Submitted:
19 November 2024
Posted:
20 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication Process
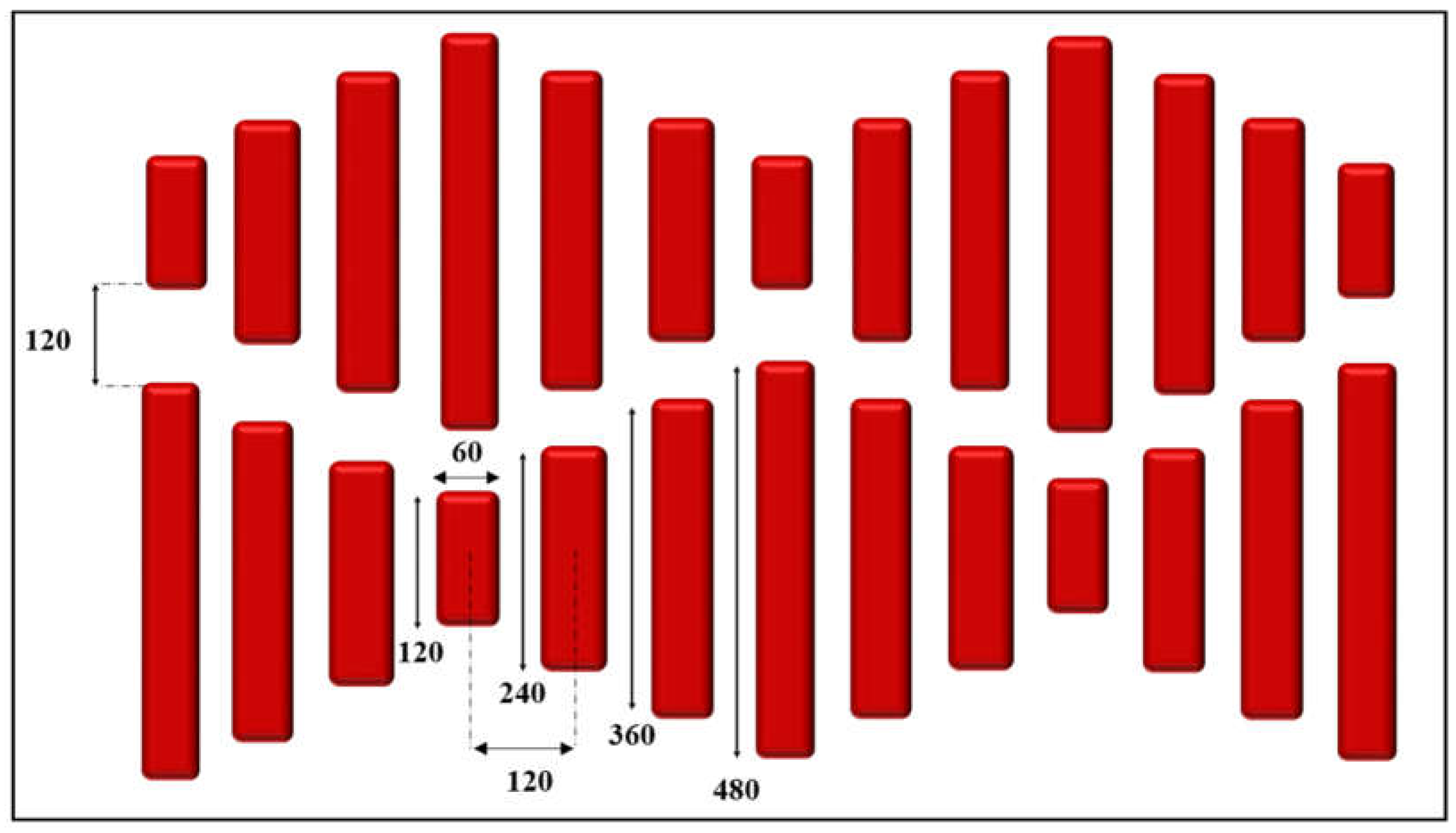
2.2.1. Femtosecond Laser Machining
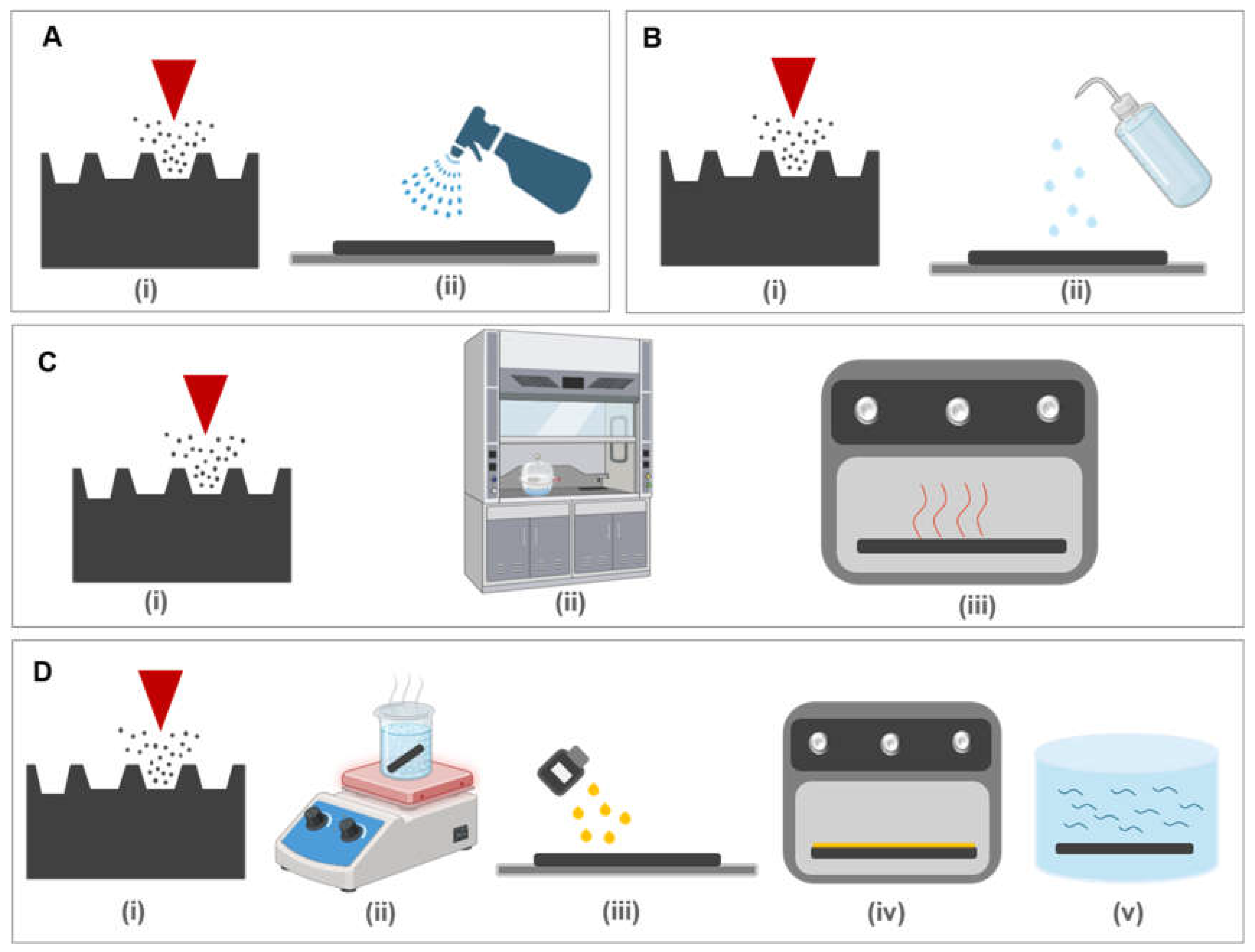
2.2.2. Post-Processing Procedure
- i.
- Spray Coating and IPA treatment
- ii.
- Silanization
- iii.
- Silicone oil Treatment
2.3. Characterization Tests
3. Results and Discussion
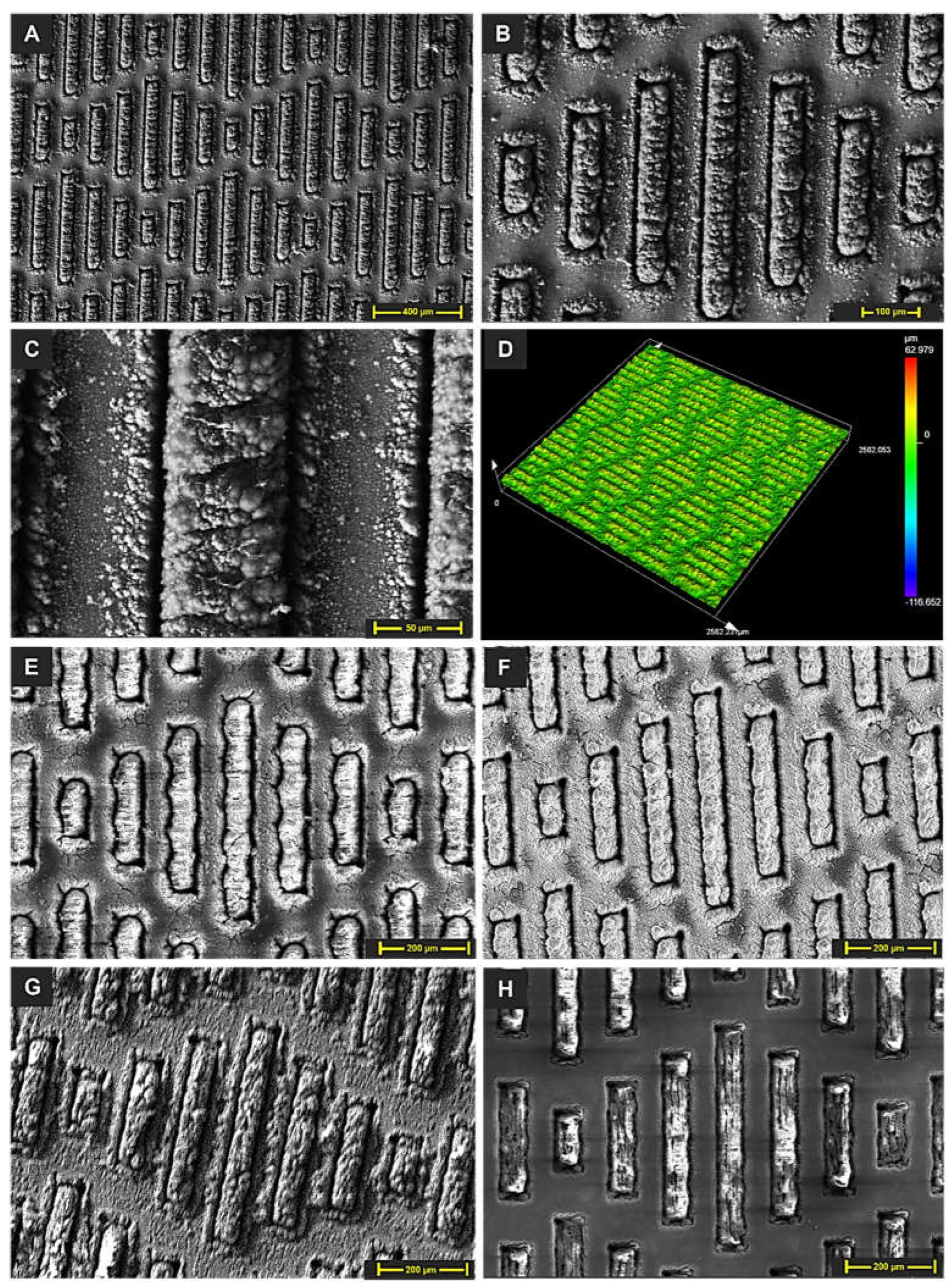
3.1. Surface Morphology
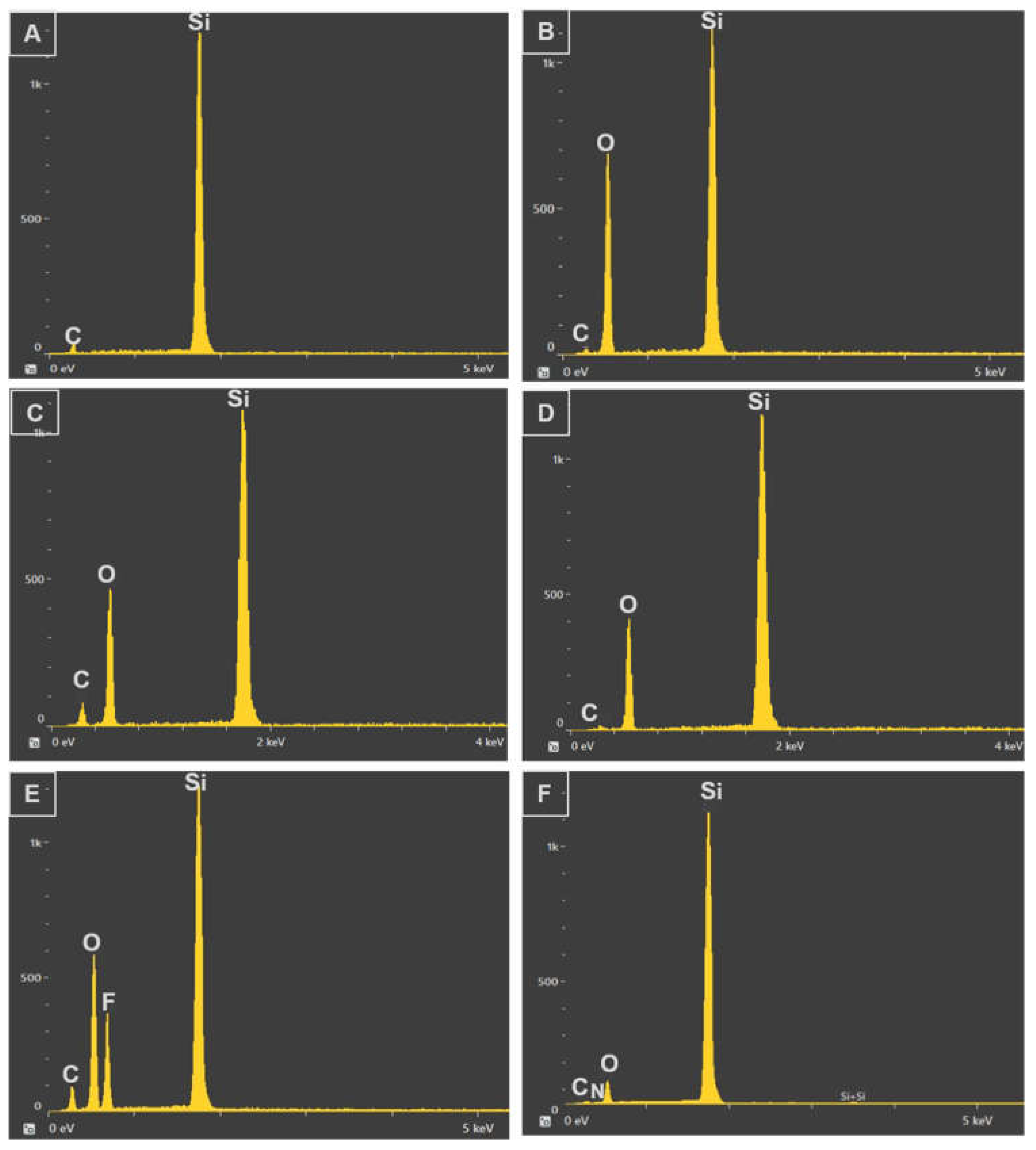
3.2. Chemical Composition
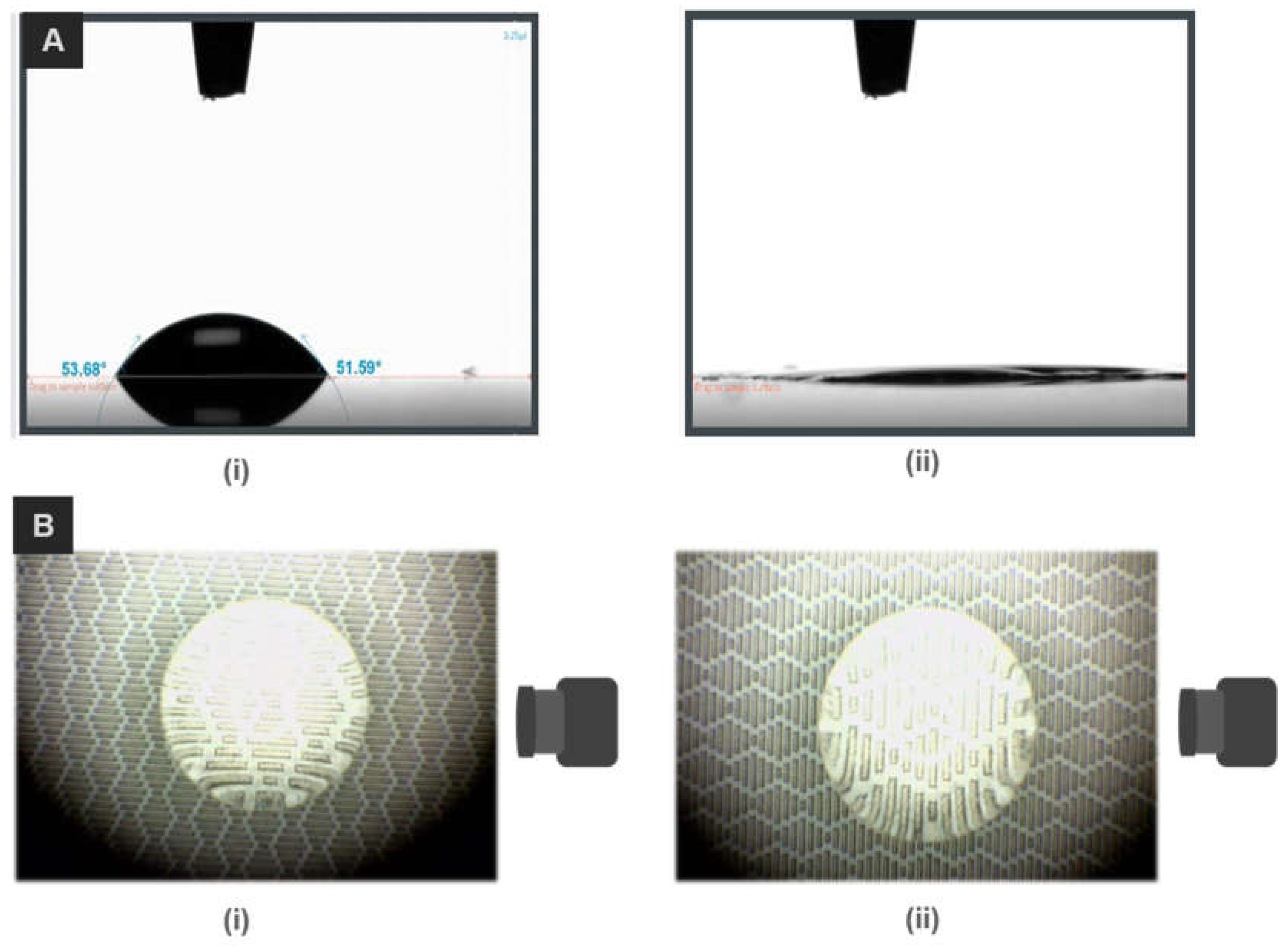
3.3. Water Contact Angle Measurement
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.Z.; Militky, J.; Petru, M.; Tomková, B.; Ali, A.; Tören, E.; Perveen, S. Recent advances in superhydrophobic surfaces for practical applications: A review. Eur. Polym. J. 2022, 178, 111481. [CrossRef]
- Darband, G.B.; Aliofkhazraei, M.; Khorsand, S.; Sokhanvar, S.; Kaboli, A. Science and engineering of superhydrophobic surfaces: review of corrosion resistance, chemical and mechanical stability. Arab. J. Chem. 2020, 13, 1763-1802. [CrossRef]
- Sotoudeh, F.; Mousavi, S.M.; Karimi, N.; Lee, B.J.; Abolfazli-Esfahani, J.; Manshadi, M.K. Natural and synthetic superhydrophobic surfaces: A review of the fundamentals, structures, and applications. Alex. Eng. J. 2023, 68, 587-609. [CrossRef]
- Shen, D.; Ming, W.; Ren, X.; Xie, Z.; Liu, X. Progress in non-traditional processing for fabricating superhydrophobic surfaces. Micromachines. 2021, 12, 1003. [CrossRef]
- Rasitha, T.; Sofia, S.; Anandkumar, B.; Philip, J. Long term antifouling performance of superhydrophobic surfaces in seawater environment: Effect of substrate material, hierarchical surface feature and surface chemistry. Colloids Surf. A: Physicochem. Eng. Asp. 2022, 647, 129194. [CrossRef]
- Sahin, F.; Celik, N.; Ceylan, A.; Pekdemir, S.; Ruzi, M.; Onses, M.S. Antifouling superhydrophobic surfaces with bactericidal and SERS activity. Chem. Eng. J. 2022, 431, 133445. [CrossRef]
- Hsu, W.-T.; Lee, N.; Yun, M.; Lee, D.; Cho, H.H. Unidirectional wicking-driven flow boiling on tilted pillar structures for high-power applications. Int. J. Heat Mass Transfer. 2022, 189, 122673. [CrossRef]
- Feng, J.; Rothstein, J.P. One-way wicking in open micro-channels controlled by channel topography. J. Colloid Interface Sci. 2013, 404, 169-178. [CrossRef]
- Ge, P.; Wang, S.; Zhang, J.; Yang, B. Micro-/nanostructures meet anisotropic wetting: from preparation methods to applications. Mater. Horiz. 2020, 7, 2566-2595.
- Zhang, M.; Chu, L.; Chen, J.; Qi, F.; Li, X.; Chen, X.; Yu, D.-G. Asymmetric wettability fibrous membranes: Preparation and biologic applications. Compos. B Eng. 2023, 111095. [CrossRef]
- Samanta, A.; Wang, Q.; Shaw, S.K.; Ding, H. Roles of chemistry modification for laser textured metal alloys to achieve extreme surface wetting behaviors. Mater.Des. 2020, 192, 108744. [CrossRef]
- Vu, H.H.; Nguyen, N.K.; Singha, P.; Walker, G.; Nguyen, N.T.; Kashaninejad, N. Exploring Wettability of Re-Entrant Microstructures: Effects of Geometry and Material Composition. Adv. Mater. Interfaces. 2024, 2400626. [CrossRef]
- Cheng, C.T.; To, S. Wetting Characteristics of Micro-patterned Surfaces Fabricated by Ultra-precision Raster Milling. In Fly Cutting Technology for Ultra-precision Machining; Springer: 2023; pp. 393-412.
- Basset, S.; Heisbourg, G.; Pascale-Hamri, A.; Benayoun, S.; Valette, S. Effect of texturing environment on wetting of biomimetic superhydrophobic surfaces designed by femtosecond laser texturing. Nanomater. 2022, 12, 3099. [CrossRef]
- Zhu, D.; Shi, Z.; Tan, X.; Zhang, J.; Zhang, S.; Zhang, X. Accelerated wetting transition from hydrophilic to hydrophobic of sputtered Cu films with micro-scale patterns. Appl. Surf. Sci. 2020, 527, 146741. [CrossRef]
- Allione, M.; Limongi, T.; Marini, M.; Torre, B.; Zhang, P.; Moretti, M.; Perozziello, G.; Candeloro, P.; Napione, L.; Pirri, C.F. Micro/nanopatterned superhydrophobic surfaces fabrication for biomolecules and biomaterials manipulation and analysis. Micromachines. 2021, 12, 1501. [CrossRef]
- Ikhsan, S.N.W.; Yusof, N.; Aziz, F.; Ismail, A.F.; Jaafar, J.; Salleh, W.N.W.; Misdan, N. Superwetting materials for hydrophilic-oleophobic membrane in oily wastewater treatment. J. Environ. Manage. 2021, 290, 112565. [CrossRef]
- Lee, E. Simple fabrication of asphalt-based superhydrophobic surface with controllable wetting transition from Cassie-Baxter to Wenzel wetting state. Colloids Surf. A: Physicochem. Eng. Asp. 2021, 625, 126927. [CrossRef]
- Čereška, D.; Žemaitis, A.; Kontenis, G.; Nemickas, G.; Jonušauskas, L. On-demand wettability via combining fs laser surface structuring and thermal post-treatment. Mater. 2022, 15, 2141. [CrossRef]
- Vorobyev, A.; Guo, C. Laser turns silicon superwicking. Opt. Express. 2010, 18, 6455-6460. [CrossRef]
- Samanta, A.; Huang, W.; Parveg, A.S.; Kotak, P.; Auyeung, R.C.; Charipar, N.A.; Shaw, S.K.; Ratner, A.; Lamuta, C.; Ding, H. Enabling superhydrophobicity-guided superwicking in metal alloys via a nanosecond laser-based surface treatment method. ACS appl. Mater. Interfaces. 2021, 13, 41209-41219. [CrossRef]
- Pu, Z.; Jing, X.; Yang, C.; Wang, F.; Ehmann, K.F. Wettability modification of zirconia by laser surface texturing and silanization. Int. J. Appl. Ceram. Technol. 2020, 17, 2182-2192. [CrossRef]
- Tran, N.G.; Chun, D.-M. Simple and fast surface modification of nanosecond-pulse laser-textured stainless steel for robust superhydrophobic surfaces. CIRP Ann. 2020, 69, 525-528. [CrossRef]
- Yan, H.; Rashid, M.R.B.A.; Khew, S.Y.; Li, F.; Hong, M. Wettability transition of laser textured brass surfaces inside different mediums. Appl. Surf. Sci. 2018, 427, 369-375. [CrossRef]
- Hwang, G.B.; Page, K.; Patir, A.; Nair, S.P.; Allan, E.; Parkin, I.P. The anti-biofouling properties of superhydrophobic surfaces are short-lived. ACS Nano. 2018, 12, 6050-6058. [CrossRef]
- Zhang, L.; Liu, G.; Chen, H.; Liu, X.; Ran, T.; Zhang, Y.; Gan, Y.; Zhang, D. Bioinspired unidirectional liquid transport micro-nano structures: A review. J. Bionic. Eng. 2021, 18, 1-29. [CrossRef]
- Sun, D.; Böhringer, K.F. Self-cleaning: From bio-inspired surface modification to MEMS/microfluidics system integration. Micromachines. 2019, 10, 101. [CrossRef]
- Gao, H.; Qian, H.; Meng, Z.; Chang, S.; Wang, X.; Han, Z.; Liu, Y. Bioinspired interlaced wetting surfaces for continuous on-demand emulsion separation. J. Hazard. Mater. 2024, 136011. [CrossRef]
- Wang, S.; Liu, K.; Yao, X.; Jiang, L. Bioinspired surfaces with superwettability: new insight on theory, design, and applications. Chem. Rev. 2015, 115, 8230-8293. [CrossRef]
- Leng, X.; Sun, L.; Long, Y.; Lu, Y. Bioinspired superwetting materials for water manipulation. Droplet. 2022, 1, 139-169. [CrossRef]
- Domel, A.G.; Saadat, M.; Weaver, J.C.; Haj-Hariri, H.; Bertoldi, K.; Lauder, G.V. Shark skin-inspired designs that improve aerodynamic performance. J. R. Soc. Interface. 2018, 15, 20170828. [CrossRef]
- Chien, H.-W.; Chen, X.-Y.; Tsai, W.-P.; Lee, M. Inhibition of biofilm formation by rough shark skin-patterned surfaces. Colloids Surf. B Biointerfaces. 2020, 186, 110738. [CrossRef]
- Tran, N.G.; Chun, D.-M. Green manufacturing of extreme wettability contrast surfaces with superhydrophilic and superhydrophobic patterns on aluminum. J. Mater. Process. Technol. 2021, 297, 117245. [CrossRef]
- Jeevahan, J.; Chandrasekaran, M.; Britto Joseph, G.; Durairaj, R.; Mageshwaran, G. Superhydrophobic surfaces: a review on fundamentals, applications, and challenges. J. Coat. Technol. Res. 2018, 15, 231-250. [CrossRef]
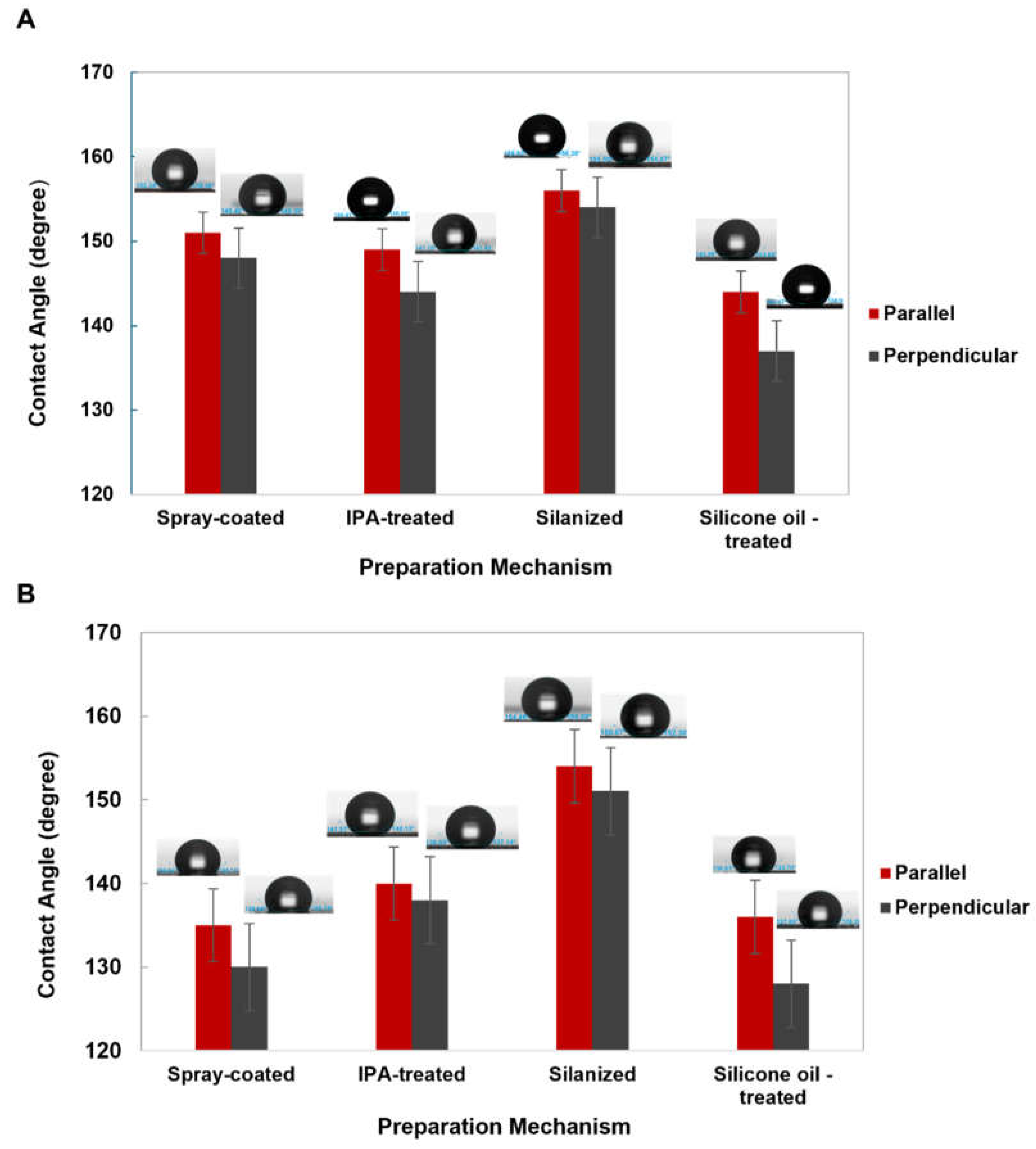
| Preparation Technique | Spray coating | IPA modification | Salinization | Silicone oil treatment | |
|---|---|---|---|---|---|
| Criteria | |||||
| Time required for wetting transition | 3 weeks | 30 Days | After experiment | After experiment | |
| Measured water contact angle | 151º ± 1 (Parallel) 148º ± 1(Perpendicular) |
149º ± 2(Parallel) 144º ± 3 (Perpendicular) |
156º ± 2 (Parallel) 154º ± 1(Perpendicular) |
144º ± 1 (Parallel) 137º ± 1(Perpendicular) |
|
| Stability of the surface’s wettability | Fourth rank in the category | Second rank in the category | First rank in the category | Third rank in the category | |
| Level of complexity of the experiment | Low | Low | Highest | Comparatively high | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





