1. Introduction
During the last few years, the prevalence of antibiotic-resistant bacteria in clinical settings has dramatically risen worldwide, alarming scientists and government agencies. According to the Center for Disease Control and Prevention’s (CDC) 2019 Antibiotic Resistance Threats Report, antibiotic-resistant bacteria and fungi cause more than 2.8 million infections and 35,000 deaths annually, with annual healthcare costs exceeding
$281 million in the United States [
1]. A pathogen of particular concern is
Acinetobacter baumannii, a “difficult -to-treat” bacterium responsible for infections with mortality rates as high as 60% (through community-acquired pneumonia) and 43.4% (through bloodstream infections).
A. baumannii remains a top priority in the latest WHO list and has been categorized as an "Urgent" threat by CDC.
Intrinsic features such as
A. baumannii’s ability to persist in clinical settings even under desiccation and nutrient starvation, as well as its ability to acquire foreign DNA, have contributed to its success as a major nosocomial pathogen [
2,
3,
4,
5]. The indiscriminate use of broad-spectrum antibiotics in hospital settings favors the selection of organisms harboring active mechanisms of horizontal gene transfer and extreme genome plasticity, such as
A. baumannii. Comparative genomic studies have revealed high variability in
Acinetobacter genome organization and the presence of foreign DNA sequences, suggesting that acquiring exogenous genetic traits has significantly contributed to the evolution and adaptation of the genus to unfavorable environmental conditions. Among numerous mechanisms that drive
A. baumannii evolution, acquiring foreign DNA by natural transformation plays a key role but is often underestimated [
3,
6]. Recent research has shown that
A. baumannii can acquire DNA and consequently, antibiotic resistance, including resistance to carbapenems from other
A. baumannii clinical strains,
Klebsiella pneumoniae, Providencia rettgeri, and methicillin-resistant
Staphylococcus aureus [
7]. The acquired DNA often includes mobile genetic elements, antimicrobial resistance genes, and operons involved in metabolism. In silico gene analysis indicates that when
A. baumannii and
K. pneumoniae share the same environment, they continuously exchange genetic material [
7]. Furthermore, natural transformation is primarily responsible for recombination events between
A. baumannii clinical isolates, leading to carbapenem resistance [
6]. Additional evidence of DNA acquisition and genomic plasticity in
A. baumannii is provided by a recent study comparing genomes of two
A. baumannii isolates recovered before and after COVID-19 pandemic. This study which proposed a unique background based on core-genome phylogeny and comparative genome analysis [
8], found that the post-COVID-19 strain acquired eight additional antimicrobial resistance genes, including
blaNDM-1.
Although
A. baumannii is known for its ability to acquire DNA fragments, certain genes remain host-specific and are rarely, if ever, found in this bacterium. For example,
A. baumannii isolates seldom carry the carbapenemases KPC, SPM-1, and VIM-2, which are typically found in Enterobacterales and
Pseudomonas spp. [
9]. Other genes with strict host specificity include the CMY-like plasmid-encoded β-lactamase genes, which are exclusively found in Enterobacterales. These genes, with 186 reported variants (
http://bldb.eu/alignment.php?align=C:CMY), are primarily located on IncC and IncI1 plasmids. Among this large group of CMY-like β-lactamase coding genes, CMY-1-like genes are the most prevalent plasmid-borne
ampC genes in Enterobacterales [
10,
11,
12]. IncI1 plasmids, often found in isolates from animals and hospitalized patients, have recently gained attention due to their ability to transfer between different bacteria. To date,
blaCMY-like genes have not been detected in
A. baumannii.
In this study, we describe A. baumannii AMA205, a highly resistant clinical strain that harbors blaCMY-6 and multiple antibiotic resistance mechanisms, including blaOXA-23 and blaNDM-1.
2. Results and Discussion
2.1. Genomic and Phylogenomic Comparative Analysis of A. baumannii AMA205 Reveals a Distinct Location of ST79
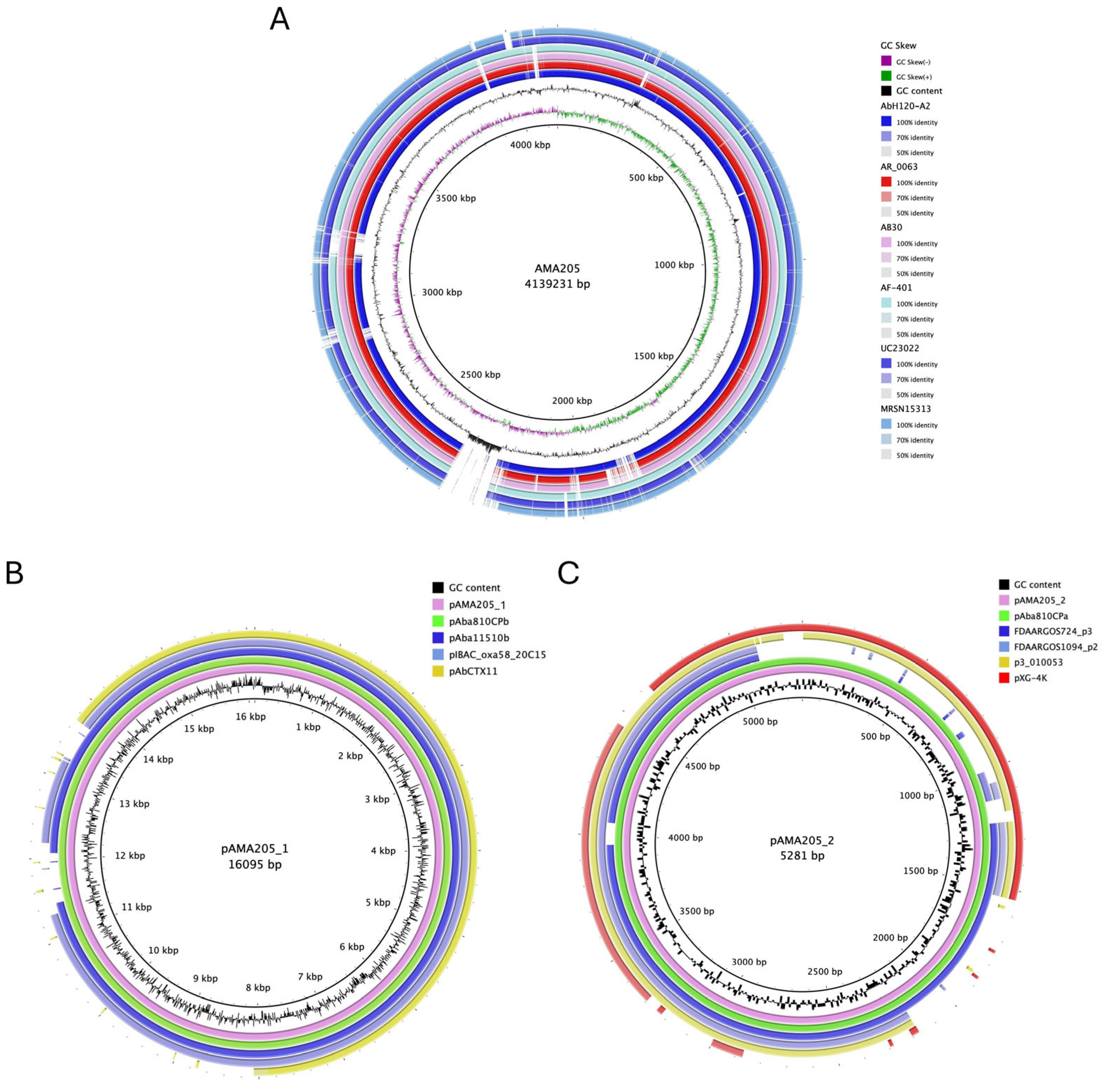
The ANI% score (e.g., 98.2± 1.80% with ATCC 17978) confirmed that the AMA205 isolate belongs to the A. baumannii species. The hybrid assembly of the AMA205 strain sequence produced a 4.139.231 bp- chromosome with a G + C content of 39.06%, and 4007 predicted protein-coding sequences with an average gene length of 900 bp (
Table 1 and
Figure 1A). Additionally, the procedure identified two plasmids, pAMA205_1 and pAMA205_2, whose sizes were 16.095 bp and 5.281 bp, respectively (
Table 1 and
Figure 1B, 1C). Comparing AMA205 plasmids with GenBank database, pAMA205_1 and pAMA205_2 were found in other A. baumannii isolates with high coverage (90-100%) (
Figure 1B and 1C). Interestingly, pAMA205_1 contains the znuB gene encoding a TonB-dependent receptor, which may play an important role in the virulence of AMA205.
Additionally, a comparison of AMA205 genome with all 246 A. baumannii ST79 genomes deposited in the GenBank database (
Table S1) revealed 1555 conserved gene families and 139 unique genes. The most clinically relevant unique genes belonged to mobile genetic elements, such as insertion sequences (ISEcp1, ISKpn8, IS103, etc), and coded for the β-lactamases NDM-1 and CMY-6 (
Table S2).
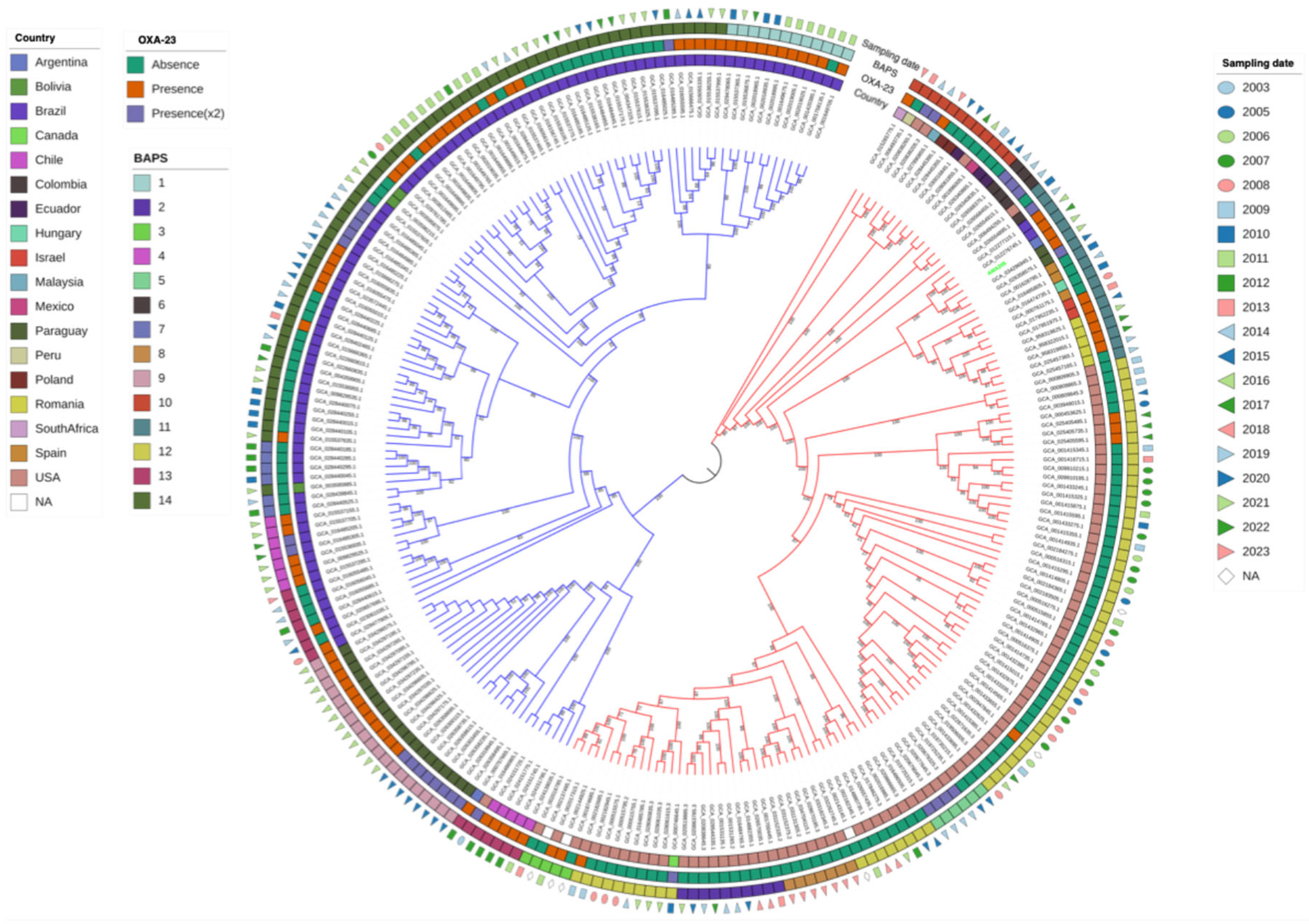
A core-genome phylogenetic analysis including A. baumannii AMA205 and all ST79 A. baumannii genomes currently in the GenBank (N = 246), which includes strains isolated worldwide between 2003 and 2023 (
Table S1), identified two major clusters: Cluster A and Cluster B. A. baumannii AMA205 was located in Cluster A, which included a diverse array of isolates from various regions worldwide, predominantly from the USA (90/123, 73.17%). Specifically, A. baumannii AMA205 was closely related to isolates from Paraguay and Brazil recovered during 2021-2022 (
Figure 2). Cluster B primarily consisted of isolates from South America, except for one isolate from the USA (GCA_016486965.1). Most isolates in Cluster B were from Brazil (94/124, 75.81%) and Paraguay (20/124, 16.13%), while Cluster A was mainly composed of isolates from the USA (90/123, 73.17%).
Population structure was inferred using BAPS clusters defined at the first stratification level, capturing the dataset's total genetic variation. This analysis divided the isolates into 14 BAPS clusters, with a significant correlation between the maximum likelihood phylogenetic tree and the BAPS clusters (
Figure 2). Cluster A included eight BAPS clusters (2, 3, 5, 6, 8, 10, 11, 12), while Cluster B comprised of six BAPS clusters (1, 4, 7, 9, 13, 14). A. baumannii AMA205 was placed within BAPS 11. These genetic clusters likely reflect common population differentiation processes, objectively defining groups of strains with similar genetic characteristics.
Further analysis revealed that the Brazilian isolates in Cluster B are divided into four populations (BAPS: 4, 7, 13, 14), while all Paraguayan isolates belong to a single population (BAPS: 9). Within the Paraguayan population, a correlation was observed between the presence of blaOXA-23, either in single or duplicated copies, and the year of isolation. Isolates from 2021 have a single blaOXA-23 copy, whereas those from 2020 have the gene is duplicated. In contrast, most isolates in Cluster A lack the blaOXA-23 gene. This low prevalence is particularly evident in isolates from the USA, where the gene is present in 12 out of 90 isolates (13.3%). Another important observation is that none of the available genomes showed the presence of blaNDM-1 in ST79. Therefore, AMA205 is the first A. baumannii strain with CMY-6 but also the first ST79 strain with the co-occurrence of blaOXA-23 and blaNDM-1.
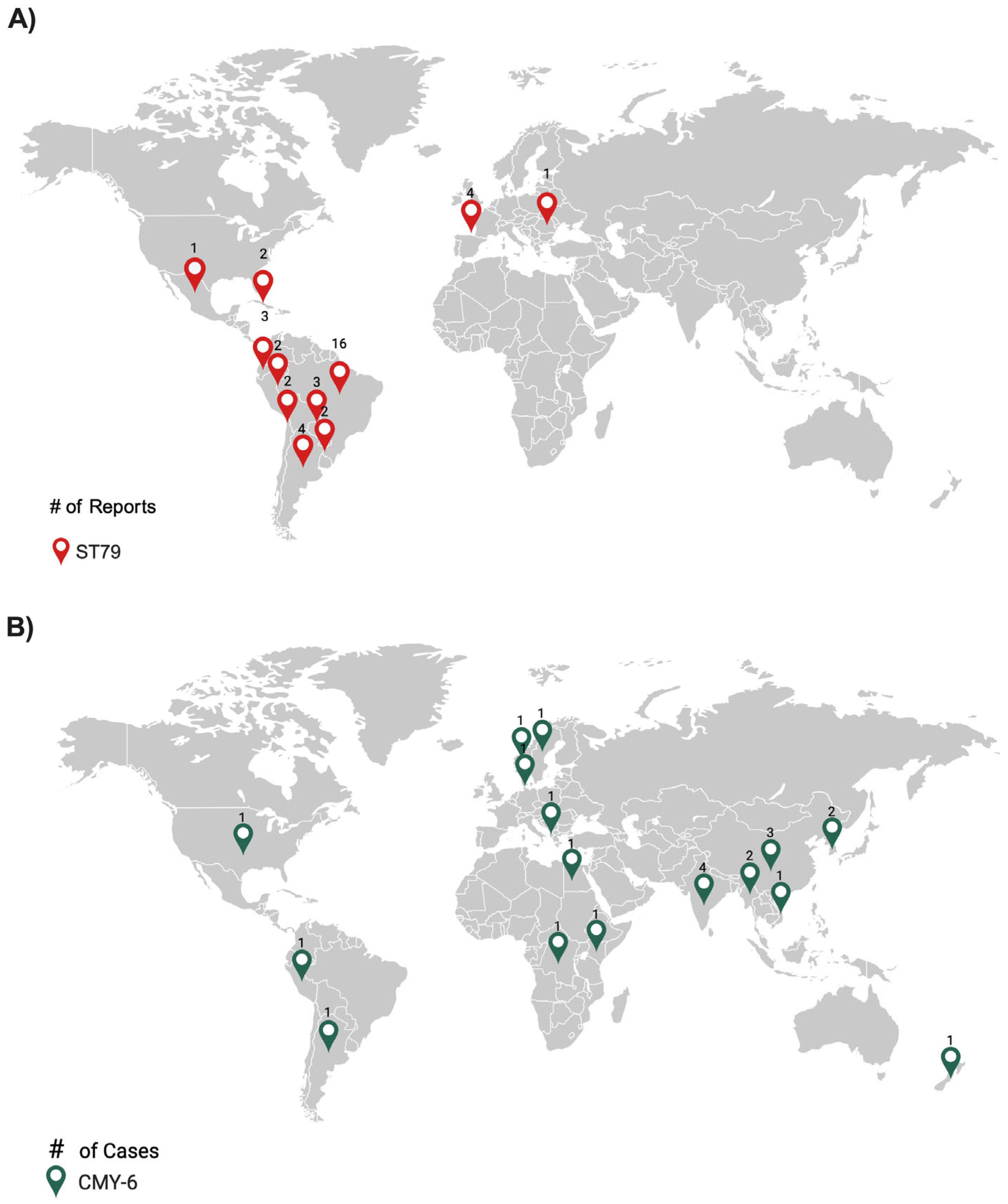
Most of the
A. baumannii ST79 strains published to date (based on a PubMed search performed in June 2024) were from Latin America (n=35, 87.5%), with additional cases in Spain (n=4, 10.0%) and a single case in Romania (n=1, 2.5%). The highest number of isolates were from Brazil (n=16, 45.7%) (
Figure 3A). Eighteen strains possessed
blaOXA-23, of which eleven were from Brazil. These results suggest that the ST79 most likely originated in Latin America, where the earliest report occurred in 2014 and the most recent in 2022. Infections caused by the sequence type outside Latin America may be attributed to infected travelers.
2.2. AMA205 Exhibits Resistance to Almost All Tested Antibiotics, Including Cefiderocol
Susceptibility testing of AMA205 to various antibiotics, including cloxacillin (CX), ceftazidime (CAZ), ceftazidime-avibactam (CZA), imipenem (IMP), meropenem (MEM), ampicillin-sulbactam (AMS), amikacin (AK), gentamicin (CN), and tigecycline (TIG) was conducted. As anticipated, the clinical isolate exhibited high resistant to most antibiotic families tested (
Table 2). The high MIC of Cefiderocol (FDC) determined by two different methodologies, showed that AMA205 is also resistant to this antibiotic. Furthermore, the presence of intracolonies (IHC) was evident in this isolate when E-strips were used. The isolate demonstrated a high level of resistance to ceftazidime, with a MIC greater than 256 mg/L, which remained unchanged with the individual use of MBL inhibitors and cephalosporinases. However, when both inhibitors were used simultaneously, the MIC was decreased to 16 mg/L. This result indicates that both
blaNDM-1 and
blaCMY-6 actively contribute to the cephalosporin resistance profile of this isolate.
The significant resistance of AMA205 is due to the presence of multiple resistance genes, including CMY-6, OXA-23, and NDM. This extensive array of resistance mechanisms complicates treatment options with currently approved drugs. It underscores the urgent need for alternative therapeutic approaches and robust antibiotic stewardship programs to manage and mitigate the spread of such resistant strains in patients.
2.3. Genomic Studies Reveal the Presence of CMY-6 and Other Antimicrobial Resistance Genes in the AMA205 Genome
The genomic analysis of
A. baumannii AMA205 revealed antibiotic resistance genes within its core (intrinsic genes) and accessory (acquired genes) genomes. The acquisition of genetic determinants, a critical factor in
A. baumannii’s evolution, occurs through mechanisms like transformation, conjugation, and transduction, involving mobile genetic elements [
13,
14]. Genes conferring resistance to trimethoprim, florfenicol, β-lactams, aminoglycosides, and sulfonamides were identified. The intrinsic genes
blaADC-25 and
blaOXA-65 were present, but not associated with flanking insertion sequences (ISs), which is linked to their basal expression, resulting in weak levels of β-lactam hydrolysis [
15,
16]. All other antibiotic resistance genes were flanked by mobile genetic elements, suggesting acquisition through horizontal gene transfer. The globally distributed
blaOXA-23 gene, found in both chromosomes and plasmids, was located within the transposon, Tn2008. This transposon along with Tn2006, is one of the most common platforms harboring
blaOXA-23. Although these transposons are typically associated with a TnAbaR4-like island [
17,
18,
19], in
A. baumannii AMA205, Tn2008 was found outside the TnAbaR-like element. A β-lactamase gene
blaTEM-1B was identified within Tn3, differing from the usual association with the TnAbaR element seen in other
A. baumannii isolates where blaTEM-1B has been detected.
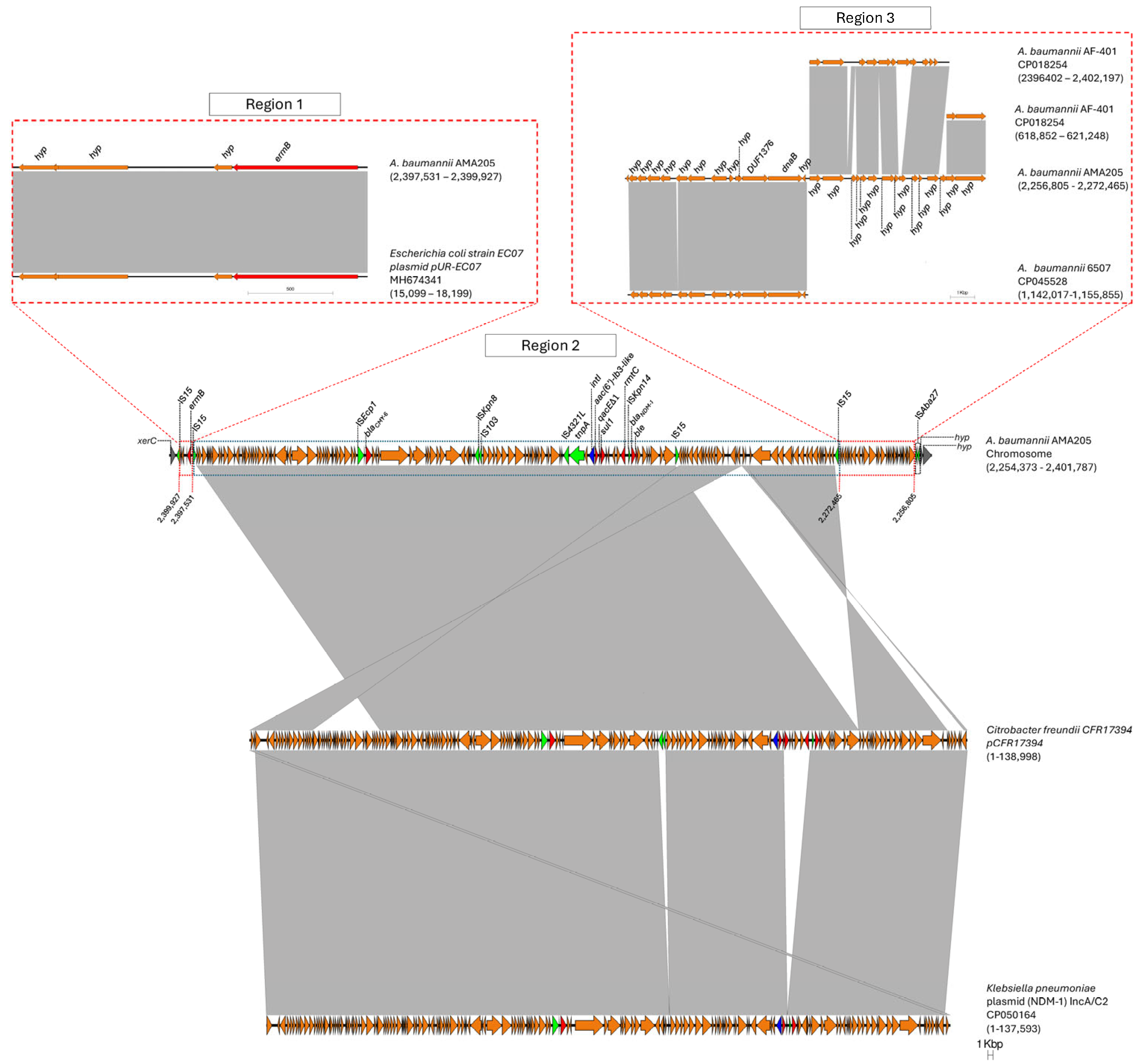
A large genomic island (GI-CMY) was identified in the AMA205 strain (
Figure 4). This 147.4-kb sequence is flanked by the
xerC gene, which encodes the XerC recombinase protein, and a gene for a hypothetical protein. The GI-CMY contains genes that confer resistance to aminoglycosides (
aac(6’)-Ib3-like, rmtC, and
ermB), β-lactams (
blaCMY-6 and
blaNDM-1), and sulfonamides (
sul1) (
Figure 4). Based on sequence homology, three regions were identified within GI-CMY. Region 1 harbors a 2396 bp fragment containing four genes (three encoding hypothetical proteins and the
emrB gene) that show high homology to a fragment of the
Escherichia coli plasmid pUR-EC07 (Coverage: 100%, Nucleotide Identity: 100%). Region 2, the largest at 125,066 bp, shows high homology with various Enterobacterales plasmids, such as those from
Citrobacter freundii (Coverage: 99, Nucleotide Identity 100) and Klebsiella pneumoniae (Coverage: 93%, Nucleotide Identity: 99%). Notably, an important region of the chromosomal insertion of the blaCMY-6 gene in AMA205 resembles the plasmid previously documented by Martino et al. [
20]. This region contains a class 1 integron with
aac(6’)-Ib3-like in the variable region and also includes the cephalosporin resistance gene
blaCMY-6 and the carbapenemase
blaNDM-1, located downstream of the insertion sequences IS
Ecp1 and IS
Kpn14, respectively (
Figure 4).
The AAC(6’)-Ib3-like variant identified shows an amino acid change at the first position (M1L), defining it as a new variant of aminoglycoside 6’N-acetyltransferase type Ib. The activity spectrum of this new variant will be investigated in future studies. Finally, region 4 (15,660 bp) contains fragments of A. baumannii sequences that show greater homology with genomes from other ST (6507) or more distantly related strains within the same sequence type (AF-401). These findings suggest that GI-CMY has undergone at least 4 to 5 recombination events, two of which likely involve the integration of DNA sequence segments commonly found in Enterobacterales (Regions 1 and 2). It is hypothesized that two intra-species recombination events may have occurred in region 3. The recombination of these regions and the potential mobilization of GI-CMY may be facilitated by the various ISs flanking each region and the overall structure of the genomic island (IS15 and ISAba27).
As of June 2024, the
blaCMY-26 gene was reported worldwide, more than half of the cases were from Asia (n=12, 54.5%), and two from Latin America (9.1%) (
Figure 3B). This gene is frequently found alongside
blaNDM-1 in various species belonging to Enterobacterales, including
E. coli, K. pneumoniae, and
Providencia vermicola.
To investigate the frequency or rarity of the blaCMY gene acquisition, a search was conducted of 120 CRAB clinical isolates, all of which tested negative. This finding suggests that the blaCMY gene is rare in this species.
The AMA205 strain, which shows resistance to multiple antibiotics, is also resistant to cefiderocol. The resistance is linked to mutations in genes involved in iron uptake, such as
pirA, piuA, and
cirA [
21,
22]. A comparison of the
pirA and
piuA genes from AMA205 with those from the cefiderocol-susceptible reference strain ATCC17978 revealed 100% amino acid identity, indicating that mutations in these genes are not responsible for resistance to cefiderocol. However, the
cirA from AMA205 showed 87% amino acid identity of 87% with 100% coverage compared to ATCC17978, including a significant deletion of six nucleotides in the gene sequence. These differences in the
cirA gene along with the presence of multiple β-lactamases, may partially explain the cefiderocol resistance phenotype.
2.4. AMA205 Genomic Analysis Revealed the Presence of a Variety of Virulence Factors
Recent research identified virulence factors in
A. baumannii [
23]. Using the VFDB database, 111 potential virulence factors coding genes were found in the
A. baumannii AMA205 strain (
Table S3).
Adherence to the host cell is the crucial initial step in bacterial colonization and infection. During this process, bacteria can form microcolonies that develop into a highly organized microbial community known as a biofilm. In
A. baumannii, the initial stage of biofilm formation is driven by elements coded for by the CsuA/BABCDE operon genes. A key factor in this process is the fimbriae chaperone, which is responsible for the assembly and production of pili that facilitate surface adhesion [
24,
25]. The regulation of this operon is controlled by a two-component system (BfmRS), comprising a kinase sensor (BfmS) and a response regulator (BfmR) [
26,
27]. The development of a mature biofilm structure involves a biofilm-associated protein (Bap), an ortholog of the protein found in Staphylococcus species, first identified in the
A. baumannii AB307-0294 strain [
28,
29]. This study confirmed the presence of the CsuA/BABCDE operon, the bfmSR regulatory system, and the bap gene in the
A. baumannii AMA205 genome (
Table S3).
The functions of TonB, ExbB, and ExbD are not limited to the acinetobactin iron uptake system. These three inner membrane proteins are involved in transporting various molecules, including heme, vitamin B12, and other iron-siderophore complexes [
30,
31,
32,
33]. Additionally, three distinct copies of
tonB have been identified,
tonB1 and
tonB3, which, along with
exbB and
exbD, form an operon, and
tonB2, a monocistronic gene. All five genes were found in the
A. baumannii AMA205 genome.
A. baumannii AMA205 also contains the bfn locus, which includes genes responsible for the biosynthesis of baumannoferrin, a siderophore first found in
A. baumannii AYE strain [
24,
33]. Baumannoferrin has a higher affinity for iron than acinetobactin. Its synthesis and internalization operate independently of the genes specific to the acinetobactin iron uptake system. The similarity of the Bfn proteins in
A. baumannii AMA205 to those in other Acinetobacter species, along with the fact that the locus is not ubiquitous to
A. baumannii [
34,
35], suggest that it was acquired through horizontal gene transfer (
Table S4).
The capsular polysaccharide is a crucial virulence factor in Gram-negative bacteria, enabling resistance to the bactericidal activity of the complement system.
A. baumannii AMA205 contains the capsular polysaccharide biosynthesis loci (KL, K locus) and LPS loci (OCL, OC locus). These loci are typically genomic "hotspots" of variability [
36,
37]. Comparative analysis of the KL structure in
A. baumannii AMA205 showed a GC content of 33.35%, 99% nucleotide identity, and 100% coverage with the KL9 type (Figure sup KL and OC). The OCL locus, responsible for O antigen synthesis, had a GC content of 36.01%, and was identified as OCL10-like (
Table S3).
3. Materials and Methods
3.1. Bacterial isolates
A. baumannii AMA205 was isolated in 2021 (Argentina) from a 30-year-old patient admitted to hospital with COVID-19, who developed sepsis and pneumonia after 11 days of hospitalization. AMA205 strain was cultured in Luria Bertani (LB) medium and was initially identified using MALDI-TOF MS [
28]. The identification was later confirmed by whole-genome sequencing (WGS) analysis. In addition, 120 CRAB strains from the National Regional Reference Laboratory for Antimicrobial Resistance (ANLIS–Dr. Carlos G. Malbrán) collection were used to search for the presence of
blaCMY by PCR amplification using primers CMY-F 5-TGGCCAGAACTGACAGGCAAA-3 and CMY-R 5-TTTCTCCTGAACGTGGCTGGC-3.
3.2. Whole Genomic Sequencing (WGS)
AMA205 DNA was extracted using the Wizard® Genomic DNA Purification Kit (Madison, USA) according to the manufacturer’s protocol. Whole genome sequencing (WGS) was conducted using the Illumina NovaSeq X Plus sequencer platform and Oxford Nanopore MinION Mk1B (Seqcenter sequencing service). Sequencing quality was evaluated using FASTQC software (
https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). De novo assembly was performed with Unicycler, and quality assessment was conducted using QUAST software. The genome annotation files can be found in the zenodo repository
https://zenodo.org/records/13741979. The Whole Genome Shotgun project has been deposited in GenBank with accession numbers CP169298 (AMA205 Chromosome) CP169299 and CP169300 (plamids pAMA205_1 and pAMA205_2, respectively).
3.3. Comparative Genomic Analysis
AMA205 genome was annotated using PROKKA [
38]. The ortholog functional assignment was done using EggNOG v2.0 (default parameter) [
38]. To validate the species identification, the average nucleotide identity (ANI) were calculated using JSpeciesWS [
39] and reference genomes of Acinetobacter available in NCBI genome database. To assess core genome phylogeny, we used 246
A. baumannii ST79 sequences from a total of 25087
A. baumannii genome available in the GenBank (
Table S1). Core genome phylogeny analysis was performed using the maximum likelihood method, implemented with IQtree2 using default parameters [
40].
Bayesian Analysis of Population Structure (BAPS) was performed using the "fastbaps" R package [
41]. This software employs a phylogeny-independent, nested Bayesian clustering method to analyze population stratification, using core-genome sequences as input data.
tRNA and ncRNA predictions were conducted using tRNAscan-SE and Infernal software, respectively [
42] and the Multilocus Sequence Typing (MLST) profile was determined using MLST scripts (
https://github.com/tseemann/mlst) AMA205 genomic DNA was extracted using the Wizard® . Antimicrobial resistance and virulence genes were identified using VFDB and Resfinder databases [
43,
44], respectively, using the BLASTp software.
3.4. Antibiotic Susceptibility Testing (AST)
AST profiles of AMA205 were performed following the Clinical and Laboratory Standards Institute (CLSI) guidelines as described in the 30th Edition informational supplement [
45]. Disk diffusion was firstly performed with the following antibiotics: 10 µg ampicillin/sulbactam, 30 µg amikacin, 30 µg cefepime, 30 µg ceftazidime 5 µg ciprofloxacin, 10 µg imipenem, 10 µg gentamicin, 10 µg meropenem, 15 µg tigecycline, 30 µg minocycline or 10 µg colistin. Broth Microdilution for Minimum inhibitory concentration (MIC) determination was conducted according to CLSI guidelines. For cefiderocol susceptibility three different methods, commercial E-strips (Liofilchem S.r.l., Roseto degli Abruzzi, Italy), ComASP ((Liofilchem S.r.l.), and broth microdilution (reference method), were used. The methods were performed according to the manufacturer's instructions and EUCAST standards (
https://www.eucast.org/clinical_breakpoints).
Each experiment was repeated at least three times for each strain. Results were interpreted using CLSI guidelines, except for colistin and tigecycline, which were interpreted using the European Committee on Antimicrobial Susceptibility Testing (EUCAST) and Food and Drug Administration (FDA) recommendations, respectively. The CLSI, EUCAST, and FDA provide guidelines for antimicrobial susceptibility testing, including standardized methods, quality control procedures, and interpretive criteria for assessing the susceptibility of microorganisms to antimicrobial agents.
To study and determine the specific contributions of both blaNDM-1 and blaCMY-6 genes to the overall resistance profile, susceptibility to ceftazidime was assessed using commercial E-strips (Etest, Biomerieux, Germany) according to the manufacturer's guidelines. This evaluation was conducted using Mueller-Hinton broth alone and with the addition of Metallo-β-lactamases (MBL) inhibitor EDTA at a final concentration of 0.4 mM, and cephalosporinases inhibitor 3-amino-phenyl-boronic acid, at a final concentration of 300 µg/ml.
5. Conclusions
The concerning resistance patterns observed in this strain, coupled with novel resistance mechanisms in A. baumannii, emphasize the necessity for global surveillance to effectively target antimicrobial therapy. Furthermore, this strain represents an emerging threat due to the known potential of ST79 strains to spread across various environments. In summary, A. baumannii’s unique ability to evolve and acquire resistance from diverse species highlights the urgent need for ongoing research and clinical efforts to address this public health threat.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1. Comparison of AMA205 genome with all 246 A. baumannii ST79 genomes deposited in the GenBank database. Table S2. The most clinically relevant unique genes found in A. baumannii AMA205 strain. Table S3. The virulence genes found in A. baumannii AMA205 strain.
Author Contributions
GMT, FP, RAB, GR, MET, and MSR conceived the study and designed the experiments. GMT, FP, AM, SM, UA, SG, CM, NF, JE, AQ, CF, EA, MRT, and MSR performed the experiments and genomics and bioinformatics analyses. GMT, FP, RAB, GR, MRT, MET, EA, and MSR analyzed the data and interpreted the results. GMT, FP, MET, AQ, CF and M.S.R. contributed reagents/materials/analysis tools. GMT, FP, MRT, RAB, GR, MET, and MSR. wrote and revised the manuscript. All authors read and approved the final manuscript.
Funding
The authors' work was supported by NIH SC3GM125556 to MSR, R01AI100560, R01AI063517, R01AI072219 to RAB, and 2R15 AI047115 to MET. This study was supported in part by funds and facilities provided by the Cleveland Department of Veterans Affairs, Award Number 1I01BX001974 to RAB from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development and the Geriatric Research Education and Clinical Center VISN 10 to RAB. NF works was funded by the U-RISE at Cal State Fullerton grant 5T34 GM149493-01. The content is solely the authors' responsibility and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans.
Institutional Review Board Statement
Not applicable
Data Availability Statement
The Whole Genome Shotgun project was deposited in GenBank with accession numbers CP169298 (Chromosome) CP169299 and CP169300 (plasmids).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Henley, S.J.; King, J.B.; German, R.R.; Richardson, L.C.; Plescia, M. Centers for Disease Control & Prevention (CDC). 2010.
- Roca, I.; Espinal, P.; Vila-Farrés, X.; Vila, J. The Acinetobacter baumannii Oxymoron: Commensal Hospital Dweller Turned Pan-Drug-Resistant Menace. Front. Microbiol. 2012, 3. [CrossRef]
- Perez, F.; Stiefel, U. The Impact of Natural Transformation on the Acquisition of Antibiotic Resistance Determinants. MBio 2022, 13. [CrossRef]
- Antunes, L.C.S.; Imperi, F.; Carattoli, A.; Visca, P. Deciphering the Multifactorial Nature of Acinetobacter baumannii Pathogenicity. PLoS One 2011, 6, e22674. [CrossRef]
- Nasr, P. Genetics, Epidemiology, and Clinical Manifestations of Multidrug-Resistant Acinetobacter baumannii. J. Hosp. Infect. 2020, 104, 4–11. [CrossRef]
- Godeux, A.-S.; Svedholm, E.; Barreto, S.; Potron, A.; Venner, S.; Charpentier, X.; Laaberki, M.-H. Interbacterial Transfer of Carbapenem Resistance and Large Antibiotic Resistance Islands by Natural Transformation in Pathogenic Acinetobacter. MBio 2022, 13. [CrossRef]
- Traglia, G.M.; Place, K.; Dotto, C.; Fernandez, J.S.; Montaña, S.; Bahiense, C. dos S.; Soler-Bistue, A.; Iriarte, A.; Perez, F.; Tolmasky, M.E.; et al. Interspecies DNA Acquisition by a Naturally Competent Acinetobacter baumannii Strain. Int. J. Antimicrob. Agents 2019, 53, 483–490. [CrossRef]
- Traglia, G.M.; Pasteran, F.; Escalante, J.; Nishimura, B.; Tuttobene, M.R.; Subils, T.; Nuñez, M.R.; Rivollier, M.G.; Corso, A.; Tolmasky, M.E.; et al. Genomic Comparative Analysis of Two Multi-Drug Resistance (MDR) Acinetobacter Baumannii Clinical Strains Assigned to International Clonal Lineage II Recovered Pre- and Post-COVID-19 Pandemic. Biology (Basel). 2023, 12, 358. [CrossRef]
- López, C.; Ayala, J.A.; Bonomo, R.A.; González, L.J.; Vila, A.J. Protein Determinants of Dissemination and Host Specificity of Metallo-β-Lactamases. Nat. Commun. 2019, 10, 3617. [CrossRef]
- Bauernfeind, A.; Stemplinger, I.; Jungwirth, R.; Wilhelm, R.; Chong, Y. Comparative Characterization of the Cephamycinase BlaCMY-1 Gene and Its Relationship with Other Beta-Lactamase Genes. Antimicrob. Agents Chemother. 1996, 40, 1926–1930. [CrossRef]
- Lee, K.; Lee, M.; Shin, J.H.; Lee, M.H.; Kang, S.H.; Park, A.J.; Yong, D.; Chong, Y. Prevalence of Plasmid-Mediated AmpC β -Lactamases in Escherichia coli and Klebsiella Pneumoniae in Korea. Microb. Drug Resist. 2006, 12, 44–49. [CrossRef]
- Sekar, R.; Mahalakshmi, D.; Srivani, R.; Shankar, E.M.; Vignesh, R. High Rate of Detection of High-Level Aminoglycoside-Resistant Enterococci from Urinary Tract Specimens in South India. Int. J. Antimicrob. Agents 2008, 31, 383–385. [CrossRef]
- Domingues, S.; Harms, K.; Fricke, W.F.; Johnsen, P.J.; da Silva, G.J.; Nielsen, K.M. Natural Transformation Facilitates Transfer of Transposons, Integrons and Gene Cassettes between Bacterial Species. PLoS Pathog. 2012, 8, e1002837. [CrossRef]
- Da Silva, G.; Domingues, S. Insights on the Horizontal Gene Transfer of Carbapenemase Determinants in the Opportunistic Pathogen Acinetobacter baumannii. Microorganisms 2016, 4, 29. [CrossRef]
- Héritier, C.; Poirel, L.; Fournier, P.-E.; Claverie, J.-M.; Raoult, D.; Nordmann, P. Characterization of the Naturally Occurring Oxacillinase of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2005, 49, 4174–4179. [CrossRef]
- Figueiredo, S.; Poirel, L.; Croize, J.; Recule, C.; Nordmann, P. In Vivo Selection of Reduced Susceptibility to Carbapenems in Acinetobacter baumannii Related to IS Aba1 -Mediated Overexpression of the Natural Bla OXA-66 Oxacillinase Gene. Antimicrob. Agents Chemother. 2009, 53, 2657–2659. [CrossRef]
- Nigro, S.; Hall, R.M. Distribution of the Bla OXA-23-Containing Transposons Tn 2006 and Tn 2008 in Australian Carbapenem-Resistant Acinetobacter baumannii Isolates. J. Antimicrob. Chemother. 2015, 70, 2409–2411. [CrossRef]
- Yoon, E.-J.; Kim, J.O.; Yang, J.W.; Kim, H.S.; Lee, K.J.; Jeong, S.H.; Lee, H.; Lee, K. The BlaOXA-23-Associated Transposons in the Genome of Acinetobacter Spp. Represent an Epidemiological Situation of the Species Encountering Carbapenems. J. Antimicrob. Chemother. 2017, 72, 2708–2714. [CrossRef]
- Traglia, G.; Chiem, K.; Quinn, B.; Fernandez, J.S.; Montaña, S.; Almuzara, M.; Mussi, M.A.; Tolmasky, M.E.; Iriarte, A.; Centrón, D.; et al. Genome Sequence Analysis of an Extensively Drug-Resistant Acinetobacter baumannii Indigo-Pigmented Strain Depicts Evidence of Increase Genome Plasticity. Sci. Rep. 2018, 8, 16961. [CrossRef]
- Martino, F.; Tijet, N.; Melano, R.; Petroni, A.; Heinz, E.; De Belder, D.; Faccone, D.; Rapoport, M.; Biondi, E.; Rodrigo, V.; et al. Isolation of Five Enterobacteriaceae Species Harbouring BlaNDM-1 and mcr-1 Plasmids from a Single Paediatric Patient. PLoS One 2019, 14, e0221960. [CrossRef]
- Malik, S.; Kaminski, M.; Landman, D.; Quale, J. Cefiderocol Resistance in Acinetobacter baumannii: Roles of β-Lactamases, Siderophore Receptors, and Penicillin Binding Protein 3. Antimicrob. Agents Chemother. 2020, 64. [CrossRef]
- Yamano, Y.; Ishibashi, N.; Kuroiwa, M.; Takemura, M.; Sheng, W.-H.; Hsueh, P.-R. Characterisation of Cefiderocol-Non-Susceptible Acinetobacter baumannii Isolates from Taiwan. J. Glob. Antimicrob. Resist. 2022, 28, 120–124. [CrossRef]
- Lucidi, M.; Visaggio, D.; Migliaccio, A.; Capecchi, G.; Visca, P.; Imperi, F.; Zarrilli, R. Pathogenicity and Virulence of Acinetobacter baumannii : Factors Contributing to the Fitness in Healthcare Settings and the Infected Host. Virulence 2024, 15. [CrossRef]
- Ramirez, M.S.; Penwell, W.F.; Traglia, G.M.; Zimbler, D.L.; Gaddy, J.A.; Nikolaidis, N.; Arivett, B.A.; Adams, M.D.; Bonomo, R.A.; Actis, L.A.; et al. Identification of Potential Virulence Factors in the Model Strain Acinetobacter baumannii A118. Front. Microbiol. 2019, 10. [CrossRef]
- Tomaras, A.P.; Dorsey, C.W.; Edelmann, R.E.; Actis, L.A. Attachment to and Biofilm Formation on Abiotic Surfaces by Acinetobacter baumannii: Involvement of a Novel Chaperone-Usher Pili Assembly System. Microbiology 2003, 149, 3473–3484. [CrossRef]
- Gaddy, J.A.; Actis, L.A. Regulation of Acinetobacter baumannii Biofilm Formation. Future Microbiol. 2009, 4, 273–278. [CrossRef]
- Kishii, K.; Hamada, M.; Aoki, K.; Ito, K.; Onodera, J.; Ishii, Y.; Tateda, K. Differences in Biofilm Formation and Transcription of Biofilm-Associated Genes among Acinetobacter baumannii Clinical Strains Belonging to the International Clone II Lineage. J. Infect. Chemother. 2020, 26, 693–698. [CrossRef]
- Hamidian, M.; Wick, R.R.; Hartstein, R.M.; Judd, L.M.; Holt, K.E.; Hall, R.M. Insights from the Revised Complete Genome Sequences of Acinetobacter baumannii Strains AB307-0294 and ACICU Belonging to Global Clones 1 and 2. Microbiol. Hamidian, RR Wick, RM Harts. LM Judd, KE Holt, RM HallMicrobial genomics, 2019•microbiologyresearch.org 2019, 5. [CrossRef]
- Fattahian, Y.; Rasooli, I.; Gargari, S.; … M.R.-M.; 2011, undefined Protection against Acinetobacter baumannii Infection via Its Functional Deprivation of Biofilm Associated Protein (Bap). Elsevier.
- Crosa, J.H. Genetics and Molecular Biology of Siderophore-Mediated Iron Transport in Bacteria. Microbiol. Rev. 1989, 53, 517–530. [CrossRef]
- Zimbler, D.L.; Penwell, W.F.; Gaddy, J.A.; Menke, S.M.; Tomaras, A.P.; Connerly, P.L.; Actis, L.A. Iron Acquisition Functions Expressed by the Human Pathogen Acinetobacter baumannii. BioMetals 2009, 22, 23–32. [CrossRef]
- Klebba, P.E.; Newton, S.M.C.; Six, D.A.; Kumar, A.; Yang, T.; Nairn, B.L.; Munger, C.; Chakravorty, S. Iron Acquisition Systems of Gram-Negative Bacterial Pathogens Define TonB-Dependent Pathways to Novel Antibiotics. Chem. Rev. 2021, 121, 5193–5239. [CrossRef]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. CARD 2017: Expansion and Model-Centric Curation of the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2017, 45, D566–D573. [CrossRef]
- Penwell, W.F.; DeGrace, N.; Tentarelli, S.; Gauthier, L.; Gilbert, C.M.; Arivett, B.A.; Miller, A.A.; Durand-Reville, T.F.; Joubran, C.; Actis, L.A. Discovery and Characterization of New Hydroxamate Siderophores, Baumannoferrin A and B, Produced by Acinetobacter baumannii. ChemBioChem 2015, 16, 1896–1904. [CrossRef]
- Sheldon, J.R.; Skaar, E.P. Acinetobacter Baumannii Can Use Multiple Siderophores for Iron Acquisition, but Only Acinetobactin Is Required for Virulence. PLOS Pathog. 2020, 16, e1008995. [CrossRef]
- Kenyon, J.J.; Hall, R.M. Variation in the Complex Carbohydrate Biosynthesis Loci of Acinetobacter baumannii Genomes. PLoS One 2013, 8, e62160. [CrossRef]
- Tickner, J.; Hawas, S.; Totsika, M.; Kenyon, J.J. The Wzi Outer Membrane Protein Mediates Assembly of a Tight Capsular Polysaccharide Layer on the Acinetobacter baumannii Cell Surface. Sci. Rep. 2021, 11, 21741. [CrossRef]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [CrossRef]
- Richter, M.; Rosselló-Móra, R.; Oliver Glöckner, F.; Peplies, J. JSpeciesWS: A Web Server for Prokaryotic Species Circumscription Based on Pairwise Genome Comparison. Bioinformatics 2016, 32, 929–931. [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [CrossRef]
- Tonkin-Hill, G.; Lees, J.A.; Bentley, S.D.; Frost, S.D.W.; Corander, J. Fast Hierarchical Bayesian Analysis of Population Structure. Nucleic Acids Res. 2019, 47, 5539–5549. [CrossRef]
- Lowe, T.M.; Eddy, S.R. TRNAscan-SE: A Program for Improved Detection of Transfer RNA Genes in Genomic Sequence. Nucleic Acids Res. 1997, 25, 955–964. [CrossRef]
- Liu, B.; Zheng, D.; Jin, Q.; Chen, L.; Yang, J. VFDB 2019: A Comparative Pathogenomic Platform with an Interactive Web Interface. Nucleic Acids Res. 2019, 47, D687–D692. [CrossRef]
- Gupta, S.K.; Padmanabhan, B.R.; Diene, S.M.; Lopez-Rojas, R.; Kempf, M.; Landraud, L.; Rolain, J.-M. ARG-ANNOT, a New Bioinformatic Tool To Discover Antibiotic Resistance Genes in Bacterial Genomes. Antimicrob. Agents Chemother. 2014, 58, 212–220. [CrossRef]
- Wayne, A. Clinical and Laboratory Standards Institute; CLSI. 2011. Perform. Stand. Antimicrob. susceptibility testing. 20th Informational Suppl. CLSI Doc. 2017.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).