Submitted:
18 November 2024
Posted:
19 November 2024
You are already at the latest version
Abstract
Muscle element fingerprint is a comprehensive reflection of the accumulated transformation of elements from different sources in muscles, and has habitat indicator functions such as stability and representativeness. Leiocassis longirostris (Ll), Coilia mystus (Cm) and Collichthys lucidus (Cl) are important fishery species in the Yangtze River Estuary with different ecological habits. In this study, the element fingerprint of three fish species (Ll, Cm, Cl) and two environmental media of water (W) and sediment (S) were analyzed. According to the PERMANOVA, significant differences (p<0.05) were observed in the elemental composition between sediment and the fish species. Through principal component analysis (PCA), it was found that the major elements ratios of Ti, V, Al, Fe, Ba, Ni, Mn, Co, Zn, and Cr to Ca in the sediment were significantly higher than those of the other groups (p<0.05). Stepwise linear discriminant analysis (LDA) explored that the differential discriminant elements ratios between the water and the fish species were Mo/Ca, Ba/Ca, and K/Ca. LDA of the muscle elemental contents among different fish species showed that Mo, Mn, and Ba were the characteristic discriminant elements of L. longirostris, C. mystus and C. lucidus, respectively, which were closely related to their different ecological habitats. In summary, the elemental compositions in the muscle of the fish species differed significantly with that of the major elements in the sediments, and that with a few discriminant elements in the water, and there are differences in characteristic discriminative elements among fish with different ecological habits. Therefore, when tracing the origin of fish with habitat changes, the same fish species should be selected as the reference for tracing according to the stability of the characteristic elements enriched in each of the fish with different ecological habits. This study has important theoretical guidance and technical support in analyzing the source of elemental composition of fishes and in the traceability discrimination of fishes based on elemental fingerprints, and it has important application value in the selection of reference media for fish species traceability.
Keywords:
1. Introduction
2. Materials and Methods
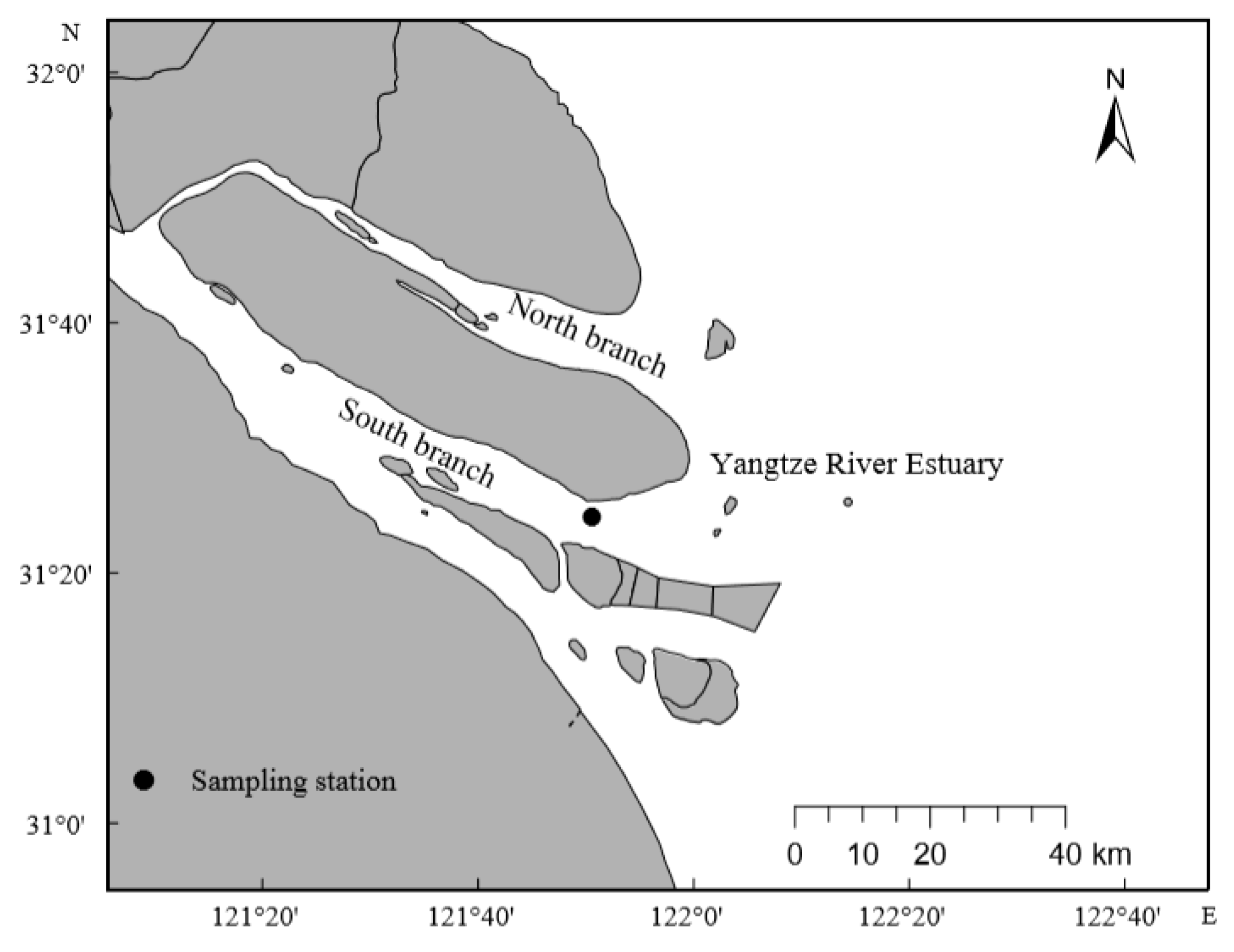
2.1. Sampling Sites, Sample Collection and Processing
2.2. Elemental Analysis
2.3. Data Analysis
3. Results
3.1. Elemental Composition of Fish and Environmental Media
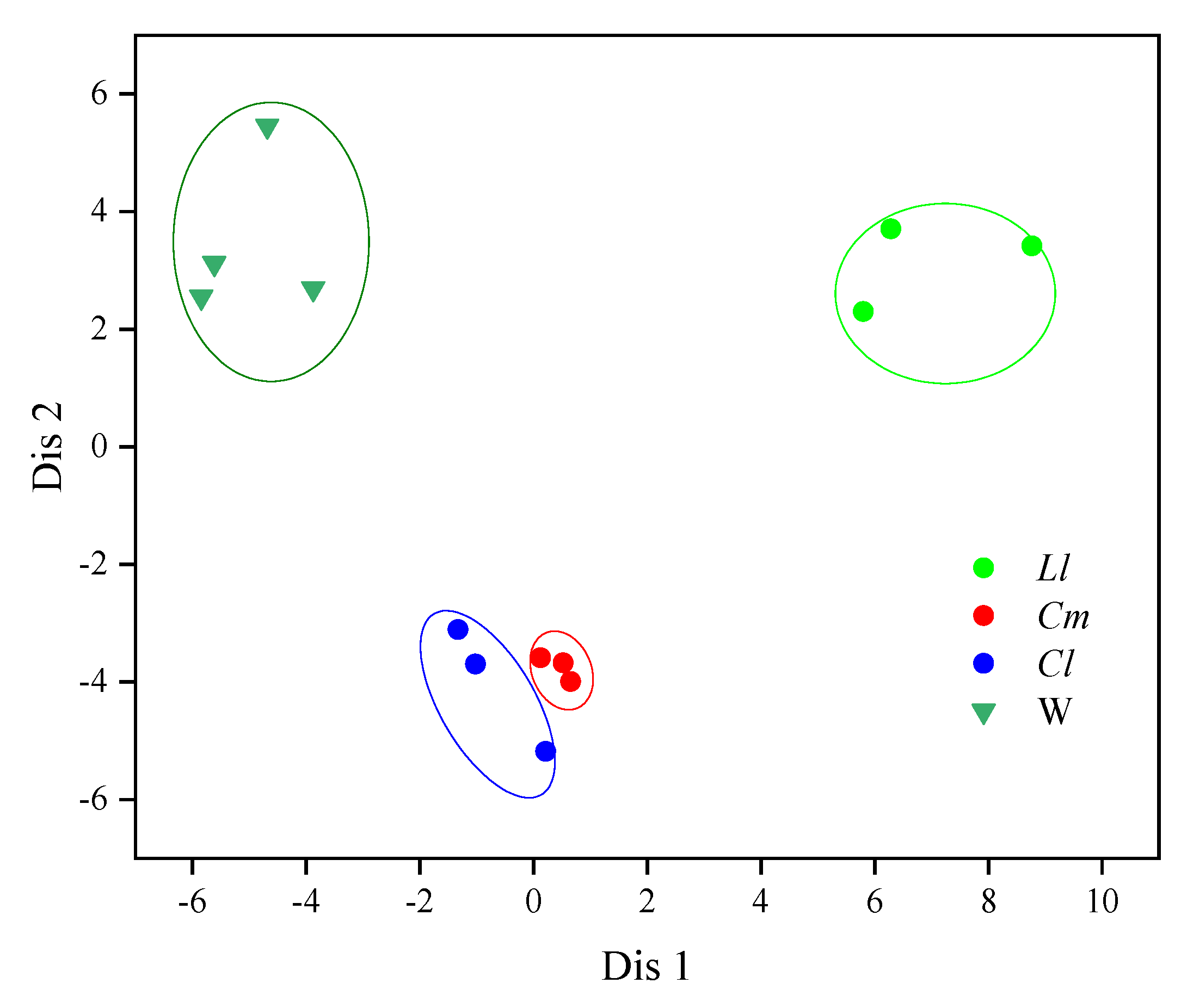
3.2. Association Analysis Between Fish and Environmental Media
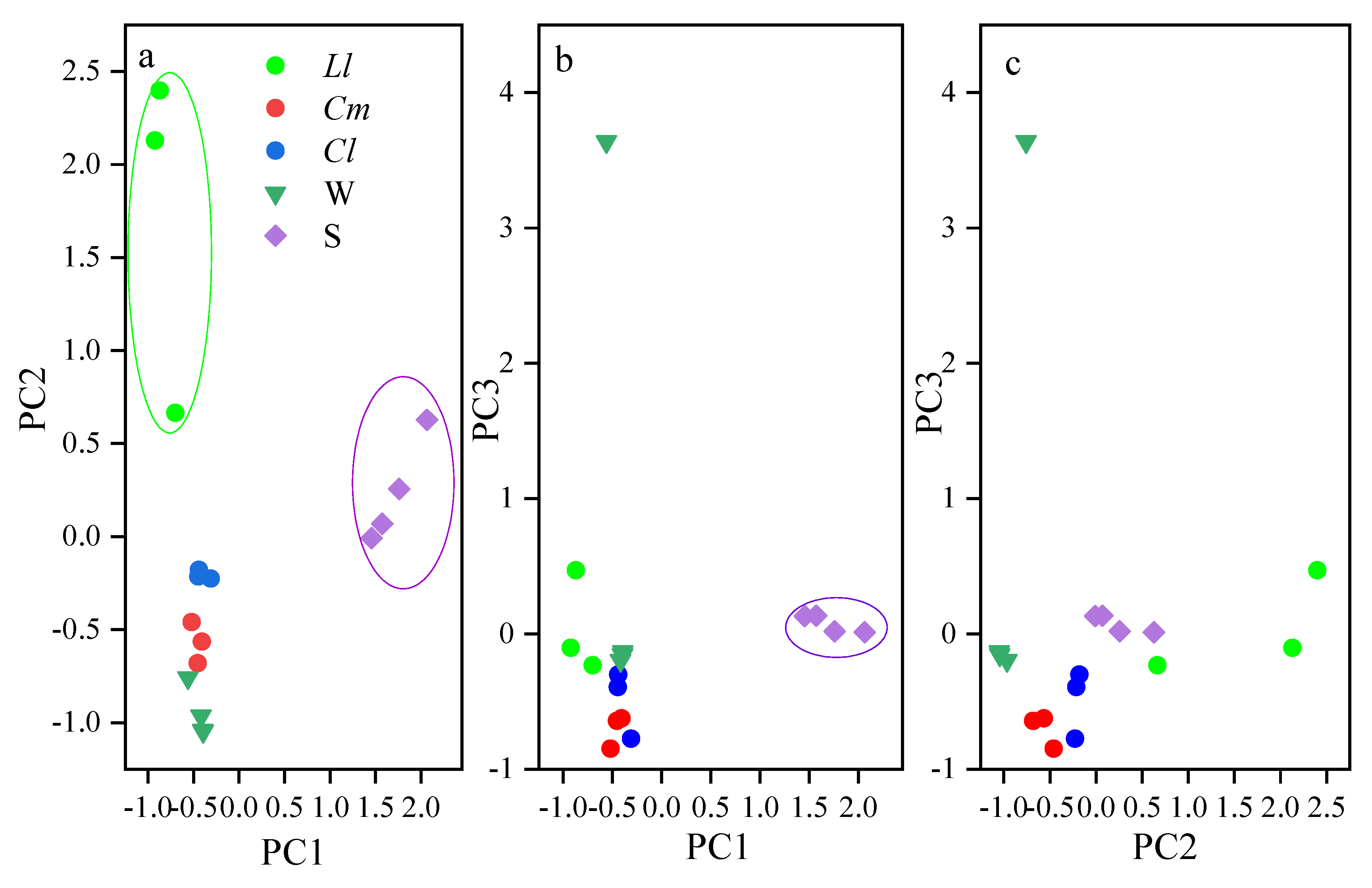
3.3. Major Element Composition in Fish and Environmental Media
3.4. Discrimination Between Fish Groups and Water Group
3.5. Traceability Discrimination among Three Different Fish Species
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhuang, P.; Wang, Y.H.; Li, S.F.; Deng, S.M.; Li, C.S.; Ni, Y.; Zhang, L.Z.; Zhang, T.; Feng, G.P.; Ling, J.Z.; et al. Fishes of the Yangtze Estuary; Shanghai Scientific and Technical Publishers: Shanghai, China, 2006; pp. 29-57.
- Zhao, Z.M.; Zhao, H.; Wang, X.Y.; Zhang, L.; Mou, C.Y.; Huang, Z.P.; Ke, H.Y.; Duan, Y.L.; Zhou, J.; Li, Q. Effects of different temperatures on Leiocassis longirostris gill structure and intestinal microbial composition. Sci. Rep. 2024,14,7150. [CrossRef]
- Li, Y.; Zou, X.X.; Jin, H.H.; Zhou, B.; Zhou, J.; Zhang, L.; Li, Z.; Ling, L.; Liu, F.; Gao, Y.; et al. Identification of genes related to growth from transcriptome profiles of the muscle and liver of Chinese longsnout catfish (Leiocassis longirostris). Comp. Biochem. Phys. D 2024, 49, 101180. [CrossRef]
- Yang, M.; Xie, Y.D.; Shi, Y.H.; Sui, C.; Xu J.B. Study on artificial breeding of Chinese longsnout catfish Leiocassis longirostris in Shanghai. Fish. Sci. Technol. Inf. 2024, 2, 77-82.
- Yang, J.; Arai, T.; Liu, H.; Miyazaki, N.; Tsukamoto, K. Reconstructing habitat use of Coilia mystus and Coilia ectenes of the Yangtze River Estuary, and of Coilia ectenes of Taihu Lake, based on otolith strontium and calcium. J. Fish Biol. 2006, 69, 1120-1135. [CrossRef]
- Zhuang, P.; Zhang, T.; Li, S.F.; Ni, Y.; Wang, Y.H.; Deng, S.M.; Zhang, L.Z.; Ling, J.Z.; Hu, F.; Yang, G.; et al. Fishes of the Yangtze Estuary. China Agriculture Press: Beijing, China, 2018; pp.124-127.
- Song, C.; Zhao, F.; Feng, G.P.; Wang, S.K.; Zhuang, P. Comparative study of nutritional composition of adults and juveniles of Coilia mystus collected from the Yangtze Estuary. Indian J. Anim. Res. 2020, 54,1103-1108. [CrossRef]
- Zhang, S. Jiang, Y.; Li, M.; Zhu, J.F.; Xu, S.N.; Chen, Z.Z. Life history of spinyhead croaker Collichthys lucidus (Sciaenidae) differentiated among populations from Chinese coastal waters. Aquat. Biol. 2022, 31, 65-76.
- Kumar, R.; Jaiswar, A.K.; Sharma, R.; Prasad, L. Quantification of morphological variations among populations of Channa gachua (Hamilton, 1822) from different geographical locations in India. Indian J. Fish. 2020, 67, 114-119. [CrossRef]
- Klobucar, S.L.; Rick, J.A.; Mandeville, E.G.; Wagner, C.E.; Budy, P. Investigating the morphological and genetic divergence of arctic char (Salvelinus alpinus) populations in lakes of arctic Alaska. Ecol. Evol. 2021, 11, 3040-3057. [CrossRef]
- Guo, H.; Zhang, X.F.; Naslund, J.; Peng, L.Y.; Liu, C.H.; Tian, M.J.; Chai, X.J.; Zhang, D.Y.; Zhang, X.M. Differences in external morphology, body composition and swimming performance between hatchery- and wild-origin marbled rockfish (Sebastiscus marmoratus). Front. Mar. Sci. 2022, 9, 912129. [CrossRef]
- Zhu, K.; Wang, H.Z.; Lu, K.X.; Zhu, W.B. Morphological variation and distinction in cultured and wild populations of Pseudosciaena crocea in the East China Sea. Appl. Ecol. Environ. Res. 2024, 22, 2065-2077. [CrossRef]
- Purrafee Dizaj, L.; Esmaeili, H.R.; Teimori, A. Comparative otolith morphology of clupeids from the Iranian brackish and marine resources (Teleostei: Clupeiformes). ACTA Zool. 2022, 103, 29-47. [CrossRef]
- Qiao, J.L.; Zhu, R.; Chen, K.; Zhang, D.; Yan, Y.Z.; He, D.K. Comparative otolith morphology of two morphs of Schizopygopsis Thermalis Herzenstein 1891 (Pisces, Cyprinidae) in a Headwater Lake on the Qinghai-Tibet Plateau. Fishes 2022, 7, 99.
- Zhou, Y.; Xu, L.; He, Z.T.; Cui, W.J.; Lu, Q.; Qin, J.G.; Su, S.Q.; He, T. Discrimination of Schizothorax Grahami (Regan, 1904) stocks based on otolith morphology. Fishes 2023, 8, 504.
- Astorga, M.P.; Valenzuela, A.; Segovia, N.I.; Poulin, E.; Vargas-Chacoff, L.; Gonzalez-Wevar, C.A. Contrasting patterns of genetic diversity and divergence between landlocked and migratory populations of fish, evaluated through mitochondrial DNA sequencing and nuclear DNA microsatellites. Front. Genet. 2022, 13, 854362. [CrossRef]
- Biton-Porsmoguer, S.; Bou, R.; Lloret, E.; Alcaide, M.; Lloret, J. Fatty acid composition and parasitism of European sardine (Sardina pilchardus) and anchovy (Engraulis encrasicolus) populations in the northern Catalan Sea in the context of changing environmental conditions. Conserv. Physiol. 2020, 8, coaa121. [CrossRef]
- Amoussou, N.; Marengo, M.; Afe, O.H.I.; Lejeune, P.; Durieux, E.D.H.; Douny, C.; Scippo, M.-L.; Gobert, S. Comparison of fatty acid profiles of two cultivated and wild marine fish from Mediterranean Sea. Aquac. Int. 2022, 30, 1435-1452. [CrossRef]
- Kang, X.M.; Zhao, Y.F.; Tan, Z.J.; Ning, J.S.; Zhai, Y.X.; Zheng, G.C. Evaluation of multivariate data analysis for marine mussels Mytilus edulis authentication in China: Based on stable isotope ratio and compositions of C, N, O and H. J. Food Compos. Anal. 2022, 111, 104627. [CrossRef]
- Xuan, Z.Y.; Wang, W.X. Trace elemental and stable isotopic signatures to reconstruct the large-scale environmental connectivity of fish populations. Mar. Ecol. Prog. Ser. 2024, 730, 95-111. [CrossRef]
- Leal, M.C.; Pimentel, T.; Ricardo, F.; Rosa, R.; Calado, R. Seafood traceability: current needs, available tools, and biotechnological challenges for origin certification. Trends Biotechnol. 2015, 33, 331-336. [CrossRef]
- Song, C.; Yang, C.Y.; Zhao, F.; Xie, J.L.; Tao, H.; Huang, X.R.; Zhuang, P. Using muscle element fingerprint analysis (EFA) to trace and determine the source of Hypophthalmichthys nobilis in the Yangtze River Basin. 2024, 9, 316. [CrossRef]
- Cabral, A.E.; Ricardo, F.; Patinha, C.; Silva, E.F.D.; Correia, M.; Palma, J.; Planas, M.; Calado, R. Successful use of geochemical tools to trace the geographic origin of long-snouted seahorse Hippocampus guttulatus raised in captivity. Animals 2021, 11, 1534. [CrossRef]
- Fu, X.S.; Hong, X.Z.; Liao, J.Y.; Ji, Q.G.; Li, C.F.; Zhang, M.Z.; Ye, Z.H.; Yu, X.P. Fingerprint approaches coupled with chemometrics to discriminate geographic origin of imported salmon in China’s consumer market. Foods 2021, 10, 2986. [CrossRef]
- Zhang, J.; Yang, R.D.; Li, Y.C.; Wen, X.F.; Peng, Y.S.; Ni, X.R. Use of mineral multi-elemental analysis to authenticate geographical origin of different cultivars of tea in Guizhou, China. J. Sci. Food Agric. 2020, 100, 3046-3055. [CrossRef]
- Qi, J.; Li, Y.Y.; Zhang, C.; Wang, C.; Wang, J.Q.; Guo, W.P.; Wang, S.W. Geographic origin discrimination of pork from different Chinese regions using mineral elements analysis assisted by machine learning techniques. Food Chem. 2021, 337, 127779. [CrossRef]
- Lai, J.; Zhao, L.J.; Fan, Y.C.; Qu, X.C.; Liu, D.Y.; Guo, Z.L.; Wang, Y.H.; Liu, Q.G.; Chen, Y.S. Using whole body elemental fingerprint analysis to distinguish different populations of Coilia nasus in a large river basin. Biochem. Syst. Ecol. 2015, 60, 249-257. [CrossRef]
- Zhang, J.H.; Li, X.C.; Guo, L.Q.; Deng, Z.M.; Wang, D.W.; Liu, L.S. Assessment of heavy metal pollution and water quality characteristics of the reservoir control reaches in the middle Han River, China. Sci. Total Environ. 2021, 799, 149472. [CrossRef]
- Pecyna-Utylska, P.; Muntean, E.; Kernert, J.; Michalski, R. Indexing methods and chemometric analysis of selected metals and metalloids for drinking water quality assessment in Upper Silesia region, Poland. Int. J. Environ. Anal. Chem. 2023, 103, 5503-5521. [CrossRef]
- Orłowski, G.; Niedzielski, P.; Karg, J.; Proch, J. Colour-assisted variation in elytral ICP-OES-based ionomics in an aposematic beetle. Sci. Rep. 2020, 10, 22262. [CrossRef]
- Di Donato, F.; Gornati, G.; Biancolillo, A.; D’Archivio, A.A. ICP-OES analysis coupled with chemometrics for the characterization and the discrimination of high added value Italian Emmer samples. J. Food Compos. Anal. 2021, 98, 103842. [CrossRef]
- Lossow, K.; Schlörmann, W.; Tuchtenhagen, M.; Schwarz, M.; Schwerdtle, T.; Kipp, A.P. Measurement of trace elements in murine liver tissue samples: Comparison between ICP-MS/MS and TXRF. J. Trace Elem. Med. Biol. 2023, 78, 127167. [CrossRef]
- Li, Y.L.; Fan, J.; Jin, H.Y.; Wei, F.; Ma, S.C. New vision for TCM quality control: Elemental fingerprints and key ingredient combination strategy for identification and evaluation of TCMs. Eur. J. Med. Chem. 2024, 281, 117006. [CrossRef]
- Danezis, G.P.; Pappas, A.C.; Zoidis, E.; Papadomichelakis, G.; Hadjigeorgiou, I.; Zhang, P.; Brusic, V.; Georgiou, C.A. Game meat authentication through rare earth elements fingerprinting. Anal. Chim. Acta 2017, 991, 46-57. [CrossRef]
- Ren, Y.F.; Feng, C.; Ye, Z.H.; Zhu, H.Y.; Hou, R.Y.; Granato, D.; Cai, H.M.; Peng, C.Y. Keemun black tea: Tracing its narrow-geographic origins using comprehensive elemental fingerprinting and chemometrics. Food Control 2022, 133, 108614. [CrossRef]
- Dehelean, A.; Cristea, G.; Puscas, R.; Hategan, A.R.; Magdas, D.A. Assigning the geographical origin of meat and animal rearing system using isotopic and elemental fingerprints. Appl. Sci. 2022, 12, 12391. [CrossRef]
- Mamede, R.; Patinha, C.; Martins, P.; Ferreira da Silva, E.; Calado, R.; Ricardo, F. Effects of H2O2 pretreatment on the elemental fingerprints of bivalve shells and their implications for the traceability of geographic origin. Heliyon 2024, 10, e25872. [CrossRef]
- Mamede, R.; Santos, A.; da Silva, E.F.; Patinha, C.; Calado, R.; Ricardo, F. New evidence of fraudulent mislabeling and illegal harvesting of Manila clams (Ruditapes philippinarum) through elemental fingerprints of their shells and chemometric analyses. Food Control 2024, 163, 110501. [CrossRef]
- Jin, Y.J.; He, K.; Xiang, P.; Wang, X.D.; Tong, L.T.; Wei, Z.; Zhang, X.Y.; Song, Z.B. Temporal genetic variation of the Chinese longsnout catfish (Leiocassis longirostris) in the upper Yangtze River with resource decline. Environ. Biol. Fish. 2022, 105, 1139-1151. [CrossRef]
- Wang, Q.; Zhou, L.; Algeo, T.J.; Soltanian, M.R.; Liu, J.; Feng, L.P.; Liu, J.C.; Peng, S.H. The geochemical behavior of molybdenum in the modern Yangtze Estuary and East China Sea Shelf. J. Hydrol. 2021, 595, 125997. [CrossRef]
- Fan, J.; Fan, D.D.; Wu, Y.J. Spatiotemporal variations of heavy metal historical accumulation records and their influencing mechanisms in the Yangtze River Estuary. Sci. Total Environ. 2023, 854, 158733. [CrossRef]
- Zhai, L.; Li, Z.G.; Hu, Y.B.; Huang, C.W.; Tian, S.Q.; Wan, R.; Pauly, D. Assessment of Coilia mystus and C. nasus in the Yangtze River Estuary, China, using a length-based approach. Fishes 2022, 7, 95.
- Zhou, D.X.; Ge Y.C.; Jiang Z.Y.; Ruan Y.Q.; Cao F.; Yang S.Y.; Zhang R.F. Seasonal variations of dissolved Mn concentration in the surface water of the Changjiang River Estuary and its adjacent area. Haiyang Xuebao, 2024, 46, 111-120.
- Xuan, W.D.; Zhang, H.L.; Zhang, H.B.; Wu, T.; Zhou, Y.D.; Zhu, W.B. Distribution characteristics and driving factors of Collichthys lucidus species in offshore waters of Zhejiang Province, China. Fishes 2024, 9, 83. [CrossRef]
- Wang, A.; Wang, Z.H.; Liu, J.K.; Xu, N.C.; Li, H.L. The Sr/Ba ratio response to salinity in clastic sediments of the Yangtze River Delta. Chem. Geol. 2021, 559, 119923. [CrossRef]
- Kong, X.B.; Chen, Q.S.; Xu, M.; Liu, Y.H.; Li, X.M.; Han, L.X.; Zhang, Q.; Wan, H.L.; Liu, L.; Zhao, X.; et al. Geographical origin identification of winter jujube (Ziziphus jujuba ‘Dongzao’) by using multi-element fingerprinting with chemometrics. J. Integr. Agric. 2024, 23, 1749-1762. [CrossRef]
| Index | Ll | Cm | Cl | W | S | p |
|---|---|---|---|---|---|---|
| Co/Ca | 39.87± 17.77 a | 19.20± 17.88a | 15.42± 5.04 a | 2.58± 0.27 a | 450.56± 55.74 b | 0.000 |
| Mo/Ca | 154.72± 26.25 a | 15.32± 3.37b | 21.42± 4.09 b | 36.08± 13.07b | 20.26± 11.09 b | 0.000 |
| Cd/Ca | 82.69± 62.16 a | 13.51± 2.06b | 30.63± 18.88b | 0.40± 0.34b | 8.71± 3.31 b | 0.014 |
| V/Ca | 102.78± 43.51 a | 28.19± 16.87 a | 32.51± 11.31a | 53.35± 4.81 a | 2919.24± 469.70 b | 0.000 |
| Hg/Ca | 427.73± 126.48 a | 36.69± 9.28b | 85.38± 60.37 b | 2.38± 0.53 b | 4.56± 1.88 b | 0.000 |
| Ni/Ca | 91.85± 65.88 a | 62.52± 45.59 a | 81.31± 34.02 a | 15.24± 5.08 a | 1062.14± 172.80 b | 0.000 |
| Ti/Ca | 258.89± 73.39 a | 68.93± 32.77 a | 182.06± 111.84 a | 12.16± 3.44 a | 98680.53± 13444.56 b | 0.000 |
| Ba/Ca | 595.17± 345.59 ab | 173.90± 42.51 a | 815.70± 109.12 ab | 1142.99± 133.72 b | 16224.68± 679.57 c | 0.000 |
| Cr/Ca | 1139.59± 444.29 a | 259.24± 86.63 a | 519.21± 93.59 a | 67.62± 35.26 a | 2574.02± 1020.62b | 0.000 |
| Pb/Ca | 397.72± 146.71 ab | 203.10± 79.26 a | 837.16± 643.09 b | 3.26± 1.32 a | 841.98± 119.68 b | 0.005 |
| Cu/Ca | 2199.02± 1382.98a | 340.53± 44.45b | 638.50± 218.53b | 41.73± 10.55 b | 607.43± 315.00b | 0.006 |
| Mn/Ca | 1364.04± 533.93 a | 595.63± 137.72 a | 574.15± 140.28 a | 12.84± 2.58 a | 24745.02± 6239.79 b | 0.000 |
| Al/Ca | 3501.45± 1007.42 a | 1753.43± 931.91 a | 3685.53± 3102.81 a | 27.49± 26.67 a | 1909440.87±137470.41 b | 0.000 |
| As/Ca | 5.47± 2.16 a | 3.83± 1.83 a | 1.06± 0.32 b | 0.04± 0.01 b | 0.37± 0.06 b | 0.000 |
| Sr/Ca | 2.54± 0.37 a | 3.61± 1.18 a | 3.59± 0.29 a | 9.32± 5.83 a | 6.75± 0.55 a | 0.053 |
| Fe/Ca | 45.62± 31.47 a | 7.65± 2.92 a | 16.75± 10.29 a | 0.14± 0.14 a | 989.69± 125.44 b | 0.000 |
| Zn/Ca | 36.67± 13.27 a | 8.37± 2.51 a | 10.36± 2.86 a | 0.04± 0.03 a | 137.09± 34.86 b | 0.000 |
| K/Ca | 23.07± 5.98 a | 3.18± 1.16 b | 9.41± 2.39 c | 0.36± 0.40 b | 0.52± 0.01b | 0.000 |
| Na/Ca | 3.84± 1.98 a | 0.54± 0.23 a | 1.30± 0.40 a | 4.32± 7.67 a | 0.41± 0.05 a | 0.546 |
| Mg/Ca | 1.55± 0.39a | 0.26± 0.09 b | 0.65± 0.15 ab | 0.69± 0.90 ab | 0.46± 0.03 ab | 0.052 |
| Groups | t | p |
|---|---|---|
| L. longirostris vs sediment | 5.110 | 0.042 |
| C. mystus vs sediment | 4.216 | 0.035 |
| C. lucidus vs sediment | 3.933 | 0.023 |
| L. longirostris vs water | 3.061 | 0.058 |
| C. mystus vs water | 2.333 | 0.156 |
| C. lucidus vs water | 2.831 | 0.063 |
| Variable | Principal Component | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Co/Ca | 0.967 | 0.208 | 0.030 |
| Mo/Ca | -0.421 | 0.826 | 0.115 |
| Cd/Ca | -0.352 | 0.810 | -0.099 |
| V/Ca | 0.981 | 0.172 | 0.046 |
| Hg/Ca | -0.454 | 0.863 | -0.010 |
| Ni/Ca | 0.972 | 0.209 | 0.016 |
| Ti/Ca | 0.985 | 0.151 | 0.038 |
| Ba/Ca | 0.974 | 0.121 | 0.059 |
| Cr/Ca | 0.803 | 0.499 | 0.016 |
| Pb/Ca | 0.532 | 0.324 | -0.226 |
| Cu/Ca | -0.180 | 0.911 | -0.002 |
| Mn/Ca | 0.968 | 0.199 | 0.026 |
| Al/Ca | 0.980 | 0.139 | 0.040 |
| As/Ca | -0.483 | 0.639 | -0.237 |
| Sr/Ca | 0.210 | -0.376 | 0.887 |
| Fe/Ca | 0.978 | 0.188 | 0.038 |
| Zn/Ca | 0.915 | 0.378 | 0.018 |
| K/Ca | -0.506 | 0.839 | -0.073 |
| Na/Ca | -0.310 | 0.138 | 0.924 |
| Mg/Ca | -0.359 | 0.633 | 0.668 |
| Characteristic Value | 10.677 | 5.331 | 2.234 |
| Contribution Rate/% | 53.387 | 26.653 | 11.172 |
| Cumulative Contribution/% | 53.387 | 80.040 | 91.212 |
| Discriminative Elements | Ll | Cm | Cl | W |
|---|---|---|---|---|
| Mo/Ca | 0.871 | 0.056 | -0.104 | 0.716 |
| Ba/Ca | 0.001 | 0.003 | 0.019 | 0.061 |
| K/Ca | -0.951 | -0.004 | 0.661 | -4.981 |
| Constant | -58.110 | -2.096 | -11.265 | -48.633 |
| Method | Groups | Prediction Category | Discriminant Accuracy | Comprehensive Discrimination rate (%) | |||
|---|---|---|---|---|---|---|---|
| Ll | Cm | Cl | W | ||||
| Stepwise Discrimination | Ll | 0 | 3 | 0 | 0 | 100.0 | 100.0 |
| Cm | 0 | 0 | 3 | 0 | 100.0 | ||
| Cl | 3 | 0 | 0 | 0 | 100.0 | ||
| W | 0 | 0 | 0 | 4 | 100.0 | ||
| Cross Verification |
Ll | 0 | 3 | 0 | 0 | 100.0 | 100.0 |
| Cm | 0 | 0 | 3 | 0 | 100.0 | ||
| Cl | 3 | 0 | 0 | 0 | 100.0 | ||
| W | 0 | 0 | 0 | 4 | 100.0 | ||
| Discriminative Elements | Ll | Cm | Cl |
|---|---|---|---|
| Mo | -19792.114 | -32523.073 | -28981.547 |
| Ba | -1110.222 | -1754.700 | -1511.046 |
| Mn | 687.021 | 1084.610 | 931.647 |
| Sr | 57.663 | 92.876 | 83.095 |
| Mg | 5.240 | 8.344 | 7.370 |
| Constant | -1973.485 | -4875.909 | -3803.379 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





