Submitted:
04 October 2024
Posted:
08 October 2024
You are already at the latest version
Abstract
Keywords:
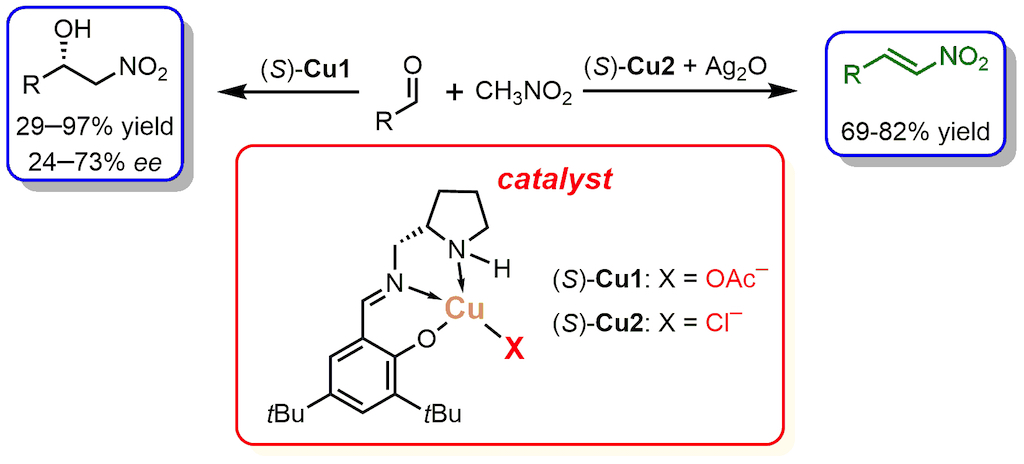
1. Introduction
2. Results and Discussion
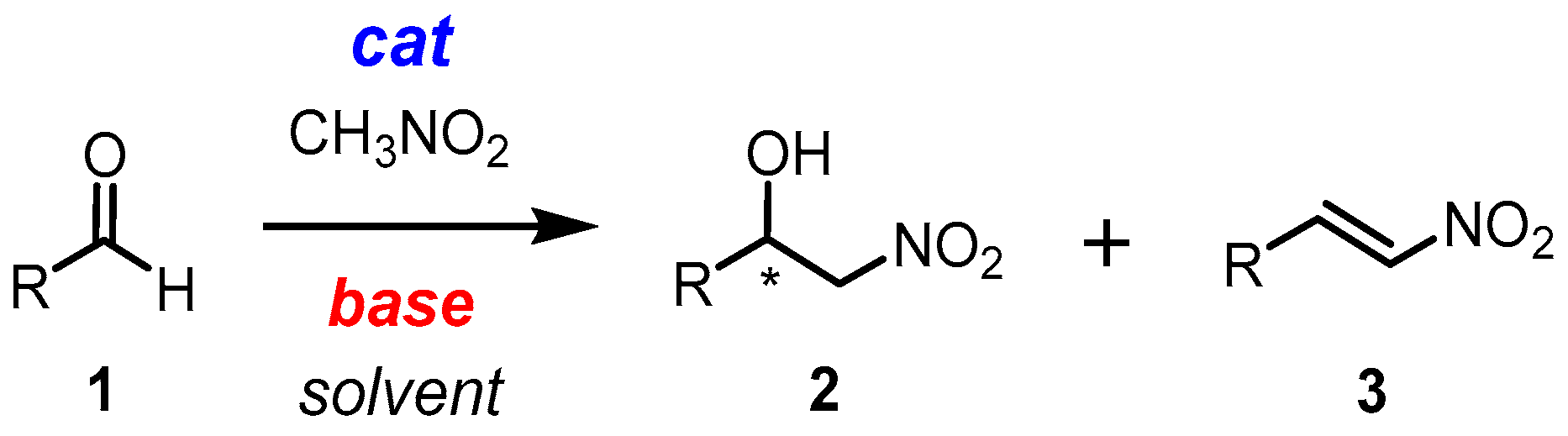
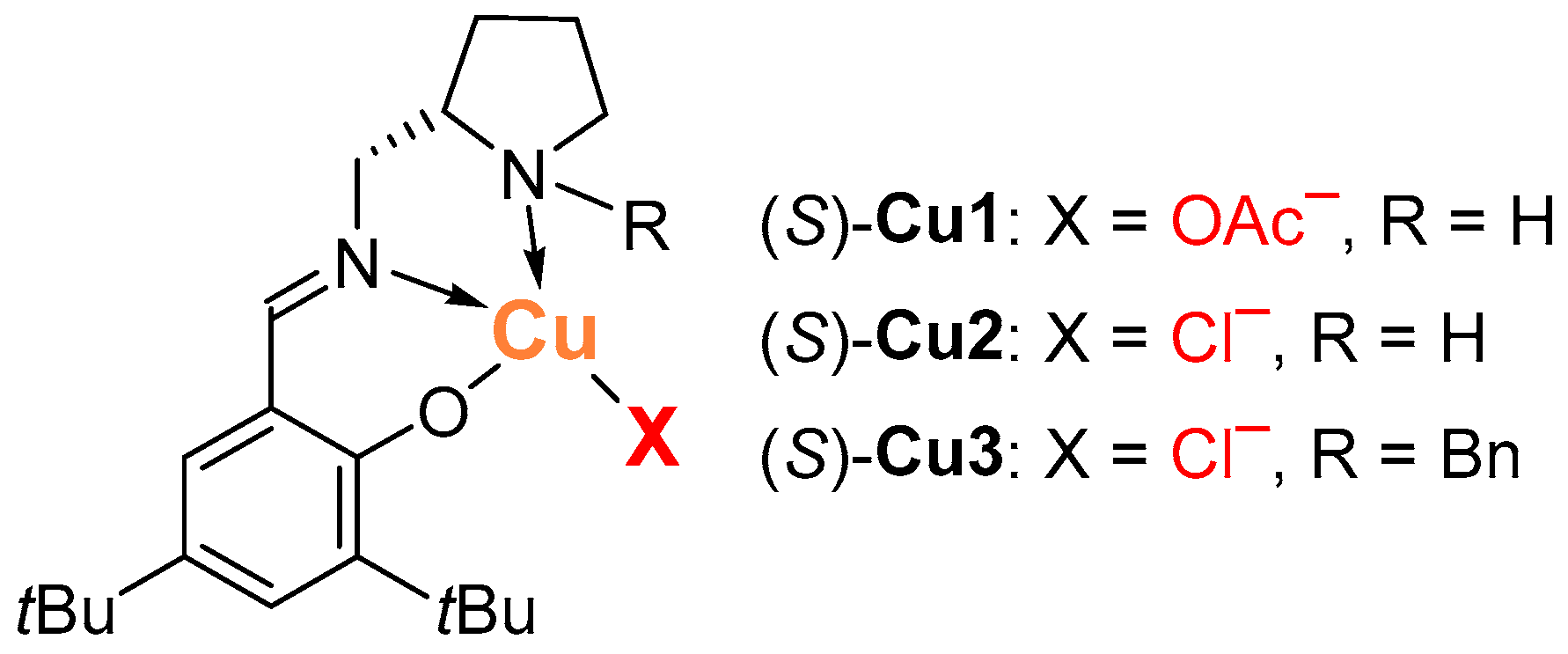
2.1. Henry Reaction Conditions Screening
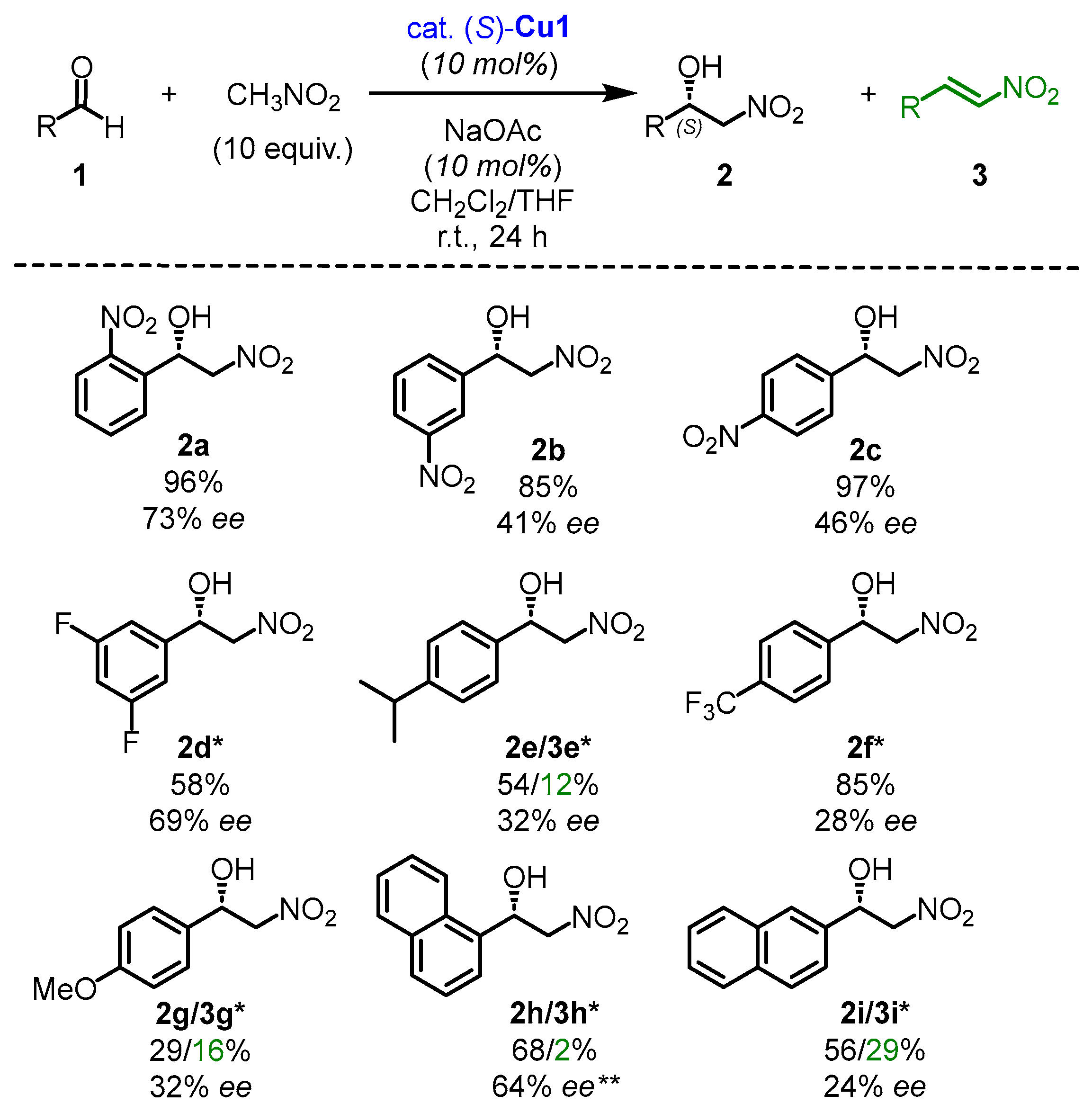
2.2. The Scope of Aldehydes in the Enantioselective Henry Reaction with the Complex (S)-Cu1
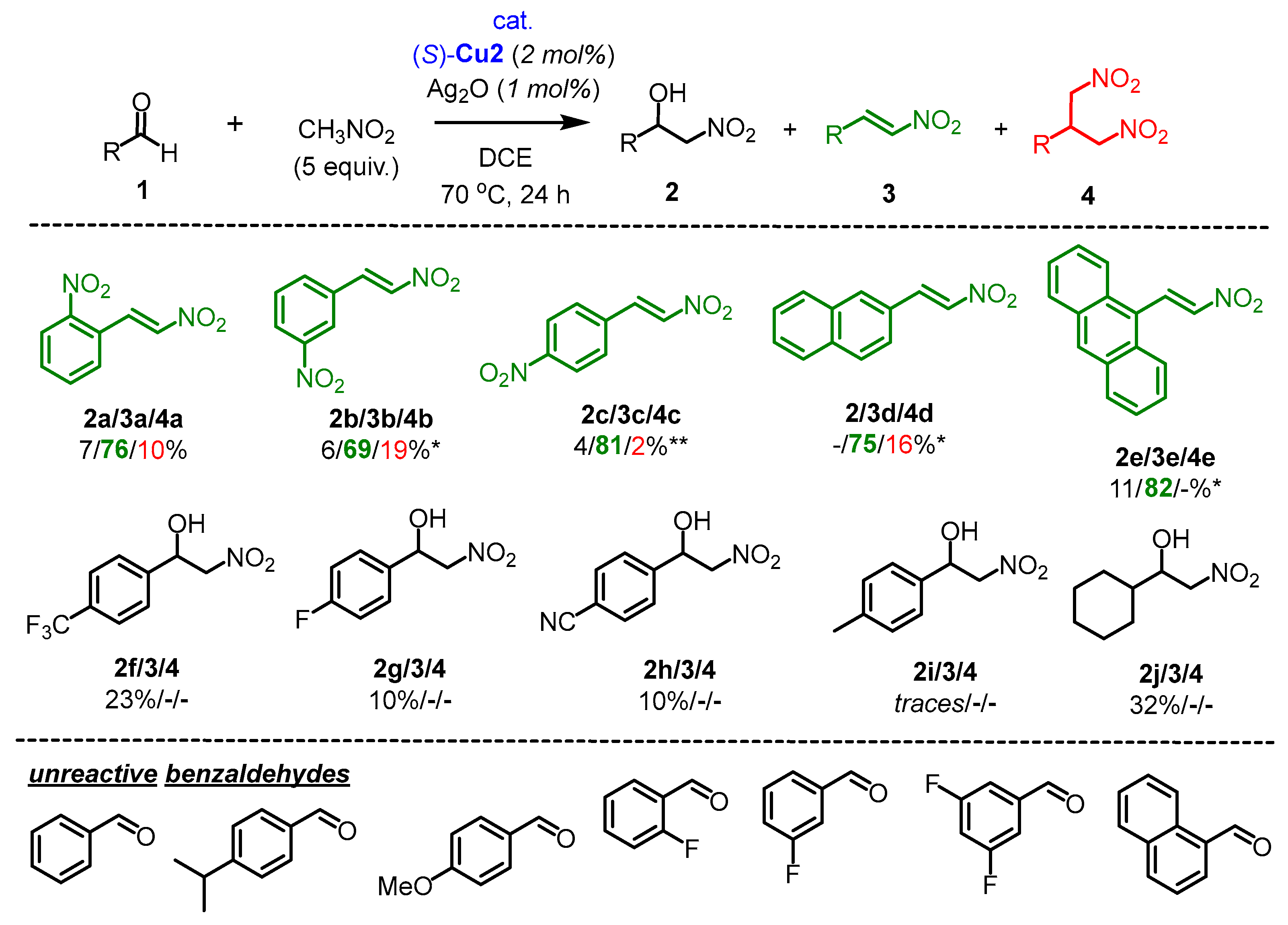
2.3. The Scope of Aldehydes in the Synthesis of β-Nitrostyrenes 3
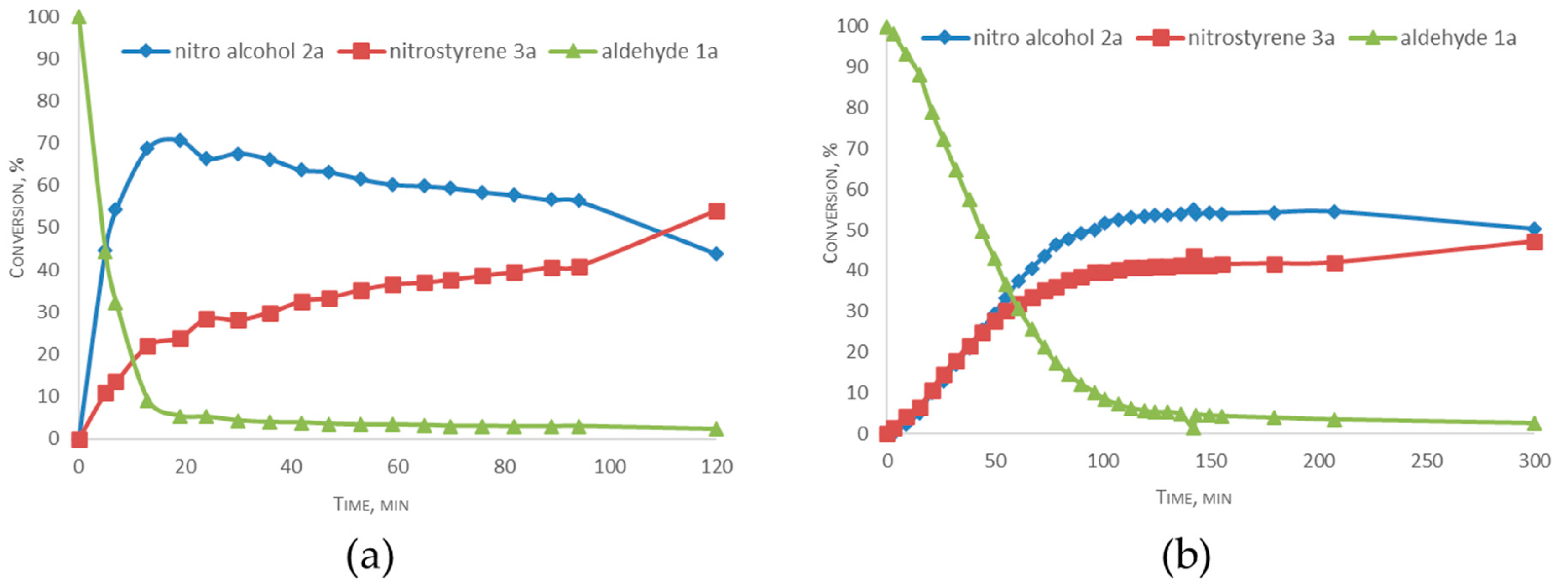
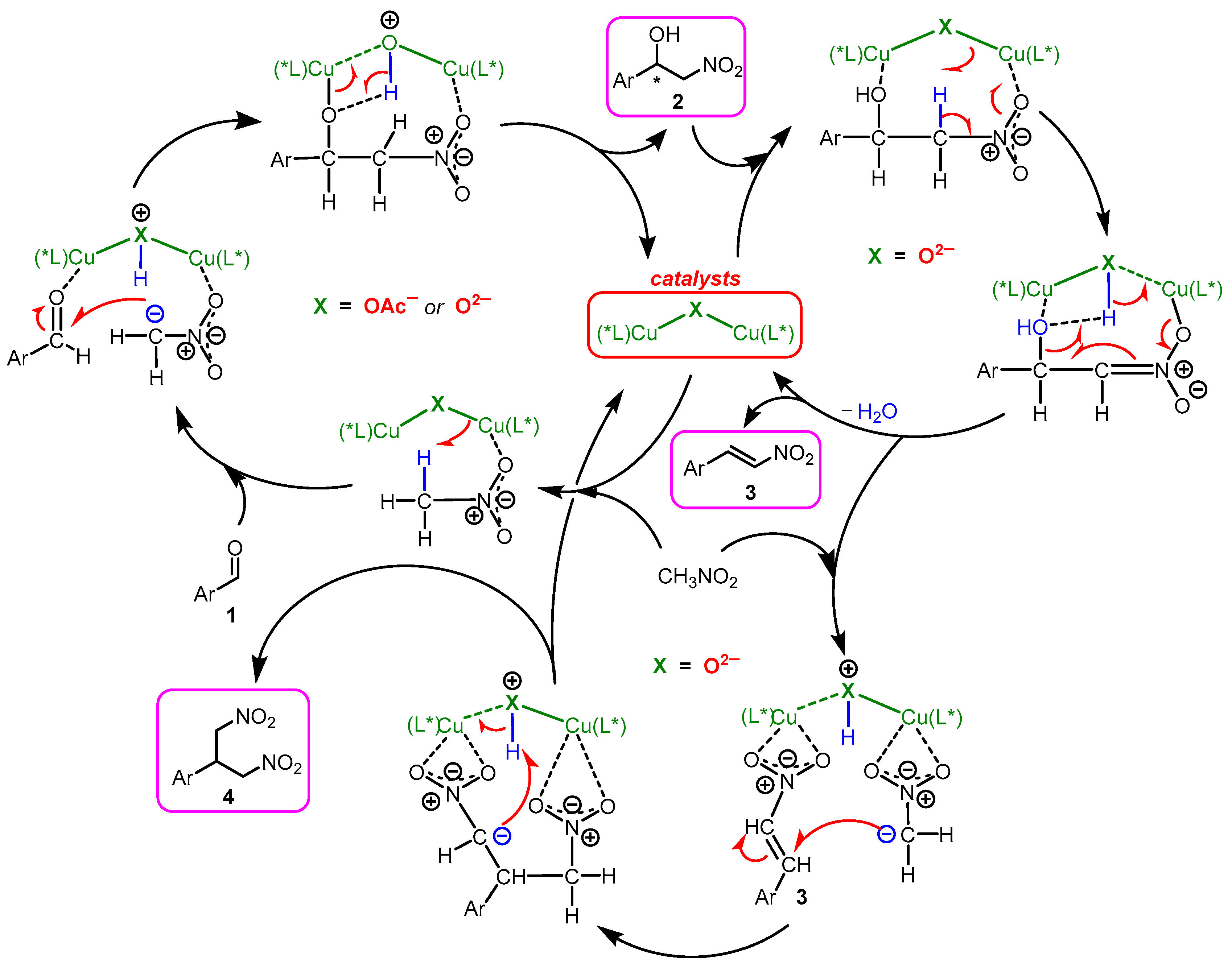
2.4. Mechanism Related Experiments
3. Materials and Methods
3.1. General Information
3.2. Instrumentation
3.3. Synthesis
3.3.1. General Procedure for the Enantioselective Henry Reaction
- 1-(2-nitrophenyl)-2-nitroethan-1-ol (2a)
- 1H NMR (400 MHz, CDCl3): δ = 8.11–8.08 (m, 1H, ArH), 7.98–7.96 (m, 1H, ArH), 7.78–7.74 (m, 1H, ArH), 7.59–7.55 (m, 1H, ArH), 6.07 (ddd, J = 8.8, 4.2, 2.2 Hz, 1H), 4.89 (dd, J = 13.9, 2.2 Hz, 1H), 4.57 (dd, J = 13.9, 8.8 Hz, 1H), 3.15 (d, J = 4.2 Hz, 1H) ppm.
- All spectroscopic data were in agreement with the literature [14b].
- The enantiomeric excess was established by HPLC analysis using a Kromasil 3-AmyCoat column, ee = 73% (conditions: heptane/isopropanol = 90:10, flow rate: 1 mL/min, 254 nm, 25 ˚C, tR(major) = 12.7 min, tR(minor) = 11.0 min).
- All spectroscopic data were in agreement with the literature [14a].
- 1-(3-nitrophenyl)-2-nitroethan-1-ol (2b)
- 1H NMR (CDCl3, 300 MHz): δ = 8.33–8.28 (m, 1H), 8.24–8.15 (m, 1H), 7.77 (d, J = 7.7 Hz, 1H), 7.64–7.56 (m, 1H), 5.66–5.55 (m, 1H), 4.68–4.54 (m, 2H), 3.46–3.40 (m, 1H) ppm.
- The enantiomeric excess was established by HPLC analysis using a Chiralcel OD-H column, ee = 41% (conditions: hexane/isopropanol = 90:10, flow rate: 1 mL/min, 254 nm, 25 ˚C, tR(major) = 28.4 min, tR(minor) = 25.0 min).
- All spectroscopic data were in agreement with the literature [17a].
- 1-(4-nitrophenyl)-2-nitroethan-1-ol (2c)
- 1H NMR (CDCl3, 300 MHz): δ = 8.26 (d, J = 8.7 Hz, 2H), 7.62 (d, J = 8.6 Hz, 2H), 5.65–5.56 (m, 1H), 4.67–4.51 (m, 2H), 3.33–3.26 (m, 1H) ppm.
- The enantiomeric excess was established by HPLC analysis using a Chiralcel OD-H column, ee = 46% (conditions: hexane/isopropanol = 90:10, flow rate: 1 mL/min, 254 nm, 25 ˚C, tR(major) = 31.4 min, tR(minor) = 25.3 min).
- All spectroscopic data were in agreement with the literature [17a].
- 1-(3,5-difluorophenyl)-2-nitroethan-1-ol (2d)
- 1H NMR (CDCl3, 300 MHz): δ = 7.02–6.91 (m, 2H), 6.85–6.74 (m, 1H), 5.51–5.41 (m, 1H), 4.62–4.46 (m, 2H), 3.14–3.07 (m, 1H) ppm. 19F NMR (282 MHz, CDCl3): δ = –107.7 (s, 2F) ppm.
- The enantiomeric excess was established by HPLC analysis using a Chiralcel OD-H column, ee = 69% (conditions: hexane/isopropanol = 90:10, flow rate: 1 mL/min, 254 nm, 25 ˚C, tR(major) = 12.5 min, tR(minor) = 10.7 min).
- All spectroscopic data were in agreement with the literature [17b].
- 1-(4-isopropylphenyl)-2-nitroethan-1-ol (2e)
- 1H NMR (CDCl3, 300 MHz): δ = 7.36–7.30 (m, 2H), 7.29–7.23 (m, 2H), 5.48–5.39 (m, 1H), 4.61 (dd, J = 13.3, 9.6 Hz, 1H), 4.50 (dd, J = 13.2, 3.1 Hz, 1H), 3.00–2.86 (m, 1H), 1.25 (d, J = 6.9 Hz, 6H) ppm.
- The enantiomeric excess was established by HPLC analysis using a Chiralcel OD-H column, ee = 32% (conditions: hexane/isopropanol = 90:10, flow rate: 1 mL/min, 254 nm, 25 °C, tR(major) = 18.2 min, tR(minor) = 12.2 min).
- All spectroscopic data were in agreement with the literature [17c].
- 1-(4-(trifluoromethyl)phenyl)-2-nitroethan-1-ol (2f)
- 1H NMR (CDCl3, 300 MHz): δ = 7.67 (d, J=8.1 Hz, 2H), 7.55 (d, J = 8.1 Hz, 2H), 5.60–5.48 (m, 1H), 4.65–4.48 (m, 2H), 3.21–3.09 (m, 1H) ppm. 19F NMR (282 MHz, CDCl3): δ = –62.7 (s, 3F) ppm.
- The enantiomeric excess was established by HPLC analysis using a Chiralcel OD-H column, ee = 28% (conditions: hexane/isopropanol = 90:10, flow rate: 1 mL/min, 254 nm, 25 ˚C, tR(major) = 14.6 min, tR(minor) = 11.5 min).
- All spectroscopic data were in agreement with the literature [17a].
- 1-(4-methoxyphenyl)-2-nitroethan-1-ol (2g)
- 1H NMR (CDCl3, 300 MHz): δ = 7.35–7.28 (m, 2H), 6.95–6.89 (m, 2H), 5.45–5.37 (m, 1H), 4.60 (dd, J = 13.2, 9.6 Hz, 1H), 4.47 (dd, J = 13.2, 3.1 Hz, 1H), 3.81 (s, 3H), 2.84–2.80 (m, 1H) ppm.
- The enantiomeric excess was established by HPLC analysis using a Chiralcel OD-H column, ee = 32% (conditions: hexane/isopropanol = 90:10, flow rate: 1 mL/min, 254 nm, 25 ˚C, tR(major) = 26.0 min, tR(minor) = 20.3 min).
- All spectroscopic data were in agreement with the literature [17a].
- 1-(naphthalen-1-yl)-2-nitroethan-1-ol (2h)
- 1H NMR (CDCl3, 300 MHz): δ = 8.04 (d, J = 8.3 Hz, 1H), 7.94–7.89 (m, 1H), 7.89–7.83 (m, 1H), 7.77 (d, J = 7.2 Hz, 1H), 7.64–7.49 (m, 3H), 6.30–6.23 (m, 1H), 4.74–4.59 (m, 2H), 2.92 (d, J=3.6 Hz, 1H) ppm.
- The enantiomeric excess was established by HPLC analysis using a Chiralcel OD-H column, ee = 64% (conditions: hexane/isopropanol = 90:10, flow rate: 1 mL/min, 254 nm, 25 ˚C, tR(major) = 24.8 min, tR(minor) = 18.0 min).
- All spectroscopic data were in agreement with the literature. [17c]
- 1-(naphthalen-2-yl)-2-nitroethan-1-ol (2i)
- 1H NMR (CDCl3, 300 MHz): δ = 7.90–7.81 (m, 4H), 7.57–7.49 (m, 2H), 7.49–7.41 (m, 1H), 5.65–5.56 (m, 1H), 4.68 (dd, J = 13.3, 9.4 Hz, 1H), 4.57 (dd, J = 13.3, 3.2 Hz, 1H), 3.16–3.10 (br. s, 1H) ppm.
- The enantiomeric excess was established by HPLC analysis using a Chiralcel OD-H column, ee = 24% (conditions: hexane/isopropanol = 90:10, flow rate: 1 mL/min, 254 nm, 25 ˚C, tR(major) = 52.5 min, tR(minor) = 37.1 min).
- All spectroscopic data were in agreement with the literature. [17b]
3.3.2. General Procedure for the Synthesis of β-Nitrostyrenes 3
- 2-nitro-β-nitrostyrene (3a)
- 1H NMR (300 MHz, CDCl3): δ = 8.54 (d, J = 13.4 Hz, 1H), 8.21 (d, J = 7.9 Hz, 1H), 7.81–7.65 (m, 2H), 7.61 (d, J = 7.3 Hz, 1H), 7.43 (d, J = 13.5 Hz, 1H) ppm.
- All spectroscopic data were in agreement with the literature [18].
- 3-nitro-β-nitrostyrene (3b)
- 1H NMR (300 MHz, CDCl3): δ = 8.42 (t, J = 2.0 Hz, 1H), 8.35 (dd, J = 8.4, 2.2 Hz, 1H), 8.06 (d, J = 13.7 Hz, 1H), 7.88 (d, J = 7.7 Hz, 1H), 7.74–7.63 (m, 2H) ppm.
- All spectroscopic data were in agreement with the literature [19].
- 4-nitro-β-nitrostyrene (3c)
- 1H NMR (300 MHz, acetone-d6): δ = 8.33 (d, J = 8.8 Hz, 2H), 8.25–8.07 (m, 4H) ppm.
- All spectroscopic data were in agreement with the literature [18].
- (E)-2-(2-nitrovinyl)naphthalene (3d)
- 1H NMR (300 MHz, CDCl3): δ = 8.15 (d, J = 13.6 Hz, 1H), 8.00 (s, 1H), 7.88 (dt, J = 9.4, 3.6 Hz, 3H), 7.69 (d, J = 13.6 Hz, 1H), 7.65–7.51 (m, 3H) ppm. 13C NMR (101 MHz, CDCl3): δ = 139.4, 137.3, 135.0, 133.3, 132.4, 129.5, 129.0, 128.5, 128.1, 127.7, 127.4, 123.4 ppm.
- (E)-9-(2-nitrovinyl)anthracene (3e)
- 1H NMR (300 MHz, CDCl3): δ = 8.98 (d, J = 13.7 Hz, 1H), 8.53 (s, 1H), 8.11 (dd, J = 39.5, 8.4 Hz, 4H), 7.76–7.39 (m, 5H) ppm. 13C NMR (101 MHz, CDCl3): δ = 142.8, 135.8, 131.2, 130.6, 130.0, 129.3, 127.6, 125.8, 124.4, 123.3 ppm.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- a) Henry, L. Formation synthétique d’alcohols nitrés. Hebd. Seances Acad. Sci. 1895, 120, 1265−1268; b) Henry, L. Bull. Soc. Chim. Fr. 1895, 13, 999.
- a) Luzzio, F.A. The Henry reaction: recent examples. Tetrahedron 2001, 57, 915−945; b) Mahrwald, R. Modern Aldol Reactions; Wiley-VCH, Weinheim, 2004; pp. 1−357; c) Geary L.M.; Hultin, P.G. The state of the art in asymmetric induction: the aldol reaction as a case study. Tetrahedron: Asymmetry 2009, 20, 131–173; d) Murugavel, G.; Sadhu, P.; Punniyamurthy, T. Copper(II)-catalyzed nitroaldol (Henry) reactions: recent developments. Chem. Rec. 2016, 16, 1906–1917; e) Zhang, S.; Li, Y.; Xu, Y.; Wang, Z. Recent progress in copper catalyzed asymmetric Henry reaction. Chin. Chem. Lett. 2018, 29, 873–883; f) Mondal, K.; Mistri, S. Schiff base based metal complexes: A review of their catalytic activity on aldol and Henry reaction. Comments on Inorganic Chemistry 2023, 43, 77–105.
- Ballini, R. New and convenient synthesis of (Z)-heneicos-6-en-11-one, the douglas fir tussock moth (Orgyia pseudotsugata) sex pheromone, and (Z)-non-6-en-2-one, the immediate precursor for the synthesis of brevicomin, the sex attractant of the western pine beetle Dentroctonus brevicomis. J. Chem. Soc. Perkin Trans. 1 1991, 6, 1419−1421.
- a) Melton, J.; McMurry, J. E. New method for the dehydration of nitro alcohols. J. Org. Chem. 1975, 40, 2138−2139; b) Denmark, S.E.; Marcin, L.R. A general method for the preparation of 2,2-disubstituted 1-nitroalkenes. J. Org. Chem. 1993, 58, 3850−3856; c) Zhang, K.; Jelier, B.; Passera, A.; Jeschke, G.; Katayev, D. Synthetic diversity from a versatile and radical nitrating reagent. Chem. Eur. J. 2019, 25, 12929−12939; d) Patra, S.; Mosiagin, I.; Giri, R.; Nauser, T.; Katayev, D. Electron-driven nitration of unsaturated hydrocarbons. Angew. Chem., Int. Ed. 2023, 62, e202300533.
- Sasai, H.; Itoh, N.; Suzuki, T.; Shibasaki, M. Catalytic asymmetric nitroaldol reaction: An efficient synthesis of (S) propranolol using the lanthanum binaphthol complex. Tetrahedron Lett. 1993, 34, 855–858. [Google Scholar] [CrossRef]
- a) Allmendiger, L.; Bauschke, G.; Paintner, F.F. Total synthesis of Sperabillin A and C. Synlett 2005, 17, 2615−2618; b) Gogoi, N.; Boruwa, J.; Barua, N. C. A total synthesis of (−)-bestatin using Shibasaki’s asymmetric Henry reaction. Tetrahedron Lett. 2005, 46, 7581−7582; c) Li, H.; Wang, B.; Deng, L. Enantioselective nitroaldol reaction of α-ketoesters catalyzed by cinchona alkaloids. J. Am. Chem. Soc. 2006, 128, 732−733; d) Blay, G.; Hernández-Olmos, V.; Pedro, J.R. Synthesis of (S)-(+)-sotalol and (R)-(−)-isoproterenol via a catalytic enantioselective Henry reaction. Tetrahedron: Asymmetry 2010, 21, 578–581; e) Guo, Z.-L.; Deng, Y.-Q.; Zhong, S.; Lu, G. Enantioselective synthesis of (R)-salmeterol employing an asymmetric Henry reaction as the key step. Tetrahedron: Asymmetry 2011, 22, 1395–1399.
- a) Sasai, H.; Takeyuki, S.; Arai, T.; Shibasaki, M. Basic character of rare earth metal alkoxides. Utilization in catalytic carbon-carbon bond-forming reactions and catalytic asymmetric nitroaldol reactions. J. Am. Chem. Soc. 1992, 114, 4418–4420; b) Evans, D.A.; Seidel, D.; Rueping, M.; Lam, H.W.; Shaw, J.T.; Downey, C.W. A new copper acetate-bis(oxazoline)-catalyzed, enantioselective Henry reaction. J. Am. Chem. Soc. 2003, 125, 12692–12693; c) Ginotra, S.K.; Singh, V.K. Enantioselective Henry reaction catalyzed by a C2-symmetric bis(oxazoline)–Cu(OAc)2·H2O complex. Org. Biomol. Chem. 2007, 5, 3932–3937; d) Cruz, H.; Aguirre, G.; Madrigal, D.; Chávez, D.; Somanathan, R. Enantioselective nitromethane addition to brominated and fluorinated benzaldehydes (Henry reaction) catalyzed by chiral bisoxazoline–copper(II) complexes. Tetrahedron: Asymmetry 2016, 27, 1217–1221; e) Pavlov, A.A.; Dalinger, A.I.; Suslov, E.V.; Ponomarev, K.Yu.; Mozhaitsev, E.S.; Vatsadze, S.Z. Investigation of the possibility of complex formation of bidentate bispidine ligands with copper(II) salts in solution by proton NMR spectroscopy. Russ. Chem. Bull. 2023, 72, 635–640.
- a) Xion, Y.; Wang, F.; Huang, X.; Wen, Y.; Feng, X. A new copper(I)–tetrahydrosalen-catalyzed asymmetric Henry reaction and its extension to the synthesis of (S)-norphenylephrine. Chem. Eur. J. 2007, 13, 829–833; b) White, J.D.; Shaw, S. A new catalyst for the asymmetric Henry reaction: synthesis of β-nitroethanols in high enantiomeric excess. Org. Lett. 2012, 14, 6270–6273; c) Kannan, M.; Punniyamurthy, T. Effect of ligand N,N-substituents on the reactivity of chiral copper(II) salalen, salan, and salalan complexes toward asymmetric nitroaldol reactions. Tetrahedron: Asymmetry 2014, 25, 1331–1339; d) Kureshy, R.I.; Das, A.; Khan, N.H.; Abdi, S.H.R.; Bajaj, H.C. Cu(II)-macrocylic [H4]Salen catalyzed asymmetric nitroaldol reaction and its application in the synthesis of α1-adrenergic receptor agonist (R)-phenylephrine. ACS Catal. 2011, 1, 1529–1535.
- a) Kowalczyk, R.; Sidorowicz, L.; Skarzewski, J. Asymmetric Henry reaction catalyzed by chiral secondary diamine-copper(II) complexes. Tetrahedron: Asymmetry 2008, 19, 2310–2315; b) Sanjeevakumar, N.; Periasamy, M. Highly enantioselective Henry reaction catalyzed by a new chiral C2-symmetric N,N′-bis(isobornyl)ethylenediamine–copper complex. Tetrahedron: Asymmetry 2009, 20, 1842–1847; c) Chunhong, Z.; Liu, F.; Gou, S. Application of chiral N,N′-dialkyl-1,2-cyclohexanediamine derivatives in asymmetric copper(II)-catalyzed Henry reactions. Tetrahedron: Asymmetry 2014, 25, 278–283; d) Malhotra, S.V.; Brown, H.C. C2-symmetric N,N′-bis(terpenyl)ethylenediamines−synthesis and application in the enantioselective nitroaldol reaction. RSC Adv. 2014, 4, 14264–14269.
- a) Jin, W.; Li, X.; Huang, Y.; Wu, F.; Wan, B. A highly effective bis(sulfonamide)–diamine ligand: a unique chiral skeleton for the enantioselective Cu-catalyzed Henry reaction. Chem. Eur. J. 2010, 16, 8259–8261; b) Jin, W.; Li, X.; Wan, B. A highly diastereo- and enantioselective copper(I)-catalyzed Henry reaction using a bis(sulfonamide)−diamine ligand. J. Org. Chem. 2011, 76, 484–491; c) Valverde-González, A.; Fernández-Seriñan, P.; Matarín, Á.; Arnanz, A.; Sánchez, F.; Iglesias, M. Porous aromatic frameworks containing binaphthyl-dihydroazepine units (cBAPAFs) as catalytic supports for asymmetric reactions. J. Catal. 2022, 413, 434–442.
- a) Maheswaran, H.; Prasanth, K.L.; Krishna, G.G.; Ravikumar, K.; Sridharb, B.; Kantam, M.L. Enantioselective nitroaldol (Henry) reaction using copper(II) complexes of (−)-sparteine. Chem. Commun. 2006, 39, 4066–4068; b) Arai, T.; Watanabe, M.; Yanagisawa, A. Practical asymmetric Henry reaction catalyzed by a chiral diamine-Cu(OAc)2 complex. Org. Lett. 2007, 9, 3595–3597; c) Arai, T.; Takashita, R.; Endo, Y.; Watanabe, M.; Yanagisawa, A. Asymmetric syn-selective Henry reaction catalyzed by the sulfonyldiamine−CuCl−pyridine system. J. Org. Chem. 2008, 73, 4903–4906; d) Qi, G.; Ji, Y.Q.; Judeh, Z.M.A. Novel chiral C1-1′,2′,3′,4′-tetrahydro-1,1′-bisisoquinolines: synthesis, resolution, and applications in catalytic enantioselective reactions. Tetrahedron 2010, 66, 4195–4205; e) Noole, A.; Lippur, K.; Metsala, A.; Lopp, M.; Kanger, T. A highly diastereo- and enantioselective copper(I)-catalyzed Henry reaction using a bis(sulfonamide)−diamine ligand. J. Org. Chem. 2010, 75, 1313–1316; f) Wei, Y.; Yao, L.; Zhang, B.; He, W.; Zhang, S. Novel Schiff base ligands derived from Cinchona alkaloids for Cu(II)-catalyzed asymmetric Henry reaction. Tetrahedron 2011, 67, 8552–8558; g) Chougnet, A.; Zhang, G.; Liu, K.; Häussinger, D.; Kägi, A.; Allmendinger, T.; Woggon, W.-D. Diastereoselective and highly enantioselective Henry reactions using C1-symmetrical copper(II) complexes. Adv. Synth. Catal. 2011, 353, 1797–1806; h) Blay, G.; Hernandez-Olmos, V.; Pedro, J.R. Development of new N,N-ligands for the enantioselective copper(II)-catalyzed Henry reaction. Synlett 2011, 9, 1195–1211; i) Ji, Y.Q.; Judeh, Z.M.A. 1,1′-Methylene-bis(1,1′,2,2′,3,3′,4,4′-octahydroisoquinoline): synthesis, reaction, resolution, and application in catalytic enantioselective transformations. Tetrahedron 2011, 67, 4086–4092; j) Ji, Y.Q.; Qi, G.; Judeh, Z.M.A. Efficient asymmetric copper(I)-catalyzed Henry reaction using chiral N-alkyl-C1-tetrahydro-1,1′-bisisoquinolines. Eur. J. Org. Chem. 2011, 25, 4892–4898; k) Zhou, Y.; Gong, Y. Asymmetric copper(II)-catalysed nitroaldol (Henry) reactions utilizing a chiral C1-symmetric dinitrogen ligand. Eur. J. Org. Chem. 2011, 30, 6092–6099; l) Filippova, L.; Stenstrøm, Y.; Hansen, T.V. Cu(II)-catalyzed asymmetric Henry reaction with a novel C1-symmetric aminopinane-derived ligand. Molecules 2015, 20, 6224–6236.
- a) Poe, S.L.; Kobašlija, M.; McQuade, D.T. Microcapsule enabled multicatalyst system. J. Am. Chem. Soc. 2006, 128, 15586–15587; b) Komura, K.; Kawamura, T.; Sugi, Y. Layered silicate PLS-1: A new solid base catalyst for C–C bond forming reactions. Catal. Commun. 2007, 8, 644–648; c) Motokura, K.; Tada, M.; Iwasawa, Y. Cooperative catalysis of primary and tertiary amines immobilized on oxide surfaces for one-pot C-C bond forming reactions. Angew. Chem. Int. Ed. 2008, 47, 9230–9235; d) Rokhum, L.; Bez, G. One-pot solid phase synthesis of (E)-nitroalkenes. Tetrahedron Lett. 2013, 54, 5500–5504; e) Ishitani, H.; Saito, Y.; Tsubogo, T.; Kobayashi, S. Synthesis of nitro-containing compounds through multistep continuous flow with heterogeneous catalysts. Org. Lett. 2016, 18, 1346−1349; f) Lee, L.-C.; Lu, J.; Weck, M.; Jones, C.W. Acid–base bifunctional shell cross-linked micelle nanoreactor for one-pot tandem reaction. ACS Catal. 2016, 6, 784−787.
- a) Luo, M.; Yan, B.; Enantioselective Henry reactions catalyzed by chiral N-metal complexes containing R(+)/S(−)-α-ethylphenyl amines. Tetrahedron Lett. 2010, 51, 5577–5580; b) Jones, M.D.; Cooper, C.J.; Mahon, M.F.; Raithby, P.R.; Apperley, D.; Wolowska, J.; Collison, D. Cu(II) homogeneous and heterogeneous catalysts for the asymmetric Henry reaction. J. Mol. Cat. A: Chemical 2010, 325, 8–14; c) Didier, D.; Magnier-Bouvier, C.; Schulz, E. Charge-transfer interactions: an efficient tool for recycling bis(oxazoline)-copper complexes in asymmetric Henry reactions. Adv. Synth. Catal. 2011, 353, 1087–1095; d) Cirujano, F.G.; López-Maya, E.; Rodríguez-Albelo, M.; Barea, E.; Navarro, J.A.R.; de Vos, D.E. Selective one-pot two-step C−C bond formation using metal–organic frameworks with mild basicity as heterogeneous catalysts. ChemCatChem 2017, 9, 4019–4023; e) El-Asaad, B.; Métay, E.; Karamé, I.; Lemaire, M. Chiral N-arylated diamine – copper complexes catalyzed asymmetric Henry reaction. Mol. Catal. 2017, 435, 76–81; f) Marquez, C.; Cirujano, F.G.; Smolders, S.; van Goethem, C.; Vankelecom, I.; de Vos, D.; de Baerdemaeker, T. Metal ion exchange in Prussian blue analogues: Cu(II)-exchanged Zn–Co PBAs as highly selective catalysts for A3 coupling. Dalton Trans. 2019, 48, 3946–3954; g) El-Atawy, M.A.; Khalil, K.D.; Bashal, A.H. Chitosan capped copper oxide nanocomposite: efficient, recyclable, heterogeneous base catalyst for synthesis of nitroolefins. Catalysts 2022, 12, 964.
- a) Larionov, V.A.; Yashkina, L.V.; Smol’yakov, A.F.; Zubavichus, Y.V.; Babievsky, K.K.; Akat’yev, N.V.; Titov, A.A.; Belokon, Y.N.; Maleev, V.I. Synthesis and investigations of chiral NNO type copper(II) coordination polymers. ChemistrySelect 2018, 3, 653–656; b) Larionov, V.A.; Yashkina, L.V.; Medvedev, M.G.; Smol’yakov, A.F.; Peregudov, A.S.; Pavlov, A.A.; Eremin, D.B.; Savel’yeva, T.F.; Maleev, V.I.; Belokon, Y.N. Henry reaction revisited. Crucial role of water in an asymmetric Henry reaction catalyzed by chiral NNO-type copper(II) complexes. Inorg. Chem. 2019, 58, 11051−11065.
- Sarkar, A.; Mistry, S.; Bhattacharya, S.; Natarajan, S. Multistep cascade catalytic reactions employing bifunctional framework compounds. Inorg. Chem. 2023, 62, 11142–11151. [Google Scholar] [CrossRef] [PubMed]
- Herold, F.; Dawidowski, M.; Wolska, I.; Chodkowski, A.; Kleps, J.; Turło, J.; Zimniak, A. The synthesis of new diastereomers of (4S,8aS)- and (4R,8aS)-4-phenyl-perhydropyrrole [1,2-α]pyrazine-1,3-dione. Tetrahedron Asymmetry 2007, 18, 2091–2098. [Google Scholar] [CrossRef]
- a) Lai, G.; Guo, F.; Zheng, Y.; Fang, Y.; Song, H.; Xu, K.; Wang, S.; Zha, Z.; Wang, Z. Highly enantioselective Henry reactions in water catalyzed by a copper tertiary amine complex and applied in the synthesis of (S)-N-trans-feruloyl octopamine. Chem. Eur. J. 2011, 17, 1114–1117; b) Cruz, H.; Aguirre, G.; Madrigal, D.; Chávez, D.; Somanathan, R. Enantioselective nitromethane addition to brominated and fluorinated benzaldehydes (Henry reaction) catalyzed by chiral bisoxazoline–copper(II) complexes. Tetrahedron Asymmetry 2016, 27, 1217–1221; c) Yao, C.; Chen, Y.; Wang, C.; Sun, R.; Chang, H.; Jiang, R.; Li, L.; Wang, X.; Li, Y.-M. Binaphthyl-proline hybrid chiral ligands: modular design, synthesis, and enantioswitching in Cu(II)-catalyzed enantioselective Henry reactions. J. Org. Chem. 2023, 88, 7651–7659.
- Qi, X.; Tian, J.; Li, Y.; Chen, L.; Yan, X. (1R,2R)-(+)-1,2-DPEN-modified Wang resin: an efficient catalyst for the asymmetric Michael addition of acetone to β-nitroolefins. Catal. Commun. 2015, 71, 70–73. [Google Scholar] [CrossRef]
- Ohe, T.; Uemura, S. Palladium(II)-catalyzed Michael-type hydroarylation of nitroalkenes using aryltins and sodium tetraarylborates. Bull. Chem. Soc. Jpn. 2003, 76, 1423–1431. [Google Scholar] [CrossRef]

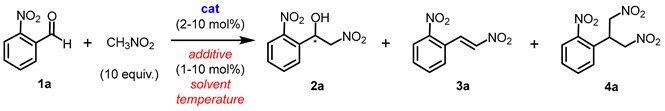 | |||||||
| entry | catalyst | additive (x mol%) |
solvent | T, ˚C | yield (ee) of 2a (%)b,c |
yield of 3a (%)b |
Yield of 4a (%)b |
| 1ref. 14b | (S)-Cu1 | – | CD2Cl2d | RT | 78 (77) | – | – |
| 2 | (S)-Cu1 | – | DCE | 50 | 43 (25) | 55 | traces |
| 3 | (S)-Cu1 | NaOAc (10) | DCE | RT | 63 (60) | 3 | – |
| 4 | (S)-Cu1 | NaOAc (10) | THF/CH2Cl2 | RT | 96 (73) | 2 | – |
| 5 | (S)-Cu2 | - | CH2Cl2 | RT | NR | – | – |
| 6f | (S)-Cu2 | PhONa (10) | CH2Cl2 | RT | 53 (12) | 40 | 3 |
| 7 | (S)-Cu2 | PhONa (10) | CH2Cl2 | –17 | 98 (39) | traces | traces |
| 8f | (S)-Cu2 | Ag2O (5) | CH2Cl2 | RT | 59 (0) | 34 | 2 |
| 9 | (S)-Cu2 | Ag2O (5) | CH2Cl2 | RT | 31 (ND) | 54 | 14 |
| 10 | (S)-Cu2 | Ag2O (5) | CH2Cl2 | –17 | 89 (56) | 4 | traces |
| 11f,g | (S)-Cu2 | Ag2O (5) | CH2Cl2 | RT | 71 (ND) | 24 | traces |
| 12g | (S)-Cu2 | Ag2O (5) | CH2Cl2 | RT | 50 (ND) | 48 | 2 |
| 13 | (S)-Cu2 | Ag2O (5) | DCE | RT | 23 (ND) | 63 | 13 |
| 14 | (S)-Cu2 | Ag2O (5) | CH3CN | RT | 72 (ND) | 16 | 11 |
| 15 | (S)-Cu2 | Ag2O (5) | EtOAc | RT | 51 (ND) | 30 | 18 |
| 16 | (S)-Cu2 | Ag2O (5) | THF | RT | 69 (ND) | 15 | 15 |
| 17 | (S)-Cu2 | Ag2O (5) | 1,4-dioxane | RT | 51 (ND) | 32 | 16 |
| 18 | (S)-Cu2 | Ag2O (5) | toluene | RT | 28 (ND) | 61 | 10 |
| 19 | (S)-Cu2 | Ag2O (5) | MeOH | RT | 99 (ND) | – | – |
| 20h | (S)-Cu2 | Ag2O (5) | - | RT | 44 (ND) | 35 | 20 |
| 21 | (S)-Cu2 | Ag2O (5) | DCE | 50 | 4 (ND) | 81 | 14 |
| 22 | (S)-Cu2 | Ag2O (5) | DCE | 70 | <1 (ND) | 81 | 18 |
| 23 | (S)-Cu2 (5) | Ag2O (2.5) | DCE | 70 | 2 (ND) | 87 | 10 |
| 24 | (S)-Cu2 (2) | Ag2O (1) | DCE | 70 | 4 (ND) | 87 | 8 |
| 25i | (S)-Cu2 (2) | Ag2O (1) | DCE | 70 | 3 (ND) | 88 | 6 |
| 26i | CuCl2*2H2O + 1,10-phen (2) |
Ag2O (1) | DCE | 70 | 33 (ND) | 23 | 4 |
| 27 | – | Ag2O (5) | DCE | 50 | NR | – | – |
| 28 | – | tBuOK (5) | DCE | 50 | 85 (0) | – | – |
| 29 | (S)-Cu3 | Ag2O (5) | DCE | 50 | 7 (ND) | 78 | 13 |
| 30 | (S)-Cu3 | NaOAc (10) | DCE | RT | 11 (ND) | 72 | 15 |
| aReaction conditions: o-nitrobenzaldehyde 1a (0.15 mmol), nitromethane (10 eq., 1.5 mmol), catalyst (10 mol%) and an additive (5 or 10 mol%) in 0.5 mL solvent were stirred for 24 h. bYields were determined by 1H NMR analysis of the crude mixture. cEnantiomeric excess was determined by chiral HPLC analysis. d0.1 equiv. of water was added. e1.0 equiv. of water was added. fThe reaction time was 3 h. gCD3NO2 was used instead of CH3NO2. h30.0 equiv. of CH3NO2 was used. i5.0 equiv. of CH3NO2 was used. DCE = 1,2-dichloroethane. NR = no reaction. | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





