1. Introduction
Age-related hearing loss (ARHL) is a complex sensory deficit that commonly affects older adults and primarily stems from the cumulative effects of ageing on the auditory system [
1]. The prevalence of ARHL increases with age, affecting approximately 40% of people over the age of 50 [
2]. With the ageing global population, the number of patients with ARHL is expected to increase sharply. Hearing loss may lead to reduced socialization, worsened depression, and eventually cognitive decline in older adults [
3,
4]. However, the pathogenesis of ARHL remains unclear, and there are currently no effective treatments to prevent its onset.
The primary role of mitochondria is to produce adenosine triphosphate (ATP) to meet cellular energy demands, and they are considered a key regulator of cell death and survival processes [
5]. During ageing, imbalances in mitochondrial biogenesis and mitophagy lead to a gradual decline in mitochondrial function, resulting in a pool of unhealthy mitochondria that impair cellular function. Mitophagy degrades damaged and dysfunctional cellular components through the formation of autophagic lysosomes. PINK1 accumulates at the surface of damaged mitochondria and activates parkin (a ubiquitin ligase), which becomes phosphorylated and migrates from the cytoplasm to the surface of the mitochondria, where it interacts with PINK1 to ubiquitinate damaged mitochondrial proteins. The ubiquitinated mitochondria are then recognised by the autophagy machinery and encapsulated in autophagosomal vesicles for eventual degradation. Mitophagy is reduced in dysfunctional aged tissues and organs, and the aggregation of damaged mitochondria leads to excessive production of reactive oxygen species (ROS), lipid accumulation, imbalance in cytokine secretion, accumulation of inflammation, and metabolic dysfunction [
1], which ultimately leads to cellular senescence or death [
6]. Reduced mitophagy and increased hearing loss have been observed in the ageing cochlea [
7], and mitophagy can effectively protect hair cells and delay the onset of ARHL [
8]. However, given the tissue specificity of auditory organs, there is still a paucity of studies on mitophagy in the auditory system.
With increasing age, ROS production in hair cells increases and antioxidant capacity decreases [
5]. Excess oxidative metabolites and an imbalance in cellular metabolism leads to an increase in the rate of apoptosis and consequently exacerbates ARHL. Sestrins are a highly conserved family of stress-induced antioxidant proteins that exist in three forms (SESN1, 2, and 3). They regulate mitophagy in response to various cellular stresses such as DNA damage, oxidative stress, and hypoxia. Among them, Sestrin2 is the most studied, with experiments showing that its expression decreases with age and is associated with various age-related diseases [
9]. Increased Sestrin2 expression improves age-related cardiac dysfunction [
10] and prevents neuromuscular dystrophy [
11]. In 2017, Sestrin2 expression was observed for the first time in the organ of Corti, with high expression levels in hair cells, which led to it being recognized as a novel gene related to ARHL [
12], but the precise underlying mechanism remains unclear. The activation of AMPK and inhibition of mTOR can promote autophagy in response to various stress-induced injuries [
13].
Based on the role of Sestrin2 in the aging process, it has been reported that Sestrin2 expression in the mouse cochlea is temporal and spatial expression specific and identified as a novel gene associated with ARHL. In modeling the aging cochlea and Sestrin2 gene regulation, we found that Sestrin2 overexpression (Sestrin2 OE) effectively delayed the progression of ARHL. Further molecular mechanistic studies revealed that Sestrin2 OE improved mitochondrial function in hair cells, counteracted hair cell ageing, and lowered the auditory brainstem response (ABR) threshold, thereby delaying the progression of ARHL. In addition, when hair cells undergo senescence stress, the body activates AMPK and inhibits mTOR to promote mitophagy in response to a cellular energy imbalance.
2. Materials and Methods
2.1. Animals
C57BL/6 mice were purchased from Beston (Zhuhai, Guangdong) and were used to construct a mouse model of ARHL [
14]. All animal care and experimental procedures strictly adhered to the relevant guidelines and regulations approved by the committee of Huateng Biomedical Technology Co, Guangzhou, China. All mice were randomly divided into six groups: Group 1 comprised 8-week-old mice; Group 2 comprised 26-week-old mice; Group 3 comprised 26-week-old+Sestrin2 OE control (empty vector) mice; Group 4 comprised 26-week-old+Sestrin2 OE mice; Group 5 comprised 26-week-old+Sestrin2 knockout (KO) control (empty vector) mice; and Group 6 comprised 26-week-old+Sestrin2 KO mice. After the mice underwent ABR testing, they were deeply anaesthetised with 1% Zoletil (VIRBAC, Shanghai City, China) and 0.5% Xylazine II (Shengda Animal Pharmaceuticals, Dunhua City, China), and their cochlea were removed.
2.2. ABR Measurement
Each test was conducted in a soundproof chamber. Prior to testing, 1% Zoletil (VIRBAC) and 0.5% Xylazine II were injected intraperitoneally into each mouse at a dose of 0.1 ml/20 g body weight. During the tests, mice were kept warm using a heating pad. Electrodes were inserted subcutaneously into the stimulated ear (left ear), midline of the skull, and inner thigh of the stimulated ear, and the ABR (SmartEP technology system) was recorded via the scalp of the mice. Sound intensity was controlled using frequency-specific burst tone stimuli (4, 8, 16, and 32 kHz) at near-threshold intervals, decreasing from 90 dB SPL in intervals of 10 dB to 20 dB SPL. The threshold was defined as the lowest stimulus level at which the peak of the visible wave response was observed in the evoked trajectory.
2.3. AAV Vector Construction and Transfection
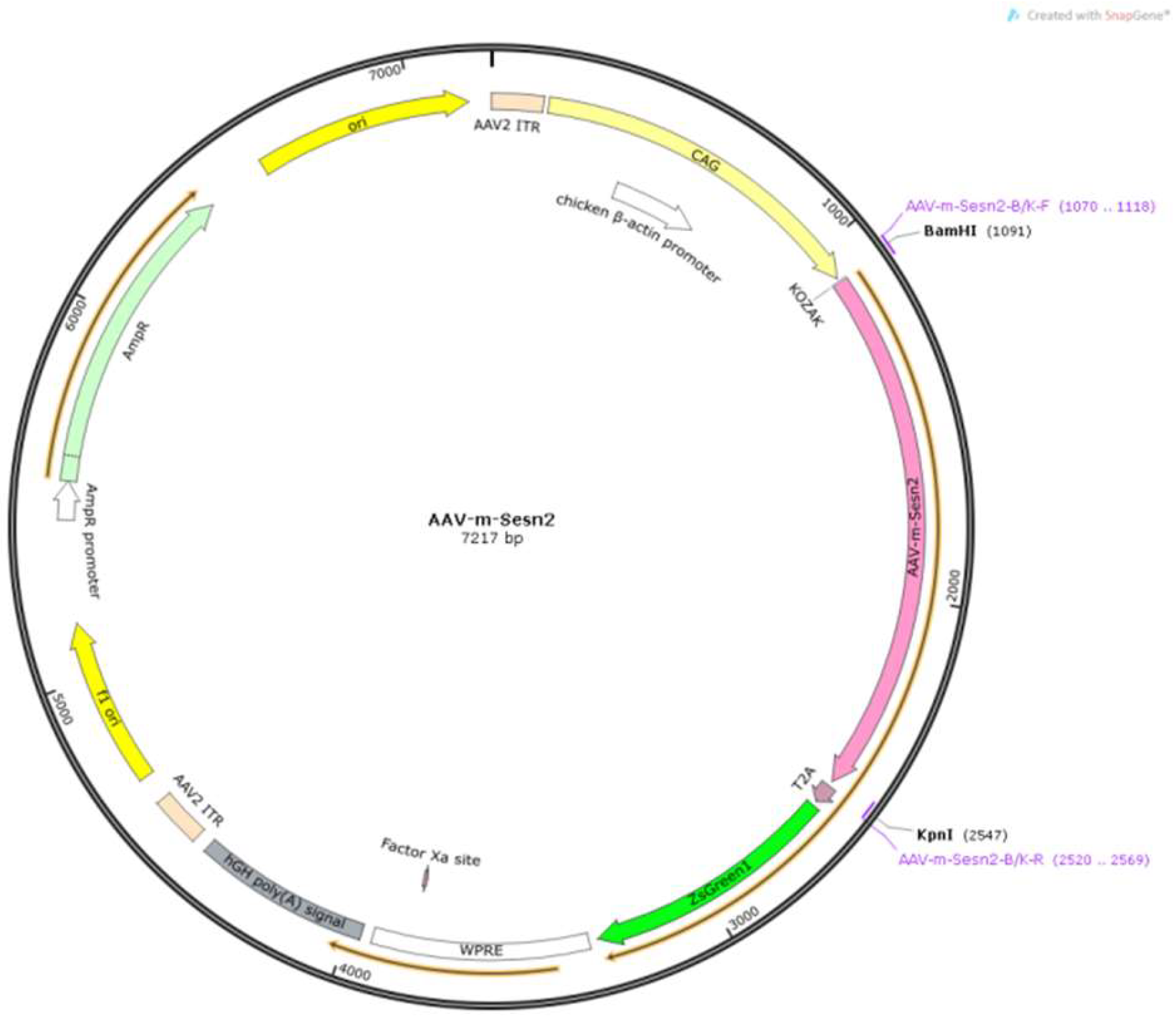
The Sestrin2 sequence was inserted into the pHBAAV-CAG-MCS-T2A-ZsGreen vector (as shown in
Figure 1) to generate AAV. After co-transfecting 293 T cells with the packaging vector, the construct was packaged and purified (HanHeng Bio, Shanghai, China). The primer sequences are listed in
Table 1. An AAV was constructed to regulate Sestrin2 expression in the cochlea of mice.
2.3. Animal Surgery: PSC Injection
Surgery was performed as described previously [
15]. After the mice were deeply anesthetized, the surgical site was shaved and disinfected. Subsequently, a small incision of approximately 2 cm was made along the posterior edge of the auricle in the left ear, the subcutaneous tissues were separated, and the PSC was gradually exposed. The virus was injected into the PSC slowly. After injection of the virus, a small piece of muscle was quickly plugged into the needle hole, the skin wound was sutured closed, and the mice were left to wake up.
2.4. In Vivo Imaging
The AniView600 in vivo imaging system (Guangzhou Boluteng Biotechnology Co., Ltd., China) was used to visualise the transfection of the cochlea in mice transfected with viruses labelled with green fluorescent proteins (eGFP and ZsGreen). Prior to imaging, the mice were anaesthetised. Owing to the temporal bone encasing the cochlea, the AniView600 in vivo imaging system cannot directly capture fluorescent signals from the cochlear region through the temporal bone. To overcome this limitation, the cochleae were dissected from the mice to observe the green fluorescence to indirectly assess whether the virus successfully reached the cochlea. Imaging parameters were set to an excitation filter of 465 nm, an emission filter of 540 nm, an exposure time of 0.19 seconds, and a light source intensity of 80%. Fluorescence imaging of the cochlea was performed and compared with that of the right ear, which had not received the virus injection, to evaluate the transfection status of the virus in the cochlear region. Subsequent histological experiments to further validate the distribution and infection of the virus in the cochlea were conducted.
2.5. Western Blotting(WB)
Total protein was extracted from the cochlea using cold RIPA buffer (Beyotime, Shanghai, China) and a proteinase inhibitor mixture (Beyotime). The protein concentration was measured using a BCA protein quantification kit (Beyotime). Approximately 20 μg of total protein from the cochlea was loaded onto 10% SDS-PAGE gel (Servicebio, Wuhan, China). After transfer to a PVDF membrane via electroblotting, the gel was blocked in 5% BSA-PBST for 120 min at room temperature and left overnight. The strips were incubated with Sestrin2, Myosin VIIa, PINK1, mTOR, Parkin, and primary antibody at 4°C overnight. Then, the membrane was hybridised with secondary antibodies (goat anti-mouse and goat anti-rabbit, Servicebio) at room temperature for 1.5 h. Strip images were captured using ChemiCapture (Clinx, Shanghai, China), and the intensity was semi-quantified using ImageJ[
16].
2.6. Confocal Microscopy and Image Analysis
Samples were fixed in 4% paraformaldehyde for 30 min. Then, the cells were incubated in permeabilisation buffer (Beyotime) for 20 min. The samples were blocked with immunostaining blocking buffer (Beyotime) for 30 min, and subsequently incubated with primary antibodies at 4°C overnight. Antibody information is presented in
Table 2. The next day, after washing three times with PBS, secondary antibodies (488-labeled goat anti-rabbit, 594-labeled goat anti-mouse, 1:100, Servicebio) were added and incubated at room temperature for 1 h. Nuclei were stained for 5 min using an anti-fade mounting medium containing DAPI (Sangon Biotech, Shanghai, China) and the samples were mounted. Images were captured using the NIS-Elements AR software.
2.7. TUNEL
Hair cell apoptosis was measured using a TUNEL assay kit (Beyotime). The test method was the same as that used for the immunofluorescence staining.
2.8. Mitochondrial Isolation and Mitochondrial Fluorescent Probe Staining Analysis
Mitochondria were extracted from the mouse cochlea using a commercial tissue mitochondrial isolation kit (Beyotime Biotechnology). Isolated mitochondrial tissues were incubated with JC-1 staining solution (1:200, Beyotime) for 20 min at 37 °C and washed twice with washing buffer according to the manufacturer's instructions. Mitochondrial membrane potential (MMP) was determined by monitoring the emission of JC1 monomers and aggregates using a multifunctional enzyme-labelling instrument (Spark, Tecan Trading AG, Switzerland). The red/green ratio was calculated using Excel, and statistical analyses were performed using normalised controls.
2.9. Statistical Analysis
Each experiment was repeated at least three times, and all data are expressed as mean ± SD. Statistical significance was analysed using the statistical software GraphPad Prism 9.5, Microsoft Excel, and SPSS (version 23). Comparisons between two groups were conducted using the independent samples t-test, and multiple comparisons were performed using one-way analysis of variance (ANOVA) followed by the LSD post hoc test. Statistical significance was set at p<0.05. *p<0.05; **p<0.01; ***p<0.001.
3. Results
3.1. Regulating Sestrin2 Expression Influences the Progression of ARHL
We delivered Sestrin2-OE and Sestrin-knockout (Sestrin KO) vectors into the inner ear of mice via the PSC using AAV to investigate the effect of Sestrin2 on the progression of ARHL (
Figure 2a). Green fluorescence in the cochlea was observed, confirming that the gene vectors had been successfully transfected into the cochlea of mice (
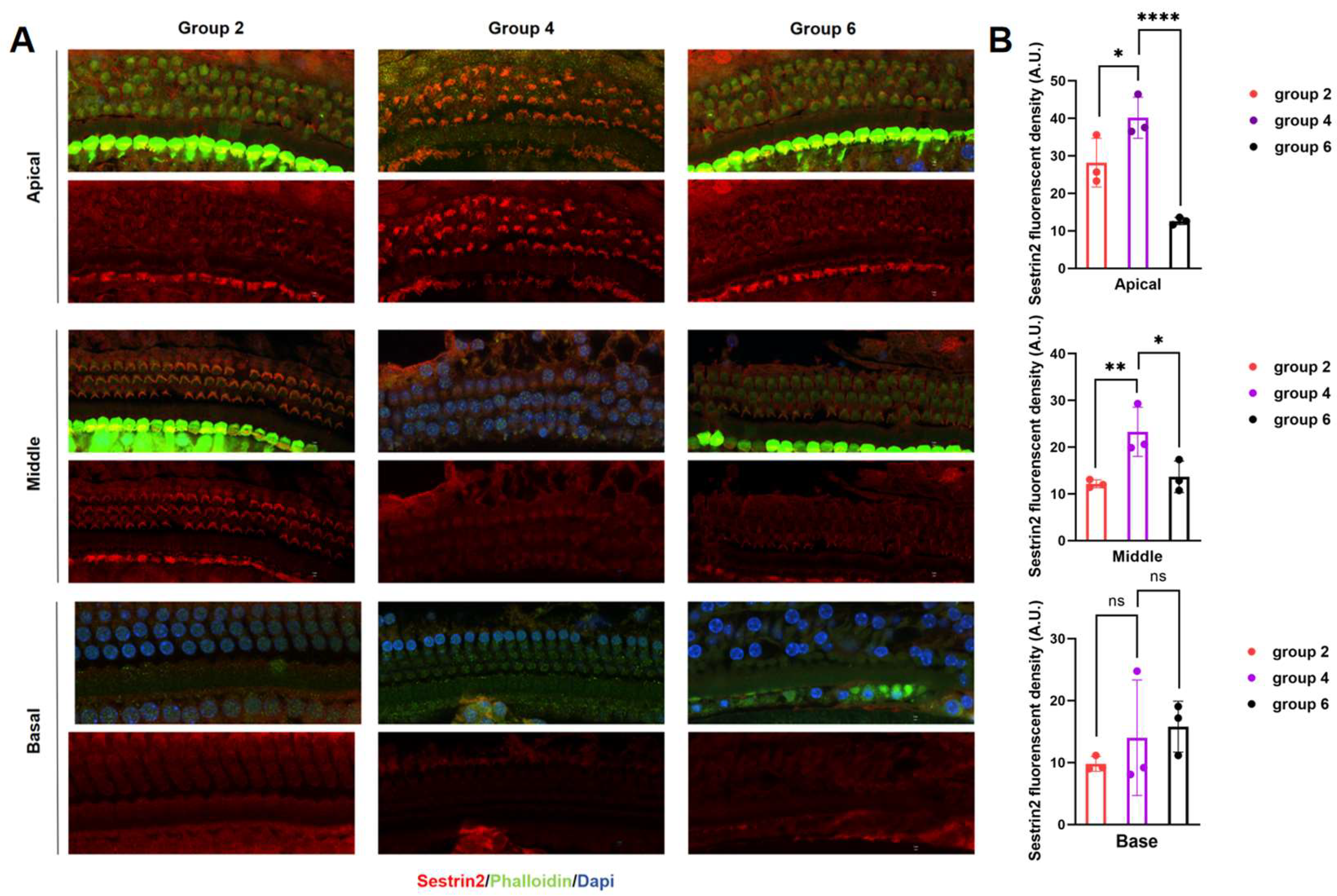
Figure 2b). Immunofluorescence results showed that the spatial expression of Sestrin2 in the apical, middle, and basal turns of the cochlea in Group 4 was higher than that in Groups 2 and 6, indicating that the Sestrin2 OE and Sestrin2 KO models were successfully constructed (
Figure 3). The results of the ABR test demonstrated a significant difference in the ABR thresholds between young untreated and aged mice across the 4–32 kHz frequency range, with Group 4 exhibiting significantly better hearing than Group 6 at low frequencies (
Figure 2c). Additionally, Groups 3 and 5 showed no significant differences in ABR thresholds at any frequency (
Figure 2d), excluding the effects of the gene or surgery on hearing. Overall, these data suggest that Sestrin2 OE delays ARHL progression.
3.2. Sestrin2 Overexpression Can Delay Mitochondrial Dysfunction and Directly Protect Hair Cells
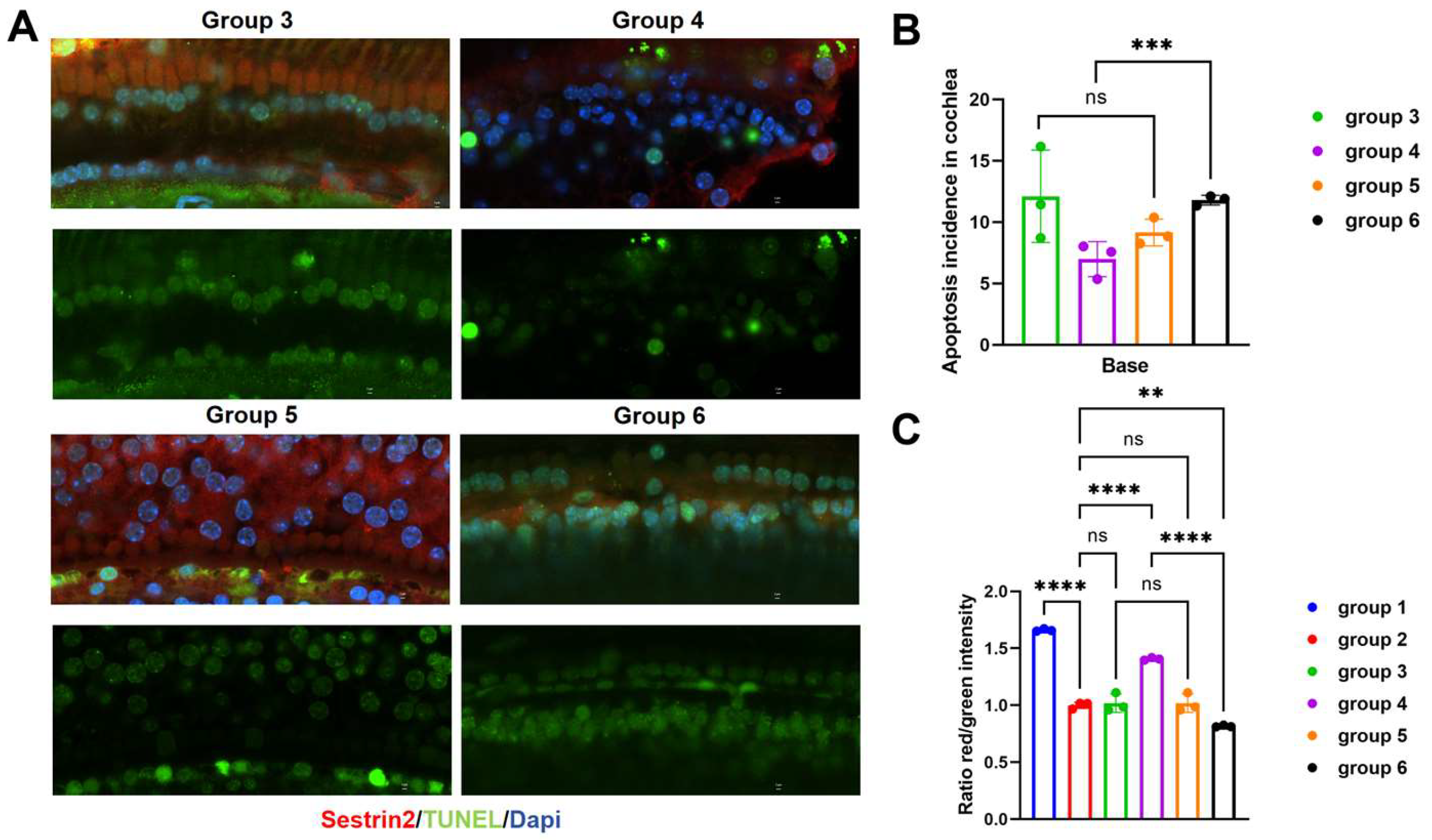
We applied TUNEL staining to detect apoptosis in the basal turn in each mouse group. Group 6 exhibited more TUNEL-positive cells than Group 4, whereas Groups 3 and 5 had similar numbers of TUNEL-positive cells(
Figure 4A, B). These data indicate that high Sestrin2 expression delays hair cell apoptosis. To explore the potential mechanisms underlying the effects of Sestrin2 in ARHL, we compared the MMP among groups. After excluding the influence of the gene vectors, we found that MMP was lower in ageing cochleae, but Group 4 displayed stronger red fluorescence, indicating a higher MMP (
Figure 4c). A higher MMP reflects healthier mitochondrial function, resulting in increased ATP production, which is crucial for maintaining normal cellular physiological functions. These results revealed that high Sestrin2 expression delayed mitochondrial dysfunction and exerted a direct protective effect on hair cells.
3.3. Activation of AMPK and Inhibition of mTOR Enhance Mitophagy
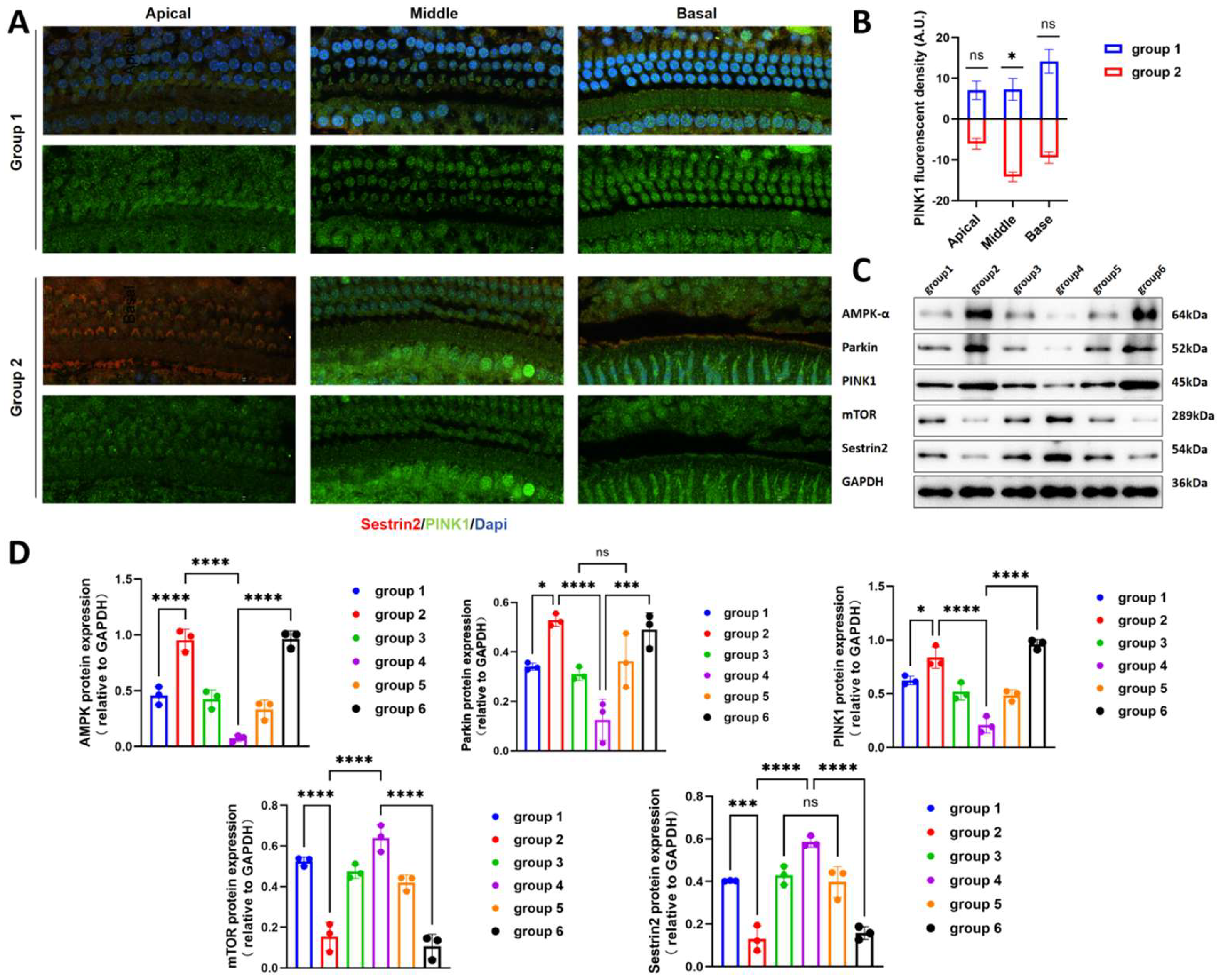
We investigated the expression of PINK1 in the cochlea using immunofluorescence staining. The results showed that the green fluorescence indicating PINK1 expression was stronger in Group 1, which had high Sestrin2 expression, compared with that in the other groups (
Figure 5a, b). AMPK is a key molecule in the regulation of cellular energy metabolism and has an antagonistic relationship with mTOR. Our western blotting results indicated that modulating the expression of Sestrin2 influenced the levels of AMPK and mTOR. Further analysis revealed that, compared to the Sestrin2 KO group, the Sestrin2 OE group exhibited decreased expression of PINK1, Parkin, and AMPK and increased mTOR expression (
Figure 5c, d). These findings suggested that Sestrin2 KO accelerated the ageing process of hair cells, and that the organism responds to this change by activating mitophagy via AMPK activation and mTOR inhibition. Conversely, Sestrin2 suppresses this process and antagonises hair cell senescence.
4. Discussion
Ageing is a risk factor for various diseases, and an ageing population will result in a significantly increased socioeconomic burden [
17]. The pathological mechanisms related to ageing are the subject of ongoing research, and it is clear that reducing pathogenesis is a key strategy in antagonistic ageing research. Sestrin2 has been shown to regulate mitophagy, alleviate oxidative stress, and regulate ageing [
10,
18]. Furthermore, Sestrin2 has been linked to age-related degeneration of cochlear sensory cells [
12], but the specific mechanisms remain unclear. Our experiments demonstrated that Sestrin2 inhibited hair cell apoptosis by improving mitochondrial function, thereby delaying ARHL. Consistent with the findings of previous studies, we observed that Sestrin2 expression in the cochlea decreases with age. Using Sestrin2 OE and Sestrin2 KO mice, we unveiled the complex relationship between mitochondrial function and inflammation during ageing, which may also partially explain why autophagy is mainly regulated by the AMPK/mTOR pathway [
19], and why the function of Sestrin2 in regulating mitophagy may be secondary. Moreover, to our knowledge, this study is the first to demonstrate that the age-related Sestrin2-AMPK/mTOR-mediated inflammatory response is physiologically conserved and that this response can be suppressed by stress-induced mitophagy.
PINK1-PRKN-mediated mitophagy is a classical pathway that plays an important role in mitophagy quality control [
20]. Mitophagy degrades damaged mitochondria via lysosomes, thereby realizing cellular self-repair and protective functions. Mitophagy has been shown to be protective under various stress conditions such as drug damage, noise exposure, and ageing and is an important indicator of mitochondrial quality [
21,
22]. However, the role of mitochondrial quality in the process of hair cell damage remains unclear [
23]. Our study showed that the MMP of the cochlea was low, whereas the fluorescence intensity of PINK1 was significantly enhanced during the ageing process. This may indicate that, during hair cell ageing, decreased mitochondrial quality triggers an increase in mitophagy, which is a stress-protective response in mice to cochlear cell senescence. However, the progression of senescence ultimately results in decreased mitophagy.
A normal MMP is required for maintaining intracellular redox homeostasis. With ageing, the accumulation of ROS can lead to a decline in the MMP, resulting in mitochondrial dysfunction, reduced ATP production, and effects on the normal transport of inner ear ions, further exacerbating ARHL [
24,
25]. In the Sestrin2 OE group, we observed a significant reduction in PINK1 and Parkin expression, accompanied by higher red fluorescence. This suggested that Sestrin2 exerted a strong protective effect on cochlear hair cell mitochondrial function and inhibited hair cell apoptosis, thereby delaying ARHL. Therefore, we propose that Sestrin2 is critical for counteracting hair cell damage during ageing, whereas mitophagy is not significantly associated with hair cell apoptosis.
AMPK activation is a classical mechanism that occurs in response to changes in cellular energy status and regulates almost all metabolic activities in the living body [
26]. By inhibiting the phosphorylation activity of mTOR, AMPK can inhibit mTOR-mediated downstream pathways and promote autophagy, which is a key mechanism in the induction of autophagy [
27]. In this study, we transfected Sestrin2-regulated genes into mouse cochlear hair cells using AAV. Compared with the Sestrin2 KO group, the Sestrin2 OE group showed a significant decrease in AMPK expression, significant increase in mTOR expression, and decrease in the expression of important markers related to mitophagy. This phenomenon indicates that AMPK inhibition of mTOR expression in response to cellular ageing promotes the protective role of stress-induced autophagy, whereas Sestrin2 suppresses this pathway to directly protect hair cells.
Based on these results, we inferred that Sestrin2 OE maintains cellular metabolic homeostasis and energy balance, thereby reducing cytotoxicity. In contrast, metabolic disruption and decreased mitochondrial function mediated by Sestrin2 KO may be the direct causes of hair cell damage and hearing loss. Both the Sestrin2 OE and KO models we constructed supported this conclusion; however, the specific mechanisms require thorough investigation. Additionally, the sample size in this experiment was relatively small. Future studies could increase the sample size to enhance the reliability of the results. To gain a deeper understanding of the dynamic protective effects of Sestrin2 and its potential time dependency, more experimental time points should be considered in future research, particularly by observing older age groups, to assess whether the effects of Sestrin2 persist or strengthen over a longer duration.
5. Conclusions
Our study showed that Sestrin2 OE not only exerted a comprehensive protective effect on hair cells by improving mitochondrial function but also prevented mitophagy by inhibiting AMPK and upregulating mTOR. In contrast, Sestrin2 KO led to severe mitochondrial dysfunction and metabolic disorders, which further exacerbated hair cell damage and hearing loss. This study not only confirmed the critical role of Sestrin2 in maintaining mitochondrial homeostasis and inhibiting hair cell apoptosis but also revealed the secondary role of mitophagy in hair cell apoptosis, thereby providing a new therapeutic direction for clinical intervention.
Author Contributions
L.W. and L.J. designed this study. L.W. performed the experiments, analysed the data, and wrote the manuscript. L.W., L.J., and S.G. revised the manuscript. Y.J. and T.N. contributed funding support and supervised the project. All the authors have read and approved the final version of the manuscript.
Funding
This work was supported by the Municipal Schools (Institutes) and Enterprises Joint Funding Theme, Guangzhou Municipal Health Commission Basic, and Applied Basic Research Project in 2023 (2023A03J0488).
Institutional Review Board Statement
All animal protocols were approved by the Institutional Animal Care and Use Committee of Guangzhou Huateng Biomedical Technology Co. (IACUC:B202308-6)(Guangzhou, China).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data used in this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bazhin, A. V. Mitochondria and Cancer. Cancers 2020, 12, 2641. [Google Scholar] [CrossRef] [PubMed]
- Slade, K.; Plack, C. J.; Nuttall, H. E. The Effects of Age-Related Hearing Loss on the Brain and Cognitive Function. Trends in Neurosciences 2020, 43, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Jafari, Z.; Kolb, B. E.; Mohajerani, M. H. Age-related hearing loss and cognitive decline: MRI and cellular evidence. Annals of the New York Academy of Sciences 2021, 1500, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Sugiura, S.; Nishita, Y.; Saji, N.; Sone, M.; Ueda, H. Age-related hearing loss and cognitive decline — The potential mechanisms linking the two. Auris Nasus Larynx 2019, 46, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Kepp, O.; Trojel-Hansen, C.; Kroemer, G. Mitochondrial Control of Cellular Life, Stress, and Death. Circulation Research 2012, 111, 1198–1207. [Google Scholar] [CrossRef]
- Zhang, J. Teaching the basics of autophagy and mitophagy to redox biologists-Mechanisms and experimental approaches. Redox Biology 2015, 4, 242–259. [Google Scholar] [CrossRef]
- Oh, J.; Youn, C. K.; Jun, Y.; Jo, E.-R.; Cho, S. I. Reduced mitophagy in the cochlea of aged C57BL/6J mice. Experimental Gerontology 2020, 137, 110946. [Google Scholar] [CrossRef]
- Xiong, H.; Chen, S.; Lai, L.; Yang, H.; Xu, Y.; Pang, J.; Su, Z.; Lin, H.; Zheng, Y. Modulation of miR-34a/SIRT1 signaling protects cochlear hair cells against oxidative stress and delays age-related hearing loss through coordinated regulation of mitophagy and mitochondrial biogenesis. Neurobiology of Aging 2019, 79, 30–42. [Google Scholar] [CrossRef]
- Sun, W.; Wang, Y.; Zheng, Y.; Quan, N. The Emerging Role of Sestrin2 in Cell Metabolism, and Cardiovascular and Age-Related Diseases. Aging and Disease 2020, 11, 154–163. [Google Scholar]
- Iglesias, M.; Wang, H.; Krause-Hauch, M.; Ren, D.; Zoungrana, L. I.; Li, Z.; Zhang, J.; Wei, J.; Yadav, N.; Patel, K.; Fatmi, M. K.; Liu, R.; Lesnefsky, E. J.; Li, J. Sestrin2 Mediates Metformin Rescued the Age-Related Cardiac Dysfunctions of Cardiorenal Syndrome Type 3. Cells 2023, 12. [Google Scholar] [CrossRef]
- Yang, X.; Xue, P.; Liu, Z.; Li, W.; Li, C.; Chen, Z. SESN2 prevents the slow-to-fast myofiber shift in denervated atrophy via AMPK/PGC-1α pathway. Cellular & Molecular Biology Letters 2022, 27, 66. [Google Scholar]
- Zhang, C.; Sun, W.; Li, J.; Xiong, B.; Frye, M. D.; Ding, D.; Salvi, R.; Kim, M.-J.; Someya, S.; Hu, B. H. Loss of sestrin 2 potentiates the early onset of age-related sensory cell degeneration in the cochlea. Neuroscience 2017, 361, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Yin, N.; Li, M. O. Sestrins Function as Guanine Nucleotide Dissociation Inhibitors for Rag GTPases to Control mTORC1 Signaling. Cell 2014, 159, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Erway, L. C.; Willott, J. F.; Archer, J. R.; Harrison, D. E. Genetics of age-related hearing loss in mice: I. Inbred and F1 hybrid strains. Hearing research 1993, 65, 125–132. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Guo, J.-Y.; Qu, T.-F.; Wei, W.; Liu, K.; Peng, Z.; Wang, G.-P.; Gong, S.-S. Cellular origin and response of flat epithelium in the vestibular end organs of mice to Atoh1 overexpression. Hearing research 2020, 391, 107953. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Rueden, C. T.; Hiner, M. C.; Eliceiri, K. W. The ImageJ ecosystem: An open platform for biomedical image analysis. Molecular Reproduction and Development 2015, 82, 518–529. [Google Scholar] [CrossRef]
- Lama, L.; Adura, C.; Xie, W.; Tomita, D.; Kamei, T.; Kuryavyi, V.; Gogakos, T.; Steinberg, J. I.; Miller, M.; Ramos-Espiritu, L.; Asano, Y.; Hashizume, S.; Aida, J.; Imaeda, T.; Okamoto, R.; Jennings, A. J.; Michino, M.; Kuroita, T.; Stamford, A.; Gao, P.; Meinke, P.; Glickman, J. F.; Patel, D. J.; Tuschl, T. Development of human cGAS-specific small-molecule inhibitors for repression of dsDNA-triggered interferon expression. Nature Communications 2019, 10, 2261. [Google Scholar] [CrossRef]
- Lee, J. H.; Budanov, A. V.; Karin, M. Sestrins orchestrate cellular metabolism to attenuate aging. Cell metabolism 2013, 18, 792–801. [Google Scholar] [CrossRef]
- Kim, Y. C.; Guan, K.-L. mTOR: a pharmacologic target for autophagy regulation. The Journal of Clinical Investigation 2015, 125, 25–32. [Google Scholar] [CrossRef]
- Pickrell, A.M.; Youle, R.J. The Roles of PINK1, Parkin, and Mitochondrial Fidelity in Parkinson’s Disease. Neuron 2015, 85, 257–273. [Google Scholar] [CrossRef]
- Li, J.; Liu, C.; Müller, U.; Zhao, B. Autophagy proteins are essential for aminoglycoside-induced hearing loss. Autophagy 2023, 19, 1599–1600. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Kuang, H.; Wang, Y.; Bao, L.; Cao, W.; Yu, L.; Qi, M.; Wang, R.; Yang, X.; Ye, Q.; Ding, F.; Ren, L.; Liu, S.; Ma, F.; Liu, S. MSC-derived exosomes protect auditory hair cells from neomycin-induced damage via autophagy regulation. Biological Research 2024, 57, 3. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhou, Y.; Yin, H.; Li, H.; Zhou, M.; Sun, G.; Cao, Z.; Man, R.; Wang, H.; Li, J. PINK1 Protects Against Gentamicin-Induced Sensory Hair Cell Damage: Possible Relation to Induction of Autophagy and Inhibition of p53 Signal Pathway. Frontiers in Molecular Neuroscience 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Zhao, C.; Yang, Z.; Gong, S.; Du, Z. D-galactose-induced mitochondrial oxidative damage and apoptosis in the cochlear stria vascularis of mice. BMC Molecular and Cell Biology 2023, 24, 27. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Guo, Q.; Wang, Z.; Shao, J. B.; Liu, K.; Du, Z. D.; Gong, S. S. D-Galactose-induced oxidative stress and mitochondrial dysfunction in the cochlear basilar membrane: an in vitro aging model. Biogerontology 2020, 21, 311–323. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Hall, M. N.; Lin, S.-C.; Hardie, D. G. AMPK and TOR: The Yin and Yang of Cellular Nutrient Sensing and Growth Control. Cell metabolism 2020, 31, 472–492. [Google Scholar] [CrossRef]
- Steinberg, G. R.; Carling, D. AMP-activated protein kinase: the current landscape for drug development. Nature Reviews Drug Discovery 2019, 18, 527–551. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).