1. Introduction
Recognizing the impact of the external exposome on allergy development is becoming increasingly urgent. Environmental exposures can be classified into three interconnected domains: the general external environment, the specific external environment, and the internal, host-dependent environment [
1]. The general external environment encompasses factors such as climate, biodiversity, and the urban, social, and economic conditions that shape human interactions with the natural world [
2,
3]. In contrast, the specific external environment involves direct exposures, including allergens, microbes, diet, tobacco, pollutants, and other toxic substances that directly impact human health. In addition, the internal environment consists of host-dependent physiological factors—such as metabolism, inflammation, and oxidative stress—that determine how individuals respond to these external exposures [
4,
5,
6]. Together, these three domains form the concept of the “meta-exposome,” which reflects the dynamic interplay between environmental exposures and human health [
7,
8].
Allergen exposure is a primary factor in allergic sensitization, a critical process in the development of conditions like asthma, allergic rhinitis, and eczema [
9,
10,
11,
12]. Airborne allergens, particularly pollen and dust mites, are the most significant risk factors for respiratory allergic diseases. Moreover, demographic factors such as age, sex, and geographical location also influence patterns of allergen sensitization [
13,
14,
15,
16].
Climate change is a major threat to humanity, driven by greenhouse gases that pose significant challenges to human health and healthcare systems, potentially reversing decades of medical progress [
17]. One notable health impact is the rise in allergic respiratory diseases, fueled by increasing atmospheric carbon dioxide and higher temperatures. These factors intensify the concentration and allergenicity of airborne particles like pollen and fungal spores, resulting in more severe symptoms [
18]. Additionally, the biodiversity hypothesis suggests that exposure to natural environments enriches the human microbiome and promotes immune balance, offering protection against allergies and inflammatory disorders. However, the loss of these immunoprotective factors in rapidly urbanizing areas experiencing biodiversity loss is an emerging health risk [
19,
20].
Understanding the external exposome’s role in allergy development is critical. Advances in Precision Allergy Molecular Diagnosis (PAMD@) now allow for more precise identification of individual IgE reactivity profiles, leading to more accurate diagnoses and personalized treatments [
21,
22,
23,
24]. The aim of this study was to investigate regional patterns of allergic sensitization in two distant regions -Tenerife, Spain, and Lima, Peru- which despite sharing similar climatic conditions, differ significantly in urbanization and socioeconomic status. Evaluating how these factors influence allergen exposure in different geographic areas is essential for developing targeted diagnostic and therapeutic tools.
2. Results
2.1. Demographic Features of Investigated Patients
All 181 subjects who met the ARIA or GINA criteria for allergic rhinitis (AR) or asthma (A) [
25,
26] tested positive for one or more aeroallergens in a skin prick test (SPT). Most participants were female (67%) with a median age of 29.7 years (range: 4–75) (
Table 1).
None of the patients had received allergen immunotherapy or biologic treatments before or during the study inclusion. Atopic comorbidities were present, with food allergies (to seafood, nuts, eggs, and/or milk) affecting 9.42% (18 patients) and drug allergies -to beta-lactam antibiotics and/or nonsteroidal anti-inflammatory drugs- in 6.62% (12 patients). Additionally, a family history of atopy was reported by 74.25% of the patients
2.2. Prevalence, sIgE Reactivity and Individual Molecular Profile According to Atopic Disease
The sensitization to aeroallergen extracts through SPT and the prevalence of the 181 patients who met the inclusion criteria are summarized in
Table 2.
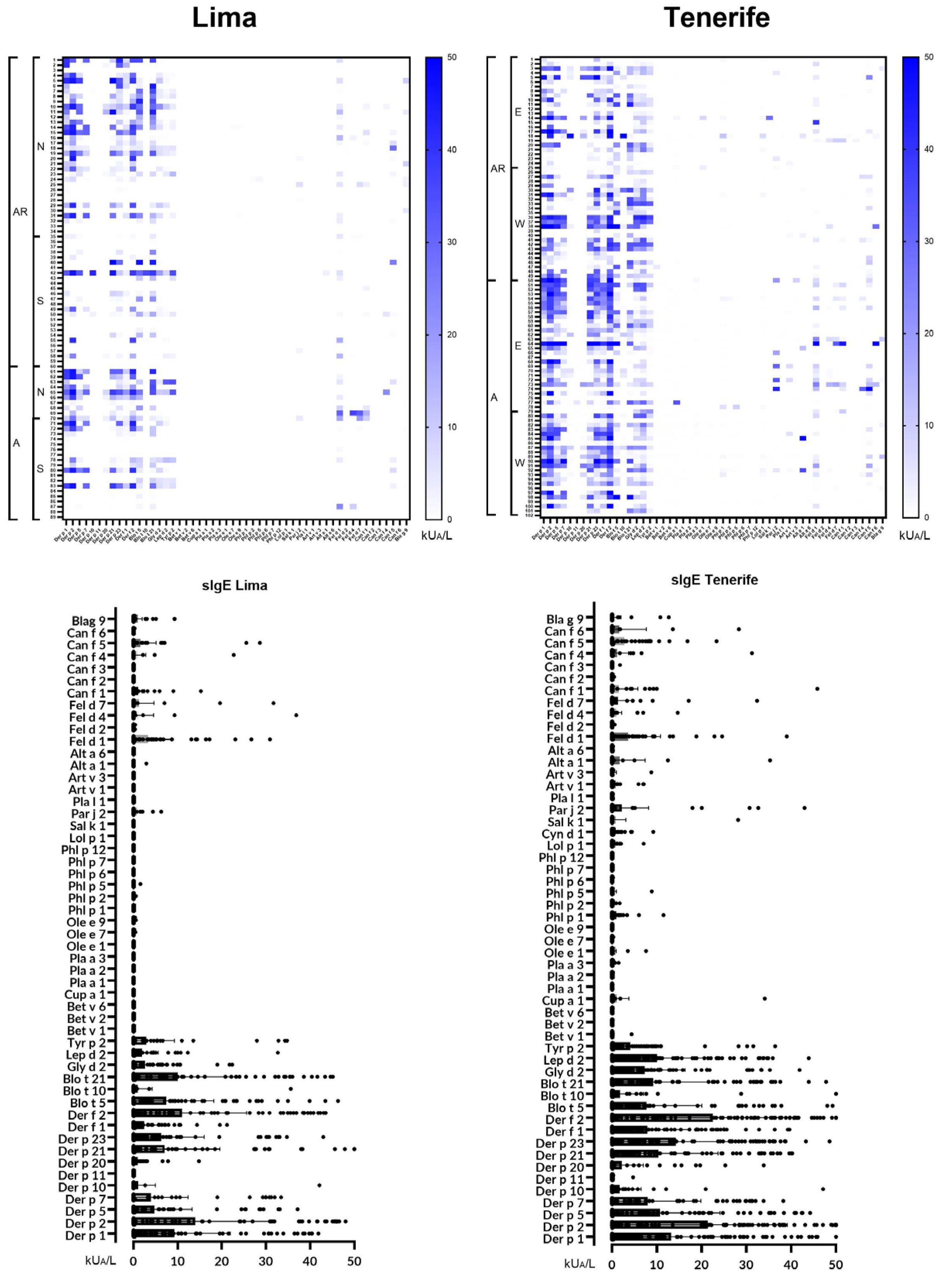
Overall, 162 out of 181 patients (89.5%) were sIgE-positive (≥ 0.35 kUA/L) to one or more of the 52 individual molecular aeroallergens included in the multiplex array (
Figure 1).
Despite having a positive SPT for at least one of the investigated aeroallergens, 19 patients (10.4%)—comprising 12 with AR (11 from Peru and 1 from Spain) and 7 with AA (6 from Peru and 1 from Spain)—did not display specific IgE (sIgE) levels above 0.35 kUA/L for any of the allergens tested in the multiplex array.
2.3. Mites
Mites were identified as the most prevalent source of sensitizing airborne allergens in both populations, regardless of the subjects’ underlying atopic condition. Sensitization to one or more of the 17 investigated mite molecular allergens was observed in 154 (85%) subjects. However, 27 patients—19 with AR (18 from Peru, 1 from Spain) and 8 with AA (7 from Peru, 1 from Spain)—were not sensitized to any of the mite allergens tested. Our findings confirmed Der p 1, Der p 2, Der f 2, and Der p 23 as major allergens (prevalence above 50%). Mid-tier allergens, with a prevalence between 20% and 50%, included Der f 1, Der p 5, Der p 7, Der p 21, Blo t 5, Blo t 21, Gly d 2, Lep d 2, and Tyr p 2. Minor allergens, with a prevalence below 20%, were Der p 10, Der p 11, Der p 20, and Blo t 10.
The overall proportion of subjects with sIgE to group 1 allergens—Der p 1 and Der f 1—was 53.1%, which was lower than to group 2 allergens—Der p 2 and Der f 2—at 63.7%, and to Der p 23 at 61.6%. Among the individual molecular allergens, Der p 2 (63.8%) was the most frequently identified with sIgE ≥0.35 kUA/L, followed by Der f 2 (63.6%), Der p 23 (61.6%), Der p 1 (59.6%), and Der f 1 (47.5%). Among the subjects sensitized to group 1 allergens (53.1%), 79.8% were positive for both Der f 1 and Der p 1 (21 subjects were exclusively sensitized to Der p 1, and 1 subject to Der f 1). Similarly, 99.1% of subjects sensitized to group 2 allergens had sIgE to both Der f 2 and Der p 2 (with only 1 subject having sIgE exclusively to Der f 2).
Most subjects (57%) recognized between 9 and 16 of the tested allergens. The most common IgE profile, comprising 11 molecules, included all five major allergens. Single sensitizations to mite allergens were rare (2.76%), with only 1 out of 181 patients (0.5%) showing exclusive sensitization to Der p 23 (
Table 3).
2.3.1. sIgE Reactivity Profiles and Basal Respiratory Allergic Diseases by Geographic Location
The overall frequency of molecular sensitization was quantitatively higher in 13 out of 17 (73.47%) molecules—Der p 1, Der p 2, Der p 5, Der p 7, Der p 11, Der p 20, Der p 21, Der p 23, Der f 1, Der f 2, Blo t 5, Lep d 2, and Tyr p 2—in patients with AA, in contrast to those with AR, who were more frequently sensitized to Der p 10, Blo t 10, Blo t 21, and Gly d 2. In addition to the high number of sIgE mite responses, significant differences in prevalence and quantification titers were observed between the basal atopic diseases and their corresponding mite molecular allergens in patients with AR and those with AA.
Individuals with AR in the Spanish cohort showed a higher overall frequency of sensitization to all 17 mite molecules compared to those in the Peruvian cohort. Interestingly, for those with AA, only one molecule (Blo t 21) showed higher sensitization in the Peruvian population compared to the Spanish population. Spanish asthmatic patients demonstrated increased sensitization to most mite allergens compared to those with AR, except for Blo t 21, Gly d 2, and Tyr p 2. Similarly, Peruvian asthmatic subjects showed a higher frequency of sensitization to most individual allergens compared to those with AR, with the exceptions of Der p 10, Blo t 10, and Tyr p 2. Spanish patients with AR had significantly higher serum titers (P < 0.05) for 6 mite molecules (35.29%)—Der p 23, Der f 1, Der f 2, Gly d 2, Lep d 2, and Tyr p 2—compared to Peruvian subjects with AR. Additionally, they had significantly higher titers for 10 allergens (58.82%)—Der p 2, Der p 5, Der p 10, Der p 21, Der p 23, Der f 1, Der f 2, Blo t 10, Gly d 2, and Lep d 2—compared to the group of Peruvian asthmatics (
Table 4).
2.3.2. Age and sIgE Reactivity Profiles
Younger patients (<21 years old, n = 76) displayed a higher frequency of sIgE binding to 10 out of 17 molecular mite allergens—specifically, Der p 1, Der p 2, Der p 5, Der p 10, Der p 23, Der f 1, Blo t 5, Blo t 10, Gly d 2, and Tyr p 2—compared to older patients (≥21 years old, n = 105). Furthermore, younger subjects had a higher frequency of sIgE binding in both AR (14 molecules) and AA (9 molecules) compared to their older counterparts. Among adults, those with asthma showed a higher frequency of sIgE binding to 16 out of 17 mite molecules (excluding Tyr p 2) compared to adult patients with AR. Regarding geographical variations, younger patients with AR in the Spanish cohort demonstrated a higher frequency of sensitization to 11 mite molecules compared to their AA peers. Conversely, in the Peruvian cohort, younger AA subjects exhibited a higher frequency of sensitization to 11 out of 17 allergens compared to those with AR.
2.4. Cat and Dog Epithelia
Overall, 87 out of 181 patients (48.06%) were sensitized to one or more of the 10 investigated epithelial molecular allergens. Among these, cat allergens were the most frequently identified, with 67 patients (37.01%) showing sensitivity, compared to dog allergens in 45 patients (24.86%). A total of 112 specific IgE (sIgE) responses to cat allergens were detected, with Fel d 1 being the most common (33.57%), followed by Fel d 7 (6.7%), Fel d 4 (6.6%), and Fel d 2 (1.0%). For dog allergens, Can f 5 was the most frequently identified (19.1%), followed by Can f 1 (9.92%), Can f 4 (4.3%), Can f 6 (2.45%), Can f 2 (0.5%), and Can f 3 (0.5%).
Asthmatics, compared to AR patients, exhibited higher frequencies of Fel d 1, Fel d 4, Fel d 7, and Can f 1 in both the Spanish and Peruvian cohorts, while Can f 6 was observed only in the Spanish cohort. In contrast, Can f 5 was more frequently found in AR patients than in those with AA in both investigated populations. In the Spanish group, the most frequent epithelial allergens among AR patients were Can f 5 (28%) and Fel d 1 (24%), followed by Can f 4 and Can f 6 (6%), Can f 1 and Fel d 4 (4%), and Fel d 2, Fel d 7, Can f 2, and Can f 3 (2%). In AA patients, the frequencies were Fel d 1 (44.2%), Can f 5 (25%), Can f 1 (15.4%), Fel d 4 (13.5%), Fel d 7 (11.5%), Can f 4 and Can f 6 (5.8%), while Fel d 2, Can f 2, and Can f 3 were undetected. In the Peruvian cohort, the most frequent allergens for AR patients were Fel d 1 (32%), Can f 5 (18%), Can f 1 (10%), Fel d 4 and Fel d 7 (4%), and Fel d 2 and Can f 4 (2%), with Can f 2, Can f 3, and Can f 6 not detected. Among AA patients, the frequencies were Fel d 1 (34.5%), Can f 5 (13.8%), Fel d 7 and Can f 1 (10.3%), Fel d 4 (6.9%), and Can f 4 (3.4%), with no detection of Fel d 2, Can f 2, Can f 3, or Can f 6.
2.5. Pollens
A total of 37 out of 181 patients (20.44%) were sensitized to one or more of the 22 pollen allergens investigated, showing 58 sIgE individual pollen responses. The Spanish subset showed a higher detection of pollen molecules (23 molecules in 12 AR patients, and 26 allergens in 13 AA subjects) compared to the Peruvian cohort (8 molecules in 6 AR patients, and 1 molecule in 1 AA subject).
In the Spanish cohort, the most frequent allergens for AR patients were Phl p 1 (10%), Lol p 1 (10%), Par j 2 (8%), Ole e 1 (4%), Art v 1 (4%), Cup a 1 (4%), Bet v 1 (2%), Art v 3 (2%), Pla a 3 (2%), Phl p 2 (2%), and Sal k 1 (2%). For AA patients, the frequencies were Pla a 3 (3.8%), Phl p 1 (9.6%), Lol p 1 (9.6%), Par j 2 (9.6%), Art v 1 (9.6%), Phl p 2 (3.8%), and Phl p 5 (1.9%). Similarly, in the Peruvian cohort, the most frequent allergens for AR patients were Par j 2 (8%), Ole e 7 (2%), Ole e 9 (2%), Phl p 2 (2%), and Phl p 5 (2%). For AA patients, Par j 2 was detected in 3.4% of cases.
2.6. Cockroach and Molds
Thirteen subjects out of 181 (7.18%) were sensitized to Bla g 9, and 7 (3.86%) to Alt a 1. None of the patients showed sensitization to Alt a 6. In the Peruvian cohort, AR patients had sensitization rates of 10% for Bla g 9 and 2% for Alt a 1. For AA patients, Bla g 9 was detected in 3.4% of cases. In the Spanish cohort, sensitization rates for AR patients were 6% for Alt a 1 and 2% for Bla g 9, while for AA patients, the rates were 11.5% for Bla g 9 and 5.8% for Alt a 1.
2.7. Regional Differences in IgE Profiles
Our investigation included 181 patients in total, with 90 individuals from Lima and 91 from Tenerife. Approximately 50% of participants from each location were from surrounding regional areas.
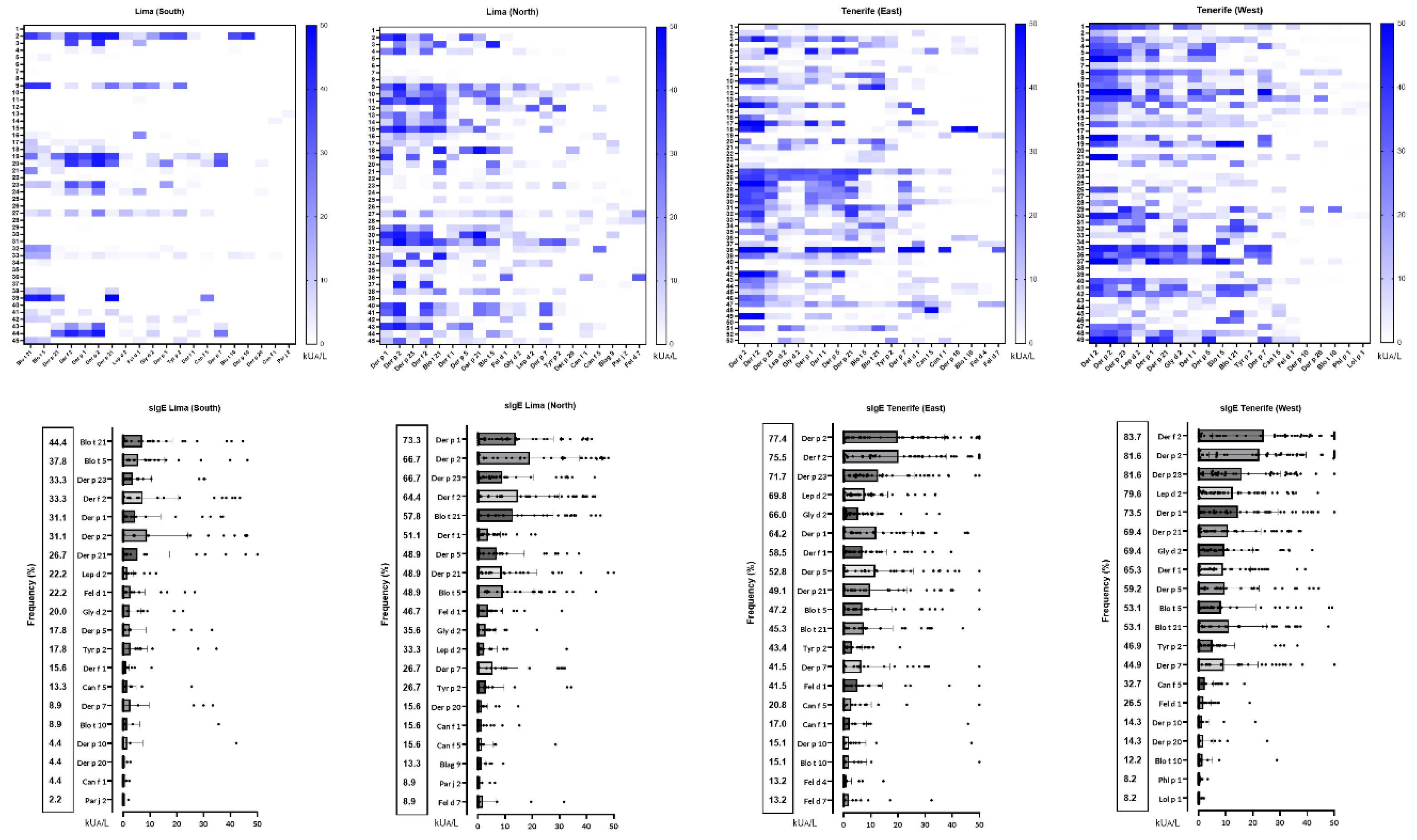
Figure 2 presents the profiles of the 20 most frequently recognized molecular allergens in each region, highlighting the serodominance of each allergen. Significant differences (p < 0.05) were observed for allergens Der p 1, Der p 2, Der p 5, Der p 23, Der f 1, and Der f 2 between North and South Lima, Peru, and for Fel d 1 between East and West Tenerife, Spain.
3. Discussion
Climate change is influencing the seasonality, production, concentration, allergenicity, and geographic spread of airborne allergens, leading to increased rates of allergic diseases [
25,
26]. This study is the first to assess aeroallergen sensitization in two populations with respiratory allergies from distinct geographical and socio-economic backgrounds—Tenerife and Lima—both of which share similar climatic conditions.
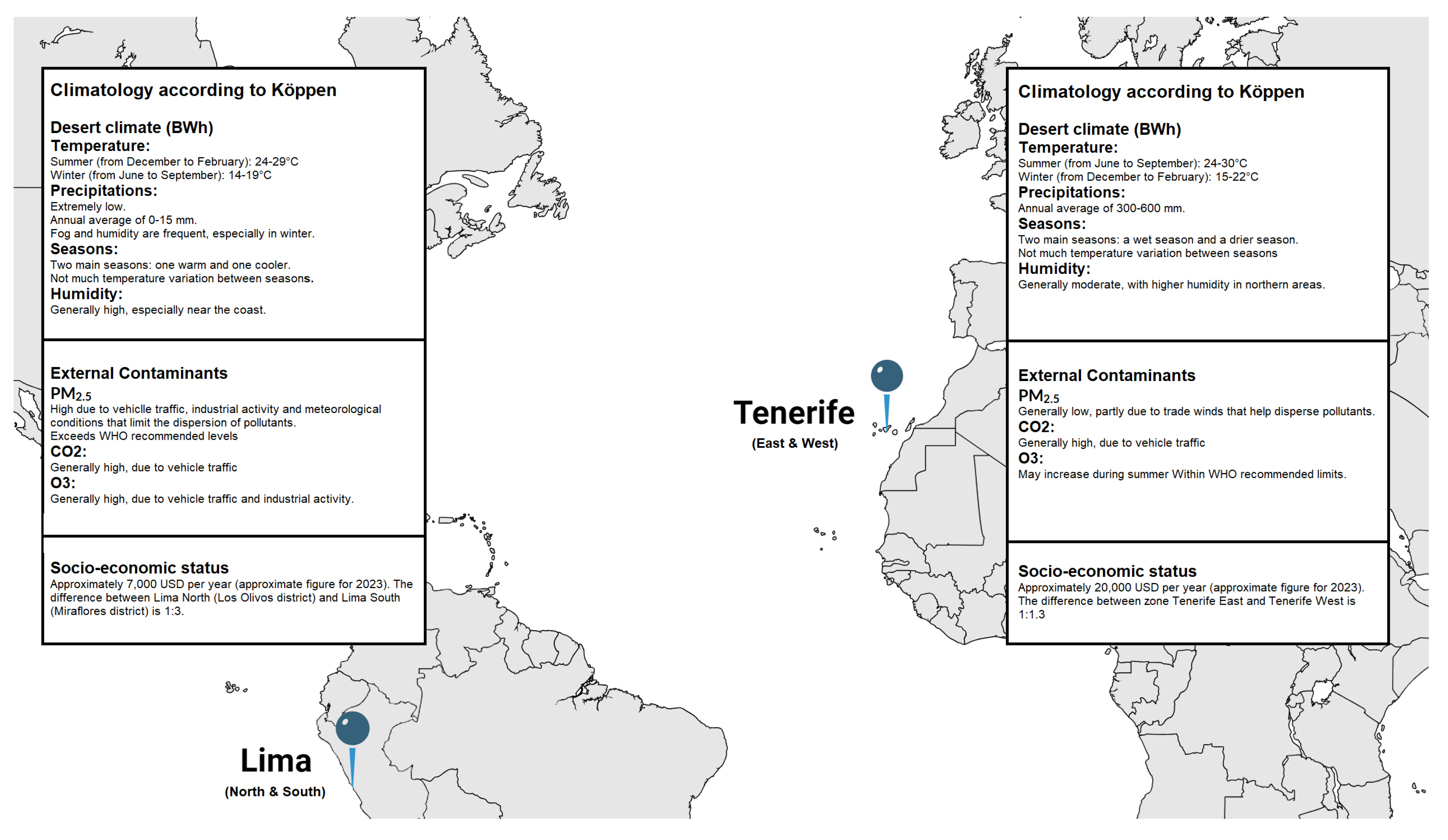
Tenerife, the largest and highest island in the Canary archipelago, has a climate influenced by the cool, humid northeast trade winds from the Azores anticyclone. While generally having lower air pollution than continental Europe, Tenerife is occasionally impacted by Saharan dust intrusions, which can cause PM10 concentrations to exceed 50 µg/m³, a level linked to adverse health effects [
27,
28]. In contrast, Lima, Peru’s capital, is a densely populated city situated between the Pacific Ocean and the Andes Mountains. With over 11 million residents, Lima is the third-most populous and second-most polluted city in the Americas, affected by an aging transportation fleet, biomass stoves, and topography that traps pollutants [
29,
30]. Both Lima and Tenerife share a desert climate (Köppen BWh) but experience different seasonal patterns: Lima’s temperatures remain stable with minimal seasonal changes, while Tenerife, with its varied elevations, has more pronounced seasonal fluctuations [
31,
32].
Lima’s pollution levels, particularly PM2.5, CO2, and O3, are higher due to heavy traffic, industrial activity, and inefficient energy use [
33,
34,
35]. Tenerife benefits from trade winds that disperse pollutants, though Saharan dust occasionally raises particulate levels. Additionally, Lima faces a significant income disparity (13:1) between its regions, leading to uneven pollution exposure, whereas Tenerife has a minimal income gap (1.1:1), reflecting more equitable socio-economic conditions (
Figure 3) [
36].
Specific IgE antibodies, detected in blood or via SPTs, are key indicators of allergic sensitization and strong predictors of respiratory symptoms in epidemiological studies [
37,
38]. In the current study, mites were consistently identified through both SPTs and serum sIgE as the most prevalent allergen affecting both populations. Among the population studied, 85% were sensitized to at least one of the 17 investigated mite molecular allergens, 48.06% to one or more of the 10 epithelial allergens, 20.44% to one or more of the 22 pollen allergens, 7.18% to Bla g 9, and 3.86% to Alt a 1. These findings highlight mites as a primary target for intervention, while also revealing a diverse spectrum of other allergens which may contribute to the overall burden of respiratory symptoms.
The production of HDM allergens is intricately connected to various environmental factors, which could potentially amplify allergenicity in the context of climate change and pollution. House dust mites (HDMs) synthesize cysteine and serine proteases, including Der p 1, and these levels fluctuate based on dietary influences and temperature variations [
39]. Additionally, lipid-binding proteins such as Der p 2 and Der p 7 play a crucial role in helping mites detect pathogens; their concentrations may rise in humid environments rich in fungal presence [
40]. Pollution significantly impacts allergen production, as exposure to diesel particulate matter has been shown to enhance the synthesis of glutathione transferase allergens like Der p 8, indicating that deteriorating air quality is associated with increased allergenicity of HDMs [
41,
42]. Furthermore, this study reaffirms that Der p 1, Der p 2, Der f 2, and Der p 23 remain prominent allergens, while simultaneously highlighting regional and disease-specific variations in sensitization patterns [
43]. Notably, these differences are particularly pronounced among patients with allergic rhinitis (AR) compared to those with allergic asthma (AA), as well as between cohorts from Spain and Peru.
Among patients with AR, the Spanish cohort exhibited significantly higher overall sensitization to six key mite allergens, including Der p 23, Der f 1, and Lep d 2, compared to their Peruvian counterparts [
22]. While some allergens, such as Der p 10, Blo t 10, Blo t 21, and Gly d 2, were frequently recognized in both populations, Spanish AR patients demonstrated higher general sensitization levels, as indicated by elevated serum IgE titers, than Peruvian patients. The differences between the cohorts became even more pronounced among those with AA. Spanish AA patients showed higher sensitization rates to 13 out of the 17 studied mite allergens, including Der p 1, Der p 2, and Der p 23. In contrast, Peruvian AA patients exhibited higher sensitization to only one allergen, Blo t 21. Additionally, Spanish AA patients had significantly higher sensitization rates to 10 allergens, including Der p 5, Blo t 10, and Gly d 2. These findings suggest significant regional differences in allergen exposure or immune responses. This is consistent with the work of Muddaluru and colleagues, who compared house dust mite sensitization profiles in allergic adults from Canada, Europe, South Africa, and the USA, finding that Spanish asthmatics are more sensitive to a broader range of allergens. Interestingly, younger patients tend to exhibit a more pronounced immune response—sIgE binding—to a wider variety of allergens, particularly among those with AR [
44]. This observation implies that age, along with geographical factors such as climate, local allergen exposure, and genetic predispositions, may influence the development of specific sensitization profiles in different populations. These findings suggest significant regional differences in allergen exposure or immune responses, as also revealed by Muddaluru and coworkers [
44] when comparing house dust mite sensitisation profiles in allergic adults from Canada, Europe, South Africa, and the USA, with Spanish asthmatics being more sensitive to a broader range of allergens. Interestingly, younger patients tend to show a more pronounced immune response—sIgE binding—to a wider range of allergens, particularly among those with AR.
This observation implies that age, along with geographical factors such as climate, local allergen exposure, and genetic predispositions, may influence the development of specific sensitization profiles across different populations. Moreover, it necessitates a reevaluation of the prevalence-based classification proposed by Posa et al. [
45] for
Dermatophagoides allergens, suggesting that it may require nuanced refinement. Specifically, it would be advisable to incorporate Der p 5, Der p 7, and Der p 21 into Group A allergens, which includes molecules with a prevalence exceeding 40% for Tenerife, as well as Der p 5 and Der p 21 for Lima. This adjustment would complement the previously recognized allergens—Der p 1, Der p 2, and Der p 23—and enhance the robustness of the classification framework.
Regarding epithelial allergens [
46,
47], Spanish patients with allergic rhinitis (AR) exhibited significant sensitivities to Can f 5 (28%) and Fel d 1 (24%), indicating a notable prevalence of both cat and dog allergens. The Peruvian cohort also showed high sensitization rates to Fel d 1 (32%) and Can f 5 (18%). Among patients with allergic asthma (AA), sensitivities varied between the cohorts. In the Spanish cohort, Fel d 1 was particularly prevalent, with 44.2% of asthma patients demonstrating sensitivity. Other common cat allergens included Fel d 4 (13.5%) and Fel d 7 (11.5%). For dog allergens, Can f 1 was found in 15.4% of asthmatic patients, while Can f 6 was unique to this cohort. In contrast, the Peruvian cohor
t’s asthmatics showed high sensitization rates to Fel d 1 (34.5%), followed by Can f 5 (13.8%) and both Fel d 7 and Can f 1 (10.3%). Certain allergens, such as Can f 2, Can f 3, and Can f 6, were undetected in this population, emphasizing geographical differences in allergen exposure and sensitization patterns. These data align with the study by Ukleja-Sokołowska N. and colleagues, which found that Fel d 1 was the primary allergen for cat-sensitized individuals, affecting 93.9% of cat-allergic patients, while Can f 1 and Can f 5 were the most common allergens among dog-sensitized individuals [
48].
Pollens such as ragweed in North America, grasses in Europe, and Japanese cedar in Japan have been shown to have prolonged or overlapping seasons, creating continuous allergenic exposure for sensitive individuals. Cross-reactivity between pollen types, such as birch and cypress in Europe, further complicates allergic responses and prolongs the duration of symptoms [
49,
50]. These findings suggest the need for region-specific allergen management and more accurate monitoring to improve outcomes for pollen-sensitized patients. Consequently, our study revealed significant differences between the Spanish and Peruvian cohorts. The Spanish cohort identified 23 pollen allergens among 12 patients with AR) and 26 allergens in 13 patients with allergic AA, whereas the Peruvian cohort detected only 8 allergens in 6 AR patients and just 1 allergen in 1 AA patient. The most frequently identified allergens among Spanish AR patients were Phl p 1 and Lol p 1 (both at 10%), while AA patients exhibited sensitivities to Pla a 3 (3.8%) and Phl p 1 (9.6%). In contrast, Peruvian AR patients were primarily sensitized to Par j 2 (8%), with other allergens such as Ole e 7 (2%) and Ole e 9 (2%) also present. These results suggest that the Spanish population is exposed to a greater variety of pollen allergens, leading to broader sensitization, while the Peruvian cohort shows a more limited range of sensitization. This contrasts with the data reported by Dramburg et al., which revealed a high prevalence of IgE responses to major allergens such as grass pollen (Phl p 1, Phl p 5, and Cyn d 1), olive (Ole e 1), and cypress (Cup a 1), along with variable responses to other airborne allergens and pan-allergens [
51].
Finally, both populations exhibited sensitivities to Bla g 9 and Alt a 1, yet the rates varied significantly, especially among allergic asthma (AA) patients in Spain, who demonstrated higher sensitization levels. Notably, the lack of sensitization to Alt a 6 in all patients indicates that this allergen may not be pertinent to these populations, underscoring the necessity for region-specific diagnostic and management strategies for allergic conditions. The study presents several limitations that may influence its findings and generalizability. First, the comparison between Spanish and Peruvian populations could introduce variability stemming from differing environmental exposures and genetic backgrounds. Additionally, the emphasis on a narrow range of allergens, primarily house dust mites, risks overlooking other significant contributors to sensitization. Furthermore, evaluating sensitization at a single time point does not account for temporal variations, and the absence of longitudinal data hampers a comprehensive understanding of allergic responses over time.
Author Contributions
Conceptualization, R.G-P and F.P; methodology, R.G-P and F.P; software, T.G and O.E; validation, P.P-G and C.G-C; formal analysis, T.G, P.P-G and I.S-M; investigation, R.G-P, P.P-G, T.G, C.G-C and O.E-C; resources, R.G-P, I.S-M and F.P; data curation, P.P-G and T.G; writing—original draft preparation, R.G-P and F.P; writing—review and editing, R.G-P, C.G-C, O.E-C-C and F.P.; visualization, R.G-P, P.P-G, I.S-M, T.G, C.G-C and O.E-C; supervision, R.G-P and F.P.; project administration, I.S-M; funding acquisition, R.G-P and F.P.
Figure 1.
Heatmap illustrating the distribution of airborne allergens in Lima (Peru) and Tenerife (Spain), accompanied by their serodominance levels. Statistically significant differences (p>0.05) were observed between Lima and Tenerife for the following allergens: Der p 2, Der p 5, Der p 10, Der p 21, Der p 23, Der f 2, Lol p 1, Pla l 1, Art v 3, and Alt a 6. AR, allergic rhinitis; A, asthma; N, north; S, south; E, east; W, west.
Figure 1.
Heatmap illustrating the distribution of airborne allergens in Lima (Peru) and Tenerife (Spain), accompanied by their serodominance levels. Statistically significant differences (p>0.05) were observed between Lima and Tenerife for the following allergens: Der p 2, Der p 5, Der p 10, Der p 21, Der p 23, Der f 2, Lol p 1, Pla l 1, Art v 3, and Alt a 6. AR, allergic rhinitis; A, asthma; N, north; S, south; E, east; W, west.
Figure 2.
Heatmap of the 20 most frequently identified molecular allergens in each region, along with their serodominance. Significant statistical differences (p<0.05) were found for Der p 1, Der p 2, Der p 5, Der p 23, Der f 1, and Der f 2 between the two zones in Lima, and for Fel d 1 between the two zones in Tenerife.
Figure 2.
Heatmap of the 20 most frequently identified molecular allergens in each region, along with their serodominance. Significant statistical differences (p<0.05) were found for Der p 1, Der p 2, Der p 5, Der p 23, Der f 1, and Der f 2 between the two zones in Lima, and for Fel d 1 between the two zones in Tenerife.
Figure 3.
Climatology, external contaminants, and socio-economic status between North and South Lima, Peru, and East and West Tenerife, Spain.
Figure 3.
Climatology, external contaminants, and socio-economic status between North and South Lima, Peru, and East and West Tenerife, Spain.
Table 1.
Descriptive statistics regarding basal comorbid conditions and associated clinical characteristics of the studied population (n=181).
Table 1.
Descriptive statistics regarding basal comorbid conditions and associated clinical characteristics of the studied population (n=181).
| |
Tenerife, Spain |
Lima, Peru |
| n=181 |
91 |
90 |
| Age (y.o.) median (range) |
29.75 (8-70) |
22.0 (4-75) |
| <21 y.o. (n= 76) |
38 (41.7%) |
38 (42.2%) |
| ≥21 y.o. (n=105) |
53 (58.2%) |
52 (57.7%) |
| Sex (F/M) |
61/30 |
66/24 |
| Allergic Rhinitis (n=135) |
46 (50.5%) |
89 (98.8%) |
| Allergic Asthma (n=75) |
45 (49.5%) |
30 (33.3%) |
| SPT+ to any aeroallergen |
91 (100%) |
90 (100%) |
| Total IgE (IU/ml) median (range) |
365 (23.88-1010) |
434.84 (46.32-762.3) |
Table 2.
Prevalence of sensitization to grouped local aeroallergens by Skin Prick Test (n= 181).
Table 2.
Prevalence of sensitization to grouped local aeroallergens by Skin Prick Test (n= 181).
| Positive SPT (n=181) |
Tenerife, Spain (n=91) |
Lima, Peru (n=90) |
| HDM and/or SM (%) |
88 (96.7) |
68 (75.5) |
| Cat and/or dog dander (%) |
34 (37.3) |
14 (15.6) |
| Pollen (%) |
12 (13.1) |
14 (15.8) |
| Cockroach (%) |
7 (7.6) |
10 (11.1) |
| Molds (%) |
6 (6.5) |
2 (2.2) |
Table 3.
Number of identified mite molecular allergens, geographical location, and corresponding basal atopic disease (allergic rhinitis (AR), and alergic asthma (AA)) in 181 patients studied with microarray.
Table 3.
Number of identified mite molecular allergens, geographical location, and corresponding basal atopic disease (allergic rhinitis (AR), and alergic asthma (AA)) in 181 patients studied with microarray.
| Number of identified mite molecular allergens |
AR Tenerife, Spain |
AR Lima, Peru |
AA Tenerife, Spain |
AA Lima, Peru |
| 0 |
0 |
18 |
3 |
6 |
| 1 |
1 |
1 |
1 |
2 |
| 2 |
0 |
3 |
1 |
1 |
| 3 |
2 |
2 |
1 |
2 |
| 4 |
5 |
7 |
1 |
2 |
| 5 |
3 |
3 |
0 |
1 |
| 6 |
5 |
5 |
4 |
2 |
| 7 |
6 |
3 |
1 |
2 |
| 8 |
3 |
2 |
4 |
2 |
| 9 |
7 |
3 |
5 |
3 |
| 10 |
4 |
5 |
3 |
0 |
| 11 |
6 |
3 |
11 |
1 |
| 12 |
5 |
2 |
5 |
1 |
| 13 |
1 |
1 |
7 |
2 |
| 14 |
1 |
2 |
2 |
1 |
| 15 |
1 |
0 |
0 |
0 |
| 16 |
0 |
0 |
1 |
0 |
| 17 |
0 |
0 |
0 |
0 |
Table 4.
Serological analysis - Mean (frequency) of specific IgE (sIgE) responses (kU/L) to mite molecular allergens in patients with allergic rhinitis (AR, n=135) and allergic asthma (AA, n=75). Bold figures indicate significant differences (P < 0.05) in mean sIgE levels to mite molecular allergens between the two atopic conditions.
Table 4.
Serological analysis - Mean (frequency) of specific IgE (sIgE) responses (kU/L) to mite molecular allergens in patients with allergic rhinitis (AR, n=135) and allergic asthma (AA, n=75). Bold figures indicate significant differences (P < 0.05) in mean sIgE levels to mite molecular allergens between the two atopic conditions.
| Molecule |
sIgE AR (Spain) |
sIgE AR (Peru) |
sIgE AA (Spain) |
sIgE AA (Peru) |
| Der p 1 |
11.7±15.6 (66) |
5.52±10 (46) |
13.6±13.4 (82.7) |
10.5±14.4 (51.7) |
| Der p 2 |
16.19±17.3 (76) |
10.7±16.7 |
25.23±18.3 |
16±19.7 |
| Der p 5 |
8.14±14.1(42) |
3.36±8.5 (28) |
12.15±13.6 (69.2) |
4.29±8.3 (34.5) |
| Der p 7 |
5.08±10.3 (30) |
2.54±7.6 (16) |
9.62±13.7 (55.8) |
5.31±10.7 (24.1) |
| Der p 10 |
1.67±7.2 (14) |
0.9±5.9 (4) |
0.65±2.2 (15.4) |
0±0 (0) |
| Der p 11 |
0±0 (0) |
0±0 (0) |
0±0 (1.9) |
0±0 (0) |
| Der p 20 |
1.72±6 (10) |
0.35±2.1 (6) |
1.47±4.7 (15.4) |
0.55±1.6 (13.8) |
| Der p 21 |
7.38±12.45 (48) |
5.61±12.9 (32) |
12.16±14.8 (69.2) |
6.58±12.1 (41.4) |
| Der p 23 |
13.44±14.8 (72) |
3.25±6.6 (42) |
13.93±14.5 (80.8) |
6.06±10.5 (51.7) |
| Der f 1 |
3.63±5.5 (50) |
0.97±3 (22) |
10.96±11.9 (73.1) |
2.38±4.7 (44.8) |
| Der f 2 |
17.17±18.3 (76) |
8.15±13.5 (44) |
26.5±18.7 (82.7) |
12.54±16.8 (51.7) |
| Blo t 5 |
7.85±12.9 (46) |
5.71±10.5 (36) |
6.39±12.9 (53.8) |
4.43±6.8 (44.8) |
| Blo t 10 |
1.78±8.1 (12) |
0.81±5 (8) |
0.61±1.9 (15.4) |
0±0 (0) |
| Blo t 21 |
10.46±13.7 (54) |
7.35±1 (46) |
6.99±11.9 (44.2) |
8.19±12.8 (51.7) |
| Gly d 2 |
7.34±10.2 (74) |
1.31±3.4 (22) |
6.11±8.8 (61.5) |
1.81±3.2 (27.6) |
| Lep d 2 |
9.92±12 (72) |
0.67±1.67 (22) |
9.12±10.1 (76.9) |
2.34±6.5 (27.6) |
| Tyr p 2 |
4.41±8.6 (46) |
1.54±5.3 (20) |
2.43±4.2 (44.2) |
3.1±8.8 (17.2) |