Submitted:
03 December 2024
Posted:
04 December 2024
You are already at the latest version
Abstract
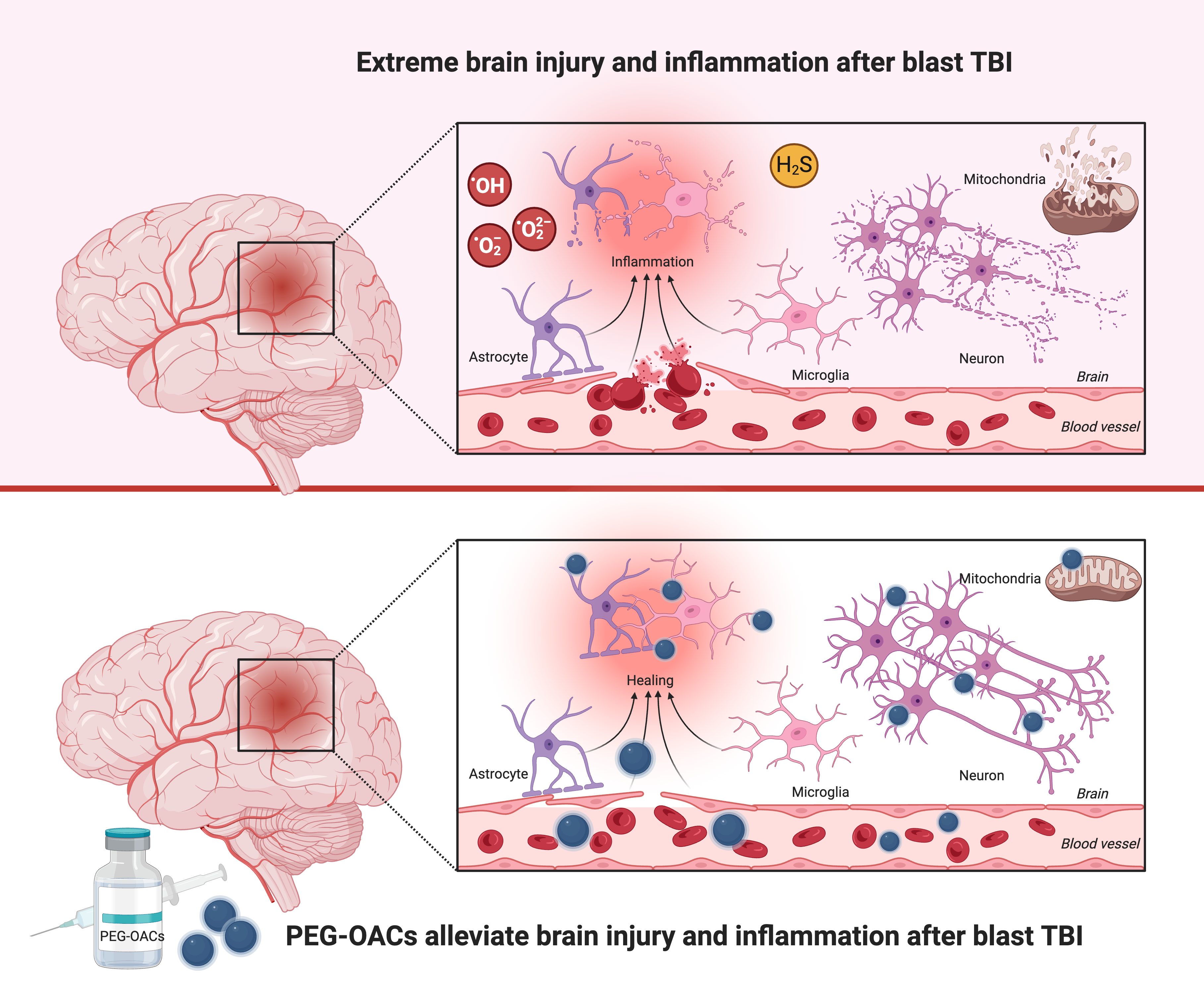
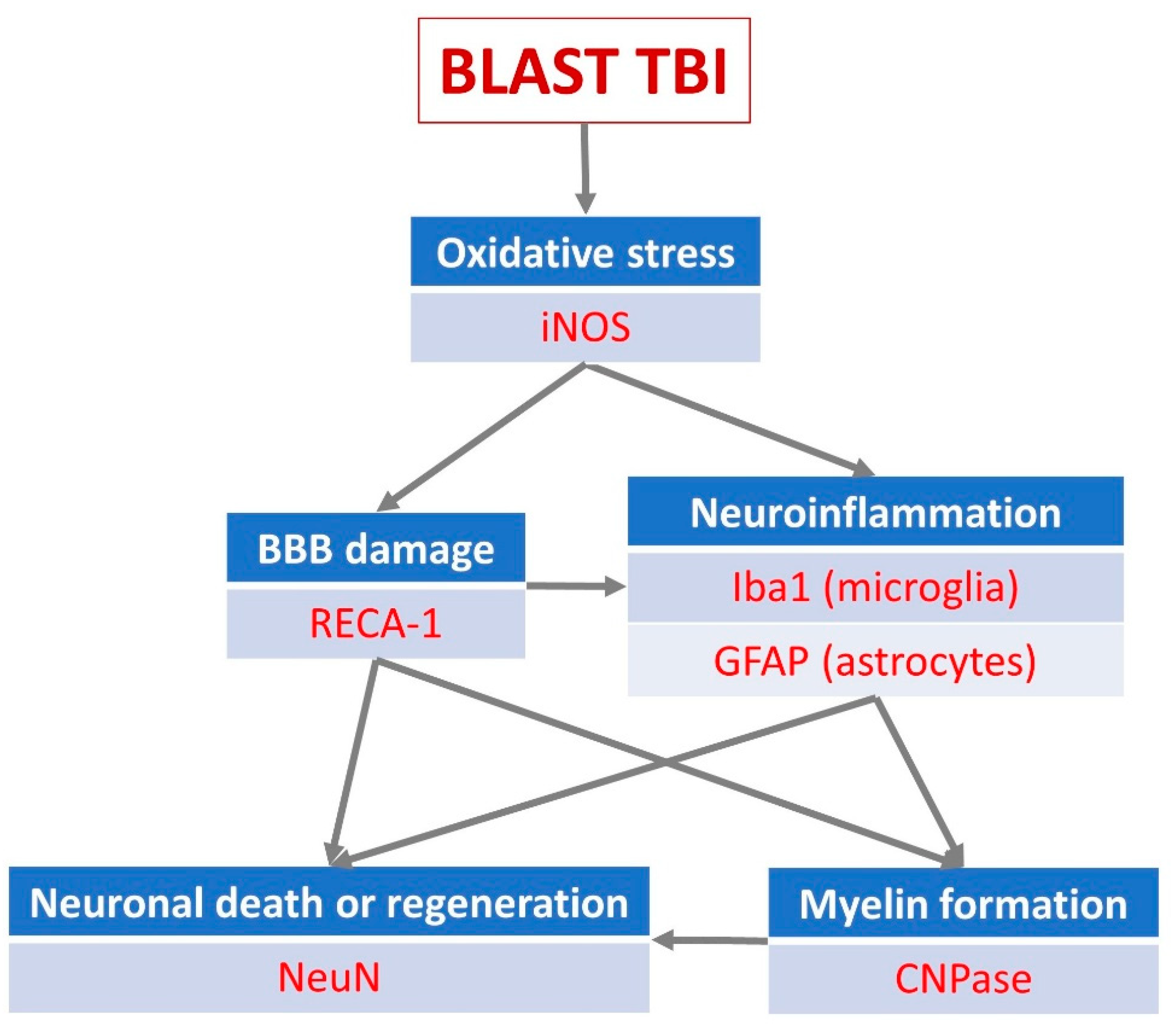
Traumatic brain injury (TBI) causes multiple cerebrovascular disruptions and oxidative stress. These pathological mechanisms are often accompanied by serious impairment in cerebral blood flow autoregulation, and neuronal and glial degeneration; Background/Objectives: Multiple biochemical cascades are triggered by brain damage resulting in reactive oxygen species production alongside blood loss and hypoxia. However, most currently available early antioxidant therapies lack capacity and hence sufficient efficacy against TBI. The aim of this study was to test a novel catalytic antioxidant nanoparticle to alleviate the damage occurring in blast TBI; Methods: TBI was elicited in an open blast rat model in which the rats are exposed to the effects of an explosive blast. Key events of the post-traumatic chain in the brain parenchyma were studied using immunohistochemistry. The application of a newly developed biologically compatible, catalytic superoxide dismutase-mimetic carbon-based nanoclusters, poly-ethylene-glycol-functionalized hydrophilic carbon clusters (PEG-HCCs), was tested post-blast to modulate the components of the TBI process; Results: PEG-HCC was shown to significantly ameliorate neuronal loss in brain cortex, the dentate gyrus and hippocampus when administered shortly after the blast. There was also a significant increase in endothelial activity to repair blood-brain barrier damage as well as modulation of microglial and astrocyte activity and an increase in inducible NO synthase in the cortex; Conclusions: We have demonstrated qualitatively and quantitatively that the previously demonstrated antioxidant properties of PEG-HCC have a neuroprotective effect after traumatic brain injury following an explosive blast acting at multiple levels of the pathological chain of events elicited by the TBI.
Keywords:
1. Introduction
2. Material and Methods
Preparation of PEG-HCC Nanoparticles
Animals
Blast Exposure
Treatment Protocol and Immediate Post-Blast Management
Chronic Post Blast Management
Immunohistochemistry
Image Analysis, Statistical Analysis and Data Presentation

3. Results
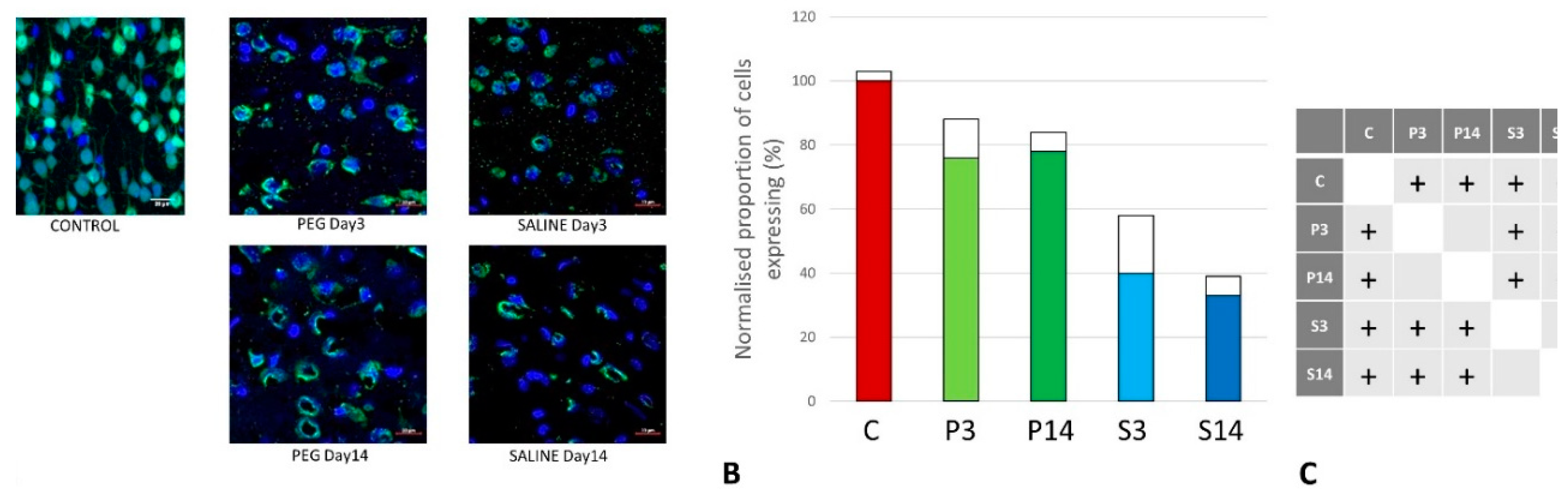
Quantification of Neuronal Loss
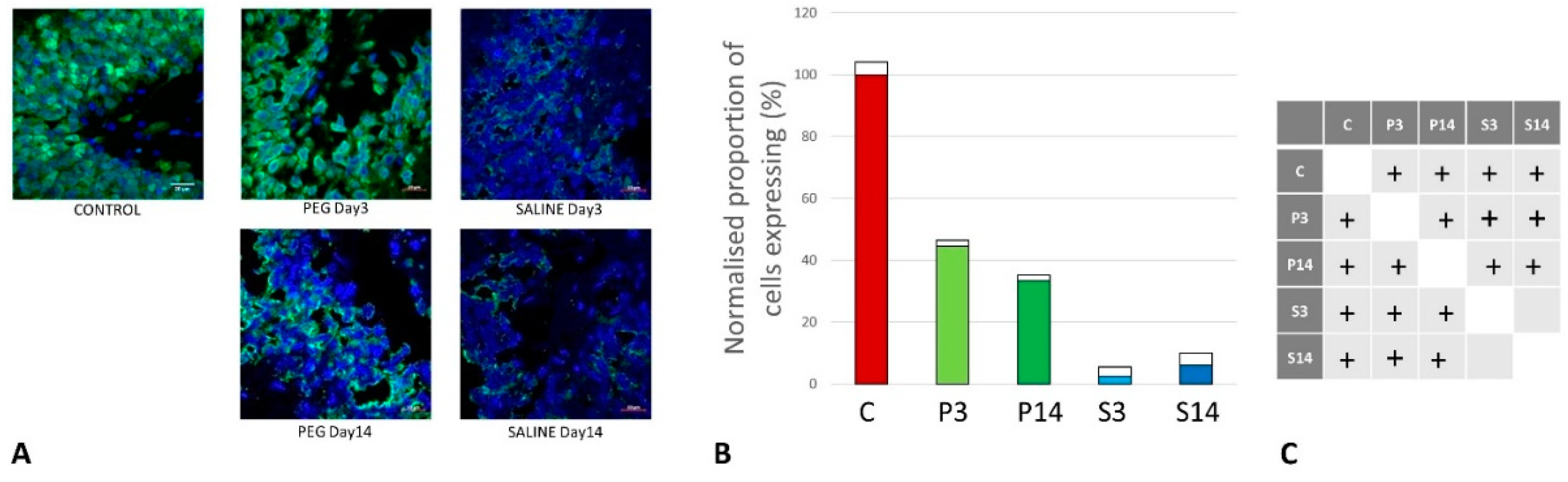
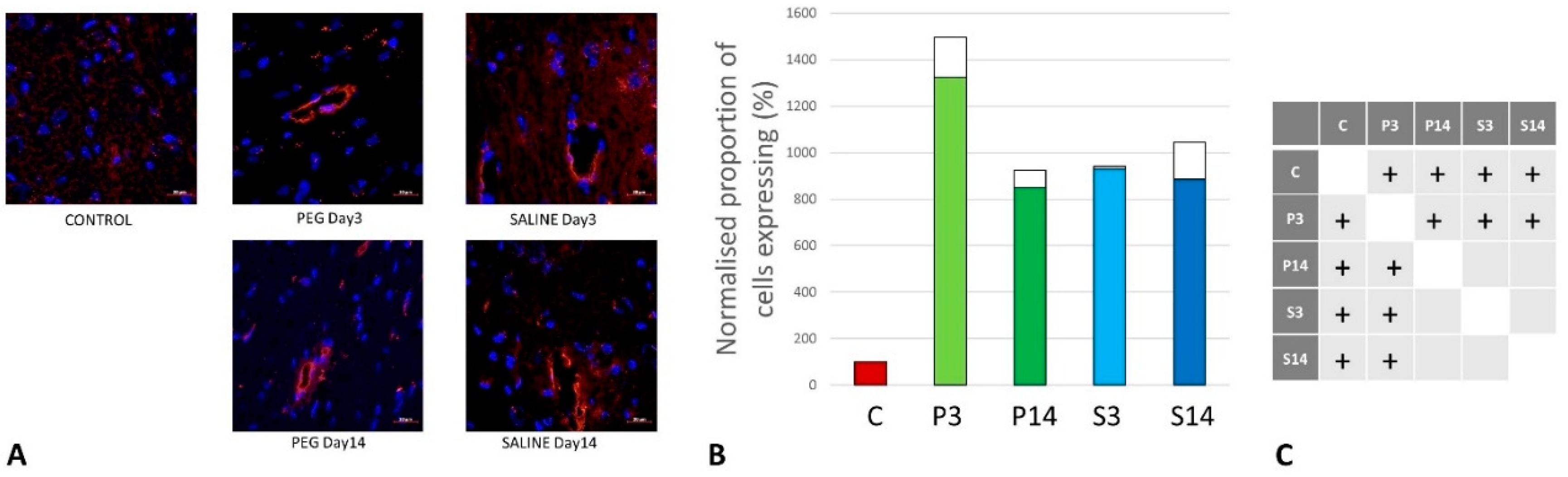
Quantification of BBB Damage
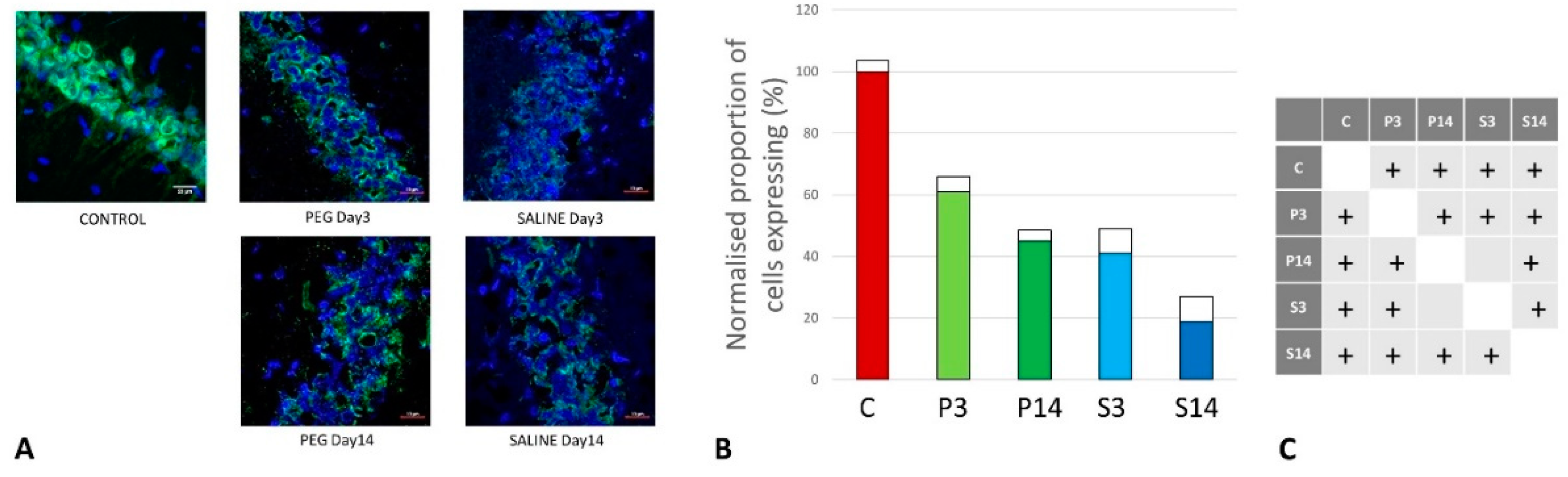
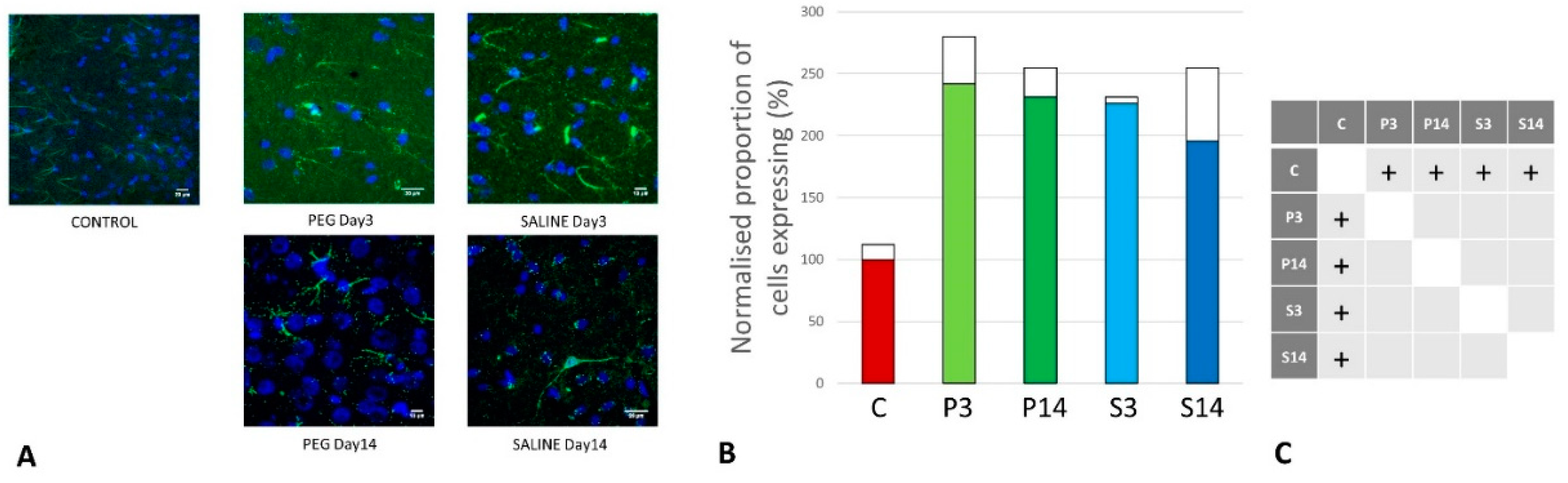
Quantification of Inflammation
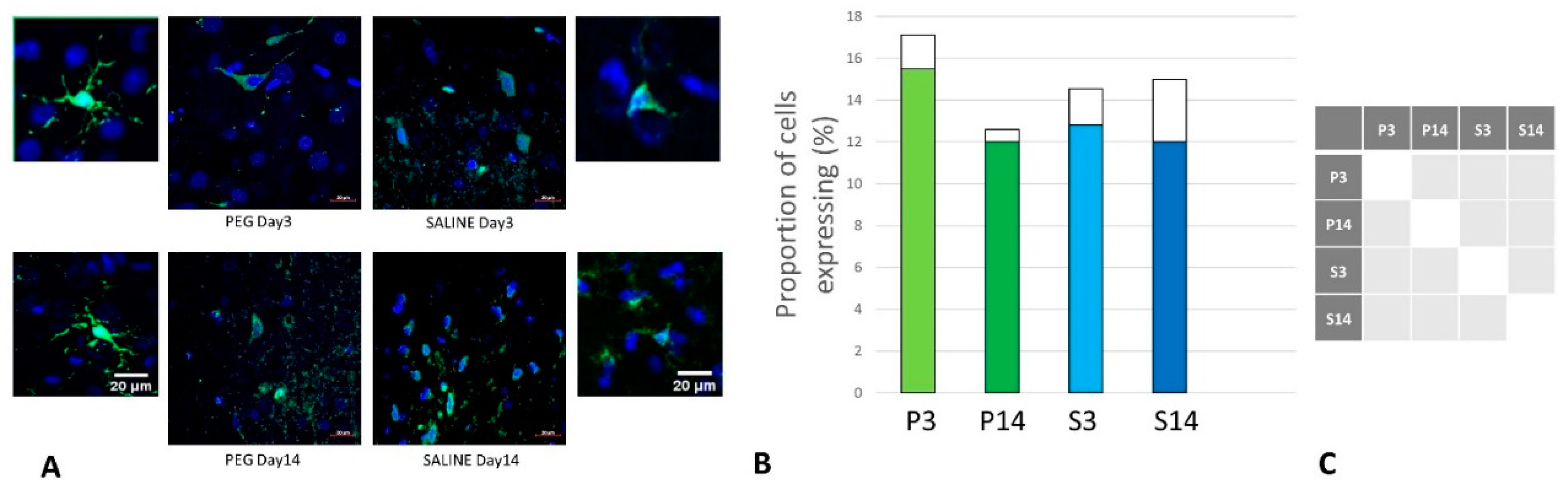
Quantification of iNOS-Positive Glial Cells
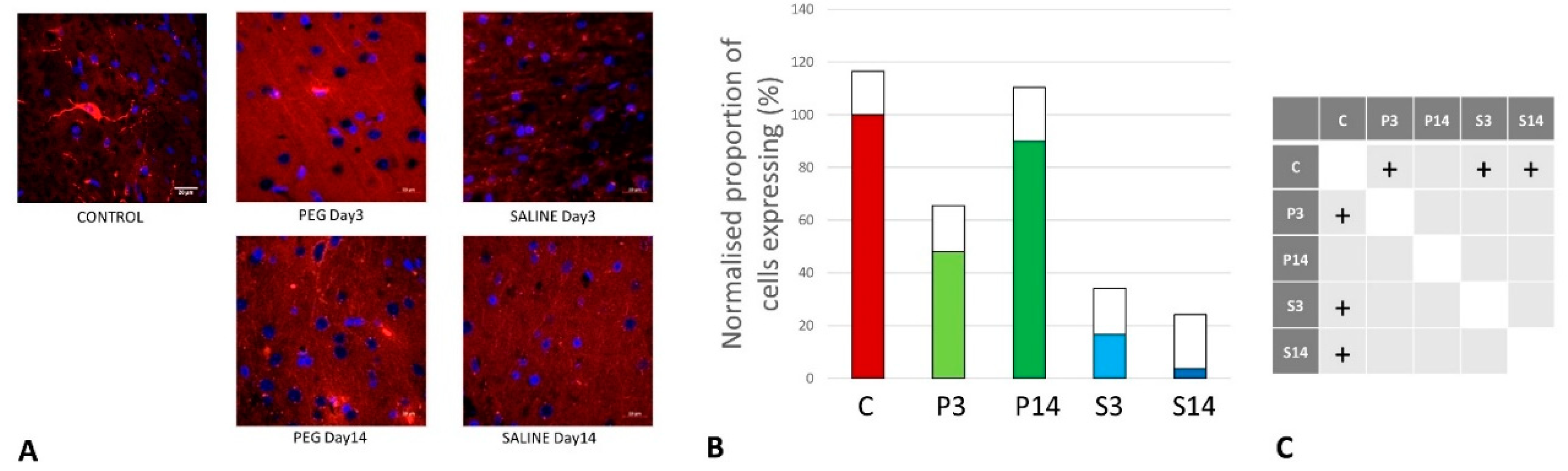
Quantification of Oligodendrocyte Regeneration
4. Discussion
5. Conclusion
Acknowledgments
Conflicts of Interest
References
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; et al.. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol 2017, 16, 987-1048. [CrossRef]
- Daugherty, J.; Waltzman, D.; Sarmiento, K.; Xu, L. Traumatic brain injury–related deaths by race/ethnicity, sex, intent, and mechanism of injury—United States, 2000–2017. Morb. Mortal. Wkly. Rep. 2019 68, 1050–1056. [CrossRef]
- DePalma, R.G.; Hoffman, S.W. . Combat blast related traumatic brain injury (TBI): Decade of recognition; promise of progress. Behav. Brain Res. 2018, 340, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, J.; Nowadly, C.D.; Schauer, S.G.; Koyfman, A.; Long, B. High risk and low prevalence diseases: Blast injuries. Am. J. Emerg. Med. 2023, 70, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Pun, P.B.L.; Kan, E. M.; Salim, A.; Li, Z.; Ng, K.C.; Moochhala, S.M.; Ling, E.A.; HongTan, M.; Lu, J. Low level primary blast injury in rodent brain. Front. Neurol., 2011, 19. [CrossRef]
- Gardner, A.J.; Zafonte, R. Neuroepidemiology of traumatic brain injury. Handb. Clin. Neurol 2016, 138, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Kabu, S.; Jaffer, H.; Petro, M.; Dudzinski, D.; Stewart, D.; Courtney, A.; Coutney, M.; Labhasetwar, V. Blast-Associated Shock Waves Result in Increased Brain Vascular Leakage and Elevated ROS Levels in a Rat Model of Traumatic Brain Injury. PLoS One 2015, 10, e0127971. [Google Scholar] [CrossRef] [PubMed]
- Meabon, J.S.; Huber, B.R.; Cross, D.J.,;Richards, T.L.; Minoshima, S.; Pagulayan, K.F.; Li, G.; Meeker, K.D.; Kraemer, B.C.; Petrie, E.C.; Raskind, M.A.; Peskind, E.R.; Cook, D.G. Repetitive blast exposure in mice and combat veterans causes persistent cerebellar dysfunction. Sci. Transl. Med. 2016, 8(321), 321ra6. [CrossRef]
- Phipps, H.; Mondello, S.; Wilson, A.; Dittmer, T.; Rohde, N.N.; Schroeder, P.J.; Nichols, J.; McGirt, C.; Hoffman, J.; Tanksley, K.; Chohan, M.; Heiderman, A.; Abbas, H.A.; Kobeissy, F.; Hinds, S. . Characteristics and Impact of U.S. Military Blast-Related Mild Traumatic Brain Injury: A Systematic Review. Front. Neurol. 2020, 11, 559318. [Google Scholar] [CrossRef]
- Georges, A.; Das, J.M. Traumatic brain injury (Archive). StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing 2024.
- Girgis, F.; Pace, J.; Sweet, J.; Miller, J.P. . Hippocampal neurophysiologic changes after mild traumatic brain injury and potential neuromodulation treatment approaches. Front. Syst. Neurosci 2016, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, S.J.; Acosta, S.; Lozano, D. Neuroinflammation in traumatic brain injury: A chronic response to an acute injury. Brain Circ. 2017, 3, 135–142. [Google Scholar] [CrossRef]
- Lu, J.; Ng, K.C.; Ling, G.; Wu, J.; Poon, D.J.F.; Kan, E.M.; Tan, M.H.; Wu, Y.J.; Li, P.; Moochhala, S.; Yap, E.; Lee, T.K.H.; Teo, M.; Yeh, I.B.; Sergio, D.M.B.; Chua, F.; Kumar, S.D.; Ling, E.A. Effect of blast exposure on the brain structure and cognition in Macaca fascicularis. J. Neurotrauma. 2012, 29, 1434–1454. [Google Scholar] [CrossRef] [PubMed]
- Bramlett, H.M.; Dietrich, W.D. Long-Term Consequences of Traumatic Brain Injury: Current Status of Potential Mechanisms of Injury and Neurological Outcomes. J. Neurotrauma 2015, 32, 1834–1848. [Google Scholar] [CrossRef]
- Gardner, R.C.; Burke, J.F.; Nettiksimmons, J.; Golgman, S.; Tanner, C.M.; Yaffe, K. Traumatic brain injury in later life increases risk for Parkinson disease. Ann. Neurol. 2015, 77, 987–995. [Google Scholar] [CrossRef]
- Graham, N.S.; Sharp, D.J. Understanding neurodegeneration after traumatic brain injury: from mechanisms to clinical trials in dementia. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Moochhala, S.; Shirhan, M.; Ng, K.C.; Teo, A.L.; Tan, M.H.; Moore, X.L.; Wong, M.C.; Ling, E.A. Neuroprotection by aminoguanidine after lateral fluid-percussive brain injury in rats: a combined magnetic resonance imaging, histopathologic and functional study. Neuropharmacology. 2003, 44, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, V.; Yakoub, K.M.; Caruso, G.; Lazzarino, G.; Signoretti, S.; Barbey, A.K.; Tavazzi, B.; Lazzarino, G.; Belli, A.; Amorini, A.M. Antioxidant Therapies in Traumatic Brain Injury. Antioxidants 2020, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Bhardwaj, K.; Nepovimova, E.; Kuca, K.; Dhanjal, D.S.; Bhardwaj, S.; Bhatia, S.K.; Verma, R.; Kumar, D. Antioxidant functionalized nanoparticles: A combat against oxidative stress. Nanomaterials 2020, 10, 1334. [Google Scholar] [CrossRef]
- Forman, H.J. ; Zhang, H Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Samuel, E.L.G.; Duong, M.T.; Bitner, B.R.; Marcano, D.C.; Tour, J.M.; Kent, T.A. Hydrophilic carbon clusters as therapeutic, high-capacity antioxidants. Trends Biotechnol. 2014, 32, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Tour, J.M.; Berlin, J.; Marcano, D.; Leonard, A.; Kent, T.A.; Pautler, R.G.; Bitner, B.; Inoue, T. Use of carbon nanomaterials with antioxidant properties to treat oxidative stress. USA Patent 2017, US9572834B2. [Google Scholar]
- Marcano, D.C.; Bitner, B.R.; Berlin, J.M.; Jarjour, J.; Lee, J.M.; Jacob, A.; Fabian, R.H.; Kent, T.A.; Tour, J.M. Design of poly (ethylene glycol)-functionalized hydrophilic carbon clusters for targeted therapy of cerebrovascular dysfunction in mild traumatic brain injury. J. Neurotrauma 2013, 30, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, K.; Derry, P.J.; Cherian, L.M.; Garcia, R.; Nilewski, L.; Goodman, J.C.; Mbye, L.; Robertson, C.S.; Tour, J.M.; Kent, T.A. Functional and Structural Improvement with a Catalytic Carbon Nano-Antioxidant in Experimental Traumatic Brain Injury Complicated by Hypotension and Resuscitation. J. Neurotrauma 2019, 36, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Samuel, E.L.G.; Marcano, D.C.; Berka, V.; Bitner, B.R.; Wu, G.; Potter, A.; Fabian, R.H.; Pautler, R.G.; Kent, T.A.; Tsai, A.-L.; Tour, J.M. Highly efficient conversion of superoxide to oxygen using hydrophilic carbon clusters. Proc. Natl. Acad. Sci. U.S.A 2015, 112, 2343–2348. [Google Scholar] [CrossRef] [PubMed]
- Derry, P.J.; Nilewski, L.G.; Sikkema, W.K.A.; Mendoza, K.; Jalilov, A.; Berka, V.; McHugh, E.A.; Tsai, A.-L.; Tour, J.M.; Kent, T.A. Catalytic oxidation and reduction reactions of hydrophilic carbon clusters with NADH and cytochrome C: features of an electron transport nanozyme. Nanoscale 2019, 11, 10791–10807. [Google Scholar] [CrossRef]
- Derry, P.J.; Liopo, A.V.; Mouli, K.; McHugh, E.A.; Vo, A.T.T.; McKelvey, A.; Suva, L.J.; Wu, G.; Gao, Y.; Olson, K.R.; Tour, J.M.; Kent, T.A. Oxidation of Hydrogen Sulfide to Polysulfide and Thiosulfate by a Carbon Nanozyme: Therapeutic Implications with an Emphasis on Down Syndrome. Adv. Mater. 2024, 36, e2211241. [Google Scholar] [CrossRef] [PubMed]
- Shi, R. Polyethylene glycol repairs membrane damage and enhances functional recovery: a tissue engineering approach to spinal cord injury. Neurosci. Bull. 2013, 29, 460–466. [Google Scholar] [CrossRef]
- Bitner, B.R.; Marcano, D.C.; Berlin, J.M.; Fabian, R.H.; Cherian, L.; Culver, J.C.; Dickinson, M.E.; Robertson, C.S.; Pautler, R.G.; Kent, T.A.; Tour, J.M. Antioxidant Carbon Particles Improve Cerebrovascular Dysfunction Following Traumatic Brain Injury. ACS Nano. 2012, 6, 8007–8014. [Google Scholar] [CrossRef]
- Fabian, R.H.; Derry, P.J.; Rea, H.C.; Dalmeida, W.V.; Nilewski, L.G.; Sikkema, W.K.A.; Mandava, P.; Tsai, A.-L.; Mendoza, K.; Berka, V.; Tour, J.M.; Kent, T.A. Efficacy of Novel Carbon Nanoparticle Antioxidant Therapy in a Severe Model of Reversible Middle Cerebral Artery Stroke in Acutely Hyperglycemic Rats. Front. Neurol. 2018, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Huq, R.; Samuel, E.L.G.; Sikkema, W.K.A.; Nilewski, L.G.; Lee, T.; Tanner, M.R.; Khan, F.S.; Porter, P.C.; Tajhya, R.B.; Patel, R.S.; Inoue, T.; Pautler, R.G.; Corry, D.B.; Tour, J.M.; Beeton, C. Preferential uptake of antioxidant carbon nanoparticles by T lymphocytes for immunomodulation. Sci. Rep. 2016, 6, 33808. [Google Scholar] [CrossRef] [PubMed]
- Sahni, D.; Jea, A.; Mata, J.A.; Marcano, D.C.; Sivaganesan, A.; Berlin, J.M.; Tatsui, C.E.; Sun, Z.; Luerssen, T.G.; Meng, S.; Kent, T.A.; Tour, J.M. Biocompatibility of pristine graphene for neuronal interface. J. Neurosurg. Pediatr. 2013, 11, 575–583. [Google Scholar] [CrossRef]
- Lerner, E.B.; Moscati, R.M. The golden hour: scientific fact or medical “urban legend”? Acad. Emerg. Med. 2001, 8, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.R.; Trooskin, S.Z.; Doshi, P.J.; Greenwald, L.; Mode, C.J. Time to laparotomy for intra-abdominal bleeding from trauma does affect survival for delays up to 90 minutes. J. Trauma 2002, 52, 420–425. [Google Scholar] [CrossRef]
- Gundersen, H.J.; Jensen, E.B. The efficiency of systematic sampling in stereology and its prediction. J. Microsc. 1987, 147 (Pt 3), 229–263. [Google Scholar] [CrossRef]
- West, M.J.; Slomianka, L.; Gundersen, H.J. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 1991, 231, 482–97. [Google Scholar] [CrossRef]
- Kaur, C.; Singh, J.; Moochhala, S.; Lim, M.K.; Lu, J.; Ling, E.A. Induction of NADPH diaphorase/nitric oxide synthase in the spinal cord motor neurons of rats following a single and multiple non-penetrative blasts. Histol. Histopathol. 1999, 14, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Gravel, M.; Peterson, J.; Yong, V.W.; Kottis, V.; Trapp, B.; Braun, P.E. Overexpression of 2',3'-cyclic nucleotide 3'-phosphodiesterase in transgenic mice alters oligodendrocyte development and produces aberrant myelination. Mol. Cell. Neurosci. 1996, 7, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Lee, K.; Qi, Z.; Wang, Z.; Xu, Z.; Wu, X.; Mao, Y. Neuroinflammation Following Traumatic Brain Injury: Take It Seriously or Not. Front. Immunol. 2022, 13, 855701. [Google Scholar] [CrossRef]
- Middeldorp, J.; Hol, E.M. GFAP in health and disease. Prog Neurobiol. 2011, 93, 421–443. [Google Scholar] [CrossRef] [PubMed]
- Eng, L.F.; Ghirnikar, R.S.; Lee, Y.L. Glial fibrillary acidic protein: GFAP-thirty-one years (1969-2000). Neurochem. Res. 2000, 25, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Cattin, A.L.; Burden, J.J.; Van Emmenis, L.; Mackenzie, F.E.; Hoving, J.J.; Garcia Calavia, N.; Guo, Y.; McLaughlin, M.; Rosenberg, L.H.; Quereda, V.; Jamecna, D.; Napoli, I.; Parrinello, S.; Enver, T.; Ruhrberg, C.; Lloyd, A.C. Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves. Cell 2015, 162, 1127–1139. [Google Scholar] [CrossRef]
- Mullen, R.J.; Buck, C.R.; Smith, A.M. NeuN, a neuronal specific nuclear protein in vertebrates. Development 1992, 116, 201–211. [Google Scholar] [CrossRef]
- Wolf, H.K.; Buslei, R.; Schmidt-Kastner, R.; Schmidt-Kastner, P.K.; Pietsch, T.; Wiestler, O.D.; Blümcke, I. . NeuN: a useful neuronal marker for diagnostic histopathology. J. Histochem. Cytochem. 1996, 44, 1167–1171. [Google Scholar] [CrossRef]
- Ito, D.; Imai, Y.; Ohsawa, K.; Nakajima, K.; Fukuuchi, Y.; Kohsaka, S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res. Mol. Brain Res. 1998, 57, 1–9. [Google Scholar] [CrossRef]
- Ohsawa, K.; Imai, Y.; Sasaki, Y.; Kohsaka, S. Microglia/macrophage-specific protein Iba1 binds to fimbrin and enhances its actin-bundling activity. J. Neurochem. 2004, 88, 844–856. [Google Scholar] [CrossRef]
- Nathan, C.; Xie, Q.W. Nitric oxide synthases: roles, tolls, and controls. Cell 1994, 78, 915–918. [Google Scholar] [CrossRef]
- Colton, C.A.; Gilbert, D.L. Production of superoxide anions by a CNS macrophage, the microglia. FEBS Lett. 1987, 223, 284–288. [Google Scholar] [CrossRef]
- Baumann, N.; Pham-Dinh, D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol. Rev. 2001, 81, 871–927. [Google Scholar] [CrossRef]
- Dyer, C.A.; Benjamins, J.A. Organization of oligodendroglial membrane sheets. I. Association of myelin basic protein and 2',3'-cyclic nucleotide 3'-phosphohydrolase with cytoskeleton. J. Neurosci. Res. 1989, 24, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Bryden, D.W.; Tilgham, J.I; Hinds, S.R. 2nd. Blast-related traumatic brain injury: Current concepts and research considerations. J. Exp. Neurosci. 2019, 13, 1179069519872213. [Google Scholar] [CrossRef]
- Cernak, I. . Chapter 45; Blast injuries and blast-induced neurotrauna: Overview of pathophysiology and experimental kwoledge models and findings. In Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects; Kobeissy, F.H., Ed.; CRC Press/Taylor & Francis: Boca Raton (FL); 2015. [Google Scholar]
- Rosenfeld, J.V.; McFarlane, A.C.; Bragge, P.; Armonda, R.A.; Grimes, J.B.; Ling, G.S. Blast-related traumatic brain injury. Lancet Neurol. 2013, 12, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Champion, H.R.; Holcomb, J.B.; Young, L.A. Injuries from explosions: Physics, biophysics, pathology, and required research focus. J. Trauma 2009, 66, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Royo, N.C.; Schouten, J.W.; Fulp, C.T.; Shimizu, S.; Marklund, N.; Graham, D.I.; McIntosh, D.K. From cell death to neuronal regeneration: building a new brain after traumatic brain injury. J. Neuropathol. Exp. Neurol. 2003, 62, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Garry, P.S.; Ezra, M.; Rowland, M.J.; Westbrook, J.; Parrinson, K.T.S. The role of the nitric oxide pathway in brain injury and its treatment--from bench to bedside. Exp. Neurol. 2015, 263, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Cheng, Y.; Peng, W.; Wu, Y.; Rui, T.; Luo, C.; Zhang, J. Targeting iNOS Alleviates Early Brain Injury After Experimental Subarachnoid Hemorrhage via Promoting Ferroptosis of M1 Microglia and Reducing Neuroinflammation. Mol. Neurobiol. 2022, 59, 3124–3139. [Google Scholar] [CrossRef]
- Fabian, R.H.; Kent, T.A. Hyperglycemia accentuates persistent "functional uncoupling" of cerebral microvascular nitric oxide and superoxide following focal ischemia/reperfusion in rats. Transl. Stroke Res. 2012, 3, 482–90. [Google Scholar] [CrossRef] [PubMed]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef] [PubMed]
- Stoll, G.; Jander, S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog. Neurobiol. 1999, 58, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef]
- Svetlov, S.I.; Prima, V.; Glushakova, O.; Svetlov, A.; Kirk, D.R.; Gutierrez, H.; Serebruany, V.L.; Curley, K.C.; Wang, K.K.W.; Hayes, R.L. Neuro-glial and systemic mechanisms of pathological responses in rat models of primary blast overpressure compared to “composite” blast. Front. Neurol. 2012, 3, 15. [Google Scholar] [CrossRef]
- Sprinkle, T.J.; Agee, J.F.; Tippins, R.B.; Chamberlain, C.R.; Faguet, G.B.; DeVries, G.H. Monoclonal antibody production to human and bovine 2’,3’-cyclic nucleotide 3’-phosphodiesterase (CNPase): high-specificity recognition in whole brain acetone powders and conservation of sequence CNP1 and CNP2. Brain Res. 1987, 426, 349–357. [Google Scholar] [CrossRef]
- Trapp, B.D.; Bernier, L.; Andrews, S.B.; Colman, D.R. Cellular and subcellular distribution of 2',3'-cyclic nucleotide 3'-phosphodiesterase and its mRNA in the rat central nervous system. J. Neurochem. 1988, 51, 859–868. [Google Scholar] [CrossRef]
- Verrier, J.D.; Jackson, T.C.; Gillespie, D.G.; Janesko-Feldman, K.; Bansal, R.; Goebbels, S.; Nave, K.-A.; Kochanek, P.M.; Jackson, E.K. Role of CNPase in the oligodendrocytic extracellular 2',3'-cAMP-adenosine pathway. Glia 2013, 61, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- French, H.M.; Reid, M.; Mamontov, P.; Simmons, R.A.; Grinspan, J.B. Oxidative stress disrupts oligodendrocyte maturation. J. Neurosci. Res. 2009, 87, 3076–3087. [Google Scholar] [CrossRef]
- Dent, K.A.; Christie, K.J.; Bye, N.; Basrai, H.S.; Turbic, A.; Habgood, M.; Cate, H.S.; Turnley, A.M. Oligodendrocyte birth and death following traumatic brain injury in adult mice. PloS One 2015, 10, e0121541. [Google Scholar] [CrossRef] [PubMed]
- Flygt, J.; Clausen, F.; Marklund, N. Diffuse traumatic brain injury in the mouse induces a transient proliferation of oligodendrocyte progenitor cells in injured white matter tracts. Restor. Neurol. Neurosci. 2017, 35, 251–263. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




