Submitted:
08 October 2024
Posted:
10 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Data Extraction
2.3. Inclusion and Exclusion Criteria
2.4. Quality Assessment
3. Results
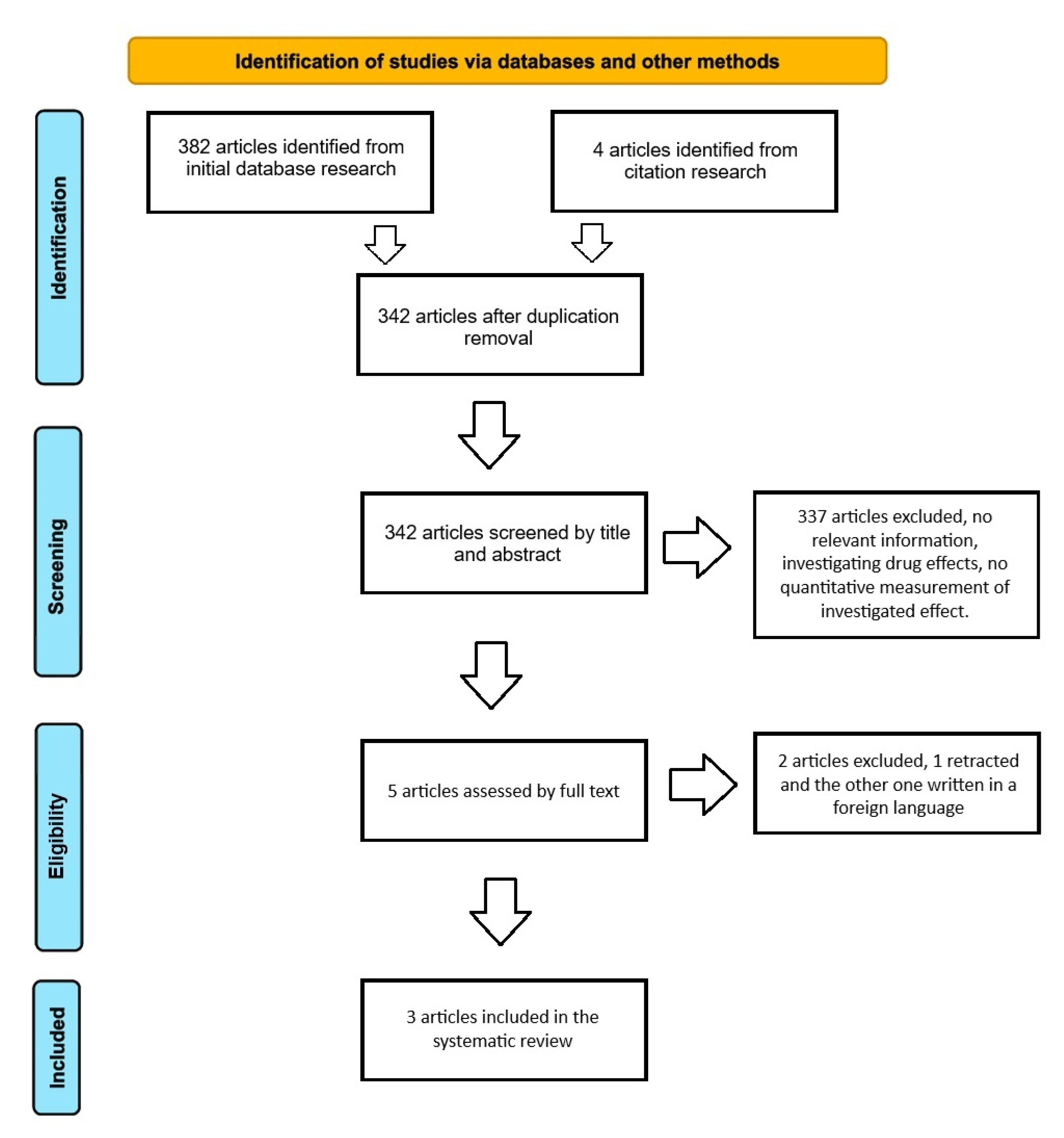
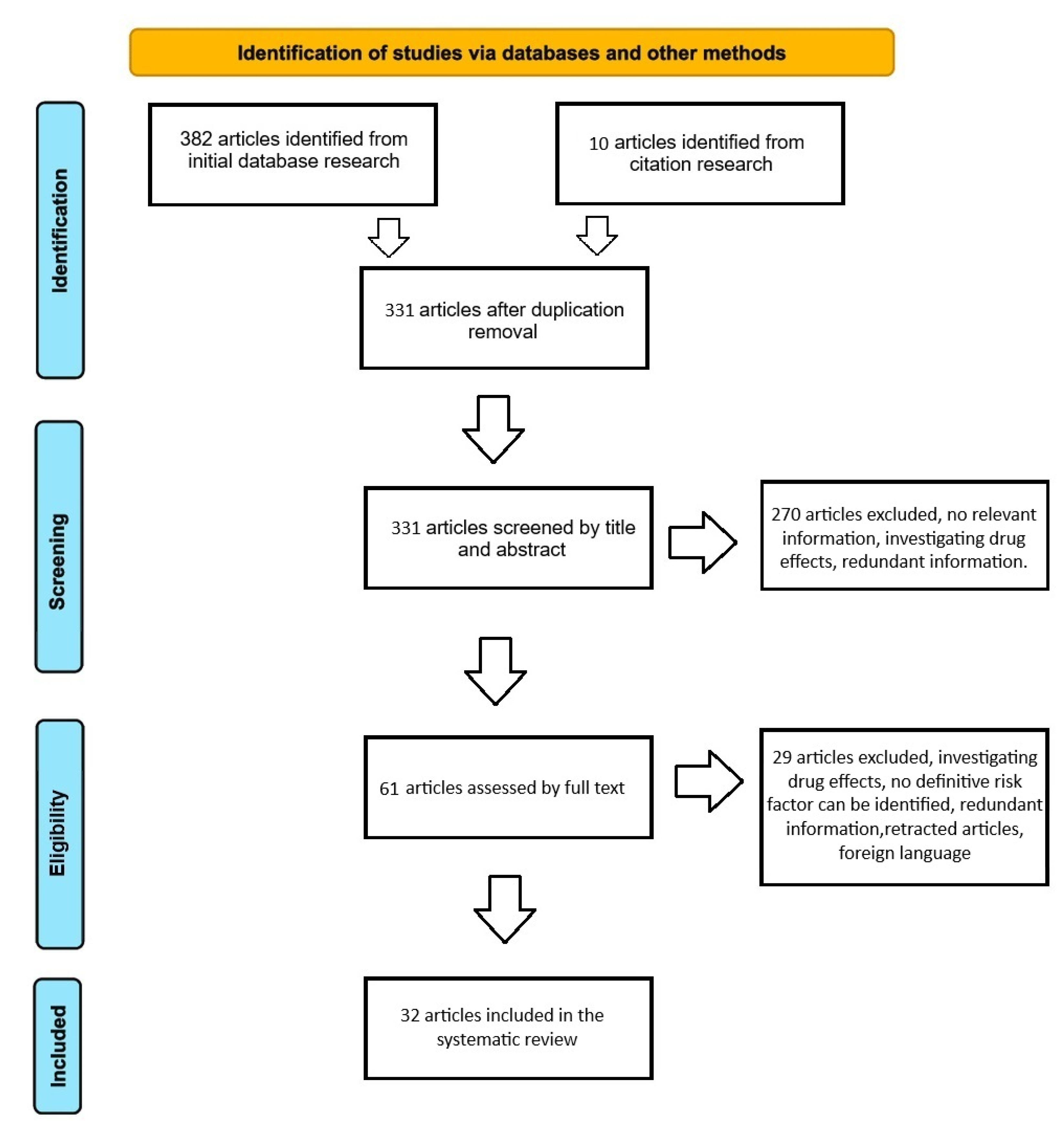
3.1. Search Results
3.2. Characteristics of the Studies and Participants – OP a Risk Factor for BM Development?
3.3. Characteristics of the Studies and Participants – Risk Factors for OP Development in Cancer Patients
3.4. Quality Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sugiura H, Yamada K, Sugiura T, Hida T, Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clinical orthopaedics and related research. 2008 Mar;466:729-36.
- Yang Y, Wang Y, Li X, Xie X. Clinical role of pretreatment albumin-to-alkaline phosphatase ratio in lung cancer: a meta-analysis. Scientific Reports. 2024 Jan 12;14(1):1166.
- Hernandez RK, Wade SW, Reich A, Pirolli M, Liede A, Lyman GH. Incidence of bone metastases in patients with solid tumors: analysis of oncology electronic medical records in the United States. BMC cancer. 2018 Dec;18:1-1.
- Zhang J, Cai D, Hong S. Prevalence and prognosis of bone metastases in common solid cancers at initial diagnosis: a population-based study. BMJ open. 2023 Oct 1;13(10):e069908.
- Office of the Surgeon General (US. Bone health and osteoporosis: a report of the surgeon general.
- Tsuzuki S, Park SH, Eber MR, Peters CM, Shiozawa Y. Skeletal complications in cancer patients with bone metastases. International Journal of Urology. 2016 Oct;23(10):825-32.
- Delea T, Langer C, McKiernan J, Liss M, Edelsberg J, Brandman J, Sung J, Raut M, Oster G. The cost of treatment of skeletal-related events in patients with bone metastases from lung cancer. Oncology. 2005 Jan 1;67(5-6):390-6.
- Groot MT, Kruger CB, Pelger RC, Uyl-de Groot CA. Costs of prostate cancer, metastatic to the bone, in the Netherlands. European urology. 2003 Mar 1;43(3):226-32.
- Andronis L, Goranitis I, Bayliss S, Duarte R. Cost-effectiveness of treatments for the management of bone metastases: a systematic literature review. Pharmacoeconomics. 2018 Mar;36:301-22.
- Drake, MT. Osteoporosis and cancer. Current osteoporosis reports. 2013 Sep;11:163-70.
- Choi HG, Lee JW, Min CY, Yoo DM, Lee SW. Analyses of the association between cervical cancer and osteoporosis/osteoporotic fracture: a cross-sectional study using KoGES HEXA data. International Journal of Clinical Oncology. 2021 Sep;26:1752-8.
- Pfeilschifter J, Diel IJ. Osteoporosis due to cancer treatment: pathogenesis and management. Journal of Clinical Oncology. 2000 Apr 7;18(7):1570-93.
- Akhtar M, Haider A, Rashid S, Al-Nabet AD. Paget’s “seed and soil” theory of cancer metastasis: an idea whose time has come. Advances in anatomic pathology. 2019 Jan 1;26(1):69-74.
- Salamanna F, Borsari V, Contartese D, Aldini NN, Fini M. Link between estrogen deficiency osteoporosis and susceptibility to bone metastases: a way towards precision medicine in cancer patients. The Breast. 2018 Oct 1;41:42-50.
- Choi IA, Umemoto A, Mizuno M, Park-Min KH. Bone metabolism–an underappreciated player. npj Metabolic Health and Disease. 2024 Jul 1;2(1):12.
- Long, F. Building strong bones: molecular regulation of the osteoblast lineage. Nature reviews Molecular cell biology. 2012 Jan;13(1):27-38.
- Wasnich RD, Miller PD. Antifracture efficacy of antiresorptive agents are related to changes in bone density. The Journal of Clinical Endocrinology & Metabolism. 2000 Jan 1;85(1):231-6.
- Borah B, Dufresne TE, Chmielewski PA, Gross GJ, Gross MC, Phipps RJ. Architecture is one of the determinants of bone strength. Journal of Bone and Mineral Research. 2003 Feb 1;18(2):38. [CrossRef]
- Dufresne TE, Chmielewski PA, Manhart MD, Johnson TD, Borah B. Risedronate preserves bone architecture in early postmenopausal women in 1 year as measured by three-dimensional microcomputed tomography. Calcified tissue international. 2003 Oct;73:423-32.
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Bmj. 2015 Jan 2;349.
- National Heart, Lung, and Blood Institute. Quality assessment tool for observational cohort and cross-sectional studies.
- Chen HM, Chen FP, Yang KC, Yuan SS. Association of bone metastasis with early-stage breast cancer in women with and without precancer osteoporosis according to osteoporosis therapy status. JAMA network open. 2019 Mar 1;2(3):e190429-.
- Lee S, Kim HY, Jung YJ, Kang SK, Kim JY, Yun MS. Is bone mineral density a prognostic factor in postmenopausal women with luminal A breast cancer?. Korean Journal of Clinical Oncology. 2023 Jun;19(1):27.
- Bowling GC, Albright JA, Maloney TJ, Quinn MS, Daniels AH, Chesnut GT. Poor Bone Mineral Density Is Associated With Increased Risk of Urological Bone Metastases. Urology. 2024 May 6. [CrossRef]
- Orrantia-Borunda E, Anchondo-Nuñez P, Acuña-Aguilar LE, Gómez-Valles FO, Ramírez-Valdespino CA. Subtypes of breast cancer. Breast Cancer [Internet]. 2022 Aug 6.
- Twiss JJ, Waltman N, Ott CD, Gross GJ, Lindsey AM, Moore TE. Bone mineral density in postmenopausal breast cancer survivors. Journal of the American Academy of Nurse Practitioners. 2001 Jun;13(6):276-84.
- Nicks KM, Fowler TW, Akel NS, Perrien DS, Suva LJ, Gaddy D. Bone turnover across the menopause transition: the role of gonadal inhibins. Annals of the New York Academy of Sciences. 2010 Apr;1192(1):153-60.
- Vehmanen L, Saarto T, Elomaa I, Mäkelä P, Välimäki M, Blomqvist C. Long-term impact of chemotherapy-induced ovarian failure on bone mineral density (BMD) in premenopausal breast cancer patients. The effect of adjuvant clodronate treatment. European Journal of Cancer. 2001 Dec 1;37(18):2373-8.
- Coleman RE, Rathbone E, Brown JE. Management of cancer treatment-induced bone loss. Nature Reviews Rheumatology. 2013 Jun;9(6):365-74.
- Saarto T, Blomqvist C, Välimäki M, Mäkelä P, Sarna S, Elomaa I. Chemical castration induced by adjuvant cyclophosphamide, methotrexate, and fluorouracil chemotherapy causes rapid bone loss that is reduced by clodronate: a randomized study in premenopausal breast cancer patients. Journal of Clinical Oncology. 1997 Apr;15(4):1341-7.
- Delmas PD, Balena R, Confravreux E, Hardouin C, Hardy P, Bremond A. Bisphosphonate risedronate prevents bone loss in women with artificial menopause due to chemotherapy of breast cancer: a double-blind, placebo-controlled study. Journal of Clinical Oncology. 1997 Mar;15(3):955-62.
- Gnant M, Mlineritsch B, Luschin-Ebengreuth G, Kainberger F, Kässmann H, Piswanger-Sölkner JC, Seifert M, Ploner F, Menzel C, Dubsky P, Fitzal F. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. The Lancet oncology. 2008 Sep 1;9(9):840-9.
- Fogelman I, Blake GM, Blamey R, Palmer M, Sauerbrei W, Schumacher M, Serin D, Stewart A, Wilpshaar W. Bone mineral density in premenopausal women treated for node-positive early breast cancer with 2 years of goserelin or 6 months of cyclophosphamide, methotrexate and 5-fluorouracil (CMF). Osteoporosis international. 2003 Dec; 14:1001-6.
- Sverrisdottir A, Fornander T, Jacobsson H, Von Schoultz E, Rutqvist L. Bone mineral density among premenopausal women with early breast cancer in a randomized trial of adjuvant endocrine therapy. Journal of Clinical Oncology. 2004 Sep 15;22(18):3694-9.
- Powles TJ, Hickish T, Kanis JA, Tidy A, Ashley S. Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy premenopausal and postmenopausal women. Journal of Clinical Oncology. 1996 Jan;14(1):78-84.
- Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, Bevers TB, Kavanah MT, Atkins JN, Margolese RG, Runowicz CD. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. Journal of the National Cancer Institute. 2005 Nov 16;97(22):1652-62.
- Eastell R, Adams J, Clack G, Howell A, Cuzick J, Mackey J, Beckmann MW, Coleman RE. Long-term effects of anastrozole on bone mineral density: 7-year results from the ATAC trial. Annals of Oncology. 2011 Apr 1;22(4):857-62.
- Coleman RE, Banks LM, Girgis SI, Vrdoljak E, Fox J, Cawthorn SJ, Patel A, Bliss JM, Coombes RC, Kilburn LS. Reversal of skeletal effects of endocrine treatments in the Intergroup Exemestane Study. Breast cancer research and treatment. 2010 Nov;124:153-61.
- Kim HY, Choi H. Bone density in patients with cervical cancer or endometrial cancer in comparison with healthy control, according to the stages. Maturitas. 2015 May 1;81(1):171.
- Altieri B, Di Dato C, Modica R, Bottiglieri F, Di Sarno A, Pittaway JF, Martini C, Faggiano A, Colao A. Bone metabolism and vitamin D implication in gastroenteropancreatic neuroendocrine tumors. Nutrients. 2020 Apr 8;12(4):1021.
- Lassemillante AC, Doi SA, Hooper JD, Prins JB, Wright OR. Prevalence of osteoporosis in prostate cancer survivors II: a meta-analysis of men not on androgen deprivation therapy. Endocrine. 2015 Nov; 50:344-54.
- Baldessari C, Pipitone S, Molinaro E, Cerma K, Fanelli M, Nasso C, Oltrecolli M, Pirola M, D’Agostino E, Pugliese G, Cerri S. Bone metastases and health in prostate cancer: from pathophysiology to clinical implications. Cancers. 2023 Feb 28;15(5):1518.
- Kwon T, Jeong IG, Park M, You D, Lee J, Kim HK, Hong S, Hong JH, Ahn H, Kim CS. Bone mineral density in prostate cancer: a comparative study of patients with prostate cancer and healthy controls using propensity score matching. Urology. 2014 Feb 1;83(2):385-92.
- Brown SA, Guise TA. Cancer treatment-related bone disease. Critical Reviews™ in Eukaryotic Gene Expression. 2009;19(1).
- Lee JE, Park CY, Lee E, Ji YI. Effect of gynecological cancer and its treatment on bone mineral density and the risk of osteoporosis and osteoporotic fracture. Obstetrics & Gynecology Science. 2020 Jul 8;63(4):470-9.
- Zhang P, Xi H, Yan R. Effects of thyrotropin suppression on lumbar bone mineral density in postmenopausal women with differentiated thyroid carcinoma. OncoTargets and therapy. 2018 Oct 9:6687-92.
- Singh B, Toohey K. The effect of exercise for improving bone health in cancer survivors—A systematic review and meta-analysis. Journal of science and medicine in sport. 2022 Jan 1;25(1):31-40.
- Melton LJ, Alothman KI, Khosla S, Achenbach SJ, Oberg AL, Zincke H. Fracture risk following bilateral orchiectomy. The Journal of urology. 2003 May;169(5):1747-50.
- Mandal, CC. High cholesterol deteriorates bone health: new insights into molecular mechanisms. Frontiers in endocrinology. 2015 Oct 23;6:165.
- Hatano T, Oishi Y, Furuta A, Iwamuro S, Tashiro K. Incidence of bone fracture in patients receiving luteinizing hormone-releasing hormone agonists for prostate cancer. BJU international. 2000 Sep;86(4):449-52.
- Salamanna F, Borsari V, Contartese D, Aldini NN, Fini M. Link between estrogen deficiency osteoporosis and susceptibility to bone metastases: a way towards precision medicine in cancer patients. The Breast. 2018 Oct 1;41:42-50.
- Ferreira Poloni P, Vespoli HD, Almeida-Filho BD, Bueloni-Dias F, Nahas-Neto J, Nahas EA. Low bone mineral density is associated with breast cancer in postmenopausal women: a case–control study. Climacteric. 2017 Sep 3;20(5):491-7.
- Ballon-Landa E, Panian J, Derweesh IH, McKay RR. Management of bone complications in patients with genitourinary malignancies. InUrologic Oncology: Seminars and Original Investigations 2020 Mar 1 (Vol. 38, No. 3, pp. 94-104). Elsevier.
- MacDonald IJ, Tsai HC, Chang AC, Huang CC, Yang SF, Tang CH. Melatonin inhibits osteoclastogenesis and osteolytic bone metastasis: implications for osteoporosis. International journal of molecular sciences. 2021 Aug 30;22(17):9435.
- Hung YC, Yeh LS, Chang WC, Lin CC, Kao CH. Prospective study of decreased bone mineral density in patients with cervical cancer without bone metastases: a preliminary report. Japanese journal of clinical oncology. 2002 Oct 1;32(10):422-4.
- CHO SH, CHO SH, LEE JA, MOON H, KIM DS. Reduced spinal bone mass in patients with uterine cervical cancer. Obstetrics & Gynecology. 1991 Oct 1;78(4):689-92.
- Nunes FA, Farias ML, Oliveira FP, Vieira L, Lima LF, Paranhos FD, Mendonça LM, Madeira M. Use of aromatase inhibitors in patients with breast cancer is associated with deterioration of bone microarchitecture and density. Archives of Endocrinology and Metabolism. 2021 Jul 28;65(4):505-11.
- Parker C, Castro E, Fizazi K, Heidenreich A, Ost P, Procopio G, Tombal B, Gillessen S. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2020 Sep 1;31(9):1119-34.
- Loibl S, André F, Bachelot T, Barrios CH, Bergh J, Burstein HJ, Cardoso MJ, Carey LA, Dawood S, Del Mastro L, Denkert C. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up☆. Annals of Oncology. 2024 Feb 1;35(2):159-82.
| ID | Year | Country | No. of Patients | No. of Controls | Study type | Cancer subtype | Comparison | Reported value |
|---|---|---|---|---|---|---|---|---|
| Hsiu-Man Chen [22] | 2019 | Taiwan | 9104 - breast cancer 14 020 - precancer osteoporosis | NA | Nationwide retrospective cohort study | Breast cancer - subtype not specified | Breast cancer group – precancer osteoporosis vs without | No additional risk of bone metastasis (aHR, 0.87; 95% CI, 0.58-1.30; P = .49) |
| Precancer osteoporosis group – osteoporosis therapy vs NUL | No association with risk of bone metastasis (bisphosphonates: aHR, 1.47; 95% CI, 1.00-2.17; P = .05; non-bisphosphonate drugs: aHR, 1.00; 95% CI, 0.72-1.39; P > .99) | |||||||
| Breast cancer group – no precancer osteoporosis vs untreated osteoporosis vs treated osteoporosis | Median time to develop bone metastasis was shorter in the untreated group vs the other two groups. (no precancer osteoporosis 2.87 years; IQR, 1.34-4.86 years) vs (untreated 1.74 years; IQR, 0.58-3.60 years; P < .001) vs (bisphosphonates: 2.34 years; IQR, 1.23-3.13 years; non-bisphosphonate drugs: 2.08 years; IQR, 0.92-4.95 years) |
|||||||
| Seungju Lee [23] | 2023 | South Korea | 348 - breast cancer | 129 – normal bone density | Retrospective study | Breast cancer – Luminal A | Breast cancer – low BMD vs normal BMD | BMD not statistically significant on five-year DFS (98.2% - low BMD group vs 95.0% - normal BMD group, P=0.33), metastases (5 y - 4 in the normal BMD group vs 2 in the low BMD group) or incidence of contralateral breast cancer |
| Breast cancer – no change in BMD over time vs improvement vs worsening BMD | DFS at 5 y was 97.0% for - no change in the BMD group, 94.6% for the BMD improvement group, and 98.4% for the BMD degradation group (P=0.79). | |||||||
| Gartrell C. Bowling [24] | 2024 | USA | 69,721 - kidney cancer, 84,755 - bladder cancer, 184,855 – prostate cancer, 3202 -testicular cancer. | Matched control cohort from a total pool of 685,066 patients with urological cancers. | PearlDiver Database - retrospective, propensity-matched cohort analysis | Kidney, bladder, prostate, and testicular cancer. | Kidney, bladder, prostate, and testicular cancer groups – PBMD vs matched control group | Strong assoiation at 1 week of cancer diagnosis (kidney: adjusted odds ratio [aOR], 2.37, P <.001; bladder: [aOR], 2.37, P <.001; prostate: [aOR], 2.84, P <.001; testicular: [aOR], 4.45, P <.001). Bisphosphonates use associated with reduced risk of kidney ([aOR], 0.46, P <.001), bladder ([aOR], 0.61, P <.001), and prostate ([aOR], 0.66, P <.001) cancer bone metastasis. |
| Year | Country | No. of patients | Study type | Cancer subtype | Risk factor | Type of effect | Reported value or conclusion | |
|---|---|---|---|---|---|---|---|---|
| Janice J. Twiss [26] | 2005 | USA | 30 | Longitudinal | Breast | Weight Age Months since diagnosis Months since menopause Daily calcium intake Cigarettes per day Daily caffeine intake |
Protective NUL NUL NUL NUL NUL NUL |
Weight and BMD gm/cm2 score at the spine (r=.417, p=.022), and at the hip (r=.458, p=.011). The subjects were all postmenopausal women. |
| Kristy M. Nicks [27] | 2010 | USA | - | Review | Breast | Gonadal inhibin of the transforming growth factor (TGF)-B superfamily levels | Protective | Bone loss in pre-menopausal women due to anti-cancer treatment – leads to low levels of inhibins |
| L Vehmanen [28] | 2001 | Finland | 73 | RCT | Breast | Adjuvant chemotherapy -CMF: cyclophosphamide, methotrexate, 5-fluorouracil | Deleterious | Chemotherapy-induced ovarian failure induced accelerated bone loss (p<0.001 in both spine and femoral neck; changes in BMD -0,3% in the menstruating group and -5,8% in the amenorrhea group) |
| Robert E. Coleman [29] | 2013 | UK | - | Review | Breast Prostate |
ADT Ovarian failure High dose glucocorticoids Fatigue-related immobility |
Deleterious Deleterious Deleterious Deleterious |
Early chemotherapy-induced bone loss might not be caused by the direct action of the chemotherapy agent but due to induced menopause, corticoid use, and immobility |
| T. Saarto [30] | 1997 | Finland | 148 | RCT | Breast | Adjuvant chemotherapy -CMF: cyclophosphamide, methotrexate, 5-fluorouracil Ovarian failure |
Deleterious Deleterious |
The mean difference at 2 years -4.6 CI (-6.9 to -2.3) in the amenorrhea group vs regular menses group at 2 years 0.0 CI (-3.9 to +3.9) |
| P. D. Delmas [31] | 1997 | France | 53 | RCT | Breast | Chemotherapy-induced ovarian failure | Deleterious | 2-y mean difference 2.5% +/- 1.2% (95% CI, 0.2 to 4.9) – (P=.041) lumbar spine, 2.6%+/-1.1% (95% CI,0.3 to 4.8) – (P=.029) femoral neck |
| Michael Gnant [32] | 2011 | Austria | 404 | RCT | Breast | OFS Anastrozole + OFS Tamoxifen + OFS |
Deleterious Deleterious |
GnRH analogs in pre-menopausal women – BMD after 3y reduced by 11.3% and 7.3% lumbar spine and trochanter – over the next 2y for the 75% of women who regained menses BMD at both sites recovered. Anastrozole + OFS – had higher bone loss than the tamoxifen + OFS – 13.6% vs -9% at 3y |
| I. Fogelman [33] | 2003 | UK | 1640 – sub-study involved 96 patients (53 in the goserelin group vs 43 in the CMF group) | RCT | Breast | OFS Adjuvant chemotherapy – CMF protocol |
Deleterious Deleterious |
Premenopausal subjects – GnRH analog adjuvant vs adjuvant CMF chemotherapy – at 2y, lumbar spine -10.5% OFS group vs -6.5% CMF group (p=.0005). Femoral neck OFS group -6.4% and -4.5% CMF group (p=.04). At 3 y partial recovery was observed in the OFS group (ovarian function regain). By contrast the BMD loss in the CMF group was persistent thus at 3y no difference was observed between the groups. |
| Á. Sverrisdóttir [34] | 2004 | Sweden | 89 | RCT | Breast | OFS vs OFS + Tamoxifen vs Tamoxifen alone |
Deleterious Deleterious Deleterious |
Mean change at 2y: -5%, P=.001 vs mean changes -1.4%, P=.02 vs -1.5%, P=.001. OFS group only showed partial recovery after 1y of cessation 1.5%, P=.02 Tamoxifen seems to counteract the effect of OFS on BMD. Different effects are based on baseline hormonal status. |
| T J Powles [35] | 1996 | UK | 179 | RCT | Breast | Tamoxifen | Deleterious in the premenopausal context Protective in the postmenopausal context |
BMD decreased progressively in the spine (P=.001) and hip(P<.05). Annual loss of BMD under Tamoxifen for premenopausal women was 1.44 Opposite effect on postmenopausal women with an annual increase in BMD of 1.17% in the spine (P=.005) and 1.71% in the hip (P<.001). |
| Bernard Fisher [36] | 2005 | USA | 13388 | RCT | Breast prevention | Tamoxifen | Protective in the postmenopausal context | 32% reduction in osteoporotic fractures at 7y follow up (RR=.68, 95%CI= .51 to .92). |
| R. Eastell [37] | 2011 | UK | 71 | RCT | Breast | Tamoxifen Anastrozole |
Detrimental even after treatment cessation Detrimental effect fades after treatment cessation |
Lumbar and total hip median BMD after 1 and 2 years of treatment cessation: -0.79% (P=.2), -0.30%(P=.9) respectively total Hip median BMD at 1 year and 2 years: -2.09%(P=.0003) respectively -2.52%(P=.0002) Lumbar spine increase: +2.35%(P=.04), at 1y +4.02%(P=.0004) at 2years Total hip median BMD: +0.71% (P=.3) at 1 year +0.5%(P=.8) at 2 years |
| Robert E. Coleman [38] | 2010 | UK | 4724 patients in the main study 206 patients in the bone sub-study |
RCT | Breast | Tamoxifen vs Exemestane |
The trend of persistence of the detrimental effect Detrimental effect fades |
At 2 years from the end of treatment with Exemestane spine BMD increased by +1.53% (P=.001) and decreased by -1.93% (P=.0002) after cessation of Tamoxifen. Changes at 2y in the two groups were similar with both treatment strategies. |
| HY Kim [39] | 2015 | Korea | 218 cervical cancer patients 85 endometrial cancer patients 259 healthy controls |
RCT | Cervical cancer Endometrial cancer |
Cervical cancer in postmenopausal status - pretreatment Endometrial cancer in postmenopausal status – pretreatment |
Detrimental No statistically relevant difference |
Osteoporosis was more frequent in the cervical cancer group 18.81% vs 10.81% in the control group and osteopenia was 38.99% vs 36.29% in the control group The endometrial cancer group also showed a higher incidence of osteoporosis (16.47%) while osteopenia was lower (28.24%) but no statistical difference (P=.228) |
| Barbara Altieri [40] | 2020 | Germany | - | Review | GEP-NET | GEP-NET Supplementation with vitamin D |
Detrimental Potentially protective |
Up to 76% of cases of GEP-NET patients present osteoporosis or osteopenia. Potential benefits of Vit. D supplementation in cases of insufficient or deficient Vit. D levels |
| Annie-Claude M. Lassemillante [41] | 2015 | Australia | 5812 studies initial search 15 articles for final review |
Meta-analysis |
Prostate cancer | Prostate cancer | Detrimental | Up to 37.8% of PC hormone naïve patients show osteoporosis, thus PC through bone resorption promotion might be a risk factor |
| Cinzia Baldessari [42] | 2023 | Italy | - | Review | Prostate cancer | LHRH analogs Older age Lower body mass index Bilateral orchiectomy |
Detrimental Detrimental Detrimental Detrimental |
The usual rate of bone loss in men varies between 0,5% and 1%. In patients with metastatic PC in the first year of ADT, bone loss varies between 2-8% at the lumbar spine level, and between 1,5-6.5% at the hip. At the end of ADT, BMD may increase in the lumbar spine while remaining low at other sites. |
| Taekmin Kwon [43] | 2014 | Korea | 3122: 502 PC group matched with 502 control group |
Retrospective propensity score matched | Prostate cancer | Prostate cancer Bone metastasis BMI |
Detrimental Detrimental Low - detrimental |
Higher osteoporosis incidence in the PC group (P=.0001) Independent predictor of osteoporosis (OR 3.45, P=.002) Continuous, OR 0.75, P<.001) |
| Sue A. Brown [44] | 2009 | USA | - | Review | Breast cancer | Tamoxifen Anastrozole Oophorectomy Chemotherapy OFS |
Detrimental/Protective Detrimental Detrimental Detrimental Detrimental |
Tamoxifen’s effect varies based on estrogen levels in premenopausal women More tissue level estrogen deprivation thus BMD losses |
| Jeong Eun Lee [45] | 2020 | Korea | 243 gynecological cancer patients: 105 cervical; 63 endometrial 75 ovarian 240 controls |
Retrospective | Cervical cancer Endometrial cancer Ovarian cancer |
Cervical cancer Endometrial cancer Ovarian cancer |
Detrimental No statistically relevant difference Detrimental |
Lower BMD for cervical cancer group -1st, 2nd lumbar and femoral neck – average score and SD, -0.9 +/- 1.4, P=.013; -0.8+/-1.5, P=.029; -0.9+/-1.0, P=.029 No statistical difference for the endometrial group Lower BMD from the 1st to the 4th lumbar at each level: -1.2+/- 1.4, P=.00; -1.1+/- 1.5, P=.001; -0.9+/- 1.5, P=.007; -0.7+/-1.5, P=.004; |
| Cervical cancer post-treatment 1y Endometrial cancer post-treatment 1y Ovarian cancer post-treatment 1y |
Detrimental Detrimental Detrimental |
Differences in BMD ofL3, L4 and femoral neck (P=.043; P=.022; P=.026) BMD of the endometrial group decreased significantly after the treatment Changes in BMD are lowest in patients who only underwent surgical treatment Highest bone loss in patients who underwent chemoradiotherapy after surgery |
||||||
| Pei Zhang [46] | 2018 | China | 225 postmenopausal women with DTC thyroid residual ablation or metastasis treatment | RCT | Differentiated thyroid carcinoma – papillary and follicular carcinoma | Postoperative thyroid–stimulating hormone suppression | No statistically relevant difference | Reduction of 1.9% in BMD in lumbar spine at 2y, but not statistically different |
| Benjamin Singh [47] | 2021 | Australia | 26 trials, interventions ranging from 12 weeks to 2 years | Meta-analysis | Cancer survivors – mostly breast cancer | Mixed-mode exercises: aerobic, resistance, mixed-mode and others | Protective | Whole body BMD, trochanter BMD, femoral neck BMD, and hip BMD were positively impacted, SMD range: .19-0.39, all p<.05) vs controls |
| L. Joseph Melton [48] | 2003 | USA | 429 | Retrospective | Prostate cancer | Bilateral orchiectomy Age Inactivity |
Detrimental Detrimental Detrimental |
Cumulative incidence of fractures after 15 years of 40% vs 19% expected (P=<.001) |
| Chandi C. Mandal [49] | 2015 | India | - | Review | Breast cancer, multiple myeloma | Statins | Protective | Levels of cellular cholesterol of cells occupying the bone microenvironment e.g. osteoblasts, osteoclasts, and metastasized cancer cells, might be a good predictor for bone health, thus agents that modulate the levels of intracellular cholesterol might improve bone health. |
| T. Hatano [50] | 2002 | Japan | 218 | RCT | Prostate cancer | ADT | Deleterious | The bone density in the fracture group was significantly lower than in the non-fracture group. There is a need to evaluate and treat secondary osteoporosis in patients receiving long-term ADT. |
| F. Salamanna [51] | 2018 | Italy | - | Review | Breast cancer Prostate cancer Lung cancer Others |
17 beta-estradiol levels | Low – deleterious | Low levels of 17 beta-estradiol due to menopause will lead to accelerated bone loss, and microarchitectural deterioration which in turn can lead to increased risk of fractures and uncoupling in the remodeling unit. |
| P. Ferreira Poloni [52] | 2017 | Brazil | 112 breast cancer survivors 224 controls |
Prospective case-control study | Breast cancer survivors | Breast cancer Chemotherapy history Regular physical activity High BMI (≥30) |
Deleterious Deleterious Protective Protective |
Higher incidence of osteopenia and osteoporosis in the femoral neck vs controls (39.3% vs 9%, P=.0005). Lumbar spine BMD did not differ between groups. OR 6.90, 95% CI 5.57-9.77 OR 0.24, 95% CI 0.06-0.98 OR 0.09, 95% CI 0.02-0.37 |
| Eric Ballon-Landa [53] | 2019 | USA | - | Review | Prostate cancer Renal cell carcinoma |
Long term ADT Nephron sparing surgery |
Deleterious Protective |
Average BMD decreased between 1.4 to 2.6% per year between 3 to 8 years of ADT treatment Significantly less osteoporosis between NSS 3.7 vs RN 7.0, P<.0001 |
| Ioana J. McDonald [54] | 2021 | Taiwan | - | Review | Breast, prostate, lung, bladder, and others (e.g. osteosarcoma | Melatonin supplementation | Protective | After 1y of treatment, femoral neck BMD increased by 0.5% with 1mg/ day supplementation and 2.3% with 3mg/day. 6 months of supplementation showed only a trend of bone resorption decrease Over 1y of treatment improved lumbar spine BMD by 4.3%. |
| Yao-Ching Hung [55] | 2002 | Taiwan | 50 cervical cancer patients 50 controls |
Prospective | Non-metastatic cervical cancer | Cervical cancer | Deleterious | Significantly lower BMD in the cervical cancer group P<.05 |
| Sooh Cho [56] | 1991 | Korea | 85 cervical cancer patients 148 controls |
Prospective | Cervical cancer patients | Cervical cancer | Deleterious | Age-adjusted and mean menopause duration-adjusted, cervical cancer group presented 12.8% lower spinal BMD, P=.0003 |
| Nunes FA [57] | 2021 | Brazil | 34 postmenopausal women with breast cancer 17 AI group 17 non-AI group |
Cross-sectional study | Breast cancer | AI exposure vs post-chemotherapy patients |
Deleterious | AI group had lower areal bone mineral density and T-scores at the hip. There is a higher incidence of osteoporosis on DXA scan, 47% in the AI group vs 17.6% in the non-AI group. |
| Hsiu-Man Chen [1] | Seungju Lee [2] | Gartrell C. Bowling [3] | |
|---|---|---|---|
| Was the research question or objective in this paper clearly stated? | 1 | 1 | 1 |
| Was the study population clearly specified and defined? | 1 | 1 | 1 |
| Was the participation rate of eligible persons at least 50%? | 0 | NA | 1 |
| Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? | 0 | 1 | 1 |
| Was a sample size justification, power description, or variance and effect estimates provided? | 0 | 0 | 0 |
| For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? | 1 | 1 | 1 |
| Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? | 1 | 1 | 1 |
| For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as a continuous variable)? | 0 | 0 | 0 |
| Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | 1 | 1 | 1 |
| Was the exposure(s) assessed more than once over time? | 1 | 1 | 1 |
| Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | 1 | 0 | 1 |
| Were the outcome assessors blinded to the exposure status of participants? | 0 | 0 | 0 |
| Was loss to follow-up after baseline 20% or less? | 0 | NA | NA |
| Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? | 1 | 0 | 1 |
| Score | 8 | 7 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




