Submitted:
18 November 2024
Posted:
19 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
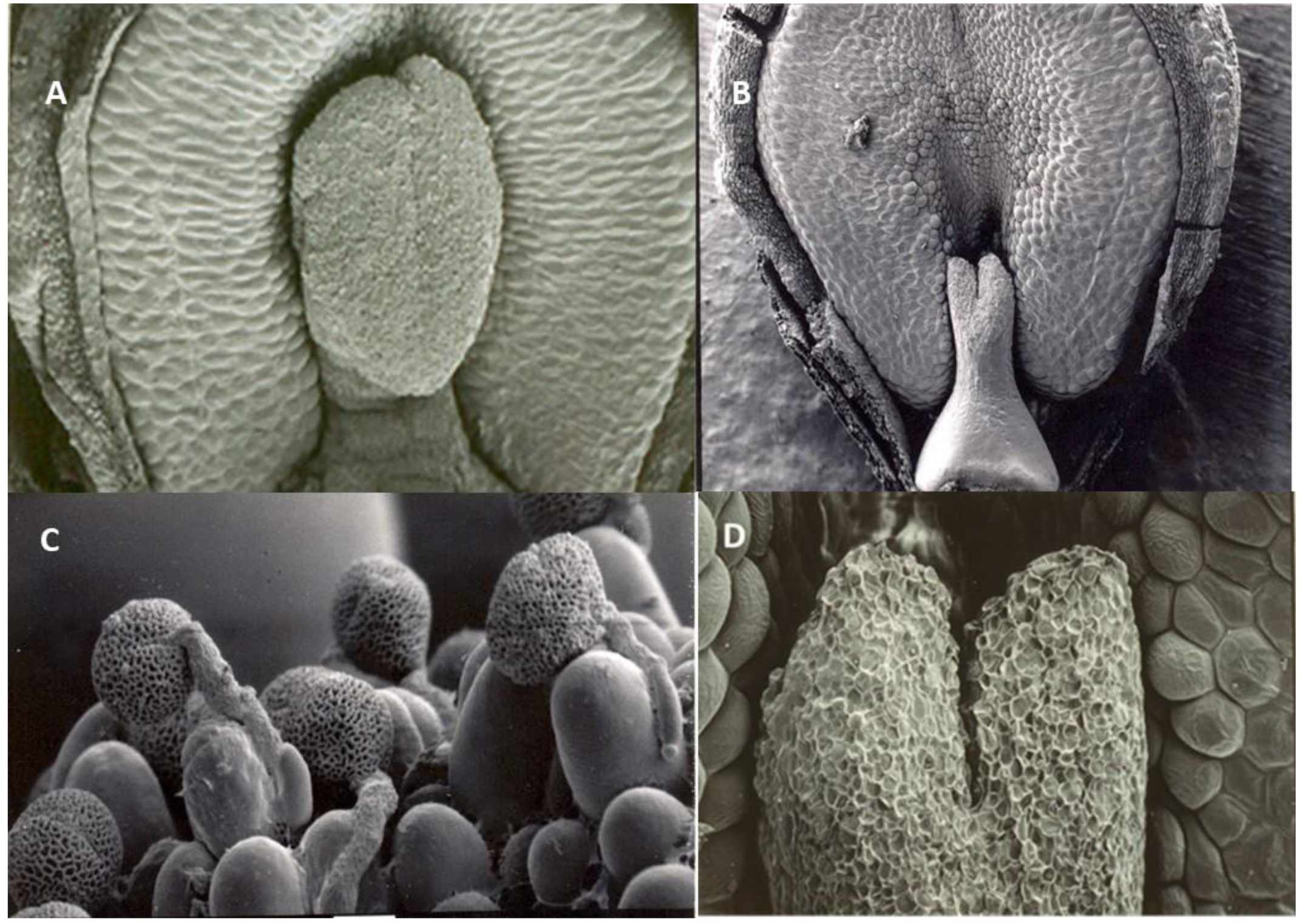
2. The Plant
3. Hypotheses to Explain Massive Fruitlet Abortion
3.1. Pollinator Attraction
3.2. Pollination Deficits
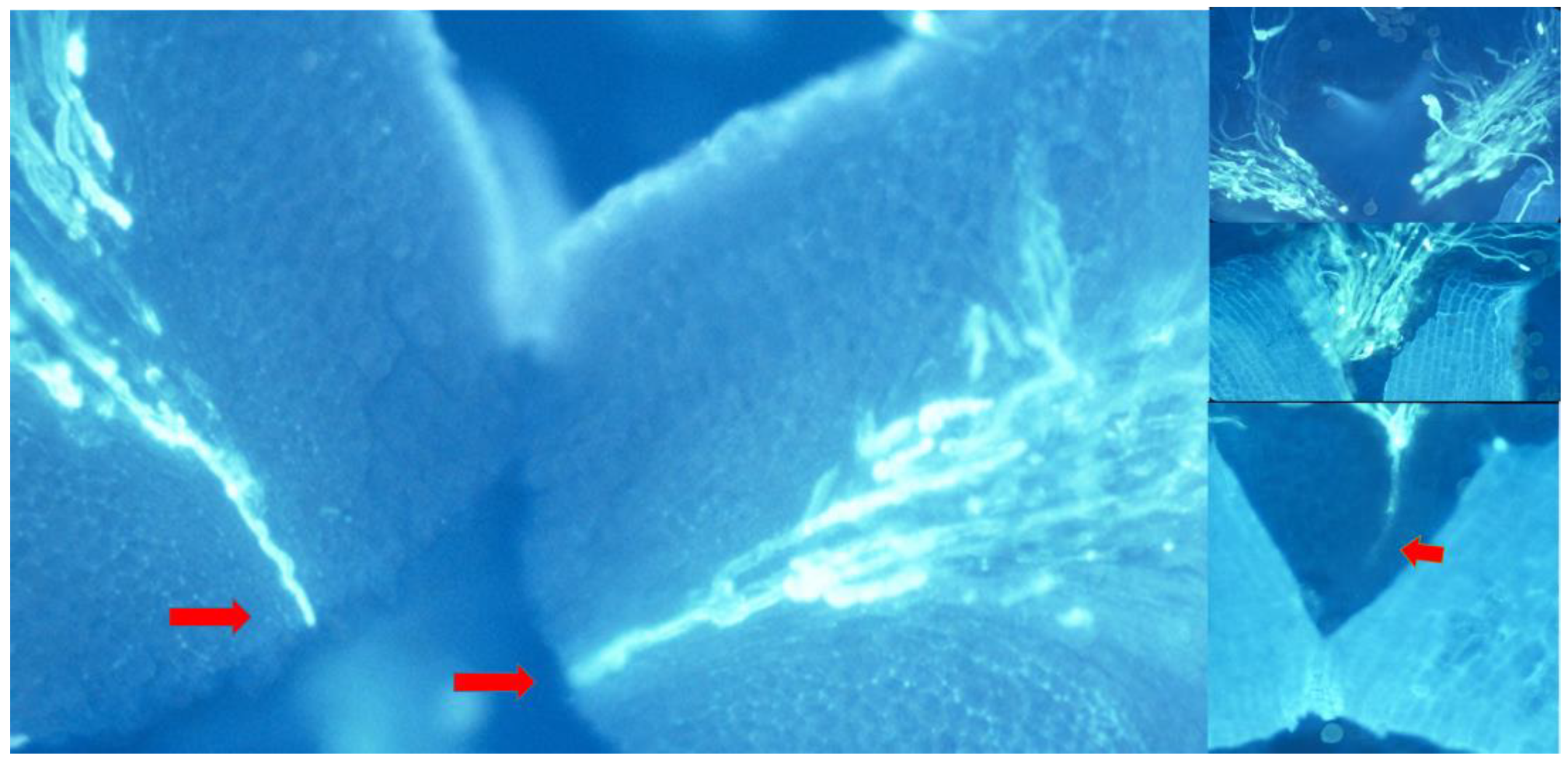
3.3. Male Function
3.4. Resource Limitation
3.5. Bet Hedging: Ovary Reserve Hypothesis
3.6. Sexual Selection, Sibling Competition and Selective Abortion
4. Consequences on Orchard Management: Future Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stephenson, A.G. Flower and fruit abortion: Proximate causes and ultimate functions. Ann. Rev. Ecol. Syst. 1981, 12, 253–279. [Google Scholar] [CrossRef]
- Sutherland, S. Patterns of fruit-set: what controls fruit-flower ratios in plants? Evolution 1986, 40, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.G. Sexual strategies in plants. I. An hypothesis of serial adjustment of maternal investment during one reproductive season. New Phytol 1980, 86, 69–80. Available online: https://www.jstor.org/stable/2434416. [CrossRef]
- Stephenson, A.G. Fruit set, herbivory, fruit reduction, and the fruiting strategy of Catalpa speciosa (Bignoniaceae). Ecology 1980, 61, 57–64. [Google Scholar] [CrossRef]
- Martin, G.C. Olive flower and fruit population dynamics. Acta Hortic. 1990, 286, 141–154. [Google Scholar] [CrossRef]
- Rapoport, H.F.; Rallo, L. Postanthesis flower and fruit abscission in ‘Manzanillo’ olive. J. Amer. Soc. Hort. Sci. 1991, 116, 720–723. [Google Scholar] [CrossRef]
- Seifi, E.; Guerin, J.; Kaiser, B.; Sedgley, M. Flowering and fruit set in olive: a review. Iran. J. Plant Physiol. 2015, 5, 1263–1272. [Google Scholar]
- Lavee, S. Olea europaea. In Handbook of Flowering; Halevy, A.H., Ed.; CRC Press: Boca Raton, Fl, USA, 1985; Volume 6, pp. 423–434. [Google Scholar]
- Cuevas, J.; Polito, V.S. The role of staminate flowers in the breeding system of Olea europaea (Oleaceae): an andromonoecious, wind-pollinated taxon. Ann. Bot. 2004, 93, 547–553. [Google Scholar] [CrossRef]
- Seifi, E.; Guerin, J.; Kaiser, B.; Sedgley, M. Inflorescence architecture of olive. Sci. Hortic. 2008, 116, 273–279. [Google Scholar] [CrossRef]
- Rallo, L.; Fernández-Escobar, R. Influence of cultivar and flower thinning within the inflorescence on competition among olive fruit. J. Amer. Soc. Hort. Sci. 1985, 110, 303–308. [Google Scholar] [CrossRef]
- Cuevas, J.; Rapoport, H.F.; Rallo, L. Relationship among reproductive processes and fruitlet abscission in 'Arbequina' olive. Adv. Hortic. Sci. 1995, 9, 92–96. [Google Scholar]
- Rapoport, H.F.; Rallo, L. Fruit set and enlargement in fertilized and unfertilized olive ovaries. HortSci. 1991, 29, 890–898. [Google Scholar] [CrossRef]
- Willson, M.F.; Price, P.W. The evolution of inflorescence size in Asclepias (Asclepiadaceae). Evolution, 1977; 31, 495–511. [Google Scholar] [CrossRef]
- Khan, S.; Kumari, P.; Verma, S. Ambophily in Olea ferruginea: a transitional state in the pollination syndrome. Plant Biosystems 2022, 157, 221–232. [Google Scholar] [CrossRef]
- Rojas-Gómez, M.; Moral, J.; López-Orozco, R.; Cabello, D.; Oteros, J.; Barranco, D.; Galán, C.; Díez, C.M. Pollen production in olive cultivars and its interannual variability. Ann. Bot, 2023; 132, 1145–1158. [Google Scholar] [CrossRef]
- Guitian, J. 1993. Why Prunus mahaleb (Rosaceae) produces more flowers than fruits. Am. J. Bot. 1993, 80, 1305–1309. [Google Scholar] [CrossRef]
- Cuevas, J.; Rallo, L.; Rapoport, H.F. Crop load effects on floral quality in olive. Sci. Hortic. 1994, 59, 123–130. [Google Scholar] [CrossRef]
- Willson, M.F.; Schemske, D.W. Pollinator limitation, fruit production, and floral display in pawpaw (Asimina triloba). Bull. Torrey Bot. Club 1980, 107, 401–408. [Google Scholar] [CrossRef]
- Bawa, K.S.; Webb, C.J. Flower, fruit and seed abortion in tropical forest trees: implications for the evolution of paternal and maternal reproductive patterns. Am. J. Bot. 1984, 71, 736–751. [Google Scholar] [CrossRef]
- Bradley, M.V.; Griggs, W.H. Morphological evidence of incompatibility in Olea europaea L. Phytomorphology 1963, 13, 141–156. [Google Scholar]
- Ateyyeh, A.F.; Stösser, R.; Qrunfleh, M. Reproductive biology of the olive (Olea europaea L.) cultivar 'Nabali Baladi'. J. App. Bot. 2000, 74, 255–270. [Google Scholar]
- Seifi, E.; Guerin, J.; Kaiser, B.; Sedgley, M. Sexual compatibility and floral biology of some olive cultivars. N. Z. J. Crop Hortic. Sci. 2011, 39, 141–151. [Google Scholar] [CrossRef]
- Sánchez-Estrada, A.; Cuevas, J. ‘Arbequina’ olive is self-incompatible. Sci. Hortic. 2018, 230, 50–55. [Google Scholar] [CrossRef]
- Sánchez-Estrada, A.; Cuevas, J. Pollen-pistil interaction in ‘Manzanillo’ olive (Olea europaea L.) under self-, free- and cross-pollination. Rev. Chapingo Ser. Hortic. 2019, 25, 141–150. [Google Scholar] [CrossRef]
- Cuevas, J.; Chiamolera, F.M.; Pinillos, V.; Rodríguez, F.; Salinas, I.; Cabello, D.; Arbeiter, A.B.; Bandelj, D.; Raboteg Božiković, M.; Vuletin Selak, G. Arbosana olive is self-incompatible, but inter-compatible with some other low-vigor olive cultivars. Horticulturae 2024, 10, 739. [Google Scholar] [CrossRef]
- Breton, C.M.; Bervillé, A. New hypothesis elucidates self-incompatibility in the olive tree regarding S-alleles dominance relationships as in the sporophytic model. C. R. Biol. 2012, 335, 563–572. [Google Scholar] [CrossRef]
- Saumitou-Laprade, P.; Vernet, P.; Vekemans, X.; Billiard, S.; Gallina, S.; Essalouh, L.; Mhaïs, A.; Moukhli, A.; El Bakkali, A.; Barcaccia, G.; Alagna, F.; Mariotti, R.; Cultrera, N.G.M.; Pandolfi, S.; Rossi, M.; Khadari, B.; Baldoni, L. Elucidation of the genetic architecture of self-incompatibility in olive: Evolutionary consequences and perspectives for orchard management. Evol. Appl. 2017, 10, 867–880. [Google Scholar] [CrossRef]
- Cuevas, J. Incompatibilidad polen-pistilo. In Variedades de olivo cultivadas en España, 1st ed.; Barranco, D., Caballero, J.M., Martín, A., Rallo, L., Del Río, C., Tous, J., Trujillo, I., Eds.; Junta de Andalucía, Mundi-Prensa and COI: Seville, Spain, 2004; pp. 303–308. [Google Scholar]
- Cuevas, J.; Polito, V. S. Compatibility Relationships in `Manzanillo' Olive. HortSci. 1997, 32, 1056–1058. [Google Scholar] [CrossRef]
- Vuletin Selak, G.; Cuevas, J.; Goreta Ban, S.; Perica, S. Pollen tube performance in assessment of compatibility in olive (Olea europaea L.) cultivars. Sci. Hortic. 2014, 165, 36–43. [Google Scholar] [CrossRef]
- Bradley, M.V.; Griggs, W.H.; Hartmann, H.T. Studies on self- and cross-pollination of olives under varying temperature conditions. Calif. Agric. 1961, 15, 4–5. [Google Scholar] [CrossRef]
- Griggs, W.H.; Hartmann, H.T.; Bradley, M.V.; Iwakiri, B.T.; Whisler, J.E. Olive pollination in California, Bulletin 869; University of California – Agricultural Experiment Station: Berkeley, USA, 1975; pp. 1–49. [Google Scholar]
- Lavee, S.; Datt, A.C. The necessity of cross-pollination for fruit set of Manzanillo olives. J. Horticult. Sci. 1978, 53, 261–266. [Google Scholar] [CrossRef]
- Willson, M.F. , Rathcke, B.J. Adaptative design of the floral display in Asclepias syriaca L. Am. Midland Naturalist 1974, 92, 47–57. [Google Scholar] [CrossRef]
- Sutherland, S.; Delph, L.F. On the importance of male fitness in plants: patterns of fruit-set. Ecology 1093, 65, 1093–1104. [Google Scholar] [CrossRef]
- Sutherland, S. Why hermaphroditic plants produce many more flowers than fruits: experimental tests with Agave mckelveyana. Evolution, 1987; 41, 750–759. [Google Scholar] [CrossRef]
- Queller, D. Pollen removal, paternity, and the male function of flowers. Am. Nat. 1997, 149, 585–594. [Google Scholar] [CrossRef]
- Queller, D. Sexual selection in a hermaphroditic plant. Nature 1983, 305, 706–707. [Google Scholar] [CrossRef]
- Lloyd, D.G. Parental strategies of angiosperms. N. Z. J. Bot. 1979, 17, 595–606. [Google Scholar] [CrossRef]
- Uriu, K. Periods of pistil abortion in the development of the olive flower. Proc. Amer. Soc. Hort. Sci. 1959, 73, 194–202. [Google Scholar]
- Cuevas, J.; Pinney, K.; Polito, V.S. Flower differentiation, pistil development and pistil abortion in olive. Acta Hortic. 1999, 474, 293–296. [Google Scholar] [CrossRef]
- Diggle, P.K. Architectural effects and the interpretation of patterns of fruit and seed development. Annu. Rev. Ecol. Evol. Syst. 1995, 26, 531–552. [Google Scholar] [CrossRef]
- Wolfe L., M.; Denton, W. Morphological constraints on fruit size in Linaria canadensis. Int. J. Plant. Soil Sci. 2002, 162, 1313–1316. [Google Scholar] [CrossRef]
- Kelly, D.; Sork, V.L. Mast seeding in perennial plants: why, how, where? Ann. Rev. Ecol. and System. 2002, 33, 427–447. [Google Scholar] [CrossRef]
- Pías, B.; Salvande, M.; Guitián, P. Variation in predispersal losses in reproductive potential in rowan (Sorbus aucuparia L. Rosaceae) in the NW Iberian Peninsula. Plant Ecol. 2007, 188, 191–203. [Google Scholar] [CrossRef]
- Lavee, S.; Rallo, L.; Rapoport, H.F.; Troncoso, A. The floral biology of the olive: effect of flower number, type and distribution on fruitset. Sci. Hortic. 1996, 66, 149–158. [Google Scholar] [CrossRef]
- Suarez, M.P.; Fernández-Escobar, R.; Rallo, L. Competition among fruits in olive II. Influence of inflorescence or fruit thinning and cross-pollination on fruit set components and crop efficiency. Acta Hortic 1984, 149, 131–143. [Google Scholar] [CrossRef]
- Rosati, A.; Zipanćič, M.; Caporali, S.; Padula, G. Fruit weight is related to ovary weight in olive (Olea europaea L.). Sci. Hortic. 2009, 122, 399–403. [Google Scholar] [CrossRef]
- Rosati, A.; Zipanćič, M.; Caporali, S.; Paoletti, A. Fruit set is inversely related to flower and fruit weight in olive (Olea europaea L.). Sci. Hortic. 2010, 126, 200–204. [Google Scholar] [CrossRef]
- Cuevas, J.; Rallo, L. Respuesta a la polinización cruzada en olivo bajo diferentes temperaturas. Actas de Horticultura 1988, 1, 203–208. [Google Scholar]
- Janzen, D.H. Escape of Cassia grandis L. beans from predators in time and space. Ecology 1971, 52, 964–979. [Google Scholar] [CrossRef]
- Ehrlen, J. Why do plants produce surplus flowers? A reserve-ovary model. Am. Nat. 1991, 138, 918–933. [Google Scholar] [CrossRef]
- Brown, A.O.; McNeil, J.N. Fruit production in cranberry (Vaccinium macrocarpon): a bet-hedging strategy to optimize reproductive effort. Am. J. Bot. 2006, 93, 910–916. [Google Scholar] [CrossRef]
- Guitián, J.; Guitián, P.; Navarro, L. Fruit set, fruit reduction, and fruiting strategy in Cornus sanguinea (Cornaceae). Am. J. Bot. 1996, 83, 744–748. [Google Scholar] [CrossRef]
- Guitian, J. Selective fruit abortion in Prunus mahaleb (Rosaceae). Am. J. Bot. 1994, 81, 1555–1558. [Google Scholar] [CrossRef]
- Mulas, M. Characterisation of olive wild ecotypes. Acta Hortic. 1999, 474, 121–124. [Google Scholar] [CrossRef]
- Khadivi, A.; Mirheidari, F.; Saeidifar, A.; Moradi, Y. Morphological characterizations of Olea europaea subsp. cuspidata. Genet. Resour. Crop Evol. 2024, 71, 1837–1853. [Google Scholar] [CrossRef]
- Darwin, C.R. 1869. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. 5th ed.; John Murray: London, Great Britain.
- Willson, M.F. Sexual selection in plants. Am. Nat. 1979, 113, 777–790. [Google Scholar] [CrossRef]
- Stephenson, A.G.; Winsor, J.A. Lotus corniculatus regulates offspring quality through selective fruit abortion. Evolution 1986, 40, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, J.; Oller, R. Olive seed set and its impact on seed and fruit weight. Acta Hortic. 2002, 586, 485–488. [Google Scholar] [CrossRef]
- Lee, T.D. Patterns of fruit maturation: a gametophyte competition hypothesis. Am. Nat. 1984, 123, 427–432. [Google Scholar] [CrossRef]
- Cuevas, J.; Rallo, L.; Rapoport, H.F. Pollen tube growth and ovule abortion in Olea europaea (Oleaceae): A case if ovule selection. In Pollination Mechanisms, Ecology and Agricultural Advances; Raskin, N.D., Vuturro, P.T., Eds.; Nova Science Publisher Inc.: New York, USA, 2011; pp. 57–72. [Google Scholar]
- Sauter, M. A. A guided tour: Pollen tube orientation in flowering plants. Chin. Sci. Bull. 2009, 54, 2376–2382. [Google Scholar] [CrossRef]
- Kanaoka, M.M. Cell–cell communications and molecular mechanisms in plant sexual reproduction. J. Plant Res. 2018, 131, 37–47. [Google Scholar] [CrossRef]
- Iwano, M.; Ngo, Q.A.; Entani, T.; Shiba, H.; Nagai, T.; Miyawaki, A.; Isogai, A.; Grossniklaus, U.; Takayama, S. Cytoplasmic Ca2+ changes dynamically during the interaction of the pollen tube with synergid cells. Development 2012, 139, 4202–4209. [Google Scholar] [CrossRef]
- Takeuchi, H.; Higashiyama, T. Attraction of tip-growing pollen tubes by the female gametophyte. Curr. Opin. Plant Biol. 2011, 14, 614–621. [Google Scholar] [CrossRef]
- Hiei, K.; Ohara, M. Variation in fruit- and seed set among and within inflorescences of Melampyrum roseum var. japonicum (Scrophulariaceae). Plant Species Biol. 2002, 17, 13–23. [Google Scholar] [CrossRef]
- Medrano, M.; Guitián, P.; Guitián, J. Patterns of fruit and seed set within inflorescences of Pancratium maritimum (Amaryllidaceae): nonuniform pollination, resource limitation, or architectural effects? Am. J. Bot. 2000, 87, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Hapuarachchi, N.S.; Kämper, W.; Hosseini Bai, S.; Ogbourne, S.M.; Nichols, J.; Wallace, H.M.; Trueman, S.J. Selective retention of cross-fertilised fruitlets during premature fruit drop of Hass avocado. Horticulturae 2024, 10, 591. [Google Scholar] [CrossRef]
- Alcaraz, M.L.; Hormaza, J.I. Fruit set in avocado: pollen limitation, pollen load size, and selective fruit abortion. Agronomy 2021, 11, 1603. [Google Scholar] [CrossRef]
- Stutte, G.W.; Martin, G.C. Effect of killing the seed on return bloom of olive. Sci. Hortic. 1986, 29, 107–113. [Google Scholar] [CrossRef]
- Stanton, M.L. Seed variation in wild radish: effect of seed size on components of seedling and adult fitness. Ecology 1984, 65, 1105–1112. [Google Scholar] [CrossRef]
- Nakamura, R. Seed abortion and seed variation within fruits of Phaseolus vulgaris: pollen donor and resource limitation effects. Am. J. Bot. 1988, 75, 1003–1010. [Google Scholar] [CrossRef]
- Zhang, J.; Maun, M.A. Seed size variation and its effects on seedling growth in Agropyron psammophilum. Bot. Gaz. 1990, 151, 106–113. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




