Submitted:
30 October 2024
Posted:
31 October 2024
You are already at the latest version
Abstract
Background. One of the most important prognostic factors in periampullary and pancreatic cancers is perineural infiltration, whose preoperative detection could be decisive in selecting which patients should not undergo upfront surgery. We evaluated CA 19.9 and the Neutrophil-to-lymphocyte ratio (NLR) as preoperative predictors of perineural invasion (PNI). Methods: Retrospective analysis including patients with periampullary and pancreatic cancers who underwent curative resection from January 2013 to August 2023 in our institution. A univariate analysis and multivariate analysis were performed to analyze the association between the CA 19.9 and NLR with the existence of PNI. Results: A total of 136 patients were included in the study. PNI was observed in 95 (69.9%) patients. The selected cut-off points were NLR: 2.2 and CA 19.9: 37 U/mL. In univariate analysis, NLR (p=0.001) and CA 19.9 (p=0.006) were statistically significantly associated with PNI. In multivariate analysis, baseline NLR levels (p=0.012; OR: 1.95; 95%CI: 1.16-3.29) as well as CA 19.9 levels (p=0.026; OR: 1.67; 95%CI: 1.06-2.64) remained independent prognostic factors for PNI. The area under de ROC curves was 0.67 for CA 19.9 (p=0.004) and 0.72 for NLR (p<0.001). A sensitivity of 65.9% with a positive predictive value of 81.7% were obtained for CA 19.9, and a sensitivity of 62.1% with a positive predictive value of 84.3% were achieved for NLR. Conclusion: Preoperative CA 19.9 and NLR levels seem to be good predictors of PNI. In patients with normal levels of CA 19.9, preoperative NLR above of 2.2 should be an indication for neoadjuvant therapy.
Keywords:
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Management of the Patient
2.3. Variables, Data Collection and Definitions
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Laboratory Values
3.3. Neo and Adjuvant Therapy
3.4. Tumor Characteristics
3.5. Surgical Data
3.6. Univariate Analysis
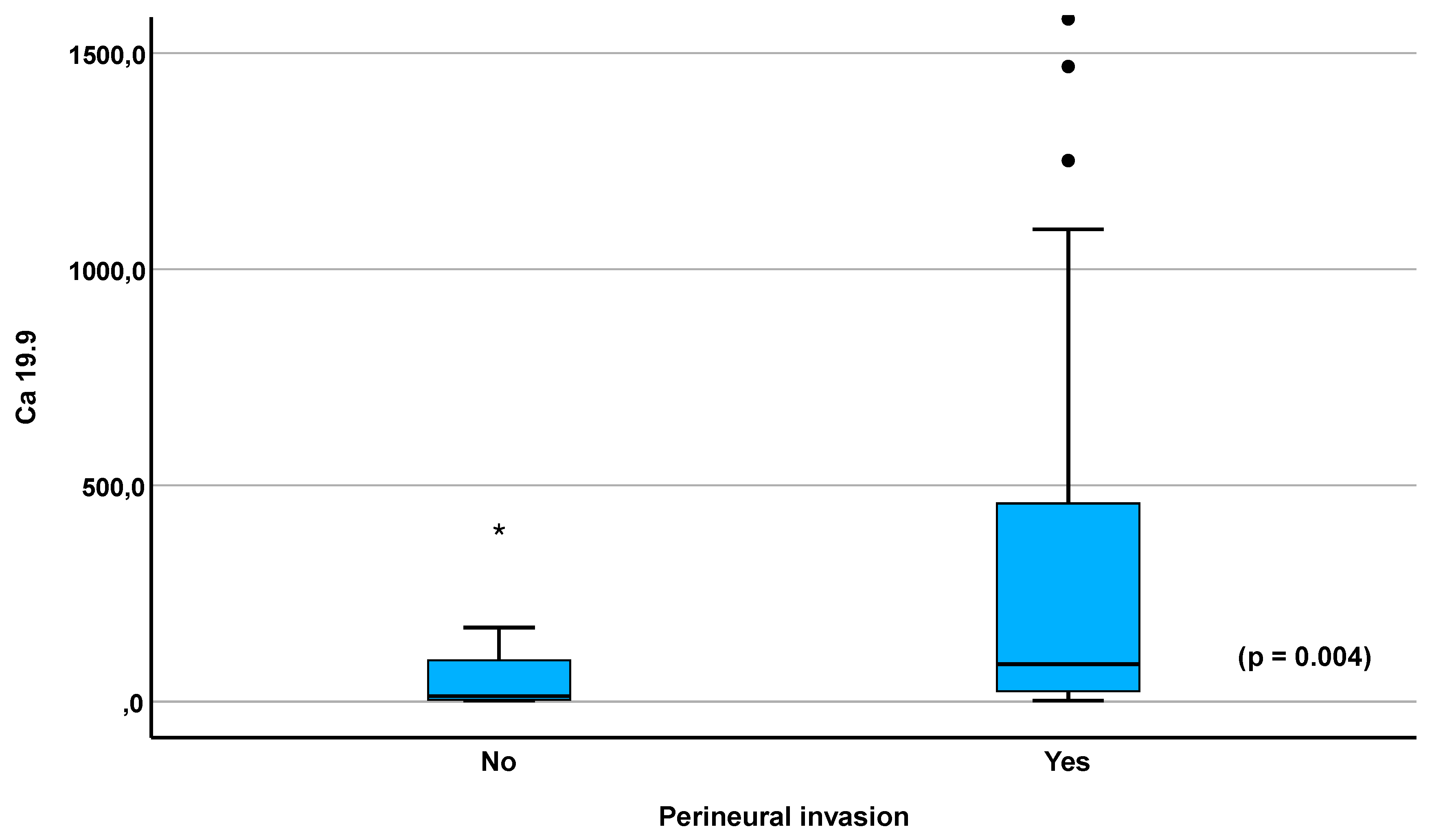
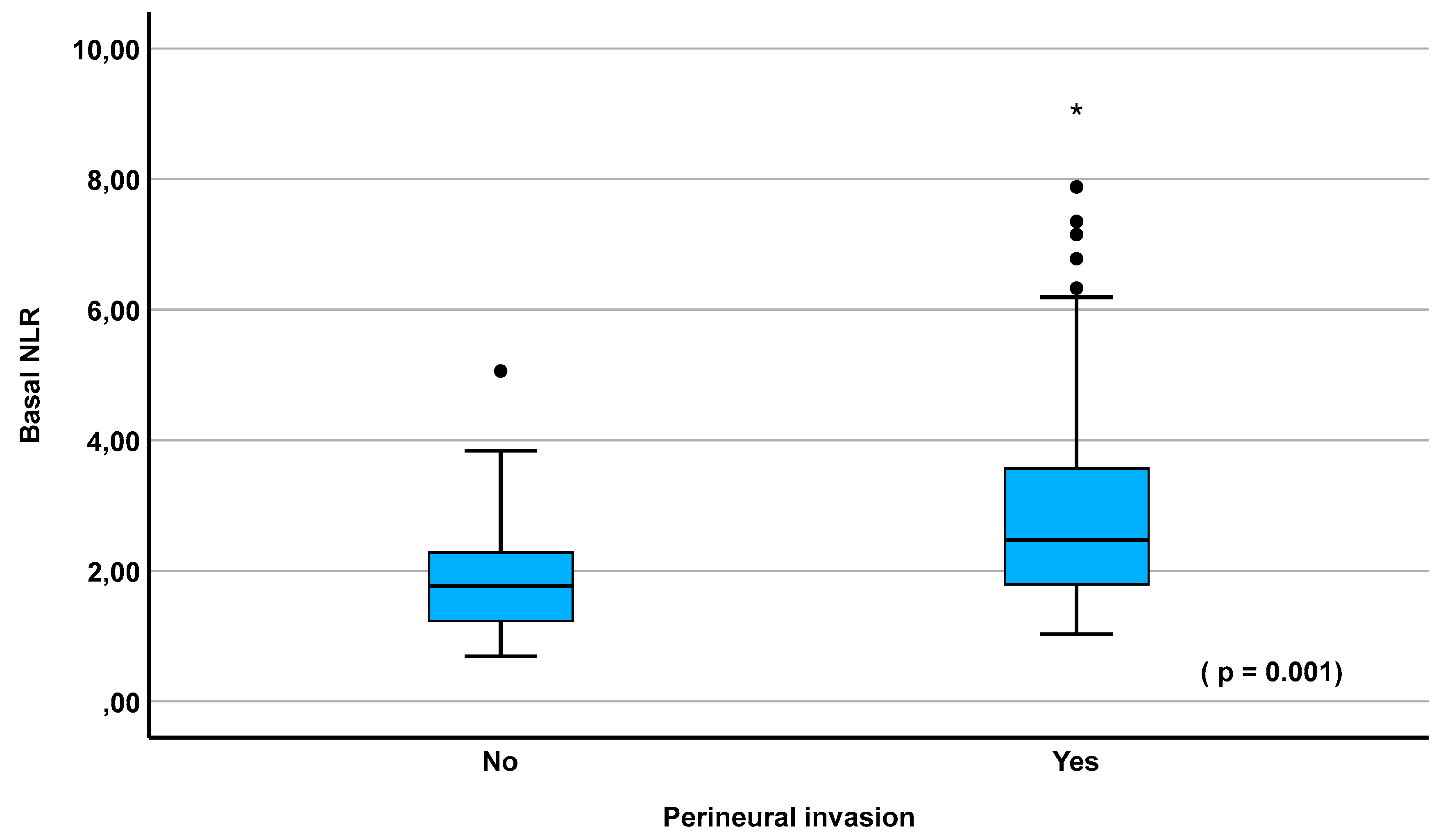
3.7. ROC Curves
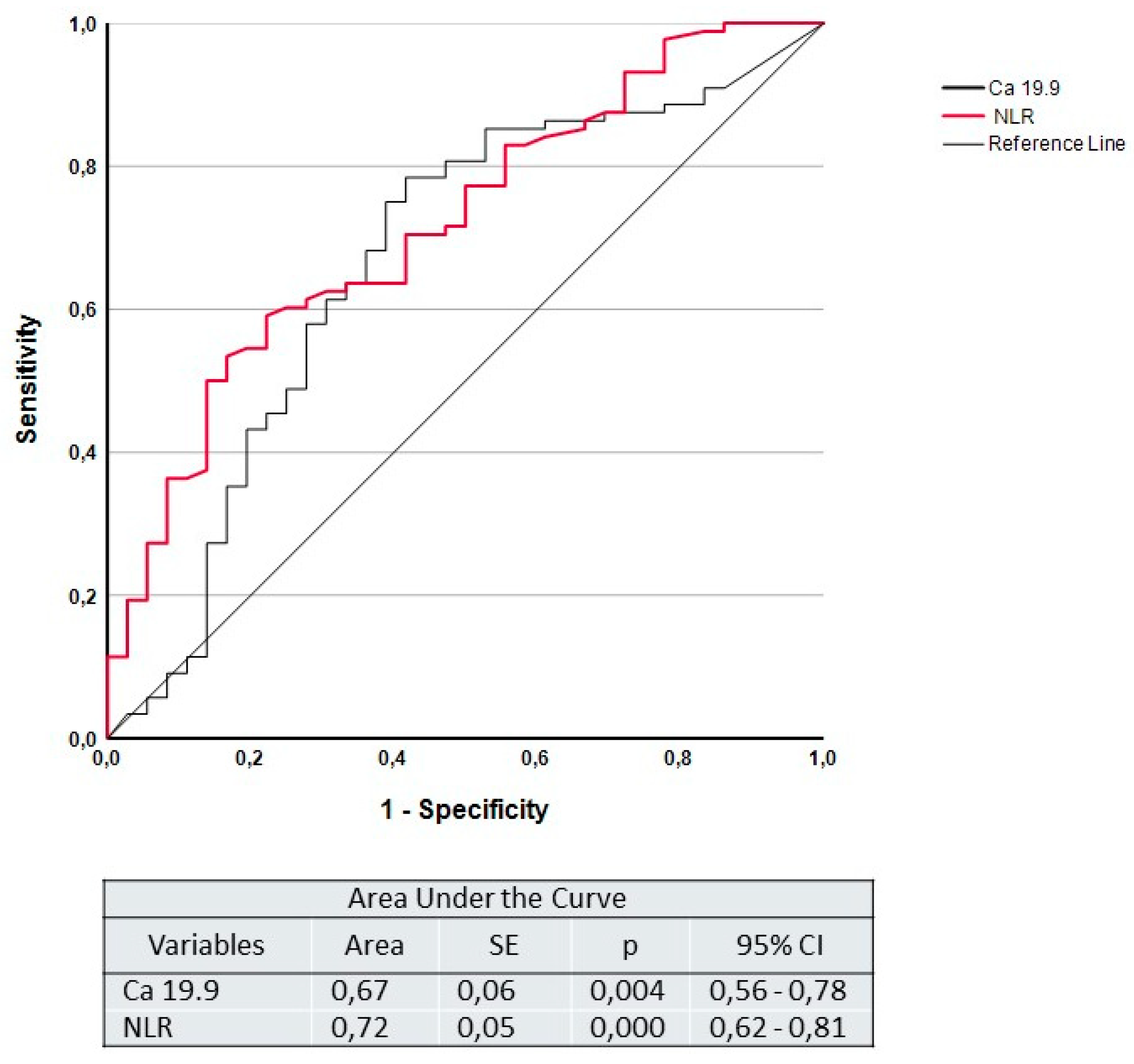
3.8. Multivariate Analysis
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
Ethical Statement
References
- He J, Ahuja N, Makary MA, Cameron JL, Eckhauser FE, Choti MA, et al. 2564 resected periampullary adenocarcinomas at a single institution: Trends over three decades. HPB. 2014;16(1):83–90. [CrossRef]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021 May;71(3):209–49.
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014 Jun;74(11):2913–21. [CrossRef]
- Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010 Apr;7(4):e1000267. [CrossRef]
- Bergquist JR, Puig CA, Shubert CR, Groeschl RT, Habermann EB, Kendrick ML, et al. Carbohydrate antigen 19-9 elevation in anatomically resectable, early stage pancreatic cancer is independently associated with decreased overall survival and an indication for neoadjuvant therapy: A national cancer database study. J Am Coll Surg. 2016 Jul 1;223(1):52–65.
- Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016 Jul;388(10039):73–85.
- Strobel O, Lorenz P, Hinz U, Gaida M, König AK, Hank T, et al. Actual Five-year Survival After Upfront Resection for Pancreatic Ductal Adenocarcinoma: Who Beats the Odds? Ann Surg. 2022 May;275(5):962–71.
- Bilici, A. Prognostic factors related with survival in patients with pancreatic adenocarcinoma. World J Gastroenterol. 2014 Aug;20(31):10802–12. [CrossRef]
- Crippa S, Pergolini I, Javed AA, Honselmann KC, Weiss MJ, Di Salvo F, et al. Implications of Perineural Invasion on Disease Recurrence and Survival After Pancreatectomy for Pancreatic Head Ductal Adenocarcinoma. Ann Surg. 2022 Aug;276(2):378–85.
- Schorn S, Demir IE, Haller B, Scheufele F, Reyes CM, Tieftrunk E, et al. The influence of neural invasion on survival and tumor recurrence in pancreatic ductal adenocarcinoma - A systematic review and meta-analysis. Surg Oncol. 2017 Mar;26(1):105–15. [CrossRef]
- Wang Y, Liu Z, Tian Y, Zhao H, Fu X. Periampullary cancer and neurological interactions: current understanding and future research directions. Front Oncol. 2024 Mar 19;14:1370111. [CrossRef]
- Felsenstein M, Lindhammer F, Feist M, Hillebrandt KH, Timmermann L, Benzing C, et al. Perineural Invasion in Pancreatic Ductal Adenocarcinoma (PDAC): A Saboteur of Curative Intended Therapies? J Clin Med. 2022 Apr;11(9).
- Schouten TJ, Kroon VJ, Besselink MG, Bosscha K, Busch OR, Crobach ASLP, et al. Perineural Invasion is an Important Prognostic Factor in Patients With Radically Resected (R0) and Node-negative (pN0) Pancreatic Cancer. Ann Surg [Internet]. 2024 May 6 [cited 2024 May 20]. Available online: https://pubmed.ncbi.nlm.nih.gov/38708885/.
- Liebl F, Demir IE, Mayer K, Schuster T, DʼHaese JG, Becker K, et al. The impact of neural invasion severity in gastrointestinal malignancies: a clinicopathological study. Ann Surg. 2014 Nov;260(5):900–7; discussion 907–8.
- Izumo W, Higuchi R, Furukawa T, Yazawa T, Uemura S, Matsunaga Y, et al. Evaluation of the Significance of Lymphatic, Microvascular and Perineural Invasion in Patients With Pancreatic Neuroendocrine Neoplasms. Cancer Diagnosis & Prognosis [Internet]. 2022 Mar 3 [cited 2024 May 20];2(2):150. [CrossRef]
- Wang PH, Song N, Shi L Bin, Zhang QH, Chen ZY. The relationship between multiple clinicopathological features and nerve invasion in pancreatic cancer. Hepatobiliary & Pancreatic Diseases International. 2013 Oct 15;12(5):546–51. [CrossRef]
- Noda Y, Pisuchpen N, Parakh A, Srinivas-Rao S, Kinowaki Y, Mino-Kenudson M, et al. Does CT overestimate extra-pancreatic perineural invasion in patients with pancreatic ductal adenocarcinoma following neoadjuvant chemoradiation therapy? Br J Radiol [Internet]. 2024 Feb 28 [cited 2024 May 16];97(1155):607–13. Available online: http://www.ncbi.nlm.nih.gov/pubmed/38305574.
- Khristenko E, Shrainer I, Setdikova G, Palkina O, Sinitsyn V, Lyadov V. Preoperative CT-based detection of extrapancreatic perineural invasion in pancreatic cancer. Sci Rep [Internet]. 2021 Jan 19 [cited 2024 May 16];11(1):1800. [CrossRef]
- Chang ST, Jeffrey RB, Patel BN, DiMaio MA, Rosenberg J, Willmann JK, et al. Preoperative Multidetector CT Diagnosis of Extrapancreatic Perineural or Duodenal Invasion Is Associated with Reduced Postoperative Survival after Pancreaticoduodenectomy for Pancreatic Adenocarcinoma: Preliminary Experience and Implications for Patient Care. Radiology. 2016 Dec;281(3):816–25. [CrossRef]
- Roxburgh CSD, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010 Jan;6(1):149–63. [CrossRef]
- Mei Z, Shi L, Wang B, Yang J, Xiao Z, Du P, et al. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: A systematic review and meta-analysis of 66 cohort studies. Cancer Treat Rev. 2017 Jul;58. [CrossRef]
- Cheng H, Long F, Jaiswar M, Yang L, Wang C, Zhou Z. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: a meta-analysis. Sci Rep. 2015 Jul;5:11026. [CrossRef]
- Yang JJ, Hu ZG, Shi WX, Deng T, He SQ, Yuan SG. Prognostic significance of neutrophil to lymphocyte ratio in pancreatic cancer: A meta-analysis. World J Gastroenterol [Internet]. 2015 Mar 7 [cited 2024 May 22];21(9):2807–15. [CrossRef]
- Mowbray NG, Griffith D, Hammoda M, Shingler G, Kambal A, Al-Sarireh B. A meta-analysis of the utility of the neutrophil-to-lymphocyte ratio in predicting survival after pancreatic cancer resection. HPB [Internet]. 2018 May 1 [cited 2024 May 22];20(5):379–84. [CrossRef]
- Neumann CCM, Schneider F, Hilfenhaus G, Vecchione L, Felsenstein M, Ihlow J, et al. Inflammation-Based Prognostic Scores in Pancreatic Cancer Patients-A Single-Center Analysis of 1294 Patients within the Last Decade. Cancers (Basel). 2023 Apr;15(8). [CrossRef]
- Park SJ, Jang S, Han JK, Kim H, Kwon W, Jang JY, et al. Preoperative assessment of the resectability of pancreatic ductal adenocarcinoma on CT according to the NCCN Guidelines focusing on SMA/SMV branch invasion. Eur Radiol [Internet]. 2021 Sep 1 [cited 2024 May 27];31(9):6889–97. [CrossRef]
- Gómez-Mateo MDC, Sabater-Ortí L, Ferrández-Izquierdo A. Pathology handling of pancreatoduodenectomy specimens: Approaches and controversies. World J Gastrointest Oncol [Internet]. 2014 Sep 15 [cited 2024 May 16];6(9):351–9. [CrossRef]
- Youden, W. Index for rating diagnostic tests. Cancer. 1950 Jan;3(1):32–5.
- Clavien PA, Strasberg SM. Severity Grading of Surgical Complications. Ann Surg. 2009 Aug;250(2):197–8. [CrossRef]
- Allen PJ, Kuk D, Castillo CF Del, Basturk O, Wolfgang CL, Cameron JL, et al. Multi-Institutional validation study of the American Joint Commission on Cancer (8th edition) changes for T and N staging in patients with pancreatic adenocarcinoma. Ann Surg [Internet]. 2017 [cited 2024 May 23];265(1):185. [CrossRef]
- Gasparini G, Pellegatta M, Crippa S, Lena MS, Belfiori G, Doglioni C, et al. Nerves and Pancreatic Cancer: New Insights into a Dangerous Relationship. Cancers (Basel). 2019 Jun;11(7). [CrossRef]
- Balaban DV, Marin FS, Manucu G, Zoican A, Ciochina M, Mina V, et al. Clinical characteristics and outcomes in carbohydrate antigen 19-9 negative pancreatic cancer. World J Clin Oncol [Internet]. 2022 Jul 24 [cited 2024 May 21];13(7):630–40. [CrossRef]
- Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J Gastrointest Oncol. 2012 Jun;3(2):105–19. [CrossRef]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008 Jul 24;454(7203):436–44.
- Liang W, Ferrara N. The Complex Role of Neutrophils in Tumor Angiogenesis and Metastasis. Cancer Immunol Res. 2016 Feb;4(2):83–91. [CrossRef]
- Coffelt SB, Wellenstein MD, De Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer [Internet]. 2016 Jun 24 [cited 2023 Jul 11];16(7):431–46. Available online: https://pubmed.ncbi.nlm.nih.gov/27282249/.
- Chawla A, Huang TL, Ibrahim AM, Hardacre JM, Siegel C, Ammori JB. Pretherapy neutrophil to lymphocyte ratio and platelet to lymphocyte ratio do not predict survival in resectable pancreatic cancer. HPB (Oxford). 2018 May;20(5):398–404. [CrossRef]
- Merlo I, Ardiles V, Sanchez-Clariá R, Fratantoni E, de Santibañes E, Pekolj J, et al. Prognostic Factors in Resected Pancreatic Ductal Adenocarcinoma: Is Neutrophil-Lymphocyte Ratio a Useful Marker? J Gastrointest Cancer. 2023 Jun;54(2):580–8.
- Stevens L, Pathak S, Nunes QM, Pandanaboyana S, Macutkiewicz C, Smart N, et al. Prognostic significance of pre-operative C-reactive protein and the neutrophil-lymphocyte ratio in resectable pancreatic cancer: a systematic review. HPB (Oxford). 2015 Apr;17(4):285–91. [CrossRef]
- Hajibandeh S, Hajibandeh S, Romman S, Parente A, Laing RW, Satyadas T, et al. Preoperative C-Reactive Protein-to-Albumin Ratio and Its Ability to Predict Outcomes of Pancreatic Cancer Resection: A Systematic Review. Biomedicines [Internet]. 2023 Jul 1 [cited 2024 May 22];11(7). [CrossRef]
- van ’t Land FR, Aziz MH, Michiels N, Mieog JSD, Bonsing BA, Luelmo SAC, et al. Increasing Systemic Immune-inflammation Index During Treatment in Patients With Advanced Pancreatic Cancer is Associated With Poor Survival: A Retrospective, Multicenter, Cohort Study. Ann Surg. 2023 Dec 1;278(6):1018–23.
- Faulkner S, Jobling P, March B, Jiang CC, Hondermarck H. Tumor Neurobiology and the War of Nerves in Cancer. Cancer Discov. 2019 Jun;9(6):702–10. [CrossRef]
- Li J, Kang R, Tang D. Cellular and molecular mechanisms of perineural invasion of pancreatic ductal adenocarcinoma. Cancer Commun [Internet]. 2021 Aug 1 [cited 2024 May 23];41(8):642. [CrossRef]
- Yi SQ, Miwa K, Ohta T, Kayahara M, Kitagawa H, Tanaka A, et al. Innervation of the pancreas from the perspective of perineural invasion of pancreatic cancer. Pancreas. 2003 Oct;27(3):225–9. [CrossRef]
- Takahashi T, Ishikura H, Motohara T, Okushiba S, Dohke M, Katoh H. Perineural invasion by ductal adenocarcinoma of the pancreas. J Surg Oncol. 1997 Jul;65(3):164–70.
- Luchini C, Veronese N, Nottegar A, Riva G, Pilati C, Mafficini A, et al. Perineural Invasion is a Strong Prognostic Moderator in Ampulla of Vater Carcinoma: A Meta-analysis. Pancreas. 2019 Jan;48(1):70–6.
- Andreasi V, Ricci C, Partelli S, Guarneri G, Ingaldi C, Muffatti F, et al. Predictors of disease recurrence after curative surgery for nonfunctioning pancreatic neuroendocrine neoplasms (NF-PanNENs): a systematic review and meta-analysis. J Endocrinol Invest [Internet]. 2022 Apr 1 [cited 2024 May 20];45(4):705–18. [CrossRef]
- Zhang JF, Hua R, Sun YW, Liu W, Huo YM, Liu DJ, et al. Influence of perineural invasion on survival and recurrence in patients with resected pancreatic cancer. Asian Pac J Cancer Prev. 2013;14(9):5133–9. [CrossRef]
- Zou W, Wu D, Wu Y, Zhou K, Lian Y, Chang G, et al. Nomogram predicts risk of perineural invasion based on serum biomarkers for pancreatic cancer. BMC Gastroenterol [Internet]. 2023 Dec 1 [cited 2024 May 23];23(1). [CrossRef]
- Shyr BS, Shyr BU, Chen SC, Shyr YM, Wang SE. Impact of tumor grade on pancreatic neuroendocrine tumors. Asian J Surg. 2022 Dec 1;45(12):2659–63. [CrossRef]
- Zhang C, Wang L, Zheng Z, Yao J, He L, Li J. Preoperative diagnosis of perineural invasion in patients with periampullary carcinoma by MSCT imaging: preliminary observations and clinical implications. Abdom Radiol (NY). 2023 Feb;48(2):601–7. [CrossRef]
- Forget P, Khalifa C, Defour JP, Latinne D, Van Pel MC, De Kock M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res Notes. 2017 Dec 3;10(1):12.
- Hasegawa S, Eguchi H, Tomokuni A, Tomimaru Y, Asaoka T, Wada H, et al. Pre-treatment neutrophil to lymphocyte ratio as a predictive marker for pathological response to preoperative chemoradiotherapy in pancreatic cancer. Oncol Lett. 2016 Feb;11(2):1560–6. [CrossRef]
- Inoue D, Ozaka M, Matsuyama M, Yamada I, Takano K, Saiura A, et al. Prognostic value of neutrophil-lymphocyte ratio and level of C-reactive protein in a large cohort of pancreatic cancer patients: a retrospective study in a single institute in Japan. Jpn J Clin Oncol. 2015 Jan;45(1):61–6. [CrossRef]
| Total N (%) 136 (100) |
Perineural Invasion | P OR (95% CI) |
||
|---|---|---|---|---|
| No N (%) 41 (30.1) |
Yes N (%) 95 (69.9) |
|||
| Age Mean ± SD |
64.7 (±9,9) |
62.7 (±10.4) | 65.6 (±9.6) | 0.120 1.03 (0.99-1.07) |
| Sex: Men Women |
76 (55.9) 60 (44.1) |
17 (41.5) 24 (58.5) |
59 (62.1) 36 (37.9) |
0.028 0.43 (0.21-0.91) |
| Charlson score Median (IQR) |
4.0 (4.0 – 6.0) |
4.0 (4.0-6.0) |
5.0 (4.0-5.3) |
0.460 1.10 (0.86-1.41) |
| BMI Mean ± SD |
25.2 (±4.8) | 26.4 (±5.2) | 24.6 (±4.6) | 0.074 0.93 (0.85-1.01) |
| CEA ng/ml Median (IQR) |
2.76 (1.7-4.7) |
2.05 (1.4 -4.0) |
2.90 (1.7-5.4) |
0.040 1.22 (1.01-1.48) |
| CA 19.9 U/mL Median (IQR) |
54.0 (8.9-338.4) |
13.4 (4.3-102.5) |
86.4 (22.9-458.7) |
0.006 1.67 (1.10-2.54) |
| Basal NLR Median (IQR) |
2.23 (1.6-3.2) |
1.81 (1.32-2.29) |
2.47 (1.79-3.57) |
0.001 2.07 (1.33-3.20) |
| Preoperative resectabilty: Resectable Borderline/Locally advanced |
122 (89.7) 14 (10.3) |
35 (85.4) 6 (14.6) |
87 (91.6) 8 (8.4) |
0.279 0.54 (0.17-1.66) |
| Neoadjuvant therapy No Yes |
120 (88.2) 16 (11.8) |
34 (82.9) 7 (17.1) |
86 (90.5) 9 (9.5) |
0.213 0.51 (0.18-1.47) |
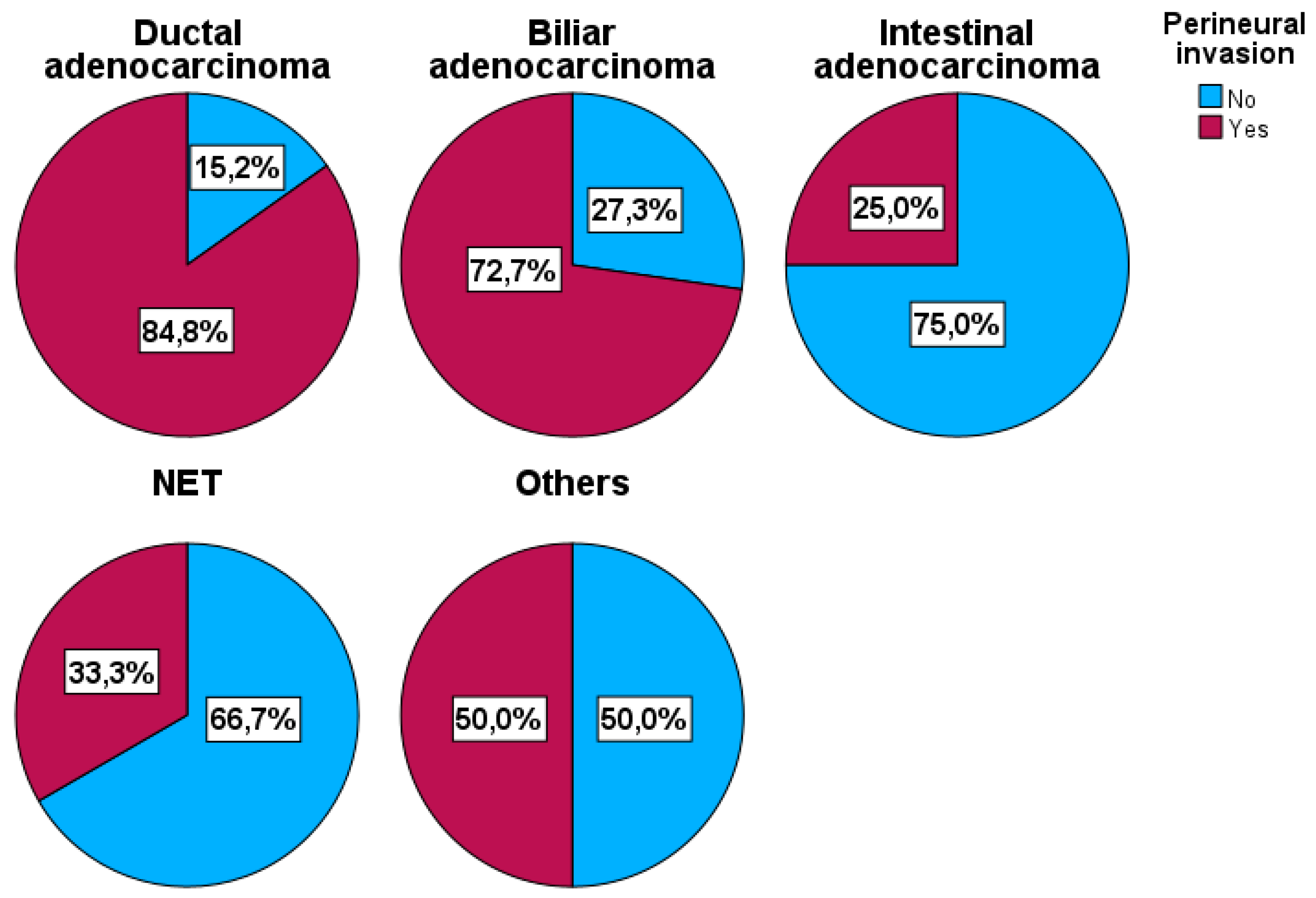
| Histopathological type: Ductal Biliary Intestinal Neuroendocrine Others |
79 (58.1) 22 (16.2) 12 (8.8) 15 (11.0) 8 (5.9) |
12 (29.3) 6 (14.6) 9 (22.0) 10 (24.4) 4 (9.8) |
67 (70.5) 16 (16.8) 3 (3.2) 5 (5.3) 4 (4.2) |
<0.001 0.64 (0.51-0.78) |
| Histopathological stage: I-II III-IV |
56 (41.2) 80 (58.8) |
25 (61.0) 16 (39.0) |
31 (32.6) 64 (67.4) |
0.003 3.23 (1.51-6.90) |
| Nodal involvement No Yes |
60 (44.1) 76 (55.9) |
28 (68.3) 13 (31.7) |
32 (33.7) 63 (66.3) |
<0.001 4.24 (1.94-9.28) |
| Resection margin R0 R1 |
94 (69.1) 42 (30.9) |
39 /95.1) 2 (4.9) |
55 (57.9) 40 (42.1) |
<0.001 14.18 (3.23-62.19) |
| Sensitivity | Specificity | PPV | PPN | |
|---|---|---|---|---|
| CA 19.9 | 65.9% | 62.9% | 81.7% | 42.3% |
| NLR | 62.1% | 73.2% | 84.3% | 45.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





