Submitted:
17 October 2024
Posted:
17 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
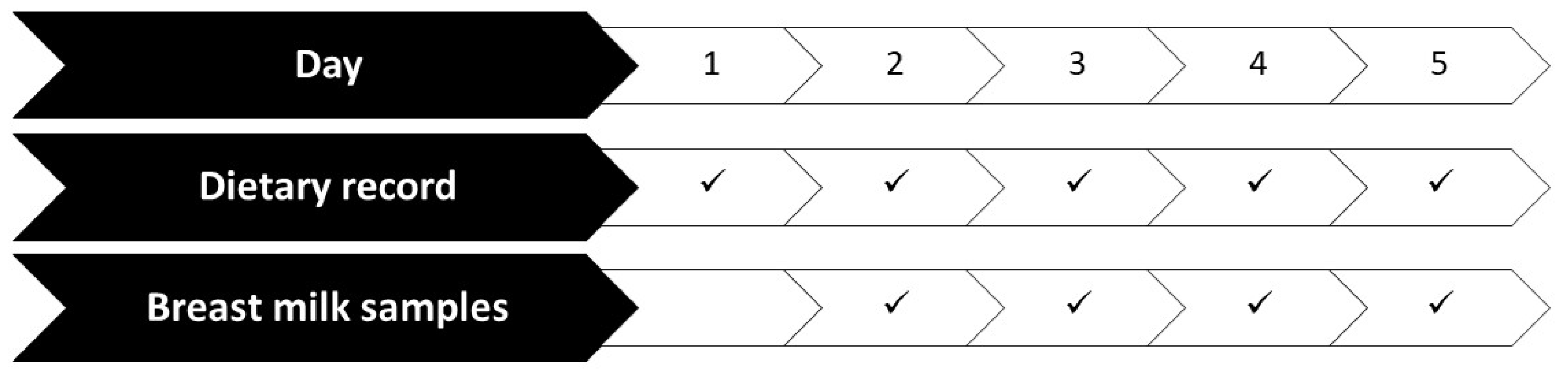
2.2. Study Protocol
2.3. Dietary Study
2.4. Micronutrient Analysis in HM
2.5. Statistics
3. Results
3.1. Characteristics of the Studied Population
3.1. Generalized Estimating Equations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Picciano, M.F. Nutrient composition of human milk. Pediatr. Clin. North. Am. 2001, 48, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Bravi, F.; Wiens, F.; Decarli, A.; Dal Pont, A.; Agostoni, C.; Ferraroni, M. Impact of maternal nutrition on breast-milk composition: A systematic review. Am. J. Clin. Nutr. 2016, 104, 646–662. [Google Scholar] [CrossRef] [PubMed]
- Keikha, M.; Bahreynian, M.; Saleki, M.; Kelishadi, R. Macro- and Micronutrients of Human Milk Composition: Are They Related to Maternal Diet? A Comprehensive Systematic Review. Breastfeed. Med. 2017, 12, 517–527. [Google Scholar] [CrossRef]
- Dror, D.K.; Allen, L.H. Overview of Nutrients in Human Milk. Adv Nutr. 2018, 9, 278S–294S. [Google Scholar] [CrossRef]
- Falize, C.; Savage, M.; Jeanes, Y.M.; Dyall, S.C. Evaluating the relationship between the nutrient intake of lactating women and their breast milk nutritional profile: a systematic review and narrative synthesis. Br. J. Nutr. 2024, 131, 1196–1224. [Google Scholar] [CrossRef]
- Petersohn, I.; Hellinga, A.H.; van Lee, L.; Keukens, N.; Bont, L.; Hettinga, K.A.; Feskens, E.J.M.; Brouwer-Brolsma, E.M. Maternal diet and human milk composition: an updated systematic review. Front. Nutr. 2024, 10, 1320560. [Google Scholar] [CrossRef]
- Keikha, M.; Shayan-Moghadam, R.; Bahreynian, M.; Kelishadi, R. Nutritional supplements and mother's milk composition: a systematic review of interventional studies. Int Breastfeed J. 2021, 16, 1. [Google Scholar] [CrossRef]
- Ureta-Velasco, N.; Keller, K.; Escuder-Vieco, D.; Serrano, J.C.E.; García-Lara, N.R.; Pallás-Alonso, C.R. Assessment of Iodine Concentration in Human Milk from Donors: Implications for Preterm Infants. Nutrients 2022, 14, 4304. [Google Scholar] [CrossRef]
- Ureta-Velasco, N.; Keller, K.; Escuder-Vieco, D.; Fontecha, J.; Calvo, M.V.; Megino-Tello, J.; Serrano, J.C.E.; Romero Ferreiro, C.; García-Lara, N.R.; Pallás-Alonso, C.R. Human Milk Composition and Nutritional Status of Omnivore Human Milk Donors Compared with Vegetarian/Vegan Lactating Mothers. Nutrients 2023, 15, 1855. [Google Scholar] [CrossRef]
- Olsen, I.E.; Groveman, S.A.; Lawson, L.M.; Clark, R.H. , Zemel, B.S. New intrauterine growth curves based on United States Data. Pediatrics 2010, 125, 214–224. [Google Scholar] [CrossRef]
- World Health Organization. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-fo-Height and Body Mass Index-for-Age: Methods and Development; World Health Organization: Geneva, Switzerland, 2006; ISBN 92-4-154693-X. [Google Scholar]
- Allen, L.H.; Carriquiry, A.L.; Murphy, S.P. Perspective: Proposed Harmonized Nutrient Reference Values for Populations. Adv. Nutr. 2020, 11, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.S.; Rahmannia, S.; Diana, A.; Leong, C.; Haszard, J.J.; Hampel, D.; Reid, M.; Erhardt, J.; Suryanto, A.H.; Sofiah, W.N.; et al. Association of maternal diet, micronutrient status, and milk volume with milk micronutrient concentrations in Indonesian mothers at 2 and 5 months postpartum. Am. J. Clin. Nutr. 2020, 112, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- urck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; et al.; EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Dietary reference values for thiamin. EFSA J. 2016, 14, 4653. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.; Mangelsdorf, I.; McArdle, H.; et al.; EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Dietary Reference Values for riboflavin. EFSA J. 2017, 15, 4919. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for niacin. EFSA J. 2014, 12, 3759. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for pantothenic acid. EFSA J. 2014, 12, 3581. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on nutrient requirements and dietary intakes of infants and young children in the European Union. EFSA J. 2013, 11, 3408. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for cobalamin (vitamin B12). EFSA J. 2015, 13, 4150. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for vitamin C. EFSA J. 2013, 11, 3418. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for vitamin A. EFSA J. 2015, 13, 4028. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on dietary reference values for vitamin D. EFSA J. 2016, 14, 4547. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for vitamin E as α-tocopherol. EFSA J. 2015, 13, 4149. [Google Scholar] [CrossRef]
- Semba, R.D.; Delange, F. Iodine in human milk: Perspectives for infant health. Nutr. Rev. 2001, 59, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Braegger, C.P. The role of iodine for thyroid function in lactating women and infants. Endocr. Rev. 2022, 43, 469–506. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for calcium. EFSA J. 2015, 13, 4101. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for phosphorus. EFSA J. 2015, 13, 4185. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for selenium. EFSA J. 2014, 12, 3846. [Google Scholar] [CrossRef]
- LASER Analytica. Comprehensive literature search and review of breast milk composition as preparatory work for the setting of dietary reference values for vitamins and minerals. EFSA Support. Publ. 2014, 11, 629E. [Google Scholar] [CrossRef]
- Kodentsova, V.M.; Vrzhesinskaya, O.A. Evaluation of the vitamin status in nursing women by vitamin content in breast milk. Bull Exp Biol Med. 2006, 141, 323–7. [Google Scholar] [CrossRef]
- Ullah, H.; Liu, G.; Yousaf, B.; Ali, M.U.; Abbas, Q.; Munir, M.A.M.; Mian, M.M. Developmental selenium exposure and health risk in daily foodstuffs: A systematic review and meta-analysis. Ecotoxicol. Environ. Saf. 2018, 149, 291–306. [Google Scholar] [CrossRef]
- Valent, F.; Horvat, M.; Mazej, D.; Stibilj, V.; Barbone, F. Maternal diet and selenium concentration in human milk from an Italian population. J. Epidemiol. 2011, 21, 285–92. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; Fente, C.; Barreiro, R.; López-Racamonde, O.; Cepeda, A.; Regal, P. Association between Breast Milk Mineral Content and Maternal Adherence to Healthy Dietary Patterns in Spain: A Transversal Study. Foods 2020, 9, 659. [Google Scholar] [CrossRef] [PubMed]
- Utri, Z.; Głąbska, D. Vitamin D Intake in a Population-Based Sample of Young Polish Women, Its Major Sources and the Possibility of Meeting the Recommendations. Foods 2020, 9, 1482. [Google Scholar] [CrossRef] [PubMed]
- Ursinyova, M.; Masanova, V.; Uhnakova, I.; Murinova, L.P.; Patayova, H.; Rausova, K.; Trnovec, T.; Stencl, J.; Gajdos, M. Prenatal and Early Postnatal Exposure to Total Mercury and Methylmercury from Low Maternal Fish Consumption. Biol Trace Elem Res. 2019, 191, 16–26. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Scientific Opinion on the risk for public health relates to the presence of mercury and methylmercury in food. EFSA J. 2012, 10, 2985. [CrossRef]
- Costa, F.; Coelho, J.P.; Baptista, J.; Martinho, F.; Pereira, M.E.; Pardal, M.A. Mercury accumulation in fish species along the Portuguese coast: Are there potential risks to human health? Mar Pollut Bull. 2020, 150, 110740. [Google Scholar] [CrossRef]
- Ureta-Velasco, N.; Montealegre-Pomar, A.; Keller, K.; Escuder-Vieco, D.; Fontecha, J.; Calvo, M.V.; Megino-Tello, J.; Serrano, J.C.E.; García-Lara, N.R.; Pallás-Alonso, C.R. Associations of Dietary Intake and Nutrient Status with Micronutrient and Lipid Composition in Breast Milk of Donor Women. Nutrients 2023, 15, 3486. [Google Scholar] [CrossRef]
- Hampel, D.; Shahab-Ferdows, S.; Islam, M.M.; Peerson, J.M.; Allen, L.H. Vitamin Concentrations in Human Milk Vary with Time within Feed, Circadian Rhythm, and Single-Dose Supplementation. J Nutr. 2017, 147, 603–611. [Google Scholar] [CrossRef]
| Micronutrients | Analytical technique |
|---|---|
| Free thiamine | UPLC tandem mass spectrometry |
| Free riboflavin | UPLC tandem mass spectrometry |
| Nicotinamide | UPLC tandem mass spectrometry |
| Pantothenic acid | UPLC tandem mass spectrometry |
| Pyridoxal | UPLC tandem mass spectrometry |
| Folic acid | UPLC tandem mass spectrometry |
| Cobalamin | Competitive immunoassay method |
| Ascorbic acid | HPLC-DAD |
| Retinol | HPLC with a fluorescence and UV detector |
| Vitamin D3 | UPLC electrospray ionization/tandem mass spectrometry |
| 25(OH)D3 | UPLC electrospray ionization/tandem mass spectrometry |
| α-tocopherol | HPLC with a fluorescence and UV detector |
| γ-tocopherol | HPLC with a fluorescence and UV detector |
| Iodine | Inductively coupled plasma-mass spectrometry |
| Calcium | Inductively coupled plasma-mass spectrometry |
| Phosphorous | Inductively coupled plasma-mass spectrometry |
| Selenium | Inductively coupled plasma-mass spectrometry |
| Characteristic | |
|---|---|
| Age (years) | 35.61 (0.39) (4.67) * |
| Weight (kg) | 61.20 (55.20, 70.60) |
| Height (cm) | 163.85 (0.53) (6.39) * |
| Pre-pregnancy BMI (kg/m2) | 22.16 (20.55, 24.92) |
| Pre-pregnancy BMI (kg/m2) category | |
| Underweight (<18.5) | 6 (4.2%) |
| Normal (18.5–24.9) | 103 (72.0%) |
| Overweight (25–29.9) | 20 (14.0%) |
| Obese (≥30) | 14 (9.8%) |
| Current BMI (kg/m2) | 22.94 (21.12, 25.29) |
| Current BMI (kg/m2) category | |
| Underweight (<18.5) | 5 (3.5%) |
| Normal (18.5–24.9) | 101 (70.6%) |
| Overweight (25–29.9) | 19 (13.3%) |
| Obese (≥ 30) | 18 (12.6%) |
| Gestational weight gain (kg) | 11.00 (8.00, 13.60) |
| Postpartum weight retention (kg) | 1.00 (-0.60, 3.00) |
| Number of living children | |
| 0a –1 | 81 (56.6%) |
| 2 | 49 (34.3%) |
| ≥3 | 13 (9.1%) |
| Country of origin: Spain | 125 (87.4%) |
| Education level | |
| Secondary studies | 6 (4.2%) |
| Technical studies | 17 (11.9%) |
| University studies | 120 (83.9%) |
| Currently working (Yes) | 65 (45.5%) |
| Physical activity | |
| Sedentary | 35 (24.5%) |
| Low activity | 72 (50.3%) |
| Active/very active | 36 (25.2%) |
| Tobacco consumption | |
| Prior to pregnancy (Yes) | 28 (19.6%) |
| During pregnancy (Yes) | 3 (2.1%) |
| Currently (Yes) | 2 (1.4%) |
| Alcohol consumption | |
| Prior to pregnancy (Yes) | 72 (50.3%) |
| During pregnancy (Yes) | 2 (1.4%) |
| Currently (Yes) | 7 (4.9%) |
| Season during the study | |
| Spring | 30 (21.0%) |
| Summer | 23 (16.1%) |
| Autumn | 52 (36.4%) |
| Winter | 38 (26.6%) |
| Characteristic | n | |
|---|---|---|
| Duration of lactation of the previous child (months) | 59 a | |
| <3 | 0 (0.0%) | |
| 3–6 | 2 (3.4%) | |
| 6–12 | 12 (20.3%) | |
| 12–24 | 25 (42.4%) | |
| >24 | 20 (33.9%) | |
| Current breastfeeding time (months) | 143 | 6.05 (4.27, 11.20) |
| Type of lactation | 142 b | |
| Exclusive | 71 (50.0%) | |
| Partial | 71 (50.0%) | |
| Sum of breastfeeding times plus daily milk pumping sessions | 143 | |
| <5 | 14 (9.8%) | |
| 5–10 | 87 (60.8%) | |
| >10 | 39 (27.3%) | |
| Missing data | 3 (2.1%) | |
| Tandem breastfeeding (Yes) | 143 | 10 (7.0%) |
| Breastfeeding twins (Yes) | 143 | 4 (2.8%) |
| Type of milk extraction * | 143 | |
| Manual | 12 (8.4%) | |
| Mechanical breast pump | 15 (10.5%) | |
| Simple electric breast pump | 101 (70.6%) | |
| Double electric breast pump | 26 (18.2%) |
| Characteristic | n | |
|---|---|---|
| Gestational age (weeks) | 143 | 39+3 (37+3, 40+2), 22+6–42+3 |
| Boy | 148a | 72 (48.6%) |
| Birth weight (grams) | 148a | 3150 (2550, 3420); 450-4640 |
| Birth weight percentile 1 | ||
| ≤25 | 49 (33.1%) | |
| 25–75 | 148a | 84 (56.8%) |
| ≥75 | 15 (10.1%) | |
| Age of infant (months) | 146b | |
| 0–6 | 73 (50.0%) | |
| 6–12 | 41 (28.1%) | |
| 12–50 | 32 (21.9%) | |
| Current weight percentile of breastfed child 2 | 146b | |
| ≤15 | 32 (21.9%) | |
| 15–85 | 94 (64.4%) | |
| ≥85 | 20 (13.5%) |
| Supplement | n (%) * | Daily Dose |
|---|---|---|
| Vitamin A, mcg | 43 (30.1%) | 640.0 (400.0, 800.0) |
| Vitamin D, mcg | 56 (39.2%) | 5.0 (3.0, 5.0) |
| Vitamin E, mg | 52 (36.4%) | 11.2 (6.3, 12.0) |
| Vitamin C, mg | 51 (35.7%) | 80.0 (48.0, 80.0) |
| Vitamin B1, thiamine, mg | 51 (35.7%) | 1.1 (0.7, 1.1) |
| Vitamin B2, riboflavin, mg | 51 (35.7%) | 1.4 (0.8, 1.4) |
| Vitamin B3, niacin, mg | 51 (35.7%) | 15.6 (9.6, 16.0) |
| Vitamin B5, pantothenic, mg | 51 (35.7%) | 5.7 (3.6, 6.0) |
| Vitamin B6, pyridoxine, mg | 51 (35.7%) | 1.4 (0.8, 1.4) |
| Vitamin B7, biotin, mcg | 51 (35.7%) | 50.0 (30.0, 50.0) |
| Vitamin B9, folic acid, mcg | 72 (50.3%) | 290.0 (165.0, 400.0) |
| Vitamin B12, cobalamin, mcg | 78 (54.5%) | 2.0 (1.6, 2.5) |
| Iodine, mcg | 75 (52.4%) | 160.0 (120.0, 200.0) |
| Calcium, mg | 38 (26.6%) | 160.0 (100.0, 200.0) |
| Iron, mg | 54 (37.8%) | 14.0 (8.4, 17.7) |
| Zinc, mg | 48 (33.6%) | 8.0 (5.3, 10.0) |
| Selenium, mcg | 46 (32.2%) | 20.0 (11.5, 45.0) |
| Nutrient | H-AR [12] | n (%) |
|---|---|---|
| Thiamine (B1), mg | 1.2 | 11 (7.7%) |
| Riboflavin (B2), mg | 1.7 | 29 (20.3%) |
| Niacin (B3), mg | 13 | 0 (0.0%) |
| Pantothenic acid (B5), mg | 5.6 | 38 (26.6%) |
| Pyridoxine (B6), mg | 1.4 | 3 (2.1%) |
| Biotin (B7), μg | 36 | 52 (36.4%) |
| Folate food + folic acid (B9), μg | 380 (DFE) | 50 (35.0%) |
| Cobalamin (B12), μg | 2.4 | 5 (3.5%) |
| Vitamin C, mg | 145 | 51 (35.7%) |
| Vitamin A, μg RAE | 1020 | 45 (31.5%) |
| Vitamin D, μg | 10 | 122 (85.3%) |
| Vitamin E, mg | 16 | 70 (49.0%) |
| Iodine, μg | 209 | 60 (42.0%) |
| Calcium, mg | 860 (19–30 y) 750 (31–50 y) |
23 (16.1%) |
| Phosphorous, mg | 580 | 0 (0.0%) |
| Selenium, μg | 59 | 4 (2.8%) |
| Nutrient 1 | Lactating women | Mature Milk Nutrient Concentration Reference | |
|---|---|---|---|
| n (o) | Concentration | ||
| Free thiamin, B1 (UPLC-MS/MS) mcg/L | 143 (570) | 17.90 (9.08, 27.90) | Free thiamin 18.5 [13] Total thiamin 180 [14] |
| Free riboflavin, B2 (UPLC-MS/MS) mcg/L | 143 (570) | 40.38 (20.45, 97.70) | Free riboflavin 11.2 [13] Total riboflavin 364 [15] |
| Nicotinamide, B3 (UPLC-MS/MS) mcg/L | 143 (570) | 43.33 (26.23, 77.15) | Nicotinamide 275 [13] Total niacin 2100 [16] |
| Pantothenic acid, B5 (UPLC-MS/MS) mcg/L | 143 (570) | 2205.76 (39.62) (473.83) | 2500 [17] 1304 [13] |
| Pyridoxal, B6 (UPLC-MS/MS) mcg/L | 143 (570) | 36.73 (24.78, 53.90) | Pyridoxal 96 [13] B6 130 [18] |
| Folic acid, B9 (UPLC-MS/MS) mcg/L | 143 (570) | 18.48 (14.25, 23.70) | |
| Cobalamin, B12 (competitive immunoassay) | 142 (565) | ||
| pM | 482.46 (448.34, 533.41) | ||
| mcg/L | 0.65 (0.61, 0.72) | 0.5 [19] | |
| Ascorbic acid (HPLC-DAD) mg/dL | 142 (566) | 4.10 (0.15) (1.84) | |
| Dehydroascorbic acid (HPLC-DAD) mg/dL | 142 (566) | 1.86 (1.15, 2.81) | |
| Vitamin C * (HPLC-DAD) | 142 (566) | ||
| mg/dL | 6.40 (5.51, 7.16) | ||
| mg/L | 63.98 (55.07, 71.61) | 35–90 [20] | |
| Retinol (HPLC with fluorescence and UV detector) | 141 (561) | ||
| mcg/dL | 45.45 (27.29, 92.23) | ||
| mcg/L | 454.50 (272.88, 922.25) | 530 [21] | |
| Vitamin D3 (UPLC–electrospray ionization/tandem MS) | 143 (566) | ||
| pg/mL | 1132.95 (279.63, 4823.43) | ||
| mcg/L | 1.13 (0.28, 4.82) | 0.25–2 [22] | |
| 25(OH)D3 (UPLC–electrospray ionization/tandem MS) | 143 (567) | ||
| pg/mL | 61.20 (27.60, 118.85) | ||
| mcg/L | 0.06 (0.03, 0.12) | ||
| α-tocopherol (HPLC with fluorescence and UV detector) | 141 (561) | ||
| mcg/dL | 441.63 (362.09, 558.06) | ||
| mg/L | 4.42 (3.62, 5.58) | 4.6 [23] | |
| γ-tocopherol (HPLC with fluorescence and UV detector) | 141 (561) | ||
| mcg/dL | 51.25 (38.01, 69.83) | ||
| mg/L | 0.51 (0.38, 0.70) | 0.45 [13] | |
| Vitamin E (as TE) ** | 141 (561) | ||
| mcg/dL | 458.91 (379.28, 580.73) | ||
| mg/L | 4.59 (3.79, 5.81) | 5.2 [13] | |
| Iodine (ICP-MS) ppb (mcg/L) | 143 (570) | 142.65 (92.93, 207.28) | 50–100 [18] 100–200 [24,25] |
| Calcium (ICP-MS) ppm (mg/L) | 143 (570) | 92.40 (61.25, 126.10) | 200–300 [26] |
| Phosphorous (ICP-MS) ppm (mg/L) | 143 (570) | 130.55 (2.18) (26.12) | 120–140 [18,27] |
| Selenium (ICP-MS) ppb (mcg/L) | 143 (570) | 11.03 (9.25, 13.08) | 18 [28] |
| Micronutrients in HM | Associated Variables | Coef | SE | z | P >|z| | 95% CI |
|---|---|---|---|---|---|---|
| Vitamin D3 + 25(OH)D3, pg/mL Mothers=143 Wald chi2(1) = 4.88 P>chi2=0.027 |
Meat, Fish, and eggs servings/day |
243.203 | 110.057 | 2.21 | 0.027 | [27.494, 458.911] |
| Free riboflavin, mcg/L Mothers=51 Wald chi2(2) =39.56 P>chi2= <0.001 |
B2 intake* mg/day |
7.312 | 11.541 | 0.63 | 0.526 | [-15.308, 29.931] |
| B2 supplement mg/day | 28.642 | 12.214 | 2.35 | 0.019 | [4.703, 52.581] | |
| Pyridoxal, mcg/L Mothers=51 Wald chi2(2) =17.08 P>chi2= <0.001 |
B6 supplement mg/day |
6.284 | 1.885 | 3.33 | 0.001 | [2.589, 9.978] |
| Fruit servings/day | 2.006 | 0.831 | 2.41 | 0.016 | [0.377, 3.634] | |
| Selenium, ppb Mothers=143 Wald chi2(1) =4.67 P>chi2=0.031 |
Meat, Fish, and eggs servings/day |
0.158 | 0.073 | 2.16 | 0.031 | [0.015, 0.301] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





