1. Introduction
Heterocyclic compounds are cyclic structures containing at least one atom other than carbon, known as a heteroatom [
1]. The most common heteroatoms are oxygen, nitrogen, and sulfur, but others like phosphorus, iron, magnesium, and selenium can also be incorporated, often within five or six-membered rings [
2,
3]. These substances occur naturally in the environment and can be synthesized in large quantities through industrial processes [
4]. Their significance lies in their widespread pharmaceutical use, including medicines, insecticides, and crop protection agents. Heterocycles can be designed to produce beneficial compounds such as hormones, antibiotics, and vitamins [
5,
6]. They also exhibit various biological activities, including antibacterial, antifungal, anti-inflammatory, anticancer, antiviral, anti-allergic, anthelmintic, antioxidant, anticonvulsant, anti-histamine, anti-leprosy, and antihypertensive properties [
7,
8,
9]. Heterocycles play a crucial role in modern drug development, enabling the modification of physicochemical properties like solubility, lipophilicity, polarity, and hydrogen bonding capacity of biologically active agents. This optimization of ADME/Tox (Absorption, Distribution, Metabolism, Excretion, and Toxicity) properties is essential for developing effective pharmaceuticals [
10,
11]. The World Health Organization (WHO) estimates that over 90% of new medications contain heterocyclic structures [
12].
However, heterocyclic compounds' widespread presence and persistence in ecosystems and their toxic, carcinogenic, and other hazardous effects pose a significant environmental concern [
13,
14]. Even trace amounts of these compounds in drinking water can disrupt endocrine function, impair development, and modulate biological processes, including intracellular calcium signaling and tumor cell proliferation. Exposure to heterocyclic pharmaceuticals can also have toxic effects on the thyroid, reproductive organs, liver, nervous system, and other body systems [
15,
16]. The United States Environmental Protection Agency (USEPA) has classified some heterocyclic compounds as priority pollutants [
17]. Pharmaceutical pollution of surface and groundwater is a well-recognized environmental hazard in many countries [
3]. The heterocyclic structure of these compounds makes them highly soluble, allowing them to contaminate groundwater readily. Heterocyclic substances have been detected in various environmental matrices at concentrations ranging from parts per billion (ppb) to parts per million (ppm), and they are often characterized by their high polarity [
18]. For example, sulfonamides, a group of antibiotics, have been consistently found in surface waters at concentrations ranging from 0.13 to 1.9 µg/L [
19]. Triadimefon, another heterocyclic compound, has also been widely detected in surface waters at elevated levels [
20].
The increasing contamination of water sources with toxic compounds is a growing concern due to the expansion of industries and the high-tech sector. A previous study estimated that approximately 38,000 chemicals are in use, with more than 300 new compounds being created annually. These components are constantly discharged into the water environment [
22,
23]. According to the United Nations report on world water development, over 80% of the wastewater generated worldwide is released untreated into the environment [
24]. Given the risks associated with heterocyclic pharmaceuticals and their widespread use, it is imperative to address the contamination of water sources, particularly wastewater from industrial operations [
25,
26]. Developing effective methods and strategies to reduce the harmful impacts of these compounds is crucial [
27].
This review aims to shed light on heterocyclic pharmaceuticals' concerns, providing detailed insights into their environmental fate, ecotoxicity, and long-term risks. By identifying knowledge gaps and exploring innovative remediation options, this review seeks to contribute to developing more effective and sustainable approaches to mitigate the harmful impacts of these compounds on ecosystems and human health.
2. Overview of Heterocyclic Pharmaceuticals
Heterocyclic compounds play a pivotal role in medicinal chemistry and the pharmaceutical industry due to their biological activity and stability. They are widely used in drug design and production, forming the core structure of numerous pharmaceutical agents [
28].
Common heterocyclic compounds encompass amino acids, vitamins, and enzyme precursors. Heterocycles with condensed ring structures exhibit diverse physiological functions and are classified based on the heteroatoms they contain [
2,
3]. The following sections will delve into the most prevalent pharmaceutical heterocyclic compounds, categorized by their heteroatoms and their applications in the pharmaceutical field.
2.1. Nitrogen-Containing Heterocycles
Nitrogen-containing heterocycles represent the most prevalent class of heterocyclic compounds employed in pharmaceuticals, comprising nearly 50% of approved new chemical entities [
28]. Over 85% of bioactive molecules possess at least one nitrogen atom [
11]. These compounds exhibit a broad spectrum of biological activities due to their structural resemblance to natural and synthetic molecules [
29]. In recent years, the primary nitrogen-containing heterocyclic building blocks utilized in medicinal chemistry have included pyrroles, indoles, triazoles, pyrimidines, imidazoles/benzimidazoles, tetrazoles, and quinolines [
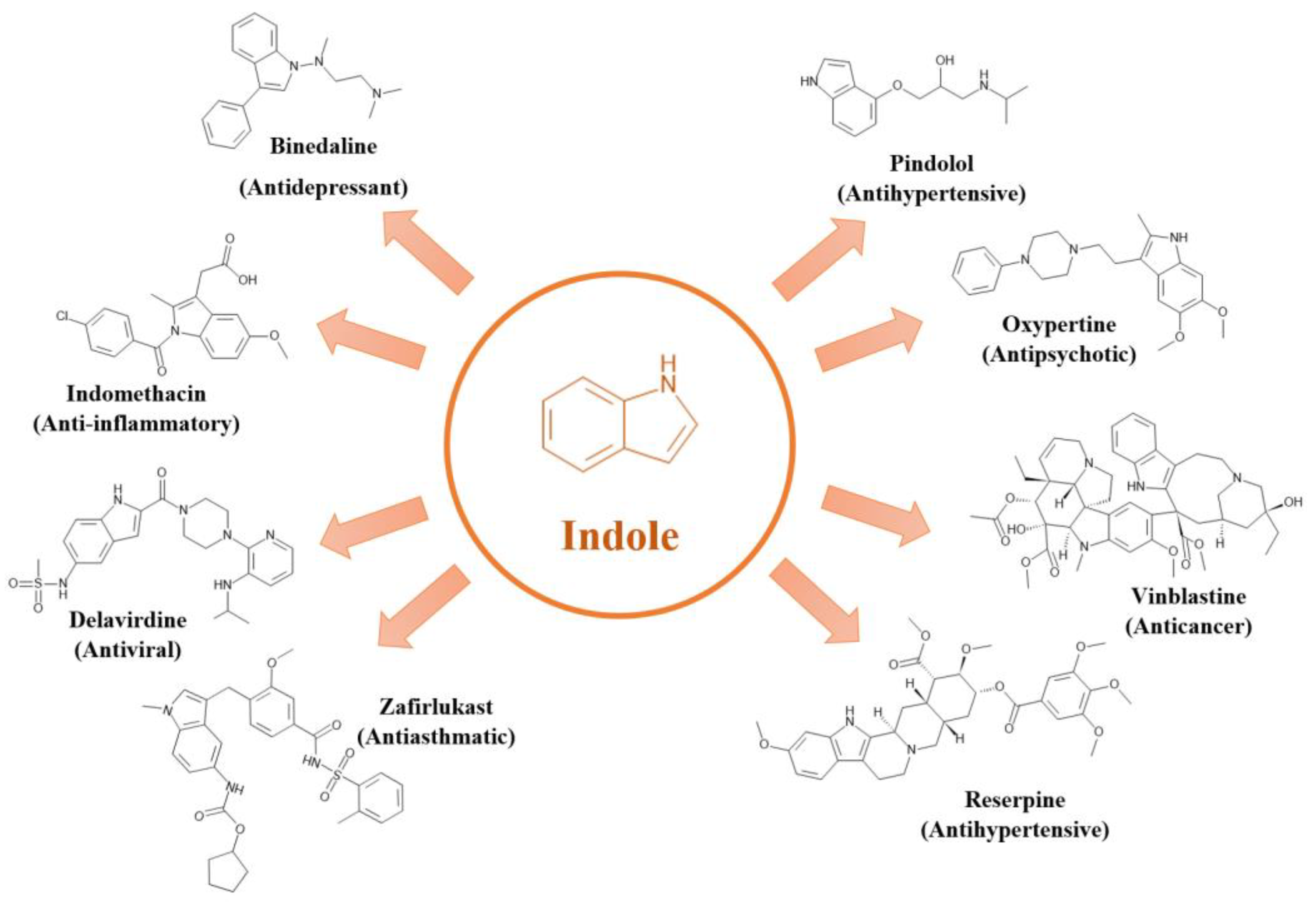
13]. Among these, the indole heterocycle is one of the most widely used therapeutics found in both natural and synthetic sources. It possesses a diverse range of medicinal and biological activities [
30,
31]. The indole's physiological activity and its demonstrated anti-inflammatory, antibacterial, antitubercular, anticonvulsant, antifungal, antimalarial, antidiabetic, antidepressant, antioxidant, anticancer, and antimicrobial effects have garnered significant attention [
16,
32,
33]. Indole and its derivatives are indispensable in medicinal chemistry.
Figure 1 illustrates the well-known basic structure of indole and its derivatives commonly used in medicines and drugs.
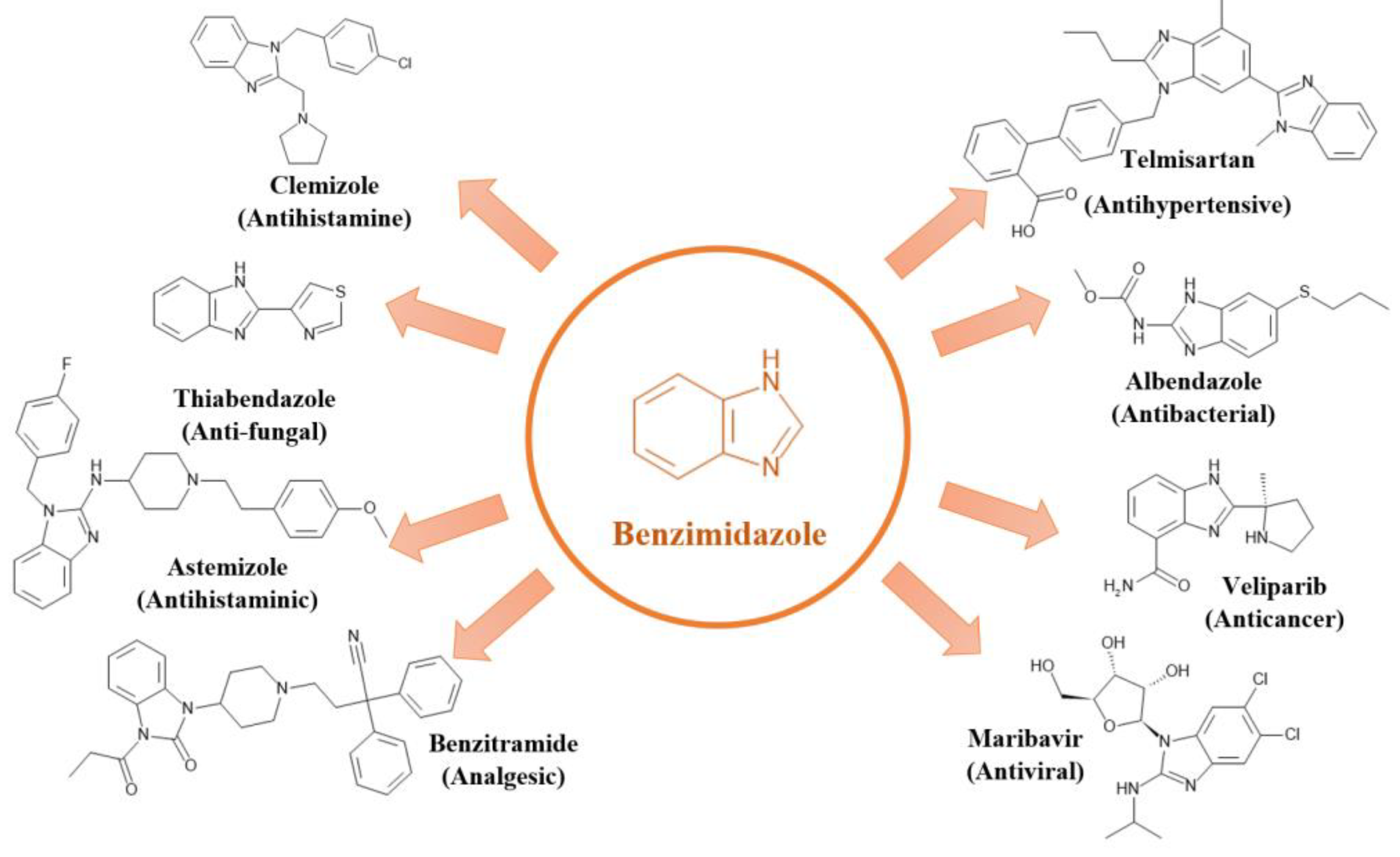
2.1.1. Benzimidazole and Imidazole
Benzimidazole and imidazole are well-recognized nitrogen-containing heterocyclic rings that exist in similar tautomeric forms [
34]. Their versatile properties have captivated medicinal chemists, leading to their widespread use in pharmaceutical drug development [
35]. Benzimidazole rings, in particular, have long served as a preferred scaffold for synthesizing therapeutic compounds with diverse pharmacological and biological applications [
36]. Notable derivatives include thiabendazole, albendazole, flubendazole, fenbendazole, mebendazole, and triclabendazole [
37]. These compounds have demonstrated significant potential in the discovery of novel agents with various pharmacological and biological activities, including antiprotozoal, antihelminthic, antiviral, antimicrobial, antimalarial, anti-inflammatory, antidiabetic, anticancer, antiparasitic, antioxidant, and anticonvulsant properties [
38,
39]. To illustrate the diverse therapeutic applications of these heterocyclic compounds,
Figure 2 presents the pharmacological activities of different benzimidazole derivatives and their corresponding names and therapeutic classes.
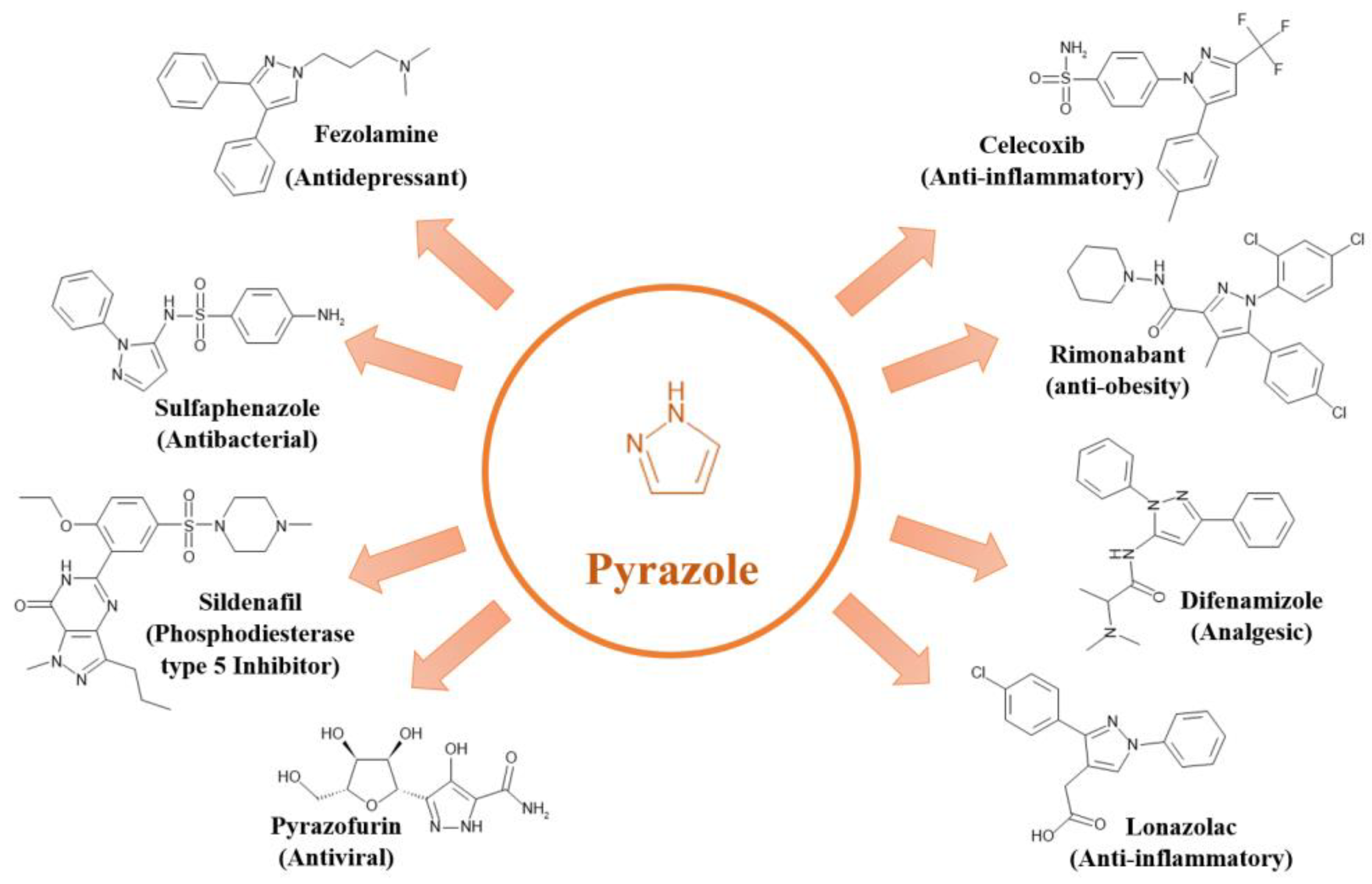
2.1.2. Pyrazole: A Versatile Nitrogen-Containing Heterocycle
Pyrazole, another nitrogen-containing heterocyclic substance, has emerged as a valuable scaffold and adaptable lead compound in pharmaceutical research due to its intriguing biological properties [
40]. Its ease of synthesis and amenability to chemical modification make pyrazole a highly attractive compound for medicinal chemistry [
41].
Pyrazole derivatives exhibit a broad range of cytoprotective and modulatory activities, leading to diverse therapeutic applications, including anticancer, antibacterial, antifungal, antinociceptive, antidepressant, and, most notably, anti-inflammatory effects [
42,
43,
44].
Figure 3 illustrates the chemical structures of the most common pyrazole heterocyclic compounds, along with their corresponding names and therapeutic classes.
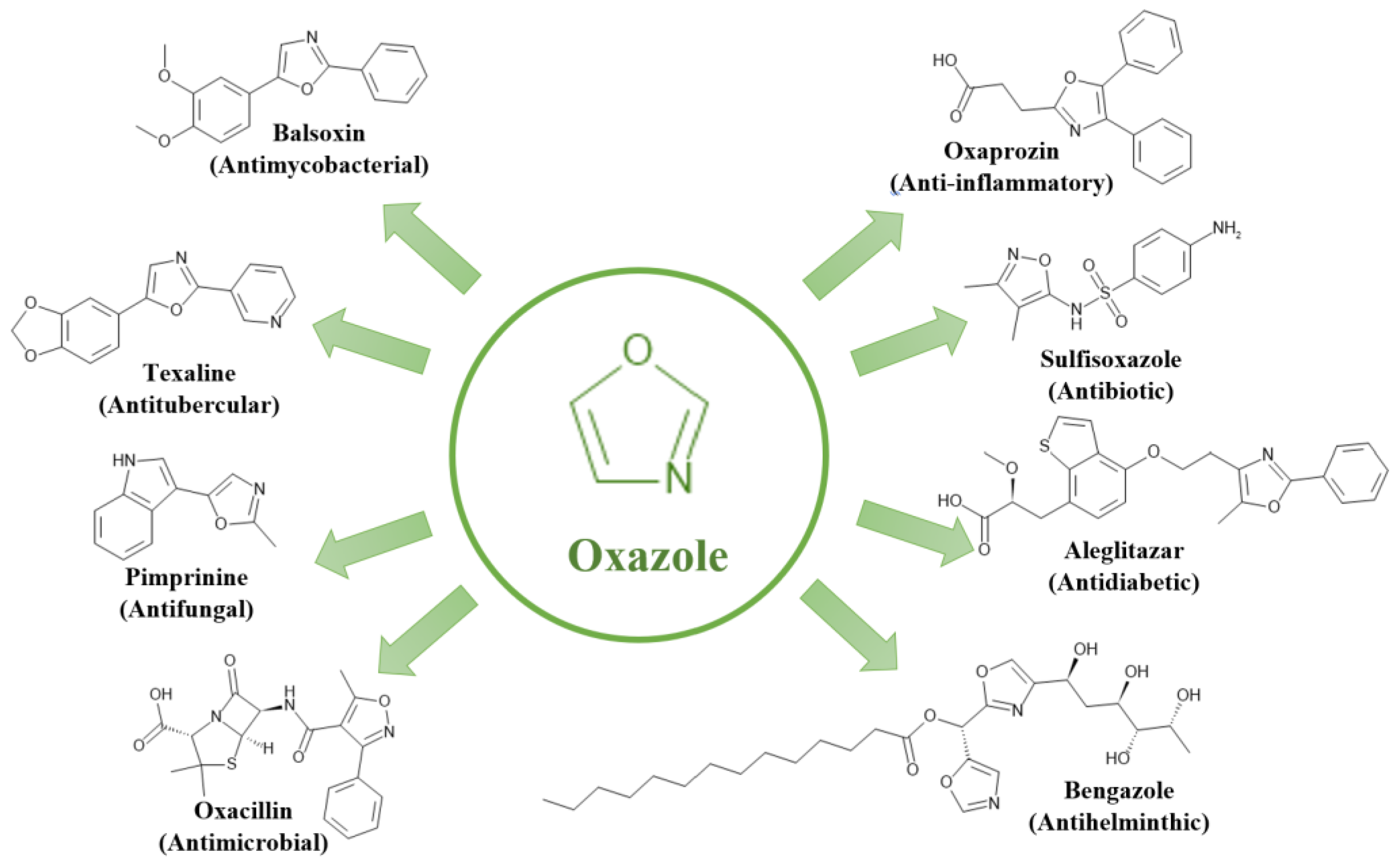
2.2. Oxygen and Nitrogen-Containing Heterocycles
Oxygen and nitrogen-containing heterocycles exhibit unique properties attributed to their ability to form diverse non-covalent interactions with enzymes and biological receptors [
45]. Among the most common examples are oxazole and isoxazole. These compounds differ in positioning their heteroatoms (oxygen and nitrogen) within their ring structures, leading to distinct chemical properties [
46].
These compounds' unique ring structures, heteroatom positions, and chemical properties contribute to their versatile biological activities. Numerous oxazole derivatives, including oxazoles, oxazolines, isoxazoles, oxazolidones, oxadiazoles, and benzoxazoles, have found applications as medicinal drugs [
47]. These compounds have demonstrated significant potential for treating various diseases, showcasing their valuable development potential and broad applicability as drugs with diverse biological activities. These activities encompass anti-inflammatory, antibacterial, antifungal, antiparasitic, anti-obesity, antitubercular, anticancer, antiviral, analgesic, anti-neuropathic, antidiabetic, and antioxidative properties, as illustrated in
Figure 4 [
45,
48].
2.3. Sulfur-Containing Heterocycles
Sulfur-containing heterocycles have garnered significant attention from pharmaceutical researchers in medicinal chemistry due to their demonstrated importance in drug discovery [
49]. These S-heterocycles serve as fundamental building blocks in various synthetic analogues, encompassing a wide range of therapeutic activities. They have been a core component of numerous FDA-approved drugs and active compounds for decades [
49,
50]. Therapeutic applications for these sulfur-containing drugs include anticancer, antiviral, antimicrobial, antidiabetic, antihypertensive, and anti-inflammatory activities [
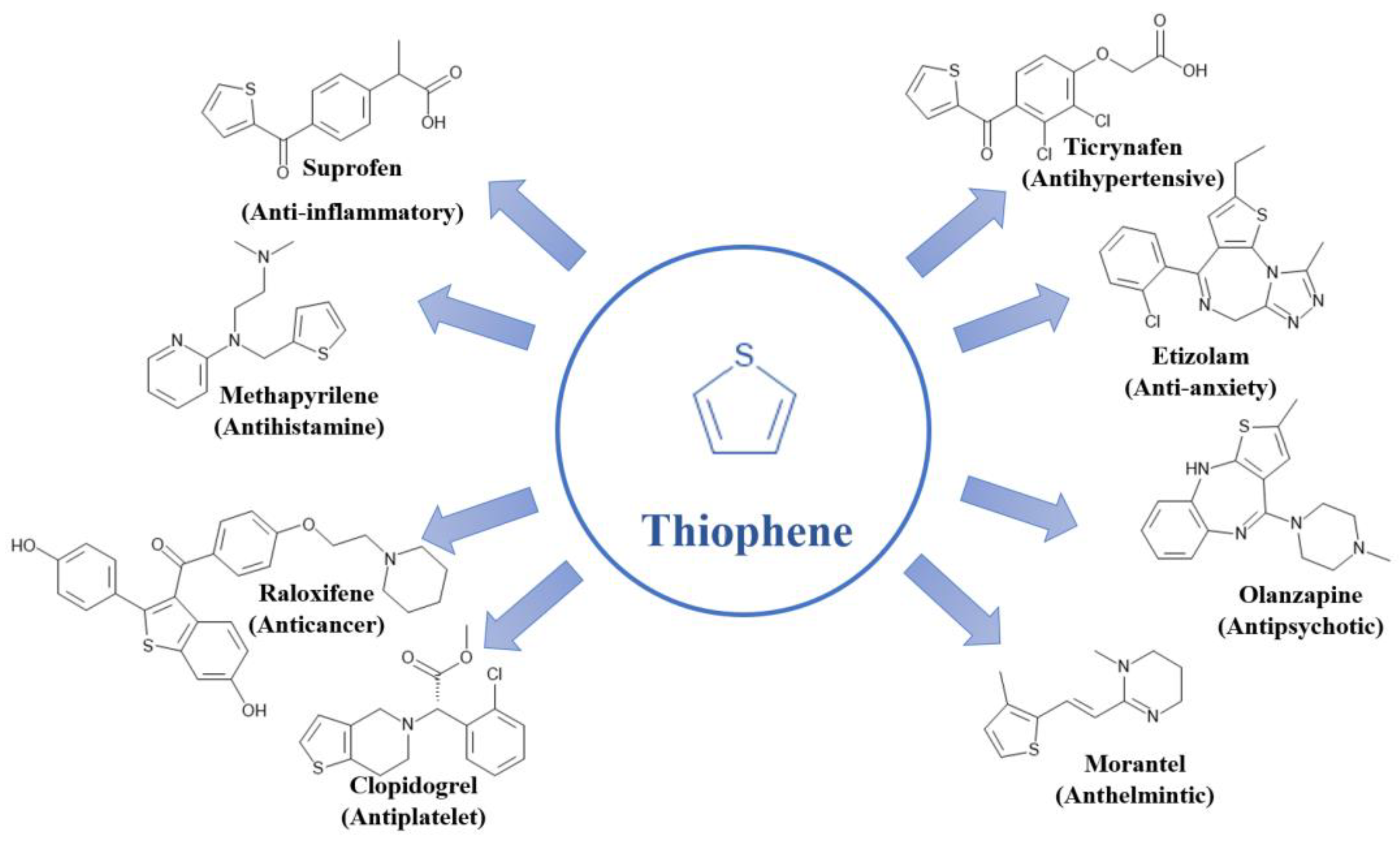
50]. Thiophene (a five-membered heterocycle) and thiopyran (a six-membered heterocycle) represent the most common S-heterocycles that do not contain nitrogen.
Numerous drugs and pharmaceuticals are derived from thiophene, finding widespread application in various medical treatments, as illustrated in
Figure 5.
2.3.1. Thiopyrans: A Less Explored Class of Sulfur-Containing Heterocycles
Compared to thiophene, thiopyrans have received less attention from researchers due to the abundance of diverse six-membered oxygen heterocycles in nature, offering an alternative scaffold [
51]. However, their potential biological activities have sparked growing interest in recent years. Thiopyrans find utility in organic synthesis and pharmaceutical chemistry, serving as versatile building blocks for producing compounds with antibacterial, anti-hyperplasia, anti-psychiatric, and anticancer properties [
52]. A well-known example of a drug derived from thiopyran is Meticrane, a diuretic.
2.4. Nitrogen and Sulfur-Containing Heterocycles
Heterocycles incorporating both nitrogen and sulfur within their structures have witnessed significant advancements in recent decades, leading to the development of novel synthetic and natural agents [
53]. S-N heterocyclic compounds commonly include thiazole, isothiazole, and thiazolidine.
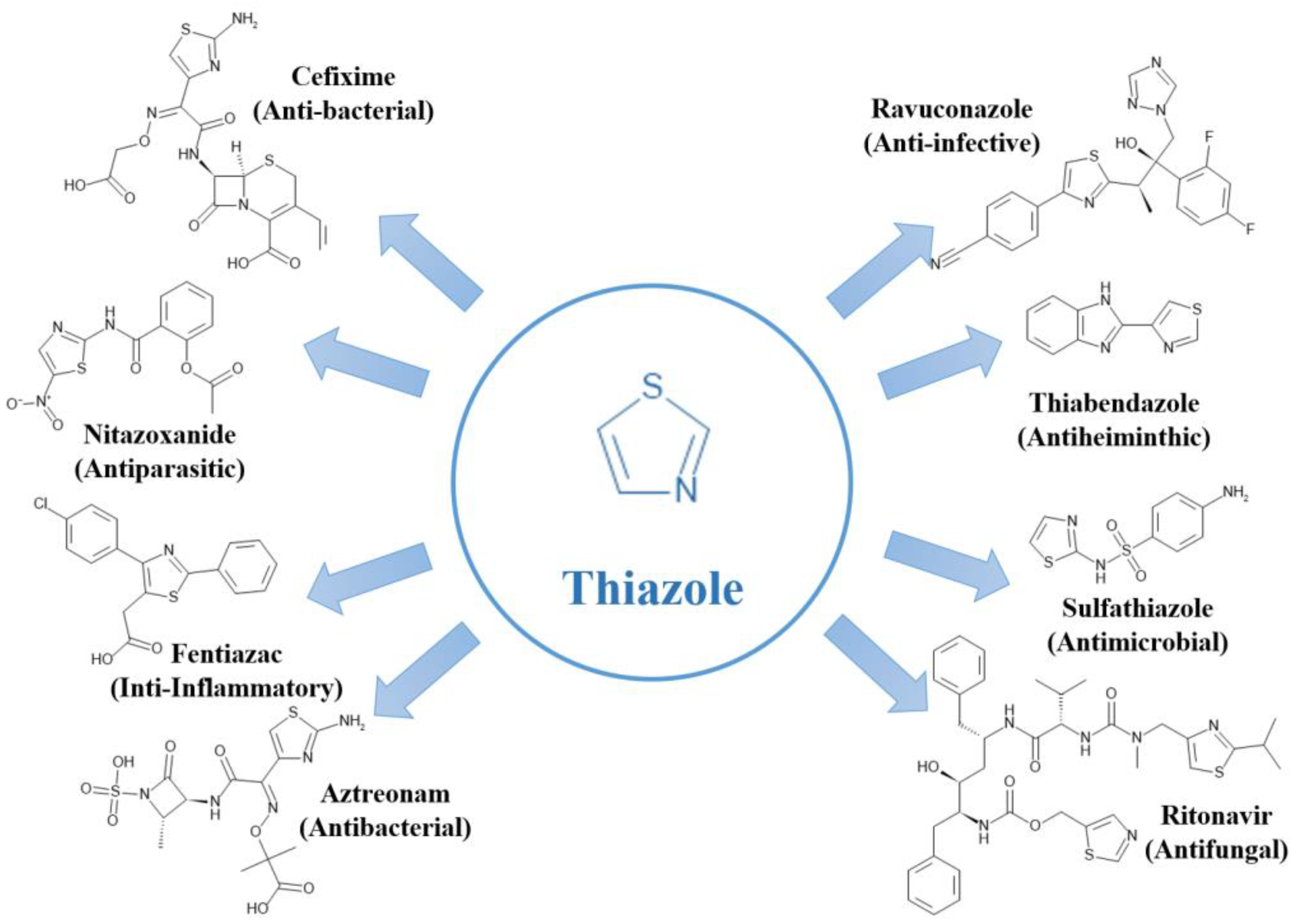
Thiazole, in particular, has garnered widespread attention in pharmaceuticals and medical treatment due to its diverse biological activities, encompassing anti-inflammatory, antibacterial, antiviral, anticancer, and antifungal properties [
54,
55,
56,
57]. The thiazole ring, composed of sulfur and nitrogen, possesses aromatic characteristics, enabling the delocalization of pi (π) electrons between bonds and providing numerous reactive sites for donor-acceptor, nucleophilic, and oxidation reactions [
53,
58,
59]. Several thiazole analogues have gained approval for treating various diseases.
Figure 6 illustrates common chemical structures of thiazole-containing drugs that serve as active pharmaceutical ingredients.
Compared to thiazole, isothiazole (a structural isomer with reversed sulfur and nitrogen positions) and thiazolidine (differing in double bonds) have found less frequent application in medicinal chemistry or therapeutic activities. However, they possess specialized uses. For instance, isothiazole is employed in psychiatric drugs such as ziprasidone, while thiazolidine is utilized in diabetic treatments like Pioglitazone and Rosiglitazone.
3. Sources and Environmental Fate of Heterocyclic Pharmaceuticals
As highlighted in the previous section, numerous pharmaceuticals and compounds used for medical purposes are derived from heterocyclic compounds. These substances, characterized by their versatile chemical structures and therapeutic properties, play a crucial role in modern medicine. Pharmaceuticals are produced in vast quantities worldwide for both human and veterinary use, totaling thousands of tons annually [
60]. Domestic use represents the most significant source of emissions [
61,
62]. Healthcare services, hospitals, industrial chemical residues, veterinary practices, agriculture, and aquaculture all contribute to releasing pharmaceuticals and drugs into the environment [
63].
Pharmaceuticals can enter the environment during manufacturing, consumption, and disposal [
64]. Improper disposal of unused or expired drugs through sinks and toilets contributes to the growing problem of pharmaceutical waste, with an estimated 3-50% of pharmaceuticals becoming waste [
65]. The presence of heterocyclic pharmaceuticals in the environment raises significant concerns due to their high toxicity and persistence, as their breakdown in environmental matrices is often incomplete [
61,
62]. If not detected and managed appropriately, disposal can lead to residue leakage and accumulation over time.
In addition to their widespread use and improper disposal, heterocyclic compounds, mainly those containing nitrogen, exhibit high solubility compared to other pharmaceutical compounds. This allows them to readily enter aquatic environments, becoming prevalent in both surface and groundwater, as well as wastewater from industrial processes [
18,
66]. These chemicals can also be transferred to the soil through irrigation and other practices. Increased quantities of heterocyclic pharmaceuticals have been detected in various aquatic environments, including surface water, groundwater, and wastewater, within a concentration range of 0.03 to 11,000 ng/L [
62]. Surface waters have been found to contain concentrations ranging from 0.03 ng/L to 11 μg/L [
18]. A 2023 study by Zhang et al. on wastewater treatment plant effluent and river samples identified 5,783 organic compounds, including pharmaceuticals [
66]. The study revealed that wastewater treatment plant effluent contributed 33.6% of these compounds to the river, highlighting the significant environmental impact of wastewater treatment plants on aquatic pollution and the need for improved treatment technologies.
Heterocyclic pharmaceuticals in aquatic systems can undergo various fates, including biodegradation, photolysis, or sorption. Microbial communities and photolysis can partially degrade these compounds, while their sorption in all environmental matrices affects bioavailability, potentially causing toxicity to the ecosystem [
67]. The resistance of pharmaceutical pollutants to conventional wastewater treatment plant methods poses a significant challenge.
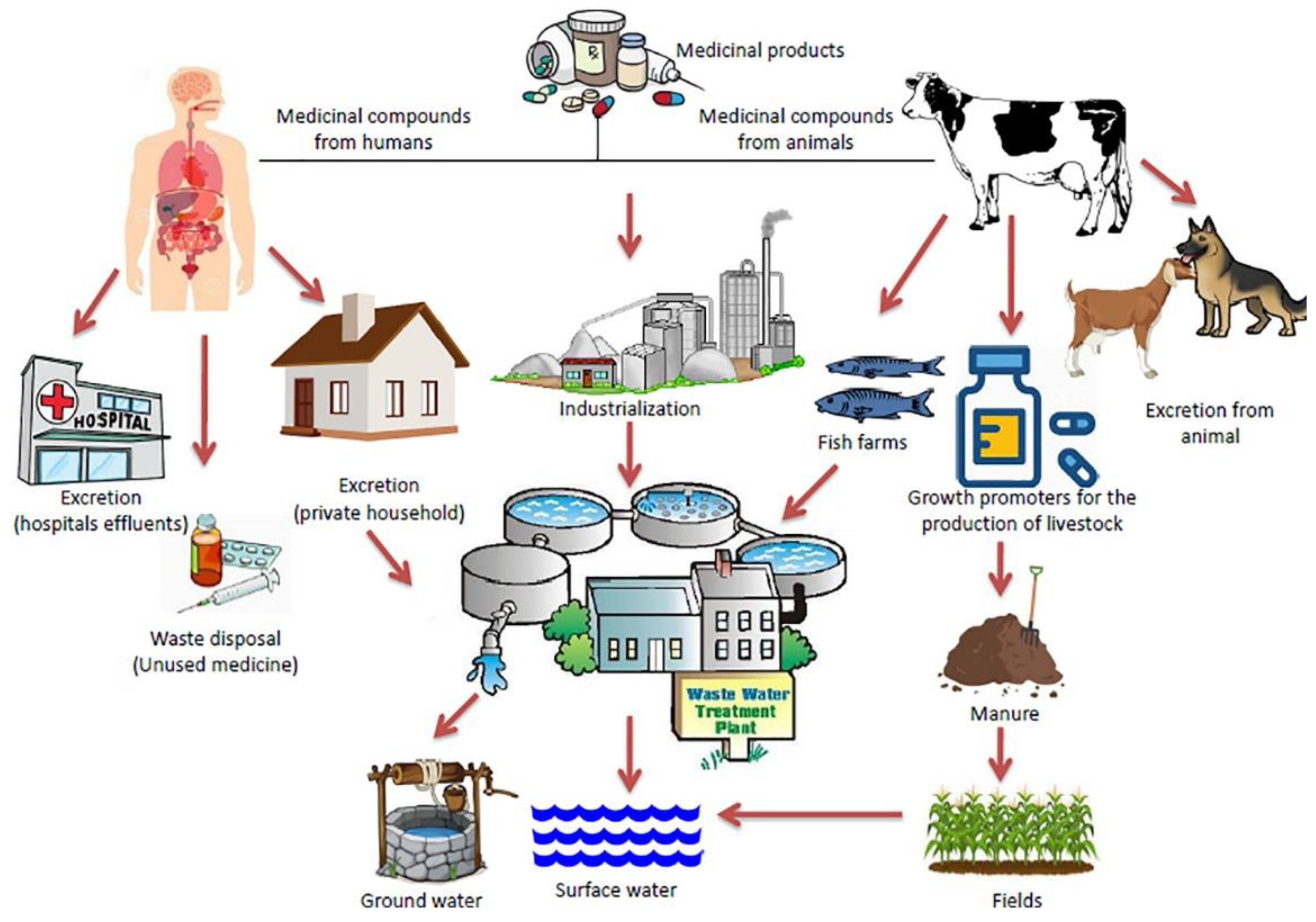
Figure 7 summarizes the pathways through which heterocyclic pharmaceuticals are released into the environment, emphasizing their environmental contamination. It focuses on the sources and fate, including industrial waste, improper human and animal waste disposal, and wastewater effluents from domestic use and hospitals.
4. Toxicity, Long-Term Risks, and Impacts on Ecosystems of Heterocyclic Pharmaceuticals
As noted in
Section 2, heterocyclic pharmaceuticals play a vital role in healthcare, offering significant therapeutic advantages and improving patients' quality of life. However, as discussed in
Section 3, their widespread usage and persistence pose a substantial environmental concern. Heterocyclic compounds present considerable health hazards due to their inherent recalcitrance and notable acute toxicity, mutagenicity, carcinogenicity, teratogenicity, and genotoxicity in diverse species, including humans, microbes, animals, and plants [
68,
69,
70]. Not only do heterocycles exhibit these effects in their structural form, but their toxicity can also increase as they decompose into more hazardous derivatives and byproducts.
In addition to these detrimental effects, heterocyclic compounds exhibit persistence in environmental matrices due to their resistance to degradation processes and possess high bioavailability. Nitrogen, oxygen, or sulfur-containing heterocycles have higher water solubility, potentially contaminating groundwater and adversely impacting soil by transporting them through water, posing environmental risks such as aquatic and terrestrial toxicity, biomagnification, and potential human health risks [
18].
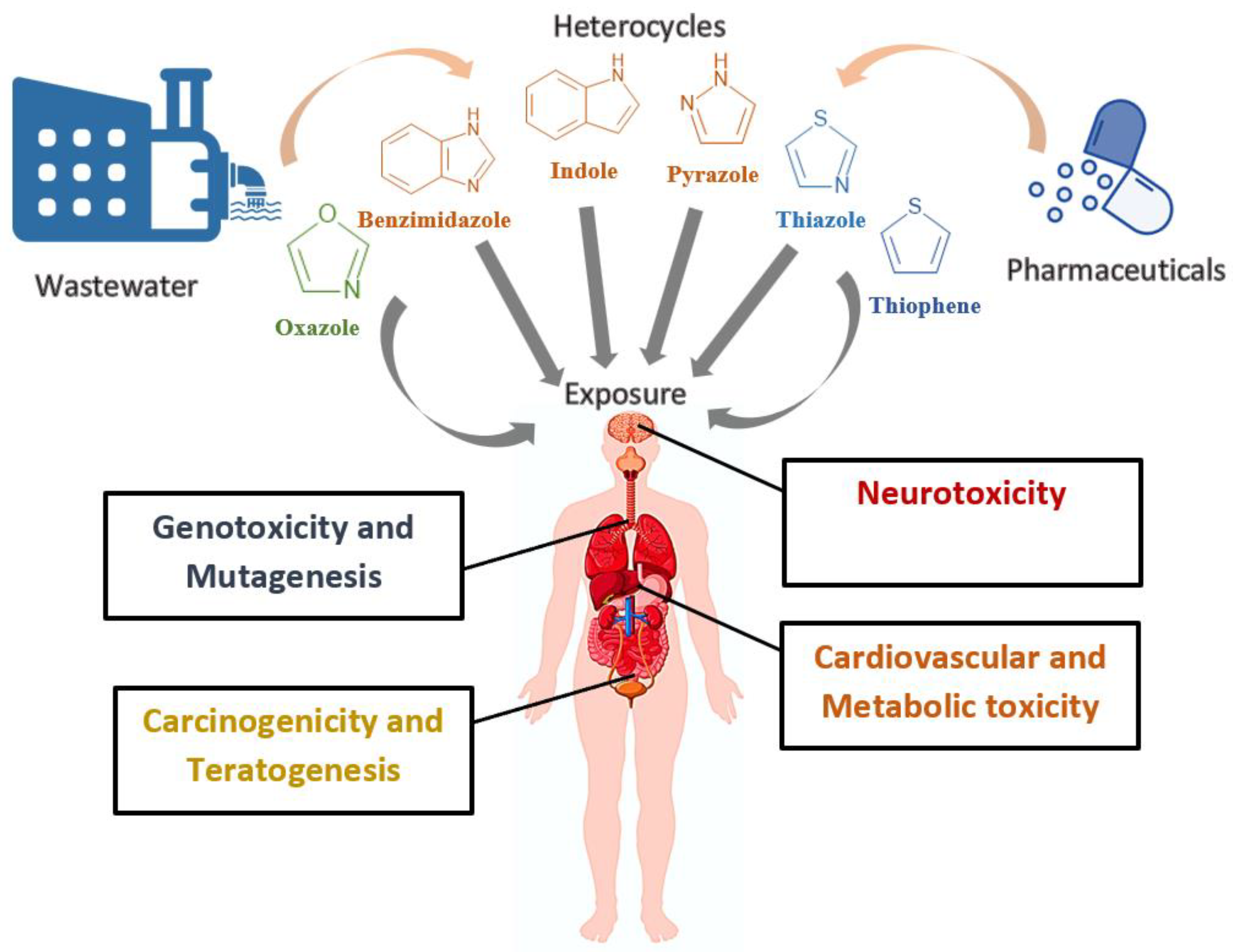
As demonstrated in the previous section, exposure to heterocyclic pharmaceuticals occurs through various pathways, including water, soil, food ingestion, inhalation, and contact [
71]. This exposure can lead to numerous toxicological impacts, such as neurotoxicity, genotoxicity and mutagenesis, cardiovascular and metabolic toxicity, carcinogenicity, and teratogenesis [
72]. Nitrogen or oxygen-containing compounds can cause neurotoxicity through oxidative damage and neurodegenerative disorders. Pyrazole, for example, can depress the central nervous system, and prolonged exposure can lead to neurological disruption, headaches, dizziness, and cognitive decline [
62]. Thiophene, an S-containing heterocycle, can also cause short-term headaches, dizziness, and unconsciousness, while long-term exposure affects the neurological and cardiovascular systems without known carcinogenic effects [
62].
Heterocyclic compounds can potentially cause DNA breakdown and mutations through genotoxicity, disrupting gene regulation, oxidative stress, mitochondrial dysfunction, and apoptosis. Mutagenesis occurs when reactive metabolites directly bind to DNA through activating aromatic hydrocarbon receptors (AhR), posing significant risks, especially with long-term exposure [
73]. Indoxyl sulfate, a metabolite of indole (one of the heterocyclic compounds), can cause cardiovascular toxicity, including myocardial necrosis, hypertrophy, fibrosis, and vasculitis, especially at high concentrations [
62]. Prolonged exposure to heterocyclic substances can disrupt normal metabolic processes through mechanisms related to oxidative stress, leading to metabolic disorders like insulin resistance or the development of type 2 diabetes [
74].
Nitrogen-containing heterocyclic substances, including pyrazole, are strongly carcinogenic and linked to various cancers like prostate and colorectal cancer. The substitution of nitrogen atoms alters their toxicological profile, making them more persistent and capable of DNA damage. Consequently, some nitrogen-containing heterocycles are classified as human carcinogens by the International Agency for Research on Cancer (IARC) [
73]. Certain heterocyclic chemicals can induce developmental defects in humans and teratogenesis, mainly when exposure occurs during pregnancy [
71,
73].
While heterocyclic pharmaceuticals offer significant medical benefits, it is evident that they pose severe risks due to their persistence and bioaccumulation. Their toxic effects, ranging from neurotoxicity to carcinogenic potential, can adversely affect critical biological functions and pathways.
Figure 8 summarizes the toxicological effects of these chemicals on human health.
4.1. Ecological Impacts of Heterocyclic Pharmaceuticals
The extensive toxicological consequences of heterocyclic compounds extend beyond human health to ecosystems. These compounds significantly threaten aquatic and terrestrial creatures, including marine species, soil organisms, and plants. Their mobility and widespread availability exacerbate this risk. The persistence of heterocyclic compounds in the environment, their potential for long-term harm, and their accumulation in aquatic environments have adverse impacts on fish, invertebrates, and algae, often resulting in lethal and sub-lethal effects such as chronic toxicity that disrupts reproductive and developmental processes [
71]. Terrestrial toxicity, which affects soil organisms, including earthworms and plants, inhibits soil ecosystem function.
Studies examining the aquatic ecotoxicity of heterocycles have utilized three species: the marine bacterium Aliivibrio fischeri, the freshwater algae Raphidocelis subcapitata, and the crustacean
Daphnia magna. Experiments have revealed that most tested heterocycles are "very toxic" to freshwater species, with EC50 values less than 1 mg/L, placing them in the acute danger category 1 [
75]. Another study focused on the toxicity of heterocycles, employing
D. magna crabs and
Saccharomyces cerevisiae yeast as test specimens. The study found that both the heterocyclic core and the halogen atoms influenced the compounds' toxicity against
D. magna [
76].
The multifaceted toxicity of heterocycles and their capacity to damage both the environment and human health underscore the urgent need for robust monitoring and regulatory measures. Comprehensive risk assessments and ongoing monitoring are essential to mitigate heterocyclic pharmaceutical residues' environmental fate, effects, and toxicity.
5. Remediation Technologies and Treatment Solutions of Heterocyclic Pharmaceutical
Heterocyclic pharmaceuticals pose significant environmental concerns due to their persistence and potential toxicity in aquatic ecosystems, as highlighted in the previous section. Addressing the long-term ecological risks associated with these pollutants requires a multifaceted approach, encompassing source reduction, monitoring techniques, analysis and detection, regulatory frameworks, and environmental treatment.
Various methods are available for monitoring these compounds in the environment, including Quantitative Structure-Activity Relationship (QSAR) and Quantitative Structure-Property Relationship (QSPR) models. These models predict the risks associated with pollutants' physicochemical properties [
77]. Developing green and sustainable approaches for producing pharmaceutical heterocyclic compounds can also serve as remediation practices, ensuring the production of physiologically active molecules for drug design while aligning with pharmaceutical production sustainability [
78,
79].
Even after monitoring and detection, the bioactivity of heterocyclic pharmaceuticals remains a concern. Their presence in water bodies can lead to long-term environmental and health implications, necessitating their removal [
80]. Conventional wastewater treatment plants often prove inadequate in eliminating these persistent organic contaminants [
81]. This has spurred interest in developing more efficient and appropriate water treatment systems to address this challenge [
80].
5.1. Evaluation of Remediation Technologies
To effectively remove these contaminants from wastewater, a variety of remediation technologies and treatment solutions have been developed, including biological treatment [
18], advanced oxidation processes (photo-Fenton and photocatalysis) [
82], and membrane and filtration technologies [
83].
Table 1 summarizes some relevant findings from the literature on the removal of pharmaceutical heterocyclic compounds.
Each remediation method possesses its own advantages and limitations. For instance, a significant concern with photocatalytic degradation is the generation of hazardous byproducts, which requires further research to elucidate reaction pathways and intermediates and identify byproducts [
69]. A study by Zhang et al. in 2023 investigated the toxicity of nitrogen-containing heterocyclic compounds after UV/H2O2 treatment using D. magna and Vibrio fischeri assays. The results revealed increased toxicity for both compounds post-treatment, suggesting that the degradation process could inadvertently generate toxic byproducts [
96]. This finding underscores the need for more effective strategies that ensure the proper degradation of these persistent pollutants while minimizing the development and toxicity of hazardous byproducts.
Combination approaches can achieve more effective results in removing emerging contaminants. Future research should prioritize innovative, sustainable treatment solutions for heterocyclic compounds to reduce toxicity and promote a more sustainable approach to pollution remediation.
6. Conclusions and Perspectives
As demonstrated in this review, heterocyclic pharmaceutical pollutants pose a significant environmental risk due to their toxic and carcinogenic properties. Their widespread use in medical therapy, constituting over 90% of new medications, and their persistence in various environmental matrices, exacerbates the risk and harms the natural ecosystem. Therefore, preventing the spread of these compounds into the environment is imperative.
This review has comprehensively examined the sources, environmental fate, toxicity, and long-term risks associated with heterocyclic pharmaceuticals, proposing potential remediation strategies. The study commenced with an overview of the diverse types of heterocyclic pharmaceuticals and their applications, focusing on the most common heteroatoms: nitrogen, oxygen, and sulfur. It subsequently explored their sources and pathways into the environment, emphasizing their resistance to biodegradation and their ability to accumulate in organisms. These chemicals can enter water systems through wastewater discharge, agricultural runoff, and improper disposal.
Furthermore, the review discussed the toxic effects and long-term consequences of exposure to heterocyclic pharmaceuticals, including neurotoxicity, genotoxicity, mutagenesis, cardiovascular and metabolic toxicity, carcinogenicity, and teratogenesis. Additionally, various remediation strategies and treatment solutions for reducing the environmental impact of these compounds were reviewed, and multiple technologies and approaches were identified for efficiently removing contaminants from wastewater, such as biological treatment, advanced oxidation processes, and membrane and filtration technologies.
This review concludes by emphasizing the urgent need for future research to develop effective strategies for reducing the formation and toxicity of hazardous byproducts from persistent pollutants. It recommends combination approaches, which have shown greater efficacy in removing emerging contaminants. Additionally, there is a critical need to innovate and implement sustainable treatment solutions for heterocyclic compounds to mitigate their toxicity and promote a more sustainable approach to pollution remediation.
The topic discussed here also invites further reflection on "Global Health and Environmental Justice." The environmental impact of heterocyclic pharmaceuticals, like other pollution sources, often disproportionately affects marginalized communities and developing countries with limited infrastructure and access to clean water and sanitation. This highlights the complexity of potential solutions. Given human, animal, and environmental health interconnectedness, the One Health approach appears to be the most appropriate.
Furthermore, to mitigate the environmental impact of heterocyclic pharmaceuticals, it is essential to develop and enforce robust international regulations governing these substances' production, use, and disposal. Increased public awareness of the environmental risks associated with the production and consumption of pharmaceuticals, including heterocyclics, is also necessary. This awareness can promote responsible consumption and disposal practices, as well as the adoption of green chemistry practices in the pharmaceutical industry, such as designing and producing compounds with reduced toxicity and persistence.
Continuous research and development of innovative wastewater treatment technologies, such as advanced oxidation processes, membrane filtration, and bioremediation, are crucial for effectively removing heterocyclic pharmaceuticals from the environment. Additionally, nanomaterial-based solutions for pollutant removal and degradation should be explored.
Author Contributions
Conceptualization, O.B. and L.S.; methodology, M.B.; validation, S.A.B., O.B. and M.B; data curation, F.L., A.Z.; writing—original draft preparation, O.B.; writing—review and editing, M.B., L.E. and S.A.B.; supervision, L.S. and L.E. Administration: L.S. for toxicity aspects, Long-Term risks, and impacts on ecosystems of pharmaceuticals, L.E. for pharmaceuticals removal methods. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data sharing is not applicable to this article as it is a review of previously published studies.
Acknowledgments
Dr. Oussama Baaloudj acknowledges his post-doctorate scholarship from MUR in the framework of the PRIMA-Partnership for Research & Innovation in the Mediterranean Area through the research project SAFE "Sustainable water reuse practices improving safety in Agriculture, Food and Environment". Project ID 1826.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kabir, E.; Uzzaman, M. A review on biological and medicinal impact of heterocyclic compounds. Results Chem. 2022, 4. [CrossRef]
- Taylor, A.P.; Robinson, R.P.; Fobian, Y.M.; Blakemore, D.C.; Jones, L.H.; Fadeyi, O. Modern advances in heterocyclic chemistry in drug discovery. Org. Biomol. Chem. 2016, 14, 6611–6637. [CrossRef]
- Aryal, N.; Wood, J.; Rijal, I.; Deng, D.; Jha, M.K.; Ofori-Boadu, A.; Khan, H.K.; Rehman, M.Y.A.; Malik, R.N.; Maculewicz, J.; et al. Transformation products of pharmaceuticals in the environment: Their fate, (eco)toxicity and bioaccumulation potential. J. Environ. Manage. 2020, 271, 1587–1594.
- Broughton, H.B.; Watson, I.A. Selection of heterocycles for drug design. J. Mol. Graph. Model. 2004, 23, 51–58. [CrossRef]
- Lamberth, C.; Dinges, J. Bioactive Heterocyclic Compound Classes: Agrochemicals. Bioact. Heterocycl. Compd. Classes Agrochem. 2012. [CrossRef]
- Lu, L.Q.; Chen, J.R.; Xiao, W.J. Development of cascade reactions for the concise construction of diverse heterocyclic architectures. Acc. Chem. Res. 2012, 45, 1278–1293. [CrossRef]
- Dua, R.; Shrivastava, S.; Sonwane, S.K.; Srivastava, S.K.; others Pharmacological significance of synthetic heterocycles scaffold: a review. Adv. Biol. Res. (Rennes). 2011, 5, 120–144.
- Moreno Ríos, A.L.; Gutierrez-Suarez, K.; Carmona, Z.; Ramos, C.G.; Silva Oliveira, L.F. Pharmaceuticals as emerging pollutants: Case naproxen an overview. Chemosphere 2022, 291. [CrossRef]
- Huanyu, T.; Jianghong, S.; Wei, G.; Jiawei, Z.; Hui, G.; Yunhe, W. Environmental fate and toxicity of androgens: A critical review. Environ. Res. 2022, 214. [CrossRef]
- josef jampilek Heterocycles in Medicinal Chemistry. Molecules 2019, 24, 1–4.
- Li Petri, G.; Holl, R.; Spanò, V.; Barreca, M.; Sardo, I.; Raimondi, M.V. Editorial: Emerging heterocycles as bioactive compounds. Front. Chem. 2023, 11. [CrossRef]
- Sadek, K.U.; Mekheimer, R.A.; Abd-Elmonem, M.; Abo-Elsoud, F.A.; Hayallah, A.M.; Mostafa, S.M.; Abdellattif, M.H.; Abourehab, M.A.S.; Farghaly, T.A.; Elkamhawy, A. Recent developments in the synthesis of hybrid heterocycles, a promising approach to develop multi-target antibacterial agents. J. Mol. Struct. 2023, 1286. [CrossRef]
- Babu, A.; Sunil, K.; Sajith, A.M.; Reddy, E.K.; Santra, S.; Zyryanov, G. V.; Venkatesh, T.; Bhadrachari, S.; Nibin Joy, M. NMI-SO2Cl2-Mediated Amide Bond Formation: Facile Synthesis of Some Dihydrotriazolopyrimidine Amide Derivatives as Potential Anti-Inflammatory and Antitubercular Agents. Pharmaceuticals 2024, 17. [CrossRef]
- Brendel, S.; Polleichtner, C.; Behnke, A.; Jessel, S.; Hassold, E.; Jennemann, C.; Einhenkel-Arle, D.; Seidel, A. Four selected high molecular weight heterocyclic aromatic hydrocarbons: Ecotoxicological hazard assessment, environmental relevance and regulatory needs under REACH. Ecotoxicol. Environ. Saf. 2018, 163, 340–348. [CrossRef]
- González-Andrés, P.; Fernández-Peña, L.; Díez-Poza, C.; Villalobos, C.; Nuñez, L.; Barbero, A. Marine Heterocyclic Compounds That Modulate Intracellular Calcium Signals: Chemistry and Synthesis Approaches. Mar. Drugs 2021, 19. [CrossRef]
- Khetan, S.K.; Collins, T.J. Human Pharmaceuticals in the Aquatic Environment: A Challenge to Green Chemistry. ChemInform 2007, 38. [CrossRef]
- Padoley, K. V.; Mudliar, S.N.; Pandey, R.A. Heterocyclic nitrogenous pollutants in the environment and their treatment options - An overview. Bioresour. Technol. 2008, 99, 4029–4043. [CrossRef]
- Ghosh, P.; Mukherji, S. Environmental contamination by heterocyclic Polynuclear aromatic hydrocarbons and their microbial degradation. Bioresour. Technol. 2021, 341. [CrossRef]
- Boreen, A.L.; Arnold, W.A.; McNeill, K. Photochemical fate of sulfa drugs in then aquatic environment: Sulfa drugs containing five-membered heterocyclic groups. Environ. Sci. Technol. 2004, 38, 3933–3940. [CrossRef]
- Hou, L.; Jin, X.; Liu, N.; Luo, Y.; Yan, Z.; Chen, M.; Liu, Y.; Xie, H.; Giesy, J.P.; Wu, F.; et al. Triadimefon in aquatic environments: occurrence, fate, toxicity, and ecological risk. Environ. Sci. Eur. 2022, 34. [CrossRef]
- Cordoba, A.; Saldias, C.; Urzúa, M.; Montalti, M.; Guernelli, M.; Focarete, M.L.; Leiva, A. On the Versatile Role of Electrospun Polymer Nanofibers as Photocatalytic Hybrid Materials Applied to Contaminated Water Remediation: A Brief Review. Nanomaterials 2022, 12. [CrossRef]
- Gupta, S.M.; Tripathi, M. A review of TiO2 nanoparticles. Chinese Sci. Bull. 2011, 56, 1639–1657. [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review. J. Hazard. Mater. 2017, 323, 274–298. [CrossRef]
- Asfaha, Y.G.; Tekile, A.K.; Zewge, F. Hybrid process of electrocoagulation and electrooxidation system for wastewater treatment: A review. Clean. Eng. Technol. 2021, 4, 100261. [CrossRef]
- Wang, J.J.; Shen, J.; Ye, D.; Yan, X.; Zhang, Y.; Yang, W.; Li, X.; Wang, J.J.; Zhang, L.; Pan, L. Disinfection technology of hospital wastes and wastewater: Suggestions for disinfection strategy during coronavirus Disease 2019 (COVID-19) pandemic in China. Environ. Pollut. 2020, 262, 114665. [CrossRef]
- Bergamasco, R.; Konradt-Moraes, L.C.; Vieira, M.F.; Fagundes-Klen, M.R.; Vieira, A.M.S. Performance of a coagulation-ultrafiltration hybrid process for water supply treatment. Chem. Eng. J. 2011, 166, 483–489. [CrossRef]
- Ashrafi, O.; Yerushalmi, L.; Haghighat, F. Wastewater treatment in the pulp-and-paper industry: A review of treatment processes and the associated greenhouse gas emission. J. Environ. Manage. 2015, 158, 146–157. [CrossRef]
- Qadir, T.; Amin, A.; Sharma, P.K.; Jeelani, I.; Abe, H. A Review on Medicinally Important Heterocyclic Compounds. Open Med. Chem. J. 2022, 16. [CrossRef]
- Sharma, S.; Kumar, D.; Singh, G.; Monga, V.; Kumar, B. Recent advancements in the development of heterocyclic anti-inflammatory agents. Eur. J. Med. Chem. 2020, 200. [CrossRef]
- KHANDALE, N.; GHODKE, M.S. Exploring Potential of Indole Derivatives: a Brief Review. Int. J. Pharm. Pharm. Sci. 2023, 1–14. [CrossRef]
- Kumari, A.; Singh, R.K. Medicinal chemistry of indole derivatives: Current to future therapeutic prospectives. Bioorg. Chem. 2019, 89. [CrossRef]
- Mushtaq, I.; Ahmed, A. Synthesis of biologically active sulfonamide-based indole analogs: a review. Futur. J. Pharm. Sci. 2023, 9. [CrossRef]
- Hao, C.; Lissemore, L.; Nguyen, B.; Kleywegt, S.; Yang, P.; Solomon, K. Determination of pharmaceuticals in environmental waters by liquid chromatography/electrospray ionization/tandem mass spectrometry. Anal. Bioanal. Chem. 2006, 384, 505–513. [CrossRef]
- Tahlan, S.; Kumar, S.; Narasimhan, B. Pharmacological significance of heterocyclic 1H-benzimidazole scaffolds: a review. BMC Chem. 2019, 13. [CrossRef]
- Gaba, M.; Mohan, C. Development of drugs based on imidazole and benzimidazole bioactive heterocycles: Recent advances and future directions. Med. Chem. Res. 2016, 25, 173–210. [CrossRef]
- Brishty, S.R.; Hossain, M.J.; Khandaker, M.U.; Faruque, M.R.I.; Osman, H.; Rahman, S.M.A. A Comprehensive Account on Recent Progress in Pharmacological Activities of Benzimidazole Derivatives. Front. Pharmacol. 2021, 12. [CrossRef]
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [CrossRef]
- Hashem, H.E.; El Bakri, Y. An overview on novel synthetic approaches and medicinal applications of benzimidazole compounds: An overview on novel synthetic approaches and medicinal applications. Arab. J. Chem. 2021, 14. [CrossRef]
- Keri, R.S.; Hiremathad, A.; Budagumpi, S.; Nagaraja, B.M. Comprehensive review in current developments of benzimidazole-based medicinal chemistry. Chem. Biol. Drug Des. 2015, 86, 799–845. [CrossRef]
- Costa, R.F.; Turones, L.C.; Cavalcante, K.V.N.; Rosa Júnior, I.A.; Xavier, C.H.; Rosseto, L.P.; Napolitano, H.B.; Castro, P.F. da S.; Neto, M.L.F.; Galvão, G.M.; et al. Heterocyclic Compounds: Pharmacology of Pyrazole Analogs From Rational Structural Considerations. Front. Pharmacol. 2021, 12. [CrossRef]
- Kumar, H.; Saini, D.; Jain, S.; Jain, N. Pyrazole scaffold: A remarkable tool in the development of anticancer agents. Eur. J. Med. Chem. 2013, 70, 248–258. [CrossRef]
- Adardour, M.; Ait Lahcen, M.; Oubahmane, M.; Ettahiri, W.; Hdoufane, I.; Bouamama, H.; Alanazi, M.M.; Cherqaoui, D.; Taleb, M.; Garcia, E.Z.; et al. Design, Synthesis, Molecular Modeling and Biological Evaluation of Novel Pyrazole Benzimidazolone Derivatives as Potent Antioxidants. Pharmaceuticals 2023, 16. [CrossRef]
- El-Helw, E.A.E.; Gado, M.M.; El-Ziaty, A.K. Synthesis and anti-rotavirus activity of some nitrogen heterocycles integrated with pyrazole scaffold. J. Iran. Chem. Soc. 2020, 17, 1479–1492. [CrossRef]
- Kabi, A.K.; Sravani, S.; Gujjarappa, R.; Garg, A.; Vodnala, N.; Tyagi, U.; Kaldhi, D.; Singh, V.; Gupta, S.; Malakar, C.C. Overview on Biological Activities of Pyrazole Derivatives. Mater. Horizons From Nat. to Nanomater. 2022, 229–306. [CrossRef]
- Zhang, H.Z.; Zhao, Z.L.; Zhou, C.H. Recent advance in oxazole-based medicinal chemistry. Eur. J. Med. Chem. 2018, 144, 444–492. [CrossRef]
- Atmaram, U.A.; Roopan, S.M. Biological activity of oxadiazole and thiadiazole derivatives. Appl. Microbiol. Biotechnol. 2022, 106, 3489–3505. [CrossRef]
- Joshi, S.; Mehra, M.; Singh, R.; Kakar, S. Review on Chemistry of Oxazole derivatives: Current to Future Therapeutic Prospective. Egypt. J. Basic Appl. Sci. 2023, 10, 218–239. [CrossRef]
- Safarzaei, M.; Maghsoodlou, M.T.; Mollashahi, E.; Hazeri, N.; Lashkari, M. Synthesis of 3-aminoisoxazolmethylnaphthols via one-pot three-component reaction under solvent-free conditions. Res. Chem. Intermed. 2018, 44, 7449–7458. [CrossRef]
- Pathania, S.; Narang, R.K.; Rawal, R.K. Role of sulphur-heterocycles in medicinal chemistry: An update. Eur. J. Med. Chem. 2019, 180, 486–508. [CrossRef]
- Archna; Pathania, S.; Chawla, P.A. Thiophene-based derivatives as anticancer agents: An overview on decade's work. Bioorg. Chem. 2020, 101. [CrossRef]
- Ingall, A.H. Thiopyrans and Fused Thiopyrans. Compr. Heterocycl. Chem. 1984, 3–7, 885–942. [CrossRef]
- Laxmikeshav, K.; Kumari, P.; Shankaraiah, N. Expedition of sulfur-containing heterocyclic derivatives as cytotoxic agents in medicinal chemistry: A decade update. Med. Res. Rev. 2022, 42, 513–575. [CrossRef]
- Arshad, M.F.; Alam, A.; Alshammari, A.A.; Alhazza, M.B.; Alzimam, I.M.; Alam, M.A.; Mustafa, G.; Ansari, M.S.; Alotaibi, A.M.; Alotaibi, A.A.; et al. Thiazole: A Versatile Standalone Moiety Contributing to the Development of Various Drugs and Biologically Active Agents. Molecules 2022, 27. [CrossRef]
- Niu, Z.X.; Wang, Y.T.; Zhang, S.N.; Li, Y.; Chen, X.B.; Wang, S.Q.; Liu, H.M. Application and synthesis of thiazole ring in clinically approved drugs. Eur. J. Med. Chem. 2023, 250. [CrossRef]
- Ayati, A.; Emami, S.; Asadipour, A.; Shafiee, A.; Foroumadi, A. Recent applications of 1,3-thiazole core structure in the identification of new lead compounds and drug discovery. Eur. J. Med. Chem. 2015, 97, 699–718. [CrossRef]
- T. Chhabria, M.; Patel, S.; Modi, P.; S. Brahmkshatriya, P. Thiazole: A Review on Chemistry, Synthesis and Therapeutic Importance of its Derivatives. Curr. Top. Med. Chem. 2016, 16, 2841–2862. [CrossRef]
- Khidre, R.E.; Radini, I.A.M. Design, synthesis and docking studies of novel thiazole derivatives incorporating pyridine moiety and assessment as antimicrobial agents. Sci. Rep. 2021, 11. [CrossRef]
- Dawood, K.M.; Raslan, M.A.; Abbas, A.A.; Mohamed, B.E.; Abdellattif, M.H.; Nafie, M.S.; Hassan, M.K. Novel Bis-Thiazole Derivatives: Synthesis and Potential Cytotoxic Activity Through Apoptosis With Molecular Docking Approaches. Front. Chem. 2021, 9. [CrossRef]
- Hussain, R.; Rehman, W.; Khan, S.; Maalik, A.; Hefnawy, M.; Alanazi, A.S.; Khan, Y.; Rasheed, L. Imidazopyridine-Based Thiazole Derivatives as Potential Antidiabetic Agents: Synthesis, In Vitro Bioactivity, and In Silico Molecular Modeling Approach. Pharmaceuticals 2023, 16. [CrossRef]
- Majumdar, A.; Pal, A. Recent advancements in visible-light-assisted photocatalytic removal of aqueous pharmaceutical pollutants. Clean Technol. Environ. Policy 2020, 22, 11–42. [CrossRef]
- Masanabo, N.; Orimolade, B.; Idris, A.O.; Nkambule, T.T.I.; Mamba, B.B.; Feleni, U. Advances in polymer-based detection of environmental ibuprofen in wastewater. Environ. Sci. Pollut. Res. 2023, 30, 14062–14090. [CrossRef]
- Ghosh, P.; Mukherji, S. Fate, detection technologies and toxicity of heterocyclic PAHs in the aquatic and soil environments. Sci. Total Environ. 2023, 892. [CrossRef]
- Caban, M.; Stepnowski, P. How to decrease pharmaceuticals in the environment? A review. Environ. Chem. Lett. 2021, 19, 3115–3138. [CrossRef]
- Baaloudj, O.; Assadi, I.; Nasrallah, N.; El, A.; Khezami, L. Simultaneous removal of antibiotics and inactivation of antibiotic-resistant bacteria by photocatalysis : A review. J. Water Process Eng. 2021, 42, 102089. [CrossRef]
- OECD Management of Pharmaceutical Household Waste; 2022;
- Zhang, Q.; Xu, H.; Song, N.; Liu, S.; Wang, Y.; Ye, F.; Ju, Y.; Jiao, S.; Shi, L. New insight into fate and transport of organic compounds from pollution sources to aquatic environment using non-targeted screening: A wastewater treatment plant case study. Sci. Total Environ. 2023, 863. [CrossRef]
- Kumar, A.; Rana, A.; Sharma, G.; Naushad, M.; Dhiman, P.; Kumari, A.; Stadler, F.J. Recent advances in nano-Fenton catalytic degradation of emerging pharmaceutical contaminants. J. Mol. Liq. 2019, 290. [CrossRef]
- Barzegar, F.; Kamankesh, M.; Mohammadi, A. Heterocyclic aromatic amines in cooked food: A review on formation, health risk-toxicology and their analytical techniques. Food Chem. 2019, 280, 240–254. [CrossRef]
- Khan, I.A.; Khan, A.; Zou, Y.; Zongshuai, Z.; Xu, W.; Wang, D.; Huang, M. Heterocyclic amines in cooked meat products, shortcomings during evaluation, factors influencing formation, risk assessment and mitigation strategies. Meat Sci. 2022, 184. [CrossRef]
- Bellamri, M.; Walmsley, S.J.; Turesky, R.J. Metabolism and biomarkers of heterocyclic aromatic amines in humans. Genes Environ. 2021, 43. [CrossRef]
- Honda, M.; Suzuki, N. Toxicities of polycyclic aromatic hydrocarbons for aquatic animals. Int. J. Environ. Res. Public Health 2020, 17. [CrossRef]
- Geng, Y.; Xie, Y.; Li, W.; Ji, J.; Chen, F.; Liao, X.; Hu, X.; Ma, L. Heterocyclic Amines in Meat and Meat Products: Occurrence, Formation, Mitigation, Health Risks and Intervention. Food Rev. Int. 2024, 40, 1503–1519. [CrossRef]
- Cao, W.; Yuan, J.; Geng, S.; Zou, J.; Dou, J.; Fan, F. Oxygenated and Nitrated Polycyclic Aromatic Hydrocarbons: Sources, Quantification, Incidence, Toxicity, and Fate in Soil—A Review Study. Processes 2023, 11. [CrossRef]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456. [CrossRef]
- Çelik, G.; Stolte, S.; Markiewicz, M. NSO-heterocyclic PAHs – Controlled exposure study reveals high acute aquatic toxicity. J. Hazard. Mater. 2023, 460. [CrossRef]
- Barbuceanu, S.F.; Rosca, E.V.; Apostol, T.V.; Socea, L.I.; Draghici, C.; Farcasanu, I.C.; Ruta, L.L.; Nitulescu, G.M.; Iscrulescu, L.; Pahontu, E.M.; et al. New Heterocyclic Compounds from Oxazol-5(4H)-one and 1,2,4-Triazin-6(5H)-one Classes: Synthesis, Characterization and Toxicity Evaluation. Molecules 2023, 28. [CrossRef]
- Samadi, A.; Pour, A.K.; Jamieson, R. Development of remediation technologies for organic contaminants informed by QSAR/QSPR models. Environ. Adv. 2021, 5. [CrossRef]
- Majee, S.; Shilpa; Sarav, M.; Banik, B.K.; Ray, D. Recent Advances in the Green Synthesis of Active N-Heterocycles and Their Biological Activities. Pharmaceuticals 2023, 16. [CrossRef]
- Martins, P.; Jesus, J.; Santos, S.; Raposo, L.R.; Roma-Rodrigues, C.; Baptista, P.V.; Fernandes, A.R. Heterocyclic anticancer compounds: Recent advances and the paradigm shift towards the use of nanomedicine's tool Box. Molecules 2015, 20, 16852–16891. [CrossRef]
- Sá, H.; Michelin, M.; Tavares, T.; Silva, B. Current Challenges for Biological Treatment of Pharmaceutical-Based Contaminants with Oxidoreductase Enzymes: Immobilization Processes, Real Aqueous Matrices and Hybrid Techniques. Biomolecules 2022, 12. [CrossRef]
- Pérez-Lucas, G.; Aatik, A. El; Aliste, M.; Navarro, G.; Fenoll, J.; Navarro, S. Removal of Contaminants of Emerging Concern from a Wastewater Effluent by Solar-Driven Heterogeneous Photocatalysis: A Case Study of Pharmaceuticals. Water. Air. Soil Pollut. 2023, 234. [CrossRef]
- Friedmann, D. A General Overview of Heterogeneous Photocatalysis as a Remediation Technology for Wastewaters Containing Pharmaceutical Compounds. Water (Switzerland) 2022, 14. [CrossRef]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total Environ. 2021, 753. [CrossRef]
- Li, R.; Kong, J.; Liu, H.; Chen, P.; Su, Y.; Liu, G.; Lv, W. Removal of indomethacin using UV–vis/peroxydisulfate: Kinetics, toxicity, and transformation pathways. Chem. Eng. J. 2018, 331, 809–817. [CrossRef]
- Wang, J.; Guo, Z.; Guo, Y.; Zhang, Y.; Yu, P.; Ye, Z.; Qian, Y.; Yoshimura, C.; Wang, T.; Zhang, L. Photochemical fate of β-blocker pindolol in riverine and its downstream coastal waters. Sci. Total Environ. 2024, 927. [CrossRef]
- Raji, A.; Pandiyaraj, K.N.; Kandavelu, V.; Vasu, D.; Saravanan, D. Efficiency evaluation of the photocatalytic degradation of telmisartan antihypertensive drug with Fenton, photo-Fenton and recyclable TiO2 heterogeneous catalyst. React. Kinet. Mech. Catal. 2020, 130, 1141–1154. [CrossRef]
- Ljubas, D.; Čizmić, M.; Vrbat, K.; Stipaničev, D.; Repec, S.; Ćurković, L.; Babić, S. Albendazole Degradation Possibilities by UV-Based Advanced Oxidation Processes. Int. J. Photoenergy 2018, 2018. [CrossRef]
- Zizzamia, A.R.; Tesoro, C.; Bianco, G.; Bufo, S.A.; Ciriello, R.; Brienza, M.; Scrano, L.; Lelario, F. Efficient photooxidation processes for the removal of sildenafil from aqueous environments: A comparative study. Case Stud. Chem. Environ. Eng. 2024, 9. [CrossRef]
- Jiménez, J.J.; Pardo, R.; Sánchez, M.I.; Muñoz, B.E. Photochemical, thermal, biological and long-term degradation of celecoxib in river water. Degradation products and adsorption to sediment. J. Hazard. Mater. 2018, 342, 252–259. [CrossRef]
- Mahlaule-Glory, L.M.; Mapetla, S.; Makofane, A.; Mathipa, M.M.; Hintsho-Mbita, N.C. Biosynthesis of iron oxide nanoparticles for the degradation of methylene blue dye, sulfisoxazole antibiotic and removal of bacteria from real water. Heliyon 2022, 8. [CrossRef]
- Giraldo, A.L.; Erazo-Erazo, E.D.; Flórez-Acosta, O.A.; Serna-Galvis, E.A.; Torres-Palma, R.A. Degradation of the antibiotic oxacillin in water by anodic oxidation with Ti/IrO2 anodes: Evaluation of degradation routes, organic byproducts and effects of water matrix components. Chem. Eng. J. 2015, 279, 103–114. [CrossRef]
- Regulska, E.; Karpińska, J. Photocatalytic degradation of olanzapine in aqueous and river waters suspension of titanium dioxide. Appl. Catal. B Environ. 2012, 117–118, 96–104. [CrossRef]
- Mansour, D.; Alblawi, E.; Alsukaibi, A.K.D.; Humaidi, J.; Tahraoui, H.; Shatat, M.; Teka, S.; Maisara, S.; Bellakhal, N.; Binous, H.; et al. Modeling and Optimization of Electrochemical Advanced Oxidation of Clopidogrel Using the Doehlert Experimental Design Combined with an Improved Grey Wolf Algorithm. Water (Switzerland) 2024, 16. [CrossRef]
- Baaloudj, O.; Nasrallah, N.; Bouallouche, R.; Kenfoud, H.; Khezami, L.; Assadi, A.A. High efficient Cefixime removal from water by the sillenite Bi12TiO20: Photocatalytic mechanism and degradation pathway. J. Clean. Prod. 2022, 330, 129934. [CrossRef]
- Hojamberdiev, M.; Vargas, R.; Madriz, L.; Yubuta, K.; Kadirova, Z.C.; Shaislamov, U.; Sannegowda, L.K.; Jędruchniewicz, K.; Typek, R.; Teshima, K.; et al. Unveiling the origin of the efficient photocatalytic degradation of nitazoxanide over bismuth (oxy)iodide crystalline phases. Environ. Sci. Nano 2023, 11, 336–350. [CrossRef]
- Zhang, X.; Guo, J.; Huang, Y.; Lu, G. Toxicity evolution and control for the UV/H2O2 degradation of nitrogen-containing heterocyclic compounds: SDZ and PMM. Chemosphere 2023, 338. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).