Submitted:
20 November 2024
Posted:
21 November 2024
You are already at the latest version
Abstract
Keywords:
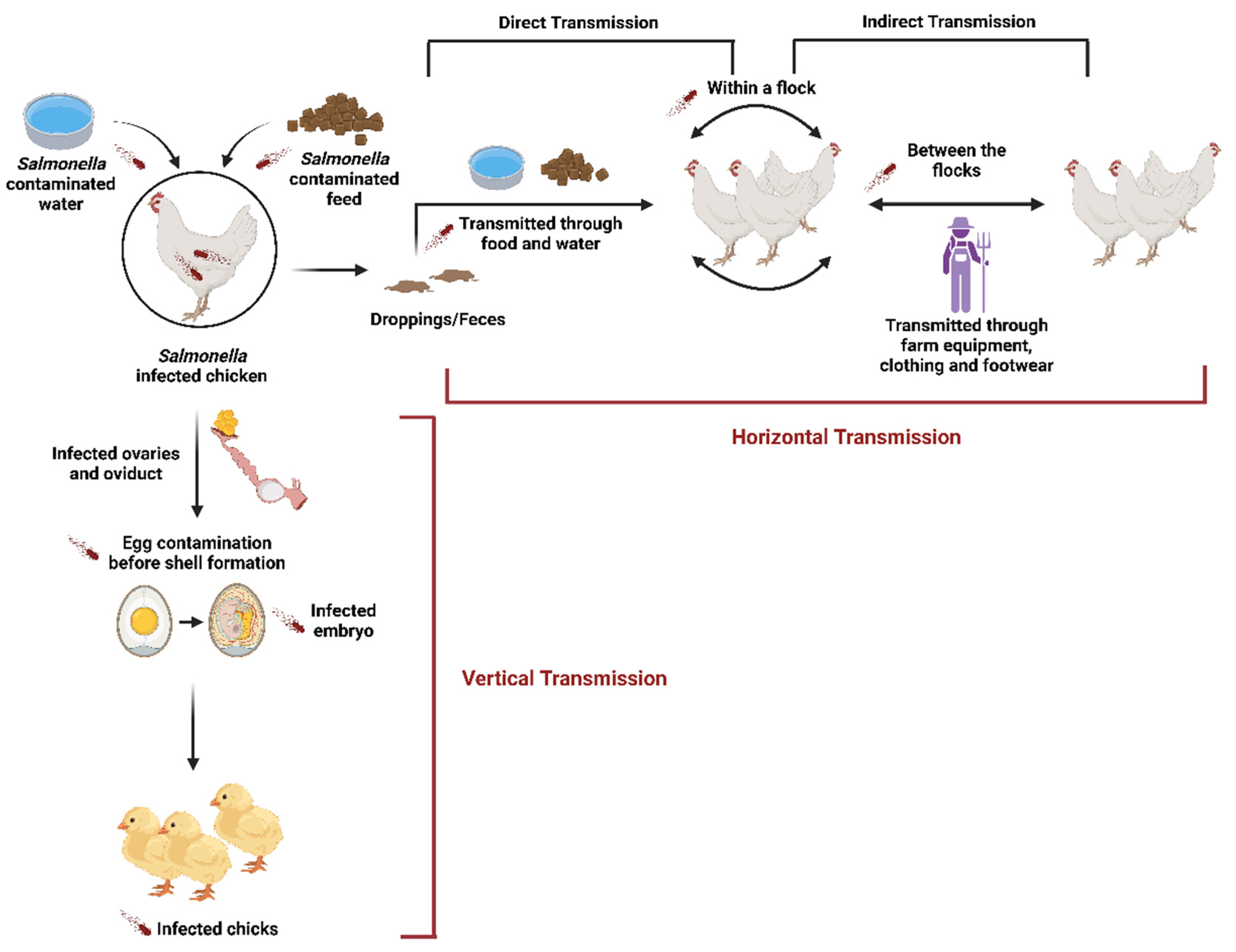
1. Introduction
2. Risk Factors Influencing Salmonella Contamination in the Pre-Harvest Stage
2.1. Environmental Factors
2.2. Farm Management Practices
2.3. Flock Density and Housing Systems
2.4. Bird Age and Immunity
2.5. Feed and Water Quality
2.6. Role of Rodents, Wild Birds, and Insects as Vectors
2.7. Antimicrobial Resistance (AMR) and its Impact on Salmonella Control
2.8. Human Interaction and Farm Personnel Practices
3. Pre-Harvest Control Strategies
3.1. Biosecurity Measures
3.2. Vaccination
3.2.1. Live Attenuated Vaccines
3.2.2. Inactivated Vaccines
3.2.3. Subunit Vaccines
3.2.4. Ghost Vaccines
| Vaccine Name | Constituents | Outcomes | Routes and frequency of administration |
|---|---|---|---|
| Live attenuated | |||
| Nobilis® SG 9R | Live attenuated S. Gallinarum (SG) 9R strain with mutant galE gene [89] | Reduced Salmonella prevalence in vaccinated flocks compared to the control group [90] | Two times via subcutaneous route; 6 weeks and 14-16 weeks of age [90] |
| Avipro® Megan Vac 1 (Elanco, USA) | S. Typhimurium cya crp mutant [89] | Reduction of Salmonella colonization in ceca and reproductive tracts of vaccinated chickens [91]. Reduction of horizontal transfer and liver, spleen ovary, and cecal colonization of S. Enteritidis [92] |
Three times via drinking water; 1 day, 2 weeks, and 5 weeks of age [91] |
| Avipro® Megan Egg (Elanco, USA) |
S. Typhimurium cya,crp mutant strain χ3985 [93] |
Reduce Salmonella colonization in the ceca, spleen, ovary, and bursa in vaccinated birds [93] | Three times via coarse spray; 2, 4, and 16 weeks of age [94] |
| Vaxsafe® ST, Bioproperties, (Australia) | Attenuated aroA deletion S. Typhimurium strain STM-1 [95] |
Reduced excretion of Salmonella in the vaccinated group [95] | Four times: on day 1 via coarse spray, 2 and 6 weeks of age via drinking water, and 12 weeks of age via intramuscular route [96,97] |
| Salmovac® SE (Ceva, France) | Attenuated S. Enteritidis strain 441/014 [98] | Reduce Salmonella colonization in ceca and invasion of internal organs [99] | Three times via drinking water; 1, 6, and 13 weeks of age [99] |
| Gallivac® SE, (Merial, France) |
S. Enteritidis Ade and His mutant [89] |
Reduced colonization of Salmonella in cecum and liver [100] |
Two times via drinking water; 1 and 15 days of age [100] |
| Poulvac® ST, Zoetis | aroA mutant S.Typhimurium [101] | 50% reduction of S. Kentucky, S. Enteritidis, S. Heidelberg, S. Typhimurium, and S. Hadar recovery from internal organs of vaccinated birds [101] | Two times: on day 1 via coarse spray and 2 weeks of age via drinking water [101] |
| Inactivated vaccines | |||
| Nobilis® Salenvac TMSD animal health, NJ, USA | Formalin killed S. Enteritidis and S. Typhimurium bacterin [102] |
Reduction of Salmonella shedding and colonization of internal organs (liver and spleen) [103] | Two times via intra-muscular route; 1 day and 4 weeks of age [103] |
| Layermune® SE (Ceva Biomune, KS, USA) | Killed S. Enteritidis [104] | Reduction of Salmonella shedding and colonization of internal organs (liver and spleen) [104] | Two times via subcutaneous route; 5 and 9 weeks of age [104] |
| Corymune® 4K and 7K (CEVA Corp., Libourne, France) | Killed S. Enteritidis [104] | Reduction of Salmonella shedding and colonization of internal organs (liver and spleen) [104] | Two times via intramuscular route; 5 and 9 weeks of age [104] |
| Poulvac® SE (Zoetis, USA) | Formalin killed S. Enteritidis, Phage Types 4, 8 and 13a [105] |
Reduction of Salmonella colonization in ceca, liver, and spleen after challenge on day 1 [106] | Two times via subcutaneous route; 12 and 20 weeks of age [106] |
| AviPro® 109 SE4 Concentrate (Elanco, USA) | Killed S. Enteritidis [107] | Reduced colonization of Salmonella in internal organs, including reproductive tract | Two times: first via subcutaneous route between 12 and 16 weeks of age and booster vaccination 4 weeks later [108] |
| Avipro® 329 ND-IB2-SE4 Concentrate (Elanco, USA) | Killed chicken bronchitis and Newcastle disease viruses and killed S. Enteritidis [109] |
Reduction of S. Enteritidis colonization in the ceca [109] | Three times: first via subcutaneous route at 12 and 16 weeks of age or intramuscular route at 13 and 17 weeks of age, followed by vaccination with S. Enteritidis monovalent vaccine 4 weeks later [94] |
3.3. Feed additives
3.3.1. Probiotics
3.3.2. Prebiotics
3.3.3. Postbiotics
| Prebiotics | Outcome | Reference |
|---|---|---|
| Mannan-rich yeast cell wall-derived preparation | Significant reductions of Salmonella recovered from ovaries and up to 1 log unit reduction of Salmonella in the ceca and of Salmonella challenged birds | [127] |
| Fructo-oligosaccharides | Dose dependent reduction of S. Enteritidis in the ceca up to 1.3 log 10 in orally challenged birds. Did not change Salmonella isolation from the internal organs (liver, gall bladder, ovary) Increase in TLR-4, IFN, and IgA expression indicating cell-mediated immune activation |
[128,129] |
| Synbiotics | ||
| Bacillus subtilis and yeast cell wall-derived glucomannan | Reduction of S. Enteritidis counts in ceca up to 0.73 log10 CFU/g | [110,111] |
|
Enterococcus faecium, Pediococcus acidilactici, Bifidobacterium animalis, Lactobacilus reuteri) + Fructo-oligosaccharides |
Improved protection provided by vaccination by reducing Salmonella counts in the cecal contents | |
| BacPack® Quality Technology International, Inc. USA. Combination of a Bacillus subtilis strain and Saccharomyces cerevisiae cell wall | Reduction of cecal S. Enteritidis counts at 11-, 15-, and 19-days post-challenge | [128,129] |
|
Bacillus subtilis, B. licheniformis + mannooligosaccharide |
Reduction of cecal S. Enteritidis counts in the ceca and ovaries of challenged birds | |
3.3.3. Organic acids, short- and medium-chain fatty acids
3.3.4. Essential oils
3.3.5. Bacteriophages
3.4. Competitive Exclusion (CE)
3.6. Genetic Approaches
3.7. Antimicrobial Use
4. Established Salmonella Control Programs
4.1. Testing and Monitoring Programs
4.2. Vaccination Requirements
4.3. Biosecurity Protocols
4.4. Certification and Quality Assurance
4.5. Surveillance and Reporting Systems
4.6. Research and Development Initiatives
4.7. Farmer Education and Outreach Programs
4.8. Implementation of Alternative Pathogen Control Methods
5. Challenges and Limitations in Pre-Harvest Control Measures
6. Future Directions and Innovations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Andino, A.; Hanning, I. Salmonella Enterica: Survival, Colonization, and Virulence Differences among Serovars. Scientific World Journal 2015, 2015. [Google Scholar] [CrossRef]
- Hoelzer, K.; Switt, A.I.M.; Wiedmann, M. Animal Contact as a Source of Human Non-Typhoidal Salmonellosis. Vet Res 2011, 42. [Google Scholar] [CrossRef]
- Pandey, M.; Sharath, E.; Goud, K. Non-Typhoidal Salmonellosis: A Major Concern for Poultry Industry, 200AD.
- Salmonella (Non-Typhoidal). Available online: https://www.who.int/news-room/fact-sheets/detail/salmonella-%28non-typhoidal%29 (accessed on 17 November 2024).
- McWhorter, A.R.; Chousalkar, K.K. A Long-Term Efficacy Trial of a Live, Attenuated Salmonella Typhimurium Vaccine in Layer Hens. Front Microbiol 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Samper-Cativiela, C.; Prieto, M.E.; Collado, S.; De Frutos, C.; Branscum, A.J.; Saez, J.L.; Alvarez, J. Risk Factors for Salmonella Detection in Commercial Layer Flocks in Spain. Animals 2023, 13. [Google Scholar] [CrossRef]
- Parsons, B.N.; Humphrey, S.; Salisbury, A.M.; Mikoleit, J.; Hinton, J.C.D.; Gordon, M.A.; Wigley, P. Invasive Non-Typhoidal Salmonella Typhimurium ST313 Are Not Host-Restricted and Have an Invasive Phenotype in Experimentally Infected Chickens. PLoS Negl Trop Dis 2013, 7. [Google Scholar] [CrossRef]
- Shaji, S.; Selvaraj, R.K.; Shanmugasundaram, R. Salmonella Infection in Poultry: A Review on the Pathogen and Control Strategies. Microorganisms 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Bangera, S.R.; Umakanth, S.; Chowdhury, G.; Saha, R.N.; Mukhopadhyay, A.K.; Ballal, M. Poultry: A Receptacle for Non-Typhoidal Salmonellae and Antimicrobial Resistance; 2019; Vol. 11.
- Wibisono, F.M.; Wibisono, F.J.; Helmi Effendi, M.; Plumeriastuti, H.; Hidayatullah, A.R.; Hartadi, E.B.; Sofiana, E.D. A Review of Salmonellosis on Poultry Farms: Public Health Importance; 2020; Vol. 11.
- Bhargavi, D. Risk Analysis of Antimicrobial Resistance among Bacterial Foodborne Pathogens from Broiler Chicken Farms IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF.
- Odoch, T.; Wasteson, Y.; L’Abée-Lund, T.; Muwonge, A.; Kankya, C.; Nyakarahuka, L.; Tegule, S.; Skjerve, E. Prevalence, Antimicrobial Susceptibility and Risk Factors Associated with Non-Typhoidal Salmonella on Ugandan Layer Hen Farms. BMC Vet Res 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Vaddu, S.; Bhumanapalli, S.; Mishra, A.; Applegate, T.; Singh, M.; Thippareddi, H. A Systematic Review and Meta-Analysis of the Sources of Salmonella in Poultry Production (Pre-Harvest) and Their Relative Contributions to the Microbial Risk of Poultry Meat. Poult Sci 2023, 102. [Google Scholar] [CrossRef]
- Denagamage, T.; Jayarao, B.; Patterson, P.; Wallner-Pendleton, E.; Kariyawasam, S. Risk Factors Associated with Salmonella in Laying Hen Farms: Systematic Review of Observational Studies. Avian Dis 2015, 59, 291–302. [Google Scholar] [CrossRef]
- Van Hoorebeke, S.; Van Immerseel, F.; Haesebrouck, F.; Ducatelle, R.; Dewulf, J. The Influence of the Housing System on Salmonella Infections in Laying Hens: A Review. Zoonoses Public Health 2011, 58, 304–311. [Google Scholar] [CrossRef]
- Bashir, A.; Lambert, P.A.; Stedman, Y.; Hilton, A.C. Combined Effect of Temperature and Relative Humidity on the Survival of Salmonella Isolates on Stainless Steel Coupons. Int J Environ Res Public Health 2022, 19. [Google Scholar] [CrossRef]
- Chinivasagam, H.N.; Tran, T.; Maddock, L.; Gale, A.; Blackall, P.J. Mechanically Ventilated Broiler Sheds: A Possible Source of Aerosolized Salmonella, Campylobacter, and Escherichia Coli. Appl Environ Microbiol 2009, 75, 7417–7425. [Google Scholar] [CrossRef] [PubMed]
- Humane Society of the United States, T. WellBeing International WellBeing International WBI Studies Repository WBI Studies Repository 2008 An HSUS Report: Human Health Implications of Intensive Poultry An HSUS Report: Human Health Implications of Intensive Poultry Production and Avian Influenza Production and Avian Influenza An HSUS Report: Human Health Implications of Intensive Poultry Production and Avian Influenza An HSUS Report: Human Health Implications of Intensive Poultry Production and Avian Influenza.
- Abo-Al-Ela, H.G.; El-Kassas, S.; El-Naggar, K.; Abdo, S.E.; Jahejo, A.R.; Al Wakeel, R.A. Stress and Immunity in Poultry: Light Management and Nanotechnology as Effective Immune Enhancers to Fight Stress. [CrossRef]
- Lara, L.J.; Rostagno, M.H. Impact of Heat Stress on Poultry Production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef] [PubMed]
- de Toledo, T. dos S.; Roll, A.A.P.; Rutz, F.; Dallmann, H.M.; Prá, M.A.D.; Leite, F.P.L.; Roll, V.F.B. An Assessment of the Impacts of Litter Treatments on the Litter Quality and Broiler Performance: A Systematic Review and Metaanalysis. PLoS One.
- Pal, A.; Bailey, M.A.; Talorico, A.A.; Krehling, J.T.; Macklin, K.S.; Price, S.B.; Buhr, R.J.; Bourassa, D. V. Impact of Poultry Litter Salmonella Levels and Moisture on Transfer of Salmonella through Associated in Vitro Generated Dust. Poult Sci 2021, 100. [Google Scholar] [CrossRef] [PubMed]
- Trampel, D.W.; Holder, T.G.; Gast, R.K. Integrated Farm Management to Prevent Salmonella Enteritidis Contamination of Eggs. Journal of Applied Poultry Research 2014, 23, 353–365. [Google Scholar] [CrossRef]
- Obe, T.; Boltz, T.; Kogut, M.; Ricke, S.C.; Brooks, L.A.; Macklin, K.; Peterson, A. Controlling Salmonella: Strategies for Feed, the Farm, and the Processing Plant. Poult Sci 2023, 102. [Google Scholar] [CrossRef] [PubMed]
- Wales, A.D.; Allen, V.M.; Davies, R.H. Chemical Treatment of Animal Feed and Water for the Control of Salmonella.
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Cesare, A. De; Herman, L.; Hilbert, F.; Lindqvist, R.; et al. Salmonella Control in Poultry Flocks and Its Public Health Impact. EFSA Journal 2019, 17. [Google Scholar] [CrossRef]
- McMullin, P. Infectious Diseases in Free-Range Compared to Conventional Poultry Production. Avian Pathology 2022, 51, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Report of the Task Force on Zoonoses Data Collection on the Analysis of the Baseline Study on the Prevalence of Salmonella in Holdings of Laying Hen Flocks of Gallus Gallus. EFSA Journal 2007, 5. [CrossRef]
- Van Hoorebeke, S.; Van Immerseel, F.; Schulz, J.; Hartung, J.; Harisberger, M.; Barco, L.; Ricci, A.; Theodoropoulos, G.; Xylouri, E.; De Vylder, J.; et al. Determination of the within and between Flock Prevalence and Identification of Risk Factors for Salmonella Infections in Laying Hen Flocks Housed in Conventional and Alternative Systems. Prev Vet Med 2010, 94, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Namata, H.; Méroc, E.; Aerts, M.; Faes, C.; Abrahantes, J.C.; Imberechts, H.; Mintiens, K. Salmonella in Belgian Laying Hens: An Identification of Risk Factors. Prev Vet Med 2008, 83, 323–336. [Google Scholar] [CrossRef]
- Carrique-Mas, J.J.; Breslin, M.; Snow, L.; McLaren, I.; Sayers, A.R.; Davies, R.H. Persistence and Clearance of Different Salmonella Serovars in Buildings Housing Laying Hens. Epidemiol Infect 2009, 137, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Dórea, F.C.; Cole, D.J.; Hofacre, C.; Zamperini, K.; Mathis, D.; Doyle, M.P.; Lee, M.D.; Maurer, J.J. Effect of Salmonella Vaccination of Breeder Chickens on Contamination of Broiler Chicken Carcasses in Integrated Poultry Operations. Appl Environ Microbiol 2010, 76, 7820–7825. [Google Scholar] [CrossRef] [PubMed]
- Zou, A.; Nadeau, K.; Wang, P.W.; Lee, J.Y.; Guttman, D.S.; Sharif, S.; Korver, D.R.; Brumell, J.H.; Parkinson, J. Accumulation of Genetic Variants Associated with Immunity in the Selective Breeding of Broilers. BMC Genet 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Olson EG, Grenda T, Ghosh A, Ricke SC. Microbial Pathogen Contamination of Animal Feed. Present Knowledge in Food Safety. Elsevier; 2023: 378-393.
- Trampel, D.W.; Holder, T.G.; Gast, R.K. Integrated Farm Management to Prevent Salmonella Enteritidis Contamination of Eggs. Journal of Applied Poultry Research 2014, 23, 353–365. [Google Scholar] [CrossRef]
- Liu, H.; Whitehouse, C.A.; Li, B. Presence and Persistence of Salmonella in Water: The Impact on Microbial Quality of Water and Food Safety. Front Public Health 2018, 6. [Google Scholar] [CrossRef]
- Abreu, R.; Semedo-Lemsaddek, T.; Cunha, E.; Tavares, L.; Oliveira, M. Antimicrobial Drug Resistance in Poultry Production: Current Status and Innovative Strategies for Bacterial Control. Microorganisms 2023, 11. [Google Scholar] [CrossRef]
- Hedman, H.D.; Vasco, K.A.; Zhang, L. A Review of Antimicrobial Resistance in Poultry Farming within Low-resource Settings. Animals 2020, 10, 1–39. [Google Scholar] [CrossRef]
- Liu, F.; Luo, Y.; Xu, T.; Lin, H.; Qiu, Y.; Li, B. Current Examining Methods and Mathematical Models of Horizontal Transfer of Antibiotic Resistance Genes in the Environment. Front Microbiol 2024, 15. [Google Scholar] [CrossRef]
- Oladeinde, A.; Abdo, Z.; Zwirzitz, B.; Woyda, R.; Lakin, S.M.; Press, M.O.; Cox, N.A.; Thomas IV, J.C.; Looft, T.; Rothrock, M.J.; et al. Litter Commensal Bacteria Can Limit the Horizontal Gene Transfer of Antimicrobial Resistance to Salmonella in Chickens. Appl Environ Microbiol 2022, 88. [Google Scholar] [CrossRef]
- Oladeinde, A.; Abdo, Z.; Press, M.O.; Cook, K.; Cox, N.A.; Zwirzitz, B.; Woyda, R.; Lakin, S.M.; Thomas, J.C.; Looft, T.; et al. Horizontal Gene Transfer Is the Main Driver of Antimicrobial Resistance in Broiler Chicks Infected with Salmonella Enterica Serovar Heidelberg.
- Biosecurity in Poultry Farming: Protecting Your Flock. Available online: https://champrix.com/articles/biosecurity-in-poultry-farming-protecting-your-flock (accessed on 14 November 2024).
- Biosecurity on the Poultry Farm. Available online: https://www.veterinariadigital.com/en/articulos/biosecurity-on-the-poultry-farm/ (accessed on 14 November 2024).
- Biosecurity for Poultry Producers. Available online: https://agriculture.vic.gov.au/biosecurity/animal-diseases/poultry-diseases/biosecurity-for-poultry-producers (accessed on 14 November 2024).
- Wegener, H.C.; Hald, T.; Wong, D.L.F.; Madsen, M.; Korsgaard, H.; Bager, F.; Gerner-Smidt, P.; Mølbak, K. Salmonella Control Programs in Denmark. Emerg Infect Dis 2003, 9, 774. [Google Scholar] [CrossRef]
- Anderson, T.C.; Nguyen, T.A.; Adams, J.K.; Garrett, N.M.; Bopp, C.A.; Baker, J.B.; McNeil, C.; Torres, P.; Ettestad, P.J.; Erdman, M.M.; et al. Multistate Outbreak of Human Salmonella Typhimurium Infections Linked to Live Poultry from Agricultural Feed Stores and Mail-Order Hatcheries, United States 2013. One Health 2016, 2, 144–149. [Google Scholar] [CrossRef]
- Ziaul Haque, A.K.M.; Akter, M.R.; Islam, S.K.S.; Alam, J.; Neogi, S.B.; Yamasaki, S.; Lutful Kabir, S.M. Salmonella Gallinarum in Small-Scale Commercial Layer Flocks: Occurrence, Molecular Diversity and Antibiogram. Vet Sci 2021, 8, 71. [Google Scholar] [CrossRef]
- Dorea, F.C.; Berghaus, R.; Hofacre, C.; Cole, D.J. Survey of Biosecurity Protocols and Practices Adopted by Growers on Commercial Poultry Farms in Georgia, U. S. A. Avian Dis 2010, 54, 1007–1015. [Google Scholar] [CrossRef]
- Meher, M.M.; Sharif, Md.A.; Bayazid, A. Al Seroprevalence of Salmonella Spp. Infection in Different Types of Poultry and Biosecurity Measures Associated with Salmonellosis. International Journal of Agriculture Environment and Food Sciences 2022, 6, 557–567. [Google Scholar] [CrossRef]
- Garber, L.; Smeltzer, M.; Fedorka-cray, P.; Ladely, S.; Garber, L.; Smeltzer, A.M.; Fedorka-cray, B.P.; Lad, C.S.; Ferrisd, K. Salmonella Enterica Serotype Enteritidis in Table Egg Layer House Environments and in Mice in U. S. Layer Houses and Associated Risk Factors Published by: American Association of Avian Pathologists Stable URL: Https://Www.Jstor.Org/Stable/1593214 Salm. Avian Dis 2003, 47, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Poirier, E.; Watier, L.; Espie, E.; Weill, F.X.; de Valk, H.; Desenclos, J.C. Evaluation of the Impact on Human Salmonellosis of Control Measures Targeted to Salmonella Enteritidis and Typhimurium in Poultry Breeding Using Time-Series Analysis and Intervention Models in France. Epidemiol Infect 2007, 136, 1217. [Google Scholar] [CrossRef]
- Jones, F.T. A Review of Practical Salmonella Control Measures in Animal Feed. Journal of Applied Poultry Research 2011, 20, 102–113. [Google Scholar] [CrossRef]
- Muckey, M.; Huss, A.R.; Yoder, A.; Jones, C. Research Note: Evaluating the Roles of Surface Sanitation and Feed Sequencing on Mitigating Salmonella Enteritidis Contamination on Animal Food Manufacturing Equipment. Poult Sci 2020, 99, 3841–3845. [Google Scholar] [CrossRef]
- Morita, T.; Kitazawa, H.; Iida, T.; Kamata, S. Prevention of Salmonella Cross-Contamination in an Oilmeal Manufacturing Plant. J Appl Microbiol 2006, 101, 464–473. [Google Scholar] [CrossRef]
- Denagamage, T.; Jayarao, B.; Patterson, P.; Wallner-Pendleton, E.; Kariyawasam, S. Risk Factors Associated with Salmonella in Laying Hen Farms: Systematic Review of Observational Studies. Avian Dis 2015, 59, 291–302. [Google Scholar] [CrossRef]
- Van Immerseel, F.; De Zutter, L.; Houf, K.; Pasmans, F.; Haesebrouck, F.; Ducatelle, R. Strategies to Control Salmonella in the Broiler Production Chain. Worlds Poult Sci J 2009, 65, 367–392. [Google Scholar] [CrossRef]
- Raccoursier, M.; Siceloff, A.T.; Shariat, N.W. In Silico and PCR Screening for a Live Attenuated Salmonella Typhimurium Vaccine Strain. https://doi.org/10.1637/aviandiseases-D-23-00051 2024, 68, 18–24. [CrossRef]
- Cogan, T.A.; Humphrey, T.J. The Rise and Fall of Salmonella Enteritidis in the UK. J Appl Microbiol 2003, 94 Suppl. [CrossRef]
- Howard, A.J.; Chousalkar, K.K.; McWhorter, A.R. In Vitro and in Vivo Efficacy of a Live Attenuated Salmonella Typhimurium Vaccine at Preventing Intestinal Colonization in Chicks. Zoonoses Public Health 2018, 65, 736–741. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, Y.; Ren, J.; Qiao, Z.; Yin, C.; Xian, H.; Yuan, Y.; Geng, S.; Jiao, X. Evaluation of the Safety and Protection Efficacy of SpiC and NmpC or RfaL Deletion Mutants of Salmonella Enteritidis as Live Vaccine Candidates for Poultry Non-Typhoidal Salmonellosis. Vaccines 2019, Vol. 7, Page 202 2019, 7, 202. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Cheng, Z.; Xu, L.; Li, Q.; Geng, S.; Pan, Z.; Jiao, X. Immunogenicity and Protective Efficacy of Salmonella Enterica Serovar Pullorum Pathogenicity Island 2 Mutant as a Live Attenuated Vaccine Candidate. BMC Vet Res 2015, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; McWhorter, A.R.; Andrews, D.M.; Underwood, G.J.; Chousalkar, K.K. Challenges in Vaccinating Layer Hens against Salmonella Typhimurium. Vaccines (Basel) 2020, 8, 1–12. [Google Scholar] [CrossRef]
- Hassan, J.O.; Curtiss, R. Development and Evaluation of an Experimental Vaccination Program Using a Live Avirulent Salmonella Typhimurium Strain to Protect Immunized Chickens against Challenge with Homologous and Heterologous Salmonella Serotypes. Infect Immun 1994, 62, 5519–5527. [Google Scholar] [CrossRef]
- Hassan, J.O.; Curtis, R.I.I.I. Efficacy of a Live Avirulent Salmonella Typhimurium Vaccine in Preventing Colonization and Invasion of Laying Hens by Salmonella Typhimurium and Salmonella Enteritidis. Avian Dis 1997, 41, 783–791. [Google Scholar] [CrossRef]
- van Immerseel, F.; Methner, U.; Rychlik, I.; Nagy, B.; Velge, P.; Martin, G.; Foster, N.; Ducatelle, R.; Barrow, P.A. Vaccination and Early Protection against Non-Host-Specific Salmonella Serotypes in Poultry: Exploitation of Innate Immunity and Microbial Activity. Epidemiol Infect 2005, 133, 959–978. [Google Scholar] [CrossRef]
- Johnson, T.J.; Flores-Figueroa, C.; Munoz-Aguayo, J.; Pinho, G.; Miller, E. Persistence of Vaccine Origin Salmonella Typhimurium through the Poultry Production Continuum, and Development of a Rapid Typing Scheme for Their Differentiation from Wild Type Field Isolates. Poult Sci 2024, 103, 103707. [Google Scholar] [CrossRef]
- Clifton-Hadley, F.A.; Breslin, M.; Venables, L.M.; Sprigings, K.A.; Cooles, S.W.; Houghton, S.; Woodward, M.J. A Laboratory Study of an Inactivated Bivalent Iron Restricted Salmonella Enterica Serovars Enteritidis and Typhimurium Challenge in Chickens. Vet Microbiol 2002, 89, 167–179. [Google Scholar] [CrossRef]
- Filho, R.A.C.P.; de Paiva, J.B.; Argüello, Y.M.S.; da Silva, M.D.; Gardin, Y.; Resende, F.; Junior, A.B.; Sesti, L. Efficacy of Several Vaccination Programmes in Commercial Layer and Broiler Breeder Hens against Experimental Challenge with Salmonella Enterica Serovar Enteritidis. Avian Pathology 2009, 38, 367–375. [Google Scholar] [CrossRef]
- Inoue, A.Y.; Berchieri, A.J.; Bernardino, A.; Paiva, J.B.; Sterzo, E. V Passive Immunity of Progeny from Broiler Breeders Vaccinated with Oil-Emulsion Bacterin Against Salmonella Enteritidis. Avian Dis 2008, 52, 567–571. [Google Scholar] [CrossRef]
- Huberman, Y.D.; Caballero-García, M.; Rojas, R.; Ascanio, S.; Olmos, L.H.; Malena, R.; Lomónaco, J.; Nievas, P.; Chero, P.; Lévano-Gracía, J.; et al. The Efficacy of a Trivalent Inactivated Salmonella Vaccine Combined with the Live S. Gallinarum 9R Vaccine in Young Layers after Experimental Infections with S. Enteritidis, S. Typhimurium, and S. Infantis. Vaccines (Basel) 2022, 10, 1113. [Google Scholar] [CrossRef]
- Barrow, P.A. Salmonella Infections: Immune and Non-Immune Protection with Vaccines. Avian pathology 2007, 36, 1–13. [Google Scholar] [CrossRef]
- Desin, T.S.; Köster, W.; Potter, A.A. Salmonella Vaccines in Poultry: Past, Present and Future. Expert Rev Vaccines 2013, 12, 87–96. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Q.; Yi, J.; Liang, K.; Hu, B.; Zhang, X.; Curtiss, R.; Kong, Q. Outer Membrane Vesicles from Flagellin-Deficient Salmonella Enterica Serovar Typhimurium Induce Cross-Reactive Immunity and Provide Cross-Protection against Heterologous Salmonella Challenge. Sci Rep 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Renu, S.; Patil, V.; Schrock, J.; Feliciano-Ruiz, N.; Selvaraj, R.; Renukaradhya, G.J. Immune Response to Salmonella Enteritidis Infection in Broilers Immunized Orally With Chitosan-Based Salmonella Subunit Nanoparticle Vaccine. Front Immunol 2020, 11. [Google Scholar] [CrossRef]
- Li, Q.; Ren, J.; Xian, H.; Yin, C.; Yuan, Y.; Li, Y.; Ji, R.; Chu, C.; Qiao, Z.; Jiao, X. ROmpF and OMVs as Efficient Subunit Vaccines against Salmonella Enterica Serovar Enteritidis Infections in Poultry Farms. Vaccine 2020, 38, 7094–7099. [Google Scholar] [CrossRef]
- Dolatyabi, S.; Renu, S.; Schrock, J.; Renukaradhya, G.J. Chitosan-Nanoparticle-Based Oral Salmonella Enteritidis Subunit Vaccine Elicits Cross-Protection against Salmonella Typhimurium in Broilers. Poult Sci 2024, 103. [Google Scholar] [CrossRef]
- Han, Y.; Renu, S.; Patil, V.; Schrock, J.; Feliciano-Ruiz, N.; Selvaraj, R.; Renukaradhya, G.J. Immune Response to Salmonella Enteritidis Infection in Broilers Immunized Orally With Chitosan-Based Salmonella Subunit Nanoparticle Vaccine. Front Immunol 2020, 11. [Google Scholar] [CrossRef]
- Renu, S.; Han, Y.; Dhakal, S.; Lakshmanappa, Y.S.; Ghimire, S.; Feliciano-Ruiz, N.; Senapati, S.; Narasimhan, B.; Selvaraj, R.; Renukaradhya, G.J. Chitosan-Adjuvanted Salmonella Subunit Nanoparticle Vaccine for Poultry Delivered through Drinking Water and Feed. Carbohydr Polym 2020, 243. [Google Scholar] [CrossRef]
- Han, Y.; Renu, S.; Schrock, J.; Acevedo-Villanuev, K.Y.; Lester, B.; Selvaraj, R.K.; Renukaradhya, G.J. Temporal Dynamics of Innate and Adaptive Immune Responses in Broiler Birds to Oral Delivered Chitosan Nanoparticle-Based Salmonella Subunit Antigens. Vet Immunol Immunopathol 2020, 228. [Google Scholar] [CrossRef]
- Han, Y.; Renu, S.; Patil, V.; Schrock, J.; Feliciano-Ruiz, N.; Selvaraj, R.; Renukaradhya, G.J. Mannose-Modified Chitosan-Nanoparticle-Based Salmonella Subunit Oralvaccine-Induced Immune Response and Efficacy in a Challenge Trial in Broilers. Vaccines (Basel) 2020, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Renu, S.; Markazi, A.D.; Dhakal, S.; Lakshmanappa, Y.S.; Shanmugasundaram, R.; Selvaraj, R.K.; Renukaradhya, G.J. Oral Deliverable Mucoadhesive Chitosan-Salmonella Subunit Nanovaccine for Layer Chickens. Int J Nanomedicine 2020, 15, 761–777. [Google Scholar] [CrossRef]
- Senevirathne, A.; Hewawaduge, C.; Park, J.Y.; Park, S.; Lee, J.H. Parenteral Immunization of Salmonella Typhimurium Ghosts with Surface-Displayed Escherichia Coli Flagellin EnhancesTLR-5 Mediated Activation of Immune Responses That Protect the Chicken against Salmonella Infection. Microb Pathog 2020, 147. [Google Scholar] [CrossRef]
- Hajam, I.A.; Kim, J.H.; Lee, J.H. Incorporation of Membrane-Anchored Flagellin into Salmonella Gallinarum Bacterial Ghosts Induces Early Immune Responses and Protection against Fowl Typhoid in Young Layer Chickens. Vet Immunol Immunopathol 2018, 199, 61–69. [Google Scholar] [CrossRef]
- Senevirathne, A.; Hewawaduge, C.; Lee, J.H. Immunization of Chicken with Flagellin Adjuvanted Salmonella Enteritidis Bacterial Ghosts Confers Complete Protection against Chicken Salmonellosis. Poult Sci 2021, 100. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.A.; Jawale, C. V.; Kim, S.W.; Lee, J.H. Construction of a Salmonella Gallinarum Ghost as a Novel Inactivated Vaccine Candidate and Its Protective Efficacy against Fowl Typhoid in Chickens. Vet Res 2012, 43. [Google Scholar] [CrossRef]
- Jawale, C. V.; Lee, J.H. Characterization of a Salmonella Typhimurium Ghost Carrying an Adjuvant Protein as a Vaccine Candidate for the Protection of Chickens against Virulent Challenge. Avian Pathology 2014, 43, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Senevirathne, A.; Hewawaduge, C.; Lee, J.H. Salmonella Enterica Serovar Enteritidis Ghosts Displaying a Surface FliC Adjuvant Elicit a Robust Immune Response and Effective Protection against Virulent Challenge. Vet Microbiol 2020, 243. [Google Scholar] [CrossRef] [PubMed]
- Senevirathne, A.; Hewawaduge, C.; Lee, J.H. Immunization of Chickens with Salmonella Gallinarium Ghosts Expressing Salmonella Enteritidis NFliC-FimAC and CD40LC Fusion Antigen Enhances Cell-Mediated Immune Responses and Protects against Wild-Type Challenges with Both Species. Dev Comp Immunol 2022, 126. [Google Scholar] [CrossRef]
- Curtiss, R. Vaccines to Control Salmonella in Poultry. Avian Dis 2023, 67, 427–440. [Google Scholar] [CrossRef]
- Feberwee, A.; Vries, T.S. de.; Hartman, E.G.; Wit, J.J. de.; Elbers, A.R.W.; Jong, W.A. de. Vaccination against Salmonella Enteritidis in Dutch Commercial Layer Flocks with a Vaccine Based on a Live Salmonella Gallinarum 9R Strain: Evaluation of Efficacy, Safety, and Performance of Serologic Salmonella Tests. Avian Dis 2001, 45, 83–91. [Google Scholar] [CrossRef]
- Dórea, F.C.; Cole, D.J.; Hofacre, C.; Zamperini, K.; Mathis, D.; Doyle, M.P.; Lee, M.D.; Maurer, J.J. Effect of Salmonella Vaccination of Breeder Chickens on Contamination of Broiler Chicken Carcasses in Integrated Poultry Operations. Appl Environ Microbiol 2010, 76, 7820–7825. [Google Scholar] [CrossRef]
- Holt, P.S.; Gast, R.K.; Kelly-Aehle, S. Use of a Live Attenuated Salmonella Typhimurium Vaccine to Protect Hens Against Salmonella Enteritidis Infection While Undergoing Molt. Avian Dis 2003, 47, 656–661. [Google Scholar] [CrossRef]
- Hassan, J.O.; Curtiss, R. Development and Evaluation of an Experimental Vaccination Program Using a Live Avirulent Salmonella Typhimurium Strain to Protect Immunized Chickens against Challenge with Homologous and Heterologous Salmonella Serotypes. Infect Immun 1994, 62, 5519–5527. [Google Scholar] [CrossRef]
- Poultry Salmonella Control.
- Alderton, M.R.; Fahey, K.J.; Coloe, P.J. Humoral Responses and Salmonellosis Protection in Chickens given a Vitamin-Dependent Salmonella Typhimurium Mutant. Avian Dis 1991, 35, 435–442. [Google Scholar] [CrossRef]
- Bioproperties - Vaxsafe ST. Available online: http://www.bioproperties.com.au/!Pages/Vaccines/VaxsafeST.html (accessed on 13 November 2024).
- Sharma, P.; Caraguel, C.; Sexton, M.; McWhorter, A.; Underwood, G.; Holden, K.; Chousalkar, K. Shedding of Salmonella Typhimurium in Vaccinated and Unvaccinated Hens during Early Lay in Field Conditions: A Randomised Controlled Trial. BMC Microbiol 2018, 18, 1–9. [Google Scholar] [CrossRef]
- Theuß, T.; Woitow, G.; Bulang, M.; Springer, S. Demonstration of the Efficacy of a Salmonella Enteritidis Live Vaccine for Chickens According to the Current European Pharmacopoeia Monograph. Heliyon 2018, 4, e01070. [Google Scholar] [CrossRef] [PubMed]
- Salmovac® - Ceva Poultry. Available online: https://poultry.ceva.com/poultry-vaccines/salmovac/?gad_source=1&gclid=CjwKCAiAudG5BhAREiwAWMlSjFs98cpFpjgtAxQt5k2SVYcOcjd8oAlkkN8cvUf-EL-83G3_0MfSzxoCuFAQAvD_BwE (accessed on 13 November 2024).
- Porcicultura, Salud Porcina, Bienestar, Enfermedades, Noticias Porcinas, Artículos, Fotos - El Sitio Porcino. Available online: https://www.elsitioporcino.com/ (accessed on 13 November 2024).
- POULVAC® ST | For Animal Healthcare Professionals. Available online: https://www.zoetisus.com/products/poultry/poulvac-st (accessed on 13 November 2024).
- Nobilis® Salenvac T - MSD Animal Health India. Available online: https://www.msd-animal-health.co.in/products/nobilis-salenvac-t/ (accessed on 13 November 2024).
- Clifton-Hadley, F.A.; Breslin, M.; Venables, L.M.; Sprigings, K.A.; Cooles, S.W.; Houghton, S.; Woodward, M.J. A Laboratory Study of an Inactivated Bivalent Iron Restricted Salmonella Enterica Serovars Enteritidis and Typhimurium Challenge in Chickens. Vet Microbiol 2002, 89, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Filho, R.A.C.P.; de Paiva, J.B.; Argüello, Y.M.S.; da Silva, M.D.; Gardin, Y.; Resende, F.; Junior, A.B.; Sesti, L. Efficacy of Several Vaccination Programmes in Commercial Layer and Broiler Breeder Hens against Experimental Challenge with Salmonella Enterica Serovar Enteritidis. Avian Pathology 2009, 38, 367–375. [Google Scholar] [CrossRef]
- Poulvac® SE | For Animal Healthcare Professionals. Available online: https://www.zoetisus.com/products/poultry/poulvac-se (accessed on 13 November 2024).
- Inoue, A.Y.; Berchieri, A.J.; Bernardino, A.; Paiva, J.B.; Sterzo, E. V Passive Immunity of Progeny from Broiler Breeders Vaccinated with Oil-Emulsion Bacterin Against Salmonella Enteritidis. Avian Dis 2008, 52, 567–571. [Google Scholar] [CrossRef] [PubMed]
- 196-278500 | Animal and Plant Health Inspection Service. Available online: https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/veterinary-biologics/product-summaries/vet-label-data/8a736d32-8d89-4f1d-b1c7-931951a9e03b (accessed on 13 November 2024).
- Poultry Salmonella Control. Available online: https://farmanimal.elanco.com/us/poultry/collection/salmonella-control (accessed on 2 November 2024).
- 196-48D711 | Animal and Plant Health Inspection Service. Available online: https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/veterinary-biologics/product-summaries/vet-label-data/17f69ae2-1ae2-46a1-815f-e33a54dae527 (accessed on 13 November 2024).
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat Rev Gastroenterol Hepatol 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Groves, P.J.; Williamson, S.L.; Ahaduzzaman, M.; Diamond, M.; Ngo, M.; Han, A.; Sharpe, S.M. Can a Combination of Vaccination, Probiotic and Organic Acid Treatment in Layer Hens Protect against Early Life Exposure to Salmonella Typhimurium and Challenge at Sexual Maturity? Vaccine 2021, 39, 815–824. [Google Scholar] [CrossRef]
- Khan, S.; Chousalkar, K.K. Salmonella Typhimurium Infection Disrupts but Continuous Feeding of Bacillus Based Probiotic Restores Gut Microbiota in Infected Hens. J Anim Sci Biotechnol 2020, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Price, P.T.; Gaydos, T.A.; Berghaus, R.D.; Baxter, V.; Hofacre, C.L.; Sims, M.D. Salmonella Enteritidis Reduction in Layer Ceca with a Bacillus Probiotic. Vet World 2020, 13, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Castillo, N.A.; Perdigán, G.; De Moreno De Leblanc, A. Oral Administration of a Probiotic Lactobacillus Modulates Cytokine Production and TLR Expression Improving the Immune Response against Salmonella Enterica Serovar Typhimurium Infection in Mice. BMC Microbiol 2011, 11. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic Mechanisms of Action. Ann Nutr Metab 2012, 61, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Marković, R.; Šefer, D.; Krstić, M.; Petrujkić, B. Effect of Different Growth Promoters on Broiler Performance and Gut Morphology. Arch Med Vet 2009, 41, 163–169. [Google Scholar] [CrossRef]
- Samanya, M.; Yamauchi, K. Histological Alterations of Intestinal Villi in Chickens Fed Dried Bacillus Subtilis Var. Natto. Comp Biochem Physiol A Mol Integr Physiol 2002, 133, 95–104. [Google Scholar] [CrossRef]
- Oh, J.K.; Pajarillo, E.A.B.; Chae, J.P.; Kim, I.H.; Kang, D.K. Protective Effects of Bacillus Subtilis against Salmonella Infection in the Microbiome of Hy-Line Brown Layers. Asian-Australas J Anim Sci 2017, 30, 1332–1339. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat Rev Gastroenterol Hepatol 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Froebel, L.K.; Froebel, L.E.; Duong, T. Refined Functional Carbohydrates Reduce Adhesion of Salmonella and Campylobacter to Poultry Epithelial Cells in Vitro. Poult Sci 2020, 99, 7027–7034. [Google Scholar] [CrossRef]
- Pandey, K.R.; Naik, S.R.; Vakil, B. V. Probiotics, Prebiotics and Synbiotics- a Review. J Food Sci Technol 2015, 52, 7577–7587. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.; Nastoh, N.; Çinar, A.; Farooq, M.; Salman, M. Advantages of the Use of Postbiotics in Poultry Production: A New Concept. Brazilian Journal of Poultry Science 2024, 26. [Google Scholar] [CrossRef]
- The Power of Postbiotics for Poultry. Available online: https://phileo-lesaffre.com/en/the-power-of-postbiotics-for-poultry/ (accessed on 17 November 2024).
- Postbiotics Effective in Reducing Salmonella Prevalence in Poultry, Says Phileo – INDUSTRY PERSPECTIVES. Available online: https://www.feedinfo.com/perspectives/postbiotics-effective-in-reducing-salmonella-prevalence-in-poultry-says-phileo-industry-perspectives/251513 (accessed on 17 November 2024).
- The Role of Postbiotics in Promoting Gut Health in Poultry. Available online: https://www.feedandadditive.com/the-role-of-postbiotics-in-promoting-gut-health-in-poultry/ (accessed on 17 November 2024).
- Mishra, B.; Mishra, A.K.; Mohanta, Y.K.; Yadavalli, R.; Agrawal, D.C.; Reddy, H.P.; Gorrepati, R.; Reddy, C.N.; Mandal, S.K.; Shamim, M.Z.; et al. Postbiotics: The New Horizons of Microbial Functional Bioactive Compounds in Food Preservation and Security. Food Production, Processing and Nutrition, 2024; 6. [Google Scholar]
- Girgis, G.; Powell, M.; Youssef, M.; Graugnard, D.E.; King, W.D.; Dawson, K.A. Effects of a Mannan-Rich Yeast Cell Wall-Derived Preparation on Cecal Concentrations and Tissue Prevalence of Salmonella Enteritidis in Layer Chickens. PLoS One 2020, 15, e0232088. [Google Scholar] [CrossRef]
- Araba, M.; Girgis, G.; McBride, H.; Lohrmann, T. Effect of a Bacillus Subtilis plus Yeast Cell Wall Synbiotic on Salmonella Enteritidis Colonization in Ceca of Layer Pullets. Poultry 2024, Vol. 3, Pages 26-35 2024, 3, 26–35. [Google Scholar] [CrossRef]
- Kimminau, E.A.; Karnezos, T.P.; Berghaus, R.D.; Jones, M.K.; Baxter, J.A.; Hofacre, C.L. Combination of Probiotic and Prebiotic Impacts Salmonella Enteritidis Infection in Layer Hens. Journal of Applied Poultry Research 2021, 30. [Google Scholar] [CrossRef]
- Gomez-Osorio, L.M.; Yepes-Medina, V.; Ballou, A.; Parini, M.; Angel, R. Short and Medium Chain Fatty Acids and Their Derivatives as a Natural Strategy in the Control of Necrotic Enteritis and Microbial Homeostasis in Broiler Chickens. Front Vet Sci 2021, 8. [Google Scholar] [CrossRef]
- Van Immerseel, F.; Russell, J.; Flythe, M.; Gantois, I.; Timbermont, L.; Pasmans, F.; Haesebrouck, F.; Ducatelle, R. The Use of Organic Acids to Combat Salmonella in Poultry: A Mechanistic Explanation of the Efficacy. [CrossRef]
- Abd El-Ghany, W.A. Applications of Organic Acids in Poultry Production: An Updated and Comprehensive Review. Agriculture (Switzerland) 2024, 14. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, L.; Guo, F.; Huang, J.; Qiao, J.; Bi, R.; Huang, J.; Zhang, K.; Guo, Y.; Wang, Z. Dietary Supplemental Coated Essential Oils and Organic Acids Mixture Improves Growth Performance and Gut Health along with Reduces Salmonella Load of Broiler Chickens Infected with Salmonella Enteritidis. J Anim Sci Biotechnol 2023, 14. [Google Scholar] [CrossRef]
- Böhme, K.; Barros-Velázquez, J.; Calo-Mata, P.; Aubourg, S.P. Antibacterial, Antiviral and Antifungal Activity of Essential Oils: Mechanisms and Applications. In Antimicrobial Compounds: Current Strategies and New Alternatives; Springer-Verlag Berlin Heidelberg, 2014; Vol. 9783642404443, pp. 51–81 ISBN 9783642404443.
- Essential Oils for Chickens: The (Science-Backed) Natural Approach For Flock Health and Productivity. Available online: Https://Rosehillfarm.ca/2020/07/31/Essential-Oils-for-Chickens/ (accessed on 12 November 2024).
- Andreatti Filho, R.L.; Higgins, J.P.; Higgins, S.E.; Gaona, G.; Wolfenden, A.D.; Tellez, G.; Hargis, B.M. Ability of Bacteriophages Isolated from Different Sources to Reduce Salmonella Enterica Serovar Enteritidis in Vitro and in Vivo. Poult Sci 2007, 86, 1904–1909. [Google Scholar] [CrossRef]
- Atterbury, R.J.; Van Bergen, M.A.P.; Ortiz, F.; Lovell, M.A.; Harris, J.A.; De Boer, A.; Wagenaar, J.A.; Allen, V.M.; Barrow, P.A. Bacteriophage Therapy to Reduce Salmonella Colonization of Broiler Chickens. Appl Environ Microbiol 2007, 73, 4543–4549. [Google Scholar] [CrossRef]
- Borie, C.; Albala, I.; Sànchez, P.; Sánchez, M.L.; Ramírez, S.; Navarro, C.; Morales, M.A.; Retamales, J.; Robeson, J. Bacteriophage Treatment Reduces Salmonella Colonization of Infected Chickens. Avian Dis 2008, 52, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Colom, J.; Cano-Sarabia, M.; Otero, J.; Cortés, P.; Maspoch, D.; Llagostera, M. Liposome-Encapsulated Bacteriophages for Enhanced Oral Phage Therapy against Salmonella Spp. Appl Environ Microbiol 2015, 81, 4841. [Google Scholar] [CrossRef]
- O’Sullivan, L.; Bolton, D.; McAuliffe, O.; Coffey, A. Bacteriophages in Food Applications: From Foe to Friend. Annu Rev Food Sci Technol 2019, 10, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, E.A.; Wojtasik, A.; Gorecka, E.; Stanczyk, M.; Dastych, J. Application of Bacteriophage Preparation Bafasal® Іn Broiler Chickens Experimentally Exposed to Salmonella Spp. Наукoвo-технічний бюлетень Державнoгo наукoвo-дoсліднoгo кoнтрoльнoгo інституту ветеринарних препаратів та кoрмoвих дoбавoк і Інституту біoлoгії тварин, 2015; 241–251. [Google Scholar]
- Clavijo, V.; Baquero, D.; Hernandez, S.; Farfan, J.C.; Arias, J.; Arévalo, A.; Donado-Godoy, P.; Vives-Flores, M. Phage Cocktail SalmoFREE® Reduces Salmonella on a Commercial Broiler Farm. Poult Sci 2019, 98, 5054. [Google Scholar] [CrossRef]
- Żbikowska, K.; Michalczuk, M.; Dolka, B. The Use of Bacteriophages in the Poultry Industry. Animals (Basel) 2020, 10, 872. [Google Scholar] [CrossRef] [PubMed]
- Nurmi, E.; Nuotio, L.; Schneitz, C. The Competitive Exclusion Concept: Development and Future. Int J Food Microbiol 1992, 15, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Gast, R.K. Serotype-Specific and Serotype-Independent Strategies for Preharvest Control of Food-Borne Salmonella in Poultry. https://doi.org/10.1637/8090-081807.1 2007, 51, 817–828. [CrossRef]
- Schneitz, C. Competitive Exclusion in Poultry––30 Years of Research. Food Control 2005, 16, 657–667. [Google Scholar] [CrossRef]
- Nisbet, D. Defined Competitive Exclusion Cultures in the Prevention of Enteropathogen Colonisation in Poultry and Swine. Antonie Van Leeuwenhoek 2002, 81, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Bar-Shira, E.; Friedman, A. Development and Adaptations of Innate Immunity in the Gastrointestinal Tract of the Newly Hatched Chick. Dev Comp Immunol 2006, 30, 930–941. [Google Scholar] [CrossRef]
- Methner, U.; Haase, A.; Berndt, A.; Martin, G.; Nagy, B.; Barrow, P.A. Exploitation of Intestinal Colonization-Inhibition between Salmonella Organisms for Live Vaccines in Poultry - Potential and Limitations. Zoonoses Public Health 2011, 58, 540–548. [Google Scholar] [CrossRef]
- Braukmann, M.; Barrow, P.A.; Berndt, A.; Methner, U. Combination of Competitive Exclusion and Immunisation with a Live Salmonella Vaccine in Newly Hatched Chickens: Immunological and Microbiological Effects. Res Vet Sci 2016, 107, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.M.; Pavic, A. Advances in Enteropathogen Control in Poultry Production. J Appl Microbiol 2010, 108, 745–755. [Google Scholar] [CrossRef]
- Ferreira, A.J.P.; Ferreira, C.S.A.; Knobl, T.; Moreno, A.M.; Bacarro, M.R.; Chen, M.; Robach, M.; Mead, G.C. Comparison of Three Commercial Competitive-Exclusion Products for Controlling Salmonella Colonization of Broilers in Brazil. J Food Prot 2003, 66, 490–492. [Google Scholar] [CrossRef]
- Calenge, F.; Kaiser, P.; Vignal, A.; Beaumont, C. Selection Evolution Open Access REVIEW BioMed Central Genetic Control of Resistance to Salmonellosis and to Salmonella Carrier-State in Fowl: A Review; 2010.
- Li, X.; Nie, C.; Liu, Y.; Chen, Y.; Lv, X.; Wang, L.; Zhang, J.; Li, K.; Jia, Y.; Ban, L.; et al. A Genome-Wide Association Study Explores the Genetic Determinism of Host Resistance to Salmonella Pullorum Infection in Chickens. Genetics Selection Evolution 2019, 51. [Google Scholar] [CrossRef]
- Jie, H.; Liu, Y.P. Breeding for Disease Resistance in Poultry: Opportunities with Challenges. Worlds Poult Sci J 2011, 67, 687–696. [Google Scholar] [CrossRef]
- Gul, H.; Habib, G.; Khan, I.M.; Rahman, S.U.; Khan, N.M.; Wang, H.; Khan, N.U.; Liu, Y. Genetic Resilience in Chickens against Bacterial, Viral and Protozoal Pathogens. Front Vet Sci 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Castro-Vargas, R.E.; Herrera-Sánchez, M.P.; Rodríguez-Hernández, R.; Rondón-Barragán, I.S. Antibiotic Resistance in Salmonella Spp. Isolated from Poultry: A Global Overview. Vet World 2020, 13, 2070–2084. [Google Scholar] [CrossRef]
- Wang, Y.; Ge, H.; Wei, X.; Zhao, X. Research Progress on Antibiotic Resistance of Salmonella. Food Quality and Safety 2022, 6. [Google Scholar] [CrossRef]
- Ban on Antibiotics as Growth Promoters in Animal Feed Enters into Effect. Https://Ec.Europa.Eu/Commission/Presscorner/Detail/En/Ip_05_1687 (Accessed on 12 November 2024).
- FDA Announces Transition of Over-the-Counter Medically Important Antimicrobials for Animals to Prescription Status. Https://Www.Fda.Gov/Animal-Veterinary/Cvm-Updates/Fda-Announces-Transition-over-Counter-Medically-Important-Antimicrobials-Animals-Prescription-Status (Accessed on 12 November 2024).
- Prevention, Detection and Control of Salmonella in Poultry. Available online: https://food.ec.europa.eu/system/files/2016-10/ia_standards_oie_78_eu-position_annex-xvii_revised-ch-6.5-salmonella-in-poultry.pdf (accessed on 14 November 2024).
- Guidelines For The Control of Nontyphoidal Salmonella Spp. in Beef and Pork Meat. Available online: Https://Www.Fao.Org/Fao-Who-Codexalimentarius/Sh-Proxy/Pt/?Lnk=1&url=https%253A%252F%252Fworkspace.Fao.Org%252Fsites%252Fcodex%252FStandards%252FCXG%2B87-2016%252FCXG_087e.Pdf (accessed on 15 November 2024).
- Control of Salmonella. Available online: https://food.ec.europa.eu/food-safety/biological-safety/food-borne-diseases-zoonoses/control-salmonella_en#:~:text=Special%20guarantees%20for%20Salmonella%20in%20certain%20EU%20countries,-When%20the%20prevalence&text=Such%20guarantees%20include%20extended%20monitoring,(table%20eggs)%20and%20Norway (accessed on 14 November 2024).
- Montoro-Dasi, L.; Lorenzo-Rebenaque, L.; Marco-Fuertes, A.; Vega, S.; Marin, C. Holistic Strategies to Control Salmonella Infantis: An Emerging Challenge in the European Broiler Sector. Microorganisms 2023, 11. [Google Scholar] [CrossRef]
- NVAP Reference Guide: National Poultry Improvement Plan. Available online: https://www.aphis.usda.gov/nvap/reference-guide/poultry/npip (accessed on 14 November 2024).
- Contains Nonbinding Recommendations Questions and Answers Regarding the Final Rule, Prevention of Salmonella Enteritidis in Shell Eggs During Production, Storage, and Transportation: Guidance for Industry .
- EggRuleAtAGlance_090710_Web (2).
- Regulation (EC) No 2160/2003 of the European Parliament and of the Council of 17 November 2003 on the Control of Salmonella and Other Specified Food-Borne Zoonotic Agents. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32003R2160 (accessed on 14 November 2024).
- NPIP Salmonella Update 2024-History of Salmonella and the NPIP .
- National Poultry Improvement Plan (NPIP). Available online: https://www.poultryimprovement.org/ (accessed on 14 November 2024).
- Food and Drug Administration-CFR - Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=118.6&utm_source=chatgpt.com (accessed on 19 November 2024).
- New Solutions for Better Salmonella Control in Europe. Available online: https://www.poultryworld.net/health-nutrition/health/new-solutions-for-better-salmonella-control-in-europe/#:~:text=Ceva%20Sant%C3%A9%20Animale%20registered%20a,Salmonella%20Enteritidis%20and%20Salmonella%20Typhimurium (accessed on 14 November 2024).
- Varmuzova, K.; Faldynova, M.; Elsheimer-Matulova, M.; Sebkova, A.; Polansky, O.; Havlickova, H.; Sisak, F.; Rychlik, I. Immune Protection of Chickens Conferred by a Vaccine Consisting of Attenuated Strains of Salmonella Enteritidis, Typhimurium and Infantis. Vet Res 2016, 47. [Google Scholar] [CrossRef]
- Ceva Introduces Its New Solutions for Better Salmonella Control in Europe. Available online: https://www.thepoultrysite.com/news/2023/10/ceva-introduces-its-new-solutions-for-better-salmonella-control-in-europe (accessed on 14 November 2024).
- Prevention of Salmonella Enteritidis in Shell Eggs During Production, Storage, and Transportation .
- How to Protect Your Bird Backyard Biosecurity. Available online: https://www.oregon.gov/oda/shared/Documents/Publications/AnimalHealth/ProtectBirdsPoster.pdf (accessed on 14 November 2024).
- Why You Should Only Buy Baby Chicks from NPIP-Certified Hatcheries. Available online: https://www.freedomrangerhatchery.com/blog/understanding-npip-certification-standards-for-poultry/#:~:text=By%20obtaining%20an%20NPIP%2Dcertified,of%20the%20birds%20they%20produce (accessed on 14 November 2024).
- Salmonella, Mycoplasma and Avian Influenza Monitoring in Parent Breeder Flocks. Available online: https://www.hyline.com/ViewFile?id=a8f0f50a-81c9-48a7-99e5-e4a84eaf40ba (accessed on 14 November 2024).
- Salmonella Control: Comparing US and EU Regulations for Poultry. Available online: https://xtalks.com/salmonella-control-comparing-us-and-eu-regulations-for-poultry-3750/#:~:text=The%20EU%20takes%20a%20different,products%20reach%20foodservice%20or%20retail (accessed on 14 November 2024).
- Salmonella. Available online: https://www.efsa.europa.eu/en/topics/topic/salmonella#:~:text=Salmonella%20is%20a%20bacterium%20that,indirectly%20between%20animals%20and%20humans (accessed on 14 November 2024).
- CDC PulseNet Outbreak Detection. Available online: https://www.cdc.gov/pulsenet/hcp/about/outbreak-detection.html#:~:text=PulseNet%20rapidly%20identifies%20potential%20outbreaks,assist%20epidemiologists%20in%20investigating%20outbreaks (accessed on 14 November 2024).
- CDC Foodborne Diseases Active Surveillance Network. Available online: https://www.cdc.gov/foodnet/reports/preliminary-data.html (accessed on 14 November 2024).
- Rizzi, V.; Boelaert, F.; Mäkelä, P.; Takkinen, J.; Ammon, A. Decrease in Human Salmonellosis in the European Union and Reduction of the Salmonella Prevalence in Poultry Populations.
- Checklist for Biosecurity Training. Available online: Https://Www.Aphis.Usda.Gov/Sites/Default/Files/2023-11/Fsc-Birds-Checklist-Biosecurity-Training.Pdf (accessed on 15 November 2024).
- Alternatives to Antibiotics (ATA) Challenges and Solutions in Animal Production. Available online: https://www.ars.usda.gov/alternativestoantibiotics/ResearchCenter/ATAindex.html (accessed on 14 November 2024).
- Kumar, M.; Sarma, D.K.; Shubham, S.; Kumawat, M.; Verma, V.; Nina, P.B.; JP, D.; Kumar, S.; Singh, B.; Tiwari, R.R. Futuristic Non-Antibiotic Therapies to Combat Antibiotic Resistance: A Review. Front Microbiol 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Ayana, G.U.; Kamutambuko, R. Probiotics in Disease Management for Sustainable Poultry Production. Advanced Gut & Microbiome Research 2024, 2024. [Google Scholar] [CrossRef]
- Antunes, P.; Mourão, J.; Campos, J.; Peixe, L. Salmonellosis: The Role of Poultry Meat. Clinical Microbiology and Infection 2016, 22, 110–121. [Google Scholar] [CrossRef]
- Halder, N.; Sunder, J.; De, A.K.; Bhattacharya, D.; Joardar, S.N. Probiotics in Poultry: A Comprehensive Review. The Journal of Basic and Applied Zoology 2024, 85. [Google Scholar] [CrossRef]
- Probiotic Intervention to Prevent Salmonella Infection in Poultry. Available online: https://today.uconn.edu/2021/06/probiotic-intervention-to-prevent-salmonella-infection-in-poultry/ (accessed on 14 November 2024).
- Hong, H.A.; Le, H.D.; Cutting, S.M. The Use of Bacterial Spore Formers as Probiotics. FEMS Microbiol Rev 2005, 29, 813–835. [Google Scholar] [CrossRef] [PubMed]
| Probiotic | Outcome | Reference |
|---|---|---|
| B. subtilis CSL2 | Re-establishment of normal gut flora abundance (phylum Firmicutes and Proteobacteria and genus Lactobacillus) that is disrupted after Salmonella infection | [118] |
| Poultry Star® Enterococcus faecium, Pediococcus acidilactici, Bifidobacterium animalis, and Lactobacillus reuteri |
Increased the efficacy of the live attenuated vaccine (aroA mutant S. Typhimurium) and reduced the cecal colonization of Salmonella | [111] |
|
Bacillus subtilis DSM 32324, Bacillus subtilis DSM 32325, and Bacillus amyloliquefaciens |
Reduction of Salmonella in cecal content and establishment of normal gut flora after Salmonella challenge | [112] |
|
Bacillus amyloliquefaciens, B. licheniformis, and B. pumilus |
Significant reduction of Salmonella in cecal content 7 days after challenge | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





