Submitted:
10 December 2024
Posted:
11 December 2024
You are already at the latest version
Abstract
The endothelium is a well-known regulator of vascular homeostasis. Several factors can influence the balance of bioavailability of active substances. This imbalance can lead to inflammation and, consequently, endothelial dysfunction, which is an underlying pathology in cardiovascular disease that commonly coexists with metabolic and chronic diseases such as metabolic dysfunction-associated steatotic liver disease (MASLD). In MASLD, a reduction in nitric oxide availability is observed, and as a result, hepatic stellate cells and liver sinusoidal endothelial cells are activated. Considering the extensive research dedicated to finding several targets with diagnostic and therapeutic effects, nuclear hormone receptors such as peroxisome proliferator-activated receptors have been highlighted as being highly influential in the gut–liver–adipose axis and are considered potential regulators of metabolism and inflammation in several pathologies. Currently, PPAR agonists are widely explored in clinical trials and experimental studies. Agents such as lanifibranor, elafibranor, daidzein, and Icariin have shown promise in improving the metabolic, hepatic, and cardiovascular health of patients with MASLD. This review aims to provide a comprehensive overview of the role of peroxisome proliferator-activated receptors in endothelial dysfunction and MASLD, exploring their mechanisms in disease progression and potential pharmacological targeting.
Keywords:
1. Introduction
2. The Function of the Endothelium
2.1. Endothelial Glycocalyx
2.2. Endothelial Functions
3. Metabolic Dysfunction-Associated Steatotic Liver Disease and Endothelial Dysfunction
3.1. MASLD Epidemiology and Pathogenesis
3.2. The Participation of Liver Sinusoidal Endothelial Cells in MASLD
3.3. The Relationship Between Endothelial Dysfunction and MASLD
4. Nuclear Receptors: Peroxisome Proliferator-Activated Receptors
4.1. PPARs and Inflammation
4.2. PPARs and Endothelium
5. PPARs as Pharmacological Targets
| Medication | Active Compound | PPAR targeted | Population | Study Design/Method | Outcomes | Adverse/Side Effects |
|---|---|---|---|---|---|---|
|
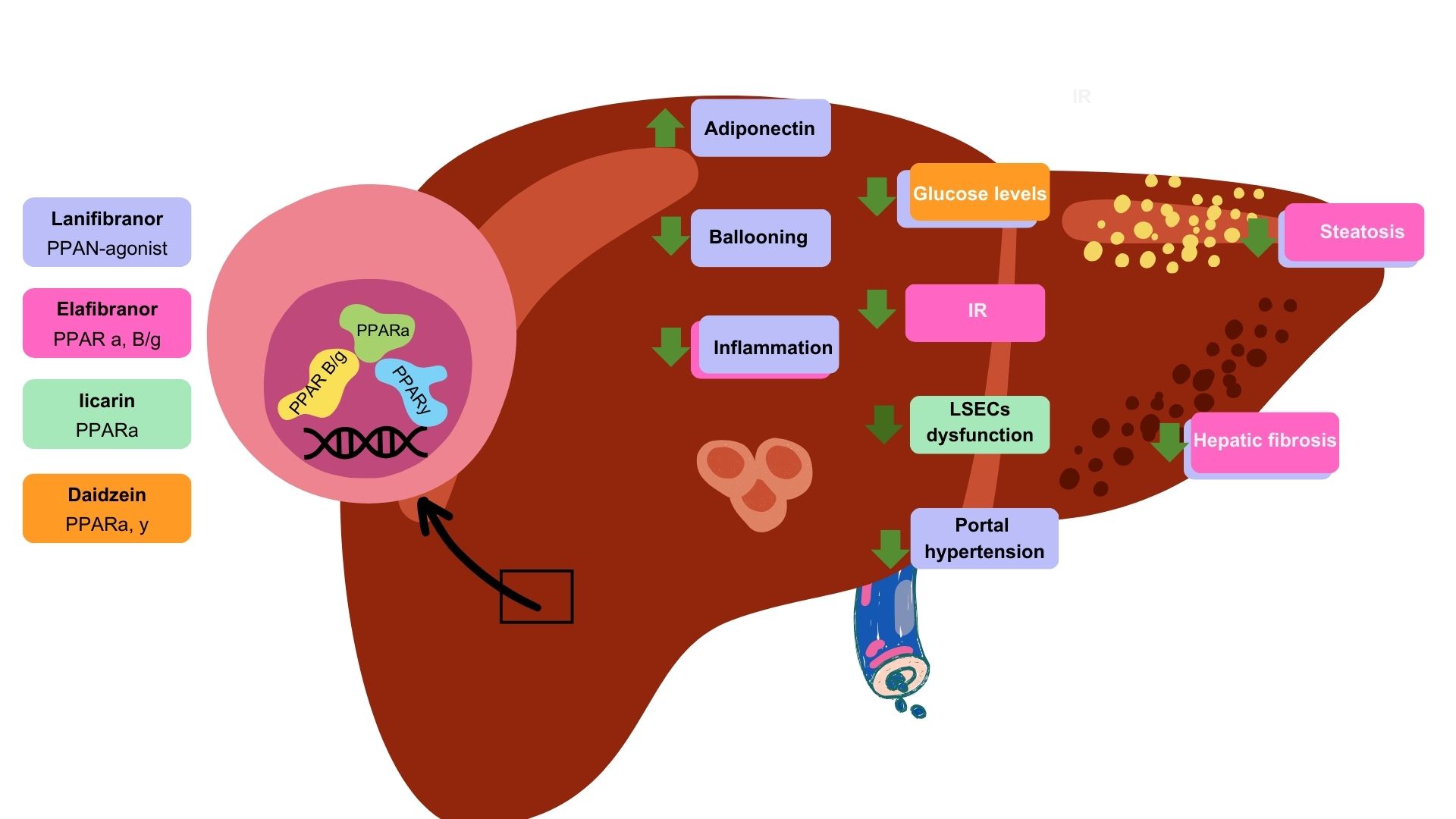
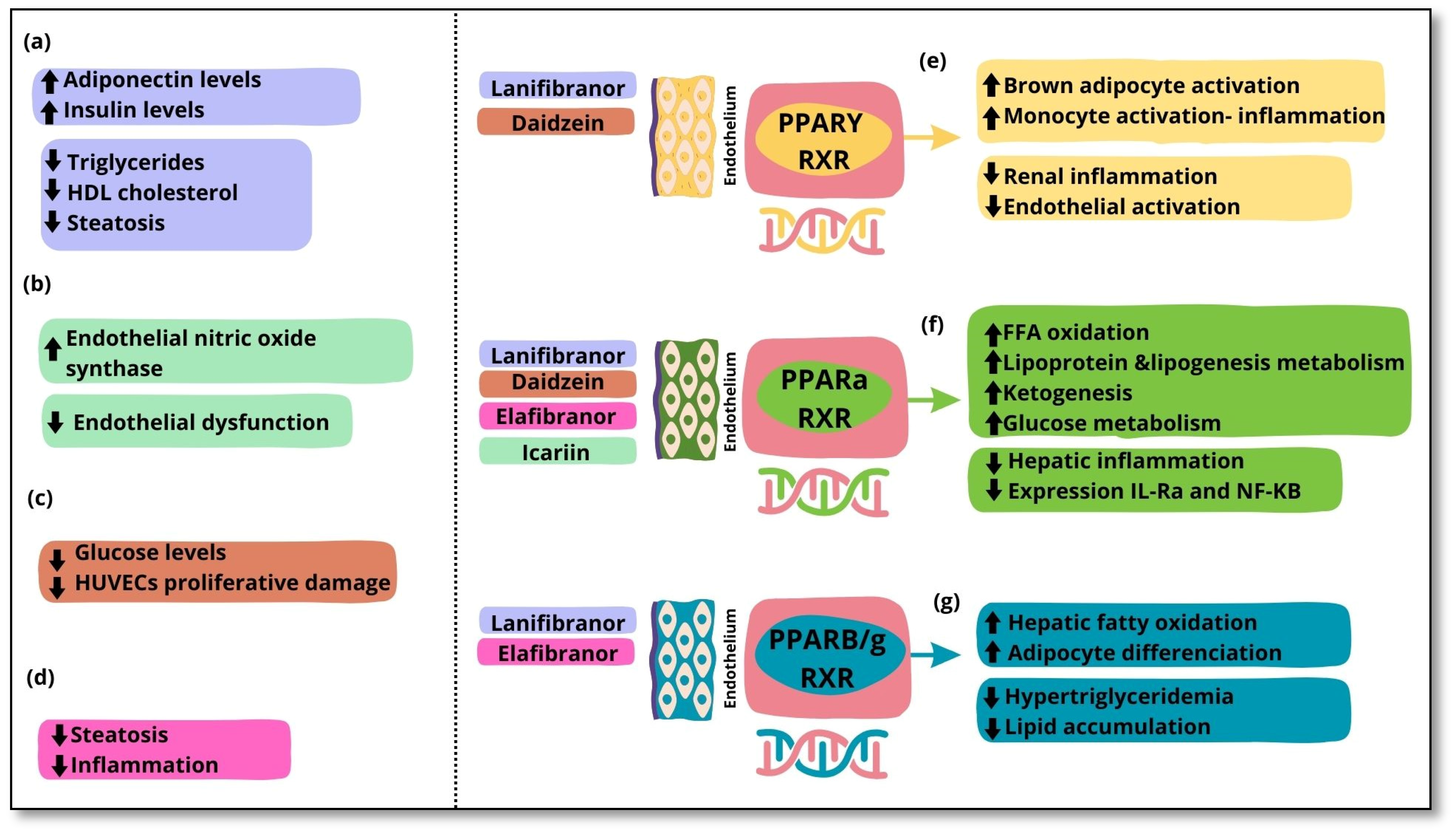
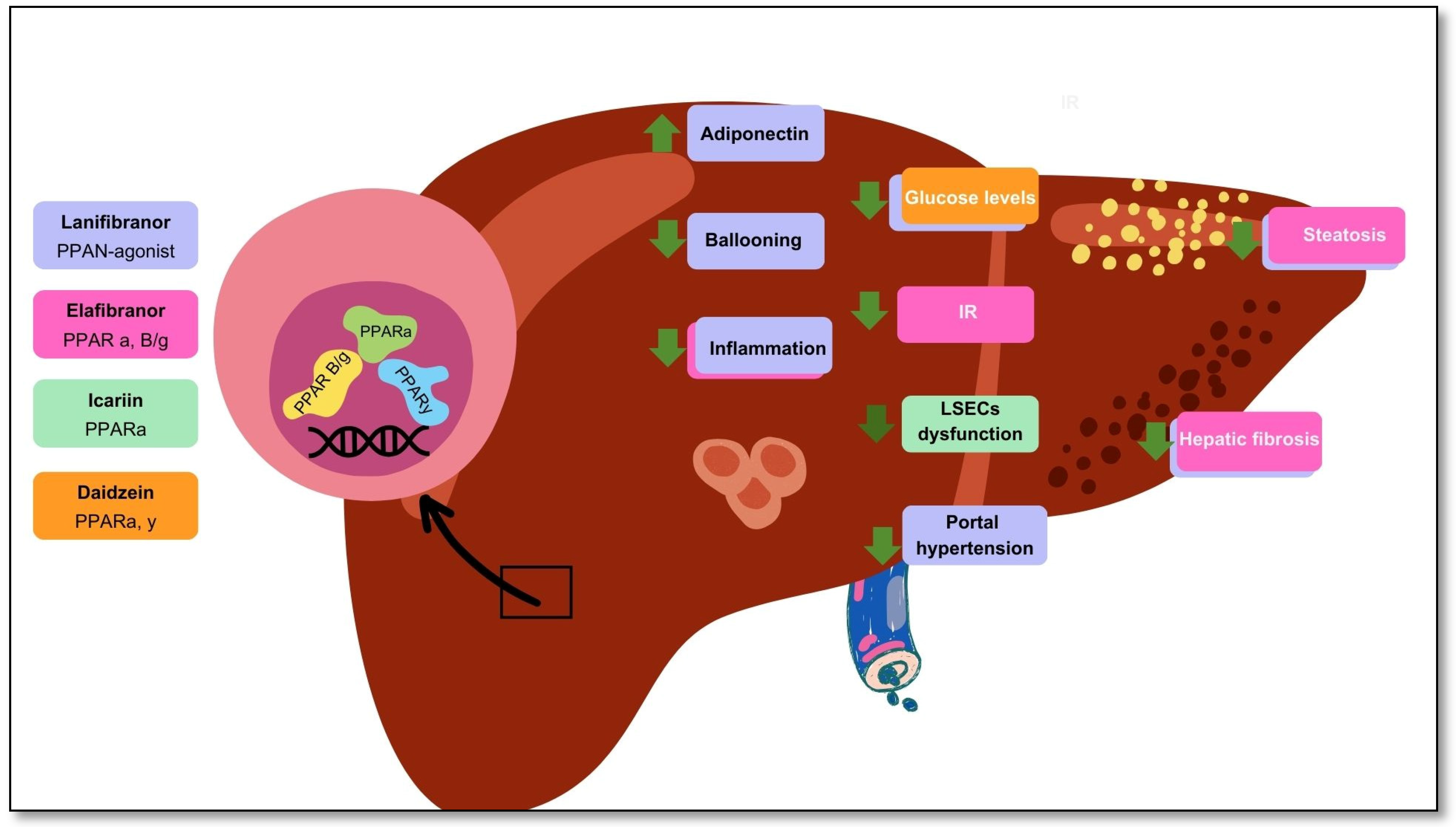
Lanifibranor [115] NATIVE trial |
PPAR agonist | PPARα PPARδ Partial activation of PPARɣ | 247 non-cirrhotic, highly active MASH patients | Double-blind randomized controlled trial | Improvements in Triglycerides, HDL cholesterol, and insulin levels and steatosis |
Gastrointestinal adverse events, peripheral edema, anemia |
| Lanifibranor [116] | PPAR agonist | PPARα PPARδ Partial activation of PPARɣ | 247 MASH patients with a poor cardiometabolic health | Clinical trial | Increased adiponectin levels Improvements in hepatic and cardiovascular health |
Gastrointestinal adverse events, peripheral edema, anemia Weight gain of 2.5 kg |
| Icariin [117] | Flavonoid glycoside | PPARα | 48 Murine models (rat) with type 1 diabetes | Experimental study | Normalization endothelial dysfunction. Inhibition of endoplasmic reticulum stress. Activation of endothelial nitric oxide synthase |
NA |
| Daidzein [119] | Isoflavone | PPARα PPARγ |
HUVECS | In vitro experimental study | Reversed high glucose levels Amelioration of HUVECs proliferative damage |
NA |
| Elafibranor [120] | PPAR agonist | PPARα PPARβ/δ |
18 Murine MASH models (mice) | In vivo and in vitro experimental study | Amelioration of steatosis and inflammation Increased (EMT)-promoting proteins |
NA |
6. Discussion
7. Conclusions
Author Contributions
Conflicts of Interest
References
- Radomski, M.W.; Palmer, R.M.J.; Moncada, S. ENDOGENOUS NITRIC OXIDE INHIBITS HUMAN PLATELET ADHESION TO VASCULAR ENDOTHELIUM. The Lancet. 1987, 330, 1057–1058. [Google Scholar] [CrossRef] [PubMed]
- Badimón, L.; Martínez-González, J. Disfunción endotelial. Revista Española de Cardiología Suplementos. 2006, 6, 21A–30A. [Google Scholar] [CrossRef]
- Lin, P.J.; Chang, C.H. Endothelium dysfunction in cardiovascular diseases. Changgeng Yi Xue Za Zhi. 1994, 17, 198–210. [Google Scholar] [PubMed]
- Poisson, J.; Lemoinne, S.; Boulanger, C.; Durand, F.; Moreau, R.; Valla, D.; et al. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. Journal of Hepatology. 2017, 66, 212–227. [Google Scholar] [CrossRef]
- Ding, B.S.; Cao, Z.; Lis, R.; Nolan, D.J.; Guo, P.; Simons, M.; et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014, 505, 97–102. [Google Scholar] [CrossRef] [PubMed]
- DeLeve, L.D. Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology. 2015, 61, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.a.; Montagnani, M.; Koh, K.K.; Quon, M.J. Reciprocal Relationships Between Insulin Resistance and Endothelial Dysfunction: Molecular and Pathophysiological Mechanisms. Circulation. 2006, 113, 1888–1904. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Dallio, M.; Masarone, M.; Persico, M.; Loguercio, C. The epidemiology of non-alcoholic fatty liver disease and its connection with cardiovascular disease: role of endothelial dysfunction. Eur Rev Med Pharmacol Sci. 2016, 20, 4731–4741. [Google Scholar]
- Evans, R.M.; Mangelsdorf, D.J. Nuclear Receptors, RXR, and the Big Bang. Cell. 2014, 157, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Magliano, D.C.; Bargut, T.C.L.; De Carvalho, S.N.; Aguila, M.B.; Mandarim-de-Lacerda, C.A.; Souza-Mello, V. Peroxisome Proliferator-Activated Receptors-Alpha and Gamma Are Targets to Treat Offspring from Maternal Diet-Induced Obesity in Mice. Huang Y, editor. PLoS ONE. 2013, 8, e64258. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, C.; Krey, G.; Keller, H.; Givel, F.; Helftenbein, G.; Wahli, W. Control of the peroxisomal β-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992, 68, 879–887. [Google Scholar] [CrossRef]
- Clyne, A.M. Endothelial response to glucose: dysfunction, metabolism, and transport. Biochemical Society Transactions. 2021, 49, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Wolinsky, H. A proposal linking clearance of circulating lipoproteins to tissue metabolic activity as a basis for understanding atherogenesis. Circ Res. 1980, 47, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Gouverneur, M.; Spaan, J.A.E.; Pannekoek, H.; Fontijn, R.D.; Vink, H. Fluid shear stress stimulates incorporation of hyaluronan into endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2006, 290, H458-452. [Google Scholar] [CrossRef] [PubMed]
- Lipowsky, H.H. Microvascular rheology and hemodynamics. Microcirculation. 2005, 12, 5–15. [Google Scholar] [CrossRef]
- Ugusman, A.; Kumar, J.; Aminuddin, A. Endothelial function and dysfunction: Impact of sodium-glucose cotransporter 2 inhibitors. Pharmacology & Therapeutics. 2021, 224, 107832. [Google Scholar] [CrossRef]
- Saunders, S.; Jalkanen, M.; O’Farrell, S.; Bernfield, M. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol. 1989, 108, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Leonova, E.I.; Galzitskaya, O.V. Structure and functions of syndecans in vertebrates. Biochemistry Moscow. 2013, 78, 1071–1085. [Google Scholar] [CrossRef] [PubMed]
- Pahakis, M.Y.; Kosky, J.R.; Dull, R.O.; Tarbell, J.M. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem Biophys Res Commun. 2007, 355, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, R.D.; Shworak, N.W.; Liu, J.; Schwartz, J.J.; Zhang, L. Heparan sulfate proteoglycans of the cardiovascular system. Specific structures emerge but how is synthesis regulated? J Clin Invest. 1997, 99, 2062–2070. [Google Scholar] [CrossRef]
- Filmus, J.; Selleck, S.B. Glypicans: proteoglycans with a surprise. J Clin Invest. 2001, 108, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Reitsma, S.; Slaaf, D.W.; Vink, H.; Van Zandvoort, M.A.M.J.; Oude Egbrink, M.G.A. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch—Eur J Physiol. 2007, 454, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.B.S.; Duling, B.R. Permeation of the luminal capillary glycocalyx is determined by hyaluronan. American Journal of Physiology-Heart and Circulatory Physiology. 1999, 277, H508–H514. [Google Scholar] [CrossRef] [PubMed]
- Gaudette, S.; Hughes, D.; Boller, M. The endothelial glycocalyx: Structure and function in health and critical illness. J Vet Emergen Crit Care. 2020, 30, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, M.D., Ph.D MP, Nelson, Ph.D RM, Mannori, M.D., Ph.D G, Cecconi, M.D O. ENDOTHELIAL-LEUKOCYTE ADHESION MOLECULES IN HUMAN DISEASE. Annu Rev Med. 1994 Feb;45(1):361–78. [CrossRef]
- Bergmeier, W.; Hynes, R.O. Extracellular Matrix Proteins in Hemostasis and Thrombosis. Cold Spring Harbor Perspectives in Biology. 2012, 4, a005132. [Google Scholar] [CrossRef] [PubMed]
- Muller, W.A. Getting Leukocytes to the Site of Inflammation. Vet Pathol. 2013, 50, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Barthel, S.R.; Gavino, J.D.; Descheny, L.; Dimitroff, C.J. Targeting selectins and selectin ligands in inflammation and cancer. Expert Opinion on Therapeutic Targets. 2007, 11, 1473–1491. [Google Scholar] [CrossRef]
- Kayal, S.; Jaïs, J.P.; Aguini, N.; Chaudière, J.; Labrousse, J. Elevated Circulating E-Selectin, Intercellular Adhesion Molecule 1, and von Willebrand Factor in Patients with Severe Infection. Am J Respir Crit Care Med. 1998, 157, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.K. Nitric oxide synthases domain structure and alignment in enzyme function and control. Front Biosci. 2003, 8, d193–d209. [Google Scholar] [CrossRef]
- Neubauer, K.; Zieger, B. Endothelial cells and coagulation. Cell Tissue Res. 2022, 387, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Widlansky, M.E.; Gokce, N.; Keaney, J.F.; Vita, J.A. The clinical implications of endothelial dysfunction. Journal of the American College of Cardiology. 2003, 42, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.S.; Ekstedt, M.; Wong, G.L.H.; Hagström, H. Changing epidemiology, global trends and implications for outcomes of NAFLD. Journal of Hepatology. 2023, 79, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Quek, J.; Chan, K.E.; Wong, Z.Y.; Tan, C.; Tan, B.; Lim, W.H.; et al. Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: a systematic review and meta-analysis. The Lancet Gastroenterology & Hepatology. 2023, 8, 20–30. [Google Scholar] [CrossRef]
- Karlsen, T.H.; Sheron, N.; Zelber-Sagi, S.; Carrieri, P.; Dusheiko, G.; Bugianesi, E.; et al. The EASL–Lancet Liver Commission: protecting the next generation of Europeans against liver disease complications and premature mortality. The Lancet. 2022, 399, 61–116. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. Journal of Hepatology. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. Journal of Hepatology. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Tilg, H. MASLD: a systemic metabolic disorder with cardiovascular and malignant complications. Gut. 2024, gutjnl-2023-330595. [CrossRef]
- Maldonado-Rojas, A.D.C.; Zuarth-Vázquez, J.M.; Uribe, M.; Barbero-Becerra, V.J. Insulin resistance and Metabolic dysfunction-associated steatotic liver disease (MASLD): Pathways of action of hypoglycemic agents. Annals of Hepatology. 2024, 29, 101182. [Google Scholar] [CrossRef] [PubMed]
- Koek, G.H.; Liedorp, P.R.; Bast, A. The role of oxidative stress in non-alcoholic steatohepatitis. Clinica Chimica Acta. 2011, 412, 1297–1305. [Google Scholar] [CrossRef]
- Yan, M.; Man, S.; Ma, L.; Guo, L.; Huang, L.; Gao, W. Immunological mechanisms in steatotic liver diseases: An overview and clinical perspectives. Clin Mol Hepatol. 2024, 30, 620–648. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. Nonalcoholic Fatty Liver Disease as a Nexus of Metabolic and Hepatic Diseases. Cell Metabolism. 2018, 27, 22–41. [Google Scholar] [CrossRef] [PubMed]
- Wehmeyer, M.H.; Zyriax, B.C.; Jagemann, B.; Roth, E.; Windler, E.; Schulze Zur Wiesch, J.; et al. Nonalcoholic fatty liver disease is associated with excessive calorie intake rather than a distinctive dietary pattern. Medicine. 2016, 95, e3887. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.B.; Lavine, J.E. Dietary fructose in nonalcoholic fatty liver disease. Hepatology. 2013, 57, 2525–2531. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.A.; Samuel, V.T. The Sweet Path to Metabolic Demise: Fructose and Lipid Synthesis. Trends in Endocrinology & Metabolism. 2016, 27, 719–730. [Google Scholar] [CrossRef]
- Schwärzler, J.; Grabherr, F.; Grander, C.; Adolph, T.E.; Tilg, H. The pathophysiology of MASLD: an immunometabolic perspective. Expert Review of Clinical Immunology. 2024, 20, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol. 2017, 18, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.K.; Peng, Z.G. Targeting Liver Sinusoidal Endothelial Cells: An Attractive Therapeutic Strategy to Control Inflammation in Nonalcoholic Fatty Liver Disease. Front Pharmacol. 2021, 12, 655557. [Google Scholar] [CrossRef] [PubMed]
- Nasiri-Ansari, N.; Androutsakos, T.; Flessa, C.M.; Kyrou, I.; Siasos, G.; Randeva, H.S.; et al. Endothelial Cell Dysfunction and Nonalcoholic Fatty Liver Disease (NAFLD): A Concise Review. Cells. 2022, 11, 2511. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Wang, L.; Li, Y.; Li, X. Liver sinusoidal endothelial cell: An important yet often overlooked player in the liver fibrosis. Clin Mol Hepatol. 2024, 30, 303–325. [Google Scholar] [CrossRef] [PubMed]
- Iwakiri, Y. Unlocking the role of liver sinusoidal endothelial cells: Key players in liver fibrosis: Editorial on “Liver sinusoidal endothelial cell: An important yet often overlooked player in the liver fibrosis. Clin Mol Hepatol. 2024, 30, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Graupera, M.; March, S.; Engel, P.; Rodés, J.; Bosch, J.; García-Pagán, J.C. Sinusoidal endothelial COX-1-derived prostanoids modulate the hepatic vascular tone of cirrhotic rat livers. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2005, 288, G763–G770. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Yadav, D.; Gupta, M.; Mishra, H.; Sharma, P. A Study of Carotid Atherosclerosis in Patients with Non-alcoholic Fatty Liver Disease. Indian J Clin Biochem. 2013, 28, 79–83. [Google Scholar] [CrossRef]
- Schreiber, R.; Taschler, U.; Preiss-Landl, K.; Wongsiriroj, N.; Zimmermann, R.; Lass, A. Retinyl ester hydrolases and their roles in vitamin A homeostasis. Biochimica et Biophysica Acta (BBA)—Molecular and Cell Biology of Lipids. 2012, 1821, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Tardelli, M.; Claudel, T.; Bruschi, F.V.; Moreno-Viedma, V.; Trauner, M. Adiponectin regulates AQP3 via PPARα in human hepatic stellate cells. Biochemical and Biophysical Research Communications. 2017, 490, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Z.; Xia, M.; Salas, S.S.; Ospina, J.A.; Buist-Homan, M.; et al. Extracellular vesicles derived from liver sinusoidal endothelial cells inhibit the activation of hepatic stellate cells and Kupffer cells in vitro. Biochimica et Biophysica Acta (BBA)—Molecular Basis of Disease. 2024, 1870, 167020. [Google Scholar] [CrossRef]
- Gibert-Ramos, A.; Sanfeliu-Redondo, D.; Aristu-Zabalza, P.; Martínez-Alcocer, A.; Gracia-Sancho, J.; Guixé-Muntet, S.; et al. The Hepatic Sinusoid in Chronic Liver Disease: The Optimal Milieu for Cancer. Cancers. 2021, 13, 5719. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Lo, H.M.; Chen, J.D. Sonographic fatty liver, overweight and ischemic heart disease. World J Gastroenterol. 2005, 11, 4838–4842. [Google Scholar] [CrossRef]
- Anderson, T.J. Assessment and treatment of endothelial dysfunction in humans. J Am Coll Cardiol. 1999, 34, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Hadi, H.A.R.; Carr, C.S.; Al Suwaidi, J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag. 2005, 1, 183–198. [Google Scholar]
- Zhang, B.; Bu, C.; Wang, Q.; Chen, Q.; Shi, D.; Qiu, H.; et al. Low molecular weight heparin promotes the PPAR pathway by protecting the glycocalyx of cells to delay the progression of diabetic nephropathy. Journal of Biological Chemistry. 2024, 300, 107493. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Barbui, C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. Journal of Hepatology. 2016, 65, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y.; Kwon, T.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Prognostic Value of Flow-Mediated Vasodilation in Brachial Artery and Fingertip Artery for Cardiovascular Events: A Systematic Review and Meta-Analysis. JAHA. 2015, 4, e002270. [Google Scholar] [CrossRef]
- Qiu, Y.Y.; Zhang, J.; Zeng, F.Y.; Zhu, Y.Z. Roles of the peroxisome proliferator-activated receptors (PPARs) in the pathogenesis of nonalcoholic fatty liver disease (NAFLD). Pharmacol Res. 2023, 192, 106786. [Google Scholar] [CrossRef] [PubMed]
- Zoete, V.; Grosdidier, A.; Michielin, O. Peroxisome proliferator-activated receptor structures: Ligand specificity, molecular switch and interactions with regulators. Biochimica et Biophysica Acta (BBA)—Molecular and Cell Biology of Lipids. 2007, 1771, 915–925. [Google Scholar] [CrossRef]
- Chandra, V.; Huang, P.; Hamuro, Y.; Raghuram, S.; Wang, Y.; Burris, T.P.; et al. Structure of the intact PPAR-γ–RXR-α nuclear receptor complex on, D. N.A. Nature. 2008, 456, 350–356. [Google Scholar] [CrossRef]
- Adeghate, E. Medicinal Chemistry and Actions of Dual and Pan PPAR Modulators. TOMCJ. 2011, 5, 93–98. [Google Scholar] [CrossRef]
- Tontonoz, P.; Spiegelman, B.M. Fat and Beyond: The Diverse Biology of PPARγ. Annu Rev Biochem. 2008, 77, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Francque, S.; Szabo, G.; Abdelmalek, M.F.; Byrne, C.D.; Cusi, K.; Dufour, J.F.; et al. Nonalcoholic steatohepatitis: the role of peroxisome proliferator-activated receptors. Nat Rev Gastroenterol Hepatol. 2021, 18, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, L.; Chen, J.; Li, L.; Zheng, Z.; Ren, J.; et al. Oleoylethanolamide, an endogenous PPAR-α ligand, attenuates liver fibrosis targeting hepatic stellate cells. Oncotarget. 2015, 6, 42530–42540. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tao, Y.; Qiu, T.; Yao, X.; Jiang, L.; Wang, N.; et al. Taurine protected As2O3-induced the activation of hepatic stellate cells through inhibiting PPARα-autophagy pathway. Chem Biol Interact. 2019, 300, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, M.V.; Lodhi, I.J.; Yin, L.; Malapaka, R.R.V.; Xu, H.E.; Turk, J.; et al. Identification of a Physiologically Relevant Endogenous Ligand for PPARα in Liver. Cell. 2009, 138, 476–488. [Google Scholar] [CrossRef]
- Todisco, S.; Santarsiero, A.; Convertini, P.; De Stefano, G.; Gilio, M.; Iacobazzi, V.; et al. PPAR Alpha as a Metabolic Modulator of the Liver: Role in the Pathogenesis of Nonalcoholic Steatohepatitis (NASH). Biology. 2022, 11, 792. [Google Scholar] [CrossRef] [PubMed]
- Manickam, R.; Duszka, K.; Wahli, W. PPARs and Microbiota in Skeletal Muscle Health and Wasting. IJMS. 2020, 21, 8056. [Google Scholar] [CrossRef] [PubMed]
- Naruhn, S.; Meissner, W.; Adhikary, T.; Kaddatz, K.; Klein, T.; Watzer, B.; et al. 15-Hydroxyeicosatetraenoic Acid Is a Preferential Peroxisome Proliferator-Activated Receptor β/δ Agonist. Mol Pharmacol. 2010, 77, 171–184. [Google Scholar] [CrossRef]
- Piqueras, L.; Reynolds, A.R.; Hodivala-Dilke, K.M.; Alfranca, A.; Redondo, J.M.; Hatae, T.; et al. Activation of PPARβ/δ Induces Endothelial Cell Proliferation and Angiogenesis. ATVB. 2007, 27, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Kohro, T.; Tanaka, T.; Kanki, Y.; Li, G.; Poh, H.M.; et al. Cross-enhancement of ANGPTL4 transcription by HIF1 alpha and PPAR beta/delta is the result of the conformational proximity of two response elements. Genome Biol. 2014, 15, R63. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.D.; Du, S.; Martin, L.; Leccia, N.; Michiels, J.F.; Wagner, N. Vascular PPARβ/δ Promotes Tumor Angiogenesis and Progression. Cells. 2019, 8, 1623. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.; Solimando, A.G.; Prete, M.; Malerba, E.; Susca, N.; Derakhshani, A.; et al. Unraveling the Role of Peroxisome Proliferator-Activated Receptor β/Δ (PPAR β/Δ) in Angiogenesis Associated with Multiple Myeloma. Cells. 2023, 12, 1011. [Google Scholar] [CrossRef] [PubMed]
- Takada, I.; Makishima, M. Peroxisome proliferator-activated receptor agonists and antagonists: a patent review (2014-present). Expert Opinion on Therapeutic Patents. 2020, 30, 1–13. [Google Scholar] [CrossRef]
- Berger, J.; Leibowitz, M.D.; Doebber, T.W.; Elbrecht, A.; Zhang, B.; Zhou, G.; et al. Novel Peroxisome Proliferator-activated Receptor (PPAR) γ and PPARδ Ligands Produce Distinct Biological Effects. Journal of Biological Chemistry. 1999, 274, 6718–6725. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tan, H.; Wan, J.; Zeng, Y.; Wang, J.; Wang, H.; et al. PPAR-γ signaling in nonalcoholic fatty liver disease: Pathogenesis and therapeutic targets. Pharmacology & Therapeutics. 2023, 245, 108391. [Google Scholar] [CrossRef]
- Patsouris, D.; Mandard, S.; Voshol, P.J.; Escher, P.; Tan, N.S.; Havekes, L.M.; et al. PPARα governs glycerol metabolism. J Clin Invest. 2004, 114, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.S.; Tan, W.R.; Low, Z.S.; Marvalim, C.; Lee, J.Y.H.; Tan, N.S. Exploration and Development of PPAR Modulators in Health and Disease: An Update of Clinical Evidence. IJMS. 2019, 20, 5055. [Google Scholar] [CrossRef]
- Desai, A.; Yang Loureiro, Z.; DeSouza, T.; Yang, Q.; Solivan-Rivera, J.; Corvera, S. PPARγ activation by lipolysis-generated ligands is required for cAMP dependent UCP1 induction in human thermogenic adipocytes. bioRxiv. 2024, 2024.08.10.607465. [CrossRef]
- Wang, W.; Zhou, X.; Kwong, J.S.W.; Li, L.; Li, Y.; Sun, X. Efficacy and safety of thiazolidinediones in diabetes patients with renal impairment: a systematic review and meta-analysis. Sci Rep. 2017, 7, 1717. [Google Scholar] [CrossRef] [PubMed]
- Bougarne, N.; Weyers, B.; Desmet, S.J.; Deckers, J.; Ray, D.W.; Staels, B.; et al. Molecular Actions of PPARα in Lipid Metabolism and Inflammation. Endocrine Reviews. 2018, 39, 760–802. [Google Scholar] [CrossRef] [PubMed]
- Stienstra, R.; Mandard, S.; Tan, N.S.; Wahli, W.; Trautwein, C.; Richardson, T.A.; et al. The Interleukin-1 receptor antagonist is a direct target gene of PPARα in liver. Journal of Hepatology. 2007, 46, 869–877. [Google Scholar] [CrossRef]
- Pawlak, M.; Baugé, E.; Bourguet, W.; De Bosscher, K.; Lalloyer, F.; Tailleux, A.; et al. The transrepressive activity of peroxisome proliferator-activated receptor alpha is necessary and sufficient to prevent liver fibrosis in mice. Hepatology. 2014, 60, 1593–1606. [Google Scholar] [CrossRef] [PubMed]
- Devchand, P.R.; Keller, H.; Peters, J.M.; Vazquez, M.; Gonzalez, F.J.; Wahli, W. The PPARα–leukotriene B4 pathway to inflammation control. Nature. 1996, 384, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Montagner, A.; Tan, N.S.; Wahli, W. Insights into the Role of PPARβ/δ in NAFLD. IJMS. 2018, 19, 1893. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Brown, J.D.; Stanya, K.J.; Homan, E.; Leidl, M.; Inouye, K.; et al. A diurnal serum lipid integrates hepatic lipogenesis and peripheral fatty acid use. Nature. 2013, 502, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Wang, C.; Li, X.; Ma, P.; Dong, L.; Shen, B.; et al. PPARβ/δ activation protects against hepatic ischaemia-reperfusion injury. Liver Int. 2023, 43, 2808–2823. [Google Scholar] [CrossRef] [PubMed]
- Morán-Salvador, E.; López-Parra, M.; García-Alonso, V.; Titos, E.; Martínez-Clemente, M.; González-Périz, A.; et al. Role for PPARγ in obesity-induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. FASEB j. 2011, 25, 2538–2550. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; et al. PPARγ signaling and metabolism: the good, the bad and the future. Nat Med. 2013, 19, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Jin, Y.; Zhang, L.; Zhou, Y.; Chen, N.; Wang, W. PPAR gamma and PGC-1alpha activators protect against diabetic nephropathy by suppressing the inflammation and NF-kappaB activation. Nephrology (Carlton). 2024. [CrossRef] [PubMed]
- Abd-Elhamid, T.H.; Althumairy, D.; Bani Ismail, M.; Abu Zahra, H.; Seleem, H.S.; Hassanein, E.H.M.; et al. Neuroprotective effect of diosmin against chlorpyrifos-induced brain intoxication was mediated by regulating PPAR-γ and NF-κB/AP-1 signals. Food Chem Toxicol. 2024, 193, 114967. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Lu, R.; Zhang, Y.; Wu, Y.; Xie, L.; Caldwell, B.A.; et al. Monocytes Reprogrammed by 4-PBA Potently Contribute to the Resolution of Inflammation and Atherosclerosis. Circ Res. 2024, 135, 856–872. [Google Scholar] [CrossRef] [PubMed]
- Okayasu, T.; Tomizawa, A.; Suzuki, K.; Manaka K ichi Hattori, Y. PPARα activators upregulate eNOS activity and inhibit cytokine-induced NF-κB activation through AMP-activated protein kinase activation. Life Sciences. 2008, 82, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Quintela, A.M.; Jiménez, R.; Piqueras, L.; Gómez-Guzmán, M.; Haro, J.; Zarzuelo, M.J.; et al. PPAR β activation restores the high glucose-induced impairment of insulin signalling in endothelial cells. British J Pharmacology. 2014, 171, 3089–3102. [Google Scholar] [CrossRef] [PubMed]
- Wakino, S.; Hayashi, K.; Kanda, T.; Tatematsu, S.; Homma, K.; Yoshioka, K.; et al. Peroxisome Proliferator-Activated Receptor γ Ligands Inhibit Rho/Rho Kinase Pathway by Inducing Protein Tyrosine Phosphatase SHP-2. Circulation Research [Internet]. 2004 Sep 3;95. Available online: https://www.ahajournals.org/doi/10.1161/01.RES.0000142313.68389.92 (accessed on 28 October 2024).
- Guixé-Muntet, S.; Biquard, L.; Szabo, G.; Dufour, J.F.; Tacke, F.; Francque, S.; et al. Review article: vascular effects of PPARs in the context of, N. A.S.H. Aliment Pharmacol Ther. 2022, 56, 209–223. [Google Scholar] [CrossRef]
- Xie, G.; Wang, X.; Wang, L.; Wang, L.; Atkinson, R.D.; Kanel, G.C.; et al. Role of Differentiation of Liver Sinusoidal Endothelial Cells in Progression and Regression of Hepatic Fibrosis in Rats. Gastroenterology. 2012, 142, 918–927.e6. [Google Scholar] [CrossRef]
- De Silva, T.M.; Li, Y.; Kinzenbaw, D.A.; Sigmund, C.D.; Faraci, F.M. Endothelial PPARγ (Peroxisome Proliferator–Activated Receptor-γ) Is Essential for Preventing Endothelial Dysfunction With Aging. Hypertension. 2018, 72, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Gui, F.; You, Z.; Fu, S.; Wu, H.; Zhang, Y. Endothelial Dysfunction in Diabetic Retinopathy. Front Endocrinol. 2020, 11, 591. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Verna, L.; Chen, N.G.; Chen, J.; Li, H.; Forman, B.M.; et al. Constitutive Activation of Peroxisome Proliferator-activated Receptor-γ Suppresses Pro-inflammatory Adhesion Molecules in Human Vascular Endothelial Cells. Journal of Biological Chemistry. 2002, 277, 34176–34181. [Google Scholar] [CrossRef] [PubMed]
- Delerive, P.; Martin-Nizard, F.; Chinetti, G.; Trottein, F.; Fruchart, J.C.; Najib, J.; et al. Peroxisome Proliferator-Activated Receptor Activators Inhibit Thrombin-Induced Endothelin-1 Production in Human Vascular Endothelial Cells by Inhibiting the Activator Protein-1 Signaling Pathway. Circulation Research. 1999, 85, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Martin-Nizard, F.; Furman, C.; Delerive, P.; Kandoussi, A.; Fruchart, J.C.; Staels, B.; et al. Peroxisome Proliferator–activated Receptor Activators Inhibit Oxidized Low-density Lipoprotein–induced Endothelin-1 Secretion in Endothelial Cells. Journal of Cardiovascular Pharmacology 2002, 40, 822–831. [Google Scholar] [CrossRef]
- Berger, J.; Moller, D.E. The Mechanisms of Action of PPARs. Annu Rev Med. 2002, 53, 409–435. [Google Scholar] [CrossRef] [PubMed]
- Fougerat, A.; Montagner, A.; Loiseau, N.; Guillou, H.; Wahli, W. Peroxisome Proliferator-Activated Receptors and Their Novel Ligands as Candidates for the Treatment of Non-Alcoholic Fatty Liver Disease. Cells. 2020, 9, 1638. [Google Scholar] [CrossRef] [PubMed]
- Cooreman, M.P.; Vonghia, L.; Francque, S.M. MASLD/MASH and type 2 diabetes: Two sides of the same coin? From single PPAR to pan-PPAR agonists . Diabetes Research and Clinical Practice. 2024, 212, 111688. [Google Scholar] [CrossRef] [PubMed]
- Francque, S.M.; Bedossa, P.; Ratziu, V.; Anstee, Q.M.; Bugianesi, E.; Sanyal, A.J.; et al. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N Engl J Med. 2021, 385, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Cooreman, M.P.; Butler, J.; Giugliano, R.P.; Zannad, F.; Dzen, L.; Huot-Marchand, P.; et al. The pan-PPAR agonist lanifibranor improves cardiometabolic health in patients with metabolic dysfunction-associated steatohepatitis. Nat Commun. 2024, 15, 3962. [Google Scholar] [CrossRef]
- Yao, W.; Wang, K.; Wang, X.; Li, X.; Dong, J.; Zhang, Y.; et al. Icariin ameliorates endothelial dysfunction in type 1 diabetic rats by suppressing ER stress via the PPARα/Sirt1/AMPKα pathway. Journal Cellular Physiology. 2021, 236, 1889–1902. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Sarkar, S.; Bordoloi, J.; Wann, S.B.; Kalita, J.; Manna, P. Daidzein, its effects on impaired glucose and lipid metabolism and vascular inflammation associated with type 2 diabetes. BioFactors. 2018, 44, 407–417. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, X.; Liu, C.; Yang, C.; Yao, S.; Qiu, H.; et al. Daidzein protects endothelial cells against high glucose-induced injury through the dual-activation of PPARα and PPARγ. gpb. 2024, 43, 153–162. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, X.; Zhu, L.; Tang, F.S. Elafibranor: A promising treatment for alcoholic liver disease, metabolic-associated fatty liver disease, and cholestatic liver disease. World J Gastroenterol. 2024, 30, 4393–4398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Barroso, E.; Ruart, M.; Peña, L.; Peyman, M.; Aguilar-Recarte, D.; et al. Elafibranor upregulates the EMT-inducer S100A4 via PPARβ/δ. Biomedicine & Pharmacotherapy. 2023, 167, 115623. [Google Scholar] [CrossRef]
- Chang, Y.; Jeong, S.W.; Jang, J.Y. Recent updates on pharmacologic therapy in non-alcoholic fatty liver disease. Clin Mol Hepatol. 2023, 30, 129–133. [Google Scholar] [CrossRef]
- An, J.; Sohn, J.H. Pharmacological advances in the treatment of nonalcoholic fatty liver diseases : focused on global results of randomized controlled trials. Clin Mol Hepatol. 2023, 29, S268–S275. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





