Submitted:
05 November 2024
Posted:
07 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
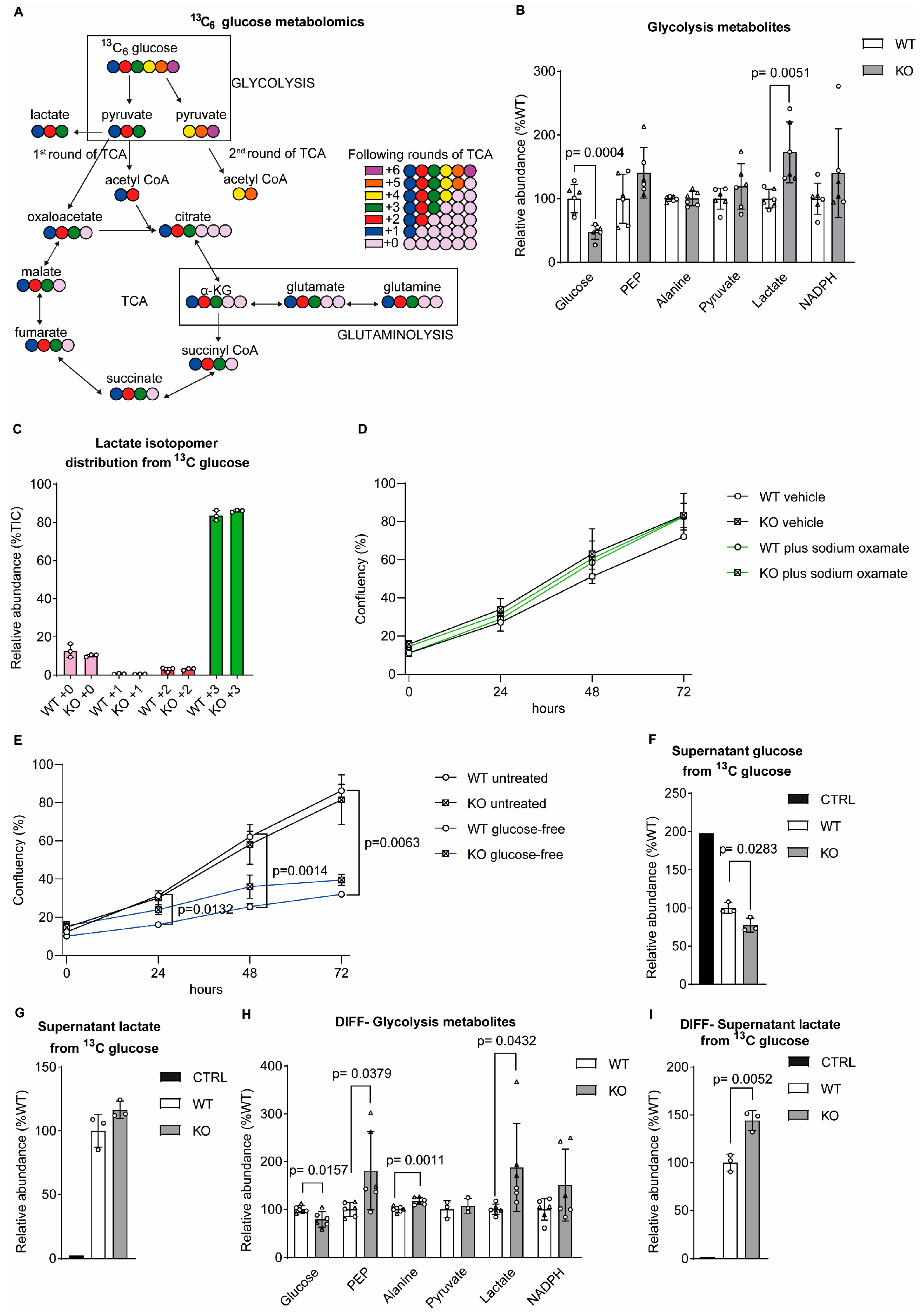
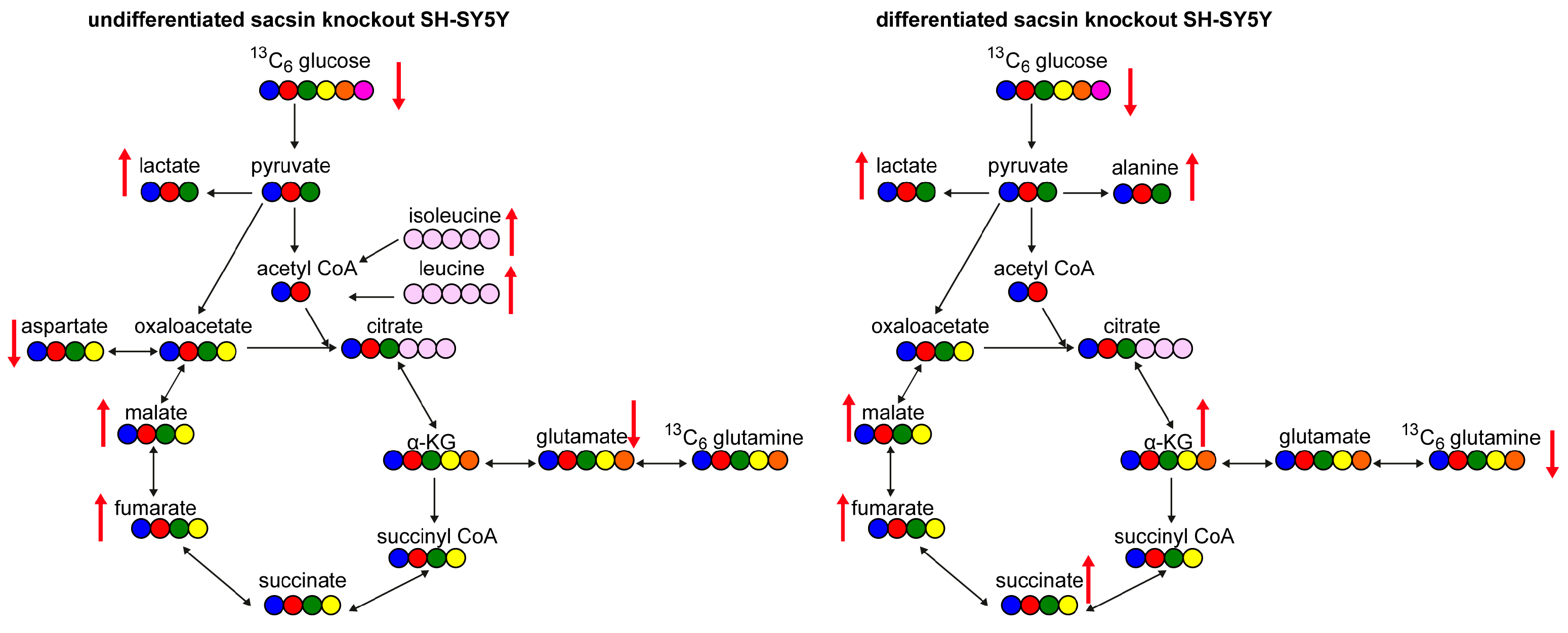
2.1. Increased Aerobic Glycolysis in Sacsin Knockout Cells
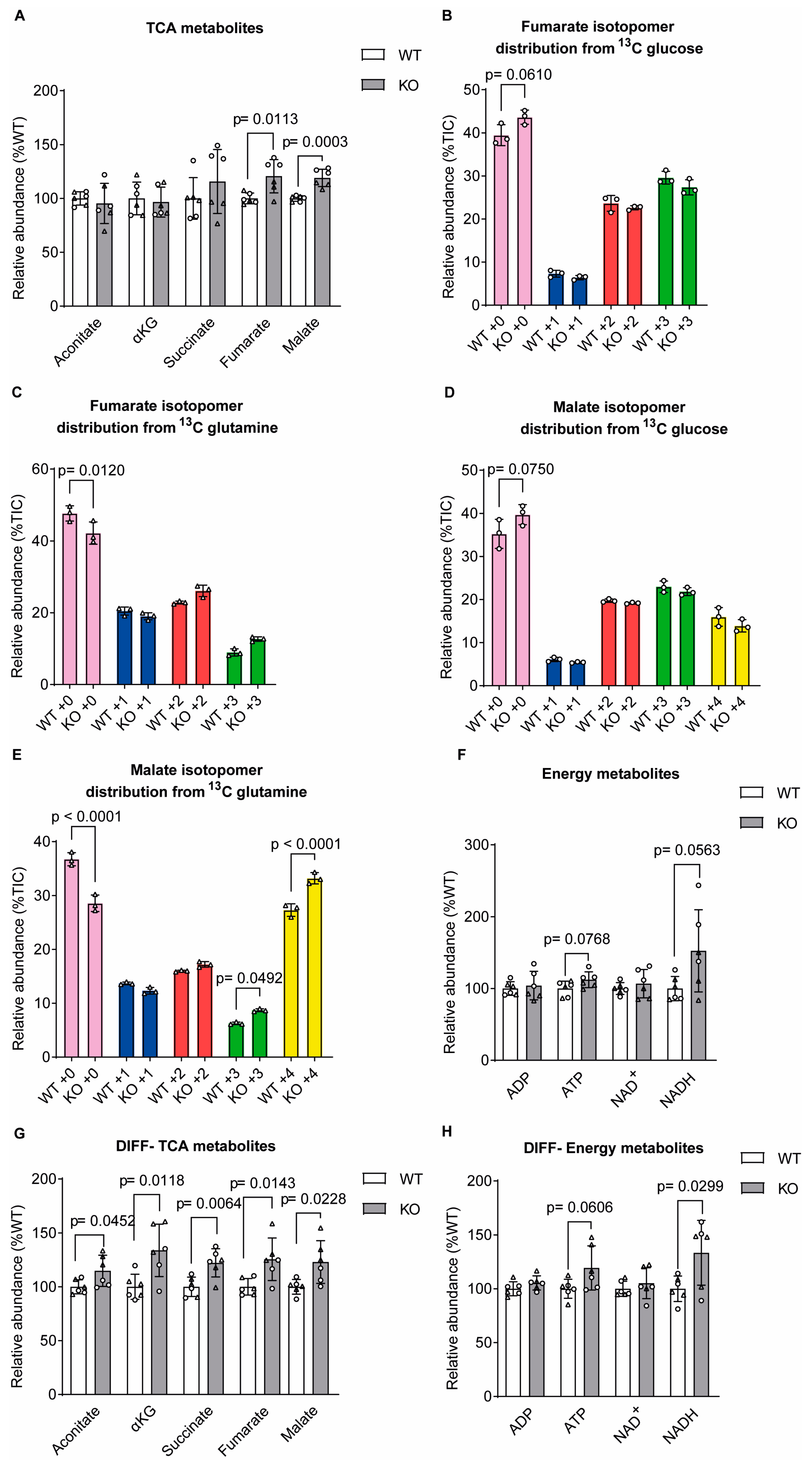
2.2. Alteration in Levels of TCA Cycle Metabolites in Sacsin Knockout Cells
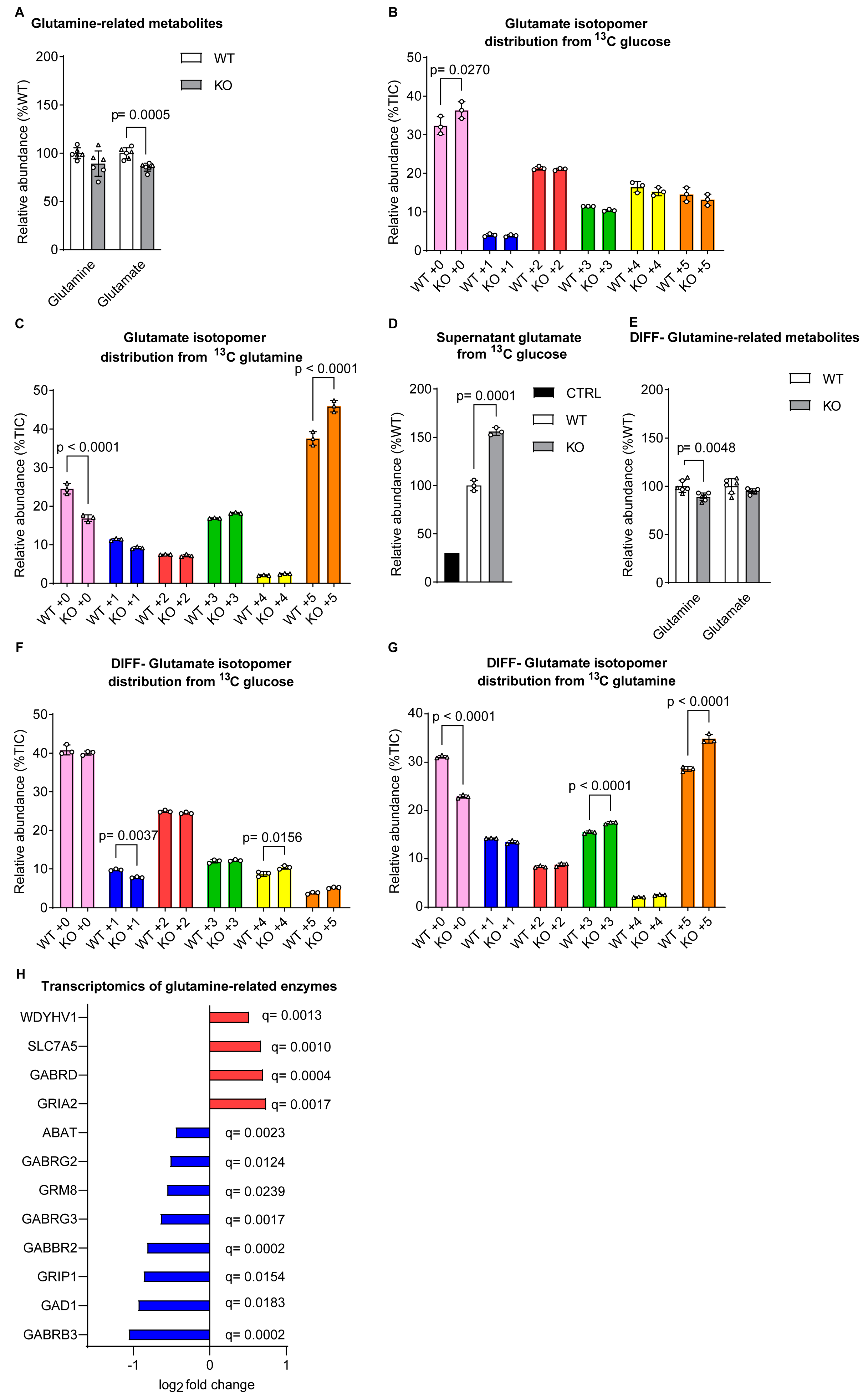
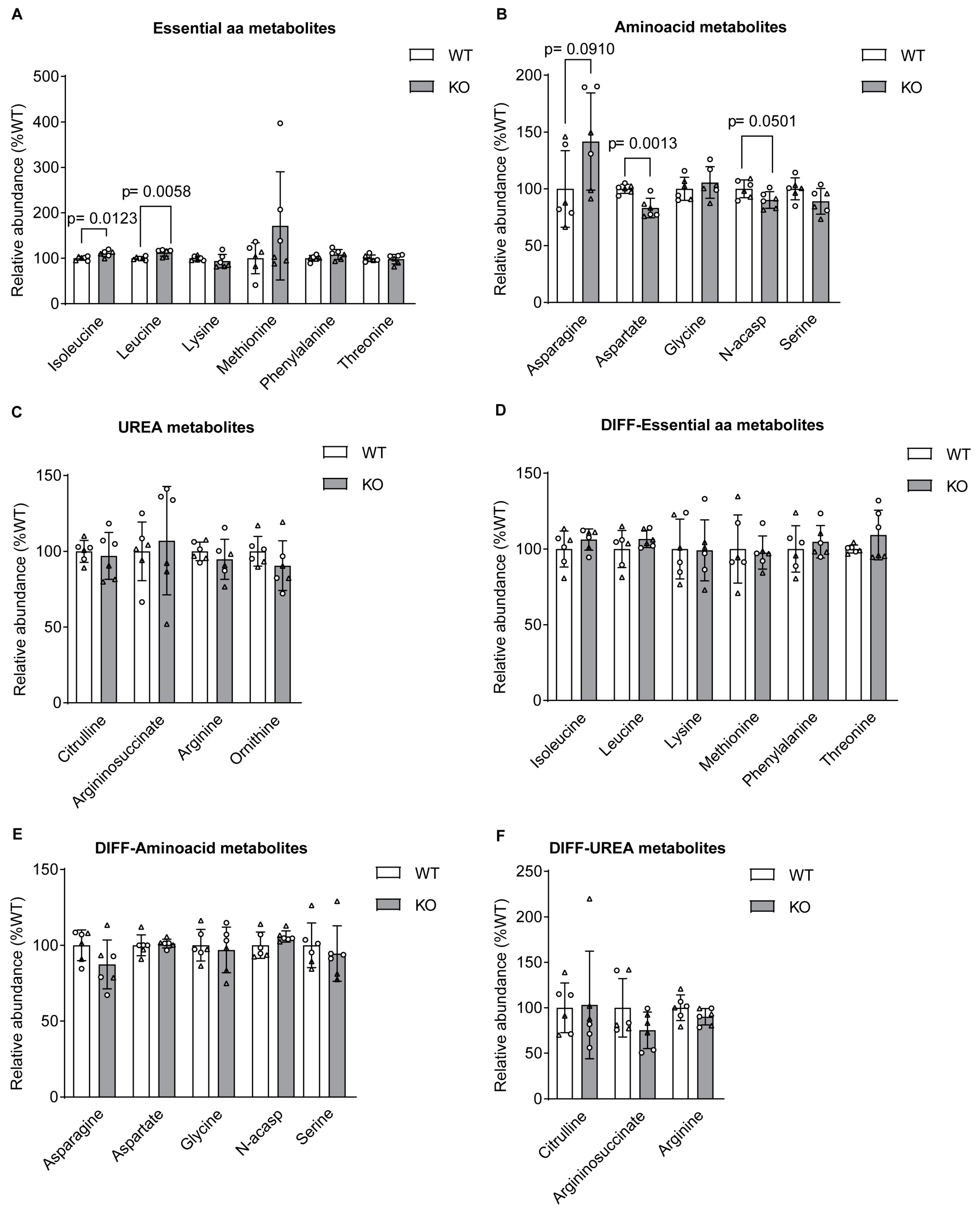
2.3. Disruption of Glutamine-Related Pathway in Sacsin Knockout Cells
3. Discussion
4. Materials and Methods
4.1. SH-SY5Y Cell Lines, Culture, and Differentiation
4.2. Metabolite Extraction
4.3. Confluency Assay
4.4. Immunoblot
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Engert, J.C., Bérubé, P., Mercier, J., Doré, C., Lepage, P., Ge, B., Bouchard, J.P., Mathieu, J., Melançon, S.B., Schalling, M., Lander, E.S., Morgan, K., Hudson, T.J., Richter, A. (2000). ARSACS, a spastic ataxia common in northeastern Québec, is caused by mutations in a new gene encoding an 11.5-kb ORF. Nat Genet. Feb;24(2):120-5. [CrossRef] [PubMed]
- Parfitt, D. A., Michael, G. J., Vermeulen, E. G., Prodromou, N. V., Webb, T. R., Gallo, J. M., . . . Chapple, J. P. (2009). The ataxia protein sacsin is a functional co-chaperone that protects against polyglutamine-expanded ataxin-1. Hum Mol Genet, 18(9), 1556-1565. [CrossRef]
- Bradshaw, T. Y., Romano, L. E., Duncan, E. J., Nethisinghe, S., Abeti, R., Michael, G. J., . . . Chapple, J. P. (2016). A reduction in Drp1-mediated fission compromises mitochondrial health in autosomal recessive spastic ataxia of Charlevoix Saguenay. Hum Mol Genet, 25(15), 3232-3244. [CrossRef]
- Girard, M., Lariviere, R., Parfitt, D. A., Deane, E. C., Gaudet, R., Nossova, N., . . . McPherson, P. S. (2012). Mitochondrial dysfunction and Purkinje cell loss in autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS). Proc Natl Acad Sci U S A, 109(5), 1661-1666. [CrossRef]
- Morani, F., Doccini, S., Sirica, R., Paterno, M., Pezzini, F., Ricca, I., . . . Santorelli, F. M. (2019). Functional Transcriptome Analysis in ARSACS KO Cell Model Reveals a Role of Sacsin in Autophagy. Sci Rep, 9(1), 11878. [CrossRef]
- Vakifahmetoglu-Norberg, H., Ouchida, A. T., & Norberg, E. (2017). The role of mitochondria in metabolism and cell death. Biochem Biophys Res Commun, 482(3), 426-431. [CrossRef]
- Martinez-Reyes, I., & Chandel, N. S. (2020). Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun, 11(1), 102. [CrossRef]
- Romano, L. E. L., Aw, W. Y., Hixson, K. M., Novoselova, T. V., Havener, T. M., Howell, S., . . . Wolter, J. M. (2022). Multi-omic profiling reveals the ataxia protein sacsin is required for integrin trafficking and synaptic organization. Cell Rep, 41(5), 111580. [CrossRef]
- Schneider, L., Giordano, S., Zelickson, B.R., Johnson, M.S., Benavides, G.A., Ouyang, X., Fineberg, N., Darley-Usmar, V.M., Zhang, J. (2011). Differentiation of SH-SY5Y cells to a neuronal phenotype changes cellular bioenergetics and the response to oxidative stress. Free Radical Biology and Medicine, 51(11). [CrossRef]
- D’Aloia, A., Pastori, V., Blasa, S., Campioni, G., Peri, F., Sacco, E., Ceriani, M., Lecchi, M., Costa, B. (2024). A new advanced cellular model of functional cholinergic-like neurons developed by reprogramming the human SH-SY5Y neuroblastoma cell line. Cell Death Discov. 10, 24. [CrossRef]
- Bhutia, Y. D., & Ganapathy, V. (2016). Glutamine transporters in mammalian cells and their functions in physiology and cancer. Biochim Biophys Acta, 1863(10), 2531-2539. [CrossRef]
- Damiani, C., Gaglio, D., Sacco, E., Alberghina, L., Vanoni,M. (2020). Systems metabolomics: from metabolomic snapshots to design principles. Current Opinion in Biotechnology, 63, 190-199. [CrossRef]
- de la Cruz-Lopez, K. G., Castro-Munoz, L. J., Reyes-Hernandez, D. O., Garcia-Carranca, A., & Manzo-Merino, J. (2019). Lactate in the Regulation of Tumor Microenvironment and Therapeutic Approaches. Front Oncol, 9, 1143. [CrossRef]
- Golikov, M. V., Valuev-Elliston, V. T., Smirnova, O. A., & Ivanov, A. V. (2022). Physiological Media in Studies of Cell Metabolism. Mol Biol, 56(5), 629-637. [CrossRef]
- Li, X., Yang, Y., Zhang, B., Lin, X., Fu, X., An, Y., Zou, Y., Wang, J.X., Wang, Z., Yu, T. (2022). Lactate metabolism in human health and disease. Sig Transduct Target Ther 7, 305. [CrossRef]
- Corkey, B. E. & Deeney, J. T. (2020). The redox communication network as a regulator of metabolism. Front. Physiol. 11, 567796.
- Yang, S. & Lian, G. (2020). ROS and diseases: Role in metabolism and energy supply. Mol. Cell Biochem. 467, 1–12.
- Cleland, N. R. W., Al-Juboori, S. I., Dobrinskikh, E., & Bruce, K. D. (2021). Altered substrate metabolism in neurodegenerative disease: new insights from metabolic imaging. J Neuroinflammation, 18(1), 248. [CrossRef]
- Aldana, B. I. (2019). Microglia-Specific Metabolic Changes in Neurodegeneration. J Mol Biol, 431(9), 1830-1842. [CrossRef]
- Nilsen, L. H., Witter, M. P., & Sonnewald, U. (2014). Neuronal and astrocytic metabolism in a transgenic rat model of Alzheimer’s disease. J Cereb Blood Flow Metab, 34(5), 906-914. [CrossRef]
- Ryan, D. G., Yang, M., Prag, H. A., Blanco, G. R., Nikitopoulou, E., Segarra-Mondejar, M., . . . Frezza, C. (2021). Disruption of the TCA cycle reveals an ATF4-dependent integration of redox and amino acid metabolism. Elife, 10. [CrossRef]
- Larivière, R., Gaudet, R., Gentil, B.J., Girard, M., Conte, T.C., Minotti, S., Leclerc-Desaulniers, K., Gehring, K., McKinney, R.A., Shoubridge, E.A., McPherson, P.S., Durham, H.D., Brais, B. (2015). Sacs knockout mice present pathophysiological defects underlying autosomal recessive spastic ataxia of Charlevoix-Saguenay. Hum Mol Genet. Feb 1;24(3):727-39. [CrossRef]
- Slemmer JE, De Zeeuw CI, Weber JT. Don’t get too excited: mechanisms of glutamate-mediated Purkinje cell death. Prog Brain Res. 2005;148:367-90. [CrossRef]
- Naef, V., Marchese, M., Ogi, A., Fichi, G., Galatolo, D., Licitra, R., . . . Santorelli, F. M. (2021). Efficient Neuroprotective Rescue of Sacsin-Related Disease Phenotypes in Zebrafish. Int J Mol Sci, 22(16). [CrossRef]
- Bremova-Ertl T, Ramaswami U, Brands M, Foltan T, Gautschi M, Gissen P, Gowing F, Hahn A, Jones S, Kay R, Kolnikova M, Arash-Kaps L, Marquardt T, Mengel E, Park JH, Reichmannová S, Schneider SA, Sivananthan S, Walterfang M, Wibawa P, Strupp M, Martakis K. (2024). Trial of N-Acetyl-l-Leucine in Niemann-Pick Disease Type C. N Engl J Med. Feb 1;390(5):421-431. [CrossRef]
- Martakis K, Claassen J, Gascon-Bayari J, Goldschagg N, Hahn A, Hassan A, Hennig A, Jones S, Kay R, Lau H, Perlman S, Sharma R, Schneider S, Bremova-Ertl T. Efficacy and Safety of N-Acetyl-l-Leucine in Children and Adults With GM2 Gangliosidoses. Neurology. 2023 Mar 7;100(10):e1072-e1083. [CrossRef]
- Feil K, Adrion C, Boesch S, Doss S, Giordano I, Hengel H, Jacobi H, Klockgether T, Klopstock T, Nachbauer W, Schöls L, Steiner KM, Stendel C, Timmann D, Naumann I, Mansmann U, Strupp M; ALCAT Study Group. Safety and Efficacy of Acetyl-DL-Leucine in Certain Types of Cerebellar Ataxia: The ALCAT Randomized Clinical Crossover Trial. JAMA Netw Open. 2021 Dec 1;4(12):e2135841. [CrossRef]
- Muddapu, V. R., Dharshini, S. A. P., Chakravarthy, V. S., & Gromiha, M. M. (2020). Neurodegenerative Diseases - Is Metabolic Deficiency the Root Cause? Front Neurosci, 14, 213. [CrossRef]
- Wang, X., Pal, R., Chen, X. W., Limpeanchob, N., Kumar, K. N., & Michaelis, E. K. (2005). High intrinsic oxidative stress may underlie selective vulnerability of the hippocampal CA1 region. Brain Res Mol Brain Res, 140(1-2), 120-126. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




