1. Introduction
Sewage and wastewater produced by population increase and industrialization has been treated with advance in technology which mainly has been relied upon microbial activated sludge process though precise operation was differently developed [
1]. Nutrient removal, started from advanced treatment, is nowadays considered into basic process for a design of plants [2-3]. Among the nutrients, nitrogen is essential target of removal because of agricultural importance in over-nutrition of soil and ecosystem conservation in terms of eutrophication [
4]. Increased nitrogen load on water and soil resulted from less-treated wastewater and sludge also has been negative impact with the viewpoint of mitigation in climate change and attainment in the carbon-neutral [5-7].
Techniques to remove nitrogen in the wastewater include physical adsorption, membrane filtration, chemical precipitation, and biological treatment [8-10]. Among the techniques, biological treatment has been widely adopted for its advantage that there needs no or less chemicals and facilities and that it based upon ecological principle with an application of existing activated sludge process [11-12]. One of the simplest biological treatment process for nitrogen removal is modified Ludzack-Ettinger (MLE) process but it shows relatively low efficiency of nitrogen removal than University of Cape Town (UCT) process or Virginia Initiative Plant (VIP) process [13-15]. High concentrations of microorganism to overcome low removal efficiency and to reduce sludge production could be reached by the addition of media for attachment of microorganism [16-17]. Materials frequently used for the immobilization were high molecular weight compound either of natural origin or synthetically manufactured product, such as natural alginate, collagen, and gelatin, or synthetic polyvinyl alcohol (PVA), polyacrylamide, and polyethylene glycol (PEG) [18-20].
Study on the nitrogen removal microorganisms has been mainly focused on bacteria divided groups of ammonia oxidizer (AOB), nitite oxidizer (NOB), and denitrifier [21-22]. Simultaneous ammonia and nitrite oxidizer and archaea (AOA and NOA) have been also discovered and studied, which made the understanding of nitrogen metabolism broad and wide [23-24]. Rapid development in the field of molecular biological techniques with bioinformatics made it possible [25-28].
Most of researches about nitrogen removal microorganisms in wastewater treatment process has been done on the bacteria with suspended growth because of their advantage in sample preparation step with easy. As processes with attached growing bacteria increase, however, the limited results from suspended growth could not resolve the true nature of microbial community involved in the nitrogen metabolism[29-30]. In this study, MLE process with the use of media and immobilized microorganism was operated and phylogentic analysis of microorganism was performed in terms of nitrogen removal with diversity in reactors.
2. Materials and Methods
2.1. Reactor Design and Immobilization of Microorganisms
A facility of modified Ludzack-Ettinger process was designed and constructed in laboratory-scales. Sewage passed through screen in J Wastewater Treatment Plant (159,000 ㎥/d of treatment capacity; located in Seoul Metropolitan City, Republic of Korea) was introduced anoxic reactor (6 L) followed by aerobic reactor (10 L). Rate of internal return from aerobic reactor to anoxic reactor was the double of inflow rate (2Q) and hydraulic retention time (HRT) was 6 hours for each of the reactors. Glucose as an additional carbon source for the adjustment of C/N ratio equals to 4.5 was added continuously after 25 days of operation. The total operation was carried out by 50 days. Mean concentrations of carbon (as Total Organic Carbon) and nitrogen (as Total Kjeldahl Nitrogen) were 15.7 mg/L and 31.2 mg/L, respectively (
Table 1).
Media for immobilization of microorganisms were constructed with polyvinyl alcohol (PVA) and polyethylene glycol (PEG). Centrifuged activated sludge from J Wastewater Treatment Plant was mixed (20%, v/v) with PVA and PEG, and activated media after formation, cross-linking, and stabilization was introduced to anoxic and aerobic reactors (
Table 2). Immobilized media was suspended in each reactor during operation with the concentrations of mixed liquor suspended solids (MLSS) at 50,000 mg/L for aerobic reactor and 40,000 mg/L for anoxic, and concentrations of mixed liquor volatile suspended solids (MLVSS) were 4,300 mg/L and 5,800 mg/L for aerobic and anoxic reactor, respectively.
2.2. Microbiological Community Analysis
Genomic DNA was extracted with PowerSoil DNA Isolation Kit (Mo Bio Laboratories, Carlsbad, CA) after biomass was detached from media. Quantification of DNA with PicoGreen (Invitrogen, Corvallis, OR) fluorescent dye and measurement of size with Agilient 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) was done with quality control procedures. Microbial community analysis by Next Generation Sequencing (NGS) was performed with Illumina 16S metagenomics sequencing library protocol under MiSeq platform (Illumina, San Diego, CA). The target region of 16S rRNA gene was V3-V4, and PCR protocols with primers used were the same with a literature [
31]. Total number of base pairs sequenced were 160 Mbp and 119 Mbp from aerobic and anoxic reactors, respectively. After paired-end sequencing, 531,188 bp from aerobic reactors and 396,844 bp from anoxic reactor was confirmed. Confirmed base pairs were assembled after MiSeq platform produced pieces of reads and sequencing bias from reads were removed through pre-processing. Finally sequences without error were clustered and used for the production of Operational Taxonomic Units (OTUs) based on sequence similarities. Total numbers of OTUs were 594 from aerobic reactor and 375 from anoxic. Identification of each OUT was carried out under phylum- and genus-level. Comparison of community diversity between aerobic and anoxic reactors were performed by algorithms provided by QIIME 2 [
32].
3. Results and Discussion
3.1. Nitrogen Removal Efficiency of MLE Process
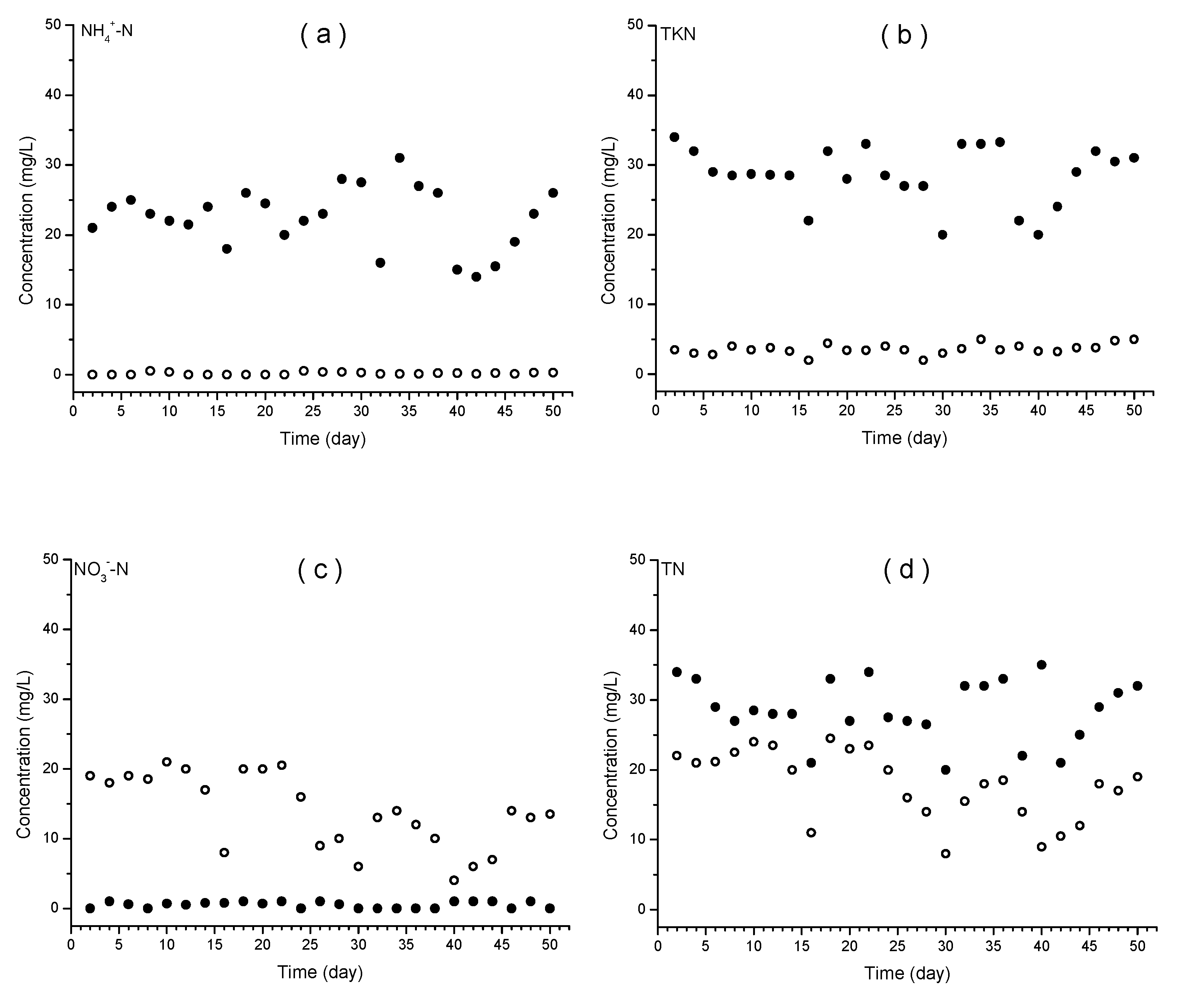
Concentrations of nitrogen components during the operation period were varied as
Figure 1. Most of ammonium in influent was oxidized and showed 0.21 mg/L of mean concentration in the effluent, which is 1% of the influent concentration. High oxidation rate of ammonia seemed to be due to high concentration of mixed liquor suspended solid, mainly microorganisms attached to media. Meanwhile concentrations of total Kjeldahl nitrogen, the sum of inorganic and organic ammonium component, showed relatively higher values than those of inorganic ammonium. Mean TKN concentration in effluent during the first 25 days when there was no adjustment of C/N ratio was 3.48 mg/L which was lower than 7.56 mg/L when C/N ratio was adjusted to 4.5. It was assumed that organic ammonium resulted from biomass of attached microorganism was rapidly produced while inorganic ammonium oxidation proceeded, so ammonium dissimilation seemed to be rate-limiting step in nitrification. In addition, the higher C/N ratio stimulated growth of media-attached microorganisms, mostly nitrifying bacteria [
33], so more rapid growth affected higher concentration of TNK in effluent.
Nitrate, oxidized product of ammonium, were increased in the aerobic reactor with the mean concentration of 18.45 mg/L during the first 25 days and decreased to 10.25 mg/L after C/N ratio adjustment. The different nitrate concentrations between with and without C/N ratio adjustment showed that denitrification activity in anoxic reactor was enhanced by increase of C/N ratio. Comparison of total nitrogen concentration between the two periods (21.9 mg/L without C/N adjustment vs. 17.8 mg/L with adjustment) supported these explanation. The mass balance of nitrogen was as follows; 24.8 mg/L of inorganic ammonium and 6.4 mg/L of organic ammonium was introduced into the reactors with 0.2 mg/L of nitrate, more than 48% of organic ammonium (with no consideration of increase in biomass) was converted into inorganic ammonium and 63% of them was oxidized to nitrate. Afterwards 30% of them, more than half of oxidized nitrate, was denitrified during first 25 days (without C/N ratio adjustment). On the other hand, after running on C/N ratio adjustment, more than 43% of nitrate was denitrified with no consideration of newly developed biomass, which showed that addition of glucose as a raising method of C/N ratio stimulated microorganism growth and activities both of nitrification and denitrification. The precise amount of biomass increase and conversion of organic to inorganic ammonium and thereafter oxidation to nitrate followed by denitrification needed to be analyzed. In this study, focus was made on the microorganism community.
The removal efficiency of nitrogen based on total nitrogen effluent to influent was calculated by 25.5% during first 25 days when there was no C/N ratio adjustment, and 48.2% after adjustment. MLE process relies on the principle that nitrogen in wastewater with oxidation state of -3 (organic and inorganic ammonium) is firstly oxidized to +5 of nitrate in the aerobic reactor, then it is returned to anoxic reactor expecting denitrifier to reduce nitrate into molecular nitrogen of 0 oxidation state [
14]. Electron donors required to reduce nitrate should be provided with influent, however simultaneous mixing of influent with internally recycled wastewater disturbs microbial metabolism both of nitrifier and denitrifier. In spite of this drawback of not-high efficiency of nitrogen removal, simple and economic aspects have been made the MLE process used widely spread [
15]. Addition of extraneous carbon source and control of internal cycling ratio can enhance efficiencies both of carbon and nitrogen removal [
13]. We could obtain 47% of increased nitrogen removal efficiency with the addition of glucose within the limit of MLE process. High rate of inorganic ammonium oxidation was another characteristics of the study. It was possible to obtain high rate of ammonium oxidation and medium rate of denitrification with the maintenance of high microorganism concentrations through media.
3.2. Microbial Communities in the Immobilized Media
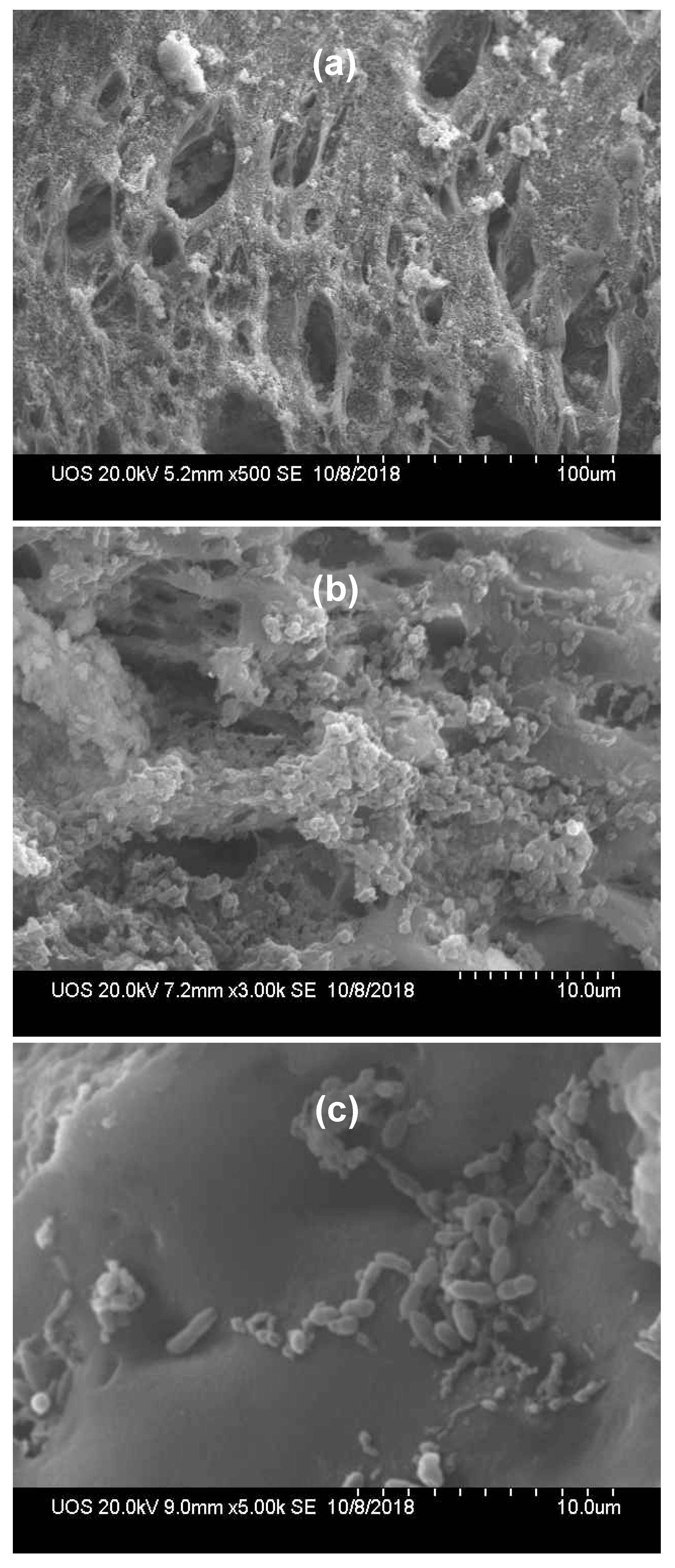
Analysis of media surface whether microbial immobilization was stable could be visualized by Scanning electron microscope (
Figure 2). Large numbers of holes in media were observed (
Figure 2a). The internal hoe size was measured from 6 ㎛ to 15 ㎛. It was announced that dominant growth of bacteria was easier than that of protozoa or metazoans in the pore size less than 15 ㎛ [
33]. Highly distributed small pore made it possible to transfer oxygen and substrates hence to stimulate growth of microorganisms [
34]. More magnified images showed growth of coccus (
Figure 1b) and rods (
Figure 1c), which supported media constructed with polyvinyl alcohol and polyethylene glycol could successfully enhance immobilization and help regional colonization of various microorganism. The metabolic activities for each microorganism visualized only could be confirmed by cultivation but it is impossible because each microorganism has different requirement in nutritional component or growth condition for isolation and cultivation. Molecular approach is the most available method to identify lots of microbial community, rapidly and simultaneously [
35]. Immobilized media was taken from each reactor and DNA extraction was done followed by NGS analysis to compare microbial community composition.
3.3. Microbial Community Analysis Using NGS
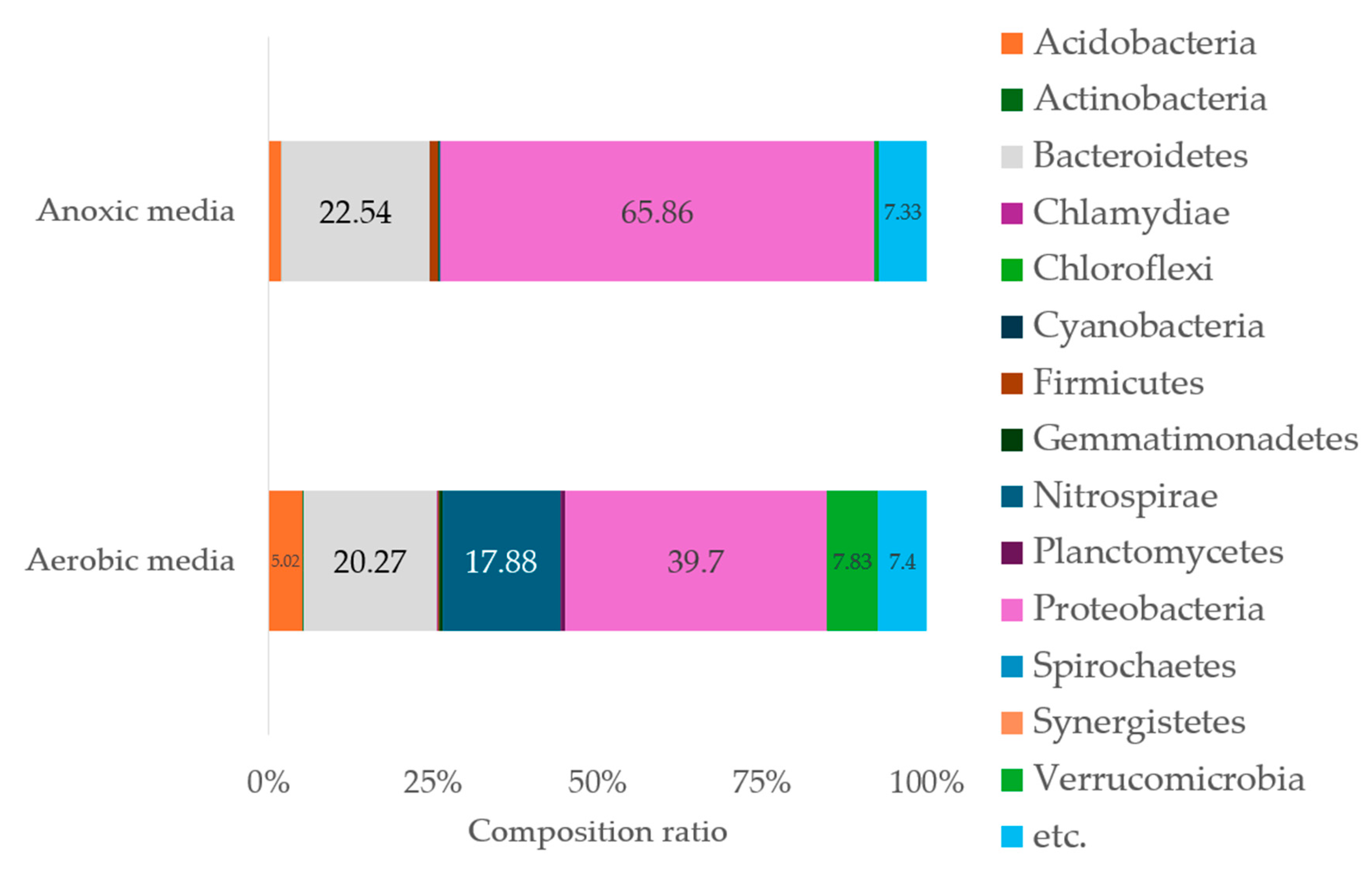
Fully sequenced and aligned taxonomic units (OTUs) by NGS were firstly classified in phylum level (
Figure 3). Proteobacteria was the dominant phylum both in aerobic and anoxic media, and the ratio was 39.7% in aerobic media and 65.9% in anoxic media. Bacteroidetes was secondly largest phylum. Mostly different characteristics between the two reactors were Nitrospirae which had relatively larger percentages of 17.9% in aerobic media than that of 0.13% in anoxic. In addition, Verrucomicrobia showed more percentage of 7.8% in aerobic media than anoxic media of 0.63%. Though true nature of microbial metabolism is incomplete with only phylum-level classification, relative abundance of nitrifying group in aerobic media and of denitrifying group in anoxic could explain different colonization phenomena. Large group of the ammonia oxidizing bacteria (AOB) and nitrite oxidizing bacteria (NOB) are contained in either phylum of Proteobacteria or Nitrospirae, whereas large species of denitrifying bacteria are classified in Proteobacteria or Bacteroidetes [
36]. Phylum-level diversity was also higher in aerobic media microorganisms which showed heterotrophic bacteria also colonized in aerobic condition.
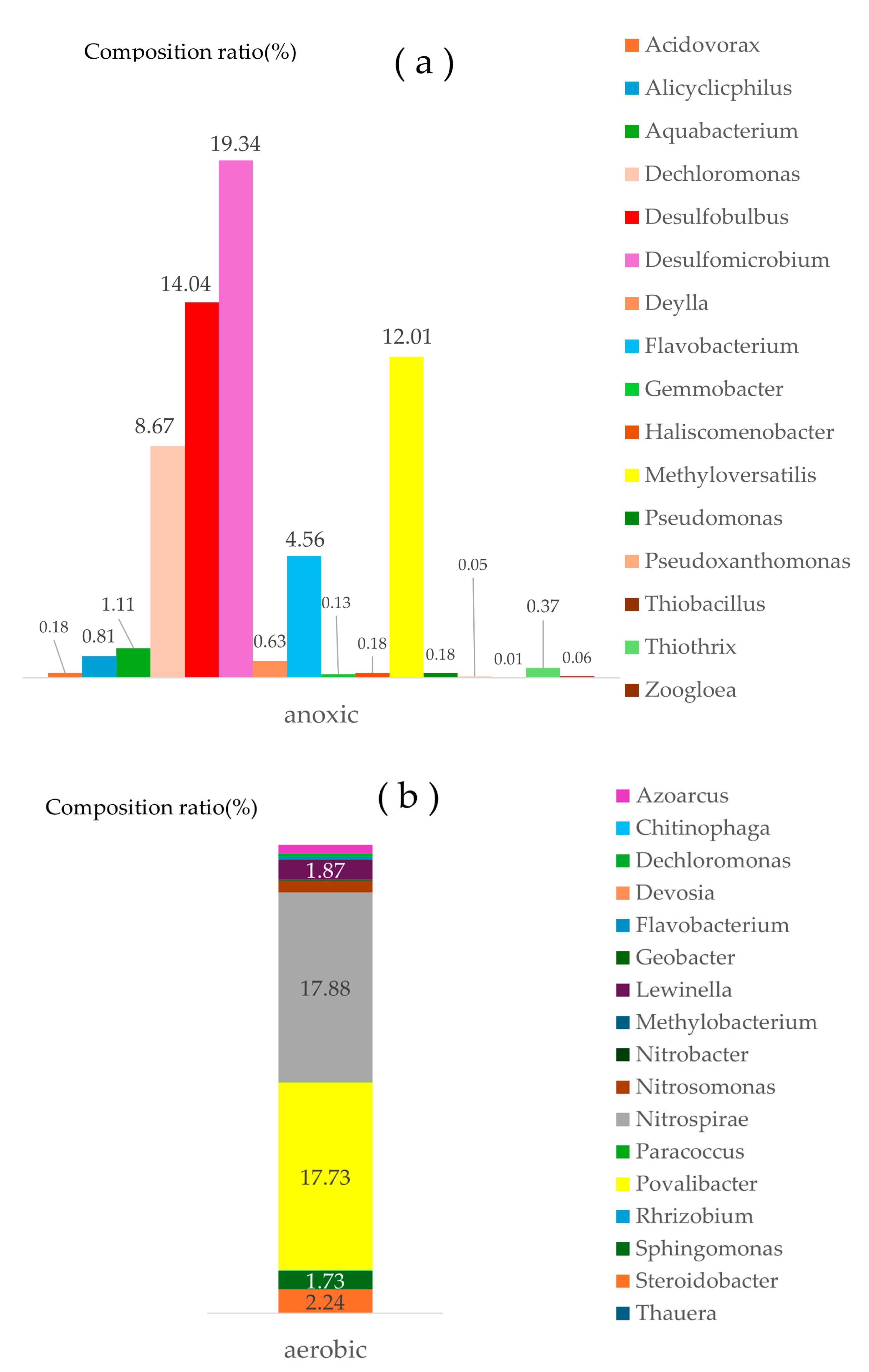
Genus-level classification could give more information to the community composition. Further comparison of microbial genera between aerobic and anoxic media was performed with same OTUs with phylum-level analysis (
Figure 4). The dominant genera in aerobic media were Nitrospira and Povalibacter (
Figure 4b). Nitrospira is one of the representative genera of nitrite oxidizing bacteria [
37], and Povalibacter is a heterotrophic bacteria of nitrogen oxidation in aerobic environment and was reported to involve in denitrification [
38]. Other nitrogen oxidizing microorganisms reported were also found in aerobic media as follows; Nitrosomonas is representative ammonia oxidizing bacteria [
39], Steroidobacter is nitrifying, denitrifying, and phosphorus-accumulating organism [
40], both Azoarcus and Geobacter are simultaneous nitrogen and phosphorus removal bacteria [
40], heterotrophic Lewinella and Chitinophaga can remove both COD and ammonium in 10 ℃ of low temperature [
41], Sphingomonas involves nitrification under low DO condition [
42], Flavobacterium announced as heterotrophic nitrifier [
43], Dechloromonas [
44], Paracoccus and Thauera [
45] have metabolic activities both of nitrification and denitrification, Rhizobium fix molecular nitrogen to organic ammonium [
46], and Methylobacterium also has similar metabolism with Rhizobium [
47]. The total percentage of these nitrification-related microorganisms was 45% though each contribution was small.
On the contrary, the dominant genera in anoxic media were Desulfomicrobium, Desulfobulbus, and Methyloversatilis in sequence of dominance. The firstly (19.3%) and secondly (14.0%) dominant genera reported widely as a sulfate reducer were also actor as a nitrate reducer when nitrate existed with sulfate in anaerobic condition because nitrate is better electron acceptor [
48]. Methyloversatilis, another dominant genus (12.0%), is also announced to involve in denitrification. Total genera related with denitrification, including Dechloromonas and Flavobacterium,[
49] amounted 63% of the total genera analyzed.
It was reported that the community composition and dominant microorganisms varied with growth status either attached or suspended, type of media if attached growth was prevalent, and characteristics of substrate. Hence new processes could be developed with use of these different and distinct microorganisms. Giraldo et al. [50-51] reported nitrification process with ammonium oxidizing archaea (AOA) in a MBR process. Study on an aerobic granular sludge (AGS) process showed that microorganisms growing in aggregated consortium structure had high resistance to toxic compound and stress on load [
52]. They also found genera of Leadbetterella, Thiotrhix, and Acetoanaerobium were dominant the structure. However there was little studies on the attached growth microorganisms, therefore this research on the immobilized aerobic and anoxic media would help future microbial studies.
3.4. Diversity of Microorganisms Immobilized to Media
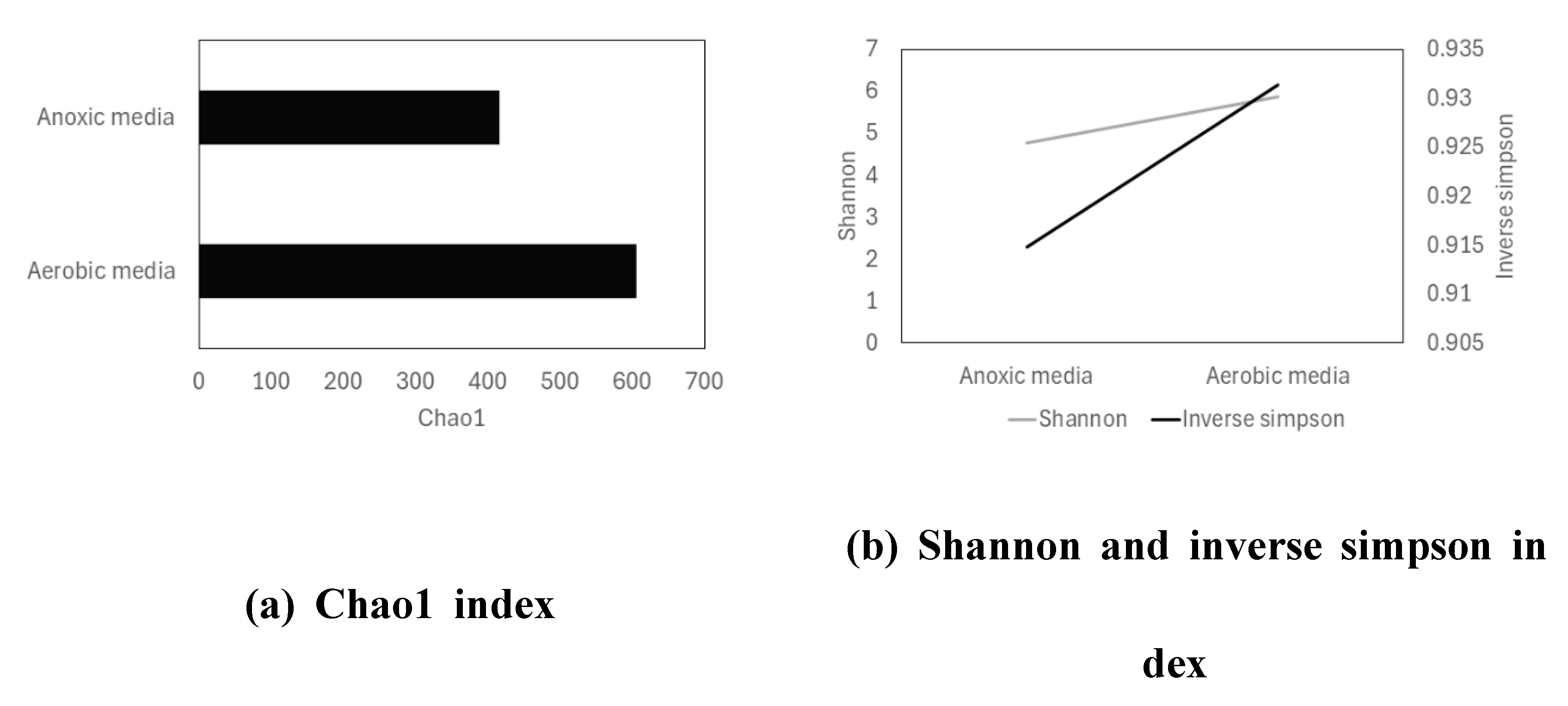
The QIIME 2 (Quantitative Insights Into Microbial Ecology) provided many open source software to analyze sequencing data including α-diversity. The analysis use various index for quantifying scores adequate to user’s purpose. Among the 18 measuring algorisms, pre-processing was performed to select best-fit measures by comparison of correlation and normality. After minimizing measures through EFA (Exploratory Factor Analysis) with z-score normalization, diversity index was calculated (
Figure 5). The chao1 index values of α-diversity were 606.05 and 415.53 for aerobic and anoxic media, respectively. These showed that total number of different microorganisms in aerobic media exceeded more than 30% in anoxic media [
53]. Other index, Shannon and Inverse Simpson, also showed higher diversity of microorganism in aerobic media than anoxic. Index imply that value of index number proportionally increase as increase in total number and distinctiveness of microorganisms for both index Shannon and Inverse Simpson; Shannon index was 5.87 in aerobic media, and 4.76 in anoxic media. Inverse Simpson index was 0.93 in aerobic and 0.91 in anoxic.
4. Conclusions
Modified Ludzack-Ettinger process with microorganisms immobilized on media were operated. Most of inorganic ammonium was oxidized to nitrate and denitrification efficiency was 48%. Addition of external glucose for adjustment of C/N ratio to 4.5 showed increased rate of nitrification and denitrification. Microbial communities were analyzed with NGS to compare microorganisms attached to aerobic and anoxic media. Phylum-level identification showed that Proteobacteria and Bacteroidetes were dominant both in aerobic and anoxic media. Nitrobacter and Povalibacter were dominant genera in aerobic media with the proportion of 17.9% and 17.7%, respectively. Both genera were known as nitrification. Other nitrogen oxidizing microorganisms including Nitrosomonas, Flavobacterium, and Nitrobacter contributed to 45% of total identified genera in aerobic media. Composition of Desulfomicrobium, Desulfobulbus, and Methyloversatilis in anoxic media was 19.3 %, 14.0 %, and 12.0 %, respectively. Other denitrification-related genera covered 63% of the total microorganisms in anoxic media. The enhanced efficiency of nitrogen removal related with increase of biomass in reactor primarily due to immobilization and most of increase proportion attributed to nitrifying microorganism in aerobic and denitrifying microorganisms in anoxic media. Diversity in microorganisms were higher in aerobic media than anoxic with the value of Chao1 of 606.05, Shannon index of 5.86, and Inverse Simpson of 0.93 in aerobic than anoxic media.
Author Contributions
Conceptualization, Dong-chul Shin; methodology, Dong-chul Shin; formal analysis, Chang-hoon Song; data curation, Chang-hoon Song; writing—original draft preparation, Chang-hoon Song; writing—review and editing, Dong-chul Shin; visualization, Chang-hoon Song; supervision, Myeong-woon Kim; funding acquisition, Myeong-woon Kim. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by DNA plus convergence technology graduate school promotion project granted funded by the Korea Ministry of Land, Infrastructure and Transport (RS-2023-00250434).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Van Loosdercht, M.C.M.; Bradjanovic, D. Anticipating the next century of wastewater treatment. Science 2014, 344, 1452-1453.
- Jetten, M.S.M.; Horn, S.J.; van Loosdrecht, M.C.M. Towards a more sustainable municipal wastewater treatment system. Water Science and Technology 1997, 35, 171-180.
- Oleszkiewicz, J.A.; Barnard, J.L. Nutrient removal technology in North America and the European Union: A review. Water Quality Research Journal of Canada 2006, 41, 449-462. [CrossRef]
- Daims; Holger; Sebastian Lücker; Michael Wagner. A New Perspective on Microbes Formerly Known as Nitrite-Oxidizing Bacteria. Trends in Microbiology 2016, 24, 699-712. [CrossRef]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; Karl, D.M.; Michaels, A.F.; Porter, J.H.; Townsend, A.R.; Vöosmarty, C.J. Nitrogen cycles: Past, present, and future. Biogeochemistry 2004, 70, 153-226. [CrossRef]
- Sutton, Mark A.; Oene Oenema; Jan William Erisman; Adrian Leip; Hans van Frinsven; Wilfried Winiwater. Too much of a good thing. Nature 2011, 472, 159-161.
- Wu, Heng; Anjie Li; Sicong Gao; Zhilin Xing; Piao Zhao. The performance, mechanism and greenhouse gas emission potential of nitrogen removal technology for low carbon source wastewater. Science of the total environment 2023, 903, 166491. [CrossRef]
- Prasse; Carsten; Daniel Stalter; Ulrike Schulte-Oehlmann; Jörg Oehlmann; Thomas A. Ternes. Spoilt for choice: A critical review on the chemical and biological assessment of current wastewater treatment technologies. Water Research 2015, 87, 237-270. [CrossRef]
- Omar A; Almomani F; Qiblawey H; Rasool K. Advances in Nitrogen-Rich Wastewater Treatment: A Comprehensive Review of Modern Technologies. Sustainability 2024, 16, 2112. [CrossRef]
- Kabuba, J; Joseph Lephallo; Hilary Rutto. Comparison of various technologies used to eliminate nitrogen from wastewater: A review. Journal of Water Process Engineering 2022, 48, 102885. [CrossRef]
- Hu; Kaiyao; Wenxuan Li; Yaning Wang; Bo Wang; Hao Mu; Shuang Ren; Kexin Zeng; Hongjuan Zhu; Jinming Liang; Ya’e Wang; Juqiang Xiao. Novel biological nitrogen removal process for the treatment of wastewater with low carbon to nitrogen ratio: A review. Journal of Water Process Engineering 2023, 53, 103673.
- Mishra; Saurabh; Virender Singh; Liu Cheng; Abid Hussain; Banu Ormeci. Nitrogen removal from wastewater: A comprehensive review of biological nitrogen removal processes, critical operation parameters and bioreactor design. Journal of Environmental Chemical Engineering 2022, 10, 107387. [CrossRef]
- Wang J-F; An Z-H; Zhang X-Y, Angelotti B; Brooks M; Wang Z-W. Effects of Nitrate Recycle on the Sludge Densification in Plug-Flow Bioreactors Fed with Real Domestic Wastewater. Processes 2023, 11, 1876. [CrossRef]
- Hollas, C.E.; Chini, A.; Antes, F.G.; do Pardo, N.V.; Bortoli, M.; Kunz, A. Modified Ludzack-Ettinger system role in efficient nitrogen removal from swine manure under high total suspended solids concentration. Int. J. Environ. Sci. Technol 2019, 16, 7715-7726. [CrossRef]
- Ghosh; Sudeshna; Timothy M LaPara. Removal of carbonaceous and nitrogenous pollutants from a synthetic wastewater using a membrane-coupled bioreactor. Journal of Industrial Microbiology and Biotechnology 2004, 31, 353-361. [CrossRef]
- Shin, Dong-chul; Su-chul Yoon; Chul-hwi Park. Biological Characteristics of microorganisms immobilization media for nitrogen removal. Journal of Water Process Engineering 2019, 32, 100979. [CrossRef]
- Gao; Yu; Liu Lou; Yun Liao; Hao Yao; Jun Fang; Gang Liu. Simultaneous nitrogen and phosphorus removal by immobilized bacterial particles of denitrifying phosphorus accumulating microorganisms and its application. Biochemical Engineering Journal 2024, 109495. [CrossRef]
- Jen, A.C.; Wake, M.C.; Mikos, A.G. Hydrogels for cell immobilization. Biotechnology and Bioengineering 1996, 50, 357-364.
- Chowdhury, M.M.I.; George Nakhla. Enhanced mainstream nitrogen removal from synthetic wastewater using gel-immobilized anammox in fluidized bed bioreactors: Process performance and disintegration mechanisms. Science of the Total Environment 2022, 811, 151373. [CrossRef]
- Min; Yitian; Liang Xu; Junfeng Su; Jiayao Ma; Amjad Ali; Xuan Li. Enhanced ammonia nitrogen and phenol removal by immobilized bacteria through composite mycelium pellet-driven quinone redox cycle. Journal of Environmental Management 2023, 345, 118893. [CrossRef]
- Kowalchuk, G.A.; Stephen, J.R. Ammonia-oxidizing bacteria: A model for molecular microbial ecology. Annual Review of Microbiology 2001, 55, 485-529. [CrossRef]
- Zhang; Dandan; Huang Yu; Xiaoli Yu; Yuchun Yang; Cheng Wang; Kun Wu; Mingyang Niu; Jianguo He; Zhili He; Qingyun Yan. Mechanisms underlying the interactions and adaptability of nitrogen removal microorganisms in freshwater sediments. Advanced Biotechnology 2024, 2.
- Yan; Yiming; Hongwei Lu; Jin Zhang; Shuguang Zhu; Yangqing Wang; Yu Lei; Rui Zhang; Liyan Song. Simultaneous heterotrophic nitrification and aerobic denitrification (SND) for nitrogen removal: A review and future perspectives. Environmental Advances 2022, 9, 100254. [CrossRef]
- James; Susan N.; Arya Vijayanandan. Recent advances in simultaneous nitrification and denitrification for nitrogen and micropollutant removal: a review. Biodegradation 2023, 34, 103-123. [CrossRef]
- Endrullar; Chrisop; Jörn Glölder; Philipp Franke; Marcus Frohme. Standardization and quality management in next-generation sequencing. Applied and Translational Genomics 2016, 10, 2-9. [CrossRef]
- Vincent; Antony T.; Nicolas Derome; Brian Boyle; Alexander I. Culley; Steve J. Charette. Next-generation sequencing (NGS) in the microbiological world: How to make the most of your money. Journal of Microbiological Methods 2017, 131, 60-71. [CrossRef]
- Haynes; Edward; Elisa Jimenez; Migual Angel Pardo; Sarah J. Helyar. The future of NGS (Next Generation Sequencing) analysis in testing food authenticity. Food Control 2019, 101, 134-143. [CrossRef]
- Yin; Yuxin; Carrie Butler; Qiuheng Zheng. Challenges in the application of NGS in the clinical laboratory. Human Immunology 2021, 82, 812-819. [CrossRef]
- Kim, Shin-seung. Prediction of Type 2 Diabetes Patients Using Alpha-diversity Score of gut microbiota. Master’s degree, Graduate School of Soongsil University, 369 Sangdo-Ro, Dongjak-Gu, Seoul, Korea (06978), November, 2020.
- Nissa; Azka Ainun; Chern-Ein Oon; Sasidharan Sreenivasan; Venugopal Balakrishnan; Deepa Rajendran; Jun-Jie Tan; Fatin Zhao; Nur Syafiqah Mohamad Nasir; Zakuan Zainy Deris; Heping Zhang; Yong-Ha Park; Guoxia Liu; Min-Tze Liong. Vaginal Infections during Pregnancy Increase Breast Milk Microbiome Alpha Diversity and Alter Taxonomic Composition. Prev. Nutri. Food. Sci 2023, 28, 1-9.
- Marungruang, N.; Tovar, J.; Björck, I.; Hållenius, F. F.. Improvement in cardiometabolic risk markers following a multifunctional diet is associated with gut microbial taxa in healthy overweight and obese subjects. Eur J Nutr 2018, 57, 2927-2936. [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; Bai, Y.; Bisanz, J.E.; Bittinger, K.; Brejnrod, A.; Brislawn, C.J.; Brown, C.T.; Callahan, B.J.; Caraballo-Rodríguez, A.M.; Chase, J.; Cope, E.K.; Da Silva, R.; Diener, C.; Dorrestein, P.C.; Douglas, G.M.; Durall, D.M.; Duvallet, C.; Edwardson, C.F.; Ernst, M.; Estaki, M.; Fouquier, J.; Gauglitz, J.M.; Gibbons, S.M.; Gibson, D.L.; Gonzalez, A.; Gorlick, K.; Guo, J.; Hillmann, B.; Holmes, S.; Holste, H.; Huttenhower, C.; Huttley, G.A.; Janssen, S.; Jarmusch, A.K.; Jiang, L.; Kaehler, B.D.; Kang, K.B.; Keefe, C.R.; Keim, P.; Kelley, S.T.; Knights, D.; Koester, I.; Kosciolek, T.; Kreps, J.; Langille, M.G.I.; Lee, J.; Ley, R.; Liu, Y.-X.; Loftfield, E.; Lozupone, C.; Maher, M.; Marotz, C.; Martin, B.D.; McDonald, D.; McIver, L.J.; Melnik, A.V.; Metcalf, J.L.; Morgan, S.C.; Morton, J.T.; Naimey, A.T.; Navas-Molina, J.A.; Nothias, L.F.; Orchanian, S.B.; Pearson, T.; Peoples, S.L.; Petras, D.; Preuss, M.L.; Pruesse, E.; Rasmussen, L.B.; Rivers, A.; Robeson, M.S.; Rosenthal, P.; Segata, N.; Shaffer, M.; Shiffer, A.; Sinha, R.; Song, S.J.; Spear, J.R.; Swafford, A.D.; Thompson, L.R.; Torres, P.J.; Trinh, P.; Tripathi, A.; Turnbaugh, P.J.; Ul-Hasan, S.; Van Der Hooft, J.J.J.; Vargas, F.; Vázquez-Baeza, Y.; Vogtmann, E.; Von Hippel, M.; Walters, W.; Wan, Y.; Wang, M.; Warren, J.; Weber, K.C.; Williamson, C.H.D.; Willis, A.D.; Xu, Z.Z.; Zaneveld, J.R.; Zhang, Y.; Zhu, Q.; Knight, R.; Caporaso, J.G. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 2019, 37, 852-857. [CrossRef]
- Shin, Dong-chul. A study on Characteristics of Advanced Sewage Treatment of Microorganisms Immobilized Media for Nitrogen Removal. Master’s degree, Department of Environmental Engineering Graduate School of University of Seoul, 163 Seoulsiripdae-Ro, Dongdaemun-Gu, Seoul, Korea (02504), August, 2018.
- Xu, X.; Lv, C.; You, X.; Wang, B.; Ji, F.; Hu, B. Nitrogen removal and microbial diversity of activated sludge entrapped in modified poly (vinyl alcohol)-sodium alginate gel. International Biodeterioration and Biodegradation 2017, 125, 243-250. [CrossRef]
- Zhao; Weihua; Xuejun Bi; Meng Bai; Yanyan Wang. Research advances of ammonia oxidation microorganisms in wastewater: metabolic characteristics, microbial community, influencing factors and process applications. Bioprocess and Biosystems Engineering 2023, 46, 621-633. [CrossRef]
- Zhang; Yu; Zehua Ji; Yuansheng Pei. Nutrient removal and microbial community structures in an artificial-natural coupled wetland system. Process Safety and Environmental Protection 2021, 147, 1160-1170. [CrossRef]
- Daims; Holger; Sebastian Lücker; Michael Wagner. A New Perspective on Microbes Formerly Known as Nitrite-Oxidizing Bacteria. Trends in Microbiology 2016, 24, 699-712. [CrossRef]
- Guo; Yanqing; Xaiotian Chen; Yuanyuan Wu; Lu Zhang; Jimin Cheng; Gehong Wei; Yanbing Lin. Natural revegetation of a semiarid habitat alters taxonomic and functional diversity of soil microbial communities. Science of The Total Environment 2018, 653, 598-606. [CrossRef]
- Soliman, M.; Eldyasti, A. Ammonia-Oxidizing Bacteria (AOB): opportunities and applications-a review. Rev Environ Sci Biotechnol 2018, 17, 285-321. [CrossRef]
- Ge; Zhibin; Dongyang Wei; Jing Zhang; Junsong Hu; Zhuo Liu; Ruihua Li. Natural pyrite to enhance simultaneous long-term nitrogen and phosphorus removal in constructed wetland: Three years of pilot study. Water Research 2019, 148, 153-161. [CrossRef]
- Zhou; Hexi; Xiangkun Li; Zhaorui Chu; Jie Zhang. Effect of temperature downshifts on a bench-scale hybrid A/O system: Process performance and microbial community dynamics. Chemosphere 2016, 153, 500-507. [CrossRef]
- Fitzgerald, C.M.; Camejo, P.; Oshlag, J.Z.; Noguera, D.R. Ammonia-oxidizing microbial communities in reactors with efficient nitrification at low-dissolved oxygen. Water Research 2015, 70, 38-51. [CrossRef]
- Chen J.; Han Y.; Wang, Y.; Gong, B.; Zhou, J.; Qing, X. Start-up and microbial communities of a simultaneous nitrogen removal system for high salinity and high nitrogen organic wastewater via heterotrophic nitrification. Bioresource technology 2016, 216, 196-202. [CrossRef]
- Sun Y.; Wang, H.; Wu, G.; Guan, Y. Nitrogen removal and nitrous oxide emission from a step-feeding multiple anoxic and aerobic process. Environmental technology 2018, 39, 814-823. [CrossRef]
- Xu, Z.; Dai, X.; Chai, X. Effect of different carbon sources on denitrification performance, microbial community structure and denitrification genes. Science of The Total Environment 2018, 634, 195-204. [CrossRef]
- Che; Rongxiao; Yongcui Deng; Fang Wang; Weijin Wang; Zhihong Xu; Yanbin Hao; Kai Xue; Biao Zhang; Li Tang; Huakun Zhou; Xiaoyong Cui. Autotrophic and symbiotic diazotrophs dominate nitrogen-fixing communities in Tibetan grassland soils. Science of The Total Environment 2018, 639, 997-1006.
- Meng, H.; Zhou, Z.; Wu, R.; Wang, Y.; Gu, J.D. Diazotrophic microbial community and abundance in acidic subtropical natural and re-vegetated forest soils revealed by high-throughput sequencing of nifH gene. Applied microbiology and biotechnology 2018, 1-11. [CrossRef]
- Jeon, Gwan Soo; Byeong Sam Park; Byeong Cheon Baek. A study on the Denitrification and Reduction Path of Nitrate ion by Sulfate Reduction Microorganisms. Journal of the Korean Society of Environmental Engineers 2001, 23, 39-49.
- Bucci; Paula; Bibiana Coppotelli; Irma Morelli; Noemí Zaritzky; Alejandro Caravelli. Heterotrophic nitrification-aerobic denitrification performance in a granular sequencing batch reactor supported by next generation sequencing. International Biodeterioration and Biodegradation 2021, 160, 105210. [CrossRef]
- Giraldo; Eugenio; Patrick Jjemba; Yangjin Liu; Swarna Muthukrishnan. Presence and Significance of Anammox spcs and Ammonia Oxidizing Archea, AOA, in Full Scale Membrane Bioreactors for Total Nitrogen Removal. Water Environment Federation 2011, 2011, 510-519. [CrossRef]
- Giraldo; Eugenio; Patrick Jjemba; Yangjin Liu; Swarna Muthukrishnan. Ammonia Oxidizing Archaea, AOA, Population and Kinetic Changes in a Full Scale Simultaneous Nitrogen and Phosphorous Removal MBR. Water Environment Federation. 2011b, 2011, 3156-3168. [CrossRef]
- Mady Elsayed; Jan Oleszkiewicz; Qiuyan Yuan. Simultaneous biological nutrients removal from wastewater with high ammonium and phosphorous loading using aerobic granular sludge. Journal of Water Process Engineering 2024, 64, 105650.
- Pérez-Prieto, Inmaculada, Abel Plaza-Florido, Esther Ubago-Guisado, Francisco B. Ortega, Signe Altmäe. Physical activity, sedentary behavior and microbiome: A systematic review and meta-analysis. Journal of Science and Medicine in Sport 2024. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).