1. Introduction
Liver cancer is the 6
th most common cancer worldwide, with an estimated 866,136 cases reported in 2022 [
1]. Hepatocellular carcinoma (HCC) accounts for 90% of all liver cancers. Hepatitis B, hepatitis C, and non-alcoholic steatohepatitis are implicated in the development of HCC. The standard systemic treatments for advanced HCC are combination therapies with immune checkpoint inhibitors and vascular endothelial growth factor (VEGF) antibodies or monotherapies of tyrosine kinase inhibitors. However, treatment options are restricted for relapse/refractory patients after these treatments. Lenvatinib is a small-molecule multi-tyrosine kinase inhibitor against VEGFRs and FGFRs and one of the first-line and second-line agents for patients with unresectable advanced HCC who are ineligible for immune checkpoint inhibitors [
2,
3]. However, varying sensitivity to lenvatinib has been reported among patients with HCC [
4], and several mechanisms of resistance to lenvatinib have been identified, such as enhanced EGFR signaling and Wnt/β-catenin signaling [
5]. Thus, the identification of novel therapeutic strategies for HCC is needed to improve the treatment of patients with HCC.
One of the causes of the unsatisfactory therapeutic response to standard treatments is the intratumor heterogeneity of HCC [
6]. The presence of cancer stem cells, which possess the abilities for self-renewal and differentiation into multiple subtypes of tumor cells, is thought to be involved in promoting intratumor heterogeneity and drug resistance [
7]. Therefore, cancer stem cells may be a target in the treatment of HCC.
Delta-like 1 homolog (DLK1) is a single transmembrane protein that is a member of the Notch ligand family. DLK1 consists of six epidermal growth factor (EGF)-like repeat sequences in the extracellular region, a disintegrin and metalloprotease (ADAM)-17 cleavage site in the juxtamembrane region, a transmembrane domain, and a short intracellular tail [
8]. The extracellular region of DLK1 is cleaved by ADAM-17, and a fragment of the extracellular region is secreted into the blood as soluble DLK1. While the involvement of DLK1 in wound healing and inhibition of adipocyte differentiation has been reported, the physiological functions of DLK1 are largely unknown [
9]. In the fetus, DLK1 is expressed in many tissues, but after birth, its expression is restricted to endocrine tissues (pituitary, adrenal, placenta, and pancreas) [
8]. DLK1 expression has been reported in tissue stem/progenitor cells such as hepatoblasts, hepatic oval cells, mesenchymal stem cells, and prostate epithelial progenitor cells [
10,
11,
12,
13].
Several reports have linked DLK1 to cancer. Immunohistochemical analysis showed that DLK1 was expressed in HCC cells, but not in other liver diseases, such as hepatitis and cirrhosis [
14]. DLK1 is one of the hepatic progenitor cell (HPC) markers and is co-expressed with other HPC markers (EpCAM, CK19, NCAM). HCC patients with higher levels of blood tumor markers (AFP, AFP-L3) have a higher expression of DLK1, along with simultaneous co-expression with other HPC markers. These patients also have a poor postoperative prognosis [
15]. In a two-step hepatic carcinogenesis mouse model, the expression of DLK1 and cancer stem cell markers was found to be upregulated in liver tumors, and knockdown of DLK1 inhibited liver tumor growth [
16,
17]. DLK1 knockdown in HCC cell lines was shown to suppress colony formation in vitro and tumor growth in vivo [
16]. In an experiment with 17 different liver cancer (mainly HCC) cell lines, the DLK1-positive sub-populations isolated from each cell line showed higher in vitro colony formation and cell proliferation activities and increased in vivo tumorigenic potential compared with DLK1-negative sub-populations [
18]. DLK1-positive sub-populations have also been reported to express stem cell and progenitor cell markers (Nanog, Oct3/4, SOX2) and exhibit cancer stem cell–like characteristics, including resistance to chemotherapeutic agents [7, 18]. Therefore, DLK1 may be a candidate cancer stem cell marker in HCC in addition to its function as a HPC marker.
CBA-1205 is a novel recombinant humanized IgG1/κ monoclonal antibody, discovered by Chiome Bioscience Inc., that specifically binds to human DLK1. The antibody-dependent cellular cytotoxicity (ADCC) of CBA-1205 was enhanced through the use of GlymaxX® technology (ProBioGen AG, Berlin, Germany), which increases ADCC activity by the modification of the glycan structure of the Fc portion at Asn 297 [
19,
20]. A phase I, first in humans, study of CBA-1205 is ongoing in patients with advanced or recurrent solid tumors (jRCT2080225288).
Here, we report the preclinical characterization of CBA-1205, including its efficacy in HCC in vitro and in vivo models [
20] and safety and toxicokinetic profiles in non-human primates. We also demonstrate that CBA-1205 showed synergistic and durable efficacy in combination with lenvatinib in HCC pre-clinical animal models [
21].
3. Discussion
In this study, we demonstrated that CBA-1205 showed specific binding to DLK1 and did not bind to any other Notch ligand family members. This suggests that CBA-1205 does not affect the various physiological functions mediated by the other ligands. DLK1 expression is restricted to specific tissues and organs, such as the hypothalamus-pituitary-adrenal axis and pancreas [
22,
23,
24]. The specific binding of CBA-1205 to DLK1 and restricted expression of DLK1 in adults suggest potential advantages in terms of reduced or limited adverse effects of CBA-1205.
While DLK1 has been reported to promote cell proliferation [
10,
11,
12,
13], CBA-1205 had no effect on the cell proliferation of DLK1-expressing cells such as HepG2 and Hep3B cells (
Figure 3). Most of the physiological functions of DLK1 identified thus far are explained by its interaction with Notch1. Previous studies showed that the EGF-like repeat 5–6 of DLK1 is involved in the interaction between DLK1 and Notch1, and the EGF-like repeat 4–5 of DLK1 is required for the interaction of DLK1 to DLK1 [
9,
25,
26,
27]. Another study showed that the juxtamembrane region after the EGF-like repeat 6 of DLK1 interacts with the C-terminal region of fibronectin to suppress Notch-independent differentiation into adipocytes [
28]. Epitope analysis revealed that CBA-1205 binds to EGF-like repeat 1–2 of DLK1 (
Figure 2). These results may suggest that CBA-1205 binds to DLK1 without affecting the physiological function of DLK1.
Our results demonstrated that CBA-1205 showed antitumor activity against HepG2 and Hep3B xenograft models as a single agent and in combination with lenvatinib, regardless of lenvatinib sensitivity (
Figure 5C,D and
Figure 6). The mechanism of the antitumor activity of CBA-1205 in vivo may be from ADCC activity against DLK1-expressing cells, as identified in the in vitro results (
Figure 4 and
Figure 5C,D).
Immune checkpoint inhibitors, multi-tyrosine kinase inhibitors, and anti-VEGF antibodies are used to treat patients with HCC. However, some patients experience resistance to these agents. The intratumor heterogeneity of HCC contributes to cancer cell proliferation, metastasis, drug resistance, and recurrence, making treatment difficult and leading to poor prognosis [
6]. The presence of tumor stem cells is thought to be one of the causative factors for intratumor heterogeneity and treatment resistance [
6,
7,
29]. Previous studies showed that DLK1-expressing sub-populations in human liver cancer cell lines exhibit cancer stem cell–like properties, such as high proliferative capacity, high invasiveness, and resistance to chemotherapy [
18]. HCC patients harboring DLK1-expressing tumors show a poor prognosis [
15]. Therefore, depletion of DLK1-expressing cancer cells by CBA-1205 may be a useful HCC treatment strategy. Our results demonstrate the antitumor activity of CBA-1205 in the liver cancer models, suggesting a potential therapeutic strategy by depletion of DLK1-positive cancer stem cells in HCC by ADCC activity of CBA-1205.
Lenvatinib is the standard of care for HCC for patients who are not suitable for immune checkpoint inhibitors [
2,
3]. In phase 3 clinical trials of HCC, lenvatinib did not show a clear combination effect when combined with anti PD-1 antibodies; the combination of lenvatinib and anti-PD-1 antibody (pembrolizumab) did not demonstrate significant efficacy over lenvatinib plus placebo [
30]. For this reason, lenvatinib has been used as a single agent in real-world clinical practice. Our results suggest that CBA-1205 has the potential for clinical use in combination with lenvatinib (
Figure 6A,B).
Various mechanisms of lenvatinib resistance in HCC have been reported [
5]. Activation of the β-catenin pathway by Frizzled-10 and IRF2 was revealed as one of the mechanisms of cancer stem cell expansion in the liver and resistance to lenvatinib [
31]. DLK1 is expressed in cancer stem cells, and its expression is regulated by Wnt/β-catenin signaling [
32,
33,
34]. Therefore, some HCC cases with resistance to lenvatinib may show increased expression of DLK1. Cancers with activated Wnt/β-catenin signaling are known as immuno-excluded tumors and are less likely to respond to immune checkpoint inhibitors [
35]. CBA-1205 may be a potential therapeutic agent targeting DLK1 in immuno-excluded cancers.
Approximately 4%–57% of HCC cases have been reported to express DLK1 [
36]. Moreover, DLK1 expression is not uniform within tumor tissue indicating intratumoral heterogeneity [
37]. Therefore, it is important to stratify patients who may benefit from CBA-1205. DLK1-expressing HCCs have been reported to show activation of RAS, Yes-associated protein (YAP), or Notch intracellular domain pathways, in addition to activation of the Myc pathway [
37,
38,
39]. In contrast, DLK1 was not expressed in HCCs with AKT pathway activation. Moreover, an increase in serum soluble DLK1 levels in HCC patients was found to correlate with tumor size [
40]. Using these characteristics as patient stratification markers, it may be possible to efficiently identify patients who may benefit from CBA-1205.
DLK1 has also been found to be expressed in lung cancer, pancreatic cancer, ovarian cancer, endocrine cancer, and pediatric cancer [
36]. An association between DLK1 expression and patient prognosis was reported in small cell lung cancer, non-small cell lung cancer [
41], ovarian cancer [
42], and gastrointestinal stromal tumor [
43]. DLK1-expressing tumor cells have a higher proliferation rate, higher invasive capacity with epithelial mesenchymal transition, and resistance to treatment compared with cancer cells not expressing DLK1 [
44]. Therefore, CBA-1205 has potential as a treatment option for cancers in addition to HCC that express DLK1.
The toxicity and toxicokinetic profiles of CBA-1205 identified in this study were favorable. DLK1 was reported to be expressed in the hypothalamus-pituitary-adrenal axis and pancreas [
22,
23,
24]. The toxicity study of CBA-1205 in monkeys revealed no abnormal findings in DLK1-expressing tissues (data not shown). While sufficient data to explain this safety finding have not been obtained, it appears to be related to the affinity of CBA-1205 for DLK1 or the amount of DLK1 expressed in these normal tissues.
4. Materials and Methods
4.1. Generation of CBA-1205
Recombinant protein corresponding to the extracellular region of human DLK1 (amino acids 26–244, accession No. NP_003827, GenBank) was mixed with the immunization adjuvant TiterMax Gold (Funakoshi Corp., Tokyo, Japan) and used to immunize female BALB/c mice. Lymphocytes from immunized mice and a mouse myeloma cell line (P3-X63-Ag8.653) were fused to establish anti-HuDLK1 monoclonal antibody–producing hybridomas. Anti-HuDLK1 antibody (clone BA-1-3D) was isolated as the parental antibody.
Humanized BA-1-3D (HuBA-1-3D) was generated by CDR grafting. The afucosylated version of HuBA-1-3D (product code name: CBA-1205) produced by GlymaxX® technology was manufactured by ProBioGen AG (Berlin, Germany).
4.2. Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA was performed using recombinant protein–coated 96-well plates. The following recombinant proteins were used at concentrations of 2.5–10 µg/mL: human DLK-1 (ab151926, Abcam, Cambridge, UK), human DLK2:Fc (AG-40A-0158-C010, AdipoGen, San Diego, CA, USA), human Jagged-1:Fc (AG-40A-0081-C010, AdipoGen), human Jagged-2:Fc (AG-40A-0155Y-C010, AdipoGen), human DLL1:Fc (AG-40A-0116Y-C010, AdipoGen), human DLL3 (His tag) (ab255797, Abcam), and human DLL4 (His-tag) (DLL4-2235H, Creative BioMart, Shirley, NY, USA). The coated plates were blocked with 1% BSA and 0.05% Tween 20 in phosphate-buffered saline (PBS) (blocking buffer) for 1 h at room temperature. CBA-1205 was added at various concentrations in 0.5% BSA and 0.025% Tween 20 in PBS (ELISA buffer), and the plates were incubated for 1 h at room temperature. After extensive washes with 0.05% Tween 20 containing PBS, the plates were incubated with Mouse Anti-Human IgG Kappa-HRP (1:4000) (SouthernBiotech, Birmingham, AL, USA) for 1 h at room temperature, washed, and incubated with TMB reagent (Nacalai Tesque, Kyoto, Japan) for 10 min before quenching with 50 µL of 1 mol/L H2SO4. The absorbance at 450 nm (655 nm as the reference) was measured with a microplate reader (iMark, Bio-Rad, Hercules, CA, USA).
4.3. Cell Culture
The 293 cell line (JCRB9068, Japanese Collection of Research Bioresources Cell Bank (JCRB), Osaka, Japan) was cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Thermo Fisher Scientific K.K, Tokyo, Japan.). We generated 293-hDLK1 cells by stably transfecting a human DLK1 expression vector [
14] into 293 cells; these cells were cultured in DMEM. HepG2 human liver cancer cells (JCRB1054, JCRB) were cultured in DMEM. Hep3B human liver cancer cells (86062703, European Collection of Authenticated Cell Cultures (ECACC)) were cultured in Eagle’s minimal essential medium (EMEM, Thermo Fisher Scientific K.K.). The culture media were supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (FBS, Hyclone, Logan, UT, USA), 50 U/mL penicillin, 50 mg/mL streptomycin, and 4 mM L-glutamine. All cell lines were cultured in a humidified atmosphere containing 5% CO
2 at 37°C.
4.4. Flow Cytometry
Cell suspensions at 0.2–1.0 × 106 cells/100 µL were incubated with CBA-1205 at 4°C. After two washes, the cells were incubated with phycoerythrin (PE)-labeled goat anti-human IgG (CAT:2040-09, SouthernBiotech) or PE-labeled goat F(ab')2 anti-human Ig (CAT:2012-09, SouthernBiotech) at 4°C. After two washes, the reactivity of CBA-1205 to DLK1 on the cell surface was analyzed using a flow cytometer (FACS Calibur, BD Biosciences, San Jose, CA, USA).
4.5. Cell Proliferation Assay
Cell proliferation of 293-hDLK1, HepG2 and Hep3B cells was evaluated using a Cell Counting Kit-8 (CCK-8) (DOJINDO, Kumamoto, Japan). Cells cultured in a 10 cm cell culture dish at 80% confluency were changed from 10% FBS–containing culture medium to serum-free medium and cultured overnight. The cells were then trypsinized, and cell suspensions were prepared with FBS-containing culture medium. Cells (2 × 104 cells/50 µL) were plated in 96-well plates, and CBA-1205 or human IgG1/κ isotype control (Bio X Cell, Lebanon, NH, USA, 659518A1) was added at various concentrations (0, 0.1, 0.3, 1, 3, and 10 µg/mL in 100 µL volume), with triplicate wells for each concentration. The positive control PD98059 was added to wells (0, 0.3, 1, 3, 10, 30, and 100 µM) in triplicate. The cells were cultured for 96 h in 5% CO2 at 37°C. WST-8 solution from the CCK-8 kit was added, and cells were incubated for 1–3 h in 5% CO2 at 37°C. The absorbance at 450 nm was measured using a plate reader (iMark, BioRad).
4.6. ADCC Assay
ADCC assays were performed at Chemicals Evaluation and Research Institute, Japan (CERI, Tokyo, Japan). The 293 (negative control), 293-hDLK1 (positive control), and liver cancer cell lines (HepG2 and Hep3B) were used as target cells. Human FcγRIIIA–expressing NK cells, TK176V (CERI), were cultured in Roswell Park Memorial Institute 1640 Medium (RPMI 1640, Thermo Fisher Scientific K.K.) containing 10% FBS, 10 ng/mL IL-2, and 1.25 µg/mL puromycin. The target cells were labeled with chromium-51 (51Cr, PerkinElmer, Inc., Waltham, MA, USA) for 1 h at 37°C. The labeling efficiency was pre-optimized as 50 mCi per 1 × 106 cells. The labeled target cells were plated in 96-well U bottom plates (BD Falcon, Franklin Lakes, NJ, USA) at 5 × 103 cells/50 µL/well. Various concentrations of CBA-1205 or human IgG1/k isotype control were added at 100 µl/well and the cells were cultured for 1 h at 37°C. Next, 2 × 105 cells (50 µL) of TK176V cells as effector cells (effector:target = 40:1) were added to the wells, and the plates were cultured for 4 h in 5% CO2 at 37°C. The amount of 51Cr release (CPM) in 50 µL culture medium was measured by a gamma counter (2480 WIZARD2 auto gamma counter, PerkinElmer, Inc.).
Human peripheral blood mononuclear cells (hPBMCs) were also used as effector cells. Three different lots of PBMCs (PBM001280, PBM001285, PBM001286) (BIOPREDIC International, Saint-Grégoire, France) were used in the experiments. hPBMCs were cultured for one day in RPMI1640 medium containing 10% FBS, 10 ng/mL human IL-2, and 500 ng/mL human GM-CSF (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). The effector:target ratio was 20:1.
ADCC activity (%) was calculated using the following formula:
cytotoxicity activity (%) = (each well CPM – spontaneous release CPM)/(max release CPM – spontaneous CPM) x 100
ADCC activity (%) = (cytotoxicity at antibody addition – cytotoxicity with no antibody)
4.7. Xenograft Experiments
Female NOD/ShiJic-scidJcl mice (NOD-SCID), 6–8 weeks old, (Japan CLEA, Tokyo, Japan) were used in this study. Hep3B (1 × 107 cells/mL) or HepG2 (5 × 107 cells/mL) cells were prepared in a 1:1 mixture of PBS and BD Matrigel, reduced growth factor, phenol-red free (BD Pharmingen, San Jose, CA, USA). Cell suspensions (100 µL) were subcutaneously transplanted into the right flank of NOD-SCID mice. When the tumor volume reached > 100 mm3, the mice were randomized to treatment groups. Vehicle (PBS), human IgG1/κ isotype control, and CBA-1205 were intraperitoneally administered to the mice at the indicated dosage (0.1, 0.3, 1, 3, and 10 mg/kg) twice a week (8 mice in each dosing group). Vehicle (purified water for lenvatinib) or lenvatinib mesylate (79A59K, Eisai, Tokyo, Japan) was orally administered once a day at the indicated dosage (3, 10, and 30 mg/kg) for the indicated periods (8 mice in each dosing group). In the combination dosing study, CBA-1205 (0.3 and 1 mg/kg, twice a week, a total of four times) was administered intraperitoneally with or without 10 mg/kg lenvatinib (daily, a total of 10 times) in Hep3B and HepG2 xenograft mice (8 animals in each group). Animals in the control group (n = 8) received vehicle (purified water orally and PBS intraperitoneally) on the same schedule.
Tumor growth was measured with calipers in two perpendicular dimensions, and tumor volume (mm3) was calculated using the formula (width2 x length) x π/6. The body weight and the general symptoms of the animals were measured and observed at the time of administration throughout the experiment.
All animal experiments in this study were approved and conducted in accordance with the Institutional Animal Care and Use Committee guidelines of Chiome Bioscience Inc. (Tokyo, Japan).
4.8. Epitope Assays
Three DLK1 expression vectors were constructed for epitope analysis. The expression vector encoding full-length HuDLK1 was previously described [
14]. The (EGF3–4) and human/mouse chimera DLK1 (human EGF 1–2 connected with mouse EGF3–6) constructs were generated by PCR. The sequences of PCR primers were as follows: Forward Primer: 5′-gcggccgcgcctgctcctcggccccc-3′, Reverse Primer: 5′-tctagagaggctgttggccacgatctcgc-3′ for HuDLK1 EGF 3-4; and Forward Primer, Y403-Not: 5′-gcggccggctgaatgcttcccggcc-3′, Reverse Primer, Y413: 5′-gtccacgcaagttccgttgttggcacaggg-3′, Forward Primer, Y444: 5′-ccctgtgccaacaacggaacttgcgtggac-3′, and Reverse Primer, Y441: 5′-tctagattagatctcctcatcacc-3′ for HuDLK1 (EGF1–2)/mouse DLK1 (EGF3-6) chimera. PCR products were verified by DNA sequencing, and the fragments were sub-cloned into the pME18SNeo plasmid, which carries the signal sequence of CD8, His tag, and transmembrane and cytoplasmic domains of FXYD5 [
14]. The three expression vectors were transiently expressed into CHO-K1 cells using Lipofectamine 2000 reagent (Thermo Fisher Scientific K.K.). At 24 h after transfection, the cells were trypsinized and subjected to flow cytometry analysis for epitope analysis of BA-1-3D antibody.
4.9. Cross-Reactivity of CBA-1205 to Human, Cynomolgus Monkey, Mouse, and Rat DLK1
The expression vector encoding human DLK1 was constructed as described previously [
14]. Mouse DLK1 cDNA was isolated as described previously [
10]. The PCR amplified cDNA fragment of mouse DLK1 was cloned into the pcDNA3 vector (Invitrogen). Rat DLK1 cDNA was synthesized using the amino acid sequence (Genbank accession No. AAI67752.1) with 5′-kozak sequences (GCCACC) and EcoRI restriction enzyme site (GAATTC) and 3′ HindIII site. The synthesized rat DLK1 cDNA was digested with EcoRI and HindIII and subcloned into the pcDNA3 vector. Cynomolgus monkey DLK1 cDNA was synthesized using amino acid sequences (Accession No. XP_015309693). The PCR-amplified cDNA of cynomolgus monkey DLK1 with a 3′-FLAG tag was subcloned into the pcDNA3 vector. The sequences of human, mouse, rat, and cynomolgus monkey DLK1 were verified by DNA sequencing.
To analyze the cross-reactivity of CBA-1205 to human, mouse, rat, and cynomolgus monkey DLK1, the expression vectors encoding human, mouse, rat, and cynomolgus monkey DLK1 were transiently expressed into Expi293F™ cells (Thermo Fisher Scientific K.K.) using the ExpiFectamine 293 Transfection Kit (Thermo Fisher Scientific K.K.) following the manufacturer’s instructions. After 48 h, the transfected cells were suspended with BAMBANKER (GC Lymphotec, Tokyo, Japan) and stored at -80°C until use. Cross-reactivity of CBA-1205 was analyzed by flow cytometry as described above.
4.10. Toxicity Study in Cynomolgus Monkeys
The general toxicity including safety pharmacology, immunotoxicity, serum cytokine profile, toxicokinetics (at 1st and 4th doses), and antibody analysis of CBA-1205 was evaluated in cynomolgus monkeys under GLP compliance. CBA-1205 was intravenously administered once weekly for 4 weeks at 0 (vehicle: 25 mM histidine-HCl buffer (pH 6.0)), 10, 30 and 100 mg/kg (dose volume: 5 mL/kg) to four male and four female cynomolgus monkeys per each dose group. Two males and two females were added to the 0 and 100 mg/kg groups to assess the reversibility of toxicity during a 4-week recovery period.
4.11. Statistical Analysis
Concentration-response curves were analyzed using GraphPad Prism version 10.2.0 (GraphPad Software, La Jolla, CA, USA), fitting a 4-parameter logistic model. EC
50 values and its 95% CI were estimated from the curves. Tumor volumes are expressed as mean ± standard deviation (SD). Statistical analysis of tumor size differences on the final study day were evaluated by the two-tailed Dunnett’s test (vs. vehicle treatment group), and a Tukey’s multiple paired comparison test when the significant results were observed in the Dunnett’s test, using the statistical program MEPHAS (
http://www.gen-info.osaka-u.ac.jp/MEPHAS/dunnett-e.html).
p < 0.05 indicated a significant difference. Toxicokinetic parameters following intravenous bolus administration of CBA-1205 were analyzed using the methods of non-compartment analysis and linear trapezoidal linear interpolation in Phoenix™ WinNonlin version 6.4 (Certara LP, Princeton, NJ, USA).
Author Contributions
Conceptualization, K.N.; data curation and investigation, K.T., I.S., T.S., Z.L. and H.Y.; formal analysis, K.N., K.T., I.S., T.S., Z.L., H.Y. and Y.T.; methodology, K.T., I.S., T.S., Z.L., H.Y. and Y.T.; supervision, K.N. and Y.T.; validation, K.N.; visualization, K.N.; writing—original draft, K.N. and Y.T.; writing—review and editing, K.N., H.Y. and Y.T. All authors have read and agreed to the published version of the manuscript.
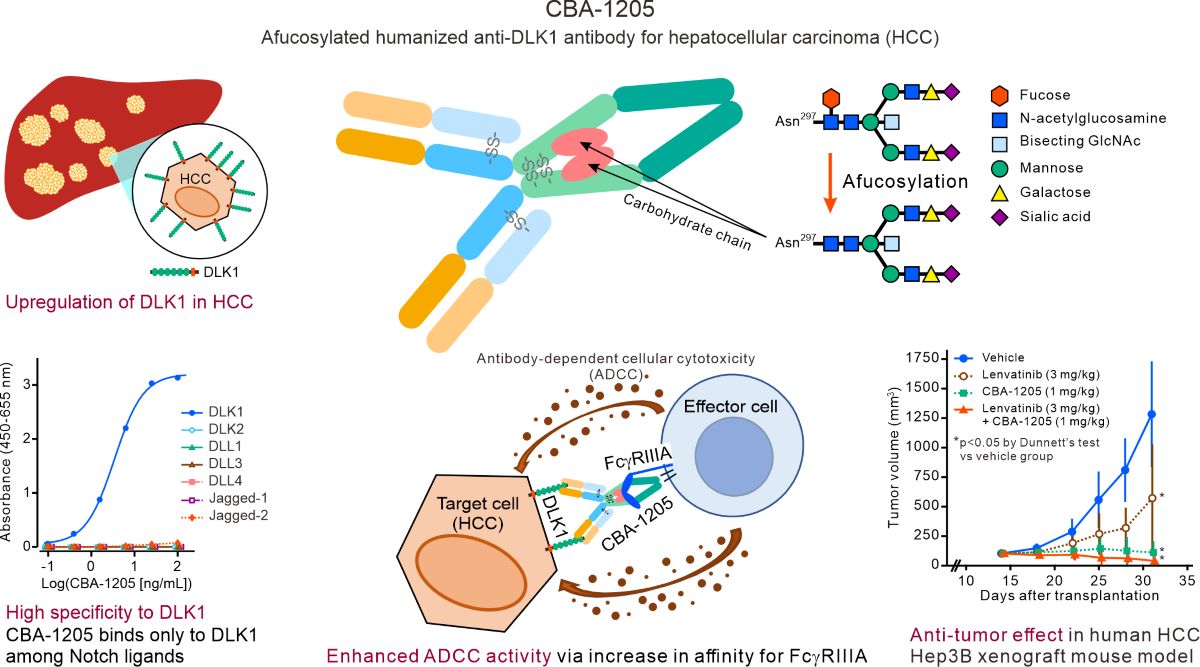
Figure 1.
Binding characteristics of CBA-1205 to human DLK1. (A) ELISA of CBA-1205 binding to Notch ligand family proteins; (B) Flow cytometric assay of CBA-1205 binding to liver cancer cell lines that express endogenous DLK1; (C) Equivalent binding affinity of CBA-1205 to human and cynomolgus monkey DLK1 (HuDLK1 and CyDLK1, respectively); (D) Species specificity of CBA-1205 binding to human DLK1, cynomolgus monkey DLK1, mouse DLK1 (MuDLK1), and rat DLK1 (RatDLK1). The expression vectors (pcDNA3) encoding human, mouse, rat, and cynomolgus monkey DLK1, with a FLAG tag at the C-terminus, were transiently expressed into Expi293F™ cells. Reactivity of CBA-1205 (30 µg/mL) to cells transiently expressing the indicated DLK1 genes was analyzed by flow cytometry. MFI, mean fluorescence intensity; SSC-H, side scatter height; FL2, second fluorescence channel (for detection of phycoerythrin-labeled secondary antibody).
Figure 1.
Binding characteristics of CBA-1205 to human DLK1. (A) ELISA of CBA-1205 binding to Notch ligand family proteins; (B) Flow cytometric assay of CBA-1205 binding to liver cancer cell lines that express endogenous DLK1; (C) Equivalent binding affinity of CBA-1205 to human and cynomolgus monkey DLK1 (HuDLK1 and CyDLK1, respectively); (D) Species specificity of CBA-1205 binding to human DLK1, cynomolgus monkey DLK1, mouse DLK1 (MuDLK1), and rat DLK1 (RatDLK1). The expression vectors (pcDNA3) encoding human, mouse, rat, and cynomolgus monkey DLK1, with a FLAG tag at the C-terminus, were transiently expressed into Expi293F™ cells. Reactivity of CBA-1205 (30 µg/mL) to cells transiently expressing the indicated DLK1 genes was analyzed by flow cytometry. MFI, mean fluorescence intensity; SSC-H, side scatter height; FL2, second fluorescence channel (for detection of phycoerythrin-labeled secondary antibody).
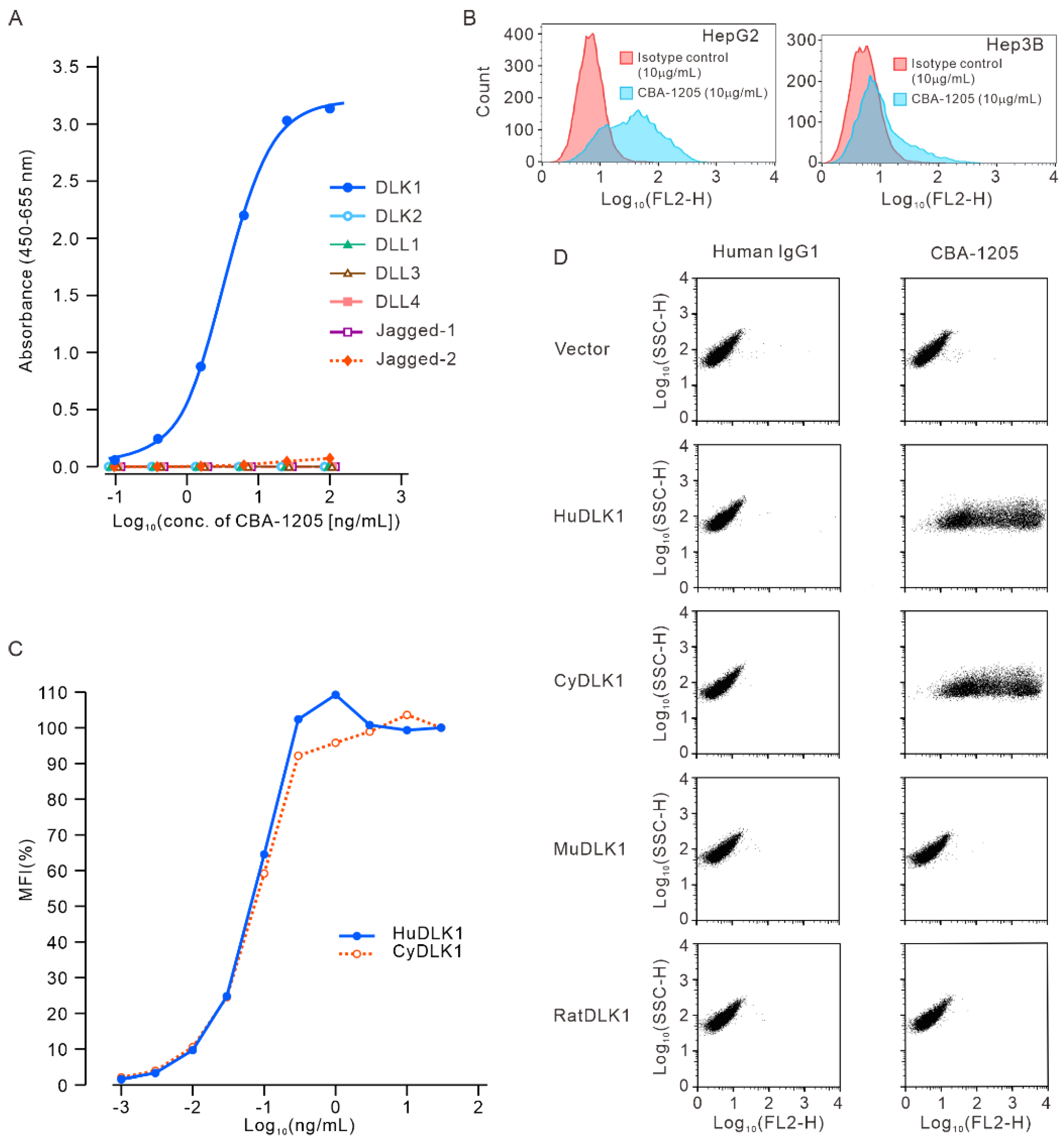
Figure 2.
Epitope analysis of CBA-1205 antibody by flow cytometry. (A) Constructs of DLK1; (B) Flow cytometric assay of CBA-1205 binding to cells expressing various DLK1 constructs. HuDLK1, human DLK1; EGF, epidermal growth factor; EGF 3–4, EGF-like repeat 3–4 (hereafter abbreviated in the same manner); MuDLK1, mouse DLK1; BA-1-3-D, mouse anti-HuDLK1 antibody, based on which CBA-1205 was humanized and afucosylated.
Figure 2.
Epitope analysis of CBA-1205 antibody by flow cytometry. (A) Constructs of DLK1; (B) Flow cytometric assay of CBA-1205 binding to cells expressing various DLK1 constructs. HuDLK1, human DLK1; EGF, epidermal growth factor; EGF 3–4, EGF-like repeat 3–4 (hereafter abbreviated in the same manner); MuDLK1, mouse DLK1; BA-1-3-D, mouse anti-HuDLK1 antibody, based on which CBA-1205 was humanized and afucosylated.
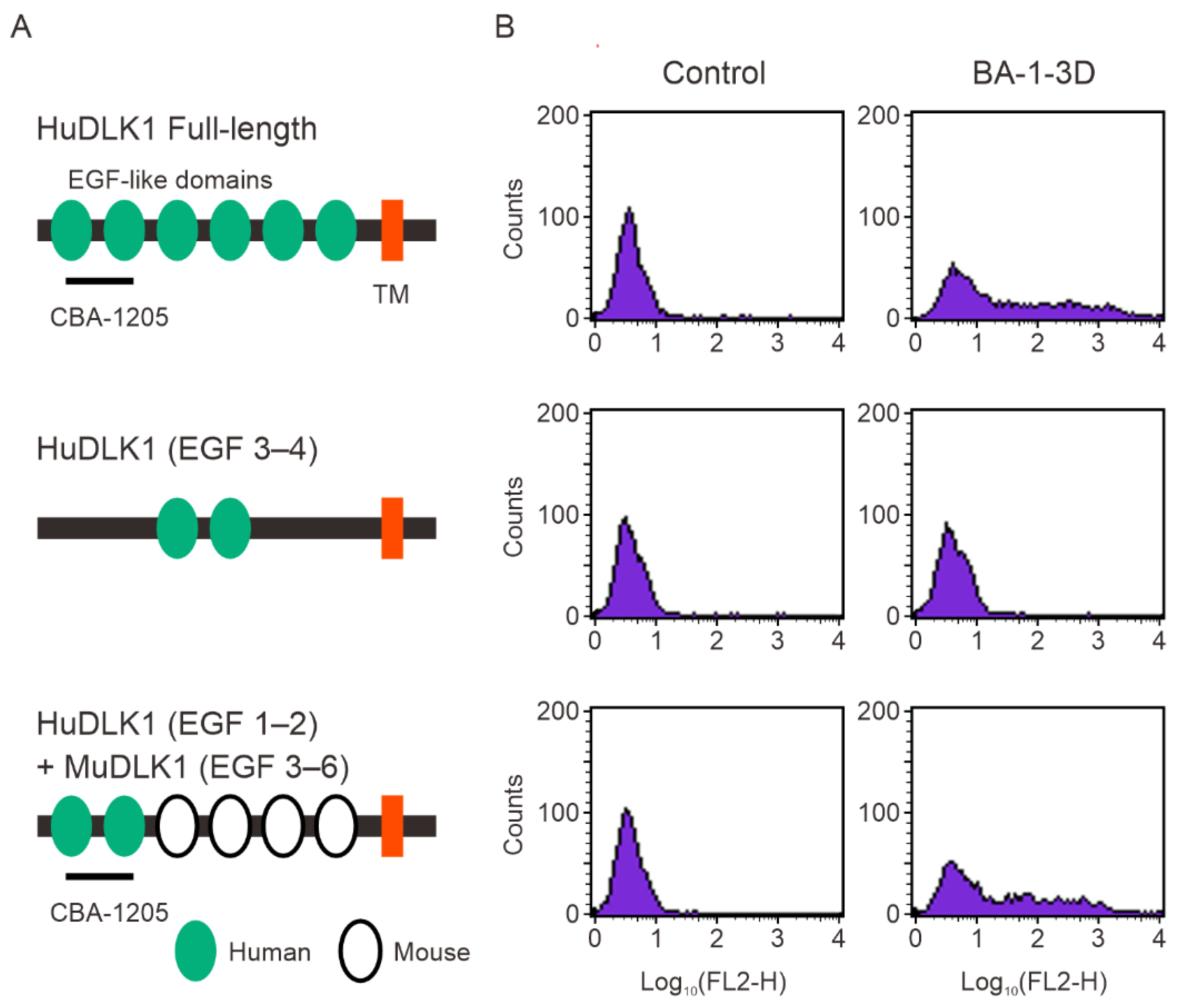
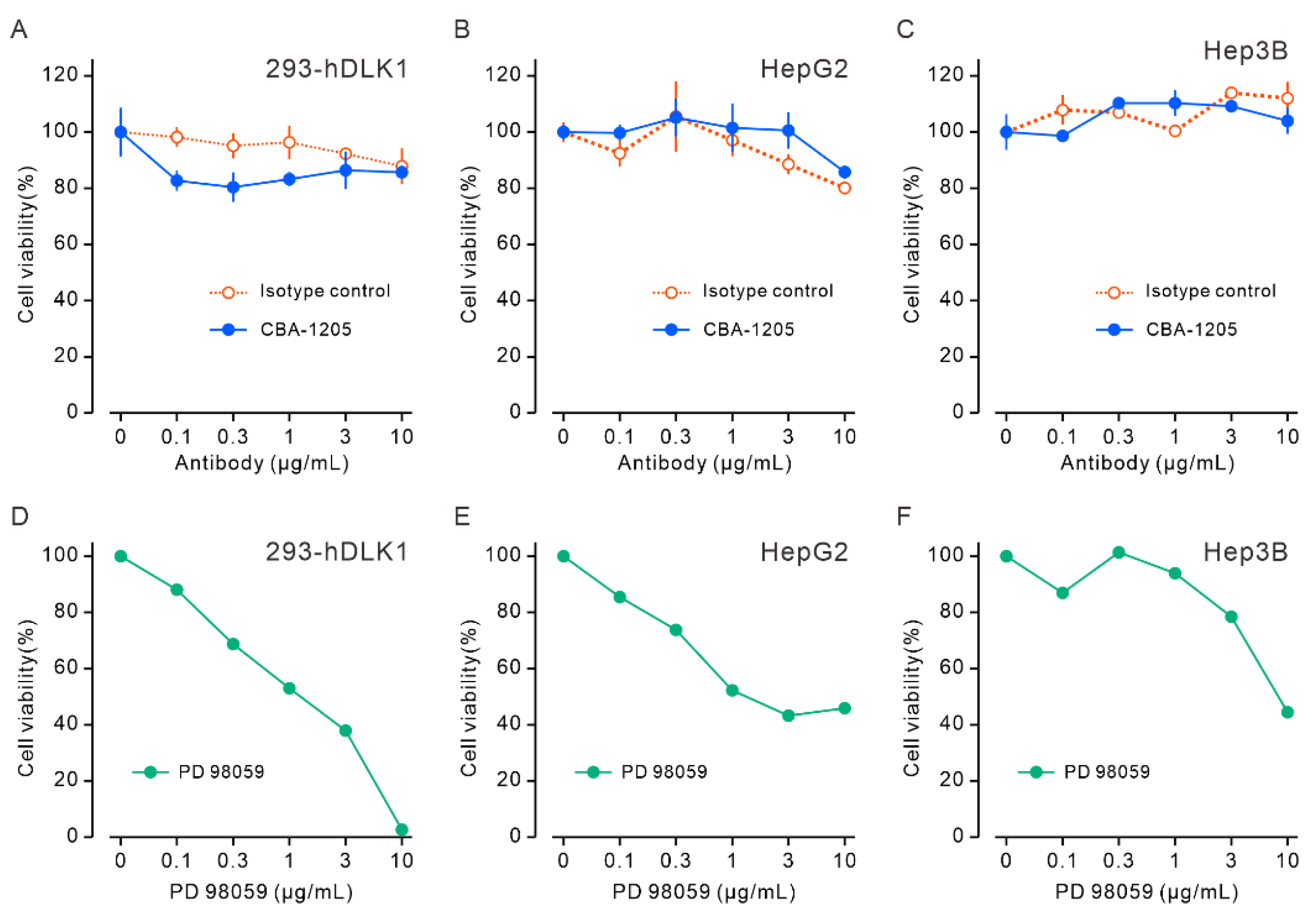
Figure 3.
CBA-1205 does not affect cell proliferation of human liver cancer cell lines in vitro. (A–C) Cells were incubated with various concentrations of CBA-1205 at 37°C in 5% CO2 for 96 h. Assays were performed in triplicate. Data are expressed as mean+ SD. (D–F) Cells were incubated with various concentrations of the MEK inhibitor PD98059 at 37°C in 5% CO2 for 96 h. (A, D) 293-hDLK1 cells stably expressing human DLK1; (B, E) HepG2 cells; (C, F): Hep3B cells.
Figure 3.
CBA-1205 does not affect cell proliferation of human liver cancer cell lines in vitro. (A–C) Cells were incubated with various concentrations of CBA-1205 at 37°C in 5% CO2 for 96 h. Assays were performed in triplicate. Data are expressed as mean+ SD. (D–F) Cells were incubated with various concentrations of the MEK inhibitor PD98059 at 37°C in 5% CO2 for 96 h. (A, D) 293-hDLK1 cells stably expressing human DLK1; (B, E) HepG2 cells; (C, F): Hep3B cells.
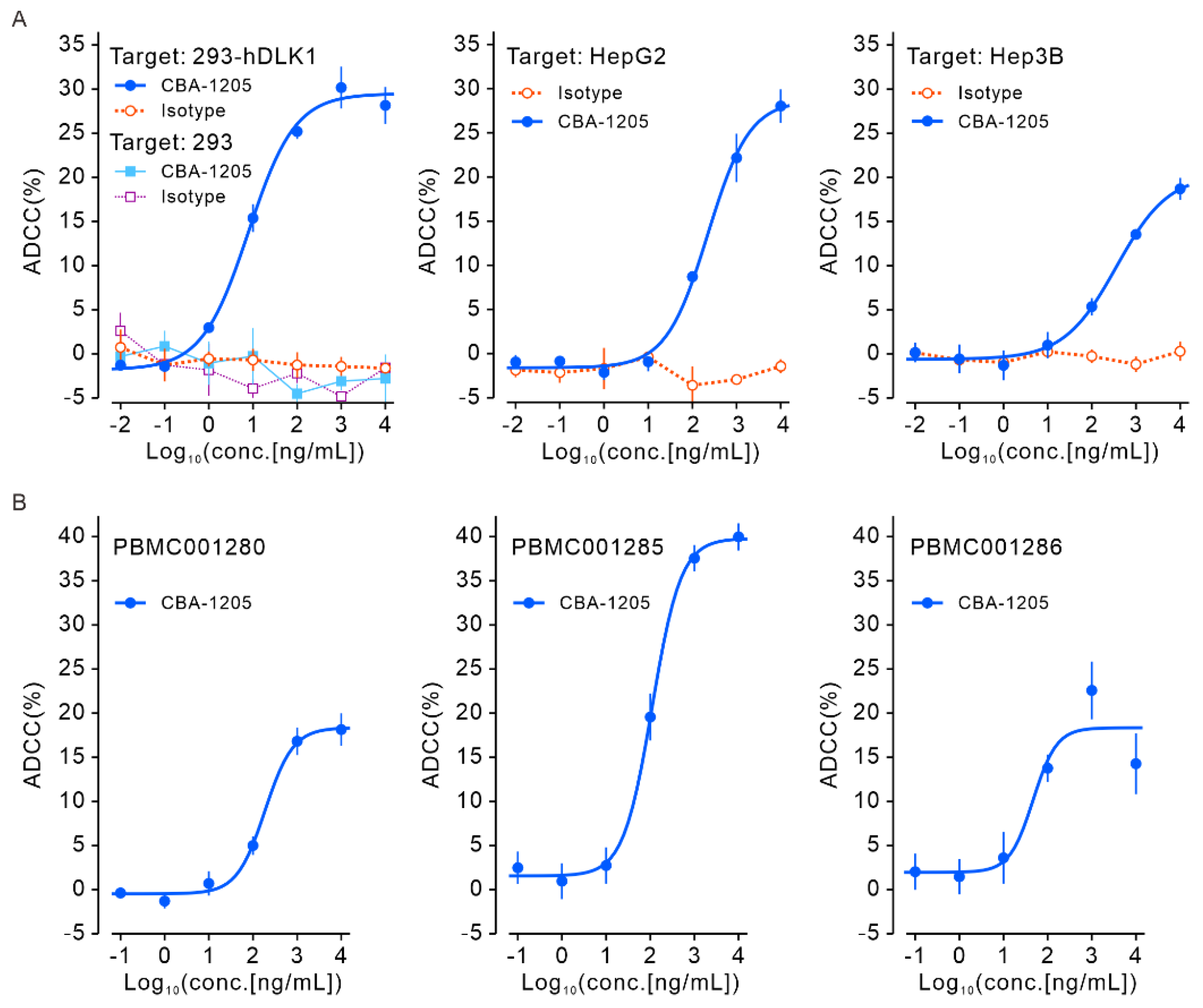
Figure 4.
ADCC activity of CBA-1205 in vitro. (A) ADCC assays with the indicated cell lines as target cells and FcγRIIIA-expressing NK cells as effector cells; the E:T ratio was 40:1. Each point is mean ± SD (n = 3). (B) ADCC assays with HepG2 and three different lots of human peripheral blood mononuclear cells (PBMCs) as effector cells; the E:T ratio was 20:1. Each point is mean ± SD (n = 3). 293-hDLK1, a stable cell line established by transfection of 293 cells with a human DLK1 expression vector; ADCC, antibody-dependent cell-mediated cytotoxicity.
Figure 4.
ADCC activity of CBA-1205 in vitro. (A) ADCC assays with the indicated cell lines as target cells and FcγRIIIA-expressing NK cells as effector cells; the E:T ratio was 40:1. Each point is mean ± SD (n = 3). (B) ADCC assays with HepG2 and three different lots of human peripheral blood mononuclear cells (PBMCs) as effector cells; the E:T ratio was 20:1. Each point is mean ± SD (n = 3). 293-hDLK1, a stable cell line established by transfection of 293 cells with a human DLK1 expression vector; ADCC, antibody-dependent cell-mediated cytotoxicity.
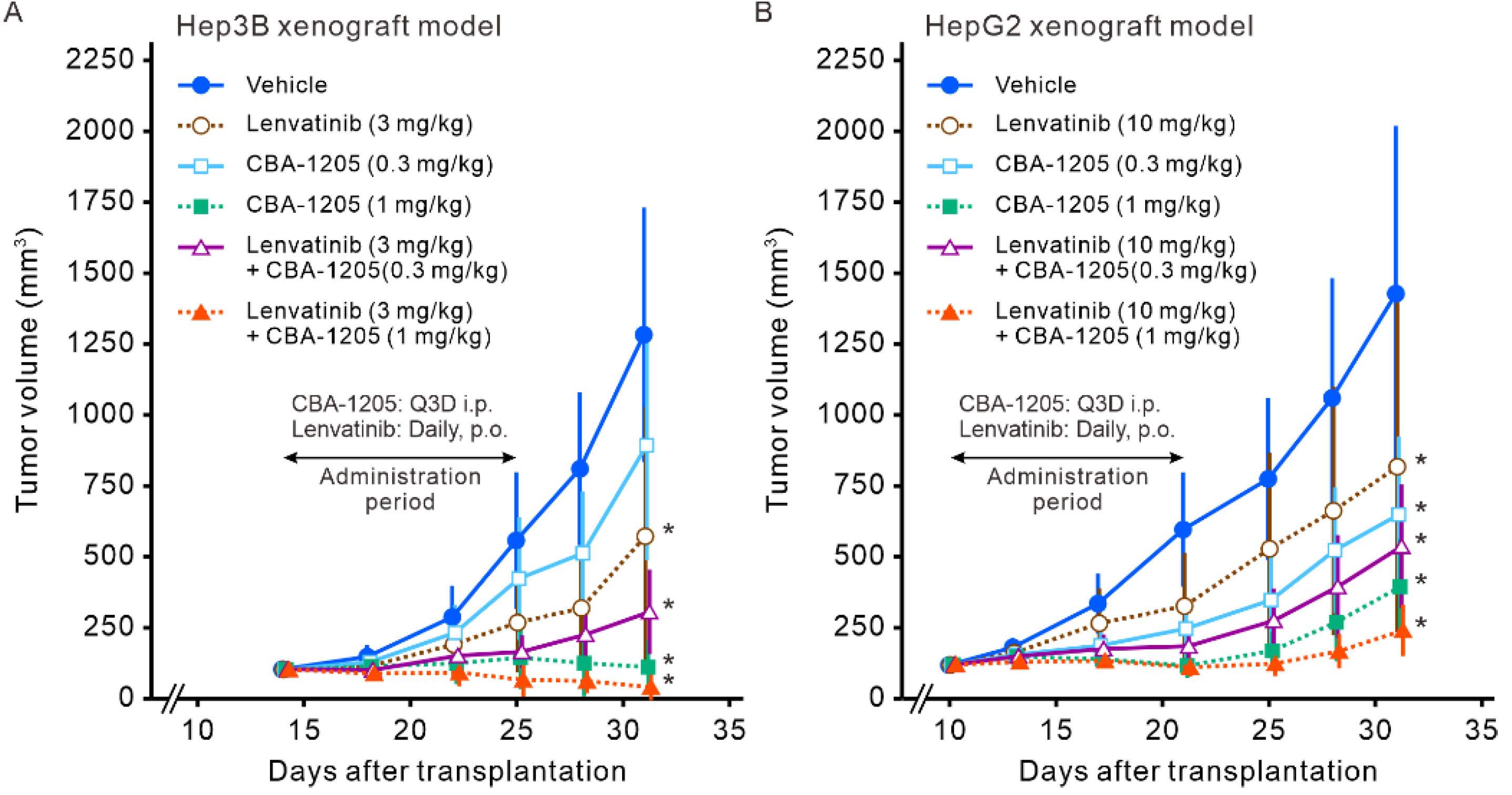
Figure 5.
Anti-tumor efficacy of CBA-1205 and lenvatinib in two different liver cancer cell xenograft mouse models. Human hepatocellular carcinoma cell lines (Hep3B, HepG2) were subcutaneously transplanted in the right flank of NOD-SCID mice. When the tumor volume reached > 100 mm3, mice were randomized to treatment groups (8 animals in each group). Lenvatinib was orally administered 5 days a week. CBA-1205 was intraperitoneally administered approximately every 3 days. (A) Hep3B model groups received vehicle (purified water) or lenvatinib alone; (B) HepG2 model groups received vehicle or lenvatinib alone; (C) Hep3B model groups received vehicle, isotype control IgG1/κ, or CBA-1205 alone on days 14, 17, 21, and 24; (D) HepG2 model groups received vehicle, isotype control IgG1/κ, or CBA-1205 alone on days 10, 14, 17, 21, and 24. Data are shown as mean ± SD Error bars indicate ± SD. * p < 0.05, by the two-tailed Dunnett’s multiple comparison test using the vehicle control group as the reference group.
Figure 5.
Anti-tumor efficacy of CBA-1205 and lenvatinib in two different liver cancer cell xenograft mouse models. Human hepatocellular carcinoma cell lines (Hep3B, HepG2) were subcutaneously transplanted in the right flank of NOD-SCID mice. When the tumor volume reached > 100 mm3, mice were randomized to treatment groups (8 animals in each group). Lenvatinib was orally administered 5 days a week. CBA-1205 was intraperitoneally administered approximately every 3 days. (A) Hep3B model groups received vehicle (purified water) or lenvatinib alone; (B) HepG2 model groups received vehicle or lenvatinib alone; (C) Hep3B model groups received vehicle, isotype control IgG1/κ, or CBA-1205 alone on days 14, 17, 21, and 24; (D) HepG2 model groups received vehicle, isotype control IgG1/κ, or CBA-1205 alone on days 10, 14, 17, 21, and 24. Data are shown as mean ± SD Error bars indicate ± SD. * p < 0.05, by the two-tailed Dunnett’s multiple comparison test using the vehicle control group as the reference group.
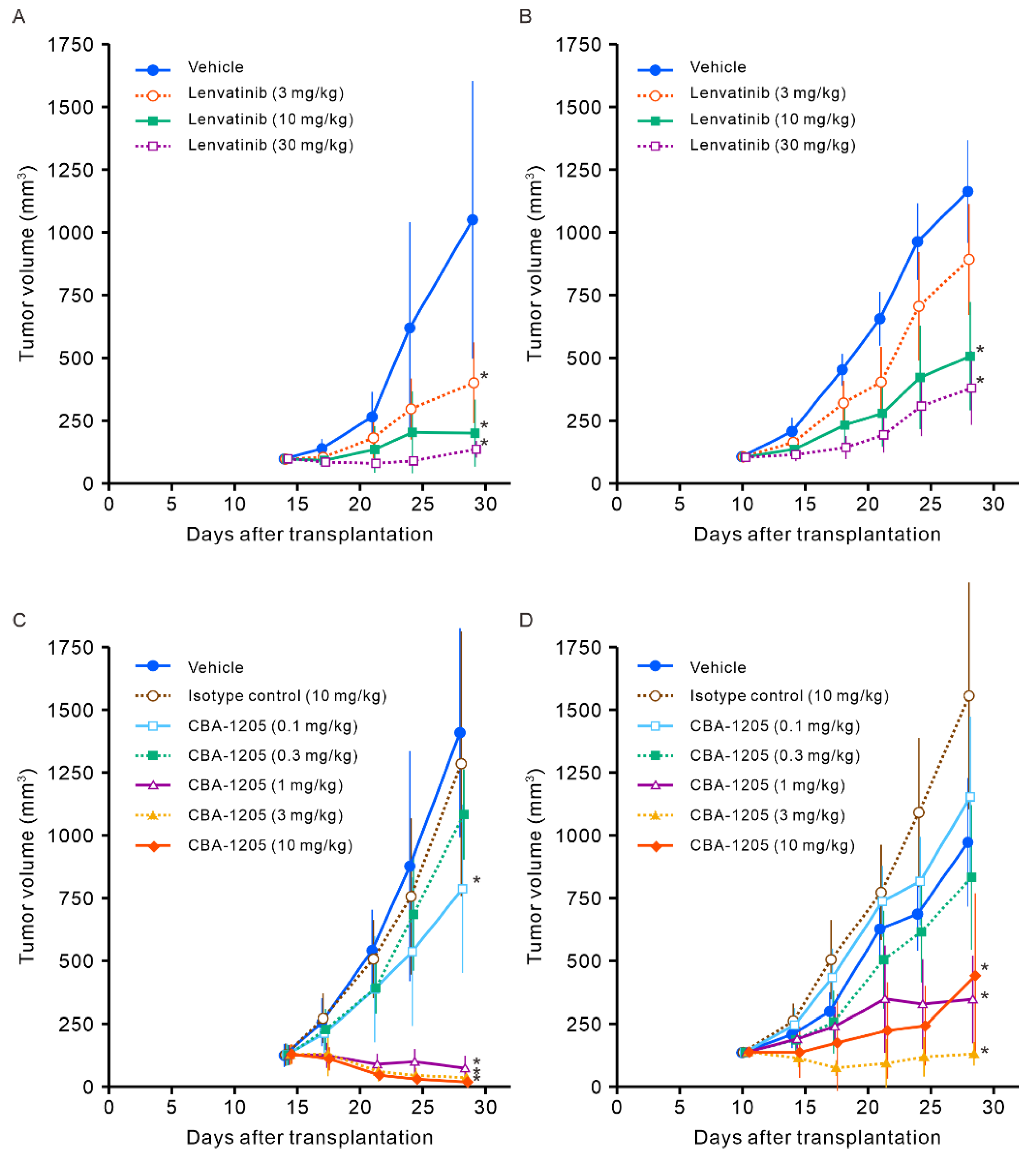
Figure 6.
Combined effect of CBA-1205 and lenvatinib in liver cancer cell xenograft mouse models. (A, B) Human hepatocellular carcinoma cell lines (Hep3B, HepG2) were subcutaneously transplanted into the right flank of NOD-SCID mice. When the tumor volume reached > 100 mm3, mice were randomized into dosing groups. CBA-1205 (0.3 and 1 mg/kg, twice a week, a total of four times) was administered intraperitoneally in combination with or without 3 mg/kg (for the Hep3B model) or 10 mg/kg (for the HepG2 model) of lenvatinib (daily, a total of 10 times) in mice (8 animals in each group). Vehicle was purified water for lenvatinib and phosphate buffered saline for CBA-1205. Data are shown as mean ± standard deviation (SD). Error bars indicate ± SD. * p < 0.05 by the two-tailed Dunnett’s multiple comparison test using the vehicle control group as the reference group.
Figure 6.
Combined effect of CBA-1205 and lenvatinib in liver cancer cell xenograft mouse models. (A, B) Human hepatocellular carcinoma cell lines (Hep3B, HepG2) were subcutaneously transplanted into the right flank of NOD-SCID mice. When the tumor volume reached > 100 mm3, mice were randomized into dosing groups. CBA-1205 (0.3 and 1 mg/kg, twice a week, a total of four times) was administered intraperitoneally in combination with or without 3 mg/kg (for the Hep3B model) or 10 mg/kg (for the HepG2 model) of lenvatinib (daily, a total of 10 times) in mice (8 animals in each group). Vehicle was purified water for lenvatinib and phosphate buffered saline for CBA-1205. Data are shown as mean ± standard deviation (SD). Error bars indicate ± SD. * p < 0.05 by the two-tailed Dunnett’s multiple comparison test using the vehicle control group as the reference group.
Table 1.
Toxicokinetic parameters of CBA-1205 in cynomolgus monkeys.
Table 1.
Toxicokinetic parameters of CBA-1205 in cynomolgus monkeys.
| |
|
|
Day 1
Mean ± SD |
Day 22
Mean ± SD |
Dose
(mg/kg) |
Sex |
n |
Cmax
(µg/mL) |
t1/2
(h) |
AUC0-168h
(µg·h/mL) |
Cmax
(µg/mL) |
t1/2
(h) |
AUC0-168h
(µg·h/mL) |
| 10 |
Male |
4 |
235
± 17 |
115
± 8 |
19,200
± 2,500 |
387
± 64 |
174
± 29 |
39,900
± 9,000 |
| |
Female |
4 |
222
± 8 |
143
± 29 |
17,400± 1,700 |
360
± 27 |
195
± 59 |
37,200
± 4,800 |
| 30 |
Male |
4 |
749
± 125 |
109
± 25 |
56,100
± 7,300 |
1,320
± 70 |
92.6
± 13.0 |
106,000
± 8,000 |
| |
Female |
4 |
702
± 114 |
100
± 10 |
56,200
± 10,700 |
1,330
± 210 |
92.9
± 17.7 |
112,000
± 20,000 |
| 100 |
Male |
6 |
2,370
± 430 |
72.9
± 11.9 |
169,000
± 28,000 |
4,480
± 740 |
88.3
± 18.1 |
325,000
± 58,000 |
| |
Female |
6 |
2,360
± 370 |
82.9
± 11.2 |
172,000
± 15,000 |
4,320
± 280 |
81.6
± 14.3 |
338,000
± 38,000 |