Submitted:
22 October 2024
Posted:
24 October 2024
You are already at the latest version
Abstract
Keywords:
1. Bacteria in Semen
1.1. Sources of Bacteria in Semen
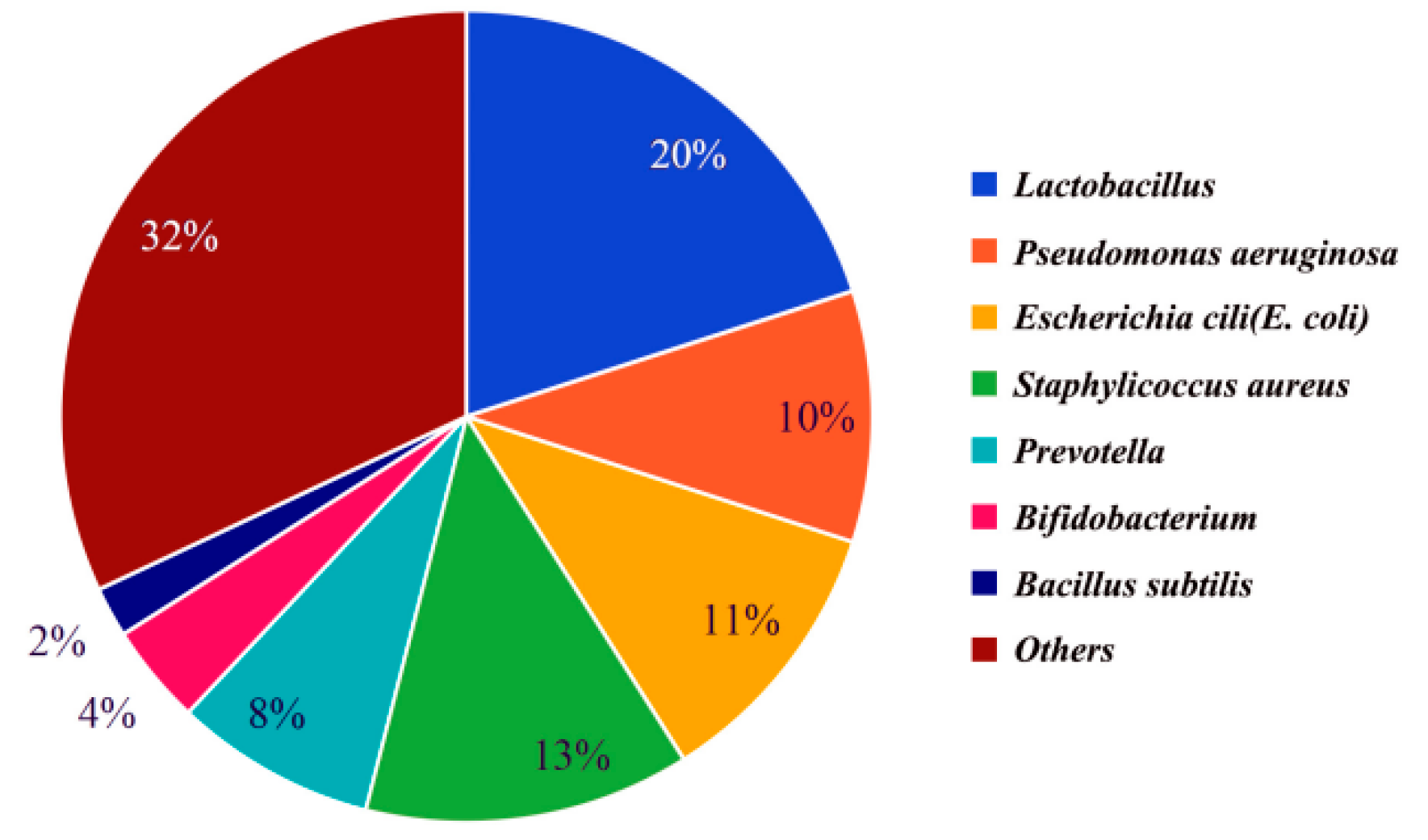
1.2. Types of Bacteria in Semen
1.3. Effects of Bacteria on Semen Quality
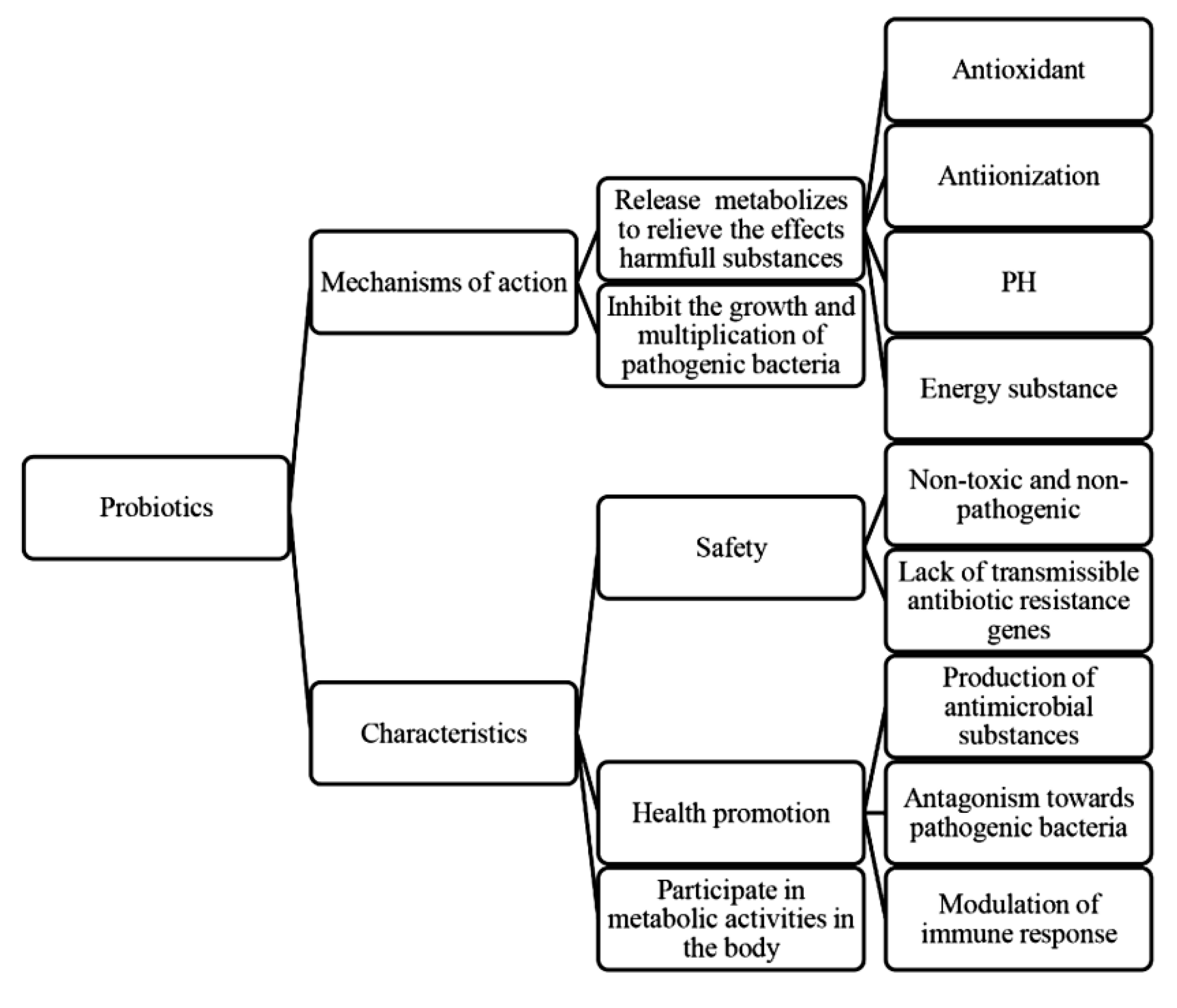
1.3.1. Effects of Probiotics on Semen Quality
1.3.2. Effects of Pathogenic Bacteria on Semen Quality
2. Natural Antimicrobial Substances in Semen
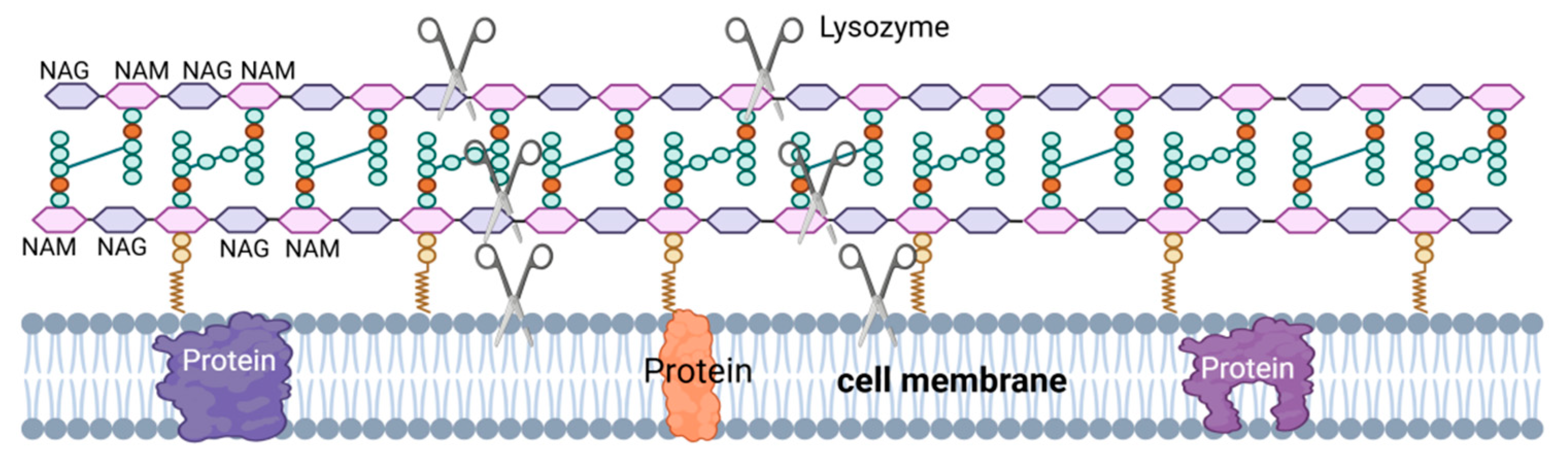
2.1. Lysozyme (LSZ)
2.2. Secretory Leukocyte Peptidase Inhibitor (SLPI)
2.3. Lactoferrin (LF)
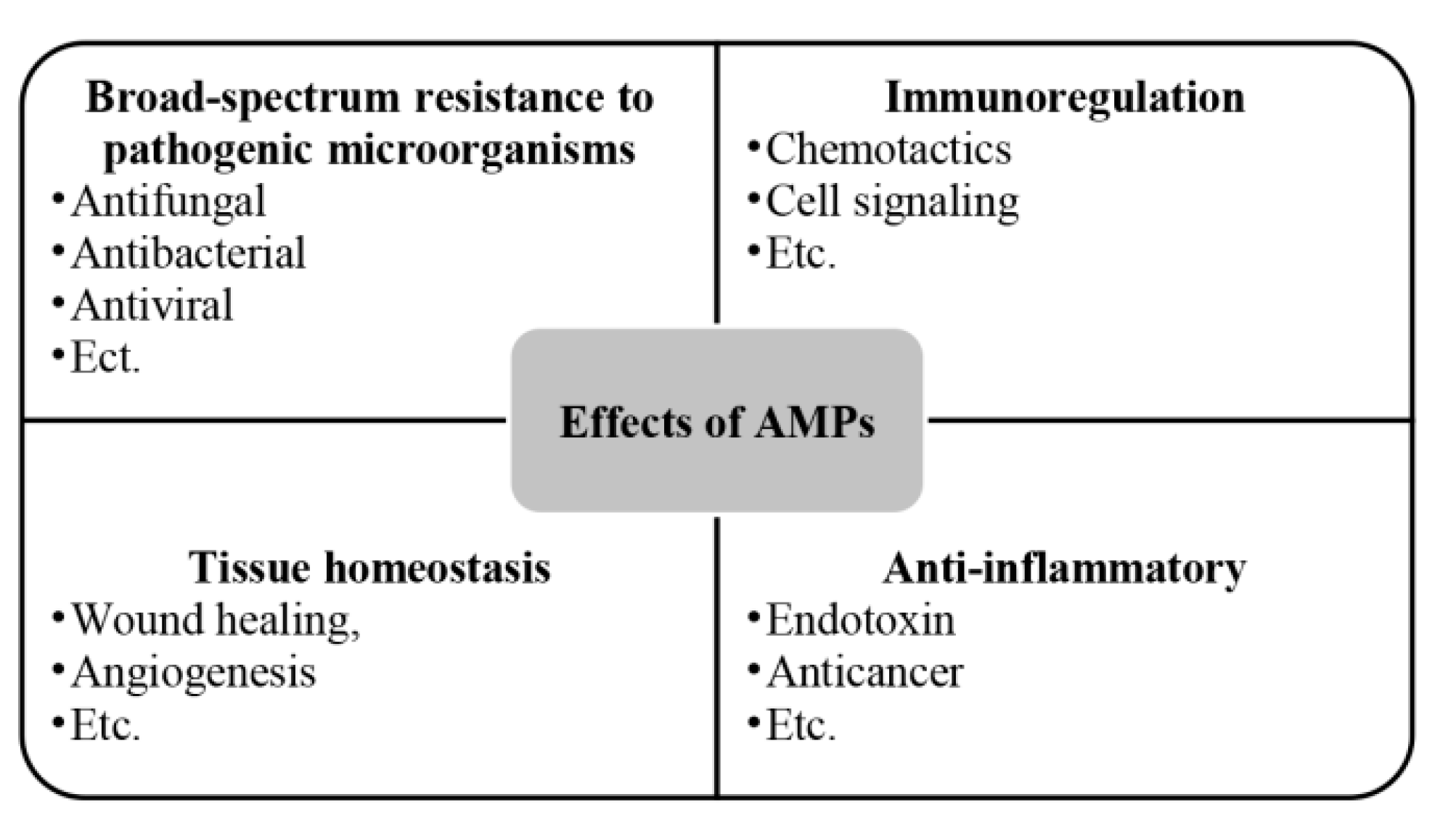
2.4. Antibacterial peptides (AMPs)
2.5. Group II Phospholipase A2 (PLA2)
2.6. Others
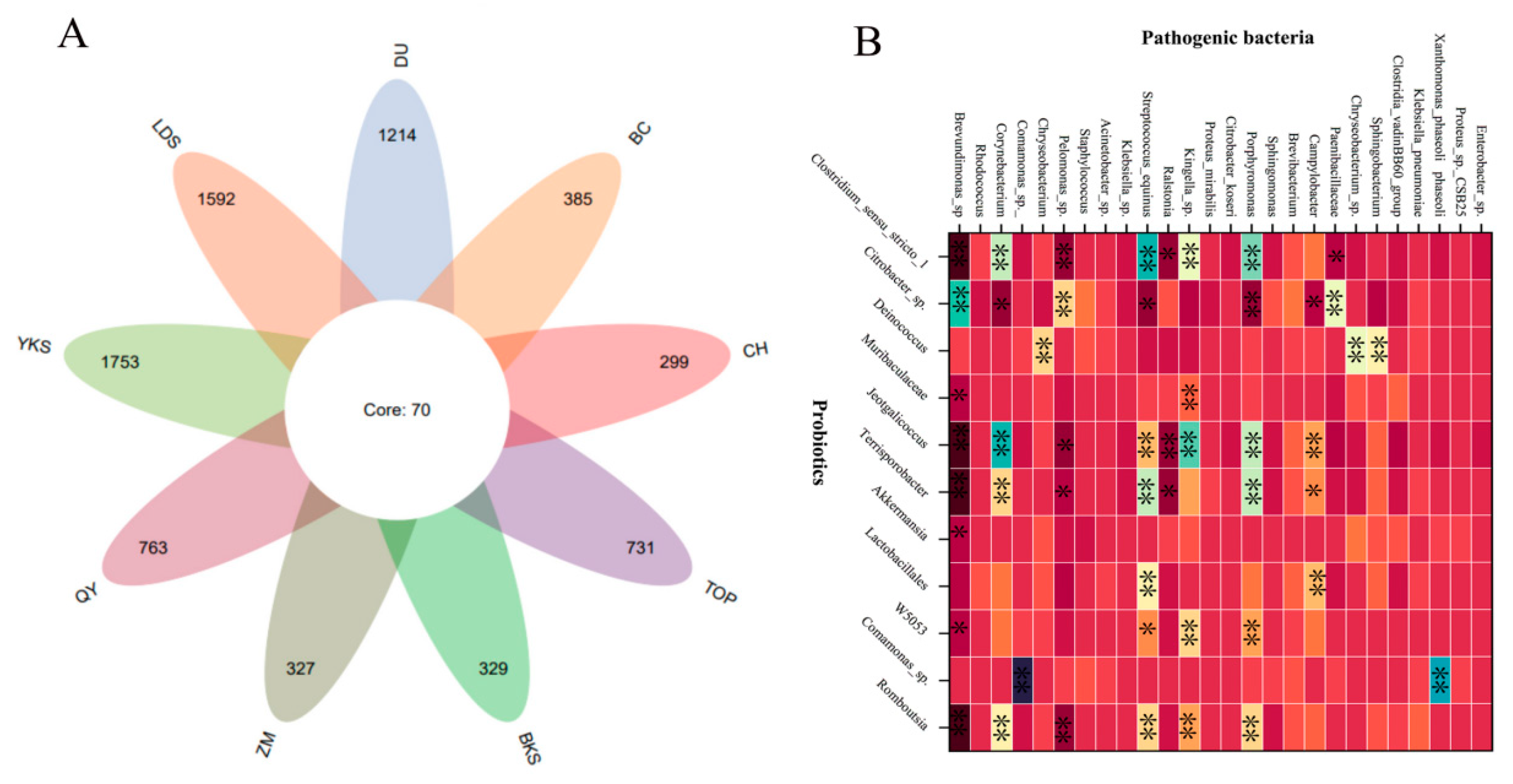
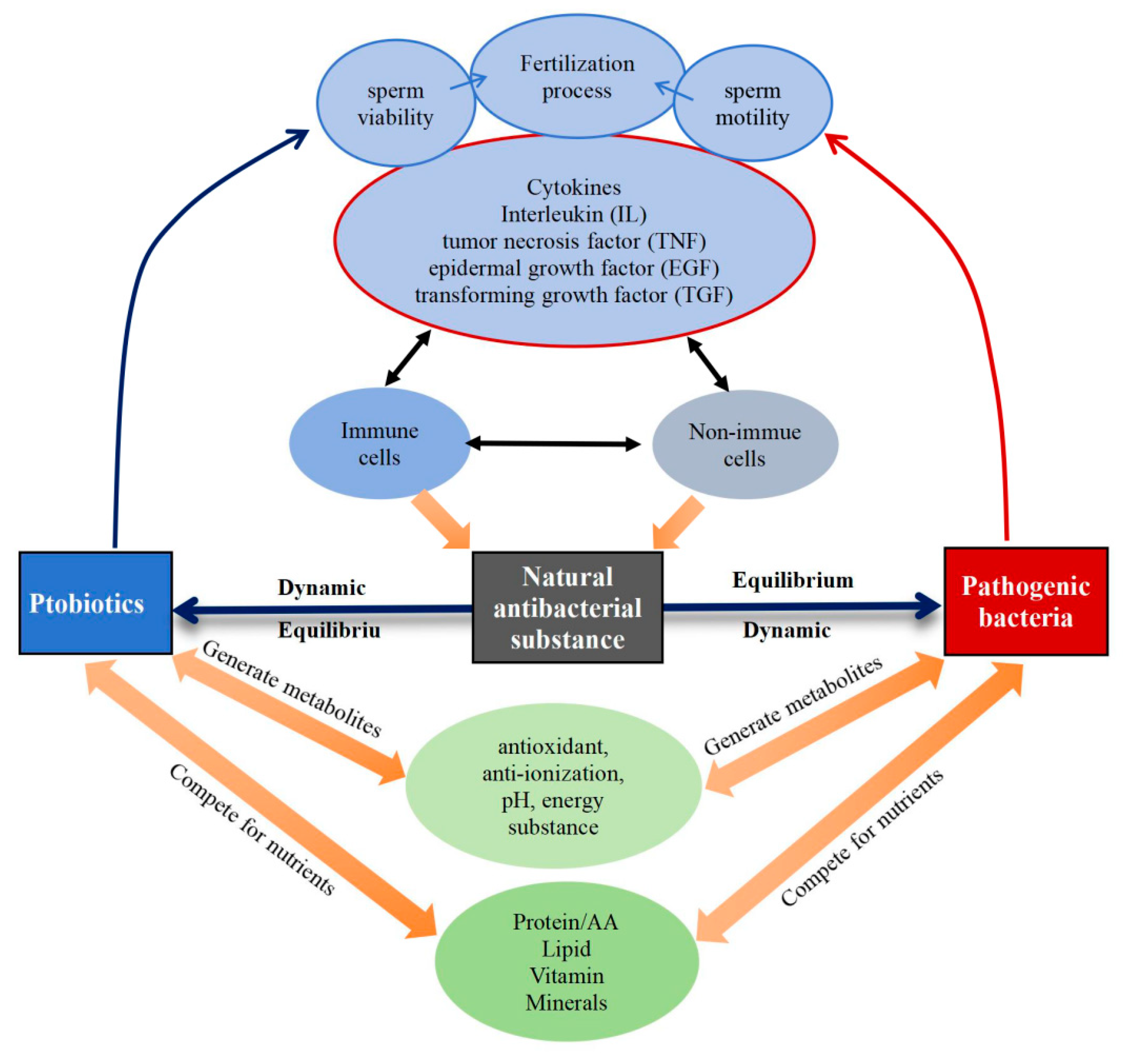
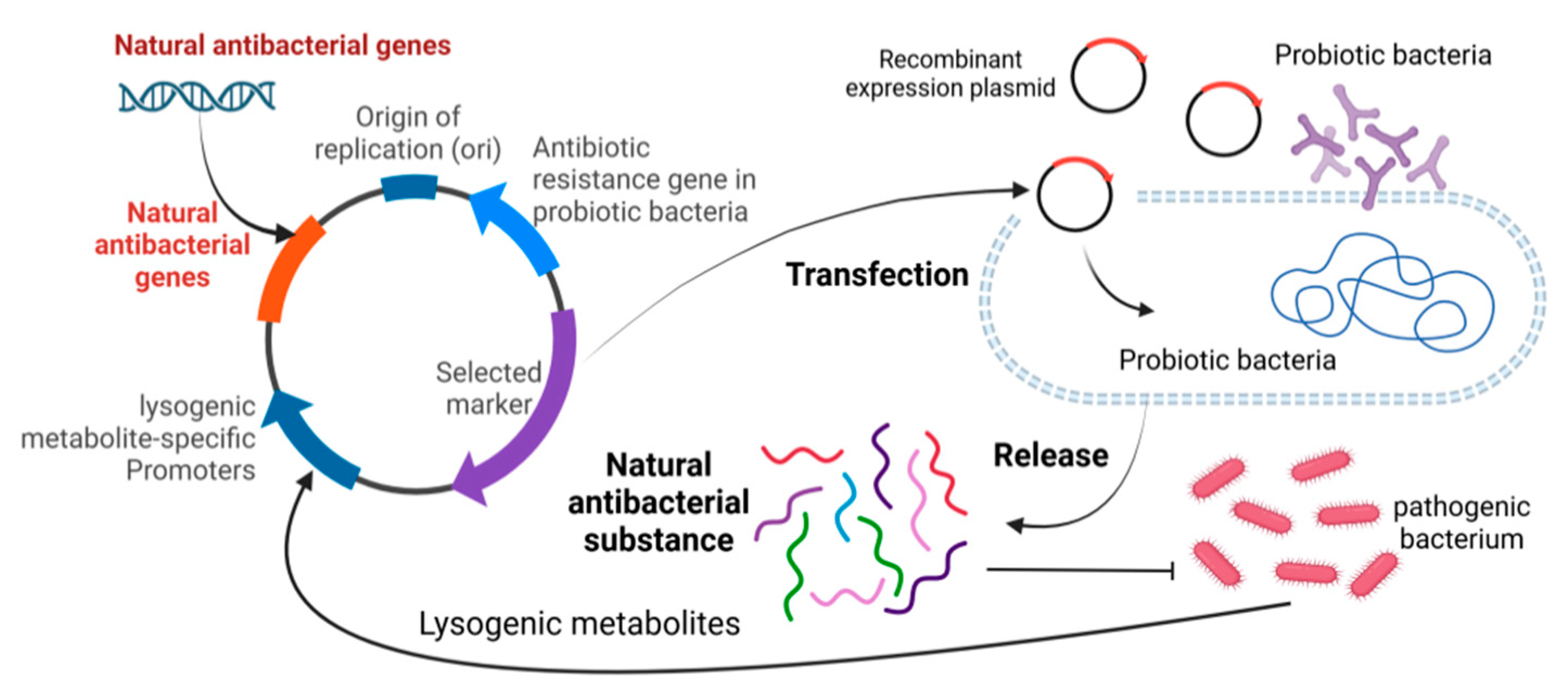
3. Interaction of Bacterial Microecosystem: Probiotics, Pathogenic Bacteria and Natural Antibacterial Substances
3.1. The Relationship Between Probiotics and Pathogenic Bacteria
3.2. The Equilibrium of Probiotics, Pathogenic Bacteria and Natural Antibacterial Substances
4. Summarization and Prospects
CRediT authorship contribution statement
Declaration of competing interest
Acknowledgments
References
- Zhang J, Liu H, Yang Q, et al. Genomic Sequencing Reveals the Diversity of Seminal Bacteria and Relationships to Reproductive Potential in Boar Sperm[J]. Frontiers in Microbiology 2020, 11, 1873. [Google Scholar]
- Sepúlveda L, Bussalleu E, Yeste M, et al. Effects of different concentrations of Pseudomonas aeruginosa on boar sperm quality[J]. Animal Reproduction Science 2014, 150, 96–106. [Google Scholar]
- Sepúlveda L, Bussalleu E, Yeste M, et al. Effect of Pseudomonas aeruginosa on sperm capacitation and protein phosphorylation of boar spermatozoa[J]. Theriogenology 2016, 85, 1421–1431. [Google Scholar] [CrossRef]
- Ďuračka M, Belić L, Tokárová K, et al. Bacterial communities in bovine ejaculates and their impact on the semen quality[J]. Systems Biology in Reproductive Medicine 2021, 67, 438–449. [Google Scholar] [CrossRef]
- Schulze M, Czirják G Á, Müller K, et al. Antibacterial defense and sperm quality in boar ejaculates[J]. Journal of Reproductive Immunology 2019, 131, 13–20. [Google Scholar] [CrossRef]
- Farsimadan M, Motamedifar M. Bacterial infection of the male reproductive system causing infertility[J]. Journal of Reproductive Immunology 2020, 142, 103183. [Google Scholar] [CrossRef] [PubMed]
- Schulze M, Jakop U, Schröter F, et al. Antibacterial defense in bull and boar semen: A putative link to the microbiome and reproductive strategy?[J]. Theriogenology 2020, 157, 335–340. [Google Scholar] [CrossRef]
- Tett A, Pasolli E, Masetti G, et al. Prevotella diversity, niches and interactions with the human host[J]. Nature Reviews. Microbiology 2021, 19, 585–599. [Google Scholar]
- Lundy S D, Sangwan N, Parekh N V, et al. Functional and Taxonomic Dysbiosis of the Gut, Urine, and Semen Microbiomes in Male Infertility[J]. European Urology 2021, 79, 826–836.
- Liang J, Wu T, Wang T, et al. Moringa oleifera leaf ethanolic extract benefits cashmere goat semen quality via improving rumen microbiota and metabolome[J]. Frontiers in Veterinary Science 2023, 10, 1049093. [Google Scholar] [CrossRef]
- Gomes I de A, Monteiro P B, Moura G A de, et al. Microbiota and seminal quality: A systematic review[J]. JBRA assisted reproduction 2023, 27, 507–513. [Google Scholar]
- Wang S, Zhang K, Yao Y, et al. Bacterial Infections Affect Male Fertility: A Focus on the Oxidative Stress-Autophagy Axis[J]. Frontiers in Cell and Developmental Biology, 2021; 9, 727812.
- Fraczek M, Kurpisz M. Mechanisms of the harmful effects of bacterial semen infection on ejaculated human spermatozoa: potential inflammatory markers in semen[J]. Folia Histochemica et Cytobiologica 2015, 53, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Pinart E, Domènech E, Bussalleu E, et al. A comparative study of the effects of Escherichia coli and Clostridium perfringens upon boar semen preserved in liquid storage[J]. Animal Reproduction Science 2017, 177, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Bonet S, Delgado-Bermúdez A, Yeste M, et al. Study of boar sperm interaction with Escherichia coli and Clostridium perfringens in refrigerated semen[J]. Animal Reproduction Science 2018, 197, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Rafiee M, Sereshki N, Alipour R, et al. The effect of probiotics on immunogenicity of spermatozoa in couples suffering from recurrent spontaneous abortion[J]. BMC immunology 2022, 23, 32. [Google Scholar]
- Baker J M, Chase D M, Herbst-Kralovetz M M. Uterine Microbiota: Residents, Tourists, or Invaders?[J]. Frontiers in Immunology 2018, 9, 208. [Google Scholar] [CrossRef]
- Jeon S J, Cunha F, Vieira-Neto A, et al. Blood as a route of transmission of uterine pathogens from the gut to the uterus in cows[J]. Microbiome 2017, 5, 109. [Google Scholar]
- Altmäe S, Franasiak J M, Mändar R. The seminal microbiome in health and disease[J]. Nature Reviews Urology 2019, 16, 703–721. [Google Scholar] [CrossRef]
- Castillo J, Jodar M, Oliva R. The contribution of human sperm proteins to the development and epigenome of the preimplantation embryo[J]. Human Reproduction Update 2018, 24, 535–555. [Google Scholar] [CrossRef]
- Alfano M, Ferrarese R, Locatelli I, et al. Testicular microbiome in azoospermic men-first evidence of the impact of an altered microenvironment[J]. Human Reproduction (Oxford, England) 2018, 33, 1212–1217. [Google Scholar] [CrossRef]
- Javurek A B, Spollen W G, Ali A M M, et al. Discovery of a Novel Seminal Fluid Microbiome and Influence of Estrogen Receptor Alpha Genetic Status[J]. Scientific Reports 2016, 6, 23027. [Google Scholar]
- Cavarretta I, Ferrarese R, Cazzaniga W, et al. The Microbiome of the Prostate Tumor Microenvironment[J]. European Urology 2017, 72, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Chen H, Luo T, Chen T, et al. Seminal bacterial composition in patients with obstructive and non-obstructive azoospermia[J]. Experimental and Therapeutic Medicine 2018, 15, 2884–2890. [Google Scholar]
- Monteiro C, Marques P I, Cavadas B, et al. Characterization of microbiota in male infertility cases uncovers differences in seminal hyperviscosity and oligoasthenoteratozoospermia possibly correlated with increased prevalence of infectious bacteria[J]. American Journal of Reproductive Immunology (New York, N.Y.: 1989) 2018, 79, e12838. [Google Scholar]
- Mändar R, Punab M, Korrovits P, et al. Seminal microbiome in men with and without prostatitis[J]. International Journal of Urology: Official Journal of the Japanese Urological Association 2017, 24, 211–216. [Google Scholar] [CrossRef]
- Liu C M, Osborne B J W, Hungate B A, et al. The semen microbiome and its relationship with local immunology and viral load in HIV infection[J]. PLoS pathogens 2014, 10, e1004262. [Google Scholar]
- McAnally B E, Smith M S, Wiegert J G, et al. Characterization of boar semen microbiome and association with sperm quality parameters[J]. Journal of Animal Science 2023, 101, skad243. [Google Scholar] [CrossRef]
- Díaz Cano J V, Argente M-J, García M-L. Effect of Postbiotic Based on Lactic Acid Bacteria on Semen Quality and Health of Male Rabbits[J]. Animals: an open access journal from MDPI 2021, 11, 1007. [Google Scholar] [CrossRef]
- Yan F, Cao H, Cover T L, et al. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism[J]. The Journal of Clinical Investigation 2011, 121, 2242–2253. [Google Scholar] [CrossRef]
- Oghbaei H, Rastgar Rezaei Y, Nikanfar S, et al. Effects of bacteria on male fertility: Spermatogenesis and sperm function[J]. Life Sciences 2020, 256, 117891. [Google Scholar] [CrossRef]
- Delgado-Bermúdez A, Bonet S, Yeste M, et al. Long-term storage of boar seminal doses contaminated with Proteus vulgaris: A dose-dependent effect on sperm motility and sperm-bacteria interaction[J]. Animal Reproduction Science 2020, 216, 106349. [Google Scholar] [CrossRef] [PubMed]
- Contreras M J, Núñez-Montero K, Bruna P, et al. Bacteria and Boar Semen Storage: Progress and Challenges[J]. Antibiotics (Basel, Switzerland) 2022, 11, 1796. [Google Scholar]
- Ivanov I B, Kuzmin M D, Gritsenko V A. Microflora of the seminal fluid of healthy men and men suffering from chronic prostatitis syndrome[J]. International Journal of Andrology 2009, 32, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Menezes T de A, Mellagi A P G, da Silva Oliveira G, et al. Antibiotic-free extended boar semen preserved under low temperature maintains acceptable in-vitro sperm quality and reduces bacterial load[J]. Theriogenology 2020, 149, 131–138. [Google Scholar] [CrossRef]
- Panacheva E, Voroshilina E, Kudryavtseva E. P-104 Impact of Semen Microbiota on the Embryo Quality[J]. Human Reproduction 2023, 38, dead093.468.
- Kuster C E, Althouse G C. The impact of bacteriospermia on boar sperm storage and reproductive performance[J]. Theriogenology 2016, 85, 21–26. [Google Scholar] [CrossRef]
- Prieto-Martínez N, Bussalleu E, Garcia-Bonavila E, et al. Effects of Enterobacter cloacae on boar sperm quality during liquid storage at 17°C[J]. Animal Reproduction Science 2014, 148, 72–82. [Google Scholar] [CrossRef]
- Sanca F M M, Blanco I R, Dias M, et al. Antimicrobial Activity of Peptides Produced by Lactococcus lactis subsp. lactis on Swine Pathogens[J]. Animals: an open access journal from MDPI 2023, 13, 2442. [Google Scholar] [CrossRef]
- Weng S-L, Chiu C-M, Lin F-M, et al. Bacterial Communities in Semen from Men of Infertile Couples: Metagenomic Sequencing Reveals Relationships of Seminal Microbiota to Semen Quality[J]. Z. Abdo. PLoS ONE 2014, 9, e110152. [Google Scholar]
- Valcarce D G, Genovés S, Riesco M F, et al. Probiotic administration improves sperm quality in asthenozoospermic human donors[J]. Beneficial Microbes 2017, 8, 193–206. [Google Scholar] [CrossRef]
- Mahiddine F Y, You I, Park H, et al. Management of dog sperm parameters and gut microbiota composition with Lactobacillus rhamnosus supplementation[J]. Veterinary Research Communications 2023, 47, 1629–1640. [Google Scholar] [CrossRef] [PubMed]
- Helli B, Kavianpour M, Ghaedi E, et al. Probiotic effects on sperm parameters, oxidative stress index, inflammatory factors and sex hormones in infertile men[J]. Human Fertility (Cambridge, England) 2022, 25, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Wolff H, Panhans A, Stolz W, et al. Adherence of Escherichia coli to sperm: a mannose mediated phenomenon leading to agglutination of sperm and E. coli[J]. Fertility and Sterility 1993, 60, 154–158. [Google Scholar] [CrossRef]
- Prabha V, Sandhu R, Kaur S, et al. Mechanism of sperm immobilization by Escherichia coli[J]. Advances in Urology 2010, 2010, 240268. [Google Scholar]
- Jones S E, Versalovic J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors[J]. BMC microbiology 2009, 9, 35. [Google Scholar]
- Ragan M V, Wala S J, Goodman S D, et al. Next-Generation Probiotic Therapy to Protect the Intestines From Injury[J]. Frontiers in Cellular and Infection Microbiology 2022, 12, 863949. [Google Scholar]
- Yan F, Cao H, Cover T L, et al. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth[J]. Gastroenterology 2007, 132, 562–575. [Google Scholar] [CrossRef]
- La Fata G, Weber P, Mohajeri M H. Probiotics and the Gut Immune System: Indirect Regulation[J]. Probiotics and Antimicrobial Proteins 2018, 10, 11–21. [Google Scholar] [CrossRef]
- Mishra V, Shah C, Mokashe N, et al. Probiotics as potential antioxidants: a systematic review[J]. Journal of Agricultural and Food Chemistry 2015, 63, 3615–3626. [Google Scholar] [CrossRef]
- Wang Y, Xie Z. Exploring the role of gut microbiome in male reproduction[J]. Andrology 2022, 10, 441–450. [Google Scholar] [CrossRef]
- Chen X L, Gong L Z, Xu J X. Antioxidative activity and protective effect of probiotics against high-fat diet-induced sperm damage in rats[J]. Animal 2013, 7, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee N, Anderson M A, Haagsman H P, et al. Antimicrobial host defence peptides: functions and clinical potential[J]. Nature Reviews. Drug Discovery 2020, 19, 311–332. [Google Scholar] [CrossRef] [PubMed]
- Bussalleu E, Sancho S, Briz M D, et al. Do antimicrobial peptides PR-39, PMAP-36 and PMAP-37 have any effect on bacterial growth and quality of liquid-stored boar semen?[J]. Theriogenology 2017, 89, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Puig-Timonet A, Castillo-Martín M, Pereira B A, et al. Evaluation of porcine beta defensins-1 and -2 as antimicrobial peptides for liquid-stored boar semen: Effects on bacterial growth and sperm quality[J]. Theriogenology 2018, 111, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Otti O, Naylor R A, Siva-Jothy M T, et al. Bacteriolytic activity in the ejaculate of an insect[J]. The American Naturalist 2009, 174, 292–295. [Google Scholar] [CrossRef]
- Doncel G F, Joseph T, Thurman A R. Role of Semen in HIV-1 Transmission: Inhibitor or facilitator?: ROLE OF SEMEN IN HIV-1 TRANSMISSION[J]. American Journal of Reproductive Immunology 2011, 65, 292–301. [Google Scholar] [CrossRef]
- Baud D, Pattaroni C, Vulliemoz N, et al. Sperm Microbiota and Its Impact on Semen Parameters[J]. Frontiers in Microbiology 2019, 10, 234. [Google Scholar]
- Farahani L, Tharakan T, Yap T, et al. The semen microbiome and its impact on sperm function and male fertility: A systematic review and meta-analysis[J]. Andrology 2021, 9, 115–144. [Google Scholar] [CrossRef]
- Yenugu S, Hamil K G, French F S, et al. Antimicrobial actions of human and macaque sperm associated antigen (SPAG) 11 isoforms: influence of the N-terminal peptide[J]. Molecular and Cellular Biochemistry 2006, 284, 25–37. [Google Scholar]
- Rowe M, Czirják G Á, McGraw K J, et al. Sexual ornamentation reflects antibacterial activity of ejaculates in mallards[J]. Biology Letters 2011, 7, 740–742. [Google Scholar] [CrossRef]
- Poiani, A. Complexity of seminal fluid: a review[J]. Behavioral Ecology and Sociobiology 2006, 60, 289–310. [Google Scholar] [CrossRef]
- Ragland S A, Criss A K. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme[J]. PLoS pathogens 2017, 13, e1006512. [Google Scholar]
- Kim K, Song M, Liu Y, et al. Enterotoxigenic Escherichia coli infection of weaned pigs: Intestinal challenges and nutritional intervention to enhance disease resistance[J]. Frontiers in Immunology 2022, 13, 885253. [Google Scholar] [CrossRef]
- Moriyama A, Shimoya K, Kawamoto A, et al. Secretory leukocyte protease inhibitor (SLPI) concentrations in seminal plasma: SLPI restores sperm motility reduced by elastase[J].
- Shugars D, C. Endogenous mucosal antiviral factors of the oral cavity[J]. The Journal of Infectious Diseases 1999, 179, S431–435. [Google Scholar] [CrossRef]
- Agnew K J, Aura J, Nunez N, et al. Effect of semen on vaginal fluid cytokines and secretory leukocyte protease inhibitor[J]. Infectious Diseases in Obstetrics and Gynecology 2008, 2008, 820845. [Google Scholar]
- Jahan M, Kracht S, Ho Y, et al. Dietary lactoferrin supplementation to gilts during gestation and lactation improves pig production and immunity[J]. C. Kanellopoulos-Langevin. PLOS ONE 2017, 12, e0185817. [Google Scholar]
- Meng Q, Li J, Wang C, et al. Biological function of resveratrol and its application in animal production: a review[J]. Journal of Animal Science and Biotechnology 2023, 14, 25. [Google Scholar]
- Schulze M, Junkes C, Mueller P, et al. Effects of cationic antimicrobial peptides on liquid-preserved boar spermatozoa[J]. PloS One 2014, 9, e100490. [Google Scholar]
- Lohner K, Blondelle S E. Molecular mechanisms of membrane perturbation by antimicrobial peptides and the use of biophysical studies in the design of novel peptide antibiotics[J]. Combinatorial Chemistry & High Throughput Screening 2005, 8, 241–256. [Google Scholar]
- Bechinger B, Gorr S-U. Antimicrobial Peptides: Mechanisms of Action and Resistance[J]. Journal of Dental Research 2017, 96, 254–260.
- Shayman J A, Tesmer J J G. Lysosomal phospholipase A2[J]. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2019, 1864, 932–940. [Google Scholar]
- Liu X, Jiang Z, Liu Y, et al. Biochemical characterization of a novel exo-oligoxylanase from Paenibacillus barengoltzii suitable for monosaccharification from corncobs[J]. Biotechnology for Biofuels 2019, 12, 190. [Google Scholar]
- Ferraboschi P, Ciceri S, Grisenti P. Applications of Lysozyme, an Innate Immune Defense Factor, as an Alternative Antibiotic[J]. Antibiotics (Basel, Switzerland) 2021, 10, 1534. [Google Scholar]
- Jiang L, Li Y, Wang L, et al. Recent Insights Into the Prognostic and Therapeutic Applications of Lysozymes[J]. Frontiers in Pharmacology, 2021; 12, 767642.
- Kalra S, Pradeep M A, Mohanty A K, et al. Structural, Functional and Phylogenetic Analysis of Sperm Lysozyme-Like Proteins[J]. PloS One 2016, 11, e0166321.
- Kwon W-S, Rahman M S, Lee J-S, et al. Discovery of predictive biomarkers for litter size in boar spermatozoa[J]. Molecular & cellular proteomics: MCP 2015, 14, 1230–1240. [Google Scholar]
- Schulze M, Nitsche-Melkus E, Hensel B, et al. Antibiotics and their alternatives in Artificial Breeding in livestock[J]. Animal Reproduction Science, 2020; 220, 106284.
- Jakop U, Hensel B, Czirják Gábor Á, et al. Bacterial killing activity and lysozymes: A stable defence mechanism in stallion seminal plasma?[J]. Reproduction in Domestic Animals 2023, 58, 73–80. [Google Scholar] [CrossRef]
- Nash J A, Ballard T N S, Weaver T E, et al. The peptidoglycan-degrading property of lysozyme is not required for bactericidal activity in vivo[J]. Journal of Immunology (Baltimore, Md.: 1950) 2006, 177, 519–526. [Google Scholar]
- Callewaert L, Michiels C W. Lysozymes in the animal kingdom[J]. Journal of Biosciences 2010, 35, 127–160. [Google Scholar] [CrossRef]
- Amann R P, Hammerstedt R H, Veeramachaneni D N. The epididymis and sperm maturation: a perspective[J]. Reproduction, Fertility, and Development 1993, 5, 361–381. [Google Scholar] [CrossRef]
- Aitken R J, Nixon B, Lin M, et al. Proteomic changes in mammalian spermatozoa during epididymal maturation[J]. Asian Journal of Andrology 2007, 9, 554–564. [Google Scholar] [CrossRef]
- Zumoffen C M, Massa E, Caille A M, et al. Effects of lactoferrin, a protein present in the female reproductive tract, on parameters of human sperm capacitation and gamete interaction[J]. Andrology 2015, 3, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi J, Sasaki A, Watanabe A, et al. Effects of exogenous lactoferrin on characteristics and functions of bovine epididymal, ejaculated and frozen-thawed sperm[J]. Animal Science Journal 2021, 92, e13538. [Google Scholar] [CrossRef] [PubMed]
- Cross N, L. Role of cholesterol in sperm capacitation[J]. Biology of Reproduction 1998, 59, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Mocé E, Blanch E, Tomás C, et al. Use of cholesterol in sperm cryopreservation: present moment and perspectives to future[J]. Reproduction in Domestic Animals = Zuchthygiene 2010, 45, 57–66. [Google Scholar] [CrossRef]
- Srakaew N, Young C D, Sae-wu A, et al. Antimicrobial host defence peptide, LL-37, as a potential vaginal contraceptive[J]. Human Reproduction (Oxford, England) 2014, 29, 683–696. [Google Scholar] [CrossRef]
- Junkes C, Harvey R D, Bruce K D, et al. Cyclic antimicrobial R-, W-rich peptides: the role of peptide structure and E. coli outer and inner membranes in activity and the mode of action[J]. European biophysics journal: EBJ 2011, 40, 515–528. [Google Scholar] [CrossRef]
- Mahlapuu M, Håkansson J, Ringstad L, et al. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents[J]. Frontiers in Cellular and Infection Microbiology 2016, 6, 194. [Google Scholar]
- Pulido D, Nogués M V, Boix E, et al. Lipopolysaccharide neutralization by antimicrobial peptides: a gambit in the innate host defense strategy[J]. Journal of Innate Immunity 2012, 4, 327–336. [Google Scholar] [CrossRef]
- Luo Y, Song Y. Mechanism of Antimicrobial Peptides: Antimicrobial, Anti-Inflammatory and Antibiofilm Activities[J]. International Journal of Molecular Sciences 2021, 22, 11401. [Google Scholar] [CrossRef]
- Branen J K, Davidson P M. Enhancement of nisin, lysozyme, and monolaurin antimicrobial activities by ethylenediaminetetraacetic acid and lactoferrin[J]. International Journal of Food Microbiology 2004, 90, 63–74. [Google Scholar] [CrossRef]
- Nelson N, Opene B, Ernst R K, et al. Antimicrobial peptide activity is anticorrelated with lipid a leaflet affinity[J]. PloS One 2020, 15, e0242907. [Google Scholar]
- Rončević T, Puizina J, Tossi A. Antimicrobial Peptides as Anti-Infective Agents in Pre-Post-Antibiotic Era?[J]. International Journal of Molecular Sciences 2019, 20, 5713. [Google Scholar] [CrossRef] [PubMed]
- Geitani R, Moubareck C A, Xu Z, et al. Expression and Roles of Antimicrobial Peptides in Innate Defense of Airway Mucosa: Potential Implication in Cystic Fibrosis[J]. Frontiers in Immunology, 2020; 11, 1198.
- He M, Zhang H, Li Y, et al. Cathelicidin-Derived Antimicrobial Peptides Inhibit Zika Virus Through Direct Inactivation and Interferon Pathway[J]. Frontiers in Immunology 2018, 9, 722. [Google Scholar] [CrossRef] [PubMed]
- Matsumura T, Sugiyama N, Murayama A, et al. Antimicrobial peptide LL-37 attenuates infection of hepatitis C virus[J]. Hepatology Research: The Official Journal of the Japan Society of Hepatology 2016, 46, 924–932. [Google Scholar] [CrossRef]
- Khan S A, Ilies M A. The Phospholipase A2 Superfamily: Structure, Isozymes, Catalysis, Physiologic and Pathologic Roles[J]. International Journal of Molecular Sciences 2023, 24, 1353. [Google Scholar] [CrossRef]
- Vanha-Perttula T, Rönkkö S, Lahtinen R. Hydrolases from bovine seminal vesicle, prostate and Cowper’s gland: Hydrolasen aus der Bläschendrüse, Prostata und Cowperschen Drüse des Bullen[J]. Andrologia 2009, 22, 10–24. [Google Scholar] [CrossRef]
- Thakkar J K, East J, Franson R C. Modulation of phospholipase A2 activity associated with human sperm membranes by divalent cations and calcium antagonists[J]. Biology of Reproduction 1984, 30, 679–686. [Google Scholar] [CrossRef]
- Schulze M, Dathe M, Waberski D, et al. Liquid storage of boar semen: Current and future perspectives on the use of cationic antimicrobial peptides to replace antibiotics in semen extenders[J]. Theriogenology 2016, 85, 39–46. [Google Scholar] [CrossRef]
- Kiattiburut W, Zhi R, Lee S G, et al. Antimicrobial peptide LL-37 and its truncated forms, GI-20 and GF-17, exert spermicidal effects and microbicidal activity against Neisseria gonorrhoeae[J]. Human Reproduction (Oxford, England) 2018, 33, 2175–2183. [Google Scholar]
- Ganz, T. Defensins and other antimicrobial peptides: a historical perspective and an update[J]. Combinatorial Chemistry & High Throughput Screening 2005, 8, 209–217. [Google Scholar]
- Sørensen O E, Follin P, Johnsen A H, et al. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3[J]. Blood 2001, 97, 3951–3959. [Google Scholar] [CrossRef]
- Cao J, Gao M, Wang J, et al. Construction of nano slow-release systems for antibacterial active substances and its applications: A comprehensive review[J]. Frontiers in Nutrition 2023, 10, 1109204. [Google Scholar] [CrossRef] [PubMed]
- Knight R, Callewaert C, Marotz C, et al. The Microbiome and Human Biology[J]. Annual Review of Genomics and Human Genetics 2017, 18, 65–86. [Google Scholar] [CrossRef]
- Fang Y, Su Y, Xu J, et al. Varicocele-Mediated Male Infertility: From the Perspective of Testicular Immunity and Inflammation[J]. Frontiers in Immunology 2021, 12, 729539. [Google Scholar] [CrossRef]
- Fritsche K L M, Ahola J K, Pinedo P J, et al. Pregnancy risk in beef and dairy cows after supplementing semen with transforming growth factor beta-1 at the time of artificial insemination[J]. Journal of Animal Science 2024, 102, skae169. [Google Scholar] [CrossRef] [PubMed]
- Schjenken J E, Sharkey D J, Green E S, et al. Sperm modulate uterine immune parameters relevant to embryo implantation and reproductive success in mice[J]. Communications Biology 2021, 4, 572. [Google Scholar]
- Kontsevaya G V, Gerlinskaya L A, Moshkin Y M, et al. The Effects of Sperm and Seminal Fluid of Immunized Male Mice on In Vitro Fertilization and Surrogate Mother-Embryo Interaction[J]. International Journal of Molecular Sciences 2021, 22, 10650. [Google Scholar] [CrossRef]
- Gao Y, Xiao X, Lui W-Y, et al. Cell polarity proteins and spermatogenesis[J]. Seminars in Cell & Developmental Biology, 2016; 59, 62–70.
- Ngcobo J N, Ramukhithi F V, Nephawe K A, et al. Flaxseed Oil as a Source of Omega n-3 Fatty Acids to Improve Semen Quality from Livestock Animals: A Review[J]. Animals: an open access journal from MDPI 2021, 11, 3395. [Google Scholar] [CrossRef]
- Adamczewska D, Słowikowska-Hilczer J, Walczak-Jędrzejowska R. The Association between Vitamin D and the Components of Male Fertility: A Systematic Review[J]. Biomedicines 2022, 11, 90. [Google Scholar]
- Vickram S, Rohini K, Srinivasan S, et al. Role of Zinc (Zn) in Human Reproduction: A Journey from Initial Spermatogenesis to Childbirth[J]. International Journal of Molecular Sciences 2021, 22, 2188. [Google Scholar] [CrossRef]
| Type | Bacterium | Effects on Sperm Quality | References |
|---|---|---|---|
| probiotics | Lactobacillus | ·Positively correlate with sperm viability parameters, structural integrity, and capacitation ·Have antagonistic effect with pathogenic bacteria |
[33,39,40] |
| Bifidobacterium | ·Improve sperm motility ·Reduce DNA fragmentation ·Reduces intracellular oxidative stress |
[41] | |
| Lactobacillus rhamnosus | ·Used in reproduction, oocyte maturation ·Supplements to improve spermatogenesis ·Enhance sperm kinematic parameters |
[42,43] | |
| Lactobacillus paracasei | ·Reduce intracellular oxidative stress ·Stop DNA breaks ·Reduce sperm DNA loss |
[43] | |
| Bacillus subtilis | ·Reduce sperm damage ·improve sperm dynamics and morphology |
[1] | |
| pathogenic bacteria | Pseudomonas aeruginosa | Associate with defective spermatogenesis, sperm DNA damage and orchitis | [2,3,4] |
| Escherichia coli (E. coli) | ·Associate with defective spermatogenesis, sperm DNA damage and orchitis ·Affect sperm motility and morphology |
[14,15,44,45] | |
| Staphylococcus aureus | ·Associate with sperm DNA damage and orchitis ·Affects sperm viability and morphology |
[5,6,7] | |
| Prevotella | Associate with defective spermatogenesis and low-quality semen | [8,9,10,11] | |
| Brucella | Orchitis | [12] | |
| Chlamydia trachomatis | ·Associate with defective spermatogenesis, sperm DNA damage and orchitis ·Affect sperm motility and morphology |
[13] | |
| Neisseria gonorrhoeae | Associate with defective spermatogenesis, sperm DNA damage and orchitis | [12] | |
| Mycoplasma urealyticum | ·Associate with inflammation, sperm DNA damage and orchitis ·Affects sperm viability and morphology |
[12,13] | |
| Staphylococcus saprophyticus | Associate with poor sperm count, decreased sperm motility, abnormal viscosity and leukocytospermia | [13] | |
| Streptococcus agalactiae | [13] | ||
| Klebsiella | [1] | ||
| Bacillus citreus | |||
| Enterobacterium | |||
| Clostridium | |||
| Enterobacter cloacae | |||
| Aeromonas hydrophila |
| Natural Antimicrobial Substances | Mechanisms of Action | References |
|---|---|---|
| Lysozyme (LSZ) | Lysozyme hydrolyzes the β-1,4 glycosidic bond between the NAM monomer and the adjacent NAG monomer. Hydrolysis of PG by lysozyme leads to cell wall instability and bacterial cell death. Lysozyme can also have a bactericidal effect through the mechanism of its cationic nature, i.e., the formation of pores in the negatively charged bacterial cell membranes by lysozyme (red columns). |
[63,64] |
| Secretory leukocyte peptidase inhibitor (SLPI) | Related to the special structure of the peptide chain, if the structure is changed, the antibacterial activity will decrease. | [65,66,67] |
| Lactoferrin (LF) | Inhibit and kill bacteria by highly binding iron, depriving them of the essential iron needed for growth. |
[68,69] |
| Antibacterial peptide (AMP) | The amphiphilic structure of AMPs, where the spatial separation of the cationic and hydrophobic components is a prerequisite for their effective interaction with bacterial membranes, is a structural feature that allows AMPs to interact with lipids of asymmetric bacterial membranes in a similar manner. | [70,71,72] |
| Group II phospholipase A2 (PLA2) | Catalyze the hydrolysis of phospholipids in the cell membrane of certain gram-positive bacteria. Activate the body immune system and kill a variety of gram-negative bacteria with the help of complement and other factors |
[73,74] |
| Zn2+, SG, SGI-derived peptides and HEL-75 protein | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




