Submitted:
09 October 2024
Posted:
10 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. The Study Population
2.2. HCC Diagnosis
2.3. Liver Function Assessment
2.4. Systemic Therapy
2.5. Assessment of Treatment Effects and Adverse Events
2.6. Statistical Analysis
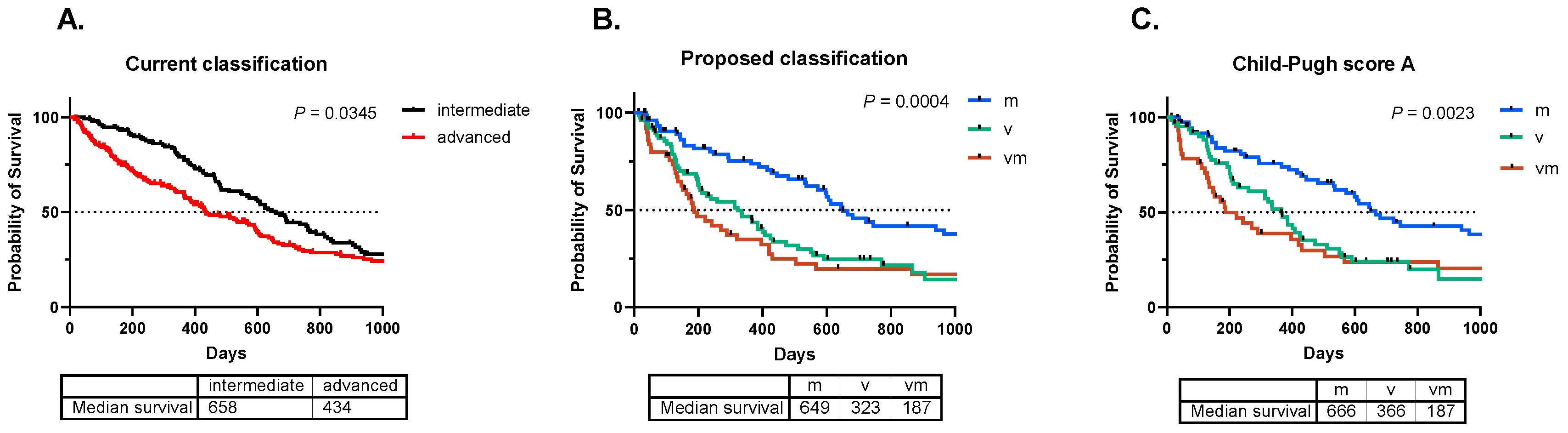
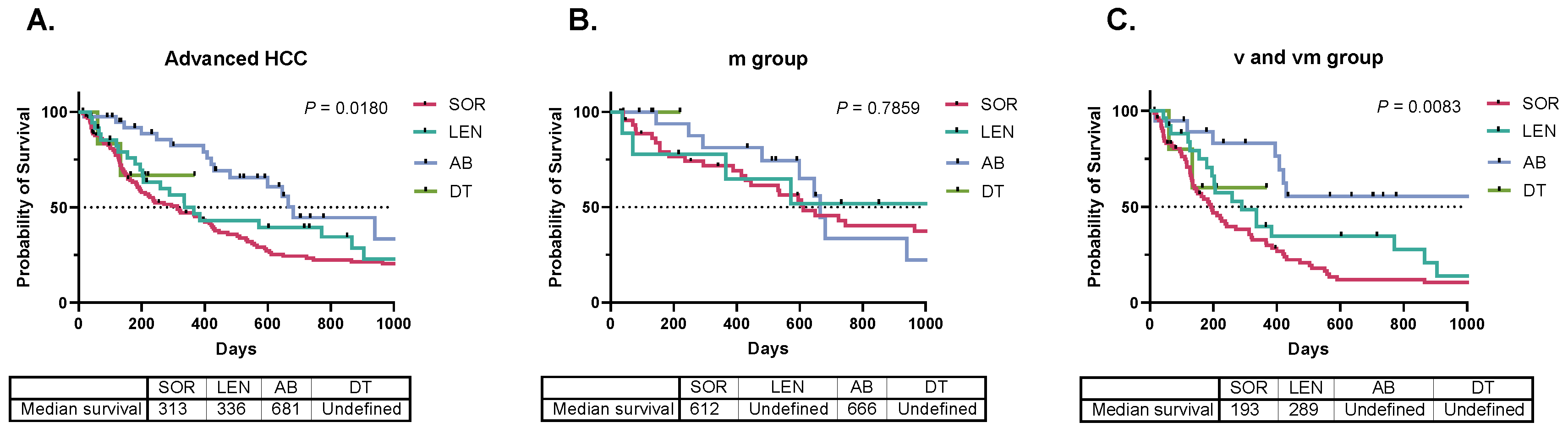
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021 May;71(3):209-249. Epub 2021 Feb 4. [CrossRef] [PubMed]
- Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021 Jan 21;7(1):6. Erratum in: Nat Rev Dis Primers. 2024 Feb 12;10(1):10. doi: 10.1038/s41572-024-00500-6. [CrossRef] [PubMed]
- Huang DQ, Mathurin P, Cortez-Pinto H, Loomba R. Global epidemiology of alcohol-associated cirrhosis and HCC: trends, projections and risk factors. Nat Rev Gastroenterol Hepatol. 2023 Jan;20(1):37-49. Epub 2022 Oct 18. [CrossRef] [PubMed] [PubMed Central]
- Shah PA, Patil R, Harrison SA. NAFLD-related hepatocellular carcinoma: The growing challenge. Hepatology. 2023 Jan 1;77(1):323-338. Epub 2022 Nov 8. [CrossRef] [PubMed] [PubMed Central]
- Kawamura Y, Arase Y, Ikeda K, Seko Y, Imai N, Hosaka T, Kobayashi M, Saitoh S, Sezaki H, Akuta N, Suzuki F, Suzuki Y, Ohmoto Y, Amakawa K, Tsuji H, Kumada H. Large-scale long-term follow-up study of Japanese patients with non-alcoholic Fatty liver disease for the onset of hepatocellular carcinoma. Am J Gastroenterol. 2012 Feb;107(2):253-61. Epub 2011 Oct 18. [CrossRef] [PubMed]
- Loomba R, Lim JK, Patton H, El-Serag HB. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology. 2020 May;158(6):1822-1830. Epub 2020 Jan 30. [CrossRef] [PubMed] [PubMed Central]
- Ioannou, GN. HCC surveillance after SVR in patients with F3/F4 fibrosis. J Hepatol. 2021 Feb;74(2):458-465. Epub 2020 Dec 7. [CrossRef] [PubMed]
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018 Mar 31;391(10127):1301-1314. Epub 2018 Jan 5. [CrossRef] [PubMed]
- Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019 Apr 11;380(15):1450-1462. [CrossRef] [PubMed]
- Brown ZJ, Tsilimigras DI, Ruff SM, Mohseni A, Kamel IR, Cloyd JM, Pawlik TM. Management of Hepatocellular Carcinoma: A Review. JAMA Surg. 2023 Apr 1;158(4):410-420. [CrossRef] [PubMed]
- Kudo M. Recent Advances in Systemic Therapy for Hepatocellular Carcinoma in an Aging Society: 2020 Update. Liver Cancer. 2020 Dec;9(6):640-662. Epub 2020 Nov 17. [CrossRef] [PubMed] [PubMed Central]
- Ganesan P, Kulik LM. Hepatocellular Carcinoma: New Developments. Clin Liver Dis. 2023 Feb;27(1):85-102. Epub 2022 Oct 18. [CrossRef] [PubMed]
- Rossari F, Tada T, Suda G, Shimose S, Kudo M, Yoo C, Cheon J, Finkelmeier F, Lim HY, Presa J, Masi G, Bergamo F, Amadeo E, Vitiello F, Kumada T, Sakamoto N, Iwamoto H, Aoki T, Chon HJ, Himmelsbach V, Iavarone M, Cabibbo G, Montes M, Foschi FG, Vivaldi C, Soldà C, Sho T, Niizeki T, Nishida N, Steup C, Hirooka M, Kariyama K, Tani J, Atsukawa M, Takaguchi K, Itobayashi E, Fukunishi S, Tsuji K, Ishikawa T, Tajiri K, Ochi H, Yasuda S, Toyoda H, Ogawa C, Nishimura T, Hatanaka T, Kakizaki S, Shimada N, Kawata K, Hiraoka A, Tada F, Ohama H, Nouso K, Morishita A, Tsutsui A, Nagano T, Itokawa N, Okubo T, Imai M, Kosaka H, Naganuma A, Koizumi Y, Nakamura S, Kaibori M, Iijima H, Hiasa Y, Persano M, Foti S, Camera S, Stefanini B, Scartozzi M, Cascinu S, Casadei-Gardini A, Rimini M. Disease Etiology Impact on Outcomes of Hepatocellular Carcinoma Patients Treated with Atezolizumab plus Bevacizumab: A Real-World, Multicenter Study. Liver Cancer. 2024 Apr 10;13(5):522-536. [CrossRef] [PubMed] [PubMed Central]
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008 Jul 24;359(4):378-90. [CrossRef] [PubMed]
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009 Jan;10(1):25-34. Epub 2008 Dec 16. [CrossRef] [PubMed]
- Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018 Mar 24;391(10126):1163-1173. [CrossRef] [PubMed]
- Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020 May 14;382(20):1894-1905. [CrossRef] [PubMed]
- Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Lim HY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Ma N, Nicholas A, Wang Y, Li L, Zhu AX, Finn RS. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022 Apr;76(4):862-873. Epub 2021 Dec 11. [CrossRef] [PubMed]
- Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, Sukeepaisarnjaroen W, Kang YK, Van Dao T, De Toni EN, Rimassa L, Breder V, Vasilyev A, Heurgué A, Tam VC, Mody K, Thungappa SC, Ostapenko Y, Yau T, Azevedo S, Varela M, Cheng AL, Qin S, Galle PR, Ali S, Marcovitz M, Makowsky M, He P, Kurland JF, Negro A, Sangro B. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022 Aug;1(8):EVIDoa2100070. Epub 2022 Jun 6. [CrossRef] [PubMed]
- Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022 Mar;76(3):681-693. Epub 2021 Nov 19. [CrossRef] [PubMed] [PubMed Central]
- Mizuno K, Imai N, Yamamoto T, Yokoyama S, Yamamoto K, Ito T, Ishizu Y, Honda T, Kuzuya T, Ishigami M, Kawashima H. Pretreatment Proteinuria Predicts the Prognosis of Patients Receiving Systemic Therapy for Unresectable Hepatocellular Carcinoma. Cancers (Basel). 2023 May 21;15(10):2853. [CrossRef] [PubMed] [PubMed Central]
- Kuzuya T, Ishigami M, Ito T, Ishizu Y, Honda T, Ishikawa T, Fujishiro M. Sorafenib vs. Lenvatinib as First-line Therapy for Advanced Hepatocellular Carcinoma With Portal Vein Tumor Thrombosis. Anticancer Res. 2020 Apr;40(4):2283-2290. [CrossRef] [PubMed]
- Yamamoto T, Imai N, Kuzuya T, Yokoyama S, Yamamoto K, Ito T, Ishizu Y, Honda T, Ishigami M. Changes in Body Composition Predict the Time to Treatment Failure of Lenvatinib in Patients with Advanced Hepatocellular Carcinoma: A Pilot Retrospective Study. Nutr Cancer. 2022;74(9):3118-3127. Epub 2022 Mar 12. [CrossRef] [PubMed]
- Shah A, Tang A, Santillan C, Sirlin C. Cirrhotic liver: What's that nodule? The LI-RADS approach. J Magn Reson Imaging. 2016 Feb;43(2):281-94. Epub 2015 May 21. [CrossRef] [PubMed]
- Terzi E, Giamperoli A, Iavarone M, Leoni S, De Bonis L, Granito A, Forgione A, Tovoli F, Piscaglia F. Prognosis of Single Early-Stage Hepatocellular Carcinoma (HCC) with CEUS Inconclusive Imaging (LI-RADS LR-3 and LR-4) Is No Better than Typical HCC (LR-5). Cancers (Basel). 2022 Jan 11;14(2):336. [CrossRef] [PubMed] [PubMed Central]
- Cai WJ, Ying M, Zheng RQ, Liao J, Luo B, Tang L, Cheng W, Yang H, Wei A, Yang Y, Wang H, Luo YC, Liu C, Zhong H, Yang Q, Yu J, Liang P. Contrast-Enhanced Ultrasound Liver Imaging Reporting and Data System in Hepatocellular Carcinoma ≤5 cm: Biological Characteristics and Patient Outcomes. Liver Cancer. 2023 Jan 24;12(4):356-371. [CrossRef] [PubMed] [PubMed Central]
- Hiraoka A, Michitaka K, Kumada T, Izumi N, Kadoya M, Kokudo N, Kubo S, Matsuyama Y, Nakashima O, Sakamoto M, Takayama T, Kokudo T, Kashiwabara K, Kudo M. Validation and Potential of Albumin-Bilirubin Grade and Prognostication in a Nationwide Survey of 46,681 Hepatocellular Carcinoma Patients in Japan: The Need for a More Detailed Evaluation of Hepatic Function. Liver Cancer. 2017 Nov;6(4):325-336. Epub 2017 Sep 22. [CrossRef] [PubMed] [PubMed Central]
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009 Jan;45(2):228-47. [CrossRef] [PubMed]
- US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) US Department of Health and Human Services; Washington, DC, USA: 2017. Version 5.0.
- Kanda, Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013 Mar;48(3):452-8. Epub 2012 Dec 3. [CrossRef] [PubMed] [PubMed Central]
- Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, Raoul JL, Sangro B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012 Nov;32(4):348-59. Epub 2013 Feb 8. [CrossRef] [PubMed]
- Kudo M, Arizumi T, Ueshima K, Sakurai T, Kitano M, Nishida N. Subclassification of BCLC B Stage Hepatocellular Carcinoma and Treatment Strategies: Proposal of Modified Bolondi's Subclassification (Kinki Criteria). Dig Dis. 2015 Oct;33(6):751-8. Epub 2015 Oct 21. [CrossRef] [PubMed]
| m | v | vm | |||||
| Number of patients | 77 | 78 | 52 | Chi-square test P value | |||
| Sex (M/F) | 64 / 13 | 62 / 16 | 43 / 9 | 0.5318 | |||
| Etiology (B/C/NBNC) | 17 / 21 /39 | 16 / 22 / 40 | 11 / 16 / 25 | 0.6178 | |||
| PS (0/1/2) | 59 / 17 / 1 | 56 / 19 / 3 | 33 / 18 / 1 | 0.0489 | |||
| Medication (SOR/LEN/AB/DT) | 47 / 9 / 20 / 1 | 44 / 18 / 12 / 4 | 35 / 8 / 8 / 1 | 0.0032 | |||
| Turkey's test P value | |||||||
| one-way ANOVA P value | m vs. v | m vs. vm | v vs. vm | ||||
| Age (year) | 72 (34 - 91) | 69 (40 - 92) | 67 (37 - 87) | 0.1654 | - | - | - |
| PFS (day) | 117 (13 - 2704) | 95 (14 - 2129) | 56 (7 - 966) | 0.0503 | - | - | - |
| TTF (day) | 216 (4 - 4071) | 119 (6 - 1146) | 88 (7 - 2370) | 0.0024 | 0.0067 | 0.0108 | 0.9850 |
| AFP (U/mL) | 22 (1 - 382175) | 289 (2 - 656984) | 1413 (2 - 3610200) | 0.1088 | - | - | - |
| DCP (mAU/mL) | 160 (0 - 603470) | 2392 (0 - 500000) | 1431 (10 - 490380) | 0.1410 | - | - | - |
| PT-INR (ratio) | 1.05 (0.91 - 1.33) | 1.08 (0.93 - 1.57) | 1.08 (0.96 - 1.31) | 0.1361 | - | - | - |
| T-bil (mg/dL) | 0.7 (0.4 - 1.6) | 0.9 (0.3 - 5.1) | 0.9 (0.4 - 4.3) | 0.0485 | 0.0529 | 0.0079 | 0.6243 |
| Alb (g/dL) | 3.8 (2.6 - 4.8) | 3.5 (2.2 - 4.4) | 3.6 (2.4 - 4.5) | 0.0040 | 0.0048 | 0.0449 | 0.8924 |
| ALBI | -2.42 (-3.24 - -1.51) | -2.20 (-2.93 - -1.16) | -2.21 (-3.21 - -1.15) | 0.0004 | 0.0009 | 0.0060 | 0.9789 |
| CPS | 5 (5 - 7) | 6 (5 - 8) | 6 (5 - 9) | 0.0030 | 0.0076 | 0.0140 | 0.9940 |
| HCC size (mm) | 23 (0 - 170) | 33 (5 - 182) | 23 (5 - 180) | 0.0075 | 0.0050 | 0.3731 | 0.3006 |
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | |
| m group | 0.50 | 0.35 - 0.71 | 0.0001 | 0.50 | 0.34 - 0.74 | 0.0004 | |
| PS | 2.59 | 1.91 - 3.51 | < 0.0001 | 2.05 | 1.46 - 2.88 | < 0.0001 | |
| CPS | 1.56 | 1.28 - 1.90 | < 0.0001 | 1.31 | 1.02 - 1.68 | 0.0294 | |
| ICI | 0.47 | 0.29 - 0.79 | 0.0038 | 0.71 | 0.42 - 1.21 | 0.2133 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





