Submitted:
16 October 2024
Posted:
17 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Assessment
2.3. Instrumental Analysis of Gait
2.4. Speech Analysis
2.5. [18F] Flutemetamol PET Study
2.6. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bove, F.; Mulas, D.; Cavallieri, F.; Castrioto, A.; Chabardès, S.; Meoni, S.; Schmitt, E.; Bichon, A.; Di Stasio, E.; Kistner, A.; et al. Long-Term Outcomes (15 Years) After Subthalamic Nucleus Deep Brain Stimulation in Patients With Parkinson Disease. Neurology 2021, 10.1212/WNL.0000000000012246, doi:10.1212/WNL.0000000000012246. [CrossRef]
- Cavallieri, F.; Fraix, V.; Bove, F.; Mulas, D.; Tondelli, M.; Castrioto, A.; Krack, P.; Meoni, S.; Schmitt, E.; Lhommée, E.; et al. Predictors of Long-Term Outcome of Subthalamic Stimulation in Parkinson Disease. Ann Neurol 2021, 89, 587–597, doi:10.1002/ana.25994. [CrossRef]
- Zampogna, A.; Cavallieri, F.; Bove, F.; Suppa, A.; Castrioto, A.; Meoni, S.; Pélissier, P.; Schmitt, E.; Bichon, A.; Lhommée, E.; et al. Axial Impairment and Falls in Parkinson’s Disease: 15 Years of Subthalamic Deep Brain Stimulation. NPJ Parkinsons Dis 2022, 8, 121, doi:10.1038/s41531-022-00383-y. [CrossRef]
- Cavallieri, F.; Campanini, I.; Gessani, A.; Budriesi, C.; Fioravanti, V.; Di Rauso, G.; Feletti, A.; Damiano, B.; Scaltriti, S.; Guagnano, N.; et al. Long-Term Effects of Bilateral Subthalamic Nucleus Deep Brain Stimulation on Gait Disorders in Parkinson’s Disease: A Clinical-Instrumental Study. J Neurol 2023, doi:10.1007/s00415-023-11780-5. [CrossRef]
- Di Rauso, G.; Cavallieri, F.; Campanini, I.; Gessani, A.; Fioravanti, V.; Feletti, A.; Damiano, B.; Scaltriti, S.; Bardi, E.; Corni, M.G.; et al. Freezing of Gait in Parkinson’s Disease Patients Treated with Bilateral Subthalamic Nucleus Deep Brain Stimulation: A Long-Term Overview. Biomedicines 2022, 10, 2214, doi:10.3390/biomedicines10092214. [CrossRef]
- Cantiniaux, S.; Vaugoyeau, M.; Robert, D.; Horrelou-Pitek, C.; Mancini, J.; Witjas, T.; Azulay, J.-P. Comparative Analysis of Gait and Speech in Parkinson’s Disease: Hypokinetic or Dysrhythmic Disorders? J Neurol Neurosurg Psychiatry 2010, 81, 177–184, doi:10.1136/jnnp.2009.174375. [CrossRef]
- Bohnen, N.I.; Yarnall, A.J.; Weil, R.S.; Moro, E.; Moehle, M.S.; Borghammer, P.; Bedard, M.-A.; Albin, R.L. Cholinergic System Changes in Parkinson’s Disease: Emerging Therapeutic Approaches. The Lancet Neurology 2022, 21, 381–392, doi:10.1016/S1474-4422(21)00377-X. [CrossRef]
- Bohnen, N.I.; Frey, K.A.; Studenski, S.; Kotagal, V.; Koeppe, R.A.; Constantine, G.M.; Scott, P.J.H.; Albin, R.L.; Müller, M.L.T.M. Extra-nigral Pathological Conditions Are Common in Parkinson’s Disease with Freezing of Gait: An in Vivo Positron Emission Tomography Study. Movement Disorders 2014, 29, 1118–1124, doi:10.1002/mds.25929. [CrossRef]
- Sauerbier, A.; Jenner, P.; Todorova, A.; Chaudhuri, K.R. Non Motor Subtypes and Parkinson’s Disease. Parkinsonism & Related Disorders 2016, 22, S41–S46, doi:10.1016/j.parkreldis.2015.09.027. [CrossRef]
- Müller, M.L.T.M.; Frey, K.A.; Petrou, M.; Kotagal, V.; Koeppe, R.A.; Albin, R.L.; Bohnen, N.I. Β-amyloid and Postural Instability and Gait Difficulty in Parkinson’s Disease at Risk for Dementia. Movement Disorders 2013, 28, 296–301, doi:10.1002/mds.25213. [CrossRef]
- Kings Parcog groupMDS Nonmotor study group; Lim, E.W.; Aarsland, D.; Ffytche, D.; Taddei, R.N.; Van Wamelen, D.J.; Wan, Y.-M.; Tan, E.K.; Ray Chaudhuri, K. Amyloid-β and Parkinson’s Disease. J Neurol 2019, 266, 2605–2619, doi:10.1007/s00415-018-9100-8. [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of Clinical Diagnosis of Idiopathic Parkinson’s Disease: A Clinico-Pathological Study of 100 Cases. J Neurol Neurosurg Psychiatry 1992, 55, 181–184, doi:10.1136/jnnp.55.3.181. [CrossRef]
- Cavallieri, F.; Gessani, A.; Merlo, A.; Campanini, I.; Budriesi, C.; Fioravanti, V.; Di Rauso, G.; Feletti, A.; Damiano, B.; Scaltriti, S.; et al. Interplay between Speech and Gait Variables in Parkinson’s Disease Patients Treated with Subthalamic Nucleus Deep Brain Stimulation: A Long-term Instrumental Assessment. Euro J of Neurology 2023, 30, 1963–1972, doi:10.1111/ene.15803. [CrossRef]
- World Medical Association World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194, doi:10.1001/jama.2013.281053. [CrossRef]
- Defer, G.L.; Widner, H.; Marié, R.M.; Rémy, P.; Levivier, M. Core Assessment Program for Surgical Interventional Therapies in Parkinson’s Disease (CAPSIT-PD). Mov Disord 1999, 14, 572–584, doi:10.1002/1531-8257(199907)14:4<572::aid-mds1005>3.0.co;2-c. [CrossRef]
- Gessani, A.; Cavallieri, F.; Fioravanti, V.; Campanini, I.; Merlo, A.; Di Rauso, G.; Damiano, B.; Scaltriti, S.; Bardi, E.; Corni, M.G.; et al. Long-Term Effects of Subthalamic Nucleus Deep Brain Stimulation on Speech in Parkinson’s Disease. Sci Rep 2023, 13, 11462, doi:10.1038/s41598-023-38555-2. [CrossRef]
- Stebbins, G.T.; Goetz, C.G.; Burn, D.J.; Jankovic, J.; Khoo, T.K.; Tilley, B.C. How to Identify Tremor Dominant and Postural Instability/Gait Difficulty Groups with the Movement Disorder Society Unified Parkinson’s Disease Rating Scale: Comparison with the Unified Parkinson’s Disease Rating Scale. Mov Disord 2013, 28, 668–670, doi:10.1002/mds.25383. [CrossRef]
- Emre, M.; Aarsland, D.; Brown, R.; Burn, D.J.; Duyckaerts, C.; Mizuno, Y.; Broe, G.A.; Cummings, J.; Dickson, D.W.; Gauthier, S.; et al. Clinical Diagnostic Criteria for Dementia Associated with Parkinson’s Disease. Movement Disorders 2007, 22, 1689–1707, doi:10.1002/mds.21507. [CrossRef]
- Litvan, I.; Goldman, J.G.; Tröster, A.I.; Schmand, B.A.; Weintraub, D.; Petersen, R.C.; Mollenhauer, B.; Adler, C.H.; Marder, K.; Williams-Gray, C.H.; et al. Diagnostic Criteria for Mild Cognitive Impairment in Parkinson’s Disease: Movement Disorder Society Task Force Guidelines. Movement Disorders 2012, 27, 349–356, doi:10.1002/mds.24893. [CrossRef]
- Grisanti, S.; Ferri, L.; Cavallieri, F.; Fioravanti, V.; Vincenzi, C.; Toschi, G.; Grisendi, I.; Sabadini, R.; Paul, J.J.; Bauer, P.; et al. Increased Stroke Risk in Patients with Parkinson’s Disease with LRRK2 Mutations. Mov Disord 2022, 37, 1117–1118, doi:10.1002/mds.28996. [CrossRef]
- Skrahina, V.; Gaber, H.; Vollstedt, E.; Förster, T.M.; Usnich, T.; Curado, F.; Brüggemann, N.; Paul, J.; Bogdanovic, X.; Zülbahar, S.; et al. The Rostock International Parkinson’s Disease ( ROPAD ) Study: Protocol and Initial Findings. Movement Disorders 2021, 36, 1005–1010, doi:10.1002/mds.28416. [CrossRef]
- Westenberger, A.; Skrahina, V.; Usnich, T.; Beetz, C.; Vollstedt, E.-J.; Laabs, B.-H.; Paul, J.J.; Curado, F.; Skobalj, S.; Gaber, H.; et al. Relevance of Genetic Testing in the Gene-Targeted Trial Era: The Rostock Parkinson’s Disease Study. Brain 2024, 147, 2652–2667, doi:10.1093/brain/awae188. [CrossRef]
- Tomlinson, C.L.; Stowe, R.; Patel, S.; Rick, C.; Gray, R.; Clarke, C.E. Systematic Review of Levodopa Dose Equivalency Reporting in Parkinson’s Disease. Mov Disord 2010, 25, 2649–2653, doi:10.1002/mds.23429. [CrossRef]
- Palmerini, L.; Mellone, S.; Avanzolini, G.; Valzania, F.; Chiari, L. Quantification of Motor Impairment in Parkinson’s Disease Using an Instrumented Timed up and Go Test. IEEE Trans Neural Syst Rehabil Eng 2013, 21, 664–673, doi:10.1109/TNSRE.2012.2236577. [CrossRef]
- Zampieri, C.; Salarian, A.; Carlson-Kuhta, P.; Aminian, K.; Nutt, J.G.; Horak, F.B. The Instrumented Timed up and Go Test: Potential Outcome Measure for Disease Modifying Therapies in Parkinson’s Disease. J Neurol Neurosurg Psychiatry 2010, 81, 171–176, doi:10.1136/jnnp.2009.173740. [CrossRef]
- Cavallieri, F.; Budriesi, C.; Gessani, A.; Contardi, S.; Fioravanti, V.; Menozzi, E.; Pinto, S.; Moro, E.; Valzania, F.; Antonelli, F. Dopaminergic Treatment Effects on Dysarthric Speech: Acoustic Analysis in a Cohort of Patients With Advanced Parkinson’s Disease. Front Neurol 2020, 11, 616062, doi:10.3389/fneur.2020.616062. [CrossRef]
- Rusz, J.; Tykalova, T.; Ramig, L.O.; Tripoliti, E. Guidelines for Speech Recording and Acoustic Analyses in Dysarthrias of Movement Disorders. Movement Disorders 2021, 36, 803–814, doi:10.1002/mds.28465. [CrossRef]
- Skodda, S.; Visser, W.; Schlegel, U. Short- and Long-Term Dopaminergic Effects on Dysarthria in Early Parkinson’s Disease. J Neural Transm 2010, 117, 197–205, doi:10.1007/s00702-009-0351-5. [CrossRef]
- Rusz, J.; Bonnet, C.; Klempíř, J.; Tykalová, T.; Baborová, E.; Novotný, M.; Rulseh, A.; Růžička, E. Speech Disorders Reflect Differing Pathophysiology in Parkinson’s Disease, Progressive Supranuclear Palsy and Multiple System Atrophy. J Neurol 2015, 262, 992–1001, doi:10.1007/s00415-015-7671-1. [CrossRef]
- Boersma, P.; Weenink, D. Praat: Doing Phonetics by Computer [Computer Program]. Version 5.3.51. (2013).
- Garon, M.; Weis, L.; Fiorenzato, E.; Pistonesi, F.; Cagnin, A.; Bertoldo, A.; Anglani, M.; Cecchin, D.; Antonini, A.; Biundo, R. Quantification of Brain β-Amyloid Load in Parkinson’s Disease With Mild Cognitive Impairment: A PET/MRI Study. Front. Neurol. 2022, 12, 760518, doi:10.3389/fneur.2021.760518. [CrossRef]
- Fiorenzato, E.; Biundo, R.; Cecchin, D.; Frigo, A.C.; Kim, J.; Weis, L.; Strafella, A.P.; Antonini, A. Brain Amyloid Contribution to Cognitive Dysfunction in Early-Stage Parkinson’s Disease: The PPMI Dataset. JAD 2018, 66, 229–237, doi:10.3233/JAD-180390. [CrossRef]
- Roberts, R.O.; Aakre, J.A.; Kremers, W.K.; Vassilaki, M.; Knopman, D.S.; Mielke, M.M.; Alhurani, R.; Geda, Y.E.; Machulda, M.M.; Coloma, P.; et al. Prevalence and Outcomes of Amyloid Positivity Among Persons Without Dementia in a Longitudinal, Population-Based Setting. JAMA Neurol 2018, 75, 970, doi:10.1001/jamaneurol.2018.0629. [CrossRef]
- Titova, N.; Chaudhuri, K.R. Nonmotor Parkinson’s and Future Directions. In International Review of Neurobiology; Elsevier, 2017; Vol. 134, pp. 1493–1505 ISBN 978-0-12-812603-5.
- Müller, M.L.T.M.; Bohnen, N.I.; Kotagal, V.; Scott, P.J.H.; Koeppe, R.A.; Frey, K.A.; Albin, R.L. Clinical Markers for Identifying Cholinergic Deficits in Parkinson’s Disease. Movement Disorders 2015, 30, 269–273, doi:10.1002/mds.26061. [CrossRef]
- Perez-Lloret, S.; Barrantes, F.J. Deficits in Cholinergic Neurotransmission and Their Clinical Correlates in Parkinson’s Disease. npj Parkinson’s Disease 2016, 2, 16001, doi:10.1038/npjparkd.2016.1. [CrossRef]
- Alves, G.; Pedersen, K.F.; Bloem, B.R.; Blennow, K.; Zetterberg, H.; Borm, G.F.; Dalaker, T.O.; Beyer, M.K.; Aarsland, D.; Andreasson, U.; et al. Cerebrospinal Fluid Amyloid-Beta and Phenotypic Heterogeneity in de Novo Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2013, 84, 537–543, doi:10.1136/jnnp-2012-303808. [CrossRef]
- Kang, J.-H. Association of Cerebrospinal Fluid β-Amyloid 1-42, T-Tau, P-Tau 181 , and α-Synuclein Levels With Clinical Features of Drug-Naive Patients With Early Parkinson Disease. JAMA Neurol 2013, doi:10.1001/jamaneurol.2013.3861. [CrossRef]
- Rochester, L.; Galna, B.; Lord, S.; Yarnall, A.J.; Morris, R.; Duncan, G.; Khoo, T.K.; Mollenhauer, B.; Burn, D.J. Decrease in Aβ42 Predicts Dopa-Resistant Gait Progression in Early Parkinson Disease. Neurology 2017, 88, 1501–1511, doi:10.1212/WNL.0000000000003840. [CrossRef]
- Buongiorno, M.; Antonelli, F.; Compta, Y.; Fernandez, Y.; Pavia, J.; Lomeña, F.; Ríos, J.; Ramírez, I.; García, J.R.; Soler, M.; et al. Cross-Sectional and Longitudinal Cognitive Correlates of FDDNP PET and CSF Amyloid-β and Tau in Parkinson’s Disease. Journal of Alzheimer’s Disease 2017, 55, 1261–1272, doi:10.3233/JAD-160698. [CrossRef]
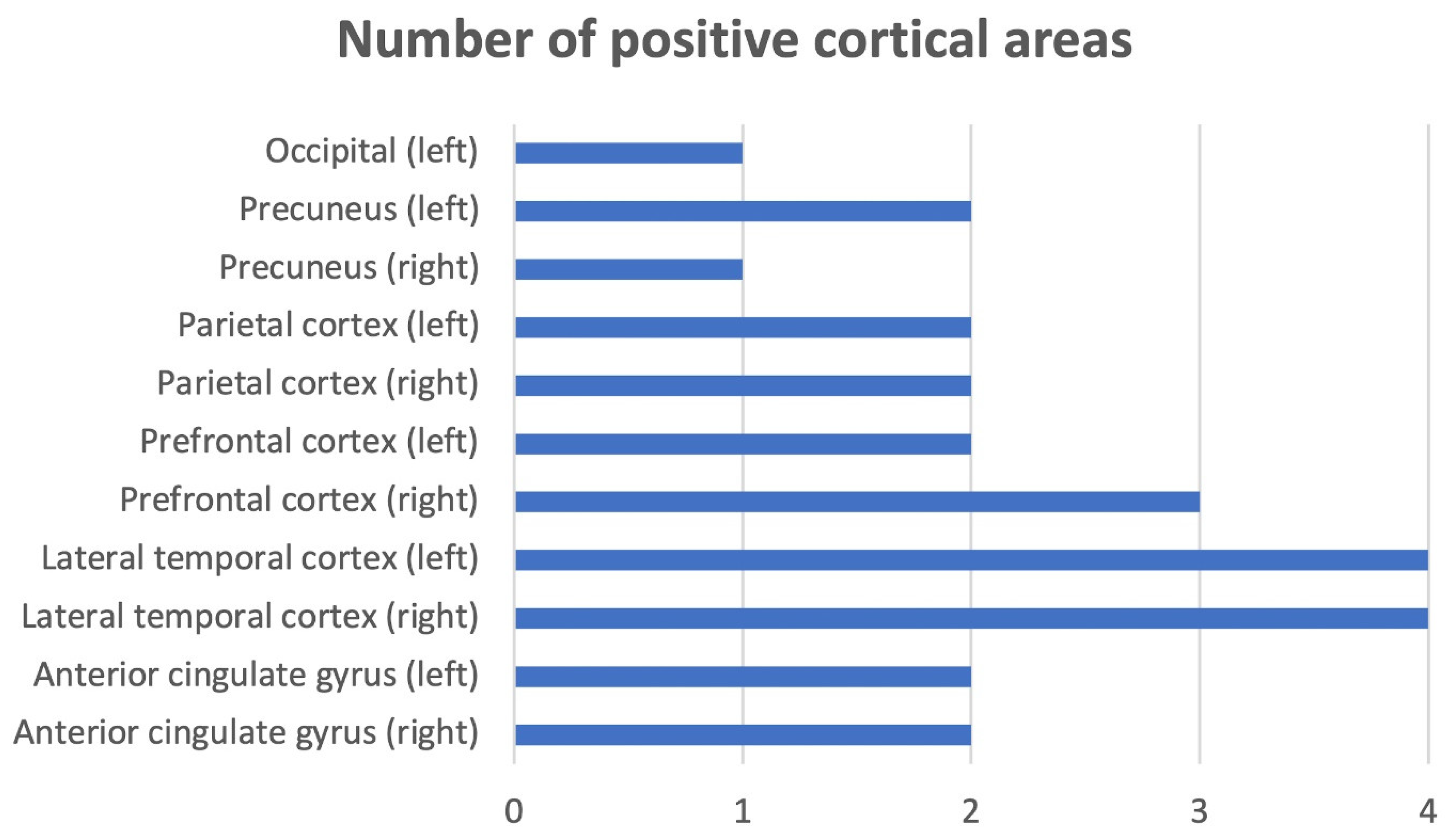
| VARIABLE | TOTAL N= 19 NO. (%), MEAN, [± SD]; MEDIAN {RANGE} |
|---|---|
|
Sex Male Female |
11 (58%) 8 (42%) |
| Preoperative assessment | |
| Age at surgery (years) | 58.89 [± 5.69]; 59.00 {46.00–71.00} |
| Age at PD onset (years) | 48.21 [± 5.93]; 47.00 {38.00-57.00} |
| Disease duration at surgery (years) | 10.47 [±4.88]; 8.00 {5.00–25.00} |
|
Genetic analysis Negative Positive |
16 (85.00%) 3 (GBA1; 15.00%) |
|
PD motor subtype PIGD Indeterminate TD |
14 (73.70%) 2 (10.50%) 3 (15.80%) |
| Levodopa equivalent daily dose (LEDD) (mg) | 924.23 [± 451.49]; 1045 {200-1898} |
| Levodopa responsiveness (%) | 58.83 [± 15.357]; 58.00 {34.00–93.94} |
| Post-operative evaluation | |
| Follow-up duration (years) | 4.73 [± 1.38]; 5 {3-7} |
| Age (years) | 63.52 [± 5.14]; 65 {53 - 74} |
| Disease duration (years) | 15.00 [± 4.78]; 13 {8-28} |
|
PD motor subtype PIGD Indeterminate TD |
17 (89.40%) 1 (5.30%) 1 (5.30%) |
| LEDD (mg) | 778.68 [±336.68]; {118.00-1460.00} |
|
PD cognitive status PD without dementia MCI-PD PDD |
9 (58%) 8 (41.40%) 2 (10.60%) |
| VARIABLES | STIMULATION/MEDICATIONS CONDITIONS | ||||
|---|---|---|---|---|---|
| mean, [±SD]; median {range} | |||||
| Off-medication | On-medication | On-stimulation/off-medication | Off-stimulation/off-medication | On-stimulation/on-medication | |
| UPDRS part-III | 36.82 [±9.86]; 34.00 {25.00-62.00} |
15.47 [±6.69]; 13.00 {6.00-31.00} |
31.05 [±13.97]; 13.97 {13.00-58.00} |
48.88 [±.12.83]; 49.00 {29.00-73.00} |
17.05 [±9.71]; 13.00 {5.00-38.00} |
| Akinesia subscore | 13.00 [±3.31]; 13.00 {7.00-18.00} |
5.05 [±3.23]; 4.00 {1.00-12.00} |
11.70 [±6.23]; 11.00 {2.00-23.00} |
18.07 [±5.69]; 18.00 {8.00-28.00} |
7.35 [±5.06]; 6.00 {1.00-17.00} |
| Tremor subscore | 4.41 [±4.30]; 3.00 {.00-14.00} |
1.35 [±2.31]; .00 {.00-9.00} |
3.11 [±2.39]; 3.00 {.00-7.00} |
4.94 [±2.90]; 4.00 {.00-10.00} |
.52 [±1.06]; .00 {.00-4.00} |
| PIGD subscore | 8.47 [±3.69]; 8.00 {3.00-16.00} |
2.82 [±1.97]; 2.00 {.00-7.00} |
8.23 [±3.43]; 9.00 {1.00-13.00} |
9.76 [±3.92]; 10.00 {1.00-15.00} |
5.52 [±3.57]; 5.00 {1.00-12.00} |
| Hoehn & Yahr | 2.86 [±.57]; 2.50 {2.00-4.00} |
2.13 [±.22]; 2.00 {2.00-2.50} |
2.86 [±.76]; 2.50 {2.00-5.00} |
3.84 [±.1.00]; 4.00 {2.00-5.00} |
2.42 [±.53]; 2.50 {2.00-4.00} |
| VARIABLE | PET positive (n= 4) | PET negative (n= 15) |
|---|---|---|
|
Sex Male Female |
2 (50%) 2 (50%) |
11 (58%) 8 (42%) |
| Follow-up duration (years) | 5.25 [± .50]; 5 {5-6} | 4.46 [± 1.50]; 4 {3-7} |
| Age (years) | 62.75 [± 4.03]; 63 {58 - 67} | 63.73 [± 5.50]; 65 {53 - 74} |
| Disease duration (years) | 13.75 [± 2.21]; 13 {12-17} | 15.33 [± 5.27]; 13 {8-28} |
|
PD motor subtype PIGD Indeterminate TD |
4 (100%) 0 (.00%) 0 (.00%) |
13 (86.70%) 1 (6.70%) 1 (6.70%) |
| Levodopa equivalent daily dose (mg) | 707.75 [±123.01] {580.00-819.00} |
797.60 [±375.30] {118.00-1460.00} |
|
PD cognitive status PD without dementia MCI-PD PDD |
1 (25.00%) 2 (50.00%) 1 (25.00%) |
8 (53.30%) 6 (40.00%) (6.70%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





