Submitted:
16 October 2024
Posted:
16 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Sensing Materials Based on Single Porphyrin Derivatives
2.1. Thin Film Formed From Only Porphyrin Molecules
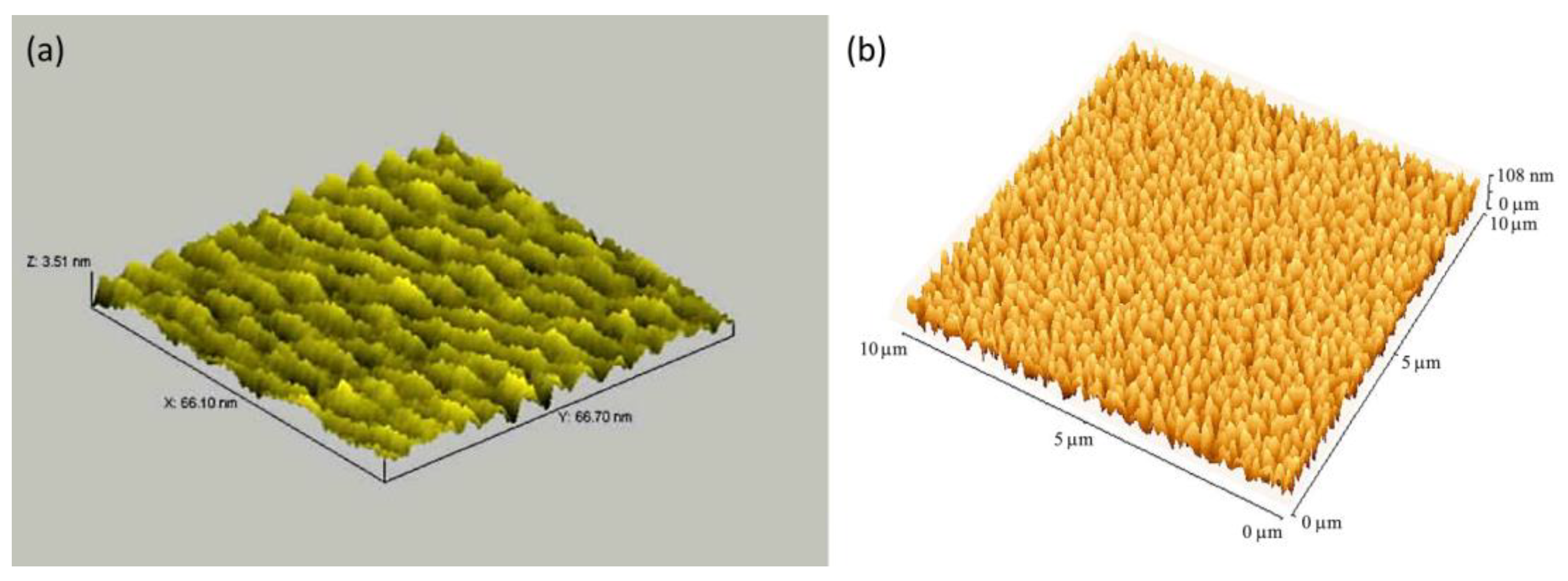
2.1.1. Dip-coating and drop-casting
2.1.2. Spin-Coating
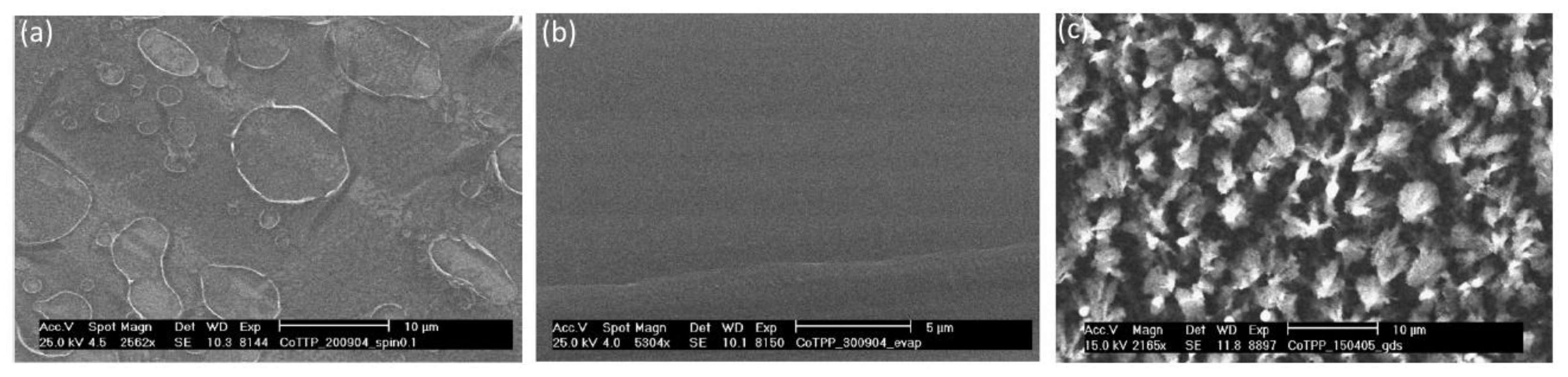
2.1.3. Vacuum Evaporation
2.1.4. Glow-Discharge-Induced Sublimation
2.1.5. Langmuir-Blodgett/Langmuir-Schäfer films
2.2. Materials Based on Oxide Matrices
2.3. Hybrid Materials Based on Organic Polymers
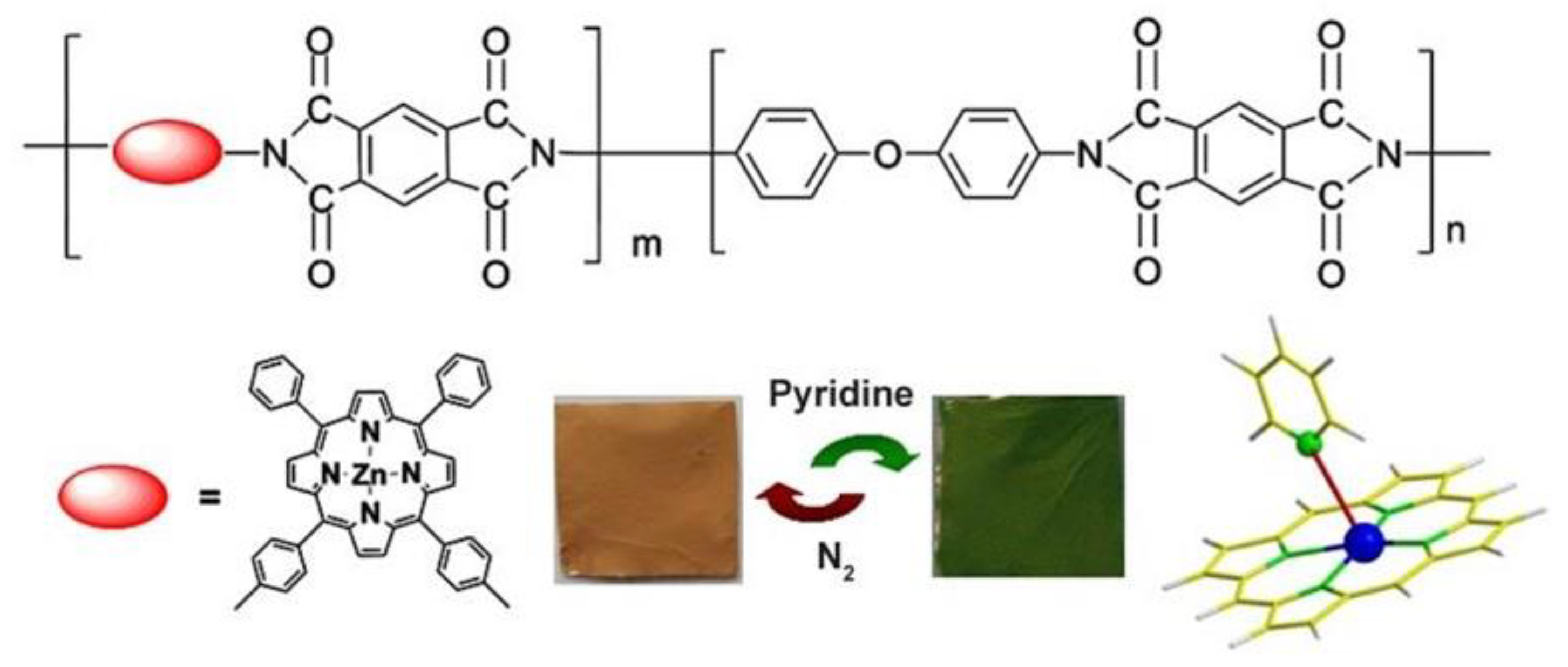
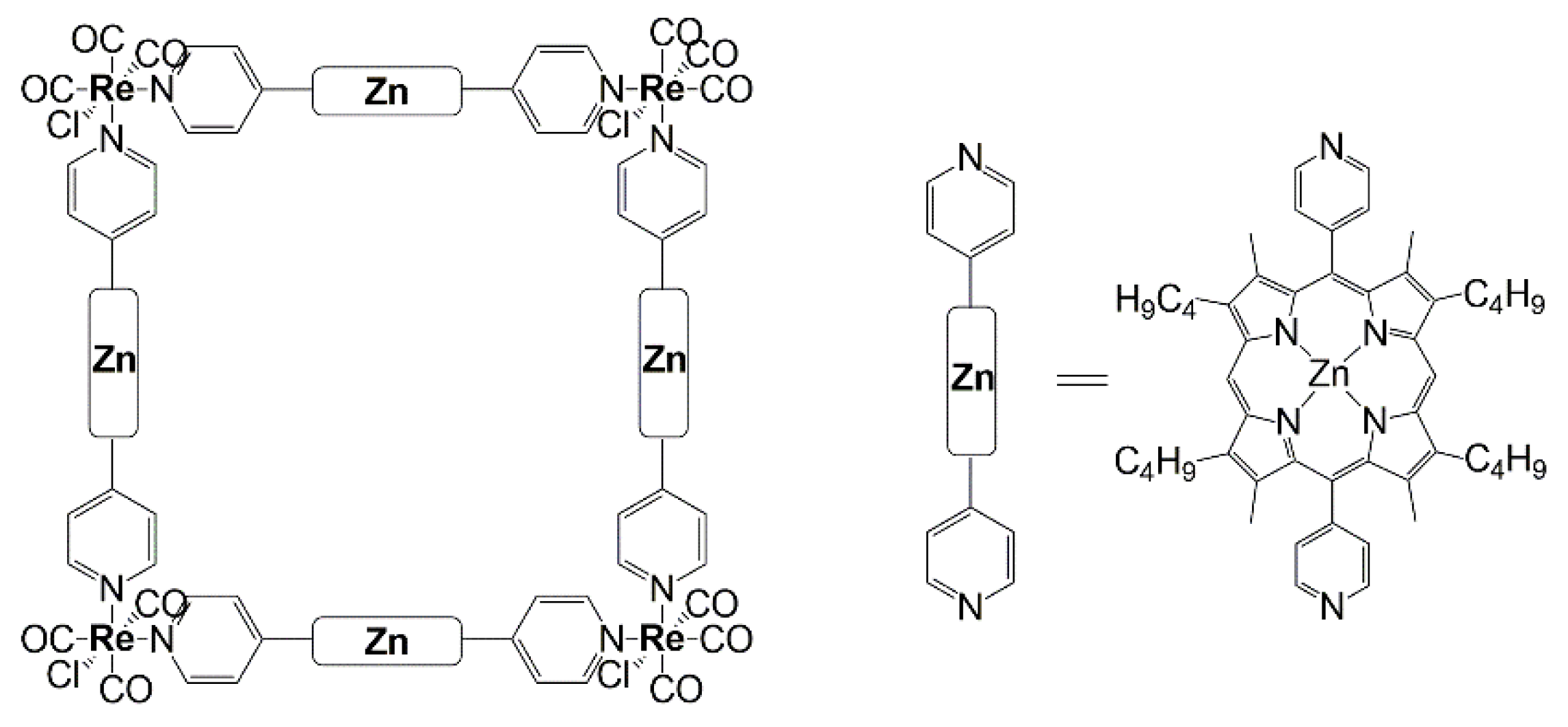
2.4. Supramolecular Assemblies and Metal-Organic Frameworks
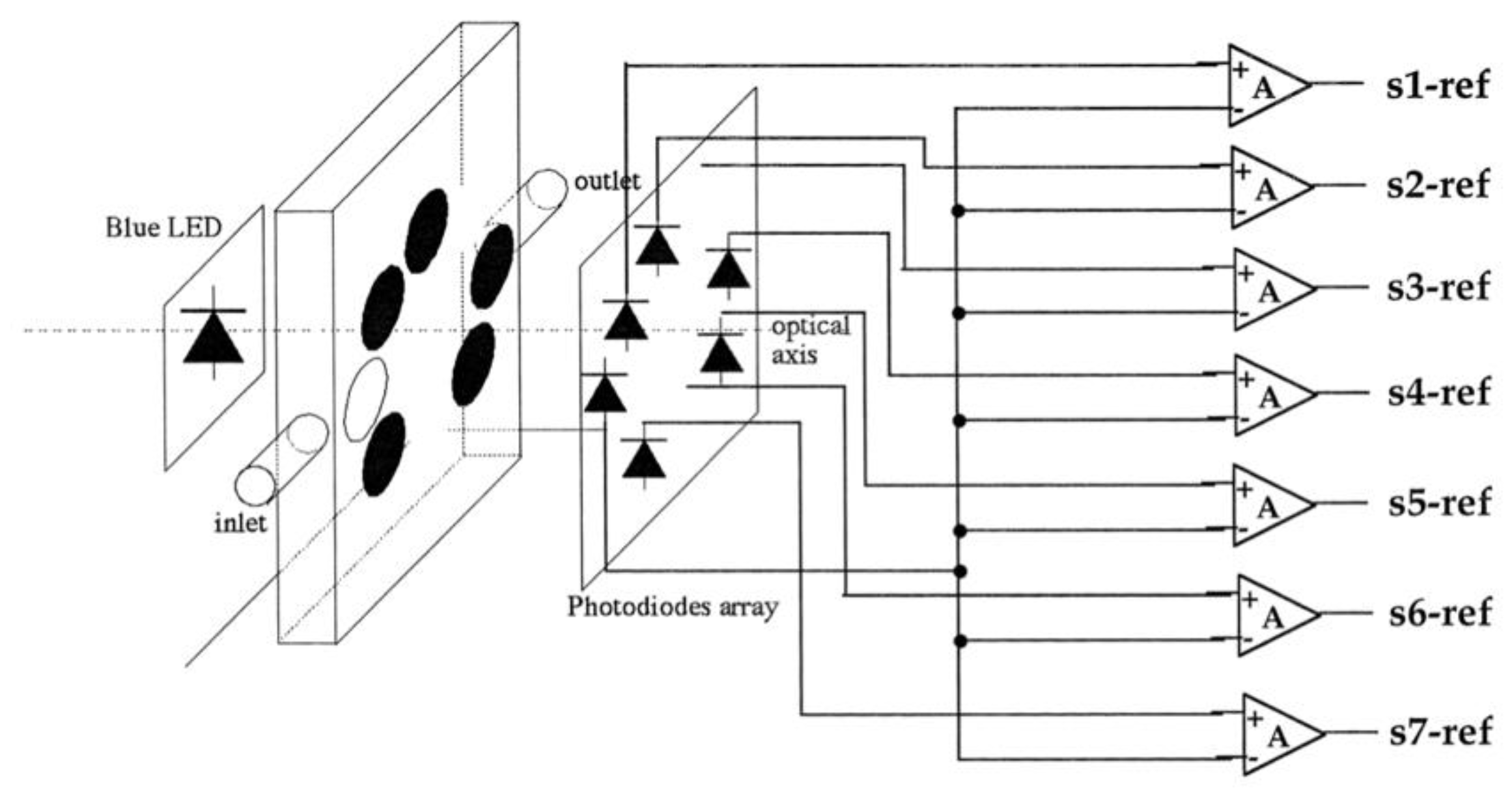
3. VOCs Sensing Based on Porphyrin Arrays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antonelli, M.; Donelli, D.; Barbieri, G.; Valussi, M.; Maggini, V.; Firenzuoli, F.; Health, P. Forest volatile organic compounds and their effects on human health: A state-of-the-art review. Int. J. Environ. Res. 2020, 17, 6506. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, X.; Wang, C.; Zhou, H. Environmental and human health impacts of volatile organic compounds: A perspective review. Chemosphere 2023, 313, 137489. [Google Scholar] [CrossRef] [PubMed]
- Salleh, M.M.; Akrajas; Yahaya, M. Optical sensing of capsicum aroma using four porphyrins derivatives thin films. Thin Solid Films 2002, 417, 162–165. [Google Scholar] [CrossRef]
- Boscher, N.D.; Bohn, T.; Heier, P.; Moisy, F.; Untereiner, B.; Heinze, K.; Choquet, P. Optical sensing responses of CrIIICl(TPP)(H2O)-based coatings obtained by an atmospheric pressure plasma method – Application to the detection of volatile amines. Sens. Actuators, B 2014, 191, 553–560. [Google Scholar] [CrossRef]
- Azzouz, A.; Vikrant, K.; Kim, K.-H.; Ballesteros, E.; Rhadfi, T.; Malik, A.K. Advances in colorimetric and optical sensing for gaseous volatile organic compounds. TrAC, Trends Anal. Chem. 2019, 118, 502–516. [Google Scholar] [CrossRef]
- Khatib, M.; Haick, H. Sensors for volatile organic compounds. ACS Nano 2022, 16, 7080–7115. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Rupam, T.H.; Chakraborty, A.; Saha, B.B. A comprehensive review on VOCs sensing using different functional materials: Mechanisms, modifications, challenges and opportunities. Renewable and Sustainable Energy Reviews 2024, 196, 114365. [Google Scholar] [CrossRef]
- McDonagh, C.; Burke, C.S.; MacCraith, B.D. Optical chemical sensors. Chem. Rev. 2008, 108, 400–422. [Google Scholar] [CrossRef]
- Russo, M.; Paolesse, R.; Bussetti, G.; Goletti, C.; Chiaradia, P.; Di Natale, C.; D'Amico, A.; Valli, L. , Exploiting gas-sensing properties of Langmuir-Blodgett/Schaeffer films by reflectance anisotropy spectroscopy. In Sensors And Microsystems, World Scientific: 2008; pp 271-277.
- Magna, G.; Monti, D.; Di Natale, C.; Paolesse, R.; Stefanelli, M. The Assembly of Porphyrin Systems in Well-Defined Nanostructures: An update. Molecules, 2019, 24, 4307. [Google Scholar] [CrossRef]
- Norvaiša, K.; Kielmann, M.; Senge, M.O. Porphyrins as colorimetric and photometric biosensors in modern bioanalytical systems. ChemBioChem 2020, 21, 1793–1807. [Google Scholar] [CrossRef]
- Purrello, R.; Gurrieri, S.; Lauceri, R. Porphyrin assemblies as chemical sensors. Coord. Chem. Rev. 1999; 190-192, 683–706. [Google Scholar]
- Monti, D.; Nardis, S.; Stefanelli, M.; Paolesse, R.; Di Natale, C.; D′ Amico, A. Porphyrin-based nanostructures for sensing applications. Journal of Sensors 2009, 2009, 856053. [Google Scholar] [CrossRef]
- Di Natale, C.; Monti, D.; Paolesse, R. Chemical sensitivity of porphyrin assemblies. Materials Today 2010, 13, 46–52. [Google Scholar] [CrossRef]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. porphyrinoids for chemical sensor applications. Chem. Rev. 2017, 117, 2517–2583. [Google Scholar] [CrossRef]
- Francis, S.; Joy, F.; Jayaraj, H.; Sunny, N.; Rajith, L.J. Recent advances in porphyrin-based optical sensing. J. Iran. Chem. Soc. 2024, 21, 13–70. [Google Scholar] [CrossRef]
- Saadh, M.J.; Ahmed, M.M.; Kamil, G.G.; Kaur, M.; Kaur, H.; Mohammed, F.; Abed, J.I.; Mahtab, A.M.; Hassan, Z.F.; Jasim, M.I.; Turki, S.A.; Kadhim, A.M. Porphyrin-based nanoarchitectures in sensing: Characterization, and applications in detecting gases, biomolecules, and environmental contaminants. Inorg. Chem. Commun. 2024, 163, 112352. [Google Scholar] [CrossRef]
- Richardson, T.H.; Dooling, C.M.; Jones, L.T.; Brook, R.A. Development and optimization of porphyrin gas sensing LB films. Adv. Colloid Interface Sci. 2005, 116, 81–96. [Google Scholar] [CrossRef]
- Di Natale, C.; Paolesse, R.; D’Amico, A. Metalloporphyrins based artificial olfactory receptors. Sensors Actuators B: Chemical 2007, 121, 238–246. [Google Scholar] [CrossRef]
- Wang, X.-d.; Wolfbeis, O.S. Optical methods for sensing and imaging oxygen: materials, spectroscopies and applications. Chem. Soc. Rev. 2014, 43, 3666–3761. [Google Scholar] [CrossRef]
- Ishihara, S.; Labuta, J.; Van Rossom, W.; Ishikawa, D.; Minami, K.; Hill, J.P.; Ariga, K. Porphyrin-based sensor nanoarchitectonics in diverse physical detection modes. Phys. Chem. Chem. Phys. 2014, 16, 9713–9746. [Google Scholar] [CrossRef]
- Li, Z.; Askim, J.R.; Suslick, K.S. The optoelectronic nose: colorimetric and fluorometric sensor arrays. Chem. Rev. 2019, 119, 231–292. [Google Scholar] [CrossRef]
- Bengasi, G.; Meunier-Prest, R.; Baba, K.; Kumar, A.; Pellegrino, A.L.; Boscher, N.D.; Bouvet, M. Molecular engineering of porphyrin-tapes/phthalocyanine heterojunctions for a highly sensitive ammonia sensor. Adv. Electron. Mater. 2020, 6, 2000812. [Google Scholar] [CrossRef]
- Magna, G.; Muduganti, M.; Stefanelli, M.; Sivalingam, Y.; Zurlo, F.; Di Bartolomeo, E.; Catini, A.; Martinelli, E.; Paolesse, R.; Di Natale, C. Light-activated porphyrinoid-capped nanoparticles for gas sensing. ACS Appl. Nano Mater. 2021, 4, 414–424. [Google Scholar] [CrossRef]
- Klyamer, D.; Shutilov, R.; Basova, T. Recent advances in phthalocyanine and porphyrin-based materials as active layers for nitric oxide chemical sensors. Sensors, 2022, 22, 895. [Google Scholar] [CrossRef]
- Basova, T. Phthalocyanine and porphyrin derivatives and their hybrid materials in optical sensors based on the phenomenon of surface plasmon resonance. Chemosensors, 2024, 12, 56. [Google Scholar] [CrossRef]
- Butt, M.A.; Voronkov, G.S.; Grakhova, E.P.; Kutluyarov, R.V.; Kazanskiy, N.L.; Khonina, S.N. Environmental monitoring: a comprehensive review on optical waveguide and fiber-based sensors. Biosensors, 2022, 12, 1038. [Google Scholar] [CrossRef]
- Ali Umar, A.; Mat Salleh, M.; Yahaya, M. Self-assembled monolayer of copper(II) meso-tetra(4-sulfanatophenyl) porphyrin as an optical gas sensor. Sens. Actuators, B 2004, 101, 231–235. [Google Scholar] [CrossRef]
- Spadavecchia, J.; Rella, R.; Siciliano, P.; Manera, M.G.; Alimelli, A.; Paolesse, R.; Di Natale, C.; D’Amico, A. Optochemical vapour detection using spin coated thin film of ZnTPP. Sens. Actuators, B 2006, 115, 12–16. [Google Scholar] [CrossRef]
- Yusoff, N.H.; Salleh, M.M.; Yahaya, M. Enhanced the performance of fluorescence gas sensor of porphyrin dye by using TiO2 nanoparticles. Adv. Mater. Res. (Durnten-Zurich, Switz.) 2008, 55, 269–272. [Google Scholar]
- Bahrampour, A.; Iadicicco, A.; De Luca, G.; Giordano, M.; Borriello, A.; Cutolo, A.; Cusano, A.; Scolaro, L.M. Porphyrin thin films on fiber optic probes through UV-light induced deposition. Opt. Laser Technol. 2013, 49, 279–283. [Google Scholar] [CrossRef]
- Elosua, C.; Matias, I.R.; Bariain, C.; Arregui, F.J. Volatile Organic Compound Optical Fiber Sensors: A Review. Sensors, 2006, 6, 1440–1465. [Google Scholar] [CrossRef]
- Di Natale, C.; Salimbeni, D.; Paolesse, R.; Macagnano, A.; D'Amico, A. Porphyrins-based opto-electronic nose for volatile compounds detection. Sens. Actuators, B 2000, 65, 220–226. [Google Scholar] [CrossRef]
- Liu, G.; Liu, P.; Liang, Y.; Xiao, Y.; Zhou, Z.; Li, Y.; Ding, L.; Peng, H.; Fang, Y. Porphyrin complex-based reversible fluorescent film sensor for differentiating and detecting sarin mimics vapor. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2024, 702, 135025. [Google Scholar] [CrossRef]
- Kladsomboon, S.; Kerdcharoen, T. A method for the detection of alcohol vapours based on optical sensing of magnesium 5,10,15,20-tetraphenylporphyrin thin film by an optical spectrometer and principal component analysis. Anal. Chim. Acta 2012, 757, 75–82. [Google Scholar] [CrossRef]
- Yang, W.Y.; Xu, J.H.; Wang, S.Y.; Chen, Y. The research of gas-sensing and optical characterization of chlorinated metallic porphyrin spin-coated films. Key Eng. Mater. 2013, 531, 58–62. [Google Scholar] [CrossRef]
- Tonezzer, M.; Quaranta, A.; Maggioni, G.; Carturan, S.; Mea, G.D. Optical sensing responses of tetraphenyl porphyrins toward alcohol vapours: A comparison between vacuum evaporated and spin-coated thin films. Sens. Actuators, B 2007, 122, 620–626. [Google Scholar] [CrossRef]
- Spadavecchia, J.; Ciccarella, G.; Stomeo, T.; Rella, R.; Capone, S.; Siciliano, P. Variation in the optical sensing responses toward vapors of a porphyrin/phthalocyanine hybrid thin film. Chem. Mater. 2004, 16, 2083–2090. [Google Scholar] [CrossRef]
- Spadavecchia, J.; Ciccarella, G.; Siciliano, P.; Capone, S.; Rella, R. Spin-coated thin films of metal porphyrin–phthalocyanine blend for an optochemical sensor of alcohol vapours. Sens. Actuators, B 2004, 100, 88–93. [Google Scholar] [CrossRef]
- Jing, L.; Jianhua, X.; Shuang, X. Volatile organic compound colorimetric array based on zinc porphyrin and metalloporphyrin derivatives. Energy Procedia 2011, 12, 625–631. [Google Scholar] [CrossRef]
- Long, J.; Xu, J.; Yang, Y.; Wen, J.; Jia, C. A colorimetric array of metalloporphyrin derivatives for the detection of volatile organic compounds. Mater. Sci. Eng. B 2011, 176, 1271–1276. [Google Scholar] [CrossRef]
- Roales, J.; Pedrosa, J.M.; Guillén, M.G.; Lopes-Costa, T.; Pinto, S.M.A.; Calvete, M.J.F.; Pereira, M.M. Optical detection of amine vapors using ZnTriad porphyrin thin films. Sens. Actuators, B 2015, 210, 28–35. [Google Scholar] [CrossRef]
- Çapan, İ.; Özkaya, C. Characterization of octaethyl porphyrin thin films with application to determination of volatile organic compounds. Anal. Lett. 2016, 49, 423–432. [Google Scholar] [CrossRef]
- Kladsomboon, S.; Thippakorn, C.; Seesaard, T. Development of organic-inorganic hybrid optical gas sensors for the non-invasive monitoring of pathogenic bacteria. Sensors 2018, 18, 3189. [Google Scholar] [CrossRef]
- Pathak, A.K.; Viphavakit, C. A review on all-optical fiber-based VOC sensors: Heading towards the development of promising technology. Sens. Actuators, A 2022, 338, 113455. [Google Scholar] [CrossRef]
- Wang, J.; Nizamidin, P.; Zhang, Y.; Kari, N.; Yimit, A. Detection of trimethylamine based on a manganese tetraphenylporphyrin optical waveguide sensing element. Anal. Sci. 2018, 34, 559–565. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Y.; Abudukeremu, H.; Maimaiti, A.; Wumaier, K.; Nizamidin, P.; Yimit, A. Detection of ethylenediamine vapor by optical waveguide sensor based on tetrakis-carboxylphenyl porphyrin film. J. Appl. Spectrosc. 2020, 87, 986–993. [Google Scholar] [CrossRef]
- Mamtmin, G.; Kari, N.; Abdurahman, R.; Nizamidin, P.; Yimit, A. 5, 10, 15, 20-tetrakis-(4-methoxyphenyl) porphyrin film/K+ ion-exchanged optical waveguide gas sensor. Opt. Laser Technol. 2020, 128, 106260. [Google Scholar] [CrossRef]
- Kutilike, B.; Yiming, K.; Tuerdi, G.; Abdurahman, R.; Nizamidin, P.; Yimit, A. A novel TiO2-modified THPP–BCP composite optical waveguide sensor for the determination of ethylenediamine at ppb level. Anal. Sci. 2024, 40, 291–300. [Google Scholar] [CrossRef]
- Maimaiti, P.; Nizamidin, P.; Kari, N.; Kutilike, B.; Yimit, A. Gas sensing properties of a maghemite-coupled porphyrin thin film–based optical waveguide sensor. Trans. Inst. Meas. Control (London) 2023, 01423312221141755. [Google Scholar] [CrossRef]
- Rankin, J.M.; Zhang, Q.; LaGasse, M.K.; Zhang, Y.; Askim, J.R.; Suslick, K.S. Solvatochromic sensor array for the identification of common organic solvents. Analyst 2015, 140, 2613–2617. [Google Scholar] [CrossRef]
- Bernini, R.; Tonezzer, M.; Mottola, F.; Zeni, L.; Quaranta, A.; Maggioni, G.; Carturan, S.; Mea, G.D. Volatile organic compounds detection using porphyrin-based metal-cladding leaky waveguides. Sens. Actuators, B 2007, 127, 231–236. [Google Scholar] [CrossRef]
- Tonezzer, M.; Maggioni, G.; Quaranta, A.; Carturan, S.; Della Mea, G. Optical sensing properties of CoTPP thin films deposited by glow-discharge-induced sublimation. Sens. Actuators, B 2007, 122, 613–619. [Google Scholar] [CrossRef]
- Tao, S.; Li, G.; Zhu, H. Metalloporphyrins as sensing elements for the rapid detection of trace TNT vapor. J. Mater. Chem. 2006, 16, 4521–4528. [Google Scholar] [CrossRef]
- Leray, I.; Vernières, M.-C.; Bied-Charreton, C. Porphyrins as probe molecules in the detection of gaseous pollutants: Detection of benzene using cationic porphyrins in polymer films. Sens. Actuators, B 1999, 54, 243–251. [Google Scholar] [CrossRef]
- Moscoso, F.G.; Romero-Guerrero, J.J.; Rodriguez-Lucena, D.; Pedrosa, J.M.; Carrillo-Carrión, C. Nanosized porphyrinic metal–organic frameworks for the construction of transparent membranes as a multiresponsive optical gas sensor. Small Sci. 2024, 2400210. [Google Scholar] [CrossRef]
- Kladsomboon, S.; Pratontep, S.; Puntheeranurak, T.; Kerdcharoen, T. An artificial nose based on M-porphyrin (M = Mg, Zn) thin film and optical spectroscopy. J. Nanosci. Nanotechnol. 2011, 11, 10589–10594. [Google Scholar] [CrossRef]
- Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef]
- Petty, M.C.; Films, L.B. , Langmuir-Blodgett films. An introduction. Cambridge University Press: : Cambridge, 1996.
- Iimura, K.-i., Langmuir and Langmuir–Blodgett monolayers having photo-responsibilities. In Stimuli-responsive interfaces. Fabrication and applications, Kawai, T.; Hashizume, M., Eds. Springer: Singapore, 2017; pp 179–186.
- Maji, S.; Alam, P.; Kumar, G.S.; Biswas, S.; Sarkar, P.K.; Das, B.; Rehman, I.; Das, B.B.; Jana, N.R.; Laskar, I.R.; Acharya, S. Induced aggregation of AIE-active mono-cyclometalated Ir(III) complex into supramolecular branched wires for light-emitting diodes. Small 2017, 13, 1603780. [Google Scholar] [CrossRef]
- Biswas, S.; Jana, D.; Kumar, G.S.; Maji, S.; Kundu, P.; Ghorai, U.K.; Giri, R.P.; Das, B.; Chattopadhyay, N.; Ghorai, B.K.; Acharya, S. Supramolecular aggregates of tetraphenylethene-cored AIEgen toward mechanoluminescent and electroluminescent devices. ACS Appl. Mater. Inter. 2018, 10, 17409–17418. [Google Scholar] [CrossRef]
- Oliveira, O.N.J.; Caseli, L.; Ariga, K. The past and the future of Langmuir and Langmuir–Blodgett films. Chem. Rev. 2022, 122, 6459–6513. [Google Scholar] [CrossRef]
- Ariga, K. Don’t forget Langmuir–Blodgett films 2020: Interfacial nanoarchitectonics with molecules, materials, and living objects. Langmuir 2020, 36, 7158–7180. [Google Scholar] [CrossRef]
- Ariga, K. Interfacial nanoarchitectonics with porphyrins and related molecules: Langmuir-Blodgett method and layer-by-layer assembly. J. Porphyrins Phthalocyanines 2023, 27, 924–945. [Google Scholar] [CrossRef]
- Liu, H.-G.; Qian, D.-J.; Feng, X.-S.; Xue, Q.-B.; Yang, K.-Z. Monolayer studies on an acetic acid substituted porphyrin and its complexes with aliphatic amines or quaternary ammonium salts at the air−liquid interface. Langmuir 2000, 16, 5079–5085. [Google Scholar] [CrossRef]
- Pedrosa, J.M.; Dooling, C.M.; Richardson, T.H.; Hyde, R.K.; Hunter, C.A.; Martín, M.T.; Camacho, L. Influence of molecular organization of asymmetrically substituted porphyrins on their response to NO2 gas. Langmuir 2002, 18, 7594–7601. [Google Scholar] [CrossRef]
- Cao, Z.; Chen, Q.; Wang, C.; Ren, Y.; Liu, H.; Hu, Y. Interfacial behaviors and aggregate structure of atropisomers of “picket-fence” porphyrin at the air/water interface. Colloids Surf. A 2011, 377, 130–137. [Google Scholar] [CrossRef]
- Stuchebryukov, S.D.; Selektor, S.L.; Silantieva, D.A.; Shokurov, A.V. Peculiarities of the reflection-absorption and transmission spectra of ultrathin films under normal incidence of light. Prot. Met. Phys. Chem. Surf. 2013, 49, 189–197. [Google Scholar] [CrossRef]
- Giancane, G.; Borovkov, V.; Inoue, Y.; Valli, L. Conformational switching in bis(zinc porphyrin) Langmuir–Schaefer film as an effective tool for selectively sensing aromatic amines. J. Colloid Interface Sci. 2012, 385, 282–284. [Google Scholar] [CrossRef]
- Umar, A.A.; Salleh, M.M.; Yahaya, M. Optical electronic nose based on Fe(III) complex of porphyrins films for detection of volatile compounds. Key Eng. Mater. 2012, 495, 75–78. [Google Scholar] [CrossRef]
- Bussetti, G.; Corradini, C.; Goletti, C.; Chiaradia, P.; Russo, M.; Paolesse, R.; Di Natale, C.; D'Amico, A.; Valli, L. Optical anisotropy and gas sensing properties of ordered porphyrin films. Phys. status solidi 2005, 242, 2714–2719. [Google Scholar] [CrossRef]
- Bussetti, G.; Cirilli, S.; Violante, A.; Chiaradia, P.; Goletti, C.; Tortora, L.; Paolesse, R.; Martinelli, E.; D’Amico, A.; Di Natale, C.; Giancane, G.; Valli, L. Optical anisotropy readout in solid-state porphyrins for the detection of volatile compounds. Appl. Phys. Lett. 2009, 95, 091906. [Google Scholar] [CrossRef]
- Bussetti, G.; Violante, A.; Yivlialin, R.; Cirilli, S.; Bonanni, B.; Chiaradia, P.; Goletti, C.; Tortora, L.; Paolesse, R.; Martinelli, E. Site-sensitive gas sensing and analyte discrimination in Langmuir−Blodgett porphyrin films. J. Phys. Chem. C 2011, 115, 8189–8194. [Google Scholar] [CrossRef]
- Evyapan, M.; Hassan, A.K.; Dunbar, A.D.F. Understanding the gas adsorption kinetics of Langmuir-Schaefer porphyrin films using two comparative sensing systems. Sens. Actuators, B 2018, 254, 669–680. [Google Scholar] [CrossRef]
- Çapan, R. Porphyrin Langmuir-Blodgett thin film for organic vapor detection. J. Phys. Sci. Appl. 2019, 9, 15–24. [Google Scholar]
- Maltceva, O.V.; Nikitin, K.S.; Kazak, A.V.; Zh, M.N.; Usol'Tseva, N.V. Sensing Ability of Zn-Tetraphenylporphyrin Langmuir-Schaefer Films. Liq. Cryst Appl. 2023, 23, 29–37. [Google Scholar] [CrossRef]
- Dunbar, A.D.F.; Richardson, T.H.; McNaughton, A.J.; Cadby, A.; Hutchinson, J.; Hunter, C.A. Optical changes induced in Zn porphyrin solutions and LB films by exposure to amines. J. Porphyrins Phthalocyanines 2006, 10, 978–985. [Google Scholar] [CrossRef]
- Wang, T.; Liu, M. Langmuir–Schaefer films of a set of achiral amphiphilic porphyrins: aggregation and supramolecular chirality. Soft Matter 2008, 4, 775–783. [Google Scholar] [CrossRef]
- Lu, G.; Zhang, X.; Cai, X.; Jiang, J. Tuning the morphology of self-assembled nanostructures of amphiphilic tetra (p-hydroxyphenyl) porphyrins with hydrogen bonding and metal–ligand coordination bonding. J. Mater. Chem. 2009, 19, 2417–2424. [Google Scholar] [CrossRef]
- Ali Umar, A.; Salleh, M.M.; Yahaya, M. Influence of surface microstructure on optical response of ruthenium-porphyrins thin films gas sensor. Eur. Phys. J. Appl. Phys. 2005, 29, 215–221. [Google Scholar] [CrossRef]
- Akrajas; Mat Salleh, M. ; Yahaya, M. Enriching the selectivity of metalloporphyrins chemical sensors by means of optical technique. Sens. Actuators, B 2002, 85, 191–196. [Google Scholar] [CrossRef]
- Dunbar, A.D.F.; Richardson, T.H.; McNaughton, A.J.; Hutchinson, J.; Hunter, C.A. Investigation of free base, Mg, Sn, and Zn substituted porphyrin LB films as gas sensors for organic analytes. J. Phys. Chem. B 2006, 110, 16646–16651. [Google Scholar] [CrossRef]
- Brittle, S.A.; Richardson, T.H.; Hutchinson, J.; Hunter, C.A. Comparing zinc and manganese porphyrin LB films as amine vapour sensing materials. Colloids Surf., A 2008, 321, 29–33. [Google Scholar] [CrossRef]
- Evyapan, M.; Dunbar, A.D.F. Improving the selectivity of a free base tetraphenylporphyrin based gas sensor for NO2 and carboxylic acid vapors. Sens. Actuators, B 2015, 206, 74–83. [Google Scholar] [CrossRef]
- Dunbar, A.D.F.; Brittle, S.; Richardson, T.H.; Hutchinson, J.; Hunter, C.A. Detection of volatile organic compounds using porphyrin derivatives. J. Phys. Chem. B 2010, 114, 11697–11702. [Google Scholar] [CrossRef]
- Brittle, S.; Richardson, T.H.; Dunbar, A.D.F.; Turega, S.; Hunter, C.A. Alkylamine sensing using Langmuir−Blodgett films of n-alkyl-N-phenylamide-substituted zinc porphyrins. J. Phys. Chem. B 2008, 112, 11278–11283. [Google Scholar] [CrossRef]
- Pedrosa, J.M.; Dooling, C.M.; Richardson, T.H.; Hyde, R.K.; Hunter, C.A.; Martín, M.T.; Camacho, L. The optical gas-sensing properties of an asymmetrically substituted porphyrin. J. Mater. Chem. 2002, 12, 2659–2664. [Google Scholar] [CrossRef]
- Gutiérrez, A.F.; Brittle, S.; Richardson, T.H.; Dunbar, A. A proto-type sensor for volatile organic compounds based on magnesium porphyrin molecular films. Sens. Actuators, B 2014, 202, 854–860. [Google Scholar] [CrossRef]
- Salleh, M.M.; Akrajas; Yahaya, M. Optical sensing of volatile organic compounds using metalloporphyrins Langmuir-Blodgett films. Int. J. Nonlinear Sci. Numer. Simul. 2002, 3, 461–464. [Google Scholar] [CrossRef]
- Colombelli, A.; Manera, M.G.; Borovkov, V.; Giancane, G.; Valli, L.; Rella, R. Enhanced sensing properties of cobalt bis-porphyrin derivative thin films by a magneto-plasmonic-opto-chemical sensor. Sens. Actuators, B 2017, 246, 1039–1048. [Google Scholar] [CrossRef]
- Yang, W.; Xu, J.; Mao, Y.; Yang, Y.; Jiang, Y. Detection of volatile organic compounds using Langmuir-Blodgett films of zinc-porphyrin and zinc-phthalocyanine. Synth. React. Inorg., Met.-Org., Nano-Met. Chem. 2016, 46, 735–740. [Google Scholar] [CrossRef]
- Brittle, S.A.; Richardson, T.H.; Dunbar, A.D.F.; Turega, S.M.; Hunter, C.A. Tuning free base tetraphenylporphyrins as optical sensing elements for volatile organic analytes. J. Mater. Chem. 2011, 21, 4882–4887. [Google Scholar] [CrossRef]
- Delmarre, D.; Bied-Charreton, C. Grafting of cobalt porphyrins in sol–gel matrices: application to the detection of amines. Sens. Actuators, B 2000, 62, 136–142. [Google Scholar] [CrossRef]
- Boscher, N.D.; Duday, D.; Heier, P.; Heinze, K.; Hilt, F.; Choquet, P. Atmospheric pressure plasma polymerisation of metalloporphyrins containing mesoporous membranes for gas sensing applications. Surf. Coat. Technol. 2013, 234, 48–52. [Google Scholar] [CrossRef]
- Heier, P.; Boscher, N.D.; Bohn, T.; Heinze, K.; Choquet, P. A new class of Zn II and Cr III porphyrins incorporated into porous polymer matrices via an atmospheric pressure plasma enhanced CVD to form gas sensing layers. J. Mater. Chem. A 2014, 2, 1560–1570. [Google Scholar] [CrossRef]
- Hanson, K.; Brennaman, M.K.; Luo, H.; Glasson, C.R.K.; Concepcion, J.J.; Song, W.; Meyer, T.J. Photostability of phosphonate-derivatized, RuII polypyridyl complexes on metal oxide surfaces. ACS Appl. Mater. Inter. 2012, 4, 1462–1469. [Google Scholar] [CrossRef]
- Roales, J.; Pedrosa, J.M.; Castillero, P.; Cano, M.; Richardson, T.H.; Barranco, Á.; González-Elipe, A.R. Selective detection of volatile organic compounds by spectral imaging of porphyrin derivatives bound to TiO2 porous films. ACS Appl. Mater. Inter. 2012, 4, 5147–5154. [Google Scholar] [CrossRef]
- Roales, J.; Pedrosa, J.M.; Cano, M.; Guillén, M.G.; Lopes-Costa, T.; Castillero, P.; Barranco, A.; González-Elipe, A.R. Anchoring effect on (tetra)carboxyphenylporphyrin/TiO2 composite films for VOC optical detection. RSC Adv. 2014, 4, 1974–1981. [Google Scholar] [CrossRef]
- Johnson, B.J.; Erickson, J.S.; Kim, J.; Malanoski, A.P.; Leska, I.A.; Monk, S.M.; Edwards, D.J.; Young, T.N.; Verbarg, J.; Bovais, C.; Russell, R.D.; Stenger, D.A. Miniaturized reflectance devices for chemical sensing. Meas. Sci. Technol. 2014, 25, 095101. [Google Scholar] [CrossRef]
- Bozkurt, S.S.; Merdivan, E.; Benibol, Y. A fluorescent chemical sensor for ethanol determination in alcoholic beverages. Microchim. Acta 2010, 168, 141–145. [Google Scholar] [CrossRef]
- Qin, W.; Parzuchowski, P.; Zhang, W.; Meyerhoff, M.E. Optical sensor for amine vapors based on dimer−monomer equilibrium of indium(III) octaethylporphyrin in a polymeric film. Anal. Chem. 2003, 75, 332–340. [Google Scholar] [CrossRef]
- Wang, Z.; Medforth, C.J.; Shelnutt, J.A. Porphyrin Nanotubes by Ionic Self-Assembly. J. Am. Chem. Soc. 2004, 126, 15954–15955. [Google Scholar] [CrossRef]
- Keefe, M.H.; O'Donnell, J.L.; Bailey, R.C.; Nguyen, S.T.; Hupp, J.T. Permeable, microporous polymeric membrane materials constructed from discrete molecular squares. Adv. Mater. 2003, 15, 1936–1939. [Google Scholar] [CrossRef]
- O'Donnell, J.L.; Thaitrong, N.; Nelson, A.P.; Hupp, J.T. Liquid/liquid interface polymerized porphyrin membranes displaying size-selective molecular and ionic permeability. Langmuir 2006, 22, 1804–1809. [Google Scholar] [CrossRef]
- Paske, A.C.; Earl, L.D.; O’Donnell, J.L. Interfacially polymerized metalloporphyrin thin films for colorimetric sensing of organic vapors. Sens. Actuators, B 2011, 155, 687–691. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, Y.; Du, Y.; Xu, J.; Wang, J. A novel porphyrin-containing polyimide nanofibrous membrane for colorimetric and fluorometric detection of pyridine vapor. Sensors 2013, 15758–15769. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Sohrabi, H.; Ghasemzadeh, S.; Ghoreishi, Z.; Majidi, M.R.; Yoon, Y.; Dizge, N.; Khataee, A. Metal-organic frameworks (MOF)-based sensors for detection of toxic gases: A review of current status and future prospects. Mater. Chem. Phys. 2023, 299, 127512. [Google Scholar] [CrossRef]
- Pal, T.K. Metal–organic framework (MOF)-based fluorescence “turn-on” sensors. Mater. Chem. Front. 2023, 7, 405–441. [Google Scholar] [CrossRef]
- Qiu, L.-G.; Li, Z.-Q.; Wu, Y.; Wang, W.; Xu, T.; Jiang, X. Facile synthesis of nanocrystals of a microporous metal–organic framework by an ultrasonic method and selective sensing of organoamines. Chem. Commun. 2008, 3642–3644. [Google Scholar] [CrossRef]
- Zou, X.; Zhu, G.; Hewitt, I.J.; Sun, F.; Qiu, S. Synthesis of a metal–organic framework film by direct conversion technique for VOCs sensing. Dalton Trans. 2009, 3009–3013. [Google Scholar] [CrossRef]
- Lu, G.; Hupp, J.T. Metal−organic frameworks as sensors: A ZIF-8 based Fabry−Pérot device as a selective sensor for chemical vapors and gases. J. Am. Chem. Soc. 2010, 132, 7832–7833. [Google Scholar] [CrossRef]
- Mines, G.A.; Tzeng, B.-C.; Stevenson, K.J.; Li, J.; Hupp, J.T. Microporous supramolecular coordination compounds as chemosensory photonic lattices. Angew. Chem., Int. Ed. 2002, 41, 154–157. [Google Scholar] [CrossRef]
- Persaud, K.; Dodd, G. Analysis of discrimination mechanisms in the mammalian olfactory system using a model nose. Nature 1982, 299, 352–355. [Google Scholar] [CrossRef]
- Kutsanedzie, F.Y.H.; Hao, L.; Yan, S.; Ouyang, Q.; Chen, Q. Near infrared chemo-responsive dye intermediaries spectra-based in-situ quantification of volatile organic compounds. Sens. Actuators, B 2018, 254, 597–602. [Google Scholar] [CrossRef]
- Askim, J.R.; Mahmoudi, M.; Suslick, K.S. Optical sensor arrays for chemical sensing: The optoelectronic nose. Chem. Soc. Rev. 2013, 42, 8649–8682. [Google Scholar] [CrossRef]
- Suslick, K.S.; Rakow, N.A.; Sen, A. Colorimetric sensor arrays for molecular recognition. Tetrahedron 2004, 60, 11133–11138. [Google Scholar] [CrossRef]
- Rakow, N.A.; Suslick, K.S. A colorimetric sensor array for odour visualization. Nature 2000, 406, 710–713. [Google Scholar] [CrossRef]
- Suslick, K.S. An optoelectronic nose: “Seeing” smells by means of colorimetric sensor arrays. MRS Bull. 2004, 29, 720–725. [Google Scholar] [CrossRef]
- Rakow, N.A.; Sen, A.; Janzen, M.C.; Ponder, J.B.; Suslick, K.S. Molecular recognition and discrimination of amines with a colorimetric array. Angew. Chem., Int. Ed. 2005, 44, 4528–4532. [Google Scholar] [CrossRef]
- Janzen, M.C.; Ponder, J.B.; Bailey, D.P.; Ingison, C.K.; Suslick, K.S. Colorimetric sensor arrays for volatile organic compounds. Anal. Chem. 2006, 78, 3591–3600. [Google Scholar] [CrossRef]
- Eaidkong, T.; Mungkarndee, R.; Phollookin, C.; Tumcharern, G.; Sukwattanasinitt, M.; Wacharasindhu, S. Polydiacetylene paper-based colorimetric sensor array for vapor phase detection and identification of volatile organic compounds. J. Mater. Chem. 2012, 22, 5970–5977. [Google Scholar] [CrossRef]
- Xiao-wei, H.; Xiao-bo, Z.; Ji-yong, S.; Zhi-hua, L.; Jie-wen, Z. Colorimetric sensor arrays based on chemo-responsive dyes for food odor visualization. Trends Food Sci. Technol. 2018, 81, 90–107. [Google Scholar] [CrossRef]
- Qu, X.; Hu, Y.; Xu, C.; Li, Y.; Zhang, L.; Huang, Q.; Sadat Moshirian-Farahi, S.; Zhang, J.; Xu, X.; Liao, M.; Fu, Y. Optical sensors of volatile organic compounds for non-invasive diagnosis of diseases. Chem. Eng. J. (Amsterdam, Neth.) 2024, 485, 149804. [Google Scholar] [CrossRef]
- Zhao, S.; Lei, J.; Huo, D.; Hou, C.; Luo, X.; Wu, H.; Fa, H.; Yang, M. A colorimetric detector for lung cancer related volatile organic compounds based on cross-response mechanism. Sens. Actuators, B 2018, 256, 543–552. [Google Scholar] [CrossRef]
- Lin, H.; Lin, J.-j.; Man, Z.-x.; Jin, H.-j.; Kutsanedzie, F.Y.H.; Chen, Q.-s. Development of colorimetric detection of 2,4,5-trimethyloxazole in volatile organic compounds based on porphyrin complexes for vinegar storage time discrimination. Food Anal. Methods 2020, 13, 2192–2203. [Google Scholar] [CrossRef]
- Guan, B.; Kang, W.; Jiang, H.; Zhou, M.; Lin, H. Freshness identification of oysters based on colorimetric sensor array combined with image processing and visible near-infrared spectroscopy. Sensors, 2022, 22, 683. [Google Scholar] [CrossRef]
- Xu, W.; He, Y.; Li, J.; Deng, Y.; Zhou, J.; Xu, E.; Ding, T.; Wang, W.; Liu, D. Olfactory visualization sensor system based on colorimetric sensor array and chemometric methods for high precision assessing beef freshness. Meat Sci. 2022, 194, 108950. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, J.; Yu, J.; Cui, B. A colorimetric sensor for qualitative discrimination and quantitative detection of volatile amines. Sensors, 2010, Sensors 2010, 10, 6463–6476. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, A.; Zhao, J.; Ouyang, Q.; Sun, Z.; Huang, L. Monitoring vinegar acetic fermentation using a colorimetric sensor array. Sens. Actuators, B 2013, 183, 608–616. [Google Scholar] [CrossRef]
- Li, L.; Xie, S.; Zhu, F.; Ning, J.; Chen, Q.; Zhang, Z. Colorimetric sensor array-based artificial olfactory system for sensing Chinese green tea’s quality: A method of fabrication. Int. J. Food Prop. 2017, 20, 1762–1773. [Google Scholar] [CrossRef]
- Lim, S.H.; Feng, L.; Kemling, J.W.; Musto, C.J.; Suslick, K.S. An optoelectronic nose for the detection of toxic gases. Nat. Chem. 2009, 1, 562–567. [Google Scholar] [CrossRef]
- Wu, Y.; Huo, D.; Hou, C.; Fa, H.; Yang, M.; Luo, X. Colorimetric artificial nose for identification of breath volatile organic compounds of patients with lung cancer. Chem. Res. Chin. Univ. 2014, 30, 572–577. [Google Scholar] [CrossRef]
- Lin, H.; Man, Z.-x.; Guan, B.-b.; Chen, Q.-s.; Jin, H.-j.; Xue, Z.-l. In situ quantification of volatile ethanol in complex components based on colorimetric sensor array. Anal. Methods 2017, 9, 5873–5879. [Google Scholar] [CrossRef]
- Guan, B.; Zhao, J.; Jin, H.; Lin, H. Determination of rice storage time with colorimetric sensor array. Food Anal. Methods 2017, 10, 1054–1062. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, G.; Zilberman, Y.; Ruan, W.; Ameri, S.K.; Zhang, Y.S.; Miller, E.; Sonkusale, S.R. Low cost smart phone diagnostics for food using paper-based colorimetric sensor arrays. Food Control 2017, 82, 227–232. [Google Scholar] [CrossRef]
- Li, J.; Hou, C.; Huo, D.; Yang, M.; Fa, H.-b.; Yang, P. Development of a colorimetric sensor array for the discrimination of aldehydes. Sens. Actuators, B 2014, 196, 10–17. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, X.; Pei, X.; Hu, J.; Zhao, W.; Chen, B.; Gu, Z. Multiplex detection of tumor markers with photonic suspension array. Anal. Chim. Acta 2009, 633, 103–108. [Google Scholar] [CrossRef]
- Xu, H.; Cao, K.-D.; Ding, H.-B.; Zhong, Q.-F.; Gu, H.-C.; Xie, Z.-Y.; Zhao, Y.-J.; Gu, Z.-Z. Spherical porphyrin sensor array based on encoded colloidal crystal beads for VOC vapor detection. ACS Appl. Mater. Inter. 2012, 4, 6752–6757. [Google Scholar] [CrossRef]
- Lv, R.; Huang, X.; Ye, W.; Aheto, J.H.; Xu, H.; Dai, C.; Tian, X. Research on the reaction mechanism of colorimetric sensor array with characteristic volatile gases-TMA during fish storage. J Food Process Eng 2019, 42, e12952. [Google Scholar] [CrossRef]
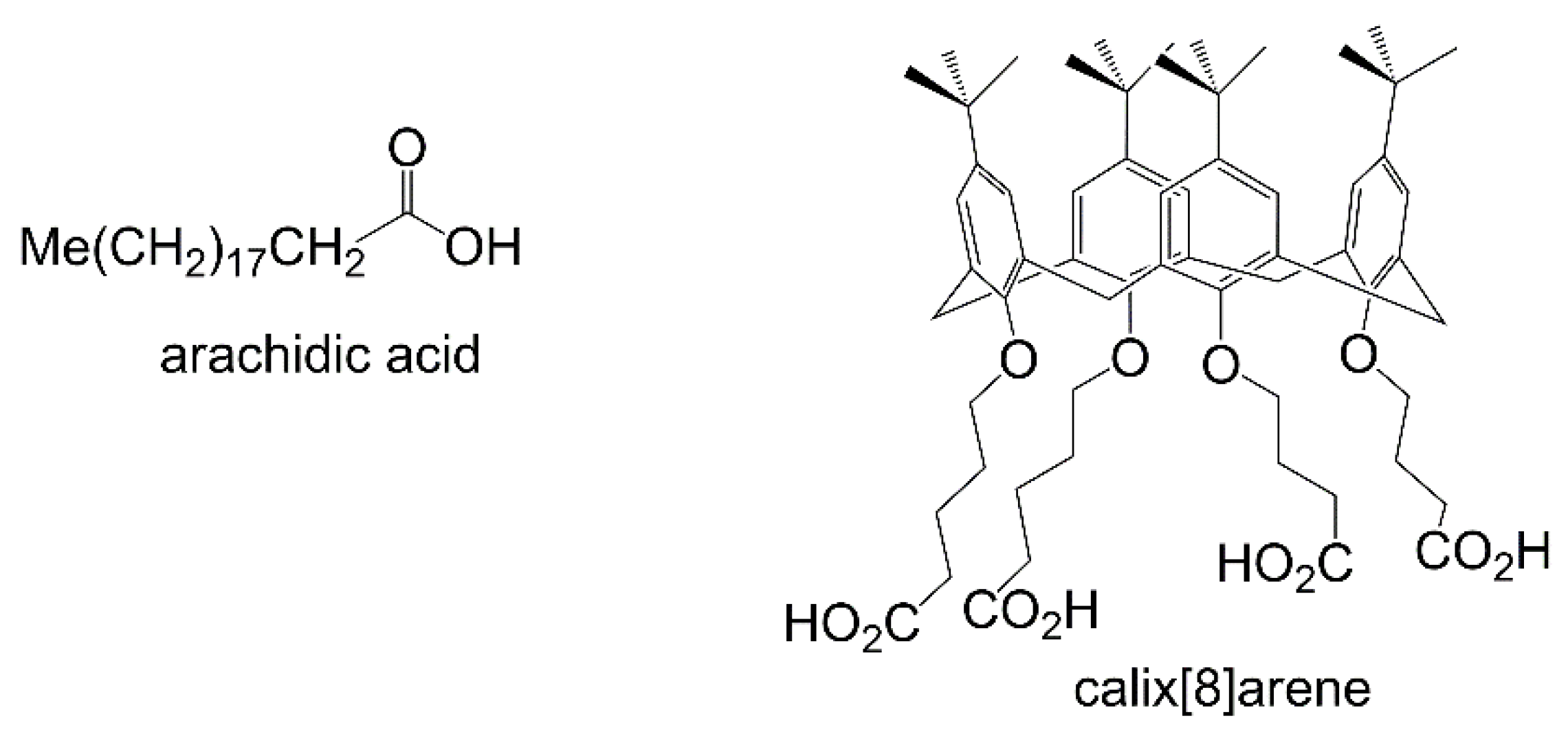
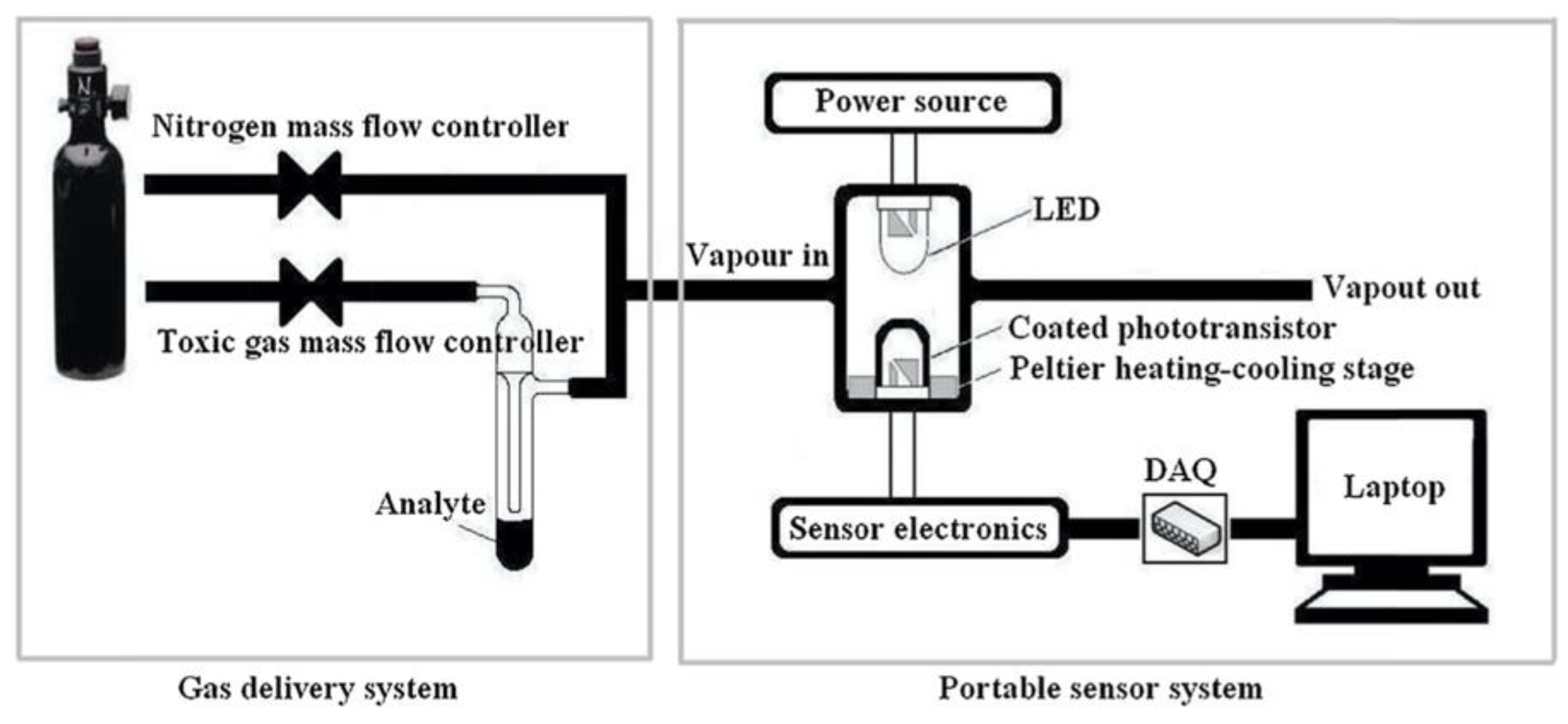
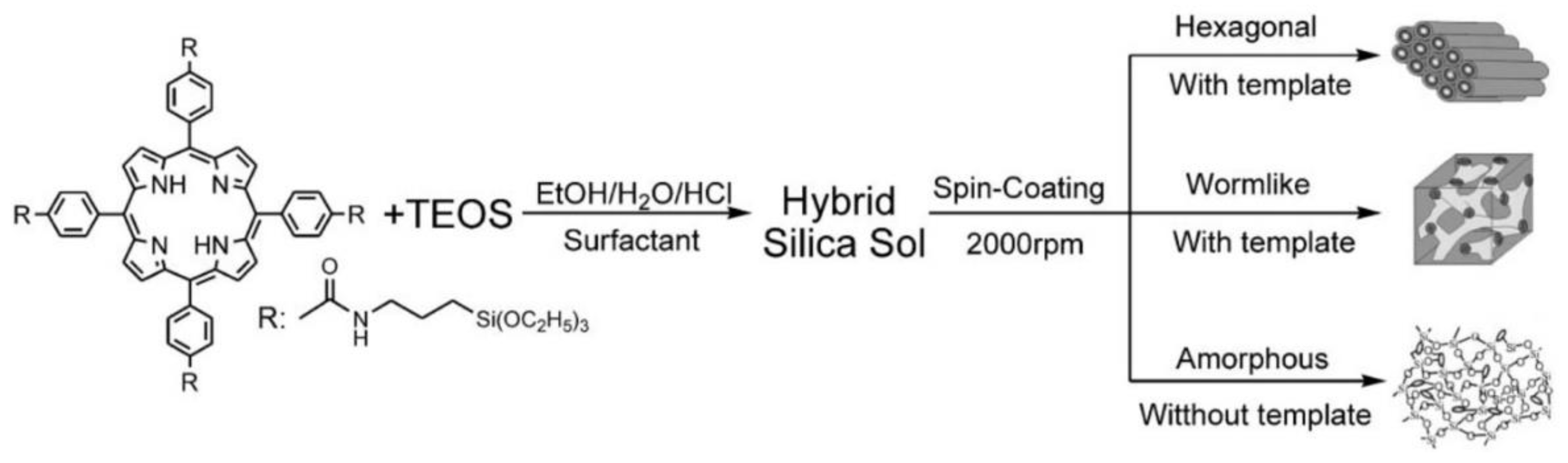
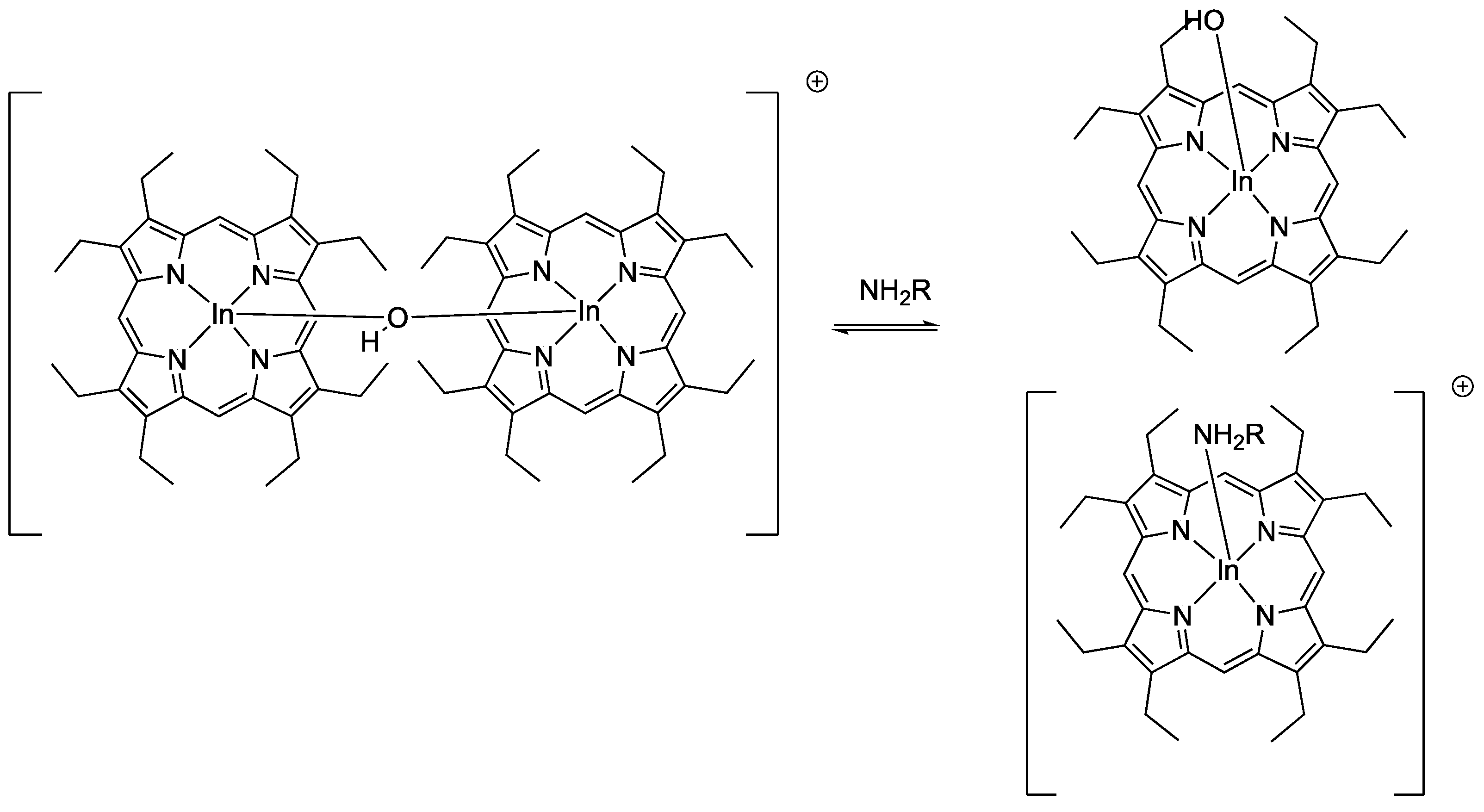
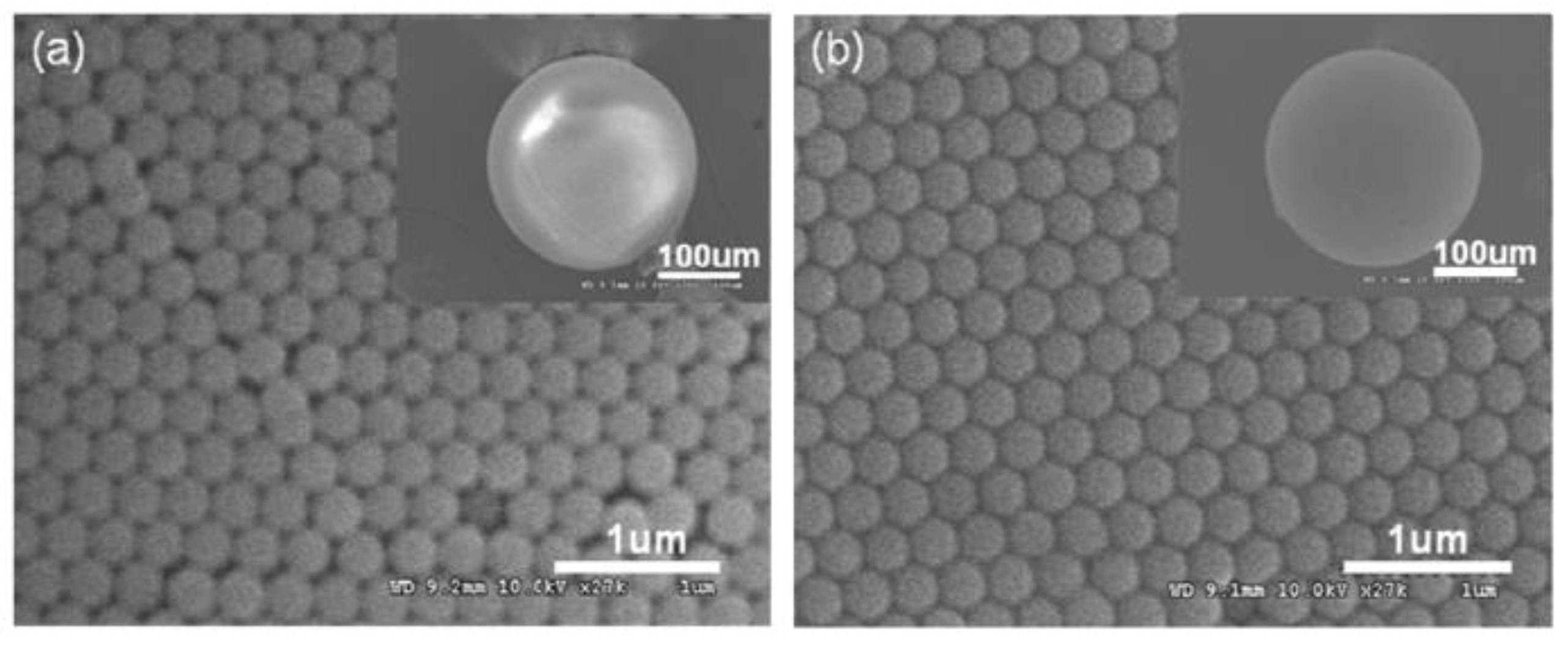
| Porphyrin precursora | Solid Support | Matrix | Optical response | VOCs | Ref |
|---|---|---|---|---|---|
| H2TPP or CoTPP or FeTPP |
P-doped (100) silicon wafer | - | UV–vis spectroscopy | MeOH, EtOH, iPrOH | 37 |
| FeTPP or MnTPP |
Glass | - | UV–vis spectroscopy | Py, NEt3, Me2NH | 36 |
| CoTPP | silica | - | UV–vis spectroscopy | EtOH | 53 |
| ZnTPP | Quartz | - | UV–vis spectroscopy | Py, MeOH, ethyl acetate | 40 |
| MgTPP | Glass | - | UV–vis spectroscopy | MeOH, PrOH, iPrOH | 35 |
| ZnTPP–NO2 | Quartz | - | UV–vis spectroscopy | Py, NEt3, MeOH, ethyl acetate | 40,41 |
| ZnTriad | Glass | - | UV–vis spectroscopy | PrNH2, BuNH2, HexNH2, PhNH2, tBuNH2 | 42 |
| MnTPP | K+-exchanged glass optical waveguide | - | UV–vis spectroscopy | NEt3 | 46 |
| H2TCPP | K+-exchanged glass optical waveguide | - | UV–vis spectroscopy | ethylenediamine | 47 |
| H2TMPP | K+-exchanged glass optical waveguide | - | UV–vis spectroscopy | ethylenediamine | 48 |
| H2TSPP | glass optical waveguides |
UV–vis spectroscopy | ethylenediamine | 50 | |
| H2TSPP | maghemite-covered glass optical waveguides |
- | UV–vis spectroscopy | ethylenediamine | 50 |
| H2THPP | TiO2-covered glass optical waveguides | - | UV–vis spectroscopy | ethylenediamine | 49 |
| H2OEP or ZnOEP |
Au- covered glass | - | SPR | CHCl3, acetone | 43 |
| H2TPP | Au-covered glass | - | Reflectance spectroscopy | EtOH, | 52 |
| H2TPP or ZnTPP or CdTPP |
Glass | SiO2 | Fluorescence spetroscopy | 2,4,6-trinitrotoluene, 2,4-dinitrotoluene, nitrobenzene | 54 |
| H2TMPyPP or H2DPyPP |
Glass substrate | PMMA PVP |
Fluorescence spetroscopy | 2,4,6-trinitrotoluene, 2,4-dinitrotoluene, nitrobenzene | 55 |
| CuTBPP +ZnPCb |
Quartz | - | UV–vis spectroscopy | MeOH, EtOH, iPrOH, acetone | 39 |
| CuTBPP +ZnPCb |
Quartz | - | UV–vis spectroscopy | MeOH, EtOH, iPrOH, PhNEt2, Py, 2-bromopyridine, Hex, acetone, tBuNH2 | 38 |
| ZnTPPP+MnTPP +ZnPC1b |
Glass | - | UV–vis spectroscopy | EtOH, acetone, MeCO2H, acetone, ethyl acetate, formaldehyde | 44 |
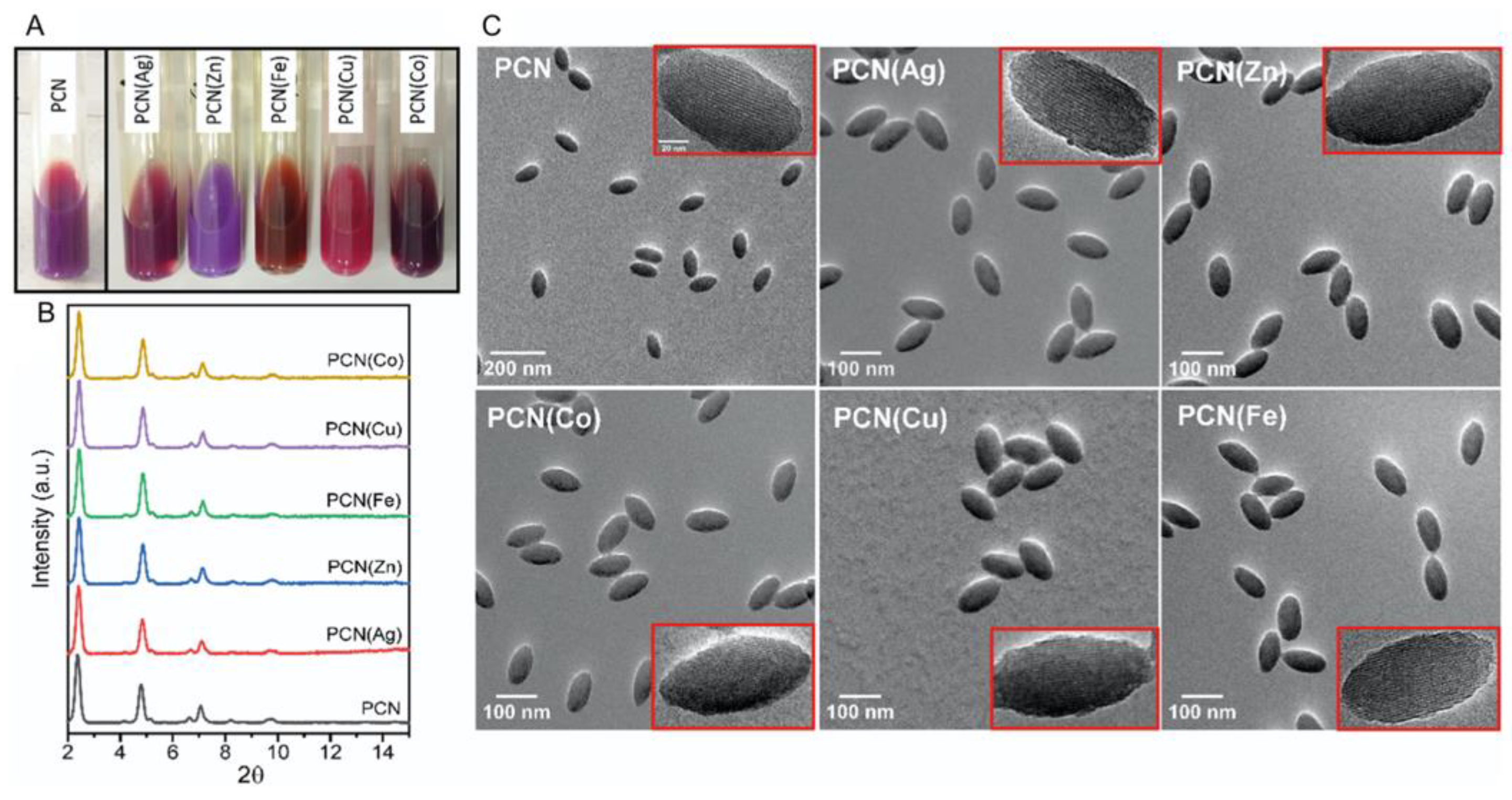
| array of M-PCN- 222c M=Ag, Zn, Fe, Cu, Co |
Glass | PDMS | UV–vis spectroscopy | acetone, CHCl3, CH2Cl2, EtOH, hexanal, BuNH2, tetrahydrofuran, toluene, 2,4-dinitrotoluene | 56 |
| array of MTPP M=Mg, Zn |
Glass | - | UV–vis spectroscopy | MeOH, EtOH, iPrOH, acetone, MeCO2H, methyl benzoate | 57 |
| Porphyrin precursor a | Method of deposition/Substrate | Optical response | VOCs | Ref |
|---|---|---|---|---|
| ZnTPP | LS/ glass | UV–vis spectroscopy | Py | 77 |
| MnTPPCl | LB/ glass | Reflectance spectroscopy | EtOH | 90 |
| RuTPP+ arachidic acidb |
LB/ glass substrate | Reflectance spectroscopy | MeOH, EtOH, iPrOH | 81 |
| H2THOPP | LB/ oxidized Si(001) | Reflectance anisotropy spectroscopy | EtOH | 72 |
| ZnTHOPP | LS/ quartz | Reflectance anisotropy spectroscopy | EtOH, Hex, NMe3 | 73 |
| ZnTHOPP | LB/ quartz | Reflectance anisotropy spectroscopy | EtOH, Hex | 74 |
| ZnEHO or ZnEHO +calix [8]- areneb |
LB/ HMDS-covered glass | UV–vis spectroscopy | MeCO2H, butanone, ethyl acetate, HexSH, HexNH2, HepCHO, OctOH, OctNH2, NEt3, P(OMe)3 | 78 |
| ZnEHO | LB/ HMDS-covered glass | UV–vis spectroscopy | PrNH2, BuNH2, PeNH2, HexNH2, HepNH2, OctNH2 | 78 |
| H2EHO | LSd/ HMDS-covered glass | UV–vis spectroscopy | MeCO2H, PrCO2H, PeCO2H | 85 |
| H2EHO, or MgEHO or SnEHO or ZnEHO or ZnEHO+calix [8]-areneb |
LB/ HMDS-covered glass | UV–vis spectroscopy | MeCO2H, butanone, ethyl acetate, HexSH, HexNH2, HepCHO, OctOH, OctNH2, NEt3, P(OMe)3 | 83 |
| MEHO M=Mg, Sn, Zn,Au,Co, Mn |
LB/ HMDS-covered glass | UV–vis spectroscopy | MeCO2H, butanone, ethyl acetate, HexSH, HexNH2, HepCHO, OctOH, OctNH2, NEt3, P(OMe)3 | 86 |
| MnEHO+calix [8]-areneb ZnEHO+calix [8]-areneb |
LB/ hydrophobic glass | UV–vis spectroscopy | PrNH2, BuNH2, PeNH2, HexNH2, HepNH2, OctNH2, NonNH2, NHEt2, NHPr2, NHBu2, NHHex2, NEt3, NPr3 |
84 |
| ZnTmBuPP ZnTmRPP |
LB/ hydrophobic glass | UV–vis spectroscopy | MeCO2H, butanone, ethyl acetate, HexSH, HepCHO, OctOH, P(OMe)3, PrNH2, BuNH2, NHPr2, NHBu2, NPr3, NBu3 | 87 |
| MgEHO | LS/ HMDS-covered glass | Phototransistor | 2-methyl-butan-2-ol | 89 |
| H2EHO | LS/ glass or Au (Au-coated glass SPR chip) |
SPR and UV–vis spectroscopy | MeCO2H, MeNH2 | 75 |
| Co-H(OEP)2 | LS/ Au (Au/Co/Au coated SPR chip) |
Magneto-optical SPR | MeOH, EtOH, iPrOH, NMe3 | 91 |
| ZnTDPP | LB/ gold coated SPR chip | SPR | benzene, toluene, ethyl benzene, xylene | 76 |
| array of ZnTPP, ZnPc2c |
LB/ quartz | UV–vis spectroscopy | pyridine, methanol | 92 |
| array of FeTPP, FeOEP, each mixed with arachidic acid |
LB/ glass | UV–vis spectroscopy | 2-propanol, acetone, cyclohexane, ethanol. | 71 |
| array of MnOEP FeOEP CoOEP RuOEP, each mixed with arachidic acid |
LB/ glass | UV–vis spectroscopy | EtOH, iPrOH, acetone, cyclohexane | 82 |
| array of FeTPP, MnTPP, CoTMPP, CoOEP, each mixed with arachidic acid |
LB/ glass | UV–vis spectroscopy | fresh capsicum annum, dried capsicum annum, fresh capsicum minimum | 3 |
| array of H2DmRDAPP, H2TmOPP, H2EHO, H2EHO, H2TmRPP, H2A2BCP, H2TZPP |
LB/ HMDS-covered glass | UV–vis spectroscopy | MeCO2H, butanone, ethyl acetate, HexSH, HexNH2, HepCHO, OctOH, OctNH2, NEt3, P(OMe)3 | 93 |
| array of H2TXPP, H2TYPP H2TPPP-Br, H2A2B2P |
LB/ HMDS-covered glass | UV–vis spectroscopy | MeCO2H, butanone, ethyl acetate, HexSH, HexNH2, HepCHO, OctOH, OctNH2, NEt3, P(OMe)3 | 93 |
| ZnTPP | LS/ glass | UV–vis spectroscopy | Py | 77 |
| MnTPPCl | LB/ glass | Reflectance spectroscopy | EtOH | 90 |
| RuTPP+ arachidic acidb |
LB/ glass substrate | Reflectance spectroscopy | MeOH, EtOH, iPrOH | 81 |
| Porphyrin precursor | Solid support | VOCs | Ref |
|---|---|---|---|
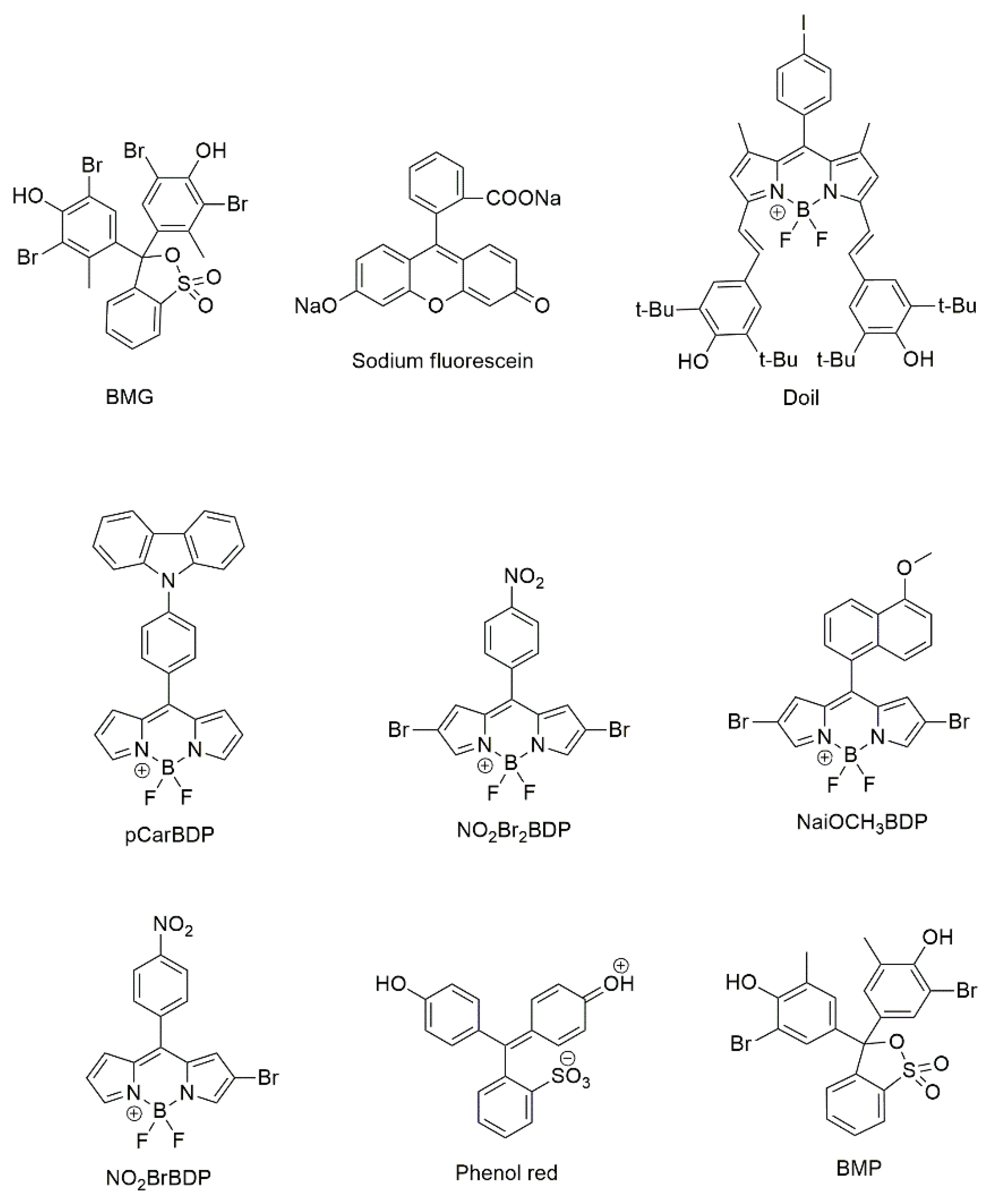
| ZnTPP, MnOEP BMGa |
reverse-phase silica gel plate | ethanol, ethyl acetate and acetic acid | 116 |
| H2TPP, ZnTPP, EuTPP, H2TTPS1, H2TF5PP Sodium fluoresceina |
PMMA plate | p-xylene, isoprene, styrene, and hexanal | 126 |
| H2TPP, CuTPP, ZnTPP, MnTPP, PdTPP, H2TPP-Cl, ZnTPP-Cl, ZnTPP-F, ZnTMPP BCGa |
reverse-phase silica gel plate | 2,4,5-Trimethyloxazole | 127 |
| FeTPP, NiTPP, CoTPP, PdTPP, MnOEP, FeTMPP, FeIITMPP, MnTSPP, H2TSPP Doila, pCarDPMa, NO2Br2BDPa, NaiOCH3BDPa, and 4 other dyes |
silica gel plate | VOCs formed in the storage process of oysters | 128 |
| H2TPP, FeTPP, MnTPP, CuTPP, VTPP, H2TMPP FeTMPP, H2OEP,MnOEP, PdOEP, Phenol reda Bromocresol purplea |
reverse-phase silica gel plate | VOCs formed in the storage process of beef | 129 |
| PCN-222b, PCN-222(Ag), PCN-222(Zn), PCN-222(Fe), PCN-222(Cu) PCN-222(Co) |
PDMSc membrane | acetone, CHCl3, CH2Cl2, EtOH, PenCHO, BuNH2, THF, toluene, DNT |
56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





