1. Introduction
Tuberous sclerosis complex (TSC) represents a multisystem genetic disorder characterized by significant clinical heterogeneity, with an estimated incidence of approximately 1 in 6,000 individuals worldwide, affecting diverse populations without predilection for gender (Northrup et al., 2013) [
1]. This relatively high prevalence positions TSC among the more commonly encountered rare genetic disorders. The pathogenesis of TSC is primarily attributable to mutations in either the TSC1 gene on chromosome 9 or the TSC2 gene on chromosome 16, which encode the proteins hamartin and tuberin, respectively. These proteins form a tumor-suppressor complex crucial for the inhibition of the mammalian target of rapamycin (mTOR) pathway, a signaling cascade integral to cellular growth, proliferation, and survival (Crino et al., 2006) [
2]. In healthy cells, the hamartin-tuberin complex exerts a negative regulatory effect on the mTOR pathway, ensuring controlled cellular proliferation. However, mutations in TSC1 or TSC2 abrogate this regulatory function, resulting in mTOR pathway hyperactivation, which precipitates the formation of benign tumors, or hamartomas, across various organ systems, including the brain, skin, kidneys, heart, and lungs (Roth et al., 2013) [
3].
The clinical manifestations of TSC are protean and widely variable, with benign tumors arising in multiple organs, resulting in a broad spectrum of physical and neurological symptoms. Central nervous system (CNS) involvement, particularly in the form of subependymal giant cell astrocytomas (SEGAs) and cortical tubers, is highly prevalent and correlates with a propensity for epilepsy, one of the most disabling and refractory symptoms associated with TSC. Approximately 80% of individuals with TSC experience seizures, frequently of an early onset and often refractory to conventional antiepileptic therapies, significantly diminishing the quality of life in affected individuals (Franz et al., 2013) [
4]. Additionally, TSC frequently manifests with neurodevelopmental and psychiatric comorbidities, including intellectual disabilities, autism spectrum disorder (ASD), and various behavioral disturbances. Studies indicate that ASD is present in approximately 40-50% of individuals with TSC, underscoring the profound neurodevelopmental impact of TSC and the multifaceted challenges it poses (Ehninger and Silva, 2008) [
5]. Besides CNS involvement, TSC-related tumors, including renal angiomyolipomas (AMLs) and dermatological lesions such as facial angiofibromas (FAs), further complicate the clinical landscape, contributing to both medical and psychosocial morbidity.
The therapeutic management of TSC has historically been limited to symptomatic treatment, encompassing anticonvulsant drugs for seizure control, surgical intervention for tumors with immediate clinical risk, and supportive strategies for neuropsychiatric symptoms. However, advancements in the understanding of TSC genetics and cellular pathophysiology have paved the way for molecularly targeted therapies, particularly those that address the aberrant mTOR pathway activity central to TSC pathogenesis. The development of mTOR inhibitors has introduced a novel therapeutic avenue that directly targets the hyperactive mTOR signaling resulting from TSC1 and TSC2 mutations. Everolimus, an orally bioavailable mTOR inhibitor, was approved in 2013 for the treatment of TSC-related SEGAs and AMLs based on robust evidence from clinical trials demonstrating its efficacy in reducing tumor volume and delaying surgical interventions (Davies et al., 2017) [
6].
Pivotal studies, including the EXIST-1 and EXIST-2 trials, provided compelling evidence supporting the efficacy of everolimus in TSC management by demonstrating significant tumor regression in both SEGA and AML cases (Bissler et al., 2016; Kingswood et al., 2014) [
7,
8]. The therapeutic impact of everolimus has been particularly transformative for individuals with SEGAs, where tumor size reduction mitigates the risk of life-threatening complications, such as obstructive hydrocephalus, without necessitating invasive surgical procedures. Beyond tumor reduction, emerging data suggest that mTOR inhibitors may also ameliorate certain neuropsychiatric manifestations of TSC. Preliminary studies have observed that everolimus may modulate social and cognitive impairments, particularly in patients presenting with ASD-related symptoms (Wataya-Kaneda et al., 2017; Krueger et al., 2013) [
9,
10]. Although interindividual variability in response is notable, these findings are significant as they highlight the potential role of mTOR dysregulation in the neurodevelopmental and behavioral phenotypes associated with TSC, warranting further investigation into mTOR inhibition as a strategy for addressing neuropsychiatric symptoms.
Despite these advancements, the therapeutic landscape of TSC remains complex. Not all individuals with TSC respond uniformly to mTOR inhibitors, and adverse effects, including immunosuppression and stomatitis, may impact treatment adherence. Furthermore, the long-term implications of sustained mTOR inhibition are not yet fully elucidated, underscoring the necessity for continued clinical surveillance and further research. Future investigations are expected to elucidate the parameters under which mTOR inhibition may optimally address neuropsychiatric symptoms and to explore combinatory approaches that may enhance treatment efficacy and mitigate adverse outcomes.
In this report, we examine the effects of everolimus treatment in an adolescent male with TSC, with particular focus on the therapeutic impact on SEGA, AML, and FAs, as well as observed improvements in ASD-related social behaviors. Given the prevalence of FAs and the psychosocial challenges they impose, this case underscores the broader therapeutic potential of systemic mTOR inhibition, suggesting that everolimus may contribute to improved physical and neurodevelopmental outcomes in TSC.
2. Case Report
The patient, an 11-year-old male, presented with clinical findings consistent with a diagnosis of TSC, identified initially in infancy following the observation of multiple hypopigmented macules, or "ash leaf" spots, on the skin. These lesions led to further genetic evaluation and imaging studies, confirming the TSC diagnosis through cutaneous and neurological markers. At 13 months, the patient developed infantile spasms, a form of epilepsy often associated with TSC, managed with adrenocorticotropic hormone (ACTH) and sodium valproate, leading to effective seizure control. By age 8, brain MRI revealed a SEGA in the right lateral ventricle and bilateral renal AMLs, consistent with multi-organ involvement typical of TSC.
At age 10, comprehensive cognitive assessments revealed an intelligence quotient (IQ) of 67, indicative of significant deficits in adaptive functioning and social cognition, consistent with the neurodevelopmental profile often observed in TSC.
At age 11, the patient underwent a minimally invasive keyhole craniotomy to achieve partial resection of the SEGA, a procedure performed to mitigate the risk of obstructive hydrocephalus associated with tumor growth. Persistent impairments in social interaction and communication subsequently led to a formal diagnosis of autism spectrum disorder (ASD), a neurodevelopmental condition frequently linked to TSC due to dysregulation of the mTOR signaling pathway.
Following formal approval by the Medical Ethics Committee of Dokkyo Medical University (approval No. 27014) and upon obtaining informed consent from the patient’s mother, oral administration of everolimus was initiated when the patient reached the age of 12. This therapeutic intervention aimed to address the dual concerns of preventing the recurrence of SEGAs and potentially mitigating autism spectrum disorder (ASD)-related symptoms, given the established role of mTOR inhibition in modulating both tumorigenic and neurodevelopmental processes. The decision to commence everolimus was based on its demonstrated efficacy in reducing SEGA progression risk in TSC patients, while also considering emerging evidence of its beneficial effects on neuropsychiatric symptoms associated with mTOR dysregulation.
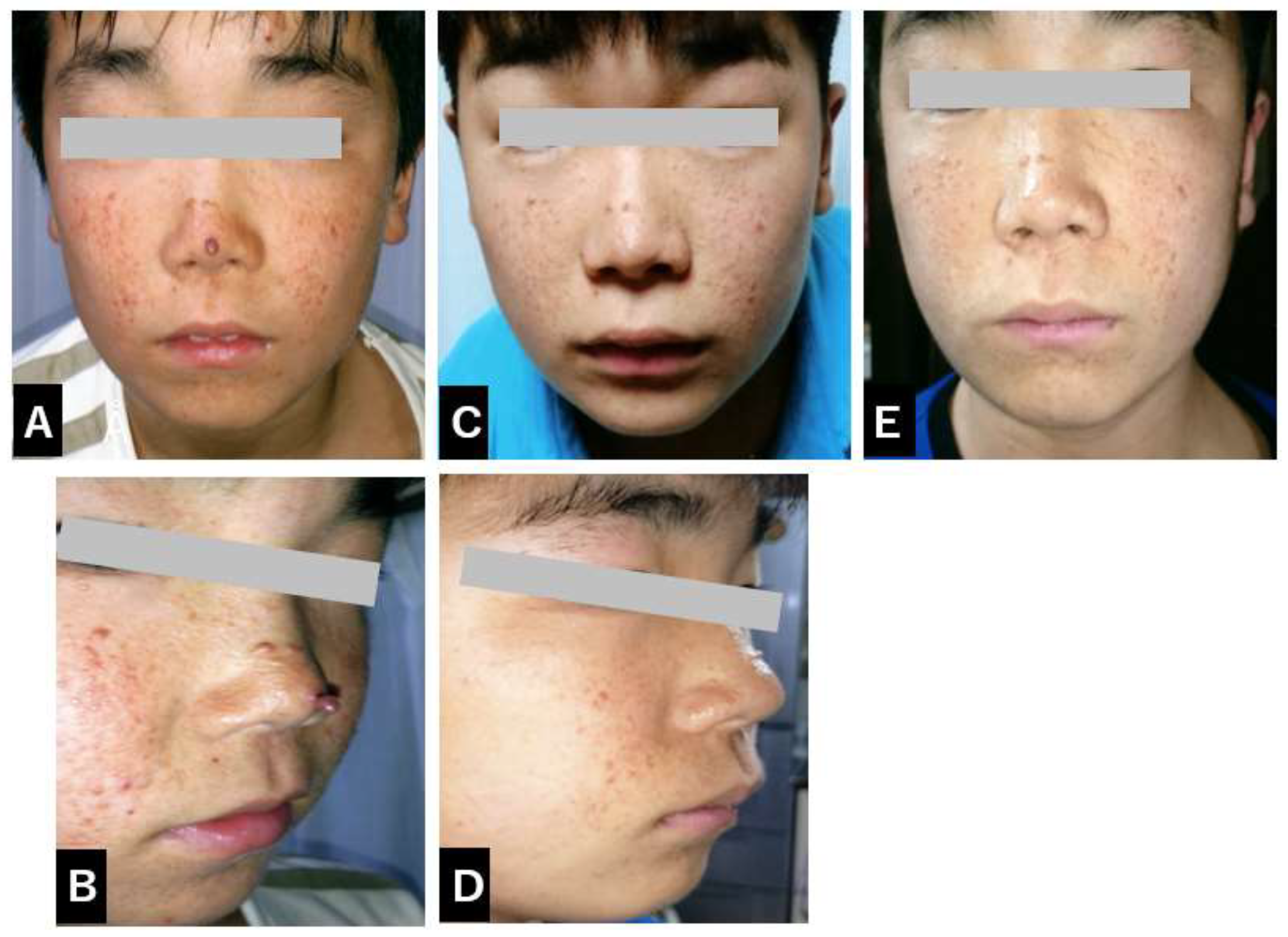
The initial prescribed dose of everolimus was 4.4 mg per day. Prior to the commencement of treatment, multiple prominent facial angiofibromas (FAs) were observed around the patient’s nose and cheeks, presenting as characteristic red papules associated with tuberous sclerosis complex and contributing to notable cosmetic and psychosocial concerns.
(Figure 1A, 1B).
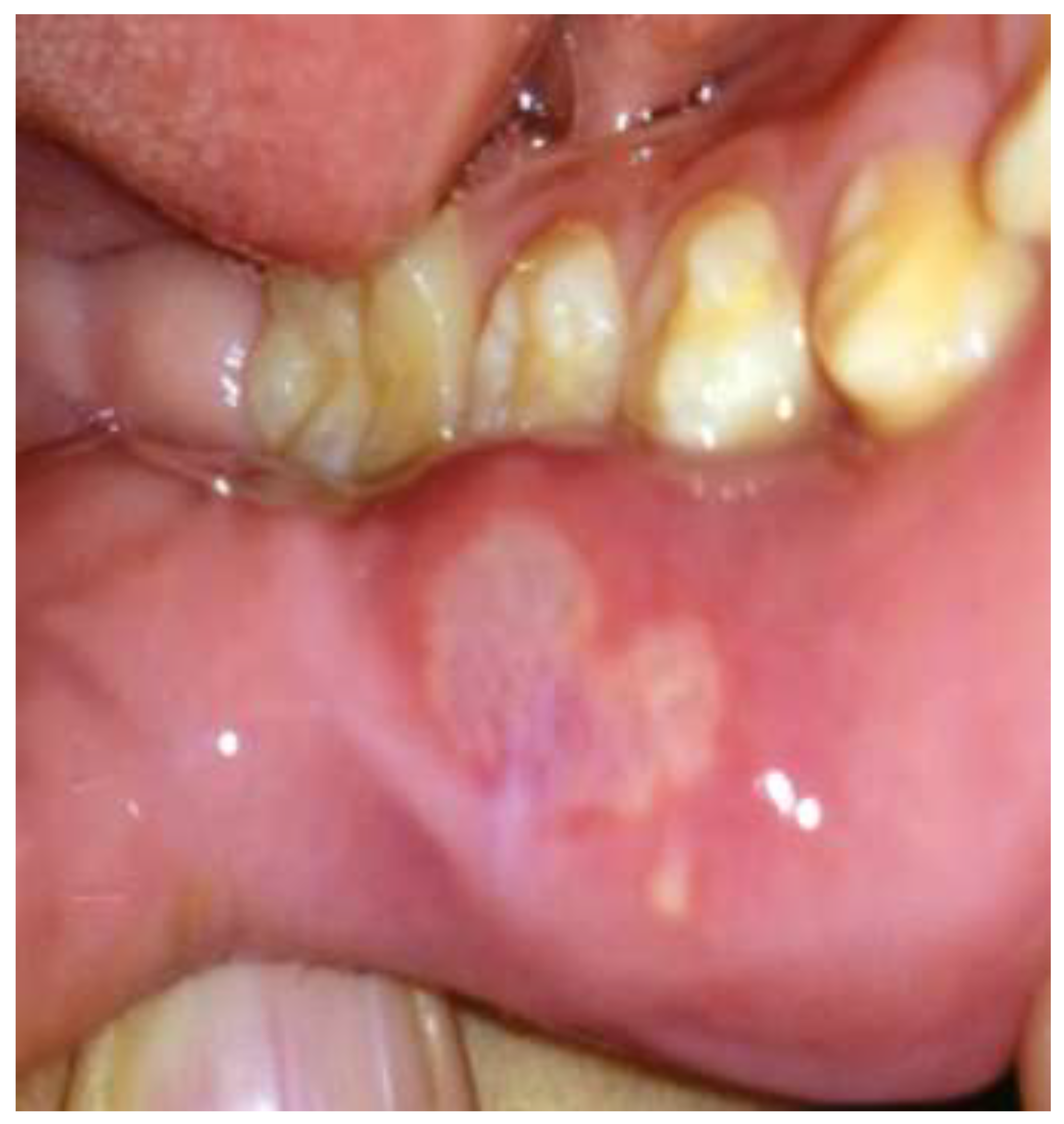
Several days following the initiation of everolimus therapy, the patient developed multiple mouth ulcers, which were identified as likely manifestations of stomatitis, a known adverse effect associated with mTOR inhibitors. These lesions were effectively managed through diligent oral hygiene practices, leading to their resolution without further complications or the necessity for additional pharmacologic intervention.
(Figure 2). Periodic dose adjustments were implemented based on therapeutic drug monitoring to ensure optimal efficacy, with serum everolimus levels carefully maintained within a therapeutic range of 7.32 to 18.1 ng/ml. By three months after the initiation of treatment, a discernible trend of improvement in the patient’s facial angiofibromas (FAs) began to emerge, indicating a positive response to systemic mTOR inhibition.
(Figure 1C, 1D). By six months into the treatment regimen, both the size and erythema of the facial angiofibromas had visibly diminished, indicating a noteworthy and somewhat unexpected therapeutic response of these lesions to systemic mTOR inhibition with everolimus.
(Figure 1E). The patient continued treatment with oral everolimus and over the months showed improvement in social behavior, including improved eye contact. A follow-up MRI scan showed a reduction in renal AML after 24 months. The patient also continued to show improvement in facial skin FAs, demonstrating the continued efficacy of everolimus in the management of TSC-related symptoms. The patient continues to receive oral everolimus therapy.
Figure 1.
Sequential Changes in Facial Appearance and Angiofibroma Development (A/B/C/D/E).
Figure 1.
Sequential Changes in Facial Appearance and Angiofibroma Development (A/B/C/D/E).
In the pre-treatment facial photograph, prominent red skin nodules and areas of diffuse blue tint are symmetrically distributed over the entire nose and extend into other facial areas. These nodules are very prominent and stand in marked contrast to the surrounding skin.
C/D: 3 months after treatment
Three months after the start of treatment, the nodules in the blue highlighted area of the nose, along with similar nodules in other facial areas, show a subtle but observable reduction in size. They are flatter and less noticeable. Additionally, the previously prominent red skin lesions on the face have almost completely disappeared. This favorable change motivated the patient to manage her own medication regimen, and adherence to her oral medications improved markedly.
E: 6 months after Everolimus treatment
Six months after the start of Everolimus treatment, the remaining nodules on the facial skin have further reduced in size and continue to flatten.
Figure 2.
Detailed Examination of the Patient’s Oral Mucosa Condition During Treatment.
Figure 2.
Detailed Examination of the Patient’s Oral Mucosa Condition During Treatment.
The patient’s oral mucosa showed characteristic signs of stomatitis, including marked mucosal pain, which appeared shortly after the start of everolimus treatment. This initial symptom is consistent with common side effects associated with mTOR inhibitors, particularly everolimus, which often induce oral discomfort and ulcers due to its effect on mucosal integrity. The patient’s symptoms improved only with oral care, such as brushing teeth, disinfecting the mouth and gargling.
3. Discussion
TSC-related gene mutations lead to PI3K/AKT/mTOR pathway dysregulation, promoting benign tumor growth and neurodevelopmental issues (Huang et al., 2008) [
11]. Everolimus, an mTORC1 inhibitor, effectively reduces SEGA and AML size, as established in the EXIST trials (Franz et al., 2013) [
4]. Additionally, evidence indicates that everolimus may benefit ASD symptoms in TSC, possibly by modifying mTOR-driven neurodevelopmental processes (Wang et al., 2015) [
12]. Animal models suggest that mTORC1 inhibition could reverse ASD-like behaviors, with clinical reports supporting these findings in human TSC cases (Yui et al., 2019) [
13].
3. 1. Everolimus for Facial Angiofibromas
Facial angiofibromas (FAs) are highly prevalent in TSC, often exacerbating social stigma (Koenig et al., 2018) [
14]. Systemic everolimus treatment provided notable FA improvement in this case, suggesting that mTOR inhibition can target both dermatologic and neurodevelopmental symptoms. While rapamycin gel has shown efficacy for FA as a topical mTOR inhibitor, systemic therapy may offer more comprehensive benefits across TSC manifestations (Pass et al., 2021) [
15]. Careful monitoring for side effects such as stomatitis is essential in systemic treatment (Devaraj et al., 2019) [
16].
3. 2. Topical vs. Systemic mTOR Inhibition
Topical rapamycin (sirolimus) gel offers localized FA management with minimal systemic absorption, though it requires caution with sun exposure due to photosensitivity (Hart et al., 2021) [
17]. By contrast, systemic everolimus addresses multiple TSC symptoms but necessitates careful monitoring to manage adverse effects. Combining both approaches could optimize outcomes in TSC, balancing localized and systemic benefits (Koenig et al., 2018) [
18]. Safety and efficacy studies on mTOR inhibitors, particularly in pediatric populations, emphasize the need for personalized approaches to treatment (Segal et al., 2020) [
19].
3. 3. Future Directions in TSC Treatment
The limitations of everolimus, particularly the common adverse effects such as stomatitis and hyperlipidemia, underscore the pressing need for ongoing research into more refined and targeted therapeutic options for TSC management. These adverse events can affect patient compliance and limit the utility of current mTOR inhibitors, especially in long-term treatment regimens, thereby emphasizing the importance of exploring alternative or adjunctive therapeutic approaches. Advances in pharmacological research have introduced dual mTORC1/mTORC2 inhibitors, which hold promise for a more comprehensive modulation of mTOR signaling by simultaneously targeting multiple aspects of the pathway. This dual inhibition approach may provide broader and more effective control over the spectrum of TSC manifestations, addressing both cellular proliferation and neurodevelopmental symptoms with greater precision (Martinez-Aguayo et al., 2020) [
20]. Moreover, the development of novel mTOR-targeting therapies, including combination regimens that pair mTOR inhibitors with other molecular agents, represents a promising direction to enhance therapeutic efficacy while potentially mitigating adverse effects. Such approaches are currently under investigation and could significantly advance treatment paradigms in TSC, ultimately offering more personalized and effective interventions for patients (Medley MA et al., 2022; Lendvai et al., 2019; Hussain et al., 2020; Bar-Peled et al., 2019; Davies DM et al., 2019) [
21,
22,
23,
24,
25].
4. Conclusions
This case highlights the multifaceted efficacy of everolimus in the management of tuberous sclerosis complex (TSC), demonstrating its therapeutic impact on both physical manifestations, such as tumor size reduction, and neurodevelopmental symptoms, including improvements in social behavior. The findings suggest that everolimus, as an mTOR inhibitor, plays a crucial role in addressing the diverse and complex symptomatology of TSC. Looking forward, continued research into the safety, long-term effects, and broader applications of mTOR inhibitors will be essential for optimizing treatment strategies.
Author Contributions
For original draft writing, G.I.; review and case report editing, S.M., K.Y., K.I., H.S. and S.Y.; supervision, K.Y. All authors have read and agreed to the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors would like to thank the staff of the Department of Pediatrics and Dermatology, Dokkyo Medical University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Northrup H, Krueger DA, et al. Tuberous sclerosis complex diagnostic criteria update. Pediatr Neurol. 2013;49(4):243-54. [CrossRef]
- Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345-56.
- Roth J, Roach ES, Bartels U, et al. Subependymal giant cell astrocytoma in tuberous sclerosis complex: guidelines for management. Pediatr Neurol. 2013;49:439-44.
- Franz DN, et al. Efficacy of everolimus for SEGAs in TSC: EXIST-1 trial. Lancet. 2013;381:125-32.
- Ehninger D, Silva AJ. Rapamycin for TSC-related disorders: mechanisms and evidence. Brain Res Rev. 2008;59(2):293-305.
- Davies DM, Johnson SR, et al. Everolimus for renal AML in TSC. Curr Med Res Opin. 2017;33(7):1271-80.
- Bissler JJ, Kingswood JC, Radzikowska E, et al. Everolimus for AML in TSC or sporadic LAM: EXIST-2 trial. Nephrol Dial Transplant. 2016;31(1):111-9.
- Kingswood JC, et al. Long-term everolimus in TSC-associated AML. Nephrol Dial Transplant. 2014;29(12):2521-9.
- Wataya-Kaneda M, et al. Efficacy of sirolimus gel for FAs in TSC. JAMA Dermatol. 2017;153(1):39-48.
- Krueger DA, et al. TSC and everolimus treatment guidelines. Am J Med Genet C Semin Med Genet. 2013;163C(2):91-9.
- Huang J, Dibble CC, et al. TSC-mTOR pathway and regulation of cell growth. Nat Rev Mol Cell Biol. 2008;9(5):339-50.
- Wang J, Peng T, Yin Q, et al. Everolimus reduces autism-like behaviors in TSC2 mice. Sci Rep. 2015;5:17109.
- Yui K, Imataka G, Sasaki H, et al. Improvement in social cognition by everolimus in TSC. Case Rep Pediatr. 2019;2019:2070619.
- Koenig MK, Bell CS, Hebert AA, et al. Efficacy and safety of topical rapamycin in TSC: TREATMENT trial. JAMA Dermatol. 2018;154(7):773-80.
- Pass C, Medley M, et al. Guidelines for rapamycin gel in TSC. Dermatol Pract Res. 2021;15(4):225-32.
- Devaraj NK, Aneesa AR, et al. Topical corticosteroids in clinical practice. Med J Malaysia. 2019;74(2):187-9.
- Hart CW, et al. Exploring sirolimus applications in TSC dermatology. Br J Dermatol. 2021;184(4):741-9.
- Koenig MK, Bell CS, et al. Everolimus and dermatologic improvements in TSC. Dermatol Online J. 2018;24(2):13030.
- Segal S, et al. Safety and efficacy of mTOR inhibitors in children. J Pediatr Pharmacol Ther. 2020;25(2):128-35.
- Martinez-Aguayo A, Rojas M, et al. Dual inhibition of mTORC1/mTORC2 in TSC. Expert Opin Investig Drugs. 2020;29(11):1125-35.
- Medley MA, et al. Current trends in mTOR inhibition for TSC. Clin Genet. 2022;101(3):203-9.
- Lendvai N, et al. Review on mTOR pathway in TSC. Curr Opin Neurol. 2019;32(5):737-43.
- Hussain Z, et al. Novel treatments targeting mTOR in TSC. Future Oncol. 2020;16(14):1035-43.
- Bar-Peled L, et al. Pharmacological advances in TSC management. Mol Cancer Ther. 2019;18(9):1455-62.
- Davies DM, et al. TSC pharmacology and management. Curr Opin Pediatr. 2019;31(5):636-42.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).