieving higher fracture strength [
13]. Notably, Cr
3C
2–NiCr coatings are known for their high hardness [
14], which is directly linked to higher particle velocities and increased coating densities when applied to a substrate at ambient temperature. Corrosion resistance in cermet coatings is also influenced by surface roughness; a rougher surface leads to greater corrosion due to the increased surface area exposed [
15]. Cr
3C
2–NiCr coatings are suitable for use in corrosive environments at service temperatures between 800 °C and 900 °C, making them ideal as protective coatings in high-temperature, corrosive settings. Various techniques, including heat treatment, sealing, and laser remelting, are employed to reduce defects in cermet coatings [
16]. However, according to literature, heat treatment has been relatively underutilized. Therefore, laboratory tests were conducted to explore the impact of heat remelting on enhancing the mechanical and anti-corrosion properties of cermet coatings on aluminum alloy substrates.
This study examined the effect of the heat remelting process on the corrosion resistance of Cr3C2-25(Ni20Cr) cermet coatings applied to an Al7075 substrate. The coatings were produced using the cold spray method. Corrosion testing in an acidic chloride solution (1.2 M Cl−) was conducted through electrochemical techniques. Additionally, supplementary methods were employed to expand the scope of the research.
2. Experimental Details
The chemical composition of the Al7075 (EN, AW-7075) alloy is as follows by weight: 5.6% Zn, 2.5% Mg, 1.6% Cu, 0.22% Cr, with minor admixtures (Mn, Fe, and Si) making up less than 0.50%, and the remainder being aluminum. As feedstock material, fine, irregular, and broken particles of Cr
3C
2-25(Ni20Cr) (Diamalloy 3004, Oerlikon Metco Inc., Westbury, NY, USA) were used. This material consists of a mixture of Cr
3C
2 and Ni
2OCr powders in a weight ratio of 75% to 25%.
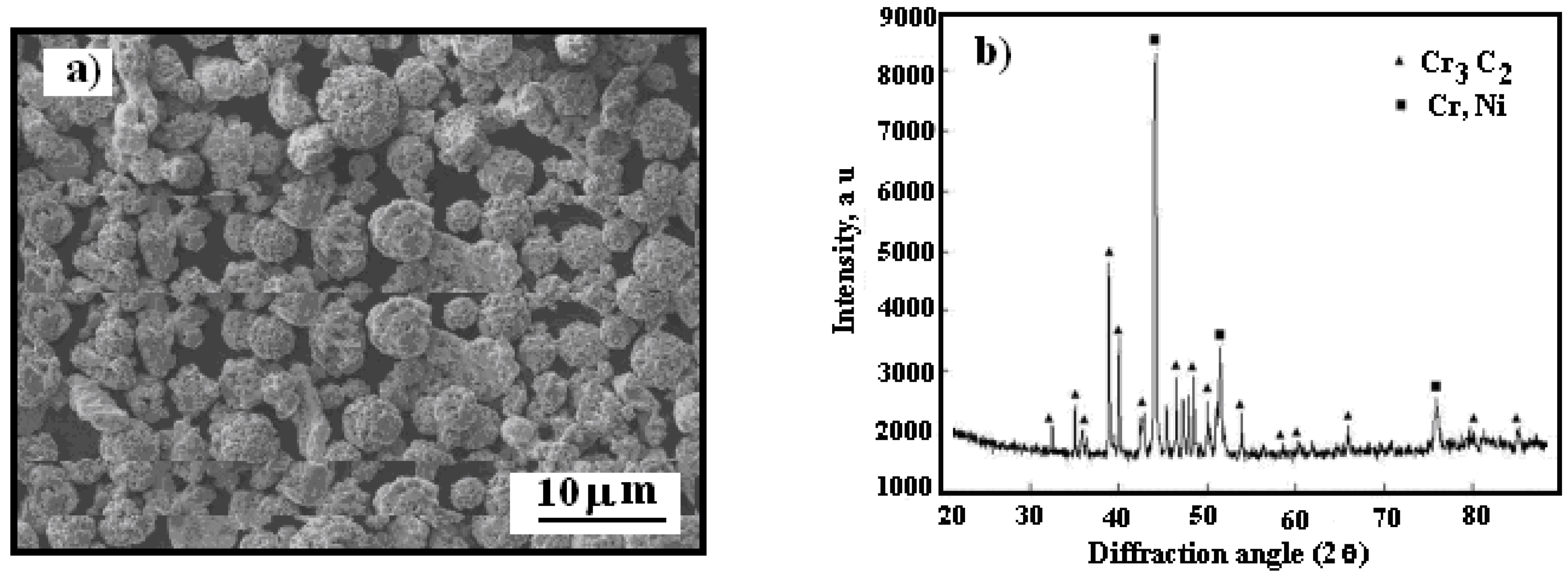
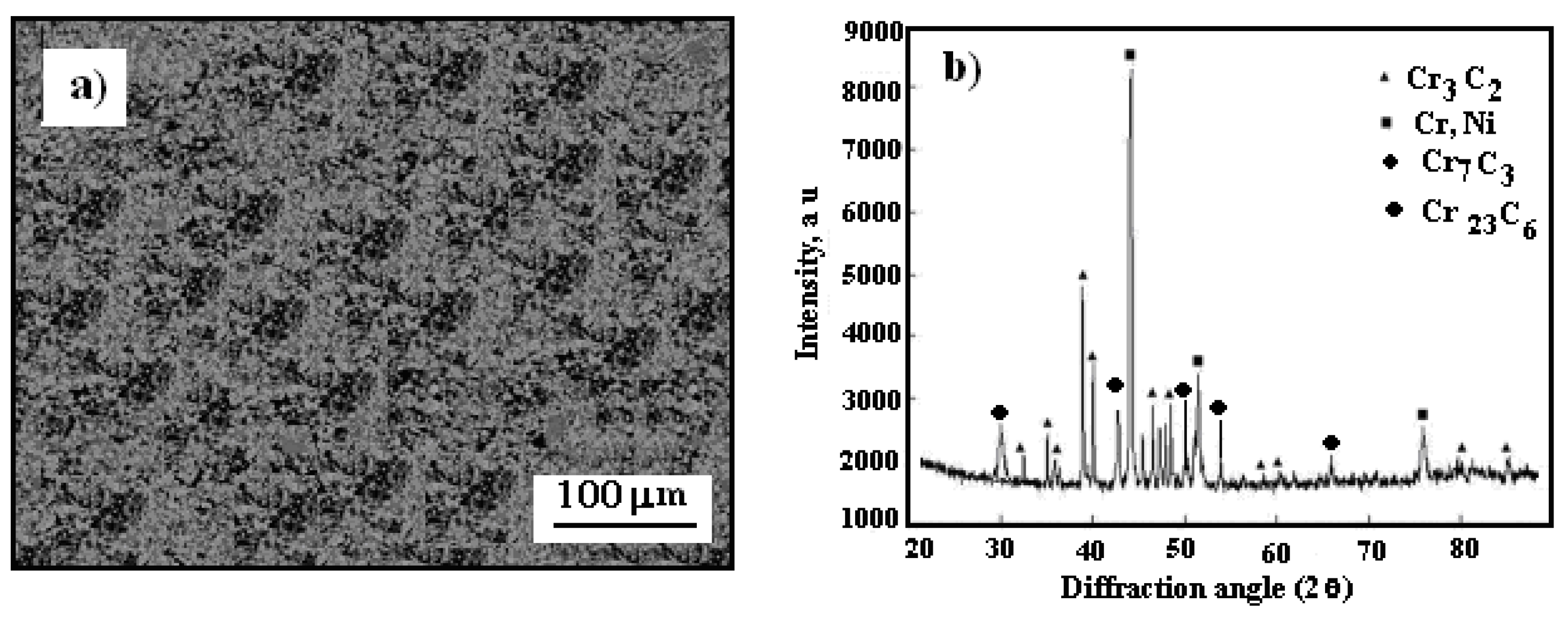
Figure 1 presents a scanning electron microscopy (SEM) image showing the morphology of the powder, alongside an X-ray diffraction pattern of the Cr
3C
2-25(Ni20Cr) powder.
The Cr
3C
2 powder particles exhibit an irregular shape, whereas the Ni20Cr particles are aspherical, as shown in
Figure 1a.
Figure 1b presents the X-ray diffraction pattern of the utilized powder. To reduce agglomeration effects, the powder was preheated to 110 °C in a convection oven for 1 hour before being loaded into the feeder system.
The Cr3C2-25(Ni20Cr) cermet coating was applied to the Al7075 substrate (Cr3C2–25(Ni20Cr)/Al7075) using the Impact Innovations 5/8 cold spraying system equipped with a Fanuc M-20iA robot (Fanuc Robotics Ltd., Oshino, Japan). The cermet coatings were produced under the following conditions: nitrogen pressure at 40 bar, nitrogen preheating to 600 °C, a spraying distance of 60 mm, a traverse speed of 40 mm/s, a step size of 2 mm between each of the 10 passes, and four coating layers. The Al7075 substrate surface was prepared by blasting with corundum particles sized between 600 and 710 μm (size 30). The substrate specimen measured 310 × 110 × 5 mm³, and the resulting coating thickness ranged from 152 μm to 158 μm. Test samples were prepared in cuboid shapes with dimensions of 30 × 10 × 5 mm³.
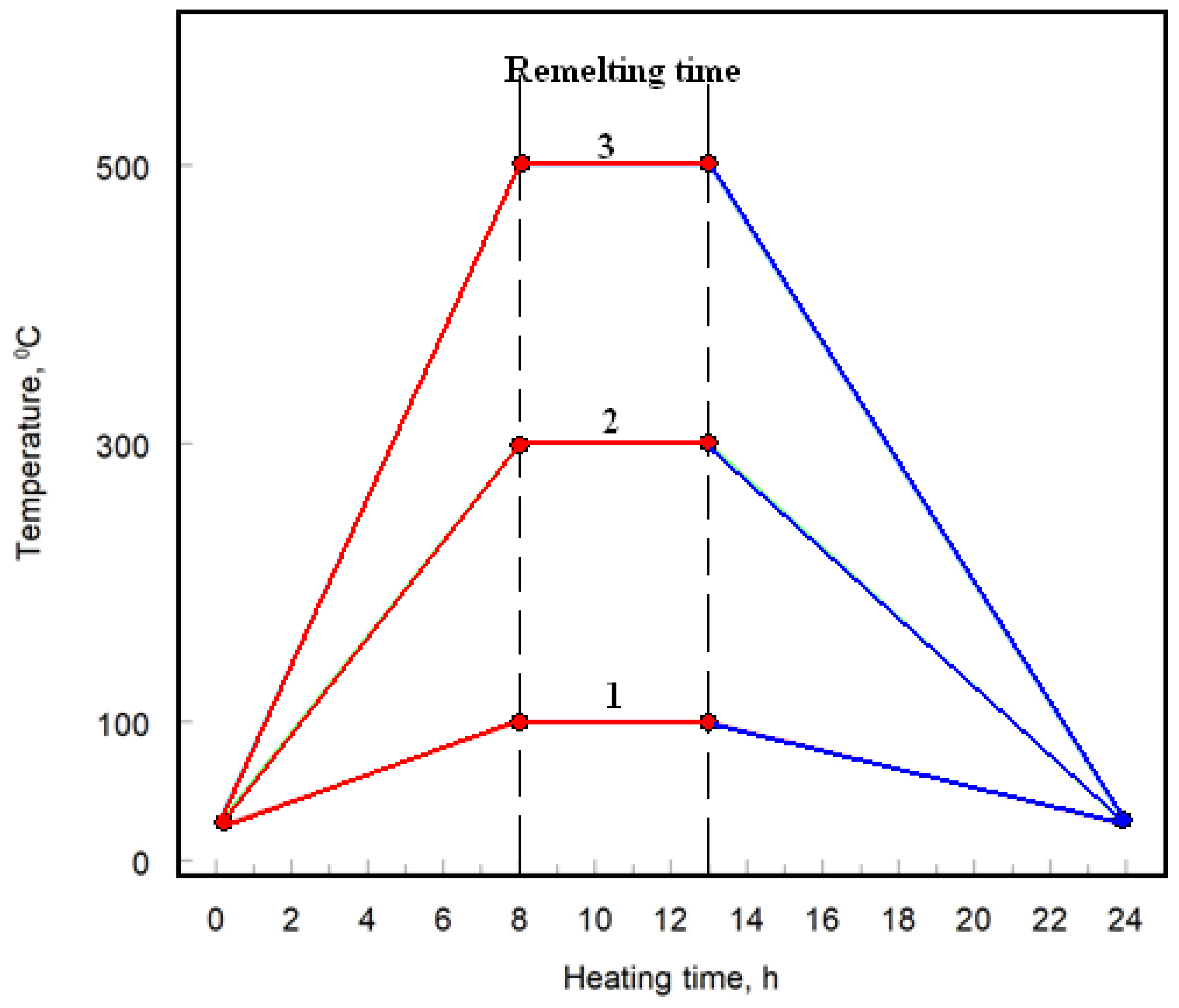
Post-coating, the samples underwent heat treatment in an electric chamber furnace (CZYLOK, Jastrzebie Zdroj, Poland, model FCF 2.5 HM). The heat treatments were conducted in air at temperatures of 100 °C, 300 °C, and 500 °C, below the aluminum melting point of 660 °C. Each Cr
3C
2–25(Ni20Cr)/Al7075 cermet-coated sample was heat-treated for 24 hours, with an additional remelting process applied to the surface for 5 hours, as illustrated in
Figure 2.
Additionally,
Table 1 provides a detailed list of other heat treatment parameters for the cermet coatings, specifically for Cr
3C
2–25(Ni20Cr) at 100 °C, 300 °C, and 500 °C.
The surface morphology and microstructure were examined using a JSM-5400 scanning electron microscope (SEM) from Joel (Tokyo, Japan) with an accelerating voltage of 20 kV. Before cross-sectional analysis, the coating samples were polished with diamond suspensions of progressively finer grains: 3 μm, 1 μm, and 0.25 μm. X-ray diffraction (XRD) was employed to analyse the phase composition of the cold-sprayed coatings, utilizing a Bruker D8 Discover diffractometer (Bruker Ltd., Malvern, UK) with Co Kα radiation at a wavelength of λ = 1.7889 Å.
The microhardness of the tested materials was measured using the Vickers hardness (HV) method on an INNOVATEST Falcon 500 hardness tester (Maastricht, The Netherlands). A diamond pyramid indenter was applied with loads ranging from 0.02 N to 20 N, resulting in an indentation depth of approximately 3 μm.
To prepare the solutions, FLUKA analytical grade sodium chloride (NaCl) and POCH analytical grade hydrochloric acid (HCl) were used. The Cl- ion concentration was set to 1.2 M, with a pH of 1.5. The electrolyte was used without deoxygenation.
The working electrode, made from Al7075 alloy coated with a Cr3C2–25(Ni20Cr) cermet layer via the cold spraying method, had a geometric surface area of 1.0 cm². A saturated calomel electrode (SCE(KCl)) served as the reference electrode and was connected to the solution through a Luggin capillary. The counter electrode, made from platinum foil (99.9% Pt), had an area of 9 cm².
Electrochemical experiments were conducted in a conventional three-electrode cell setup. All measurements were taken using a PGSTAT 128N potentiostat/galvanostat (AutoLab, Amsterdam, The Netherlands) with NOVA 1.7 software. Potentiodynamic polarization (LSV) curves were recorded over a potential range from -900 mV to +200 mV vs. SCE(KCl), with a potential scan rate of 1 mV/s and a holding time of 30 seconds at -900 mV. These LSV curves were used to determine electrochemical corrosion parameters, including corrosion potential (Ecorr), corrosion current density (jcorr), and the slopes of the cathodic (-bc) and anodic (ba) branches of the polarization curves. The Tafel Slope Analysis method was applied to further assess the corrosion parameters and polarization resistance (Rp) of the materials. More details on the Tafel method can be found in our previous publications [
17,
18]. According to the Tafel equations, the logarithm of current density varies linearly with potential (Tafel plots). The corrosion parameters for each material were determined as average values from three measurements.
Chronoamperometric (ChA) curves were obtained at specific potential values selected based on the potentiodynamic polarization curves. These potentials for the working electrode were carefully chosen to capture changes in current density at characteristic points on the LSV curves. For each tested material, three potentials were selected: one for the cathodic process and two for the anodic process. This approach enables the assessment of the anti-corrosive properties of the cermet coatings on the Al7075 substrate.
All measurements were conducted at a controlled temperature of 25 ± 0.5 °C, maintained by an air thermostat.
3. Results and Discussion
3.1. Surface Morphology
Figure 3 displays the surface morphology and X-ray diffraction pattern of the cermet coating on the Al7075 substrate in its as-sprayed state.
The surface of the cold-sprayed cermet coating on the Al7075 substrate appears compact yet uneven and undulating (
Figure 3a). As shown in
Figure 3b, the primary diffraction peaks correspond to Cr
3C
2 and (Cr, Ni) phases, resembling the X-ray diffraction pattern of Cr
3C
2–25(Ni20Cr) powder (
Figure 1b).
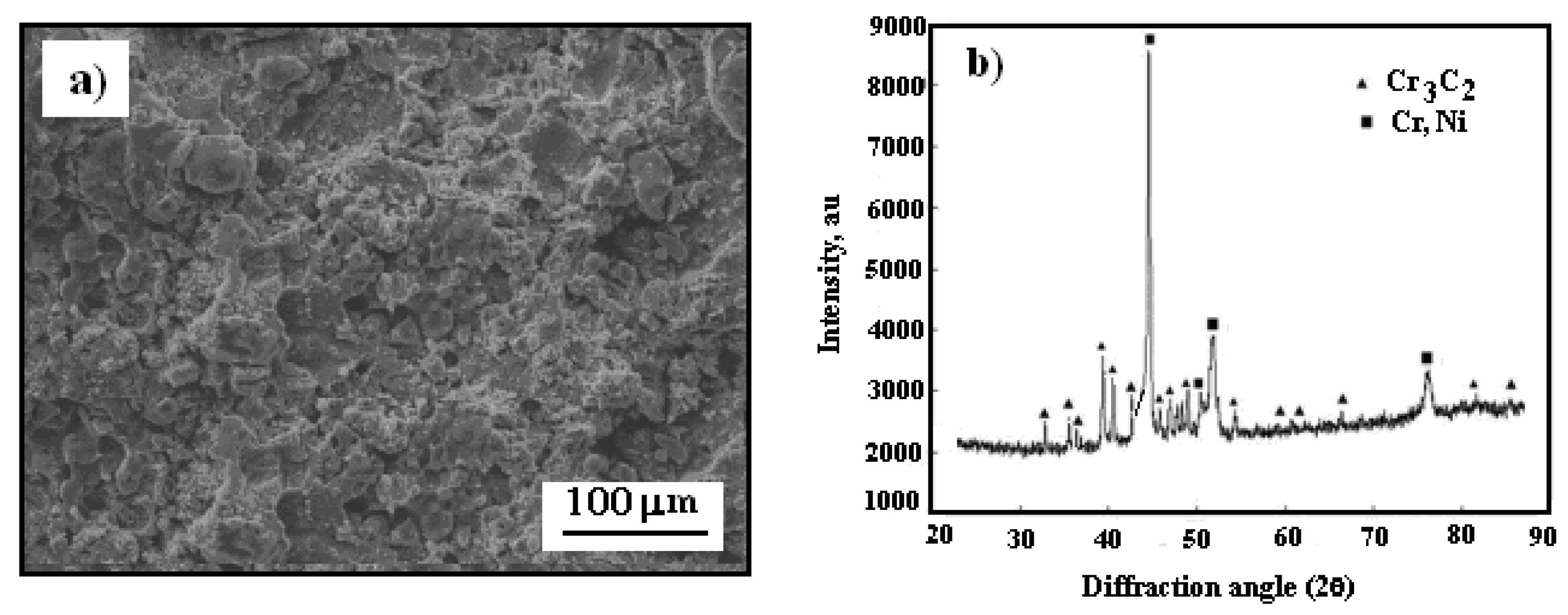
In
Figure 4, the surface morphology of the cold-sprayed cermet coating on the Al7075 substrate after heat treatment at 300 °C, along with its X-ray diffraction pattern, is presented.
It is noteworthy that the surface of the Cr
3C
2–25(Ni20Cr) coating on the Al7075 substrate became smoother following heat treatment at 300 °C in a hot air atmosphere (
Figure 4a). However, the post-heat treatment surface remains compact and tight, which should effectively shield the substrate from corrosive environments. Additionally, a new diffraction peak for the Cr
7C
3 phase was detected at 2θ = 44.17° on the cermet coating surface (
Figure 4b), suggesting the formation of this new phase through the restructuring and partial decarburization of Cr
3C
2 as per the following reaction:
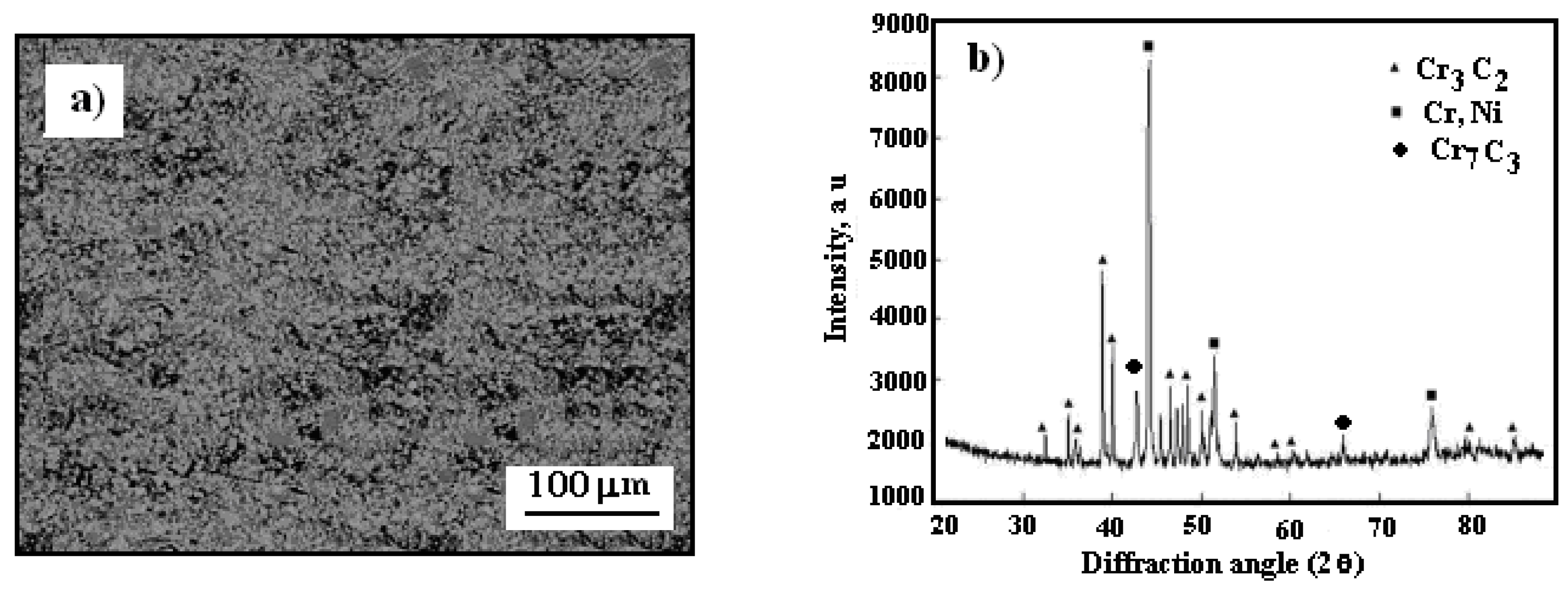
Figure 5 illustrates the surface morphology and X-ray diffraction pattern of the cold-sprayed cermet coating on the Al7075 substrate after heat treatment at 500 °C.
The high-temperature heat treatment at 500 °C resulted in noticeable changes in the surface structure of the cermet coating, as shown in
Figure 5a. Numerous depressions formed on the Cr
3C
2–25(Ni20Cr)/Al7075 surface, which considerably compromised the coating’s integrity and likely reduced its anti-corrosion effectiveness. However, under these elevated temperature conditions, a new phase appeared on the surface of the Cr
3C
2–25(Ni20Cr)/Al7075 coating, as observed in
Figure 5b, which likely formed due to the following reaction:
In the hot air atmosphere, further decarburization occurs on the Cr3C2–25(Ni20Cr)/Al7075 surface:
As a result, metastable carbides (Cr
7C
3 and Cr
23C
6) may form at the interface between Cr
3C
2 and Ni-Cr phases. Consequently, under high-temperature conditions (500 °C), structural transformations are observed on the Cr
3C
2–25(Ni20Cr)/Al7075 surface due to reactions (1) – (3), leading to a reduction in the anti-corrosion effectiveness of the cermet coating. Additionally, carbon is released as CO or CO
2 gases through reactions (4) and (5). Similar conclusions were reached by [
19], though this issue will be explored in further detail later in this study.
3.2. Microstructure of Cermet Coatings
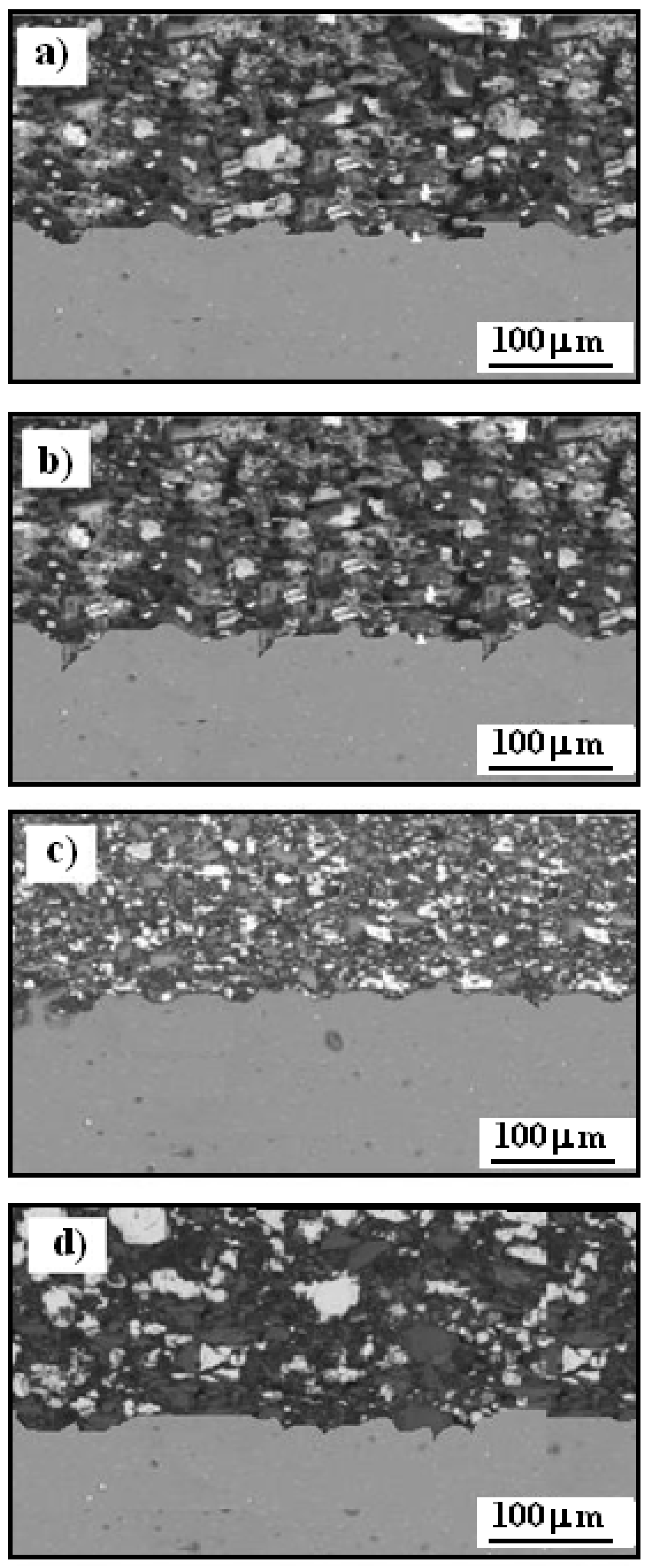
Figure 6 presents the scanning electron microscopy (SEM) cross-sectional microstructure of Cr
3C
2–25(Ni20Cr) cermet coatings on an Al7075 substrate, both before and after heat treatment. The coatings were produced using the cold spraying method, resulting in a strong bond with the substrate, with no visible cracks and densely packed carbide particles. The microstructure shows distinct contrast zones—dark gray, medium gray, and light gray—highlighted by the varying shades in the coating.
The dark gray regions in the Cr
3C
2–25(Ni20Cr)/Al7075 coatings consist of NiCr and carbon, and these areas are identified as carbide zones. The light gray regions are primarily associated with chromium carbides, specifically Cr
3C
2 and Cr
7C
3, while the medium gray phase represents the more complex chromium carbide, Cr
23C
6. This distribution of phases can be observed throughout the coating microstructure in
Figure 6.
Figure 6a shows the cross-section of the Cr
3C
2-25(Ni20Cr) cermet coating on the Al7075 substrate prior to heat treatment, while
Figure 6b illustrates the cross-section after heat treatment at 100 °C. No substantial structural changes were observed in the Cr
3C
2-25(Ni20Cr) coating at this temperature. However, after heat treatment at 300 °C, noticeable structural modifications occurred, as shown in
Figure 6c. The fine structure of chromium carbides became more distinct in the Cr
3C
2-25(Ni20Cr) coating cross-section, with heat treatment reducing microcracks and pores on the Cr
3C
2-25(Ni20Cr)/Al7075 surface.
When the temperature was raised to 500 °C, the fine-crystalline structure of chromium carbides was no longer visible, as illustrated in
Figure 6d. Additionally, elemental carbon appeared within the coating structure, which likely contributed to a significant reduction in the anti-corrosion properties of the Cr
3C
2-25(Ni20Cr) coating.
3.3. Microhardness
Table 2 illustrates the impact of heat treatment on the microhardness (HV10) of the Cr
3C
2–25(Ni20Cr) cermet coatings on the Al7075 substrate, comparing values before and after heat treatment.
The measurement results in
Table 2 reveal significant variability in HV10 microhardness values due to the heterogeneous nature of the cold-sprayed Cr
3C
2-25(Ni20Cr) coating surface. This variability arises from particles with differing deformation levels, influenced by a broad particle size distribution and varying positions in the spray jet, resulting in indentation values that depend on particle size. Heat treatment was found to alter the surface microhardness of the cermet coating on the Al7075 substrate. At 300 °C, the HV10 microhardness of the Cr
3C
2-25(Ni20Cr)/Al7075 coating nearly doubled compared to the untreated coating. However, further increasing the heat treatment temperature to 500 °C led to a substantial decrease in microhardness by approximately 150 units on the HV10 scale compared to the coating treated at 300 °C (as shown in
Table 2). This indicates that heat treatment affects the Cr
3C
2-25(Ni20Cr)/Al7075 surface structure by smoothing and hardening it, with the most pronounced effect observed at 300 °C. At lower heat treatment temperatures, such as 100 °C, the Cr
3C
2-25(Ni20Cr) coating experiences only superficial melting, leading to minimal structural and mechanical changes in the surface of the cermet coating.
3.4. Corrosion Test
The corrosion resistance of the Cr
3C
2-25(Ni20Cr) cermet coatings on the Al7075 substrate was evaluated through electrochemical testing in an acidic chloride solution (1.2 M Cl-).
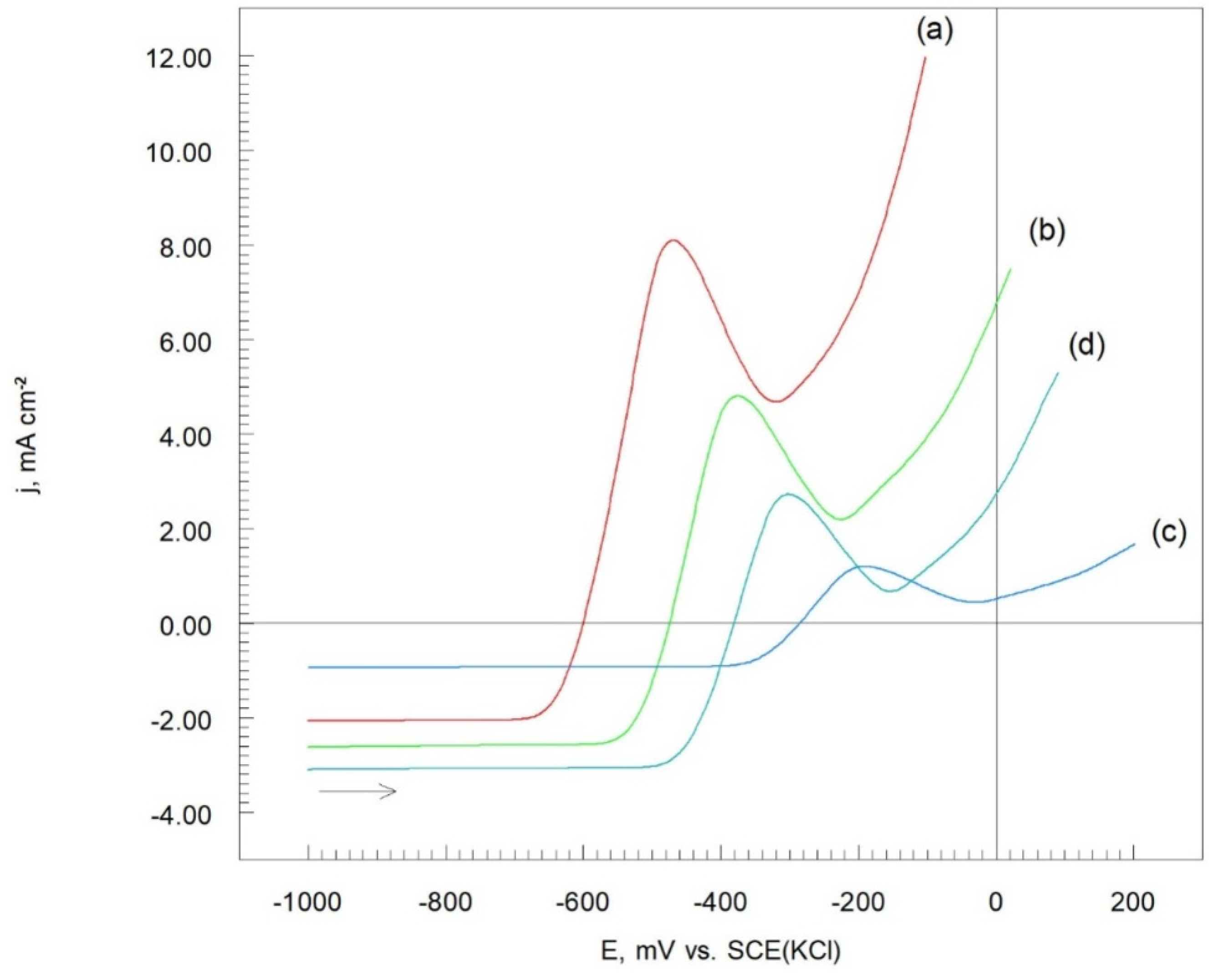
Figure 7 displays the potentiodynamic polarization (LSV) curves for the Cr
3C
2-25(Ni20Cr)/Al7075 coatings, both before and after heat treatment.
Hydrogen depolarization occurs in the cathodic region of the potentiodynamic polarization curves. In the acidic corrosive environment, the cathodic branches of the LSV curves represent the simplified reduction of hydrogen ions [
9,
13]:
where Me means the Cr, Ni and other metals.
Conversely, the anodic reaction proceeds as follows:
In this context, "(MeO)ads" refers to oxides such as (Cr
2O
3)ads and (NiO)ads. It was found that the anodic current density values vary based on the heat treatment temperature of the cermet coatings on the Al7075 substrate, as shown in
Figure 7. Notably, the lowest anodic current density values were observed for the Cr
3C
2-25(Ni20Cr)/Al7075 coating heat-treated at 300 °C (
Figure 7c). When the heat treatment temperature was increased to 500 °C, the anodic current density also increased (
Figure 7d). Therefore, the most effective anti-corrosion properties of the Cr
3C
2-25(Ni20Cr)/Al7075 coating were achieved with heat treatment at 300 °C in a hot air atmosphere.
Characteristic peaks were observed at -480 mV, -370 mV, -200 mV, and -305 mV on the anodic segments of the potentiodynamic polarization curves for the Cr
3C
2-25(Ni20Cr) coatings on the Al7075 substrate (
Figure 7). This suggests that the working electrode surface was primarily covered with a layer of (Cr
2O
3)ads and (NiO)ads oxides, indicating that the Cr
3C
2-25(Ni20Cr)/Al7075 coating was passivated under the test conditions, with the oxides adhering well to the surface. Additionally, it appears that this adsorbed oxide layer can be further stabilized by the adsorption of Cl- ions [
13]:
The adsorption layer (MeClOH)
ads in the acidic chloride solution dissolves according to the following chemical reaction:
This dissolution leads to a further sharp increase in anodic current density as the electrode surface undergoes oxidation (
Figure 7).
3.4.1. Corrosion Electrochemical Parameters
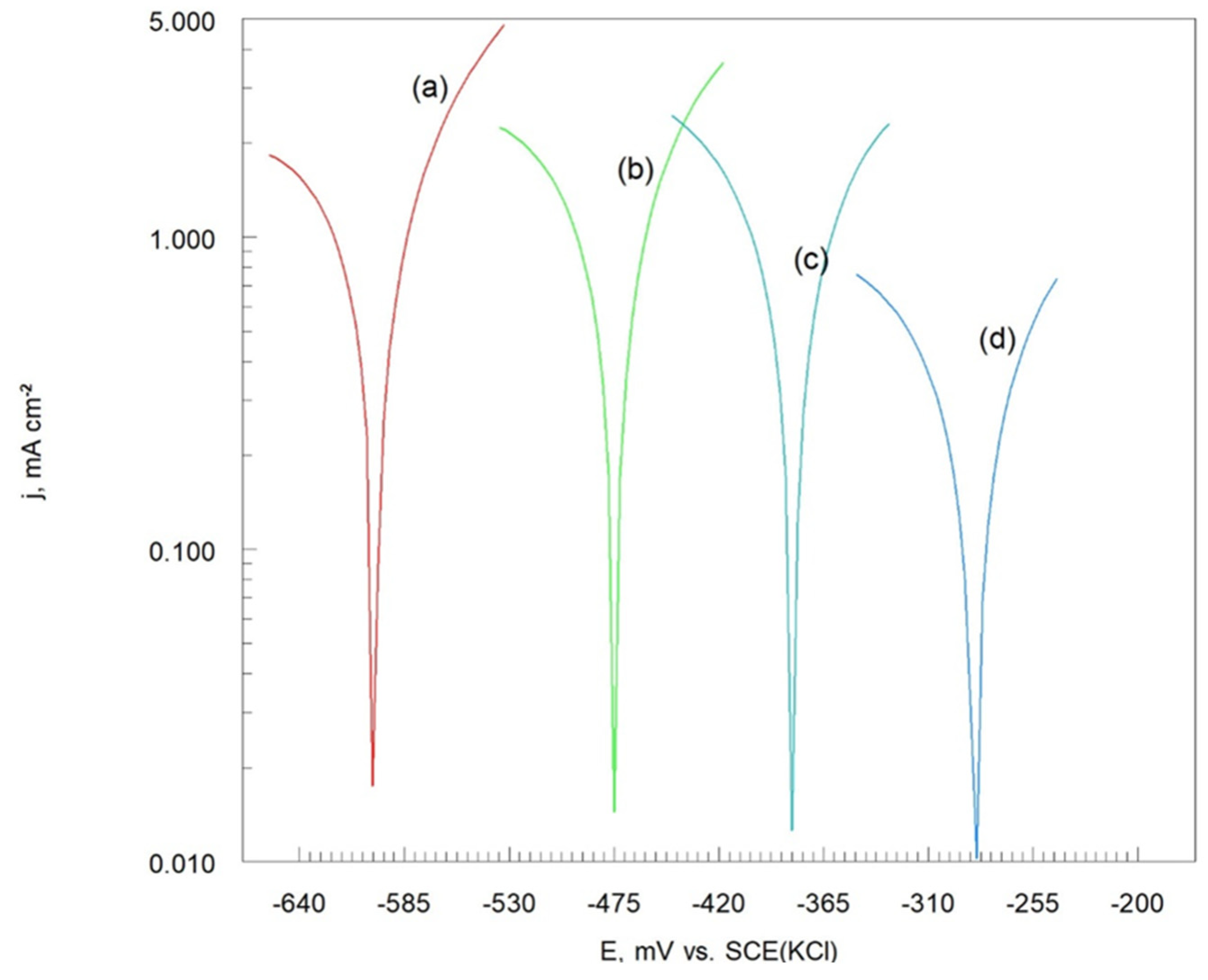
The potentiodynamic polarization curves of the Cr
3C
2-25(Ni20Cr) cermet coatings on the Al7075 substrate were analysed to determine the electrochemical corrosion parameters of the tested coatings, as shown in
Figure 8.
It was observed that the corrosion potential (E
corr) values of the tested materials shift towards more positive values with increasing heat treatment temperature, as shown in
Table 3. This indicates that heat treatment enhances the corrosion resistance of the cermet coatings on the Al7075 substrate. However, once the temperature reaches 500 °C, the corrosion potential shifts to more negative values, suggesting a loss of anti-corrosion properties in the Cr
3C
2–25(Ni20Cr)/Al7075 coating.
At the same time, the lowest corrosion current density j
corr=1.95 mA/cm
2 observed for the Cr
3C
2–25(Ni20Cr) coating treated at 300 °C suggests optimal anti-corrosion performance at this temperature (
Table 3). Notably, the slopes of the Tafel polarization curves, specifically (−b
c) and (b
a), remain relatively unchanged with increasing heat treatment temperature (
Figure 8,
Table 3). This stability in slope values indicates that the corrosion mechanism of the Cr
3C
2–25(Ni20Cr)/Al7075 cermet coatings is not significantly affected by surface heat treatment.
3.4.2. Polarization Resistance and Corrosion Rate
The polarization resistance (R
p) of the Cr
3C
2–25(Ni20Cr)/Al7075 coatings was calculated based on the slope values (
Table 3) derived from the Tafel potentiodynamic polarization curves (
Figure 8). Additionally, for the reaction:
The corrosion rate (CR) of the materials was calculated using the following equation, as described by Scendo et al. (2017, 2014):
The R
p and CR values for the cermet coatings on the Al7075 alloy are summarized in
Table 4.
The highest polarization resistance (R
p) value, 3817 kΩ cm², was observed for the Cr
3C
2–25(Ni20Cr)/Al7075 coating that underwent heat treatment at 300 °C. This high R
p value (
Table 4) indicates a significant impediment to mass and electric charge exchange between the working electrode and the solution. Additionally, the corrosion rate (CR) for the Cr
3C
2–25(Ni20Cr) coating treated at 300 °C was the lowest, at 0.018 mm/y. These findings confirm the earlier conclusion that the Cr
3C
2–25(Ni20Cr) coating on the Al7075 substrate exhibits optimal anti-corrosion properties when heat-treated in an air atmosphere at 300 °C.
3.4.3. Chronoamperometric Measurements
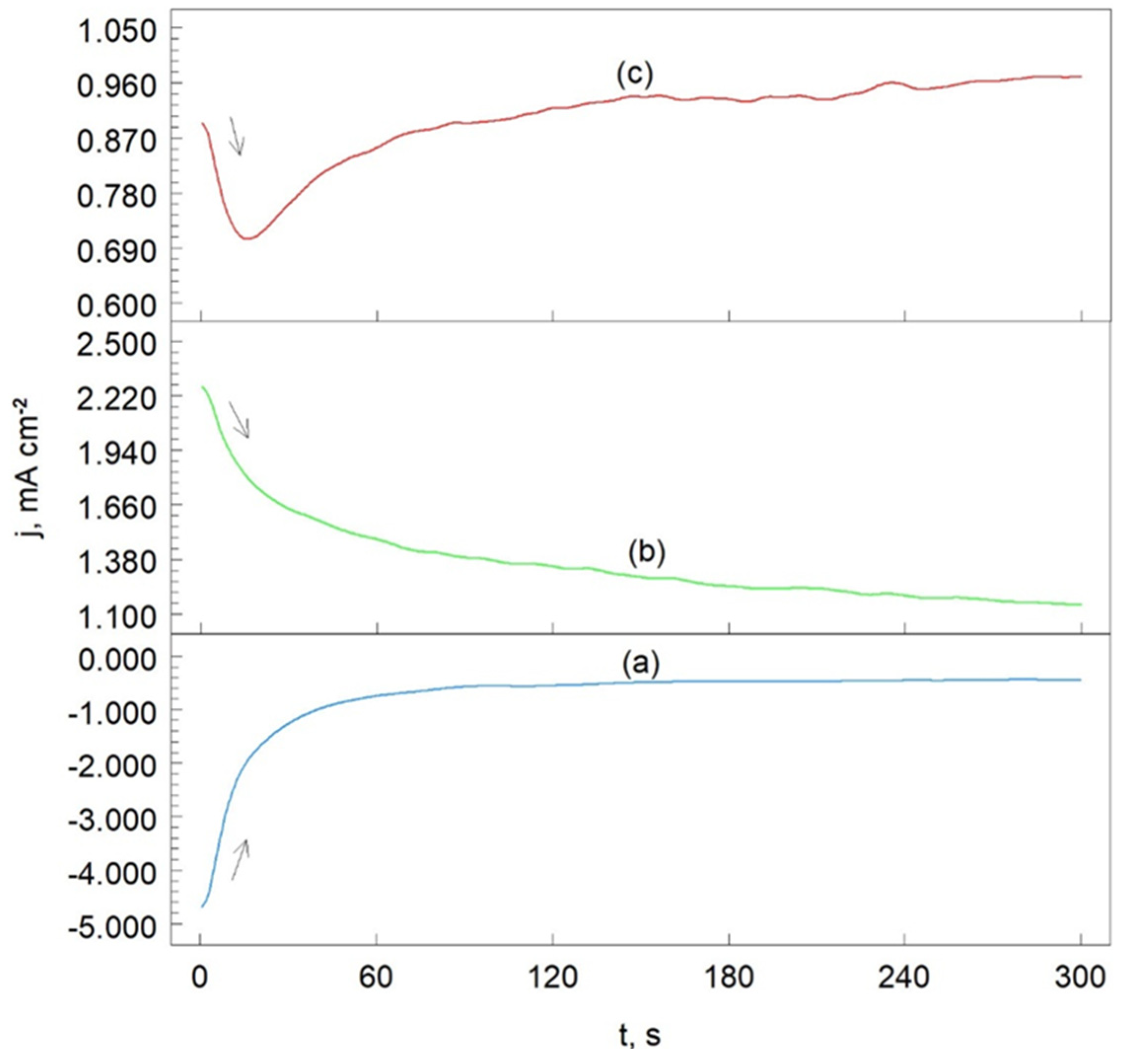
Chronoamperometry (ChA) is the study of current response over time at a specifically chosen potential.
Figure 9 presents the chronoamperometric curves for the Cr
3C
2-25(Ni20Cr) cermet coating on the Al7075 substrate after heat treatment at 300 °C in an acidic chloride solution. Similar ChA curves were also obtained for the Cr
3C
2-25(Ni20Cr)/Al7075 coatings subjected to heat treatments at 100 °C and 500 °C.
The working electrode potentials were chosen based on the potentiodynamic polarization curve (
Figure 7, curve (c)). At a potential of -800 mV vs. SCE(KCl), the reduction of H+ ions (reaction (6)) occurs on the electrode surface (
Figure 9, curve (a)). Initially, the cathodic current density decreases and then stabilizes at approximately -0.8 mA cm
2, indicating that the H+ reduction process is stable under these experimental conditions.
At -200 mV vs. SCE(KCl) (
Figure 9, curve (b)), oxidation of the Cr
3C
2-25(Ni20Cr) cermet coating surface is observed (reaction (7)). Here, the anodic current density systematically decreases, as the oxide layer on the Cr
3C
2-25(Ni20Cr)/Al7075 surface becomes sealed through the adsorption of (Cr
2O
3)
ads, and (NiO)
ads oxides due to reaction (12). This adsorbed layer of chromium and nickel oxides effectively seals the Cr
3C
2-25(Ni20Cr)/Al7075 surface. In the chloride environment (1.2 M Cl
-), the passive oxide layer can be further sealed through the adsorption of Cl
- ions, as described by reaction (8).
For a more positive working electrode potential of +100 mV, the initial oxidation current density of the Cr
3C
2-25(Ni20Cr)/Al7075 surface decreases (up to 20 s), then gradually increases with extended electrolysis time (
Figure 9, curve (c)). The initial decrease in anodic current density results from the dissolution of the (MeClOH)
ads layer as described by reaction (9). This suggests that in the acidic chloride solution, the protective layer adsorbed on the electrode surface is gradually dissolved, leading to the onset of the corrosion process in the Cr
3C
2(Ni20Cr) coating on the Al7075 substrate.
3.5. Surface Morphology After Corrosion Test
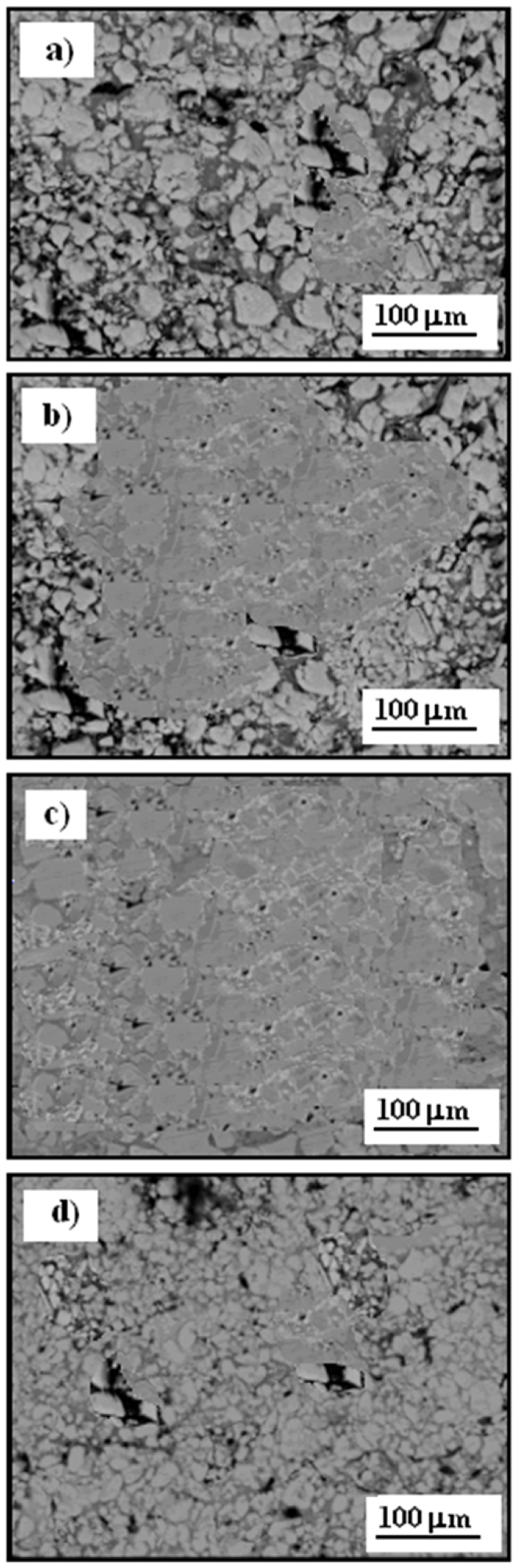
Figure 10 presents scanning electron microscopy (SEM) images of the surface morphology of the Cr
3C
2-25(Ni20Cr) cermet coatings on the Al7075 substrate following a corrosion test in an acidic chloride solution (1.2 M Cl
−) for an exposure time of 5 hours. The oxide layer on the test specimens was subsequently removed using diluted nitric acid, with an exposure time of approximately three minutes.
Figure 10a shows the surface of the Cr
3C
2-25(Ni20Cr) cermet coating without heat treatment, where extensive surface damage is visible due to prolonged exposure to the electrolyte. Numerous deep pits formed as a result of the corrosion process, significantly diminishing the mechanical and aesthetic qualities of the material. Slightly less corrosion damage is observed on the Cr
3C
2-25(Ni20Cr) surface after heat treatment at 100 °C, as shown in
Figure 10b, indicating that low-temperature heat treatment does not substantially improve the coating’s anti-corrosion properties.
The least corrosion damage is observed in
Figure 10c, representing the Cr
3C
2-25(Ni20Cr)/Al7075 surface treated at 300 °C. In this case, the heat treatment effectively hardened and sealed the coating, which significantly slowed the corrosion process and enhanced the material’s resistance in the corrosive environment.
Conversely, after heat treatment at 500 °C, the Cr
3C
2-25(Ni20Cr)/Al7075 surface showed low resistance to the corrosive environment. Extended exposure led to the formation of numerous deep pits on the Cr
3C
2-25(Ni20Cr) surface on the Al7075 substrate due to electrochemical corrosion in the chloride environment, as shown in
Figure 10d. Therefore, the tested cermet coating, when heat-treated at this higher temperature, does not effectively protect the Al7075 substrate from corrosive contact.
4. Conclusions
This paper presents research findings on the impact of heat treatment on the anti-corrosion properties of cold-sprayed Cr3C2-25(Ni20Cr) coatings on an Al7075 substrate in an acidic chloride solution. The results led to the following conclusions:
The heat treatment at 300 °C resulted in the most uniform and smooth structure for the cermet coating.
Annealing introduced new phases, specifically Cr7C3 and Cr23C6, which formed on the cermet surface due to the restructuring and partial decarburization of Cr3C2.
The highest microhardness value was achieved for the coating annealed at 300 °C.
The electrochemical corrosion mechanism of the cermet coatings involves multiple stages, with Cr2O3 and NiO particles as the primary corrosion products. However, this oxide layer did not effectively shield the substrate from the penetration of the corrosive solution.
The Cr3C2-25(Ni20Cr)/Al7075 coating heat-treated in an air atmosphere at 300 °C exhibited the highest polarization resistance and lowest corrosion rate, significantly reducing the exchange of mass and electric charge between the electrode and the electrolyte solution.
Coatings annealed at 100 °C and 500 °C suffered severe damage from electrochemical corrosion after exposure to the aggressive environment, indicating compromised resistance.
Author Contributions
Conceptualization, M.S. and W.Ż.; Formal analysis, M.S.; Funding acquisition, M.S. and W.Ż.; Investigation, M.S.; Methodology, M.S.; Project administration, W.Ż.; Resources, W.Ż.; Supervision, M.S. and W.Ż.; Validation, M.S. and W.Ż.; Visualization, M.S.; Writing—original draft, M.S.; Writing—review & editing, M.S. and W.Ż.; All authors have read and agreed to the published version of the manuscript.
Funding
The work reported herein was supported by project No. 01.1.05.00/1.02.001/ SUBB.MCKN.24.003 funded by the Ministry of Education and Science.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bolelli, G.; Cannillo, V.; Lusvarghi, L.; Montorsi, M.; Mantini, F.P.; Barletta, M. Microstructural and tribological comparison of HVOF-sprayed and post-treated M-Mo-Cr-Si (M = Co, Ni) alloy coatings. Wear 2007, 263, 1397–1416. [Google Scholar] [CrossRef]
- Toma, D.; Brandl, W.; Marginean, G. Wear and corrosion behaviour of thermally sprayed cermet coatings. Surf. Coatings Technol. 2001, 138, 149–158. [Google Scholar] [CrossRef]
- Klinkov, S.V.; Kosarev, V.F.; Rein, M. Cold spray deposition: Significance of particle impact phenomena. Aerosp. Sci. Technol. 2005, 9, 582–591. [Google Scholar] [CrossRef]
- Raletz, F.; Vardelle, M.; Ezo'O, G. Critical particle velocity under cold spray conditions. Surf. Coatings Technol. 2006, 201, 1942–1947. [Google Scholar] [CrossRef]
- Pattison, J.; Celotto, S.; Khan, A.; O'Neill, W. Standoff distance and bow shock phenomena in the Cold Spray process. Surf. Coatings Technol. 2007, 202, 1443–1454. [Google Scholar] [CrossRef]
- Samareh, B.; Stier, O.; Lüthen, V.; Dolatabadi, A. Assessment of CFD Modeling via Flow Visualization in Cold Spray Process. J. Therm. Spray Technol. 2009, 18, 934–943. [Google Scholar] [CrossRef]
- Meyer, M.; Yin, S.; Lupoi, R. Particle In-Flight Velocity and Dispersion Measurements at Increasing Particle Feed Rates in Cold Spray. J. Therm. Spray Technol. 2016, 26, 60–70. [Google Scholar] [CrossRef]
- Meyer, M.; Yin, S.; McDonnell, K.; Stier, O.; Lupoi, R. Feed rate effect on particulate acceleration in Cold Spray under low stagnation pressure conditions. Surf. Coatings Technol. 2016, 304, 237–245. [Google Scholar] [CrossRef]
- Scendo, M.; Staszewska-Samson, K. Effect of Standoff Distance on Corrosion Resistance of Cold Sprayed Titanium Coatings. Coatings 2022, 12, 1853. [Google Scholar] [CrossRef]
- Scendo, M.; Zorawski, W.; Staszewska-Samson, K.; Makrenek, M.; Goral, A. Influence of Surface Pretreatment on the Corrosion Resistance of Cold-Sprayed Nickel Coatings in Acidic Chloride Solution. J. Mater. Eng. Perform. 2018, 27, 1725–1737. [Google Scholar] [CrossRef]
- Sevillano, F.; Poza, P.; Múnez, C.J.; Vezzù, S.; Rech, S.; Trentin, A. Cold-Sprayed Ni-Al2O3 Coatings for Applications in Power Generation Industry. J. Therm. Spray Technol. 2013, 22, 772–782. [Google Scholar] [CrossRef]
- Luo, X.-T.; Li, Y.-J.; Li, C.-J. A comparison of cold spray deposition behavior between gas atomized and dendritic porous electrolytic Ni powders under the same spray conditions. Mater. Lett. 2016, 163, 58–60. [Google Scholar] [CrossRef]
- Scendo, M.; Zorawski, W.; Staszewska-Samson, K.; Goral, A. Influence of laser treatment on the corrosion resistance of Cr3C2-25(Ni20Cr) cermet coating. Materials 2021, 14, 4078–4096. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, T.S.; Prakash, S.; Agrawal, R.D. ; Characterizations of HVOF sprayed NiCrBSi coatings on Ni- and Fe-based superalloys and evaluation of cyclic oxidation behaviour of some Ni-based superalloys in molten salt environment. Thin Solid Film. 2006, 515, 95–105. [Google Scholar] [CrossRef]
- Souza, R.C.; Voorwald, H.J.C.; Cioffi, M.O.H. Fatigue strength of HVOF sprayed Cr3C2/25CrNi and WC-10Ni on AISI 4340 steel. Surf. Coat. Technol. 2008, 203, 191–198. [Google Scholar] [CrossRef]
- Sundararajan, G.; Sudharshan, P.P.; Jyothirmayi, A.; Gundarkaram, R.C. The influence of heat treatment on the micro structural, mechanical and corrosion behaviour of cold sprayed SS 316L coatings. J. Mater. Sci. 2009, 44, 2320–2326. [Google Scholar] [CrossRef]
- Scendo, M.; Staszewska-Samson, K. Effect of Surface Modification on Corrosion Resistance of Uncoated and DLC Coated Stainless Steel Surface. J. Mater. Eng. Perform. 2017, 26, 3946–3953. [Google Scholar] [CrossRef]
- Scendo, M.; Trela, J.; Antoszewski, B.; Kargul, T. Corrosion Resistance of the Joint of Stainless Steels in Aggressive Solution. Innov. Corros. Mater. Sci. (Formerly Recent Patents Corros. Sci. 2015, 4, 118–126. [Google Scholar] [CrossRef]
- Shi, Ch.; Liu, S.; Gong, I.Q.; Wang, H.; Hu, M. Deposition mechanisms and characters of nano-modified multimodal Cr3C2-NiCr coatings sprayed by HVOF. Rev. Adv. Mater. Sci. 2022, 61, 526–538. [Google Scholar] [CrossRef]
Figure 1.
SEM micrograph: a) Cr3C2-25(Ni20Cr) powder morphology, b) X-ray diffraction pattern of powder.
Figure 1.
SEM micrograph: a) Cr3C2-25(Ni20Cr) powder morphology, b) X-ray diffraction pattern of powder.
Figure 2.
Heat treatment diagram of cermet coatings: 1, 2, 3 heating programs.
Figure 2.
Heat treatment diagram of cermet coatings: 1, 2, 3 heating programs.
Figure 3.
Surface morphology of cold sprayed cermet coating onto Al7075 substrate: a) Cr3C2–25(Ni20Cr)/Al7075, b) X-ray diffraction pattern of as-sprayed coating.
Figure 3.
Surface morphology of cold sprayed cermet coating onto Al7075 substrate: a) Cr3C2–25(Ni20Cr)/Al7075, b) X-ray diffraction pattern of as-sprayed coating.
Figure 4.
Surface morphology of cold sprayed cermet coating onto Al7075 substrate after heat treatment at 300 oC: a) Cr3C2–25(Ni20Cr)/Al7075, b) X-ray diffraction pattern of coating.
Figure 4.
Surface morphology of cold sprayed cermet coating onto Al7075 substrate after heat treatment at 300 oC: a) Cr3C2–25(Ni20Cr)/Al7075, b) X-ray diffraction pattern of coating.
Figure 5.
Surface morphology of cold sprayed cermet coating onto Al7075 substrate after heat treatment at 500 oC: a) Cr3C2–25(Ni20Cr)/Al7075, b) X-ray diffraction pattern of coating.
Figure 5.
Surface morphology of cold sprayed cermet coating onto Al7075 substrate after heat treatment at 500 oC: a) Cr3C2–25(Ni20Cr)/Al7075, b) X-ray diffraction pattern of coating.
Figure 6.
SEM of cross-section of Cr3C2-25(Ni20Cr) cermet coatings onto Al7075 substrate: a) before, and after heat treatment at: b) 100 oC, c) 300 oC, and d) 500 oC.
Figure 6.
SEM of cross-section of Cr3C2-25(Ni20Cr) cermet coatings onto Al7075 substrate: a) before, and after heat treatment at: b) 100 oC, c) 300 oC, and d) 500 oC.
Figure 7.
Potentiodynamic polarization curves of Cr3C2-25(Ni20Cr) cermet coatings onto Al7075 substrate: (a) before, and after heat treatment at: (b) 100 oC, (c) 300 oC, and (d) 500 oC. Solution contained 1.2 M Cl-, pH 1.5, dE/dt 1 mV/s.
Figure 7.
Potentiodynamic polarization curves of Cr3C2-25(Ni20Cr) cermet coatings onto Al7075 substrate: (a) before, and after heat treatment at: (b) 100 oC, (c) 300 oC, and (d) 500 oC. Solution contained 1.2 M Cl-, pH 1.5, dE/dt 1 mV/s.
Figure 8.
Potentiodynamic polarization curves on a semi-logarithmic (Tafel scale) of Cr3C2-25(Ni20Cr) cermet coatings onto Al7075 substrate: (a) before, and after heat treatment at: (b) 100 oC, (c) 300 oC, and (d) 500 oC. Solution contained 1.2 M Cl-, pH 1.5.
Figure 8.
Potentiodynamic polarization curves on a semi-logarithmic (Tafel scale) of Cr3C2-25(Ni20Cr) cermet coatings onto Al7075 substrate: (a) before, and after heat treatment at: (b) 100 oC, (c) 300 oC, and (d) 500 oC. Solution contained 1.2 M Cl-, pH 1.5.
Figure 9.
Chronoamperometric curves obtained after heat treatment at 300 oC of Cr3C2-25(Ni20Cr) cermet coating onto Al7075 substrate. The potential values were as follows: (a) -800 mV, (b) -200 mV, and (c) +100 mV. Solutions contained 1.2 M Cl-, pH 1.5.
Figure 9.
Chronoamperometric curves obtained after heat treatment at 300 oC of Cr3C2-25(Ni20Cr) cermet coating onto Al7075 substrate. The potential values were as follows: (a) -800 mV, (b) -200 mV, and (c) +100 mV. Solutions contained 1.2 M Cl-, pH 1.5.
Figure 10.
SEM of surface morphology of Cr3C2-25(Ni20Cr) cermet coatings onto Al7075 substrate after corrosion test: (a) before, and after heat treatment at: (b) 100 oC, (c) 300 oC, and (d) 500 oC. Solutions contained 1.2 M Cl-, pH 1.5. Exposure time was 5 h.
Figure 10.
SEM of surface morphology of Cr3C2-25(Ni20Cr) cermet coatings onto Al7075 substrate after corrosion test: (a) before, and after heat treatment at: (b) 100 oC, (c) 300 oC, and (d) 500 oC. Solutions contained 1.2 M Cl-, pH 1.5. Exposure time was 5 h.
Table 1.
Parameters of heat treatment of cermet coatings.
Table 1.
Parameters of heat treatment of cermet coatings.
Program
number |
Heating rate
oC/h |
Temperature
oC |
Cooling rate
oC/h
|
| 1 |
12.5 |
100 |
9.1 |
| 2 |
37.5 |
300 |
27.3 |
| 3 |
62.5 |
500 |
45.5 |
Table 2.
Microhardness of Cr3C2–25(Ni20Cr) coatings onto Al7075 substrate before, and after heat treatment.
Table 2.
Microhardness of Cr3C2–25(Ni20Cr) coatings onto Al7075 substrate before, and after heat treatment.
| Sample name |
Microhardness
HV10 |
| Cr3C2–25(Ni20Cr) |
326 ± 4 |
| Cr3C2–25(Ni20Cr) - 100 |
478 ± 2 |
| Cr3C2–25(Ni20Cr) - 300 |
695 ± 1 |
| Cr3C2–25(Ni20Cr) - 500 |
549 ± 3 |
Table 3.
Corrosion electrochemical parameters of Cr3C2–25(Ni20Cr) coatings onto Al7075 substrate before, and after heat treatment.
Table 3.
Corrosion electrochemical parameters of Cr3C2–25(Ni20Cr) coatings onto Al7075 substrate before, and after heat treatment.
| Sample name |
Ecorr
mV vs. SCE |
jcorr
mA cm-2
|
-bc
|
ba
|
| mV dec-1
|
| Cr3C2–25(Ni20Cr) |
-602 |
10.23 |
62 |
34 |
| Cr3C2–25(Ni20Cr) - 100 |
-475 |
7.94 |
48 |
34 |
| Cr3C2–25(Ni20Cr) - 300 |
-285 |
1.95 |
40 |
30 |
| Cr3C2–25(Ni20Cr) - 500 |
-379 |
4.79 |
32 |
28 |
Table 4.
Polarization resistance and corrosion rate of Cr3C2–25(Ni20Cr) coatings onto Al7075 substrate before, and after heat treatment.
Table 4.
Polarization resistance and corrosion rate of Cr3C2–25(Ni20Cr) coatings onto Al7075 substrate before, and after heat treatment.
| Sample name |
Rp
kΩ cm2
|
CR
mm/y |
| Cr3C2–25(Ni20Cr) |
932 |
0.081 |
| Cr3C2–25(Ni20Cr) - 100 |
1088 |
0.073 |
|
| Cr3C2–25(Ni20Cr) - 300 |
3817 |
0.018 |
|
| Cr3C2–25(Ni20Cr) - 500 |
1354 |
0.044 |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).