1. Introduction
The Akchatau molybdenum-tungsten ore deposit [
1,
2,
3] was developed until the 90s of the 20th century but is still represented by the remains of tungsten, molybdenum and beryllium reserves in categories - C1, C2 and off-balance ores. The resumption of mining, processing and metallurgical production of tungsten from the ores of this deposit requires geological exploration and mining works to confirm reserves, as well as the choice of technology for beneficiation and processing of concentrates, taking into account modern achievements.
The average tungsten content in the Akchatau deposit ore is tenths of a percent (0.1-0.3%). Beneficiation of ore samples from this deposit by gravity and flotation made it possible to obtain rich concentrates containing 60-62% WO
3 [
3,
4,
5,
6].
Well-known industrial technologies intended to process tungsten raw materials include the following operations: sintering of concentrates with alkaline reagents; leaching of alloy or sinter; purification of sodium tungstate solutions from impurities; obtaining of technical tungsten acid, precipitation of CaWO4 or ammonium paratungstate, and finally obtaining of tungsten trioxide, metallic tungsten or tungsten carbide [
7,
8,
9,
10,
11,
12,
13].
Belskiy S.S. in article [
14] presented the results of research for the processing of wolframite concentrate by sintering it with soda, nitrate and potassium carbonate. The resulting cake was crushed and sent for leaching to obtain a solution of sodium tungstate. “Artificial scheelite” was precipitated from the solution. It was decomposed with hydrochloric acid to produce tungstic acid. During processing of wolframite concentrate, it was found that the charge must be sintered at 450-500 ºC and the ratio of components: wolframite concentrate, soda, potassium carbonate and nitrate 1:0.45:0.9:1.
The results of assessment of the mechanism and kinetics of the thermochemical interaction of wolframite and concentrates with sodium and potassium carbonates were presented in the works of Selivanov E.N. et al. [
15]. It has been shown with the use of the methods of differential thermal and phase analysis of sintering products that the interaction of wolframite with sodium and potassium carbonates begins above 723-743 K, and is accompanied by the formation of alkali metal tungstates and ferrites.
Publication [
16] presents the rationale for the technology intended to process low-quality wolframite concentrates based on sintering with Na
2CO
3 and K
2CO
3, and conducted model experiments with the use of the mathematical planning method. The authors determined particular dependencies linking the degree of extraction (γ) of tungsten into the solution during leaching with the amount (Q) of introduced alkali metal carbonates at the sintering stage, temperature (T) and process duration (τ).
The composition of the initial concentrates and literature data confirm that for Akchatau tungsten concentrates the most appropriate technology is sintering with soda, leaching of the cake with water and separation of tungsten acid with the subsequent production of tungsten trioxide and tungsten carbide.
2. Materials and Methods
The interaction reactions of tungsten minerals with soda for concentrates from Akchatau ores begin at temperatures above 520-550 oC and proceed intensively in the range of 750-850 oC was found by us.
The concentrate with soda was sintered in a muffle furnace and carborundum crucibles, at temperatures of 750, 800, 850
oC and an excess of soda from 1.05 to 1.2 in order to study the temperature sintering regimes for concentrates with soda and the effect of leaching factors on the extraction of sodium tungstate into aqueous solutions,. The chemical and phase composition of the wolframite concentrate was studied with the use of an SPM-35 X-ray spectrometer and an Empyrean multichannel diffractometer in the laboratory “Research Center KSP Steel” LLP. The data obtained are shown in
Table 1 and
Table 2 [
3].
As a result of structural analysis, the minerals FeWO4 (Ferberite), FeWO4 (Manganese Wolframate) and tungsten oxides WO2, WO3, WO2*H2O were identified.
Black melts were obtained as a result of sintering. The cakes were crushed and ground in a laboratory rod vibrating mill, followed by classification on sieves, allocation in classes with average particle size in microns: 815, 635, 365, 100.
Cakes were leached with distilled water in laboratory thermostats at temperatures from 65 to 95 °C and stirred with a paddle-type stirrer at a rotation speed of 240 to 420 rpm.
In the source [
17] (according to E.P. Bogomilskaya and Sh.I. Matusevich) shows the composition and specific gravity of sodium tungstate solutions in the form of
Table 3.
Expansion of the data in
Table 3 due to interpolation through density units equal to 0.01 made it possible to obtain an almost ideal dependence of the WO
3 content in the range of 21.73 -232.93 g/l at a density of 1.02-1.26 kg/l in the form of a linear function with regression coefficient close to unity:
where Y is the content of sodium tungstate and metal in the solution in terms of WO
3, g/l;
X is the density of the sodium tungstate solution, kg/l.
The density of the resulting solutions was measured at 20 °C with the use of a standard set of hydrometers and the pycnometric method [
18] to calculate the extraction of sodium tungstate into solution, and optical and adsorption density measurements were also used by the photocalorimetric method with a Jenway 6300 spectrophotometer.
The WO3 content in solutions was calculated from the measured density, taking into account the density of the solution due to excess soda. Leaching was performed at a ratio of L:S = 5:1 with 20 g weighed amounts of cake to eliminate the effect of the solubility of sodium tungstate and evaluate the effect of factors. The pulp was filtered using paper filters; the pulp is relatively easily settled and filtered.
Experiments with cakes obtained at temperatures of 750-850 °C were performed taking into account 5 factors at 4 levels according to probabilistic design of experiments [
19]. The assessment of the extraction degree of tungsten into the solution was controlled by the composition and weight of the insoluble residue; the effect of the transfer of silicon impurities into the solution, etc., is insignificant due to their low content in the cake.
3. Results and Discussion
The decomposition of wolframite concentrate by sintering it with soda in the presence of oxygen proceeds according to reactions [
3,
7,
8,
14,
15].
During leaching of the cake [
3,
7,
8,
14], sodium tungstate and soluble impurities pass into the solution (Na
2SiО
3, Na
2HPО
4, Na
2HAsО
4, Na
2MoО
4, Na
2SО
4), as well as excess of Na
2CО
3.
The conditions for leaching experiments of cakes obtained at different firing temperatures and the extraction of WO
3 into solution are specified in
Table 4
The density of the resulting sodium tungstate solutions was in the range of 1105-1128 g/l, and the content of the extracted component in terms of WO
3 was 93-110 g/l, the cake yield after leaching was 4.1-6.1 g. Composition of the cake obtained at 750 °C and soda excess coefficient Kc=1.2, as well as the mass and composition of the cake obtained during leaching are given in
Table 5.
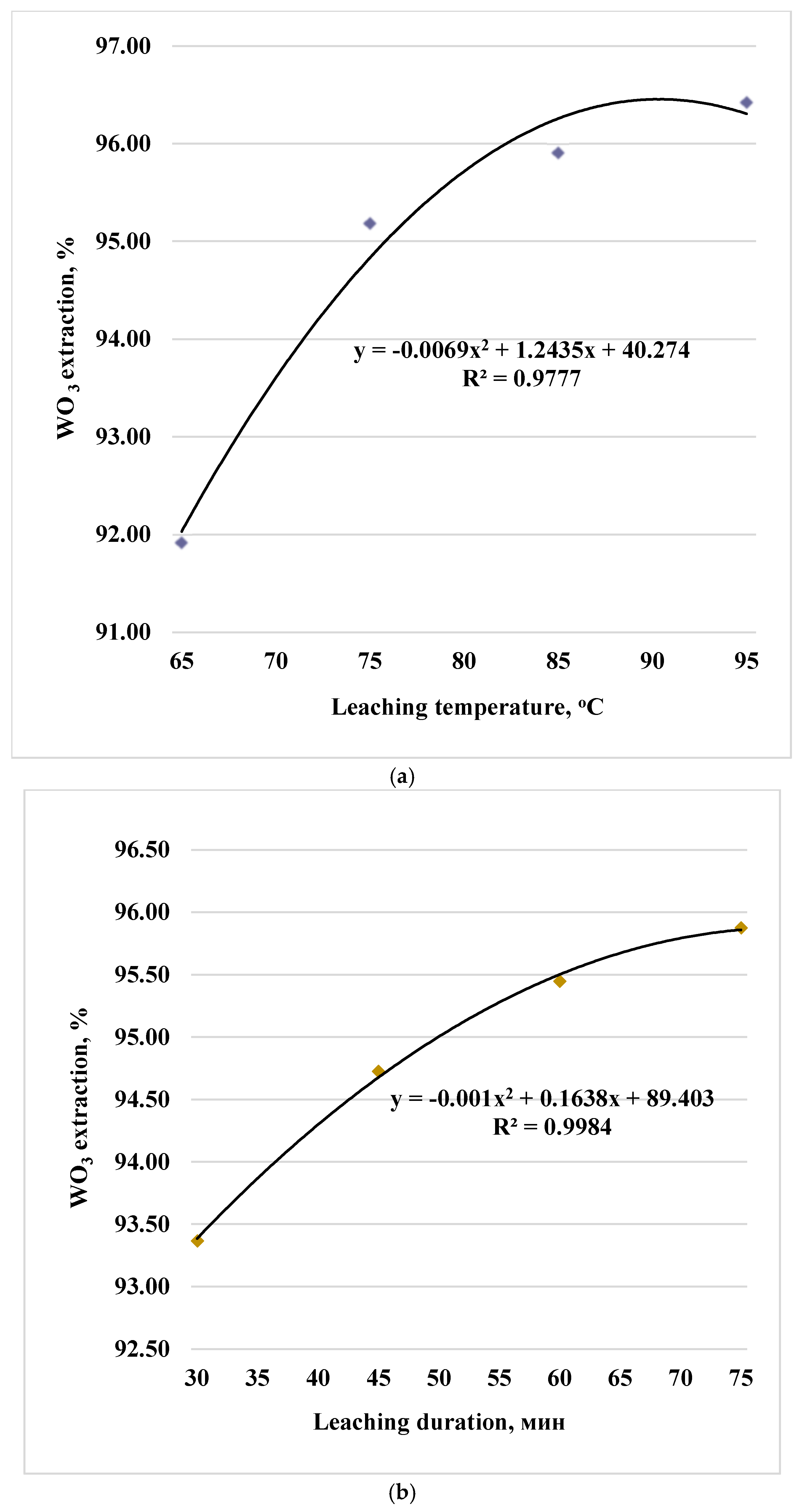
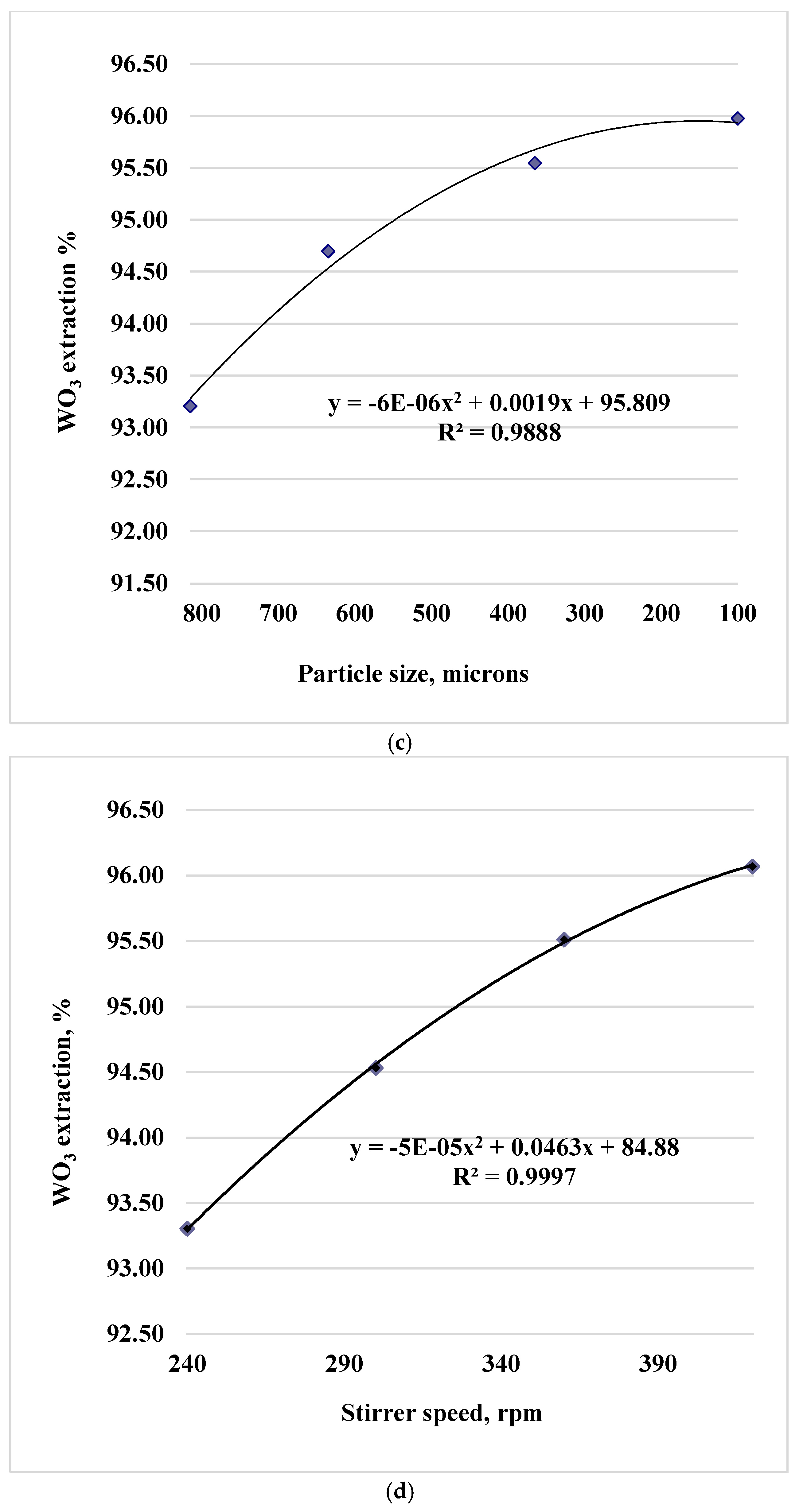
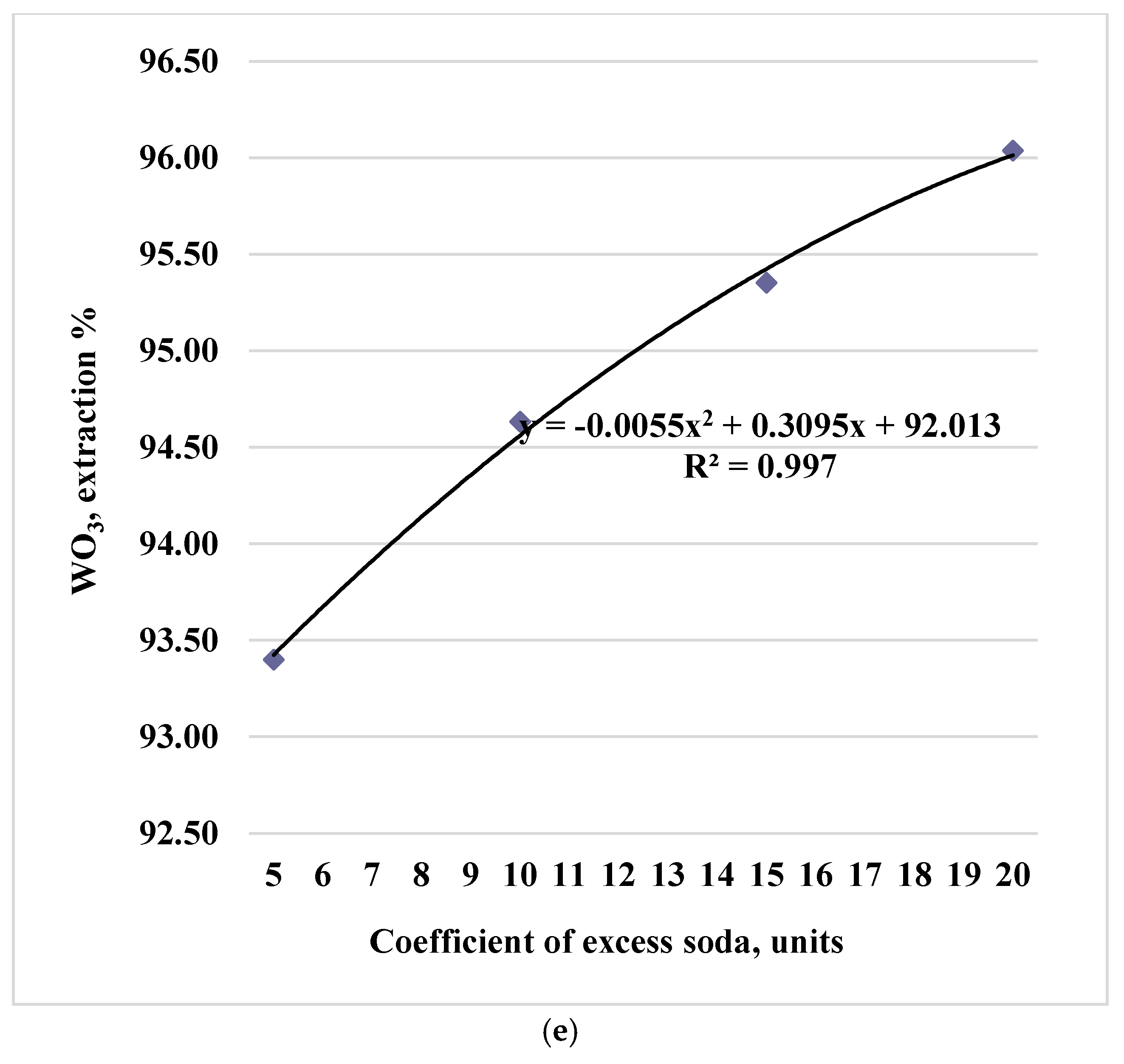
Processing of experimental data made it possible to identify the dependences of tungsten trioxide leaching into solution and the trend equations presented for the sinter obtained at 750 °C in Figures 1 (a, b, c, d, e).
Figure 1.
Paired dependences of into solution on factors: temperature (a), duration (b), particle size (c), stirrer speed (d) and soda excess coefficient (e).
Figure 1.
Paired dependences of into solution on factors: temperature (a), duration (b), particle size (c), stirrer speed (d) and soda excess coefficient (e).
Experimental data are described by polynomials of the 2nd degree (the x-values correspond to the levels of factors in codes 1,2,3,4 in the equations). During water leaching of cakes for firing temperatures of 800 and 850 °C, similar dependences of the degree of tungsten extraction into solution (in terms of WO
3) are obtained, shown in
Table 6.
Balance experiments for cake leaching. Experiments were performed with 100 g weighed cake samples and a L:S ratio of 2:1 to assess the material balance of cake leaching.
An approximate calculated balance of leaching of cake obtained at a charge firing temperature of 750 °C is specified in
Table 7.
The concentrate is mixed with a screw mixer with the revolutions after leaching the cake, air separator dust and soda ash in the traditional industrial process flow. The WO
3 content in the charge is reduced to 20-22% due to the turnover of leaching tailings to avoid melting of the charge and the formation of deposits in the drum furnace [
3,
7,
8].
It is proposed to burn the granules in a vertical shaft-type reactor with hot air supplied from a heater, instead of firing in a drum furnace, which requires supplying fuel and air to the burners of the furnace for combustion, as well as air for the oxidation reaction which significantly increases the amount of exhaust dust-laden gases. It is possible to eliminate sintering and burn the granular charge without diluting it with revolutions, and process the leaching cake in a separate circuit during firing granules in a mine reactor, by regulation of temperatures along the height of the unit.
The productive solution is purified from impurities using known reagents, with the exception of the consumption of inorganic acids for neutralization operations and the precipitation of tungstic acid. In this work, preliminary experiments have shown the possibility to use electrodialysis in a 2-chamber apparatus with separation of the anode and cathode spaces by a cation exchange membrane during purification of sodium tungstate solutions from impurities and isolation of commercial sodium tungstate at the final stage.
The possibility of electrodialysis of sodium tungstate solutions was reported in [
20,
21,
22]. The process intended to remove sodium ions from sodium tungstate solutions with the use of membrane electrodialysis processes allows one to reduce the use of mineral acids, as well as prevent the formation of salt waste.
4. Conclusions
The Akchatau deposit is represented by the remained reserves of tungsten, molybdenum and beryllium in categories - C1, C2 and off-balance ores. The production of tungsten from the ores of this deposit is possible based on the choice of beneficiation and processing process flows for concentrates. Samples of ore materials were taken to develop the technology. They were beneficiated to obtain rich concentrates, and the chemical and mineralogical composition of the samples was studied with the use of an SPM-35 X-ray spectrometer and an Empyrean multichannel diffractometer.
The concentrate was sintered with soda at 750, 800, 850 oC and an excess soda ratio of 1.05 to 1.2 in order to study the effect of sintering temperature and leaching parameters on the sodium tungstate extraction into solutions. The chemical and phase composition of the wolframite concentrate was studied with the use of an SPM-35 X-ray spectrometer and an Empyrean multichannel diffractometer.
The cakes after crushing and grinding were leached with distilled water in thermostats at 65-95 oC, stirrer speed 240-420 rpm and duration 30-75 minutes. The experiments were performed with the use of probabilistic-deterministic design of experiments for 4 levels, taking into account 5 factors. The extraction of tungsten into the solution was 89.49-99.65%, for sinter fired at 850 °C, the density of the resulting sodium tungstate solutions was in the range of 1105-1128 g/l, and the content of the extracted component in terms of WO3 was 93-110 g/l l.
Dependencies for the extraction of tungsten trioxide into solution and equations in the form of polynomials of the 2nd degree were obtained. Experiments were performed with 100 g sinter samples and a L:S ratio of 2:1 and a calculated balance of sinter leaching allowing to estimate the distribution of components.
It is proposed to burn the granules in a shaft-type reactor with hot air supplied from a heater to prevent the melting of the charge and the formation of deposits. During firing granules, it is possible to eliminate sintering and burn the granular charge without diluting it with revolutions, and process the leaching cake in a separate circuit by regulation of temperatures along the height of the unit.
The productive solution is purified from impurities using known reagents, with the exception of the consumption of inorganic acids for neutralization operations and the precipitation of tungstic acid. Preliminary experiments have shown the possibility to use electrodialysis in a 2-chamber apparatus with separation of the anode and cathode spaces by a cation exchange membrane during purification of sodium tungstate solutions from impurities and isolation of commercial sodium tungstate.
Author Contributions
Conceptualization, B.B.; Data curation, Y.T.; Formal analysis, A.D.; Investigation, A.T.; Methodology, G.M.; Project administration, B.B.; Resources, E.Y.; Software, Y.T., A.Y.; Visualization, A.Y., A.D. and A.T.; Writing—original draft, B.B.; Writing—review and editing, Y.T., G.M. All authors have read and agreed to the published version of the manuscript.
Funding
The research was carried out within the framework of grant funding of the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan for 2023–2025 in the priority area “Rational use of natural resources, including water resources, geology, processing, new materials and technologies, safe products and structures” project No. AP19677216 “Research and development of technologies, equipment for electrodialysis of solutions of tungstate, sodium sulfate with the regeneration of alkalis, acids”.
Data Availability Statement
All data to support the results of this study are included in this article.
Conflicts of Interest
The author Elena Yakob worked at KSPSteel LLP. The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Bogolepov, V.G., Shirkunov, M.V., Gulyayeva, N. Structure, composition and distribution of mineralization in the main greisen bodies of the Akchatau rare metal deposit. Mineralogy and geochemistry of tungsten deposits. L.: pub. LSU. 1971, 96-104.
- Bychkov, A.; Matveeva, S. Thermodynamic model of the formation of ore bodies at the Akchatau wolframite greisenvein deposit. Geochem. Int. 2008, 46, 867–886. [CrossRef]
- Baimbetov, B.; Moldabayeva, G.; Yeleuliyeva, A.; Jumankulova, S.; Taimassova, A.; Adilzhan, Z.; Baisultanov, R.; Yakob, E.; Serikbayev, V. Prospects of Processing Tungsten Ores from the Akchatau Deposit. Processes. 2024, 12, 77. [CrossRef]
- Wang, X.; Qin, W.; Jiao, F.; Dong, L.; Guo, J.; Zhang, J.; Yang, C. Review of tungsten resource reserves, tungsten concentrate production and tungsten beneficiation technology in China. Transactions of Nonferrous Metals Society of China. 2022, 32. 7 2318-2338. [CrossRef]
- Mountain encyclopedia: /http://www.mining-enc.ru/v/volframovaya-promyshlennost.
- Yang, X. Beneficiation studies of tungsten ores–A review. Minerals Engineering. 2018, 125, 111-119. [CrossRef]
- Zelikman A.N., Korshunov B.G. Metallurgy of rare metals. Moscow: Metallurgy, 1991, 432 p. https://reallib.org/reader?file=412937.
- Plyushcheeva, V.E. (Translator) Handbook of Rare Metals; Mir: Moscow, Russia, 1965; p. 922.
- Zhao, Z.; Li, J.; Wang, S.; Li, H.; Liu, M.; Sun, P.; Li, Y. Extracting tungsten from scheelite concentrate with caustic soda by autoclaving process. Hydrometallurgy. 2011, 108, 1–2, 152-156. [CrossRef]
- Sun, Pm., Li, Hg., Li, Yj. et al. Decomposing scheelite and scheelite-wolframite mixed concentrate by caustic soda digestion. J Cent. South Univ. Technol. 2003, 10, 297–300. [CrossRef]
- Queneau, P.; Huggins, D.; Beckstead, L. Autoclave Soda Digestion of Refractory Scheelite Concentrates. U.S. Patent 4320095A, 16 March 1982. https://patents.google.com/patent/US4320095A/en.
- Premchand. Processing of low grade tungsten ore concentrates by hydrometallurgical route with particular reference to India. Bull. Mater. Sci. 1996, 19, 295–312. [CrossRef]
- Lei, Y.; Sun, F.; Liu, X.; Zhao, Zh. Understanding the wet decomposition processes of tungsten ore: Phase, thermodynamics and kinetics, Hydrometallurgy. 2022. 213, 105928. [CrossRef]
- Belskiy S.S. Processing of wolframite concentrate. Bulletin of the Irkutsk State Technical University. 2018, 22, 1, 186-193. [CrossRef]
- Selivanov, E.N. Kinetics and mechanism of natural wolframite interactions with sodium carbonate. International Journal of Minerals, Metallurgy and Materials. 2019, 26, 11, 1364-1371. [CrossRef]
- Sitdikov, F.G. Preparation of calcium tungstate from low-quality concentrates. Materials of the international scientific and practical conference “Innovations in the complex processing of mineral raw materials”, January 21-22, 2016. - Kazakhstan, Almaty. 548-553.
- All about metallurgy: https://metal-archive.ru/redkie-metally/4200-razlozhenie-volframitovyh-koncentratov-sposobom-spekaniya-ili-splavleniya-s-sodoy.html.
- Mettler Toledo: https://www.mt.com/int/en/home/products/Laboratory_Analytics_Browse/density-meter/densitometer/D6.html.
- V.P. Malyshev. Probabilistic and deterministic planning of experiments. Almaty: Science, 1981, 116 p. https://z-lib.io/book/16011236.
- N.M. Smirnova, L.P. Glukhova, I.S. Rusakova. Method for decarbonization of tungstate-soda solutions and regeneration of sodium hydroxide by electrodialysis. SU 1750719 A1, 30 July 1992. https://patents.su/3-1750719-sposob-dekarbonizacii-volframatno-sodovykh-rastvorov-i-regeneracii-gidroksida-natriya-ehlektrodializom.html.
- Butukhanov V.L., Lebukhova N.V., Yershova T.B. Method for production of tungstic acid. RF Patent 2073644C1, 20 February 1997. https://patents.google.com/patent/RU2073644C1/ru.
- Verkhoturov A.D., Makienko V.M., Butukhanov V.L. Possibilities to process tungsten raw materials in environmentally friendly conditions. Scientific notes of Komsomolsk-on-Amur State Technical University. 2013, 1 (15), 102-106.
Table 1.
Chemical composition of wolframite concentrate, % (wt.).
Table 1.
Chemical composition of wolframite concentrate, % (wt.).
| Element content, % |
|---|
| WO3
|
Fe2O3
|
SiO2
|
Al2O3
|
P2O5
|
S |
СаО |
MnO |
MgO |
MoO3
|
Re |
С |
| 68.8 |
6.52 |
2.96 |
2.51 |
0.037 |
1.94 |
5.51 |
7.31 |
2.65 |
0.35 |
0.06 |
0.73 |
Table 2.
Phase (structural) analysis of wolframite concentrate, % (wt.).
Table 2.
Phase (structural) analysis of wolframite concentrate, % (wt.).
| Quantum distribution, % |
|---|
| WO3
|
FeWO4
|
MnWO4
|
SiO2
|
CaO |
Al2O3
|
MgO |
MoO3
|
S |
C |
Fe2O3
|
MoO3
|
| 25 |
27 |
31 |
3 |
6 |
3 |
3 |
- |
14 |
- |
- |
- |
Table 3.
Composition of sodium tungstate solutions by leaching stages.
Table 3.
Composition of sodium tungstate solutions by leaching stages.
| Leaching stage |
Specific gravity of solution |
WO3, g/l |
SiO2, g/l |
SiO2/WO3,% |
| 1 |
1.26 |
230 |
0.18 |
0.08 |
| 1+2 |
1.19 |
172 |
0.17 |
0.1 |
| 1+2+3 |
1.12 |
104 |
0.14 |
0.13 |
| 4 |
1.014 |
15 |
0.08 |
0.5 |
| Process waters |
1 |
4 |
0.03 |
0.8 |
Table 4.
Conditions of experiments on cake leaching for 4 levels and 5 factors.
Table 4.
Conditions of experiments on cake leaching for 4 levels and 5 factors.
| Experiment No. |
1. Leaching temperature, °C |
2.Duration,
min |
3. Particle size, µm |
4. Stirrer speed, rpm |
5. Coefficient of excess soda, units |
Yec, %
(Sinter 750 oC) |
Yec, %
(Sinter 800 oC) |
Yec, %
(Sinter 850 oC) |
| 1 |
65.00 |
30.00 |
815 |
240 |
1.05 |
85.77 |
86.83 |
89.49 |
| 2 |
65.00 |
45.00 |
635 |
300 |
1.1 |
91.08 |
92.95 |
93.38 |
| 3 |
65.00 |
60.00 |
365 |
360 |
1.15 |
94.35 |
96.19 |
95.17 |
| 4 |
65.00 |
75.00 |
100 |
420 |
1.2 |
96.45 |
97.72 |
95.68 |
| 5 |
75.00 |
30.00 |
635 |
360 |
1.2 |
94.98 |
97.36 |
94.61 |
| 6 |
75.00 |
45.00 |
365 |
420 |
1.05 |
95.69 |
96.75 |
95.45 |
| 7 |
75.00 |
60.00 |
100 |
240 |
1.1 |
94.18 |
92.57 |
97.00 |
| 8 |
75.00 |
75.00 |
815 |
300 |
1.15 |
95.88 |
95.55 |
95.27 |
| 9 |
85.00 |
30.00 |
635 |
360 |
1.2 |
95.9 |
95.88 |
97.23 |
| 10 |
85.00 |
45.00 |
365 |
420 |
1.05 |
95.61 |
95.93 |
98.18 |
| 11 |
85.00 |
60.00 |
100 |
240 |
1.1 |
96.25 |
99.65 |
96.59 |
| 12 |
85.00 |
75.00 |
815 |
300 |
1.15 |
95.86 |
96.93 |
97.79 |
| 13 |
95.00 |
30.00 |
635 |
360 |
1.2 |
96.82 |
97.98 |
99.44 |
| 14 |
95.00 |
45.00 |
365 |
420 |
1.05 |
96.53 |
99.03 |
97.48 |
| 15 |
95.00 |
60.00 |
100 |
240 |
1.1 |
97.02 |
98.02 |
98.56 |
| 16 |
95.00 |
75.00 |
815 |
300 |
1.15 |
95.32 |
98.03 |
98.75 |
Table 5.
Composition of cake obtained at 750 °C and water leaching cake.
Table 5.
Composition of cake obtained at 750 °C and water leaching cake.
| |
Weight, gram |
Content of chemical elements, % |
| WO3
|
Fe2O3
|
SiO2
|
Al2O3
|
P2O5
|
S |
СаО |
MnO |
MgO |
| 1. Sinter |
20 |
54.67 |
5.18 |
2.35 |
1.99 |
0.03 |
1.54 |
4.38 |
5.81 |
2.11 |
| 2. Cake |
5.19 |
11.31 |
19.96 |
9.06 |
7.68 |
0.11 |
5.94 |
16.86 |
22.37 |
8.11 |
Table 6.
Paired dependences of tungsten extraction into solution.
Table 6.
Paired dependences of tungsten extraction into solution.
| No. |
Factor influencing leaching, interval |
Function type |
Approximation coefficient |
| |
Leaching dependences for sinter obtained at 800 °C |
| 1 |
Temperature, 75-95 oC |
y = -0.0024x2 + 0.5477x + 68.054 |
R2 = 0/9998 |
| 2 |
Duration, 30-75 min. |
y = -0/0013x2 + 0/1941x + 89/952 |
R2 = 0.98 |
| 3 |
Particle size, 100-815 microns |
y = -9E-06x2 + 0.0047x + 96.578 |
R2 = 0.9927 |
| 4 |
Stirrer speed, 240-420 rpm |
y = -8E-05x2 + 0.0672x + 82.523 |
R2 = 0.9999 |
| 5 |
Excess soda in the charge, 5-20% (wt.) |
y = -0.0060x2 + 0/3242x + 93/1631 |
R2 = 1.0000 |
| |
Leaching dependences for sinter obtained at 800 °C |
| 1 |
Temperature, 75-95 oC |
y = -0.0026x2 + 0.5895x + 66.102 |
R2 = 0.9993 |
| 2 |
Duration, 30-75 min. |
y = -0.001x2 + 0.1419x + 91.802 |
R2 = 0.9947 |
| 3 |
Particle size, 100-815 microns |
y = -3E-06x2 + 0.0003x + 96.918 |
R2 = 0.9772 |
| 4 |
Stirrer speed, 240-420 rpm |
y = -6E-05x2 + 0.0437x + 88.139 |
R2 = 0.9944 |
| 5 |
Excess soda in the charge, 5-20% (wt.) |
y = -0.0124x2 + 0.412x + 93.424 |
R2 = 0.9926 |
Table 7.
Calculated balance of leaching of cake.
Table 7.
Calculated balance of leaching of cake.
| Materials received |
Total, g |
WO3 |
Fe2O3 |
SiO2 |
Al2O3 |
P2O5 |
S |
CaO |
MnO |
MgO |
H2O |
Others |
| Sinter No. 1 (750 oС) |
100 |
55.47 |
5.18 |
2.35 |
1.99 |
0.03 |
1.54 |
4.38 |
5.81 |
2.11 |
0 |
20.07 |
| Content, % |
55.47 |
5.18 |
2.35 |
1.99 |
0.03 |
1.54 |
4.38 |
5.81 |
2.11 |
0 |
20.07 |
| Water |
400 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
400 |
0 |
| Content, % |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
100 |
0 |
| TOTAL |
500 |
55.47 |
5.18 |
2.35 |
1.99 |
0.03 |
1.54 |
4.38 |
5.81 |
2.11 |
400 |
20.07 |
| Products received |
Total, g |
WO3 |
Fe2O3 |
SiO2 |
Al2O3 |
P2O5 |
S |
CaO |
MnO |
MgO |
H2O |
Others |
| Cake-1 after washing |
32.313 |
3.023 |
5.18 |
2.326 |
1.99 |
0.028 |
1.517 |
4.38 |
5.81 |
2.11 |
0.029 |
4.853 |
| Content, % |
9.355 |
16.031 |
7.199 |
6.159 |
0.086 |
4.693 |
13.555 |
17.98 |
6.53 |
0.088 |
15.02 |
| Extraction, % |
5.45 |
100 |
98.99 |
100 |
93.01 |
98.48 |
100 |
100 |
100 |
0.01 |
24.18 |
| Productive solution |
242.077 |
47.47 |
0 |
0.024 |
0 |
0.002 |
0.023 |
0 |
0 |
0 |
186.81 |
7.745 |
| Content, % |
19.61 |
0 |
0.01 |
0 |
0.001 |
0.01 |
0 |
0 |
0 |
77.17 |
3.199 |
| Extraction, % |
85.58 |
0 |
1.01 |
0 |
6.98 |
1.52 |
0 |
0 |
0 |
46.7 |
38.59 |
| Process water - 1 |
225.525 |
4.966 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
213.09 |
7.469 |
| Content, % |
2.202 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
94.486 |
3.312 |
| Extraction, % |
8.95 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
53.27 |
37.22 |
| TOTAL |
499.915 |
55.459 |
5.18 |
2.35 |
1.99 |
0.03 |
1.54 |
4.38 |
5.81 |
2.11 |
399.928 |
20.067 |
| Residual |
-0.085 |
-0.011 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
-0.072 |
-0.003 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).