Submitted:
26 September 2024
Posted:
27 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
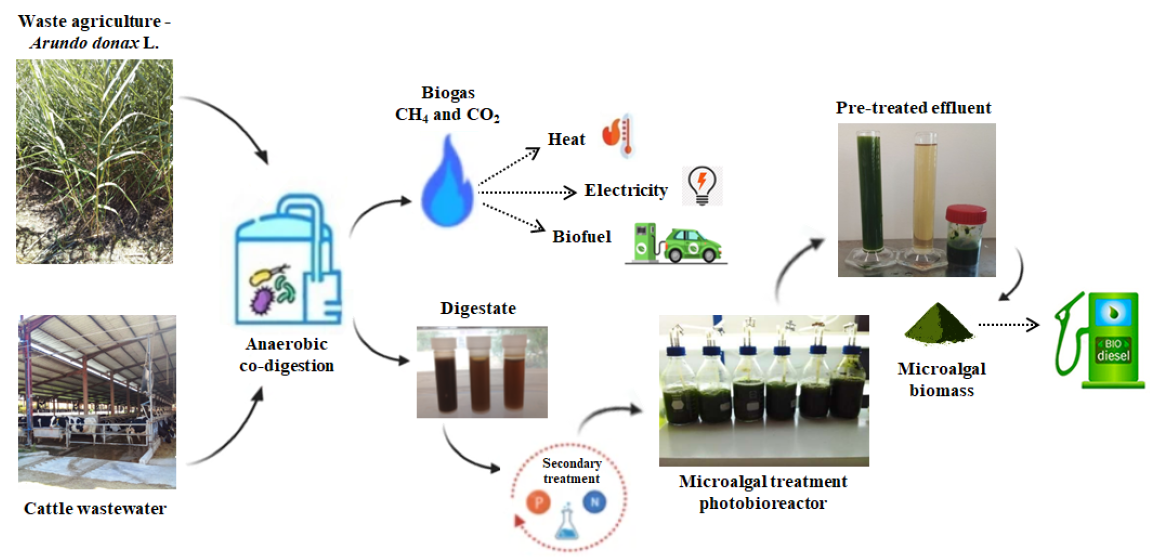
2. Materials and Methods
2.1. Substrates and Inoculum
2.2. Analytical Methods
2.3. Anaerobic Co-Digestion of DCW with Grass of Arundo donax L.
2.4. Microalgae
2.5. Digestate Treatment with Microalgal Cultivation in Photobioreactors
2.6. Biomass Processing and Biochemical Analysis
2.7. Kinetic Modeling
3. Results and Discussion
3.1. Phase I: Co-Digestion
3.1.1. pH, Alkalinity and Solids Removal
3.1.2. Removal of Total and Soluble COD
3.1.3. Nitrogen and Phosphate Compounds
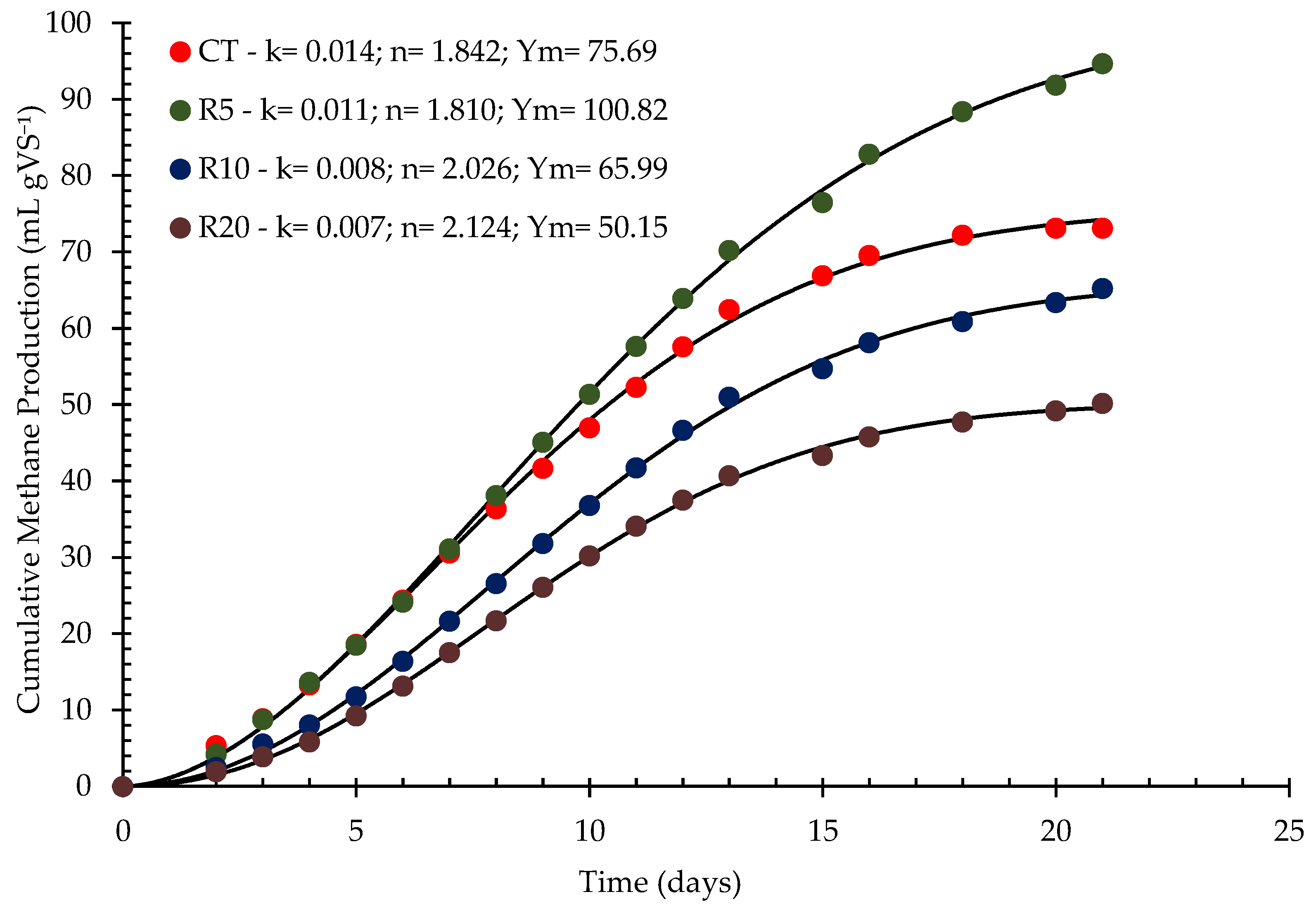
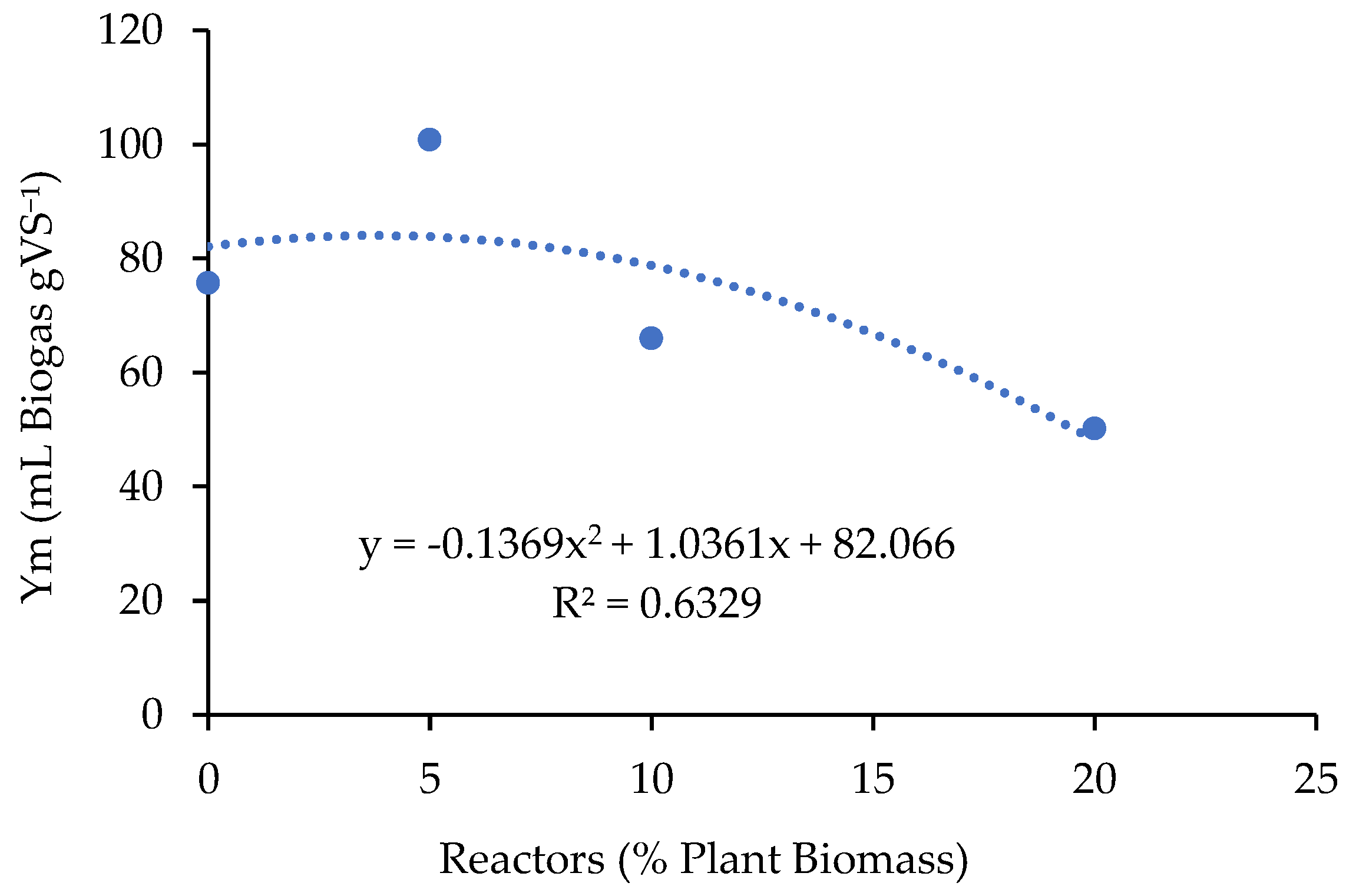
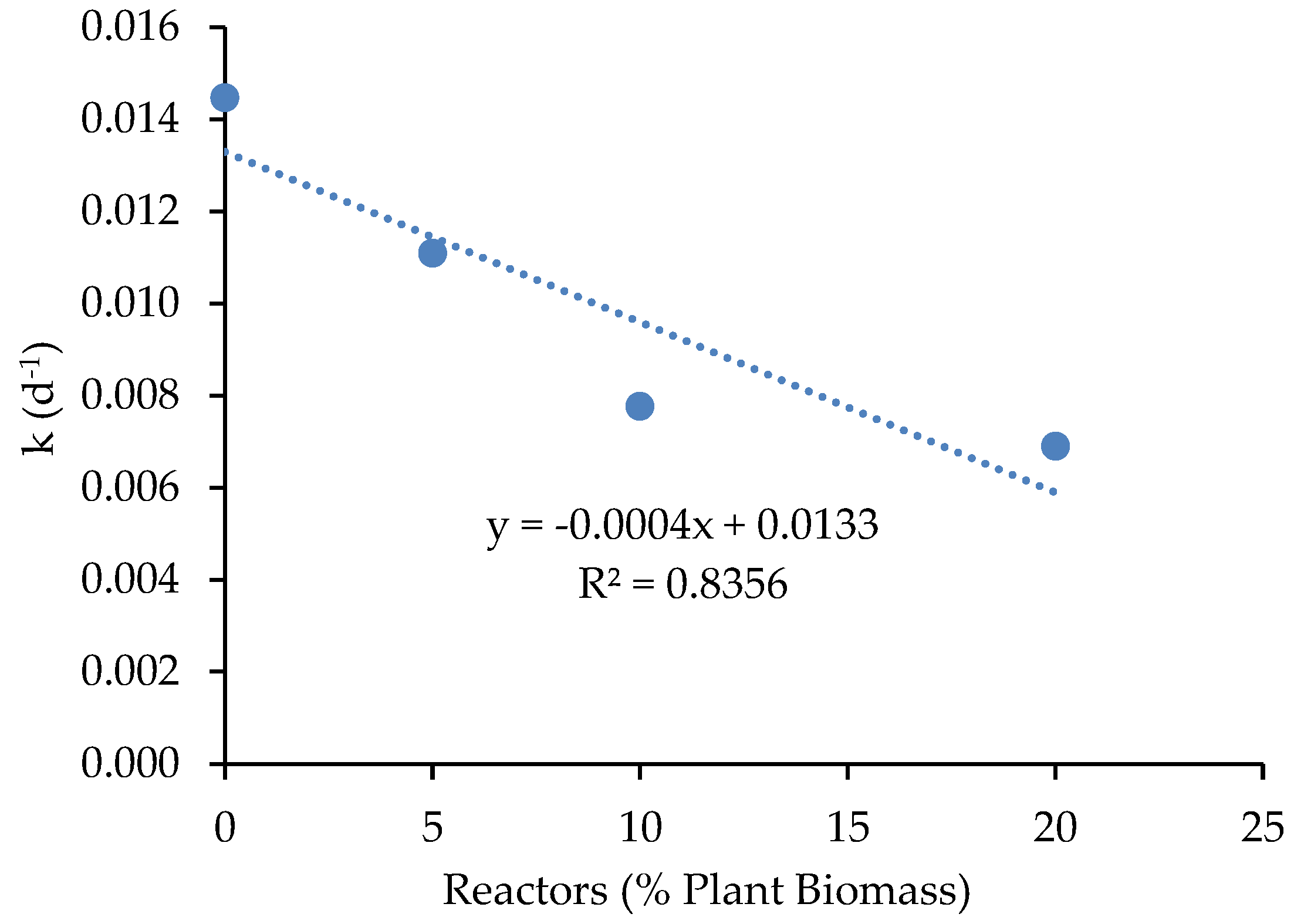
3.1.4. Biogas Production
3.1.5. Digestate
3.2. Phase II: Photobioreactors - Secondary Treatment, Microalgal Culture in the Digestate
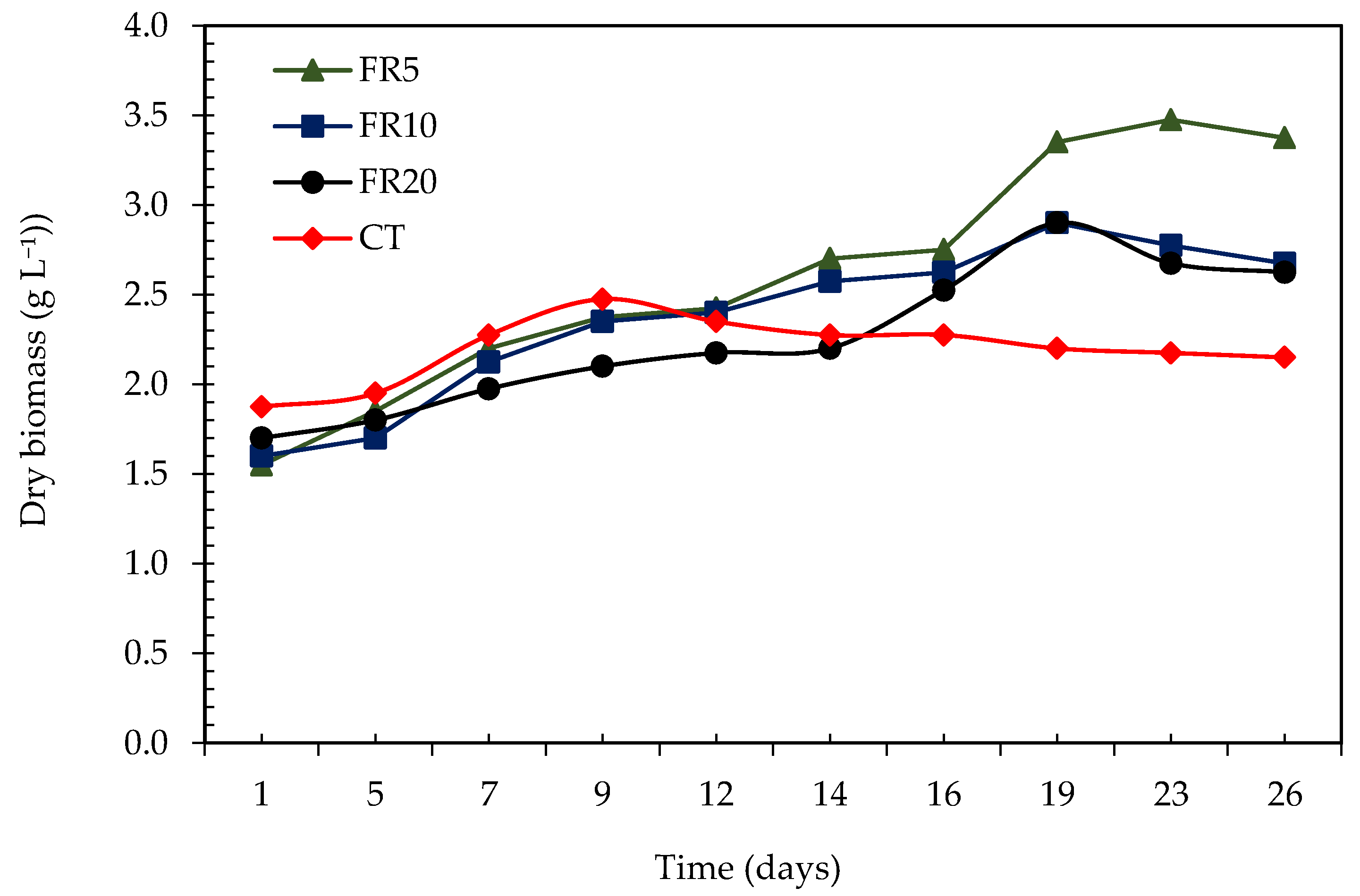
3.2.1. Dry biomass and Volumetric Productivity
3.2.2. Bioremediation: Removal of Organic Matter and Nutrients
3.3. Macromolecular Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Wang, X.; Zhu, W.; Zhao, Y.; Wang, N.; Gao, M.; Wang, Q. Anaerobic fermentation of organic solid waste: Recent updates in substrates, products, and the process with multiple products co-production. Environ. Res. 2023, 233, 116444. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, K.; Loizidou, M.; Rehan, M.; Nizami, A.S. A review of recent developments in renewable and sustainable energy systems: Key challenges and future perspective. Renew. Sustain. Energy Rev. 2020, 119, 109418. [Google Scholar] [CrossRef]
- Sallem, M. Possibility of utilizing agriculture biomass as a renewable and sustainable future energy source. Heliyon 2022, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, H.V.; Ometto, J.P.H.B.; Otenio, M.H. Production of Energy and Biofertilizer from Cattle Wastewater in Farms with Intensive Cattle Breeding. Water Air Soil Pollut. 2017, 228, 72. [Google Scholar] [CrossRef]
- Mendonça, H.V.; Ometto, J.P.H.B.; Otenio, M.H.; Dos Reis, A.J.D.; Marques, I.P.R. Bioenergy recovery from cattle wastewater in an UASB-AF hybrid reactor. Water Sci. Technol. 2017, 76, 2268–2279. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, T.; Chowdhury, H.; Hossain, N.; Ahmed, A.; Hossen, M.S.; Chowdhury, P.; Thirugnanasambandam, M.; Saidur, R. Latest advancements on livestock waste management and biogas production: Bangladesh’s perspective. J. Clean. Prod. 2020, 272, 122818. [Google Scholar] [CrossRef]
- Ardebili, S.M.S. Green electricity generation potential from biogas produced by anaerobic digestion of farm animal waste and agriculture residues in Iran. Renew. Energy 2020, 154, 29–37. [Google Scholar] [CrossRef]
- Bedoić, R.; Cucek, L.; Cosic, B.; Krajnc, D.; Smoljanic, G.; Kravanja, Z.; Ljubas, D.; Puksec, T.; Duic, N. Green biomass to biogas – A study on anaerobic digestion of residue grass. J. Clean. Prod. 2019, 213, 700–709. [Google Scholar] [CrossRef]
- Song, Y.; Pei, L.; Chen, G.; Mu, L.; Yan, B.; Li, H.; Zhou, T. Recent advancements in strategies to improve anaerobic digestion of perennial energy grasses for enhanced methane production. Sci. Total Environ. 2023, 861, 160552. [Google Scholar] [CrossRef]
- Abreu, M.; Silva, L.; Ribeiro, B.; Ferreira, A.; Alves, L.; Paixão, S.M.; Gouveia, L.; Moura, P.; Carvalheiro, F.; Duarte, L.C.; Fernando, A.L.; Reis, A.; Gírio, F. Low Indirect Land Use Change (ILUC) Energy Crops to Bioenergy and Biofuels—A Review. Energies 2022, 15, 4348. [Google Scholar] [CrossRef]
- Antal, G. Giant reed (Arundo donax L.) from ornamental plant to dedicated bioenergy spe-cies: Review of economic prospects of biomass production and utilization. Int. J. Hortic. Sci. 2018, 24, 39–46. [Google Scholar] [CrossRef]
- Oginni, O.; Singh, K. Pyrolysis characteristics of Arundo donax harvested from a re-claimed mine land. Ind. Crop. Prod. 2019, 13, 44–53. [Google Scholar] [CrossRef]
- Vasmara, C.; Gallett, S.; Cianchetta, S.; Ceotto, E. Advancements in Giant Reed (Arundo donax L.) Biomass Pre-Treatments for Biogas Production: A Review. Energies 2023, 16, 949. [Google Scholar] [CrossRef]
- Vasmara, C.; Cianchetta, S.; Marchetti, R.; Ceotto, E.; Gallett, S. Potassium Hydroxyde Pre-Treatment Enhances Methane Yield from Giant Reed (Arundo donax L.). Energies 2021, 14, 630. [Google Scholar] [CrossRef]
- Silva, G.H.; Barros, N.O.; Santana, L.A.R.; Carneiro, J.C.; Otenio, M.H. Shifts of acidogenic bacterial group and biogas production by adding two industrial residues in anaerobic co-digestion with cattle manure. J. Environ. Sci. Health, Part A, 2021, 1, 1–9. [Google Scholar] [CrossRef]
- González, R.; Peña, D.C.; Gómez, X. Anaerobic Co-Digestion of Wastes: Reviewing Current Status and Approaches for Enhancing Biogas Production. Appl. Sci. 2022, 12, 8884. [Google Scholar] [CrossRef]
- Frankowski, J.; Czekala, W. Agricultural Plant Residues as Potential Co-Substrates for Biogas Production. Energies 2023, 16, 4396. [Google Scholar] [CrossRef]
- Mendonça, H.V.; Ometto, J.P.H.B.; Otenio, M.H.; Marques, I.P.R.; Dos Reis, A.J.D. Microalgae-mediated bioremediation and valorization of cattle wastewater previously digested in a hybrid anaerobic reactor using a photobioreactor: Comparison between batch and continuous operation. Sci. Total Environment 2018, 633, 1–11. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for High-Value Products Towards Human Health and Nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef]
- Roy, U.K.; Radu, T.; Wagner, J.L. Carbon-negative biomethane fuel production: Integrating anaerobic digestion with algae-assisted biogas purification and hydrothermal carbonisation of digestate. Biomass Bioenergy 2021, 148, 106029. [Google Scholar] [CrossRef]
- Mendonça, H.V.; Otenio, M.H.; Marchão, L.; Lomeu, A.; Souza, D.S.; Reis, A. Biofuel recovery from microalgae biomass grown in dairy wastewater treated with activated sludge: The next step in sustainable production. Sci. Total Environ. 2022, 824, 153838. [Google Scholar] [CrossRef] [PubMed]
- Marangon, B.B.; Castro, J.S.; Assemany, P.P.; Couto, E.A.; Calijuri, M.L. Environmental performance of microalgae hydrothermal liquefaction: Life cycle assessment and improvement insights for a sustainable renewable diesel. Renew. Sustain. Energy Rev. 2022, 155, 111910. [Google Scholar] [CrossRef]
- Ayre, J.M.; Moheimani, N.R.; Borowitzka, M.A. Growth of microalgae on undiluted anaerobic digestate of piggery effluent with high ammonium concentrations. Algal Res. 2017, 24, 218–226. [Google Scholar] [CrossRef]
- Nagarajan, D.; Kusmayadi, A.; Yen, H.W.; Dong, C.D.; Lee, D.J.; Chang, J.S. Current advances in biological swine wastewater treatment using microalgae-based processes. Bioresour. Technol. 2019, 289, 121718. [Google Scholar] [CrossRef]
- Fernández, F.G.A.; Reis, A.; Wijffels, R.H.; Barbosa, M.; Verdelho, V.; Llamas, B. The role of microalgae in the bioeconomy. N.Biotechnol. 2021, 61, 99–107. [Google Scholar] [CrossRef]
- Ahn, Y.; Park, S.; Ji, M.K.; Ha, G.S.; Jeon, B.H.; Choi, J. Biodiesel production potential of microalgae, cultivated in acid mine drainage and livestock wastewater. J. Environ. Manage. 2022, 314, 115031. [Google Scholar] [CrossRef] [PubMed]
- Siddiki, S.Y.A.; Mofijur, M.; Kumar, P. S.; Ahmed, S.F.; Inayat, A.; Kusumo, F.; Badruddin, I.A.; Khan, T.M.Y.; Nghiem, L.D.; Ong, H.C.; Mahlia, T.M.I. Microalgae biomass as a sustainable source for biofuel, biochemical and biobased value-added products: An integrated biorefinery concept. Fuel 2022, 307, 121782. [Google Scholar] [CrossRef]
- Steinmetz, R.L.R.; Mezzari, M.P.; Silva, M.L.B.; Kunz, A.; Amaral, A.C.; Tápparo, D.C.; Soares, H.M. Enrichment and acclimation of an anaerobic mesophilic microorganism’s inoculum for standardization of BMP assays. Bioresour. Technol. 2016, 219, 21–28. [Google Scholar] [CrossRef]
- APHA; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater. 24th Edition American Pub-lic Health Association, Washington, DC, USA, 2023.
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 165–275. [Google Scholar] [CrossRef]
- Moré, J.J. The Levenberg-Marquardt algorithm: Implementation and theory. Anal. Lect. Notes Math. 1978, 630, 105–116. [Google Scholar]
- Pečar, D.; Pohleven, F.; Goršek, A. Kinetics of methane production during anaerobic fermentation of chicken manure with sawdust and fungi pre-treated wheat straw. Waste Manage. 2020, 102, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Bedoić, R.; Špehar, A.; Puljko, J.; Čuček, L.; Ćosić, B.; Pukšec, T.; Duić, N. Opportunities and challenges: Experimental and kinetic analysis of anaerobic co-digestion of food waste and rendering industry streams for biogas production. Renew. Sustain. Energy Rev. 2020, 130, 109951. [Google Scholar] [CrossRef]
- Cook, S.M.; Skerlos, S.J.; Raskin, L.; Love, N.G. A stability assessment tool for anaerobic codigestion. Water Res. 2017, 112, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Wu, S.; Zhang, W.; Dong, R. Effects of organic loading rate and effluent recirculation on the performance of two-stage anaerobic digestion of vegetable waste. Bioresour. Technol. 2013, 146, 556–561. [Google Scholar] [CrossRef]
- Issah, A.A.; Kabera, T.; Kemausuor, F. Biogas optimisation processes and effluent quality: A review. Biomass and Bioenergy 2020, 133, 105449. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Y.; Wang, S.; Zhang, Y.; Hu, Y.; Hu, Z.; Wu, G.; Zhan, X. Impact of total solids content on anaerobic co-digestion of pig manure and food waste: Insights into shifting of the methanogenic pathway. Waste Manage. 2020, 114, 96–106. [Google Scholar] [CrossRef]
- Shrestha, B.; Hernandez, R.; Fortela, D.L.B.; Sharp, W.; Chistoserdov, A.; Gang, D.; Revellame, E.; Holmes, W.; Zappi, M. A Review of Pretreatment Methods to Enhance Solids Reduction during Anaerobic Digestion of Municipal Wastewater Sludges and the Resulting Digester Performance: Implications to Future Urban Biorefineries. Appl. Sci. 2020, 10 (24), 9141. [CrossRef]
- Han, F.; Yun, S.; Zhang, C.; Xu, H.; Wang, Z. Steel slag as accelerant in anaerobic digestion for nonhazardous treatment and digestate fertilizer utilization. Bioresour. Technol. 2019, 282, 331–338. [Google Scholar] [CrossRef]
- Wang, K.; Yun, S.; Ke, T.; An, J.; Abbas, Y.; Liu, X.; Zou, M.; Liu, L.; Liu, J. Use of bag-filter gas dust in anaerobic digestion of cattle manure for boosting the methane yield and digestate utilization. Bioresour. Technol. 2022, 348, 126729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yun, S.; Li, X.; Wang, Z.; Xu, H.; Du, T. Low-cost composited accelerants for anaerobic digestion of dairy manure: focusing on methane yield, digestate utilization and energy evaluation. Bioresour. Technol. 2018, 263, 517–524. [Google Scholar] [CrossRef]
- Akhiar, A.; Battimelli, A.; Torrijos, M.; Carrere, H. Comprehensive characterization of the liquid fraction of digestates from full-scale anaerobic co-digestion. Waste Manage. 2017, 59, 118–128. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Yenigün, O.; Demirel, B. Ammonia inhibition in anaerobic digestion: A review. Process Biochemistry, 2013, 48, 5–6. [Google Scholar] [CrossRef]
- González-Suárez, A.; Pereda-Reyes, I.; Oliva-Merencio, D.; Suárez-Quiñones, T.; Silva, A.J.; Zaiat, M. Bioavailability and dosing strategies of mineral in anaerobic mono-digestion of maize straw. Eng. Life Sci. 2018, 18, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Koyama, M.; Yamamoto, S.; Ishikawa, K.; Ban, S.; Toda, T. Inhibition of anaerobic digestion by dissolved lignin derived from alkaline pre-treatment of an aquatic macrophyte. Chem. Eng. J. 2017, 311, 55–62. [Google Scholar] [CrossRef]
- Kamperidou, V.; Terzopoulou, P. Anaerobic Digestion of Lignocellulosic Waste Materials. Sustainability, 2021, 13, 12810. [Google Scholar] [CrossRef]
- Mirabi, M.; Karrabi, M.; Shahnavaz, B. Anaerobic co-digestion of lignocellulosic/lipidic wastes with cattle manure: Investigating biogas production and methane yield. Fuel 2024, 366, 131286. [Google Scholar] [CrossRef]
- Masih-Das, J.; Tao, W. Anaerobic co-digestion of foodwaste with liquid dairy manure or manure digestate: Co-substrate limitation and inhibition. J. Environ. Manage. 2018, 223, 917–924. [Google Scholar] [CrossRef]
- Bella, K.; Rao, P.V. Anaerobic digestion of dairy wastewater: effect of different parameters and co-digestion options—a review. Biomass Convers. Biorefin. 2023, 13, 2527–2552. [Google Scholar] [CrossRef]
- Cong, W.F.; Moset, V.; Feng, L.; Møller, H.B.; Eriksen, J. Anaerobic co-digestion of grass and forbs – Influence of cattle manure or grass-based inoculum. Biomass and Bioenergy 2018, 119, 90–96. [Google Scholar] [CrossRef]
- Ulukardesler, A.H. Anaerobic co-digestion of grass and cow manure: kinetic and GHG calculations. Sci. Rep. 2023, 13, 6320. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Kobayashi, T.; Li, Y.Y.; Xu, K.; Zhao, Y. Mesophilic anaerobic co-digestion of waste activated sludge and Egeria densa: Performance assessment and kinetic analysis. Appl. Energy 2015, 148, 78–86. [Google Scholar] [CrossRef]
- Strömberg, S.; Nistor, M.; Liu, J. Early prediction of Biochemical Methane Potential through statistical and kinetic modelling of initial gas production. Bioresour. Technol. 2015, 176, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Tjørve, K.M.C.; Tjørve, E. The use of Gompertz models in growth analyses, and new Gompertz-model approach: An addition to the Unified-Richards Family. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Ware, A.; Power, N. Modelling methane production kinetics of complex poultry slaughterhouse wastes using sigmoidal growth functions. Renew. Energy 2017, 104, 50–59. [Google Scholar] [CrossRef]
- Wojnarowska, A.; Pałka, S.; Otwinowska-Mindur, A.; Ptak, E. Comparison of two nonlinear functions describing the growth of Popielno White and New Zealand White rabbits. Anim. Sci. Genet. 2022, 18, 1–13. [Google Scholar] [CrossRef]
- Howell, G.; Bennett, C.; Materić, D. A comparison of methods for early prediction of anaerobic biogas potential on biologically treated municipal solid waste. J. Environ. Manage. 2019, 232, 887–8. [Google Scholar] [CrossRef]
- Soares, B.S.; Borges, A.C.; Matos, A.T.; Barbosa, R.B.G.; Silva, F.F. Exploring the Removal of Organic Matter in Constructed Wetlands Using First Order Kinetic Models. Water 2022, 14, 472. [Google Scholar] [CrossRef]
- Sobolewska, E.; Borowski, S.; Nowicka-Krawczyk, P. Effect of solar and artificial lighting on microalgae cultivation and treatment of liquid digestate. J. Environ. Manage. 2023, 344, 118445. [Google Scholar] [CrossRef]
- Calijuri, M.L.; Silva, T.A.; Magalhães, I.B.; Pereira, A.S.A.P.; Marangon, B.B.; Assis, L.R.; Lorentz, J.F. Bioproducts from microalgae biomass: Technology, sustainability, challenges and opportunities. Chemosphere 2022, 305, 135508. [Google Scholar] [CrossRef]
- Ayre, J.M.; Mickan, B.S.; Jenkins, S.N.; Moheimani, N.R. Batch cultivation of microalgae in anaerobic digestate exhibits functional changes in bacterial communities impacting nitrogen removal and wastewater treatment. Algal Res. 2021, 57, 102338. [Google Scholar] [CrossRef]
- Vadiveloo, A.; Foster, L.; Kwambai, C.; Bahri, P.A.; Moheimani, N.R. Microalgae cultivation for the treatment of anaerobically digested municipal centrate (ADMC) and anaerobically digested abattoir effluent (ADAE). Sci. Total Environ. 2021, 775, 145853. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Figueiredo, D.; Ferreira, F.; Marujo, A.; Bastos, C.R.V.; Martin-Atanes, G.; Ribeira, B.; Štěrbová, K.; Marques-dos-Santos, C.; Acién, G.; Gouveia, L. From piggery wastewater to wheat using microalgae towards zero waste. Algal Res. 2023, 72, 103153. [Google Scholar] [CrossRef]
- Khoo, K.S.; Ahmad, I.; Chew, K.W.; Iwamoto, K.; Bhatnagar, A.; Show, P.L. Enhanced microalgal lipid production for biofuel using different strategies including genetic modification of microalgae: A review. Prog. Energ. Combust. Sci. 2023, 96, 101071. [Google Scholar] [CrossRef]
- Mendonça, H.V.; Assemany, P.; Abreu, M.; Couto, E.; Maciel, A.M.; Duarte, R.L.; Santos, M.G.B.; Reis, A. Microalgae in a global world: New solutions for old problems? Renew. Energy 2021, 165(Part 1), 842–862. [Google Scholar] [CrossRef]
- Marcilhac, C.; Sialve, B.; Pourcher, A.M.; Ziebal, C.; Bernet, N.; Béline, F. Digestate color and light intensity affect nutrient removal and competition phenomena in a microalgal-bacterial ecosystem. Water Res. 2014, 64, 278–287. [Google Scholar] [CrossRef]
- Tawfik, A.; Hasanan, K.; Abdullah, M.; Badr, O.A.; Awad, H.M.; Elsamadony, M.; El-Dissouky, A.; Qyyum, M.A.; Nizami, A.S. Graphene enhanced detoxification of wastewater rich 4-nitrophenol in multistage anaerobic reactor followed by baffled high-rate algal pond. J. Hazard. Mater. 2022, 424, 127395. [Google Scholar] [CrossRef]
- Ferreira, A.; Melkonyan, L.; Carapinha, S.; Ribeiro, B.; Figueiredo, D.; Avetisova, G.; Gouveia, L. Biostimulant and biopesticide potential of microalgae growing in piggery wastewater. Environ. Adv. 2021, 4, 100062. [Google Scholar] [CrossRef]
- Molinuevo-Salces, B.; Mahdy, A.; Ballesteros, M.; González-Fernández, C. From piggery wastewater nutrients to biogas: Microalgae biomass revalorization through anaerobic digestion. Renew. Energy 2016, 96, 1103–1110. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, H.; Lee, E. Influence of Ammonia Stripping Parameters on the Efficiency and Mass Transfer Rate of Ammonia Removal. Appl. Sci. 2021, 11, 441. [Google Scholar] [CrossRef]
- Chai, W.S.; Chew, C.H.; Munawaroh, H.S.H.; Ashokkumar, V.; Cheng, C.K.; Park, Y.K.; Show, P.L. Microalgae and ammonia: A review on inter-relationship. Fuel 2021, 303, 121303. [Google Scholar] [CrossRef]
- Choi, H.J. Dairy wastewater treatment using microalgae for potential biodiesel application. Environ. Eng. Res. 2016, 21, 393–400. [Google Scholar] [CrossRef]
- Chen, B.; Wan, C.; Mehmood, A.M.; Chang, J.S.; Bai, F.; Zhao, X. Manipulating environmental stresses and stress tolerance of microalgae for enhanced production of lipids and value-added products-A review. Bioresour. Technol. 2017, 244, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Gao, Z.; Shi, T.Q.; Song, P.; Ren, L.J.; Huang, H.; Ji, X.J. Reactive Oxygen Species-Mediated Cellular Stress Response and Lipid Accumulation in Oleaginous Microorganisms: The State of the Art and Future Perspectives. Fron. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Hena, S. , Fatimah, S., Tabassum, S. Cultivation of algae consortium in a dairy farm wastewater for biodiesel production. Water Resour. Industry 2015, 10, 1–14. [Google Scholar] [CrossRef]
- Mousavi, S.; Najafpour, G.D.; Mohammadi, M.; Seifi, M.H. Cultivation of newly isolated microalgae Coelastrum sp. in wastewater for simultaneous CO2 fixation, lipid production and wastewater treatment. Bioprocess Biosyst. Eng. 2018, 41, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Koutra, E.; Grammatikopoulos, G.; Kornaros, M. Selection of microalgae intended for valorization of digestate from agro-waste mixtures. Waste Manage. 2018, 73, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Pradhan, S.; Patel, A.; Ghosh, U.K. An approach for dairy wastewater remediation using mixture of microalgae and biodiesel production for sustainable transportation. J. Environ. Manage. 2021, 297, 113210. [Google Scholar] [CrossRef]
- Mandotra, S.K.; Kuma, P.; Suseela, M.R.; Nayala, S.; Ranteke, P.W. Evaluation of fatty acid profile and biodiesel properties of microalga Scenedesmus abundans under the influence of phosphorus, pH and light intensities. Bioresour. Technol. 2016, 201, 222–229. [Google Scholar] [CrossRef]
- Zhu, L.D.; Li, Z.H.; Guo, D.B.; Huang, F.; Nugroho, Y.; Xia, K. Cultivation of Chlorella sp. with livestock waste compost for lipid production. Bioresour. Technol. 2017, 223, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.G.B.; Duarte, R.L.; Maciel, A.M.; Abreu, M.; Reis, A.; Mendonça, H.V. Microalgae Biomass Production for Biofuels in Brazilian Scenario: A Critical Review. BioEnergy Res. 2021, 14, 23–42. [Google Scholar] [CrossRef]
- Chhandama, M.V.L.; Satyan, K.B.; Changmai, B.; Vanlalveni, C.; Rokhum, S.L. Microalgae as a feedstock for the production of biodiesel: A review. Bioresour. Technol. Reports, 2021, 15, 100771. [Google Scholar] [CrossRef]
- Morales, M.; Aflalo, C.; Bernard, O. Microalgal lipids: A review of lipids potential and quantification for 95 phytoplankton species. Biomass Bioenerg. 2021, 150, 106108. [Google Scholar] [CrossRef]
- Sohail, N.F. ; Zeshan; Iftikhar, R. ; Saleem, S. Microalgal treatment of high-nutrient wastewater using twin layer cultivation system. J. Environ. Chem. Eng. 2023, 11, 2–109248. [Google Scholar] [CrossRef]
- Oliveira, C.Y.B.; Oliveira, C.D.L.; Prasad, R.; Ong, H.C.; Araujo, E.S.; Shabnam, N.; Gálvez, A.O. A multidisciplinary review of Tetradesmus obliquus: a microalga suitable for large-scale biomass production and emerging environmental applications. Rev. Aquac. 2021, 13, 3–1594. [Google Scholar] [CrossRef]
- Gupta, S. K.; Ansari, F.A.; Shriwastav, A.; Sahoo, N.K.; Rawat, I.; Bux, F. Dual role of Chlorella sorokiniana and Scenedesmus obliquus for comprehensive wastewater treatment and biomass production for biofuels. J. Clean. Prod. 2016, 115, 1–255. [Google Scholar] [CrossRef]
- Trivedi, J.; Agrawal, D.; Atray, N.; Ray, A. Enhanced lipid production in Scenedesmus obliquus via nitrogen starvation in a two-stage cultivation process and evaluation for biodiesel production. Fuel 2022, 316, 123418. [Google Scholar] [CrossRef]

| Model | Equation |
|---|---|
| First-order | ) (5) |
| Logistic Model | (6) |
| Cone | (7) |
| Modified Gompertz | (8) |
| FOMT | ) (9) |
| FOIT | (10) |
| Treatments | TKN (mg L-1) | NH4+ (mg L-1) | NOrg (mg L-1) | NO-3 (mg L-1) | PO4-3 (mg L-1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| In | Out | In | Out | In | Out | In | Out | In | Out | |
| CT | 1481 (30.8) | 1640 (0.0) | 924 (0.0) | 1134 (14) | 557 | 506 | 405 (5.35) | 362 (19.33) | 34 (4.5) | 39 (0.75) |
| R5 | 1526 (2.8) | 1568 (0.0) | 966 (14) | 1050 (14) | 560 | 518 | 424 (5.45) | 386 (5.20) | 22 (0.0) | 43 (1.0) |
| R10 | 1587 (2.8) | 1657 (0.0) | 938 (14) | 1162 (14) | 649 | 495 | 610 (6.94) | 375 (12.64) | 23 (0.25) | 44 (0.0) |
| R20 | 1630 (2.8) | 1836 (0.0) | 896 (0.0) | 1204 (0.0) | 734 | 632 | 800 (1.19) | 360 (0.5) | 24 (0.0) | 46 (2.0) |
| Model | R2 (%) | rRMSE (%) |
|---|---|---|
| First-order | 96,6 | 11,2 |
| Logistic Model | 99,7 | 3,4 |
| Cone | 99,7 | 3,7 |
| Modified Gompertz | 99,9 | 2,2 |
| FOMT | 99,9 | 2,1 |
| FOIT | 99,2 | 5,6 |
| Treatments | COD (mg L-1) | N-NH4+ (mg L-1) | PO4-3 (mg L-1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| In | Out | Rem (%) | In | Out | Rem (%) | In | Out | Rem (%) | |
| CT | 3860 (0.0) | 645 (0.0) | 83.3 | 565 (8.5) | 28 (0.0 | 95.0 | 19 (0.4) | 4.6 (0.37) | 75.8 |
| FR5 | 4770 (0.0) | 887 (80.6) | 81.4 | 522 (7.5) | 42 (14) | 92.0 | 21 (0.5) | 3.5 (0.0) | 83.3 |
| FR10 | 4992 (0.0) | 564 (80.6) | 88.7 | 577 (3.0) | 42 (14) | 92.7 | 22 (0.0) | 4.3 (0.25) | 80.5 |
| FR20 | 6588 (0.0) | 403 (80.6) | 93.8 | 600 (1.5) | 28 (0.0) | 95.3 | 23 (1.3) | 13.4 (2.87) | 41.7 |
| Substrate | Strain | Operation mode | COD (%) | NH4+ (%) | PO4-3 (%) | Reference |
|---|---|---|---|---|---|---|
| Treated aerobic dairy farm wastewater | Mixa | Batch | 98.8 | 100 | 98.8 | Hena et al. [76] |
| DCW anaerobically digested by hybrid reactor and sedimented | Tetradesmus obliquus | Batch | 65-70 | 98-99 | 69-77.5 | Mendonça et al. [18] |
| Cont. | 57-61 | 94-96 | 65-70 | |||
| DCW diluted with sterile distilled water | Coelastrum sp. | Semi-batch | 42 | >80 | 100 | Mousavi et al. [77] |
| Digestate of agro-industrial wastes diluted with water at 10% (v/v) | Parachlorella kessleri | Batch | 39.1-59.4 | >98 | 59 -88.4 | Koutra et al. [78] |
| Acutodesmus obliquus | ||||||
| Chlorella vulgaris | ||||||
| Tetraselmis tetrathele | ||||||
| Dairy wastewater diluted (70%) | Mixb | Batch | 61 | NR | 84 | Chandra et al. [79] |
| Piggery wastewater diluted (1:20) | Synechocystis sp. | NR | 61.6 | 92.4 | 90.1 | Ferreira et al. [69] |
| Tetradesmus obliquus | 73.1 | 87.5 | 98.1 | |||
| Chlorella protothecoides | 68.4 | 92.0 | 98.5 | |||
| Chlorella vulgaris | 79.2 | 79.4 | 98.6 | |||
| Piggery wastewater pre-treated with photo-Fenton | Tetradesmus obliquus | Batch | 48.6 | 37.3 | 100 | Ferreira et al. [64] |
| DCW was pre-treated in an activated sludge | Tetradesmus obliquus | Batch | 74 | 100 | 100 | Mendonça et al.[21] |
| Cont. | 78 | 94 | 74 | |||
| Chlorella vulgaris | Batch | 50 | 100 | 100 | ||
| Cont. | 60 | 92 | 61 | |||
| Digestate diluted (1:1) originating from the anaerobic co-digestion of the plant biomass Arundo donax L. and DCW | Tetradesmus obliquus | Batch - FR5 | 81.4 | 92.0 | 83.3 | Present work |
| FR10 | 88.7 | 92.7 | 80.5 | |||
| FR20 | 93.8 | 95.3 | 41.7 | |||
| CT | 83.3 | 95.0 | 75.8 |
| Treatments | Carbohydrates (%) | Lipids (%) | Proteins (%) | Ash (%) | |||
|---|---|---|---|---|---|---|---|
| AB (%) | Prod. g L-1 | AB (%) | Prod. g L-1 | AB (%) | Prod. g L-1 | ||
| CT | 29.7 (1.31) | 0.73 | 33.4 (1.3) | 0.82 | 11.6 (0.43) | 0.28 | 9.4 (0.27) |
| FR5 | 34.7 (0.54) | 1.20 | 42.7 (2.4) | 1.48 | 7.9 (0.62) | 0.27 | 8.0 (0.19) |
| FR10 | 33.6 (1.44) | 0.97 | 40.3 (1.6) | 1.16 | 7.1 (0.13) | 0.20 | 8.1 (0.14) |
| FR20 | 32.6 (1.88) | 0.94 | 36.5 (0.4) | 1.05 | 7.0 (0.18) | 0.20 | 8.9 (0.38) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





