Submitted:
15 October 2024
Posted:
16 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
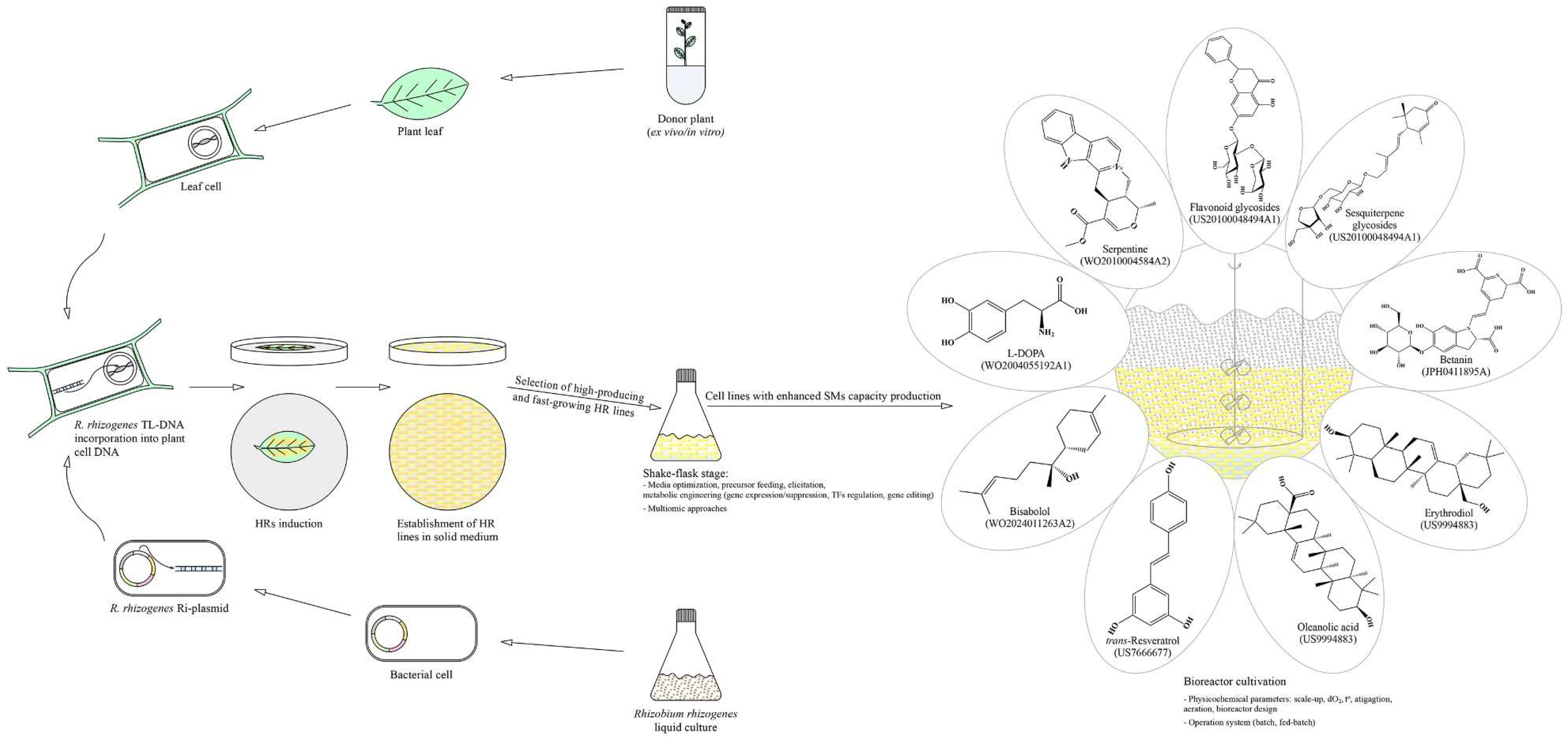
2. Rhizobium Rhizogenes Mediated Genetic Transformation and Hairy Roots Untapped Potential
3. Plant Biotechnology Toolkit to Enhance Specialized Metabolite Production
3.1. Bacterial Strains
3.2. Nutrient Medium Optimization
3.3. Precursor Feeding
3.4. Elicitation Treatment
| Metabolite(s) | Plant species | Metabolite level, mg/g DW | Yield increase, folds | Elicitor, µM | Reference |
|---|---|---|---|---|---|
| Alkaloids | |||||
| Ajmalicine | C. roseus | 15.4 | 2.5 | MeJA, 108.85 + JA, 134.08 | [68] |
| Codeine | Papaver armeniacum (L.) DC. | 0.12 | 2. | MeJA, 100 | [69] |
| Hyoscyamine |
D. stramonium L. D. innoxia Mill D. tatula L. |
11.58 8.89 17.94 |
1.4 2.8 2.1 |
ASA, 100 | [64] |
| Indole alkaloids | Isatis tinctoria L. | 3.15 | 5.9 | SA, 142.61 | [70] |
| Morphine | P. armeniacum | 0.15 | 4.0 | MeJA, 100 | [69] |
| Paclitaxel | Taxus x media var. Hicksii | 1.43 | 3.0 | MeJA, 100 | [71] |
| Scopolamine | A. luridus Link | 0.068 | 2.0 | ASA, 100 | [72] |
| Scopolamine | Atropa acuminata Royle ex Lindl. | 10.95 | 10.0 | COR, 0.5 | [73] |
| Solasodine | Solanum trilobatum L. | 9.33 | 1.9 | MeJA, 4 | [74] |
| Wilforine | Tripterygium wilfordii Hook. f. | 0.23 | 6.7 | MeJA, 50 | [75] |
| Terpenes | |||||
| Astragaloside | Astragalus membranaceus Bunge | 5.5 | 2.1 | MeJA, 157.4 | [76] |
| Baccatin III | T. media | 0.076 | 3.0 | COR, 1 | [71] |
| Cryptotanshinone | Salvia miltiorrhiza Bunge | 0.65 |
6.2 |
MeJA, 100 | [77] |
| Friedelin Еpifriedelanol | Cannabis sativa L. | 1.56 5.02 |
2.88 5.22 |
SA, 50 SA, 100 |
[78] |
| Ginsenosides | Panax ginseng C.A.Mey. | 0.42 | 3.8 | MeJA, 100 | [79] |
| Glycyrrhizin | Glycyrrhiza inflata Batalin | 34.79 | 5.7 | MeJA, 100 | [80] |
| Madecassoside | Centella asiatica (L.) Urban | 116 125 |
80 90 |
COR, 1 COR,1 + MeJA, 100 |
[81] |
| Oleanolic acid glycosides | Calendula officinalis L. | 52.52 | 3.0 | MeJA, 100 | [82] |
| Rhinacanthin | Rhinacanthus nasutus (L.) Kurz | 6.3 | 1.7 | MeJA, 10 | [83] |
| Tanshinone I | S. miltiorrhiza | 0.46 | 3.4 | MeJA, 100 | [77] |
| Tanshinones | S. miltiorrhiza | 11.33 | 3.1 | MeJA, 100 | [84] |
| Triptolide |
T. wilfordii | 0.04 |
2.0 |
MeJA, 50 | [75] |
| Triterpenoid saponins | C. asiatica | 60 | 6 | MeJA, 400 | [85] |
| Triterpenoid saponins | Psammosilene tunicoides W.C.Wu & C.Y.Wu | 15.0 | 4.55 | CS, 131.01 | [86] |
| Withaferin A | W. somnifera | 13.21 | 5.6 | MeJA, 15 | [87] |
| Withaferin A | W. somnifera | 9.57 17.45 |
6.8 12.5 |
MeJA, 100 β-CD 5.0 mM + MeJA, 100 |
[88] |
| Phenolic compounds | |||||
| Apigenin | Ficus carica cv. Siah | 0.12 | 51.96 | MeJA, 300 | [89] |
| Caffeic acid | Mentha spicata L. | 0.16 | 1.47 | MeJA, 100 | [90] |
| Caffeic acid | F. carica | 0.18 | 9.0 | MeJA, 100 | [89] |
| Caffeic acid | S. miltiorrhiza | 4.55 | 14.06 | COR, 0.1 | [91] |
| Chlorogenic acid | F. carica | 6.5 | 10.5 | MeJA, 100 | [89] |
| Chlorogenic acid | M. spicata | 0.015 | 1.7 | MeJA, 100 | [90] |
| Cinanmic acid | M. spicata | 0.043 | 1.98 | MeJA, 100 | [90] |
| Gallic acid | F. carica | 0.71 | 5.9 | MeJA, 100 | [89] |
| Salvianolic acid | S. miltiorrhiza | 2.15 | 7.96 | COR, 0.1 | [91] |
| Salvianolic acid | S. przewalskii | 21.5 | 8.4 | MeJA, 400 | [92] |
| Salvianolic acid | S. virgata | 2.11 | 3.76 | MeJA, 100 | [93] |
| Rosmarinic acid | F. carica | 1.84 | 12.3 | MeJA, 200 | [89] |
| Rosmarinic acid |
M. spicata | 11.84 |
0.06 |
MeJA, 100 |
[90] |
| Rosmarinic acid | Prunella vulgaris L. | 58.3 | 1.66 | MeJA, 50 | [94] |
| Rosmarinic acid | S. miltiorrhiza | 8.01 | 18.63 | COR, 0.1 | [91] |
| Rosmarinic acid |
S. przewalskii | 67.13 | 1.9 | MeJA, 400 | [92] |
| Rosmarinic acid | S. virgata | 18.45 |
1.81 |
MeJA, 100 | [93] |
| Rutin | F. carica | 0.03 |
3.34 |
MeJA, 200 |
[89] |
| Quercetin | F. carica | 0.2 |
6.91 | MeJA, 300 | [89] |
| ASA – acetylsalicylic acid; β-CD – β-cyclodextrin; COR – coronatine; JA – jasmonic acid; MeJA – methyl jasmonate; SA – salicylic acid | |||||
3.5. Molecular Tools for Engineering Plant Secondary Metabolism
3.5.1. Metabolic Engineering
3.5.2. CRISPR/Cas9
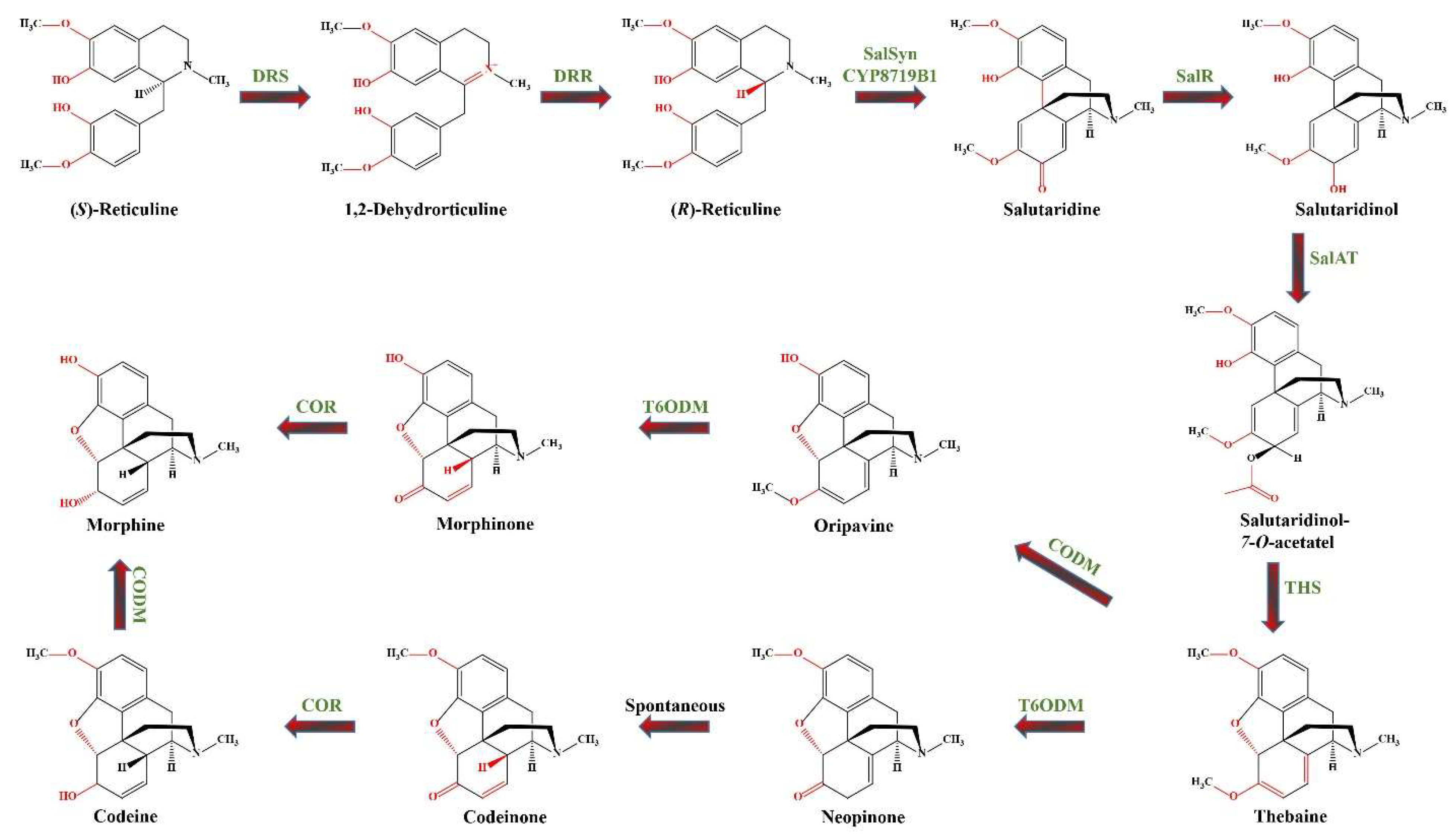
3.5.3. Case Study of the Morphinan Alkaloids Biosynthetic Pathway
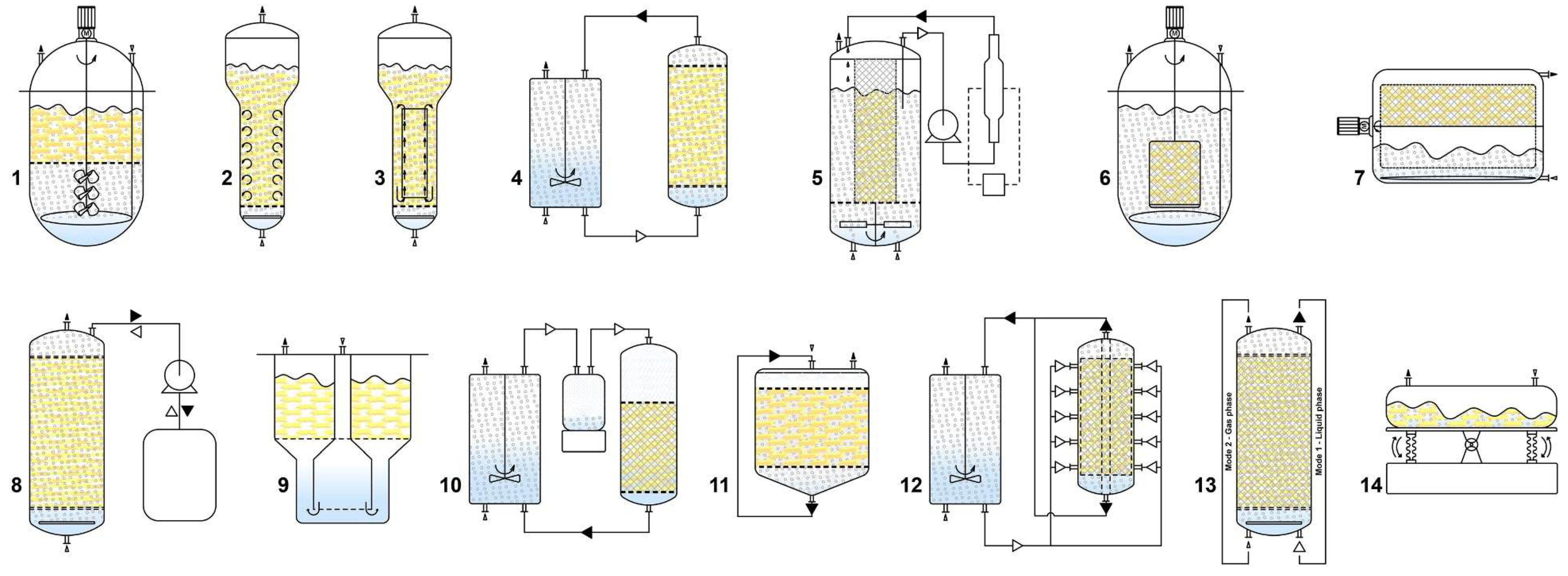
4. Bioreactor Technology for Hairy Roots Cultivation
4.1. Bioreactor Design and Process Intensification
4.2. Hairy Roots Multiple Applications, Recent Patents and Commercial Examples
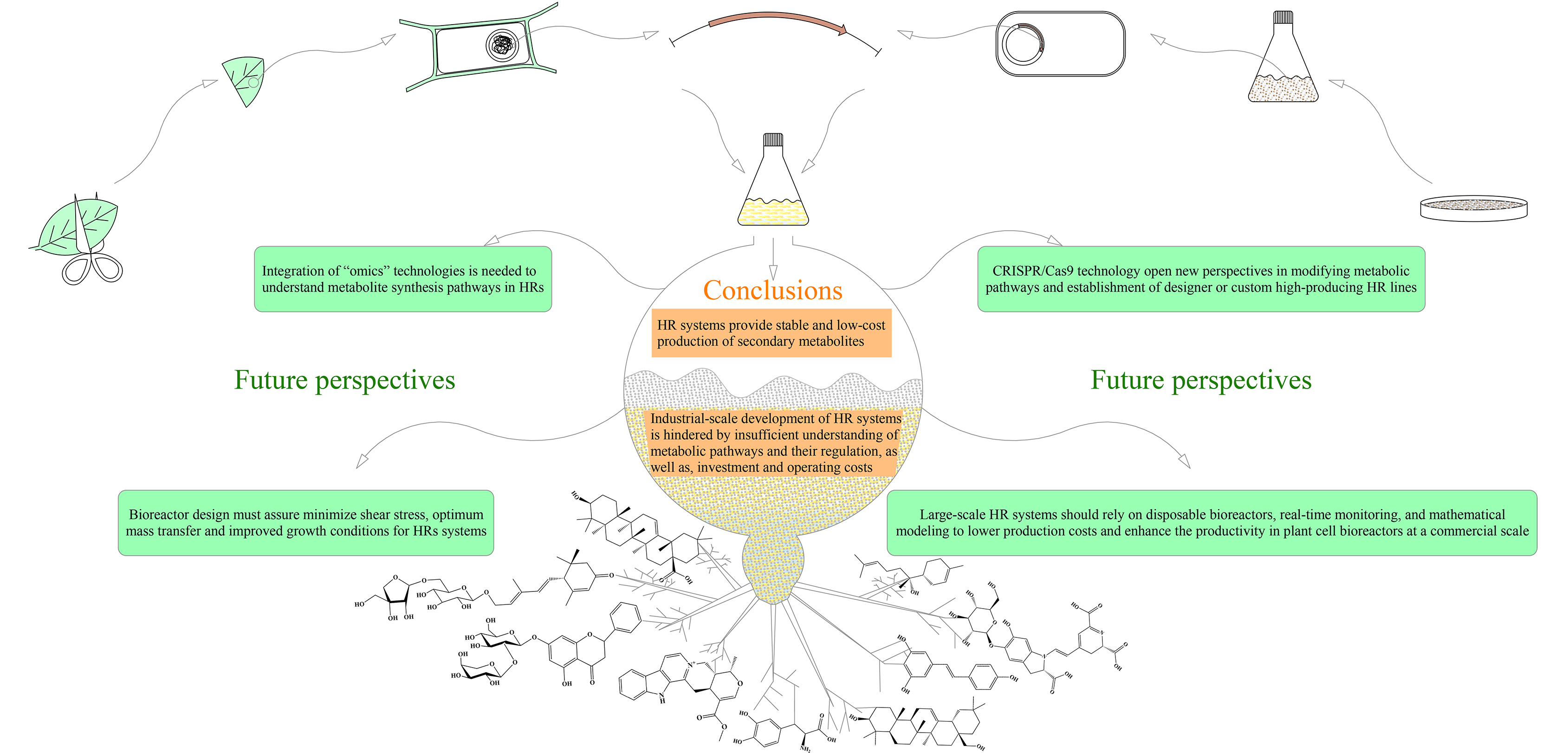
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALR | Airlift reactor |
| ASA | Acetylsalicylic acid |
| BCR | Bubble column reactor |
| CFR | Convective flow reactor |
| COR | Coronatine |
| CRISPR/Cas9 | Clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 |
| CS | Chitosan |
| DW | Dry weight |
| EFBR | Ebb and flow reactor |
| EMA | European medicinal agency |
| FDA | Food and drug administration |
| hGH1 | Human growth hormone |
| HRs | Hairy roots |
| JA | Jasmonic acid |
| L-DOPA | L-dihydroxyphenylalanine |
| MeJA | Methyl jasmonate |
| NPs | Natural products |
| ORFs | Open reading frames |
| RDR | Rotating drum reactor |
| RFR | Radial flow reactor |
| Ri-plasmid | Root-inducing plasmid |
| SA | Salicylic acid |
| SMs | Specialized metabolites |
| STR | Stirred tank reactor |
| TBR | Trickle bed reactor |
| T-DNA | Transfer DNA |
| TIAs | Terpenoid indole alkaloids |
| TFs | Transcription factors |
References
- Halder, M.; Sarkar, S.; Jha, S. Elicitation: A Biotechnological Tool for Enhanced Production of Secondary Metabolites in Hairy Root Cultures. Eng. Life Sci. 2019, 19, 880–895. [Google Scholar] [CrossRef] [PubMed]
- Biswas, D.; Chakraborty, A.; Mukherjee, S.; Ghosh, B. Hairy Root Culture: A Potent Method for Improved Secondary Metabolite Production of Solanaceous Plants. Front. Plant Sci. 2023, 14, 1197555. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Pati, P.K. Stress-Induced Metabolite Production Utilizing Plant Hairy Roots. In Hairy Roots; Srivastava, V., Mehrotra, S., Mishra, S., Eds.; Springer Singapore: Singapore, 2018. [Google Scholar] [CrossRef]
- Trehan, S.; Michniak-Kohn, B.; Beri, K. Plant Stem Cells in Cosmetics: Current Trends and Future Directions. Future Sci. OA 2017, 3, FSO226. [Google Scholar] [CrossRef] [PubMed]
- Katyal, P. Plant Secondary Metabolites: Functions in Plants and Pharmacological Importance. In Plant Secondary Metabolites; Sharma, A.K., Sharma, A., Eds.; Springer Nature Singapore: Singapore, 2022. [Google Scholar] [CrossRef]
- Koehn, F.E.; Carter, G.T. The Evolving Role of Natural Products in Drug Discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef]
- Agarwal, G.; Carcache, P. J. B.; Addo, E. M.; Kinghorn, A. D. Current Status and Contemporary Approaches to the Discovery of Antitumor Agents from Higher Plants. Biotechnol. Adv. 2020, 38, 107337. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef]
- Sharma, S. B.; Gupta, R. Drug Development from Natural Resource: A Systematic Approach. Mini-Rev. Med. Chem. 2015, 15, 52–57. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products As Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Newman, D. J.; Cragg, G. M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Namdeo, A. G.; Ingawale, D.K. Ashwagandha: Advances in Plant Biotechnological Approaches for Propagation and Production of Bioactive Compounds. J. Ethnopharmacol. 2021, 271, 113709. [Google Scholar] [CrossRef]
- Han, J.-E.; Park, Y.-J.; Lee, H.; Jeong, Y.-J.; Park, S.-Y. Increased Brazzein Expression by Abiotic Stress and Bioreactor Culture System for the Production of Sweet Protein, Brazzein. Plant Biotechnol. Rep. 2020, 14, 459–466. [Google Scholar] [CrossRef]
- Schmitz, C.; Fritsch, L.; Fischer, R.; Schillberg, S.; Rasche, S. Statistical Experimental Designs for the Production of Secondary Metabolites in Plant Cell Suspension Cultures. Biotechnol. Lett. 2016, 38, 2007–2014. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. W.; Wu, J. Y. Effective Elicitors and Process Strategies for Enhancement of Secondary Metabolite Production in Hairy Root Cultures. In Biotechnology of Hairy Root Systems; Advances in Biochemical Engineering/Biotechnology; Doran, P. M., Ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Häkkinen, S.T.; Oksman-Caldentey, K.-M. Progress and Prospects of Hairy Root Research. In Hairy Roots; Srivastava, V., Mehrotra, S., Mishra, S., Eds.; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Srivastava, S.; Srivastava, A.K. Hairy Root Culture for Mass-Production of High-Value Secondary Metabolites. Crit. Rev. Biotechnol. 2007, 27, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Paolis, A. D.; Frugis, G.; Giannino, D.; Iannelli, M.A.; Mele, G.; Rugini, E.; Silvestri, C.; Sparvoli, F.; Testone, G.; Mauro, M. L.; et al. Plant Cellular and Molecular Biotechnology: Following Mariotti’s Steps. Plants 2019, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Valdes, N.; Häkkinen, S. T.; Lemasson, C.; Guillet, M.; Oksman-Caldentey, K.-M.; Ritala, A.; Cardon, F. Hairy Root Cultures—A Versatile Tool With Multiple Applications. Front. Plant Sci. 2020, 11, 33. [Google Scholar] [CrossRef]
- Rawat, J.M.; Bhandari, A.; Raturi, M.; Rawat, B. Agrobacterium Rhizogenes Mediated Hairy Root Cultures: A Promising Approach for Production of Useful Metabolites. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 103–118. [Google Scholar] [CrossRef]
- Mauro, M.L.; Costantino, P.; Bettini, P.P. The Never Ending Story of Rol Genes: A Century After. Plant Cell Tissue Organ Cult. PCTOC 2017, 131, 201–212. [Google Scholar] [CrossRef]
- Bahramnejad, B.; Naji, M.; Bose, R.; Jha, S. A Critical Review on Use of Agrobacterium Rhizogenes and Their Associated Binary Vectors for Plant Transformation. Biotechnol. Adv. 2019, 37, 107405. [Google Scholar] [CrossRef]
- Kim, Y.; Wyslouzil, B.E.; Weathers, P.J. Secondary Metabolism of Hairy Root Cultures in Bioreactors. Vitro Cell. Dev. Biol. - Plant 2002, 38, 1–10. [Google Scholar] [CrossRef]
- Mishra, B.N.; Ranjan, R. Growth of Hairy-root Cultures in Various Bioreactors for the Production of Secondary Metabolites. Biotechnol. Appl. Biochem. 2008, 49, 1–10. [Google Scholar] [CrossRef]
- Häkkinen, S. T.; Ritala, A. Medicinal Compounds Produced in Plant Cell Factories. In Medicinal plant biotechnology; Arora, R., Ed.; CABI: UK, 2010. [Google Scholar] [CrossRef]
- Stoger, E.; Fischer, R.; Moloney, M.; Ma, J. K.-C. Plant Molecular Pharming for the Treatment of Chronic and Infectious Diseases. Annu. Rev. Plant Biol. 2014, 65, 743–768. [Google Scholar] [CrossRef]
- Banerjee, S.; Singh, S.; Rahman, L.U. Biotransformation Studies Using Hairy Root Cultures — A Review. Biotechnol. Adv. 2012, 30, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Ono, N.N.; Tian, L. The Multiplicity of Hairy Root Cultures: Prolific Possibilities. Plant Sci. 2011, 180, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Talano, M.; Laura Wevar Oller, A.; Gonzalez, P.S.; Agostini, E. Hairy Roots, Their Multiple Applications and Recent Patents. Recent Pat. Biotechnol. 2012, 6, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Du, M. Hairy Root and Its Application in Plant Genetic Engineering. J. Integr. Plant Biol. 2006, 48, 121–127. [Google Scholar] [CrossRef]
- Halder, M.; Roychowdhury, D.; Jha, S. A Critical Review on Biotechnological Interventions for Production and Yield Enhancement of Secondary Metabolites in Hairy Root Cultures. In Hairy Roots; Srivastava, V., Mehrotra, S., Mishra, S., Eds.; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Chen, Y.-K.; Li, X.-S.; Yang, G.-Y.; Chen, Z.-Y.; Hu, Q.-F.; Miao, M.-M. Phenolic Compounds from Nicotiana Tabacum and Their Biological Activities. J. Asian Nat. Prod. Res. 2012, 14, 450–456. [Google Scholar] [CrossRef]
- Mishra, B. B.; Gautam, S.; Sharma, A. Purification and Characterisation of Polyphenol Oxidase (PPO) from Eggplant (Solanum Melongena). Food Chem. 2012, 134, 1855–1861. [Google Scholar] [CrossRef]
- Tusevski, O.; Stanoeva, J.; Stefova, M.; Kungulovski, D.; Pancevska, N.; Sekulovski, N.; Panov, S.; Simic, S. Hairy Roots of Hypericum Perforatum L.: A Promising System for Xanthone Production. Open Life Sci. 2013, 8, 1010–1022. [Google Scholar] [CrossRef]
- Anton, D.; Bender, I.; Kaart, T.; Roasto, M.; Heinonen, M.; Luik, A.; Püssa, T. Changes in Polyphenols Contents and Antioxidant Capacities of Organically and Conventionally Cultivated Tomato (Solanum Lycopersicum L.) Fruits during Ripening. Int. J. Anal. Chem. 2017, 2017, 2367453. [Google Scholar] [CrossRef]
- Sena, L.M.; Zappelli, C.; Apone, F.; Barbulova, A.; Tito, A.; Leone, A.; Oliviero, T.; Ferracane, R.; Fogliano, V.; Colucci, G. Brassica Rapa Hairy Root Extracts Promote Skin Depigmentation by Modulating Melanin Production and Distribution. J. Cosmet. Dermatol. 2018, 17, 246–257. [Google Scholar] [CrossRef]
- Ritala, A.; Dong, L.; Imseng, N.; Seppänen-Laakso, T.; Vasilev, N.; Van Der Krol, S.; Rischer, H.; Maaheimo, H.; Virkki, A.; Brändli, J.; et al. Evaluation of Tobacco (Nicotiana Tabacum L. Cv. Petit Havana SR1) Hairy Roots for the Production of Geraniol, the First Committed Step in Terpenoid Indole Alkaloid Pathway. J. Biotechnol. 2014, 176, 20–28. [Google Scholar] [CrossRef]
- Rogowska, A.; Szakiel, A. Enhancement of Phytosterol and Triterpenoid Production in Plant Hairy Root Cultures—Simultaneous Stimulation or Competition? Plants 2021, 10, 2028. [Google Scholar] [CrossRef] [PubMed]
- El Jaber-Vazdekis, N.; Barres, M. L.; Ravelo, Á. G.; Zárate, R. Effects of Elicitors on Tropane Alkaloids and Gene Expression in Atropa Baetica Transgenic Hairy Roots. J. Nat. Prod. 2008, 71, 2026–2031. [Google Scholar] [CrossRef] [PubMed]
- Kai, G.; Yang, S.; Luo, X.; Zhou, W.; Fu, X.; Zhang, A.; Zhang, Y.; Xiao, J. Co-Expression of AaPMT and AaTRI Effectively Enhances the Yields of Tropane Alkaloids in Anisodus Acutangulus Hairy Roots. BMC Biotechnol. 2011, 11, 43. [Google Scholar] [CrossRef]
- Dehghan, E.; Reed, D.W.; Covello, P.S.; Hasanpour, Z.; Palazon, J.; Oksman-Caldentey, K.-M.; Ahmadi, F.S. Genetically Engineered Hairy Root Cultures of Hyoscyamus Senecionis and H. Muticus: Ploidy as a Promising Parameter in the Metabolic Engineering of Tropane Alkaloids. Plant Cell Rep. 2017, 36, 1615–1626. [Google Scholar] [CrossRef]
- Banerjee, S. Voyaging through Chromosomal Studies in Hairy Root Cultures towards Unravelling Their Relevance to Medicinal Plant Research: An Updated Review. The Nucleus 2018, 61, 3–18. [Google Scholar] [CrossRef]
- Grzegorczyk, I.; Królicka, A.; Wysokińska, H. Establishment of Salvia Officinalis L. Hairy Root Cultures for the Production of Rosmarinic Acid. Z. Für Naturforschung C 2006, 61, 351–356. [Google Scholar] [CrossRef]
- Wang, Q.; Pi, Y.; Hou, R.; Jiang, K.; Huang, Z.; Hsieh, M.; Sun, X.; Tang, K. Molecular Cloning and Characterization of 1-Hydroxy-2-Methyl-2-(E)-Butenyl 4-Diphosphate Reductase (CaHDR) from Camptotheca Acuminata and Its Functional Identification in Escherichia Coli. BMB Rep. 2008, 41, 112–118. [Google Scholar] [CrossRef]
- Ya-ut, P.; Chareonsap, P.; Sukrong, S. Micropropagation and Hairy Root Culture of Ophiorrhiza Alata Craib for Camptothecin Production. Biotechnol. Lett. 2011, 33, 2519–2526. [Google Scholar] [CrossRef]
- Kochan, E.; Szymańska, G.; Szymczyk, P. Effect of Sugar Concentration on Ginsenoside Biosynthesis in Hairy Root Cultures of Panax Quinquefolium Cultivated in Shake Flasks and Nutrient Sprinkle Bioreactor. Acta Physiol. Plant. 2014, 36, 613–619. [Google Scholar] [CrossRef]
- Kochan, E.; Szymczyk, P.; Kuźma, Ł.; Szymańska, G. Nitrogen and Phosphorus as the Factors Affecting Ginsenoside Production in Hairy Root Cultures of Panax Quinquefolium Cultivated in Shake Flasks and Nutrient Sprinkle Bioreactor. Acta Physiol. Plant. 2016, 38, 149. [Google Scholar] [CrossRef]
- Kochan, E.; Szymczyk, P.; Kuźma, Ł.; Szymańska, G.; Wajs-Bonikowska, A.; Bonikowski, R.; Sienkiewicz, M. The Increase of Triterpene Saponin Production Induced by Trans-Anethole in Hairy Root Cultures of Panax Quinquefolium. Molecules 2018, 23, 2674. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.; Furukawa, H.; Fujioka, T.; Fujii, H.; Mihashi, K.; Shimomura, K.; Ishimaru, K. Flavone Production in Transformed Root Cultures of Scutellaria Baicalensis Georgi. Phytochemistry 1999, 52, 885–890. [Google Scholar] [CrossRef]
- Praveen, N.; Murthy, H.N. Withanolide A Production from Withania Somnifera Hairy Root Cultures with Improved Growth by Altering the Concentrations of Macro Elements and Nitrogen Source in the Medium. Acta Physiol. Plant. 2013, 35, 811–816. [Google Scholar] [CrossRef]
- Javid, A.; Gampe, N.; Gelana, F.; György, Z. Enhancing the Accumulation of Rosavins in Rhodiola Rosea L. Plants Grown In Vitro by Precursor Feeding. Agronomy 2021, 11, 2531. [Google Scholar] [CrossRef]
- Grech-Baran, M.; Sykłowska-Baranek, K.; Giebułtowicz, J.; Wroczyński, P.; Pietrosiuk, A. Tyrosol Glucosyltransferase Activity and Salidroside Production in Natural and Transformed Root Cultures of Rhodiola Kirilowii (Regel) Regel Et Maximowicz. Acta Biol. Cracoviensia Ser. Bot. 2013, 55. [Google Scholar] [CrossRef]
- Grech-Baran, M.; Sykłowska-Baranek, K.; Krajewska-Patan, A.; Wyrwał, A.; Pietrosiuk, A. Biotransformation of Cinnamyl Alcohol to Rosavins by Non-Transformed Wild Type and Hairy Root Cultures of Rhodiola Kirilowii. Biotechnol. Lett. 2014, 36, 649–656. [Google Scholar] [CrossRef]
- Böhm, H.; Mäck, G. Betaxanthin Formation and Free Amino Acids in Hairy Roots of Beta Vulgaris Var. Lutea Depending on Nutrient Medium and Glutamate or Glutamine Feeding. Phytochemistry 2004, 65, 1361–1368. [Google Scholar] [CrossRef]
- Peebles, C.A.M.; Hong, S.; Gibson, S.I.; Shanks, J.V.; San, K. Effects of Terpenoid Precursor Feeding on Catharanthus Roseus Hairy Roots Over-expressing the Alpha or the Alpha and Beta Subunits of Anthranilate Synthase. Biotechnol. Bioeng. 2006, 93, 534–540. [Google Scholar] [CrossRef]
- Peebles, C.A.M.; Sander, G.W.; Hughes, E.H.; Peacock, R.; Shanks, J.V.; San, K.-Y. The Expression of 1-Deoxy-d-Xylulose Synthase and Geraniol-10-Hydroxylase or Anthranilate Synthase Increases Terpenoid Indole Alkaloid Accumulation in Catharanthus Roseus Hairy Roots. Metab. Eng. 2011, 13, 234–240. [Google Scholar] [CrossRef]
- Shameh, S.; Hosseini, B.; Palazon, J. Genetic Engineering of Tropane Alkaloid Biosynthesis of Hyoscyamus Reticulatus L. Hairy Roots by Pmt Gene Overexpression and Feeding with Putrescine. Ind. Crops Prod. 2021, 170, 113716. [Google Scholar] [CrossRef]
- Ooi, C. T.; Syahida, A.; Stanslas, J.; Maziah, M. The Influence of Methyl Jasmonate, Cholesterol and l -arginine on Solasodine Production in Hairy Root Culture of Solanum Mammosum. Eng. Life Sci. 2016, 16, 432–442. [Google Scholar] [CrossRef]
- Shakya, P.; Marslin, G.; Siram, K.; Beerhues, L.; Franklin, G. Elicitation as a Tool to Improve the Profiles of High-Value Secondary Metabolites and Pharmacological Properties of Hypericum Perforatum. J. Pharm. Pharmacol. 2018, 71, 70–82. [Google Scholar] [CrossRef]
- Radman, R.; Saez, T.; Bucke, C.; Keshavarz, T. Elicitation of Plants and Microbial Cell Systems. Biotechnol. Appl. Biochem. 2003, 37, 91–102. [Google Scholar] [CrossRef]
- Ochoa-Meza, L.C.; Quintana-Obregón, E.A.; Vargas-Arispuro, I.; Falcón-Rodríguez, A.B.; Aispuro-Hernández, E.; Virgen-Ortiz, J.J.; Martínez-Téllez, M.Á. Oligosaccharins as Elicitors of Defense Responses in Wheat. Polymers 2021, 13, 3105. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.; Palazon, J. Elicitation, an Effective Strategy for the Biotechnological Production of Bioactive High-Added Value Compounds in Plant Cell Factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Seybold, H.; Trempel, F.; Ranf, S.; Scheel, D.; Romeis, T.; Lee, J. Ca 2+ Signalling in Plant Immune Response: From Pattern Recognition Receptors to Ca 2+ Decoding Mechanisms. New Phytol. 2014, 204, 782–790. [Google Scholar] [CrossRef]
- Harfi, B.; Khelifi, L.; Khelifi-Slaoui, M.; Assaf-Ducrocq, C.; Gontier, E. Tropane Alkaloids GC/MS Analysis and Low Dose Elicitors’ Effects on Hyoscyamine Biosynthetic Pathway in Hairy Roots of Algerian Datura Species. Sci. Rep. 2018, 8, 17951. [Google Scholar] [CrossRef]
- Hashemi, S.M.; Naghavi, M.R. Production and Gene Expression of Morphinan Alkaloids in Hairy Root Culture of Papaver Orientale L. Using Abiotic Elicitors. Plant Cell Tissue Organ Cult. PCTOC 2016, 125, 31–41. [Google Scholar] [CrossRef]
- Karami, M.; Naghavi, M.R.; Nasiri, J.; Farzin, N. Methyl Jasmonate and β-Cyclodextrin Shake Hands to Boost Withaferin A Production from the Hairy Root Culture of Withania Somnifera. Preprint from Research Square. [CrossRef]
- Sivanandhan, G.; Kapil Dev, G.; Jeyaraj, M.; Rajesh, M.; Arjunan, A.; Muthuselvam, M.; Manickavasagam, M.; Selvaraj, N.; Ganapathi, A. Increased Production of Withanolide A, Withanone, and Withaferin A in Hairy Root Cultures of Withania Somnifera (L.) Dunal Elicited with Methyl Jasmonate and Salicylic Acid. Plant Cell Tissue Organ Cult. PCTOC 2013, 114, 121–129. [Google Scholar] [CrossRef]
- Thakore, D.; Srivastava, A.K.; Sinha, A.K. Model Based Fed Batch Cultivation and Elicitation for the Overproduction of Ajmalicine from Hairy Roots of Catharanthus Roseus. Biochem. Eng. J. 2015, 97, 73–80. [Google Scholar] [CrossRef]
- Sharifzadeh Naeini, M.; Naghavi, M. R.; Bihamta, M. R.; Sabokdast, M.; Salehi, M. Production of Some Benzylisoquinoline Alkaloids in Papaver Armeniacum L. Hairy Root Cultures Elicited with Salicylic Acid and Methyl Jasmonate. Vitro Cell. Dev. Biol. - Plant 2021, 57, 261–271. [Google Scholar] [CrossRef]
- Gai, Q.-Y.; Jiao, J.; Wang, X.; Zang, Y.-P.; Niu, L.-L.; Fu, Y.-J. Elicitation of Isatis Tinctoria L. Hairy Root Cultures by Salicylic Acid and Methyl Jasmonate for the Enhanced Production of Pharmacologically Active Alkaloids and Flavonoids. Plant Cell Tissue Organ Cult. PCTOC 2019, 137, 77–86. [Google Scholar] [CrossRef]
- Sykłowska-Baranek, K.; Pilarek, M.; Bonfill, M.; Kafel, K.; Pietrosiuk, A. Perfluorodecalin-Supported System Enhances Taxane Production in Hairy Root Cultures of Taxus x Media Var. Hicksii Carrying a Taxadiene Synthase Transgene. Plant Cell Tissue Organ Cult. PCTOC 2015, 120, 1051–1059. [Google Scholar] [CrossRef]
- Qin, B.; Ma, L.; Wang, Y.; Chen, M.; Lan, X.; Wu, N.; Liao, Z. Effects of Acetylsalicylic Acid and UV-B on Gene Expression and Tropane Alkaloid Biosynthesis in Hairy Root Cultures of Anisodus Luridus. Plant Cell Tissue Organ Cult. PCTOC 2014, 117, 483–490. [Google Scholar] [CrossRef]
- Fattahi, F.; Shojaeiyan, A.; Palazon, J.; Moyano, E.; Torras-Claveria, L. Methyl-β-cyclodextrin and Coronatine as New Elicitors of Tropane Alkaloid Biosynthesis in Atropa Acuminata and Atropa Belladonna Hairy Root Cultures. Physiol. Plant. 2021, 172, 2098–2111. [Google Scholar] [CrossRef]
- Shilpha, J.; Satish, L.; Kavikkuil, M.; Joe Virgin Largia, M.; Ramesh, M. Methyl Jasmonate Elicits the Solasodine Production and Anti-Oxidant Activity in Hairy Root Cultures of Solanum Trilobatum L. Ind. Crops Prod. 2015, 71, 54–64. [Google Scholar] [CrossRef]
- Zhu, C.; Miao, G.; Guo, J.; Huo, Y.; Zhang, X.; Xie, J.; Feng, J. Establishment of Tripterygium Wilfordii Hook. f. Hairy Root Culture and Optimization of Its Culture Conditions for the Production of Triptolide and Wilforine. J. Microbiol. Biotechnol. 2014, 24, 823–834. [Google Scholar] [CrossRef]
- Jiao, J.; Gai, Q.-Y.; Wang, W.; Luo, M.; Zu, Y.-G.; Fu, Y.-J.; Ma, W. Enhanced Astragaloside Production and Transcriptional Responses of Biosynthetic Genes in Astragalus Membranaceus Hairy Root Cultures by Elicitation with Methyl Jasmonate. Biochem. Eng. J. 2016, 105, 339–346. [Google Scholar] [CrossRef]
- Wang, C.H.; Zheng, L.P.; Tian, H.; Wang, J.W. Synergistic Effects of Ultraviolet-B and Methyl Jasmonate on Tanshinone Biosynthesis in Salvia Miltiorrhiza Hairy Roots. J. Photochem. Photobiol. B 2016, 159, 93–100. [Google Scholar] [CrossRef]
- Mahendran, G.; Vimolmangkang, S. Effect of Carbon Source and Elicitors on Biomass Production and Accumulation of Friedelin and Epifriedelanol in Hairy Roots of Hemp (Cannabis Sativa L.). Plant Cell Tissue Organ Cult. PCTOC 2024, 156, 61. [Google Scholar] [CrossRef]
- Kim, O.T.; Yoo, N.H.; Kim, G.S.; Kim, Y.C.; Bang, K.H.; Hyun, D.Y.; Kim, S.H.; Kim, M.Y. Stimulation of Rg3 Ginsenoside Biosynthesis in Ginseng Hairy Roots Elicited by Methyl Jasmonate. Plant Cell Tissue Organ Cult. PCTOC 2013, 112, 87–93. [Google Scholar] [CrossRef]
- Wongwicha, W.; Tanaka, H.; Shoyama, Y.; Putalun, W. Methyl Jasmonate Elicitation Enhances Glycyrrhizin Production in Glycyrrhiza Inflata Hairy Roots Cultures. Z. Für Naturforschung C 2011, 66, (7–8). [Google Scholar] [CrossRef]
- Alcalde, M. A.; Cusido, R. M.; Moyano, E.; Palazon, J.; Bonfill, M. Metabolic Gene Expression and Centelloside Production in Elicited Centella Asiatica Hairy Root Cultures. Ind. Crops Prod. 2022, 184, 114988. [Google Scholar] [CrossRef]
- Alsoufi, A. S. M.; Pączkowski, C.; Szakiel, A.; Długosz, M. Effect of Jasmonic Acid and Chitosan on Triterpenoid Production in Calendula Officinalis Hairy Root Cultures. Phytochem. Lett. 2019, 31, 5–11. [Google Scholar] [CrossRef]
- Cheruvathur, M.K.; Thomas, T.D. Effect of Plant Growth Regulators and Elicitors on Rhinacanthin Accumulation in Hairy Root Cultures of Rhinacanthus Nasutus (L.) Kurz. Plant Cell Tissue Organ Cult. PCTOC 2014, 118, 169–177. [Google Scholar] [CrossRef]
- Hao, X.; Shi, M.; Cui, L.; Xu, C.; Zhang, Y.; Kai, G. Effects of Methyl Jasmonate and Salicylic Acid on Tanshinone Production and Biosynthetic Gene Expression in Transgenic S Alvia Miltiorrhiza Hairy Roots. Biotechnol. Appl. Biochem. 2015, 62, 24–31. [Google Scholar] [CrossRef]
- Baek, S.; Ho, T.-T.; Lee, H.; Jung, G.; Kim, Y. E.; Jeong, C.-S.; Park, S.-Y. Enhanced Biosynthesis of Triterpenoids in Centella Asiatica Hairy Root Culture by Precursor Feeding and Elicitation. Plant Biotechnol. Rep. 2020, 14, 45–53. [Google Scholar] [CrossRef]
- Qiu, H.; Su, L.; Wang, H.; Zhang, Z. Chitosan Elicitation of Saponin Accumulation in Psammosilene Tunicoides Hairy Roots by Modulating Antioxidant Activity, Nitric Oxide Production and Differential Gene Expression. Plant Physiol. Biochem. 2021, 166, 115–127. [Google Scholar] [CrossRef]
- Saxena, P.; Ahlawat, S.; Ali, A.; Khan, S.; Abdin, M. Z. Gene Expression Analysis of the Withanolide Biosynthetic Pathway in Hairy Root Cultures of Withania Somnifera Elicited with Methyl Jasmonate and the Fungus Piriformospora Indica. Symbiosis 2017, 71, 143–154. [Google Scholar] [CrossRef]
- Karami, M.; Naghavi, M. R.; Nasiri, J.; Farzin, N.; Ignea, C. Enhanced Production of Withaferin A from the Hairy Root Culture of Withania Somnifera via Synergistic Effect of Methyl Jasmonate and β-Cyclodextrin. Plant Physiol. Biochem. 2024, 208, 108440. [Google Scholar] [CrossRef] [PubMed]
- Amani, S.; Mohebodini, M.; Khademvatan, S.; Jafari, M.; Kumar, V. Modifications in Gene Expression and Phenolic Compounds Content by Methyl Jasmonate and Fungal Elicitors in Ficus Carica. Cv. Siah Hairy Root Cultures. BMC Plant Biol. 2024, 24, 520. [Google Scholar] [CrossRef] [PubMed]
- Yousefian, S.; Lohrasebi, T.; Farhadpour, M.; Haghbeen, K. Effect of Methyl Jasmonate on Phenolic Acids Accumulation and the Expression Profile of Their Biosynthesis-Related Genes in Mentha Spicata Hairy Root Cultures. Plant Cell Tissue Organ Cult. PCTOC 2020, 142, 285–297. [Google Scholar] [CrossRef]
- Rastegarnejad, F.; Mirjalili, M. H.; Bakhtiar, Z. Enhanced Production of Tanshinone and Phenolic Compounds in Hairy Roots Culture of Salvia Miltiorrhiza Bunge by Elicitation. Plant Cell Tissue Organ Cult. PCTOC 2024, 156, 4. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Luo, L.; Cao, F.; Yang, B.; Gao, J.; Yan, Y.; Zhang, G.; Peng, L.; Hu, B. Increased Phenolic Acid and Tanshinone Production and Transcriptional Responses of Biosynthetic Genes in Hairy Root Cultures of Salvia Przewalskii Maxim. Treated with Methyl Jasmonate and Salicylic Acid. Mol. Biol. Rep. 2020, 47, 8565–8578. [Google Scholar] [CrossRef]
- Attaran Dowom, S.; Abrishamchi, P.; Radjabian, T.; Salami, S.A. Elicitor-Induced Phenolic Acids Accumulation in Salvia Virgata Jacq. Hairy Root Cultures. Plant Cell Tissue Organ Cult. PCTOC 2022, 148, 107–117. [Google Scholar] [CrossRef]
- Ru, M.; An, Y.; Wang, K.; Peng, L.; Li, B.; Bai, Z.; Wang, B.; Liang, Z. Prunella Vulgaris L. Hairy Roots: Culture, Growth, and Elicitation by Ethephon and Salicylic Acid. Eng. Life Sci. 2016, 16, 494–502. [Google Scholar] [CrossRef]
- Zaheer, M.; Reddy, V. D.; Giri, C. C. Enhanced Daidzin Production from Jasmonic and Acetyl Salicylic Acid Elicited Hairy Root Cultures of Psoralea Corylifolia L. (Fabaceae). Nat. Prod. Res. 2016, 30, 1542–1547. [Google Scholar] [CrossRef]
- Gai, Q.-Y.; Jiao, J.; Luo, M.; Wang, W.; Gu, C.-B.; Fu, Y.-J.; Ma, W. Tremendous Enhancements of Isoflavonoid Biosynthesis, Associated Gene Expression and Antioxidant Capacity in Astragalus Membranaceus Hairy Root Cultures Elicited by Methyl Jasmonate. Process Biochem. 2016, 51, 642–649. [Google Scholar] [CrossRef]
- Martin, K. P.; Sabovljevic, A.; Madassery, J. High-Frequency Transgenic Plant Regeneration and Plumbagin Production through Methyl Jasmonate Elicitation from Hairy Roots of Plumbago Indica L. J. Crop Sci. Biotechnol. 2011, 14, 205–212. [Google Scholar] [CrossRef]
- Gharechahi, J.; Khalili, M.; Hasanloo, T.; Salekdeh, G.H. An Integrated Proteomic Approach to Decipher the Effect of Methyl Jasmonate Elicitation on the Proteome of Silybum Marianum L. Hairy Roots. Plant Physiol. Biochem. 2013, 70, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Theboral, J.; Sivanandhan, G.; Subramanyam, K.; Arun, M.; Selvaraj, N.; Manickavasagam, M.; Ganapathi, A. Enhanced Production of Isoflavones by Elicitation in Hairy Root Cultures of Soybean. Plant Cell Tissue Organ Cult. PCTOC 2014, 117, 477–481. [Google Scholar] [CrossRef]
- Zaheer, M.; Giri, C.C. Enhanced Diterpene Lactone (Andrographolide) Production from Elicited Adventitious Root Cultures of Andrographis Paniculata. Res. Chem. Intermed. 2017, 43, 2433–2444. [Google Scholar] [CrossRef]
- Liang, Z.-S.; Yang, D.-F.; Liang, X.; Zhang, Y.-J.; Liu, Y.; Liu, F.-H. Roles of Reactive Oxygen Species in Methyl Jasmonate and Nitric Oxide-Induced Tanshinone Production in Salvia Miltiorrhiza Hairy Roots. Plant Cell Rep. 2012, 31, 873–883. [Google Scholar] [CrossRef]
- Piątczak, E.; Kuźma, Ł.; Wysokińska, H. The Influence of Methyl Jasmonate and Salicylic Acid on Secondary Metabolite Production in Rehmannia Glutinosa Libosch. Hairy Root Culture. Acta Biol. Cracoviensia Bot. 2016, 58, 57–65. [Google Scholar] [CrossRef]
- Yu, H.-S.; Ma, L.-Q.; Zhang, J.-X.; Shi, G.-L.; Hu, Y.-H.; Wang, Y.-N. Characterization of Glycosyltransferases Responsible for Salidroside Biosynthesis in Rhodiola Sachalinensis. Phytochemistry 2011, 72, 862–870. [Google Scholar] [CrossRef]
- Lan, X.; Chang, K.; Zeng, L.; Liu, X.; Qiu, F.; Zheng, W.; Quan, H.; Liao, Z.; Chen, M.; Huang, W.; et al. Engineering Salidroside Biosynthetic Pathway in Hairy Root Cultures of Rhodiola Crenulata Based on Metabolic Characterization of Tyrosine Decarboxylase. PLoS ONE 2013, 8, e75459. [Google Scholar] [CrossRef]
- Liu, X.; Tang, Y.; Zeng, J.; Qin, J.; Lin, M.; Chen, M.; Liao, Z.; Lan, X. Biochemical Characterization of Tyrosine Aminotransferase and Enhancement of Salidroside Production by Suppressing Tyrosine Aminotransferase in Rhodiola Crenulata. Ind. Crops Prod. 2021, 173, 114075. [Google Scholar] [CrossRef]
- Marchev, A. S.; Yordanova, Z. P.; Georgiev, M. I. Green (Cell) Factories for Advanced Production of Plant Secondary Metabolites. Crit. Rev. Biotechnol. 2020, 40, 443–458. [Google Scholar] [CrossRef]
- Marchev, A. S.; Georgiev, M. I. Plant In Vitro Systems as a Sustainable Source of Active Ingredients for Cosmeceutical Application. Molecules 2020, 25, 2006. [Google Scholar] [CrossRef]
- Ni, X.; Wen, S.; Wang, W.; Wang, X.; Xu, H.; Kai, G. Enhancement of Camptothecin Production in Camptotheca Acuminata Hairy Roots by Overexpressing ORCA3 Gene. J. Appl. Pharm. Sci. 2011, 85–88. [Google Scholar]
- Wang, C.-T.; Liu, H.; Gao, X.-S.; Zhang, H.-X. Overexpression of G10H and ORCA3 in the Hairy Roots of Catharanthus Roseus Improves Catharanthine Production. Plant Cell Rep. 2010, 29, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Tan, H.; Lv, Z.; Ji, Q.; Huang, Y.; Liu, J.; Chen, D.; Diao, Y.; Si, J.; Zhang, L. Targeted Expression of Vitreoscilla Hemoglobin Improves the Production of Tropane Alkaloids in Hyoscyamus Niger Hairy Roots. Sci. Rep. 2018, 8, 17969. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhu, T.; Zhang, R.; Bu, Q.; Yin, J.; Zhang, L.; Chen, W. Molecular Cloning and Functional Analysis of Hyoscyamine 6β-Hydroxylase (H6H) in the Poisonous and Medicinal Plant Datura Innoxia Mill. Plant Physiol. Biochem. 2020, 153, 11–19. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Kim, J.K.; Kim, Y.B.; Lee, S.; Kim, S.-U.; Park, S.U. Enhanced Accumulation of Phytosterol and Triterpene in Hairy Root Cultures of Platycodon Grandiflorum by Overexpression of Panax Ginseng 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase. J. Agric. Food Chem. 2013, 61, 1928–1934. [Google Scholar] [CrossRef]
- Kai, G.; Xu, H.; Zhou, C.; Liao, P.; Xiao, J.; Luo, X.; You, L.; Zhang, L. Metabolic Engineering Tanshinone Biosynthetic Pathway in Salvia Miltiorrhiza Hairy Root Cultures. Metab. Eng. 2011, 13, 319–327. [Google Scholar] [CrossRef]
- Sitarek, P.; Kowalczyk, T.; Rijo, P.; Białas, A. J.; Wielanek, M.; Wysokińska, H.; Garcia, C.; Toma, M.; Śliwiński, T.; Skała, E. Over-Expression of AtPAP1 Transcriptional Factor Enhances Phenolic Acid Production in Transgenic Roots of Leonurus Sibiricus L. and Their Biological Activities. Mol. Biotechnol. 2018, 60, 74–82. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Krzemińska, M.; Grąbkowska, R.; Gomulski, J.; Żekanowski, C.; Gaweda-Walerych, K. Accumulation of Polyphenols and Associated Gene Expression in Hairy Roots of Salvia Viridis Exposed to Methyl Jasmonate. Int. J. Mol. Sci. 2024, 25, 764. [Google Scholar] [CrossRef]
- Dong Hui, L.; Wei Wei, R.; Li Jie, C.; Li Da, Z.; Xiao Fen, S.; Ke Xuan, T. Enhanced Accumulation of Catharanthine and Vindoline in Catharanthus Roseus Hairy Roots by Overexpression of Transcriptional Factor ORCA2. Afr. J. Biotechnol. 2011, 10, 3260–3268. [Google Scholar] [CrossRef]
- Goklany, S.; Rizvi, N. F.; Loring, R. H.; Cram, E. J.; Lee-Parsons, C. W. T. Jasmonate-dependent Alkaloid Biosynthesis in Catharanthus Roseus Hairy Root Cultures Is Correlated with the Relative Expression of Orca and Zct Transcription Factors. Biotechnol. Prog. 2013, 29, 1367–1376. [Google Scholar] [CrossRef]
- Shi, M.; Gong, H.; Cui, L.; Wang, Q.; Wang, C.; Wang, Y.; Kai, G. Targeted Metabolic Engineering of Committed Steps Improves Anti-Cancer Drug Camptothecin Production in Ophiorrhiza Pumila Hairy Roots. Ind. Crops Prod. 2020, 148, 112277. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Kim, Y.B.; Uddin, M.R.; Lee, S.; Kim, S.-U.; Park, S.U. Enhanced Triterpene Accumulation in Panax Ginseng Hairy Roots Overexpressing Mevalonate-5-Pyrophosphate Decarboxylase and Farnesyl Pyrophosphate Synthase. ACS Synth. Biol. 2014, 3, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Kim, O. T.; Kim, S. H.; Ohyama, K.; Muranaka, T.; Choi, Y. E.; Lee, H. Y.; Kim, M. Y.; Hwang, B. Upregulation of Phytosterol and Triterpene Biosynthesis in Centella Asiatica Hairy Roots Overexpressed Ginseng Farnesyl Diphosphate Synthase. Plant Cell Rep. 2010, 29, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Luo, X.; Ju, G.; Li, L.; Huang, S.; Zhang, T.; Wang, H.; Kai, G. Enhanced Diterpene Tanshinone Accumulation and Bioactivity of Transgenic Salvia Miltiorrhiza Hairy Roots by Pathway Engineering. J. Agric. Food Chem. 2016, 64, 2523–2530. [Google Scholar] [CrossRef]
- Shi, M.; Luo, X.; Ju, G.; Yu, X.; Hao, X.; Huang, Q.; Xiao, J.; Cui, L.; Kai, G. Increased Accumulation of the Cardio-Cerebrovascular Disease Treatment Drug Tanshinone in Salvia Miltiorrhiza Hairy Roots by the Enzymes 3-Hydroxy-3-Methylglutaryl CoA Reductase and 1-Deoxy-d-Xylulose 5-Phosphate Reductoisomerase. Funct. Integr. Genomics 2014, 14, 603–615. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, W.; Chen, J.; Tan, H.; Xiao, Y.; Li, Q.; Ji, Q.; Gao, S.; Chen, L.; Chen, S.; et al. SmMYC2a and SmMYC2b Played Similar but Irreplaceable Roles in Regulating the Biosynthesis of Tanshinones and Phenolic Acids in Salvia Miltiorrhiza. Sci. Rep. 2016, 6, 22852. [Google Scholar] [CrossRef]
- Zhou, W.; Shi, M.; Deng, C.; Lu, S.; Huang, F.; Wang, Y.; Kai, G. The Methyl Jasmonate-Responsive Transcription Factor SmMYB1 Promotes Phenolic Acid Biosynthesis in Salvia Miltiorrhiza. Hortic. Res. 2021, 8, 10. [Google Scholar] [CrossRef]
- Sun, M.; Shi, M.; Wang, Y.; Huang, Q.; Yuan, T.; Wang, Q.; Wang, C.; Zhou, W.; Kai, G. The Biosynthesis of Phenolic Acids Is Positively Regulated by the JA-Responsive Transcription Factor ERF115 in Salvia Miltiorrhiza. J. Exp. Bot. 2019, 70, 243–254. [Google Scholar] [CrossRef]
- Hao, X.; Pu, Z.; Cao, G.; You, D.; Zhou, Y.; Deng, C.; Shi, M.; Nile, S. H.; Wang, Y.; Zhou, W.; et al. Tanshinone and Salvianolic Acid Biosynthesis Are Regulated by SmMYB98 in Salvia Miltiorrhiza Hairy Roots. J. Adv. Res. 2020, 23, 1–12. [Google Scholar] [CrossRef]
- Zakaria, M.M.; Kruse, L.H.; Engelhardt, A.; Ober, D. Seeing Double: Two Different Homospermidine Oxidases Are Involved in Pyrrolizidine Alkaloid Biosynthesis in Different Organs of Comfrey ( Symphytum Officinale ). Plant J. 2024, tpj.16847. [Google Scholar] [CrossRef]
- Zhang, F.; Qiu, F.; Zeng, J.; Xu, Z.; Tang, Y.; Zhao, T.; Gou, Y.; Su, F.; Wang, S.; Sun, X.; et al. Revealing Evolution of Tropane Alkaloid Biosynthesis by Analyzing Two Genomes in the Solanaceae Family. Nat. Commun. 2023, 14, 1446. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, G.; De Meyer, M.; Pollier, J.; Molina-Hidalgo, F. J.; Ceulemans, E.; Venegas-Molina, J.; De Milde, L.; Fernández-Calvo, P.; Ron, M.; Pauwels, L.; et al. The Basic helix–loop–helix Transcription Factors MYC1 and MYC2 Have a Dual Role in the Regulation of Constitutive and stress-inducible Specialized Metabolism in Tomato. New Phytol. 2022, 236, 911–928. [Google Scholar] [CrossRef] [PubMed]
- Hasebe, F.; Yuba, H.; Hashimoto, T.; Saito, K.; Funa, N.; Shoji, T. CRISPR/Cas9-Mediated Disruption of the PYRROLIDINE KETIDE SYNTHASE Gene Reduces the Accumulation of Tropane Alkaloids in Atropa Belladonna Hairy Roots. Biosci. Biotechnol. Biochem. 2021, 85, 2404–2409. [Google Scholar] [CrossRef]
- Zakaria, M. M.; Schemmerling, B.; Ober, D. CRISPR/Cas9-Mediated Genome Editing in Comfrey (Symphytum Officinale) Hairy Roots Results in the Complete Eradication of Pyrrolizidine Alkaloids. Molecules 2021, 26, 1498. [Google Scholar] [CrossRef]
- Fossati, E.; Narcross, L.; Ekins, A.; Falgueyret, J.-P.; Martin, V. J. J. Synthesis of Morphinan Alkaloids in Saccharomyces Cerevisiae. PLOS ONE 2015, 10, e0124459. [Google Scholar] [CrossRef]
- Khaldari, I.; Naghavi, M. R.; Motamedi, E.; Zargar, M. The Effects of Green and Chemically-Synthesized Copper Oxide Nanoparticles on the Production and Gene Expression of Morphinan Alkaloids in Oriental Poppy. Sci. Rep. 2024, 14, 6000. [Google Scholar] [CrossRef]
- Allen, R.S.; Miller, J.A.C.; Chitty, J.A.; Fist, A.J.; Gerlach, W.L.; Larkin, P.J. Metabolic Engineering of Morphinan Alkaloids by Over-expression and RNAi Suppression of Salutaridinol 7- O -acetyltransferase in Opium Poppy. Plant Biotechnol. J. 2008, 6, 22–30. [Google Scholar] [CrossRef]
- Sharafi, A.; Sohi, H.H.; Mousavi, A.; Azadi, P.; Razavi, K.; Ntui, V.O. A Reliable and Efficient Protocol for Inducing Hairy Roots in Papaver Bracteatum. Plant Cell Tissue Organ Cult. PCTOC 2013, 113, 1–9. [Google Scholar] [CrossRef]
- Hagel, J.M.; Facchini, P.J. Dioxygenases Catalyze the O-Demethylation Steps of Morphine Biosynthesis in Opium Poppy. Nat. Chem. Biol. 2010, 6, 273–275. [Google Scholar] [CrossRef]
- Beaudoin, G.A.W.; Facchini, P.J. Benzylisoquinoline Alkaloid Biosynthesis in Opium Poppy. Planta 2014, 240, 19–32. [Google Scholar] [CrossRef]
- Sato, F. Plant Alkaloid Engineering. In Comprehensive Natural Products III; Elsevier: Amsterdam, The Netherlands, 2020; pp. 700–755. [Google Scholar] [CrossRef]
- Mishra, S.; Triptahi, V.; Singh, S.; Phukan, U.J.; Gupta, M.M.; Shanker, K.; Shukla, R.K. Wound Induced Tanscriptional Regulation of Benzylisoquinoline Pathway and Characterization of Wound Inducible PsWRKY Transcription Factor from Papaver Somniferum. PLoS ONE 2013, 8, e52784. [Google Scholar] [CrossRef]
- Apuya, N.R.; Park, J.; Zhang, L.; Ahyow, M.; Davidow, P.; Van Fleet, J.; Rarang, J.C.; Hippley, M.; Johnson, T.W.; Yoo, H.; et al. Enhancement of Alkaloid Production in Opium and California Poppy by Transactivation Using Heterologous Regulatory Factors. Plant Biotechnol. J. 2008, 6, 160–175. [Google Scholar] [CrossRef]
- Dezfooli, S. A. F.; Darvish, F.; Omidi, M.; Alizadeh, H.; Rezazadeh, S. The Study of Transient Co-over Expression of Cor and TYDC Genes on Morphine Accumulation in Papaver Somniferum L. 2012.
- Wijekoon, C.P.; Facchini, P.J. Systematic Knockdown of Morphine Pathway Enzymes in Opium Poppy Using Virus-induced Gene Silencing. Plant J. 2012, 69, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.S.; Millgate, A.G.; Chitty, J.A.; Thisleton, J.; Miller, J.A.C.; Fist, A.J.; Gerlach, W.L.; Larkin, P. J. RNAi-Mediated Replacement of Morphine with the Nonnarcotic Alkaloid Reticuline in Opium Poppy. Nat. Biotechnol. 2004, 22, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Larkin, P.J.; Miller, J.A.C.; Allen, R.S.; Chitty, J.A.; Gerlach, W.L.; Frick, S.; Kutchan, T.M.; Fist, A.J. Increasing Morphinan Alkaloid Production by Over-expressing Codeinone Reductase in Transgenic Papaver Somniferum. Plant Biotechnol. J. 2007, 5, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, A.; Sohi, H. H.; Mousavi, A.; Azadi, P.; Khalifani, B. H.; Razavi, K. Metabolic Engineering of Morphinan Alkaloids by Over-Expression of Codeinone Reductase in Transgenic Hairy Roots of Papaver Bracteatum, the Iranian Poppy. Biotechnol. Lett. 2013, 35, 445–453. [Google Scholar] [CrossRef]
- Park, S.-U. Agrobacterium Rhizogenes-Mediated Transformation of Opium Poppy, Papaver Somniferum L., and California Poppy, Eschscholzia Californica Cham., Root Cultures. J. Exp. Bot. 2000, 51, 1005–1016. [Google Scholar] [CrossRef]
- Rostampour, S.; Hashemi Sohi, H.; Jourabchi, E.; Ansari, E. Influence of Agrobacterium Rhizogenes on Induction of Hairy Roots and Benzylisoquinoline Alkaloids Production in Persian Poppy (Papaver Bracteatum Lindl.): Preliminary Report. World J. Microbiol. Biotechnol. 2009, 25, 1807–1814. [Google Scholar] [CrossRef]
- Huang, P.; Xia, L.; Liu, W.; Jiang, R.; Liu, X.; Tang, Q.; Xu, M.; Yu, L.; Tang, Z.; Zeng, J. Hairy Root Induction and Benzylisoquinoline Alkaloid Production in Macleaya Cordata. Sci. Rep. 2018, 8, 11986. [Google Scholar] [CrossRef]
- Sharafi, A.; Hashemi Sohi, H.; Mousavi, A.; Azadi, P.; Dehsara, B.; Hosseini Khalifani, B. Enhanced Morphinan Alkaloid Production in Hairy Root Cultures of Papaver Bracteatum by Over-Expression of Salutaridinol 7-o-Acetyltransferase Gene via Agrobacterium Rhizogenes Mediated Transformation. World J. Microbiol. Biotechnol. 2013, 29, 2125–2131. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, H.; Du, Q.; Wang, L.; Liu, X.; Liu, C. Genome-Wide Identification of Circular RNAs Potentially Involved in the Biosynthesis of Secondary Metabolites in Salvia Miltiorrhiza. Front. Genet. 2021, 12, 645115. [Google Scholar] [CrossRef]
- Aghaali, Z.; Naghavi, M. R.; Zargar, M. Collaboration of Hairy Root Culture and Scale-up Strategies for Enhancing the Biosynthesis of Medicinal and Defensive Alkaloids in Papaver Sp. Curr. Plant Biol. 2024, 40, 100381. [Google Scholar] [CrossRef]
- Hussain, M. J.; Abbas, Y.; Nazli, N.; Fatima, S.; Drouet, S.; Hano, C.; Abbasi, B. H. Root Cultures, a Boon for the Production of Valuable Compounds: A Comparative Review. 2022.
- Mehrotra, S.; Mishra, S.; Srivastava, V. Bioreactor Technology for Hairy Roots Cultivation.
- Cuello, J. L.; Yue, L. C. Ebb-and-Flow Bioreactor Regime and Electrical Elicitation: Novel Strategies for Hairy Root Biochemical Production. 2008.
- Xu, J.; Zhang, N. On the Way to Commercializing Plant Cell Culture Platform for Biopharmaceuticals: Present Status and Prospect. Pharm. Bioprocess. 2014, 2, 499–518. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Farkya, S.; Srivastava, A. K.; Bisaria, V. S. Bioprocess Considerations for Production of Secondary Metabolites by Plant Cell Suspension Cultures. Biotechnol Bioprocess Eng 2002, 7. [Google Scholar]
- Su, W. W.; Lee, K.-T. Chapter 10. Plant Cell and Hairy Root Cultures – Process Characteristics, Products, and Applications.
- Srikantan, C.; Srivastava, S. Bioreactor Design and Analysis for Large- Scale Plant Cell and Hairy Root Cultivation.
- Ruffoni, B.; Pistelli, L.; Bertoli, A.; Pistelli, L. Plant Cell Cultures: Bioreactors for Industrial Production. Adv. Exp. Med. Biol. 2010, 698, 203–221. [Google Scholar] [CrossRef]
- Ochoa-Villarreal, M.; Howat, S.; Hong, S.; Jang, M. O.; Jin, Y.-W.; Lee, E.-K.; Loake, G. J. Plant Cell Culture Strategies for the Production of Natural Products. BMB Rep. 2016, 49, 149–158. [Google Scholar] [CrossRef]
- Stiles, A. R.; Liu, C.-Z. Hairy Root Culture: Bioreactor Design and Process Intensification. Adv. Biochem. Eng. Biotechnol. 2013, 134, 91–114. [Google Scholar] [CrossRef]
- Choi, Y.-E.; Kim, Y.-S.; Paek, K.-Y. TYPES AND DESIGNS OF BIOREACTORS FOR HAIRY ROOT CULTURE.
- Giri, A.; Narasu, M. L. Transgenic Hairy Roots. Recent Trends and Applications. Biotechnol. Adv. 2000, 18, 1–22. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Merecz-Sadowska, A.; Picot, L.; Brčić Karačonji, I.; Wieczfinska, J.; Śliwiński, T.; Sitarek, P. Genetic Manipulation and Bioreactor Culture of Plants as a Tool for Industry and Its Applications. Mol. Basel Switz. 2022, 27, 795. [Google Scholar] [CrossRef]
- Morey, K. J.; Peebles, C. A. M. Hairy Roots: An Untapped Potential for Production of Plant Products. Front. Plant Sci. 2022, 13, 937095. [Google Scholar] [CrossRef]
- Gupta, R.; Pandey, P.; Singh, S.; Singh, D. K.; Saxena, A.; Luqman, S.; Bawankule, D. U.; Banerjee, S. Advances in Boerhaavia Diffusa Hairy Root Technology: A Valuable Pursuit for Identifying Strain Sensitivity and up-Scaling Factors to Refine Metabolite Yield and Bioactivity Potentials. Protoplasma 2016, 253, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Mathur, A. K.; Shanker, K. Growth, alkaloid production, rol genes integration, bioreactor up-scaling and plant regeneration studies in hairy root lines of Catharanthus roseus. Plant Biosyst. - Int. J. Deal. All Asp. Plant Biol. 2012, 146, 27–40. [Google Scholar] [CrossRef]
- Patra, N.; Srivastava, A. K. Enhanced Production of Artemisinin by Hairy Root Cultivation of Artemisia Annua in a Modified Stirred Tank Reactor. Appl. Biochem. Biotechnol. 2014, 174, 2209–2222. [Google Scholar] [CrossRef] [PubMed]
- Patra, N.; Srivastava, A. K. Use of Model-Based Nutrient Feeding for Improved Production of Artemisinin by Hairy Roots of Artemisia Annua in a Modified Stirred Tank Bioreactor. Appl. Biochem. Biotechnol. 2015, 177, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.-T.; Park, D.-H.; Hwang, B.; Park, K.; Kim, S.-W.; Woo, J.-C. Studies on Mass Production of Transformed Panax Ginseng Hairy Roots in Bioreactor. Appl. Biochem. Biotechnol. 2002, 98–100, 98–100. [Google Scholar] [CrossRef]
- Singh, S.; Pandey, P.; Akhtar, M. Q.; Negi, A. S.; Banerjee, S. A New Synthetic Biology Approach for the Production of Curcumin and Its Glucoside in Atropa Belladonna Hairy Roots. J. Biotechnol. 2021, 328, 23–33. [Google Scholar] [CrossRef]
- Srivastava, S.; Srivastava, A. K. In Vitro Azadirachtin Production by Hairy Root Cultivation of Azadirachta Indica in Nutrient Mist Bioreactor. Appl. Biochem. Biotechnol. 2012, 166, 365–378. [Google Scholar] [CrossRef]
- Verma, P.; Khan, S. A.; Mathur, A. K.; Shanker, K.; Lal, R. K. Regulation of Vincamine Biosynthesis and Associated Growth Promoting Effects through Abiotic Elicitation, Cyclooxygenase Inhibition, and Precursor Feeding of Bioreactor Grown Vinca Minor Hairy Roots. Appl. Biochem. Biotechnol. 2014, 173, 663–672. [Google Scholar] [CrossRef]
- Cardillo, A. B.; Otálvaro, A. Á. M.; Busto, V. D.; Talou, J. R.; Velásquez, L. M. E.; Giulietti, A. M. Scopolamine, Anisodamine and Hyoscyamine Production by Brugmansia Candida Hairy Root Cultures in Bioreactors. Process Biochem. 2010, 45, 1577–1581. [Google Scholar] [CrossRef]
- Department of Biochemical Engineering and Biotechnology, IIT Delhi, Hauz Khas, New Delhi – 110016, India.; Patra, N.; Srivastava, A. K.; Department of Biotechnology and Medical Engineering, National Institute of Technology Rourkela, Odisha, India – 769008. Mass Scale Artemisinin Production in a Stirred Tank Bioreactor Using Hairy Roots of Artemisia Annua. Int. J. Biosci. Biochem. Bioinforma. 2014, 467–474. [CrossRef]
- Verma, P. C.; Singh, H.; Negi, A. S.; Saxena, G.; Rahman, L.; Banerjee, S. Yield Enhancement Strategies for the Production of Picroliv from Hairy Root Culture of Picrorhiza Kurroa Royle Ex Benth. Plant Signal. Behav. 2015, 10, e1023976. [Google Scholar] [CrossRef]
- Kim, Y.; Wyslouzil, B.; Weathers, P. A Comparative Study of Mist and Bubble Column Reactors in the in Vitro Production of Artemisinin. Plant Cell Rep. 2001, 20, 451–455. [Google Scholar] [CrossRef]
- Srivastava, S.; Srivastava, A. K. Production of the Biopesticide Azadirachtin by Hairy Root Cultivation of Azadirachta Indica in Liquid-Phase Bioreactors. Appl. Biochem. Biotechnol. 2013, 171, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Kareem, Z. J.; Su, L.; Rathgeb, A.; Sirrenberg, A.; Hadacek, F.; Rashid, A. H. A. H.; Karlovsky, P. Small-Scale Bioreactor for Sterile Hydroponics and Hairy Roots: Metabolic Diversity and Salicylic Acid Exudation by Hairy Roots of Hyoscyamus Niger. Appl. Sci. 2019, 9, 3044. [Google Scholar] [CrossRef]
- Jaremicz, Z.; Luczkiewicz, M.; Kokotkiewicz, A.; Krolicka, A.; Sowinski, P. Production of Tropane Alkaloids in Hyoscyamus Niger (Black Henbane) Hairy Roots Grown in Bubble-Column and Spray Bioreactors. Biotechnol. Lett. 2014, 36, 843–853. [Google Scholar] [CrossRef]
- Savitha, B. C.; Thimmaraju, R.; Bhagyalakshmi, N.; Ravishankar, G. A. Different Biotic and Abiotic Elicitors Influence Betalain Production in Hairy Root Cultures of Beta Vulgaris in Shake-Flask and Bioreactor. Process Biochem. 2006, 41, 50–60. [Google Scholar] [CrossRef]
- Suresh, B.; Thimmaraju, R.; Bhagyalakshmi, N.; Ravishankar, G. A. Polyamine and Methyl Jasmonate-Influenced Enhancement of Betalaine Production in Hairy Root Cultures of Beta Vulgaris Grown in a Bubble Column Reactor and Studies on Efflux of Pigments. Process Biochem. 2004, 39, 2091–2096. [Google Scholar] [CrossRef]
- Thakore, D. Mass Production of Ajmalicine by Bioreactor Cultivation of Hairy Roots of Catharanthus Roseus.
- Pal Bais, H.; Suresh, B.; Rachavarao, K. S. M. S.; Ravishankar, G. A. Performance of Hairy Root Cultures of Cichorium Intybus L. In Bioreactors of Different Configurations. Vitro Cell. Dev. Biol. - Plant 2002, 38, 573–580. [Google Scholar] [CrossRef]
- Patra, N.; Srivastava, A. K. Artemisinin Production by Plant Hairy Root Cultures in Gas- and Liquid-Phase Bioreactors. Plant Cell Rep. 2016, 35, 143–153. [Google Scholar] [CrossRef]
- Huerta-Heredia, A. A.; Marín-López, R.; Ponce-Noyola, T.; Cerda-García-Rojas, C. M.; Trejo-Tapia, G.; Ramos-Valdivia, A. C. Oxidative Stress Induces Alkaloid Production in Uncaria Tomentosa Root and Cell Cultures in Bioreactors. Eng. Life Sci. 2009, 9, 211–218. [Google Scholar] [CrossRef]
- Abbasi, B. H.; Liu, R.; Saxena, P. K.; Liu, C.-Z. Cichoric Acid Production from Hairy Root Cultures of Echinacea Purpurea Grown in a Modified Airlift Bioreactor. J Chem Technol Biotechnol 2009.
- Caspeta, L.; Nieto, I.; Zamilpa, A.; Alvarez, L.; Quintero, R.; Villarreal, Ma. L. Solanum chrysotrichum Hairy Root Cultures: Characterization, Scale-Up and Production of Five Antifungal Saponins for Human Use. Planta Med. 2005, 71, 1084–1087. [Google Scholar] [CrossRef]
- Du, M.; Wu, X. J.; Ding, J.; Hu, Z. B.; White, K. N.; Branford-White, C. J. Astragaloside IV and Polysaccharide Production by Hairy Roots of Astragalus Membranaceus in Bioreactors. Biotechnol. Lett. 2003, 25, 1853–1856. [Google Scholar] [CrossRef] [PubMed]
- Shin, K. S.; Murthy, H. N.; Ko, J. Y.; Paek, K. Y. Growth and Betacyanin Production by Hairy Roots of Beta Vulgaris in Airlift Bioreactors.
- Sivakumar, G.; Yu, K. W.; Hahn, E. J.; Paek, K. Y. Optimization of Organic Nutrients for Ginseng Hairy Roots Production in Large-Scale Bioreactors. Curr. Sci. 2005, 89. [Google Scholar]
- Malabadi, R. Alkaloid Biosynthesis Influenced by Agrobacterium Rhizogenes Mediated-Transformation and Bioreactor in Clitoria Ternatea (Linn.).
- Caspeta, L.; Quintero, R.; Villarreal, M. L. Novel Airlift Reactor Fitting for Hairy Root Cultures: Developmental and Performance Studies. Biotechnol. Prog. 2005, 21, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Kintzios, S.; Makri, O.; Pistola, E.; Matakiadis, T.; Shi, H. P.; Economou, A. Scale-up Production of Puerarin from Hairy Roots of Pueraria Phaseoloides in an Airlift Bioreactor. Biotechnol. Lett. 2004, 26, 1057–1059. [Google Scholar] [CrossRef]
- López, E. G.; Ramírez, E. G. R.; Gúzman, O. G.; Calva, G. C.; Ariza-Castolo, A.; Pérez-Vargas, J.; Rodríguez, H. G. M. MALDI-TOF Characterization of hGH1 Produced by Hairy Root Cultures of Brassica Oleracea Var. Italica Grown in an Airlift with Mesh Bioreactor. Biotechnol. Prog. 2014, 30, 161–171. [Google Scholar] [CrossRef]
- Peraza-Luna, F.; Rodríguez-Mendiola, M.; Arias-Castro, C.; Bessiere, J. M.; Calva-Calva, G. Sotolone Production by Hairy Root Cultures of Trigonella Foenum-Graecum in Airlift with Mesh Bioreactors. J. Agric. Food Chem. 2001, 49, 6012–6019. [Google Scholar] [CrossRef]
- Suresh, B.; Bais, H. P.; Raghavarao, K. S. M. S.; Ravishankar, G. A.; Ghildyal, N. P. Comparative Evaluation of Bioreactor Design Using Tagetes Patula L. Hairy Roots as a Model System. Process Biochem. 2005, 40, 1509–1515. [Google Scholar] [CrossRef]
- Liu, C.; Towler, M. J.; Medrano, G.; Cramer, C. L.; Weathers, P. J. Production of Mouse Interleukin-12 Is Greater in Tobacco Hairy Roots Grown in a Mist Reactor than in an Airlift Reactor. Biotechnol. Bioeng. 2009, 102, 1074–1086. [Google Scholar] [CrossRef]
- Mišić, D. Secoiridoid Glycosides Production by Centaurium Maritimum (L.) Fritch Hairy Root Cultures in Temporary Immersion Bioreactor. Process Biochem. 2013. [Google Scholar] [CrossRef]
- Vinterhalter, B.; Banjac, N.; Vinterhalter, D.; Krstić-Milošević, D. Xanthones Production in Gentiana Dinarica Beck Hairy Root Cultures Grown in Simple Bioreactors. Plants Basel Switz. 2021, 10, 1610. [Google Scholar] [CrossRef]
- Kozłowska, W.; Piątczak, E.; Kolniak-Ostek, J.; Kochan, E.; Pencakowski, B.; Stafiniak, M.; Bielecka, M.; Płachno, B. J.; Strzemski, M.; Matkowski, A.; et al. Upscaling Biomass Production of Rosmarinic Acid-Rich Hairy Root Cultures of Agastache Rugosa (Fisch. & C.A.Mey.) Kuntze. Plant Cell Tissue Organ Cult. PCTOC 2024, 156, 41. [Google Scholar] [CrossRef]
- Bartczak, M.; Wierzchowski, K.; Pilarek, M. Mixing Performance in a Litre-Scale Rocking Disposable Bioreactor: DoE-Based Investigation of Mixing Time Dependence on Operational Parameters. Chem. Eng. J. 2022, 431, 133288. [Google Scholar] [CrossRef]
- Eibl, R.; Werner, S.; Eibl, D. Disposable Bioreactors for Plant Liquid Cultures at Litre-scale. Eng. Life Sci. 2009, 9, 156–164. [Google Scholar] [CrossRef]
- Oosterhuis, N. M. G.; Hudson, T.; D’Avino, A.; Zijlstra, G. M.; Amanullah, A. Disposable Bioreactors. In Comprehensive Biotechnology; Elsevier, 2017; pp 361–373. [CrossRef]
- Palazón, J.; Mallol, A.; Eibl, R.; Lettenbauer, C.; Cusidó, R. M.; Piñol, M. T. Growth and Ginsenoside Production in Hairy Root Cultures of Panax Ginseng Using a Novel Bioreactor. Planta Med. 2003, 69, 344–349. [Google Scholar] [CrossRef]
- Wierzchowski, K.; Kawka, M.; Sykłowska-Baranek, K.; Pilarek, M. Intensification of Rindera Graeca Transgenic Roots Proliferation and Deoxyshikonin Secretion in Wave-Agitated Disposable Bioreactor. Chem. Eng. Process. - Process Intensif. 2024, 203, 109905. [Google Scholar] [CrossRef]
- Sivakumar, G.; Liu, C.; Towler, M. J.; Weathers, P. J. Biomass Production of Hairy Roots of Artemisia Annua and Arachis Hypogaea in a Scaled-up Mist Bioreactor. Biotechnol. Bioeng. 2010, 107, 802–813. [Google Scholar] [CrossRef]
- San, K; Chung, I; Ahmad, A. Natural Products from Vinca. US20100048494A1, 2010. https://patents.google.com/patent/US20100048494A1/en?oq=US20100048494A1.
- Asis, D; Samir, B; Nikas, P; Jayanti, S; Suman, D; Anindita, B; Jyoti, B. Process of production of anti-diabetic compound in root culture of Catharanthus roseus. WO2010004584A2, 2010. https://patents.google.com/patent/WO2010004584A2/en?oq=WO2010004584A2.
- Minamii, Y; Miura, Y; Inoue, H; Matsui, C. Production of betanin. JPH0411895A, 1992. https://patents.google.com/patent/JPH0411895A/en?oq=JPH0411895A.
- Gokare, R; Bhamidi, S; Rao, R. Process for preparation of dopa and dopamine from hairy root cultures of Beta vulgaris. WO2004055192A1, 2002. https://patents.google.com/patent/WO2004055192A1/en?oq=WO2004055192A1.
- Goossens, A; Moses, T; Pollier, J; Romero, J. Triterpenoid sapogenin production in plant and microbial cultures. US9994883, 2018. https://patents.google.com/patent/US9994883B2/en?oq=US9994883.
- Medina-Bolivar, L; Dolan, M; Bennett, S; Condori, J; Hubstenberger, J. Production of stilbenes in plant hairy root cultures. US7666677, 2010. https://patents.google.com/patent/US7666677B2/en?oq=US7666677.
- Jishuang, C; Mingliang, J; Benhou, Z; Pingkai, O; Leilei, Jin. Method for continuously obtaining secondary ginseng hairy root metabolites through tissue culture. CN104026019A, 2014. https://patents.google.com/patent/CN104026019A/en?oq=CN104026019A.
- Cardon, F; Lemasson, C; Guillet, M. Optimized hairy roots bioreactor. WO2023187185A1, 2023. https://patents.google.com/patent/WO2023187185A1/en?
- Thimmaraju, R; Ravishankar, G; Bhagyalakshmi, N; Suresh, B; Narayan, M. WO2003080819A1, 2003. https://patents.google.com/patent/WO2003080819A1/en?oq=WO2003080819A1.
- Demorset, Z; Dudley, Q; Mayers, J; Kurtz, B; Harrison, L; Jordan, A; Lapham, R. Producing sesquiterpenes and other terpenes using plant-based biomasses. WO2024011263A2, 2024. https://patents.google.com/patent/WO2024011263A2/en?oq=WO2024011263A2.
- Qantang, Y; Zongshen, Z; Tongxiang, L; Zhenyan, Y;Zhicheng, L; Gangchun, S; Zuhong, W. External circulation mist-type bioreactor for large-scale cultivation of plant hairy roots. CN102783414A. https://patents.google.com/patent/CN102783414A/en?oq=CN102783414A.
- Weathers, P; Towler, M. Scalable wall bioreactor for culture of plant and animal tissues,. US2009017529A1, 2009. https://patents.google.com/patent/US20090017529A1/en?oq=US2009017529A1.
- Shiau, Y. Bioreactor for growing fungus, plant cell, tissue, organ, hairy roots and plantlet. US7531350, 2009. https://patents.google.com/patent/US7531350B2/en?oq=US7531350.
- Brillmann, M; Krautwaschl, P; Reichelt, W; Herwig, C. Bioreactors for root organ cultures. US12018242, 2024. https://patents.google.com/patent/US12018242B2/en?oq=US12018242.
- Mibellebiochemistry. Available online: https://mibellebiochemistry.com/rootbiotectm-hw (accessed on 26.08.2024).
- Sunumbra. Available online: https://www.sunumbra.com/basilicum-hairy-root.html (accessed on 26.08.2024).
- Vitalabactive. Available online: https://vitalabactive.com/project/radiant-skin/ (accessed on 26.08.2024).
- Gutierrez-Valdes, N.; Häkkinen, S. T.; Lemasson, C.; de Groot, J.; Ele-Ekouna, J.-P.; Guillet, M.; Cardon, F.; Ritala, A. Improving Yield of a Recombinant Biologic in a Brassica Hairy Root Manufacturing Process. Biotechnol. Bioeng. 2022, 119, 2831–2841. [Google Scholar] [CrossRef]
- Cardon, F.; Pallisse, R.; Bardor, M.; Caron, A.; Vanier, J.; Ele Ekouna, J. P.; Lerouge, P.; Boitel-Conti, M.; Guillet, M. Brassica Rapa Hairy Root Based Expression System Leads to the Production of Highly Homogenous and Reproducible Profiles of Recombinant Human Alpha-L-Iduronidase. Plant Biotechnol. J. 2019, 17, 505–516. [Google Scholar] [CrossRef]
- Samabriva. Available online: https://samabriva.com/the-process/ (accessed on 26.08.2024).
| Metabolite(s) | Plant species | Metabolite level, mg/g DW | Yield increase, folds | Genes/TFs | Strategy | Reference |
|---|---|---|---|---|---|---|
| Alkaloids | ||||||
| Camptothecin | C. accuminata | 1.12 | 1.5 | ORCA3 | Overexpression | [108] |
| Catharanthine | C. roseus | 1.96 | 6.5 | G10H, ORCA3 | Overexpression | [109] |
| Hyoscyamine | A. cutangulus | 1.9 | 2.3 | TRI,PMT | Overexpression | [40] |
| Hyoscyamine | H. niger | 0.2 | 1.25 | PMT, H6H | Overexpression | [110] |
| Hyoscyamine |
H. reticulatus | 0.13 |
3.0 |
PMT, H6H | Overexpression | [57] |
| Scopolamine | Datura innoxia Mill | 0.54 | 2.16 | H6H | Overexpression | [111] |
| Scopolamine | H. niger | 0.31 | 1.20 | PMT, H6H | Overexpression | [110] |
| Scopolamine | H. reticulatus |
0.16 |
2.6 |
PMT, H6H | Overexpression | [57] |
| Terpenes | ||||||
| Platycosides | Platycodon grandiflorum A. DC | 1.6 | 2.5 | PgHMGR | Overexpression | [112] |
| Tanshinones | S. miltiorrhiza | 2.73 | 4.74 | SmHMGR/SmGGPPS | Overexpression | [113] |
| Withaferin A | W. somnifera | 17.45 | 12.5 | HMGR, SQS, SMT-1, SDS/CYP710A | Overexpression | [88] |
| Phenolic compounds | ||||||
| Apigenin | F. carica | 0.16 | 51.96 | CHS, F3’H, PAL, UFGT, MYB3, bHLH | Overexpression | [89] |
| Caffeic acid | Leonurus sibirica L. | 11.4 | 2.7 | AtPAP1 | Overexpression | [114] |
| Chlorogenic acid |
L. sibirica L. | 19.4 |
4.75 |
AtPAP1 | Overexpression | [114] |
| Gallic acid |
F. carica | 0.71 |
5.9 |
CHS, F3’H, PAL, UFGT, MYB3, bHLH | Overexpression | [89] |
| Total phenolics | S. viridis L. | 38.65 | 1.3 | TAT, HPPR, PAL, C4H, 4CL, RAS | Overexpression | [115] |
| G10H – geraniol-10-hydroxylase; TRI – tropinone reductase I; PMT – putrescine N-methyltransferase; H6H – hyoscyamine 6β-hydroxylase; HMGR – 3-hydroxy-3-methylglutaryl-coenzyme A reductase; GGPPS – geranylgeranyl diphosphate synthase; SQS – squalene synthase; SMT-1 – sterol methyltransferase 1, SDS/CYP710A – sterol-22-desaturase; CHS – chalcone synthase, F3’H – flavonoid 3′-hydroxylase, PAL – phenylalanine ammonia-lyase, UFGT – UDP-glucose flavonoid 3-O-glucosyltransferase, TAT – tyrosine aminotransferase, HPPR – 4 hydroxyphenylpyruvate reductase, C4H – cinnamic acid 4-hydroxylase , 4CL – 4-coumarate-CoA ligase, RAS – rosmarinic acid synthase | ||||||
| Characteristics | Cell suspension | Hairy roots | Microbial cells | Animal cells |
|---|---|---|---|---|
| Shape | Spherical/ cylindrical | Highly branched root tissues | Spherical/ cylindrical | Spherical |
| Size, µM | 10-200 | 10-200 | 1-10 | 10-100 |
| Growth pattern | Small aggregates | Large aggregates | Individual cells/small aggregates | Support required for growth |
| Doubling time, h | 20-100 | 20-100 | 0.5-4 | 24-48 |
| Cultivation time | Days | Weeks | Weeks | Weeks |
| Cell aggregation | Cell clusters <100 μm > 2 mm |
Cell clusters <100 μm > 2 mm |
Single cells | Single cells |
| Shear sensitivity | High | High | Low | Very high |
| Inoculum size, % | 5-10 | 5-10 | 1-2 | 5-10 |
| Oxygen uptake rate, mmoL/L/h | 2-10 | 2-10 | 10-200 | 0.05-10 |
| Damage by aeration | Low | Low | Very low | High |
| Genetic and biochemical stability | Variable | Stable | Stable | Stable |
| Product localization | Intracellular | Intracellular | Extracellular/ Intracellular |
Extracellular/ Intracellular |
|
Posttranslational modifications |
Yes | Yes | No | Yes |
| Required kLa value in bioreactor cultivation, h | 10-150 | 10-50 | 100-1000 | 0.25-10 |
| Culture scale up | Easy | Difficult | Easy | Difficult |
| Bioreactor type | Features and modifications | Advantages | Disadvantages |
|---|---|---|---|
| Stirred tank (STR) | Equipped with impeller or turbine blades and sparger to facilitate mass transfer; modified impellers and addition of mesh supports to avoid tissue damage are applied | Prevents cell aggregation; suitable for batch and continuous operation; improved nutrient and oxygen transfer compared to BCR | High shear forces and tissue damage; complicate configuration and increased exposure to contamination; high energy consumption and insufficient control of concentration gradients near dense root clumps |
| Bubble column (BCR) | Vertical cylindrical vessel with a sparger at the bottom for aeration and mixing; optionally the vessel can be segmented and provided with mesh supports | Low shear stress; simple design, operation and maintenance; low chance for contamination and energy requirements | Non-uniform fluid pattern; not suitable for high viscosity liquids and high density cell cultures; sedimentations of the cells; insufficient mass transfer due to gas flow channeling around the root clumps |
| Airlift (ALR) | Vertical cylindrical vessel with an external or internal draft tube (to improve the circulation and oxygen transfer) and a sparger at the bottom | Low shear stress; defined flow pattern due to the draft tube; enhanced mass transfer; less bubble coalescence | Greater air supply needed; formation of excessive foaming; higher energy requirements |
| Nutrient mist (NMR) | Consists of a growth chamber equipped with a mesh for HRs immobilization; a mist generator delivers medium in the form of mist generated by ultrasonic methods on the top (droplet sizes 0.01-10 μm), drained on the bottom of the vessel and recirculated again | Abundant oxygen and nutrient availability; better gas exchange and reduced shear stress | Complex construction; high energy consumption; labor intensive set-up |
| Trickle bed (TBR) | Has a similar structure to the NMR except the mist generator and the droplet size is bigger, > 10 µm | Abundant oxygen supply; low energy consumption | May produce a viscous liquid film on the roots creating a high mass transfer barrier; labor intensive set-up |
| Metabolite(s) | Plant species | Bioreactor volume and configuration | Operating conditions | Metabolite content | Reference |
|---|---|---|---|---|---|
| Stirred tank reactor (STR) | |||||
| Boeravinone B Eupalitin |
Boerhaavia diffusa L. | 10-L; central positioned nylon mesh | 25 ºC, 75 rpm agitation, 2.5 L/min flow rate | 0.0299 mg/g DW 0.0031 mg/g DW |
[166] |
| Saponins | P. ginseng | 7-L; cylindrical stainless steel mesh | 25 ºC, 70 rpm agitation, 0.5 vvm flow rate | 20.03 mg/g DW | [170] |
| Artemisinin | Artemisia annua L. | 3-L; a perforated Teflon mesh at the bottom | 25 ºC, 125 rpm agitation, 0.25 vvm flow rate; 16/8 h light/dark; batch mode | 4.63 mg/L | [168] |
| Artemisinin | A. annua L. | 3-L; a perforated Teflon mesh at the bottom | 25 ºC, 125 rpm agitation, 0.25 vvm flow rate; 16/8 h light/dark; fed-batch mode | 13.68 g/L | [169] |
| Curcumin | Atropa belladonna L. | 10-L; central positioned mesh | 25 ºC, 75 rpm agitation, 2.5 L/min flow rate | 0.112 mg/g DW | [171] |
| Azadirachtin | Azadirachta indica A. Juss. | 3-L; polyurethane foam disk for support | 26 ºC, 0.2 vvm flow rate | 97.28 mg/L | [172] |
| Indole alkaloids | Vinca minor L. | 5-L; nylon mesh support | 25 ºC, 100 rpm agitation, 2.0 L/min flow rate, 3000 lx illumination | 4.1 mg/g DW | [173] |
| Tropane alkaloids | Brugmansia candida Pers | 1.5-L; a plastic mesh placed forming a zigzag arrangement around the baffle | 24 ºC, 50 rpm agitation, 0.5 vvm flow rate | 3.72 mg/L | [174] |
| Artemisinin | Artemisia annua L. | 3-L; no modifications | 25 ºC, 125 rpm agitation, 40% dO2 | 0.32 mg/g | [175] |
| Picroliv | Picrorhiza kurroa Royle ex Benth. | 5-L; no modifications | 25 ºC, 50 rpm agitation, 0.5 L/min flow rate | 6.65 mg/g DW | [176] |
| Bubble column reactor (BCR) | |||||
| Saponins | P. ginseng | 1.6-L; four compartment stages separated by stainless steel mesh | 25 ºC, 0.5 vvm flow rate | 19.89 mg/g DW | [170] |
| Artemisinin | A. annua L. | 1.5-L; stainless steel mesh | 23 ºC, 0.167 vvm flow rate | 2.64 µg/g DW | [177] |
| Azadirachtin | A. indica | 3-L; polyurethane foam disk for support | 25 ºC, 0.2 vvm flow rate | 20.23 mg/L | [178] |
| Salicylic acid | Hyoscyamus niger L | 0.5-L; polypropylene mesh | 25 ºC, 670 mL/min flow rate; 12/12 h light/dark | 60 µg/L | [179] |
| Scopolamine, Hyoscyamine Cuscohygrine | H. niger | 0.6-L; immobilization basket in the centre | 25 ºC, 0.8 vvm flow rate | 5.3 mg/g DW 1.6 mg/g DW 26.5 mg/g DW |
[180] |
| Betalains | Beta vulgaris L. | 3-L; plastic basket | 23 ºC, 33.4 cm3/s flow rate | 280 mg/L | [181] |
| Betalains | B. vulgaris | 3-L; plastic basket | 23 ºC, 33.4 cm3/s flow rate | 36.13 mg/g DW | [182] |
| Ajmalicine | C. roseus | 3-L; no modifications | 23 ºC, 0.3 vvm flow rate | 15.5 mg/L | [183] |
| Ajmalicine | C. roseus | 5-L; polypropylene mesh | 23 ºC, 0.3 vvm flow rate | 30.0 mg/L | [183] |
| Ajmalicine | C. roseus | 5-L; polyurethane foam | 23 ºC, 0.3 vvm flow rate | 34.0 mg/L | [183] |
| Esculin | Cichorium intybus L. | 1.75-L; no modification | 25 ºC, 33.4 cm3/s flow rate | 18.5 g/L | [184] |
| Azadirachtin | A. indica | 3-L; no modifications | 23 ºC, 0.2 vvm flow rate | 10.7 mg/L | [172] |
| Artemisinin | A. annua L. | 3-L; perforated Teflon disc | 25 ºC, 0.3 vvm flow rate; 16/8 h light/dark | 5.68 g/L | [185] |
| Airlift reactors (ALR) | |||||
| Monoterpenoid oxindole alkaloids | Uncaria tomentosa Willd | 2-L; internal-loop draft tube | 25 ºC, 0.1 vvm flow rate; 15 µmol/m2/s continuous light | 9.06 mg/L | [186] |
| Cichoric acid | Echinacea purpurea Moench | 1.7-L; vertical stainless steel mesh cylinder |
25 ºC, 0.002-0.004 m3/h flow rate; 600 µmol/m2/s continuous light | 178.2 mg/L | [187] |
| Saponins | Solanum chrysotrichum Schltdl | 2-L; internal loop draft-tube | 26-27 ºC, 0.1 vvm flow rate; 8-10 µmol/m2/s continuous light | 0.7 mg/g DW | [188] |
| Astragaloside IV |
Astragalus mongholicus Bunge |
30-L; no modifications | 26-27 ºC, 400 mL/min flow rate | 1.4 mg/g DW | [189] |
| Betacyanin | B. vulgaris | 5-L cone type ALR | 25 ºC, 0.35 vvm flow rate; 60 µmol/m2/s continuous light | 34 mg/g DW | [190] |
| Nutrient mist reactor (NMR) | |||||
| Artemisinin | A. annua L. | 5-L; stainless steel mesh | 23 ºC, 0.167 vvm flow rate; two stage operation: as a BCR and NMR; mist mode: 5/15 min on/off | 2.64 µg/g DW | [177] |
| Esculin | C. intybus | 1.75-L; two-tier nylon mesh | 25 ºC, 33.4 cm3/s flow rate | 13.8 g/L | [184] |
| Azadirachtin | A. indica | 3-L; no modifications | 23 ºC, 8.6 L/min flow rate | 27.24 mg/L | [172] |
| Artemisinin | A. annua L. | 3-L; perforated Teflon disc | 25 ºC, 0.3 vvm flow rate; 16/8 h light/dark; two stage operation: as a BCR and NMR; mist mode: 5/30 sec on/off | 23.02 g/L | [185] |
| Rotating drum reactor | |||||
| Ajmalicine | C. roseus | 7-L; cylindrical mesh | 23 ºC, 0.3 vvm flow rate | 4.6 mg/L | [183] |
| Hybrid bioreactors | |||||
| Anisodamine | H. niger | 0.6-L; bubble-column/spray bioreactor | 25 ºC, 0.8 vvm flow rate (7 days workin in a BCR mode and then switches to spraying mode) | 0.67 mg/g DW | [180] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





