Submitted:
19 September 2024
Posted:
22 September 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Equations
2. Notes between the Laws
Derivation of Equations
Kinetic Energy Equation
5. The Hypothesis on Which the Equation Is Based
- The problems with Bohr model began when it could not explain the following:
- Well, this scientific paper will answer that and will also explain quantum entanglement. As a result of the movement of electrons on the fabric of space-time in a straight line. Because the straight line passes around the nucleus. Then the electron passes, forming a great circle around the nucleus. Therefore, the electron moves in a straight line and the electron does not need to expend energy. This makes the electron not, emit energy and be in a stable state.
- How quantum entanglement occurs?
- This hypothesis was based on scientific foundations, the most important of which is:
4. Method
- This part of the research will explain the spectrum of the hydrogen atom in a new way, as the results presented in these tables from previous research match the results extracted from the equation, and this is consistent with the validity of this equation. Because the new equation is consistent with the photon energy equation. We will discuss that part of the research in the results and discussion.
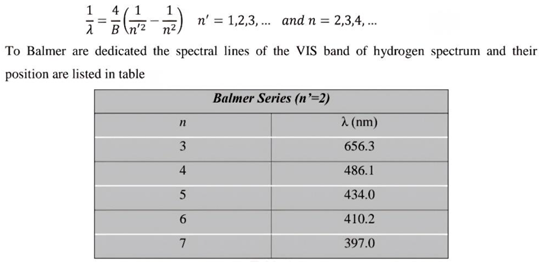
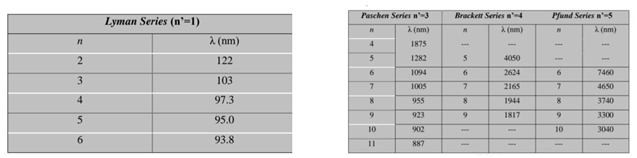
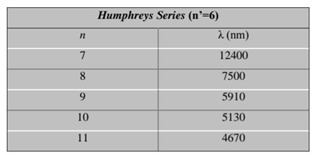
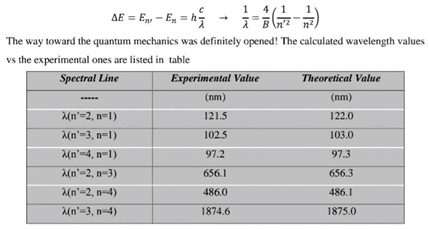
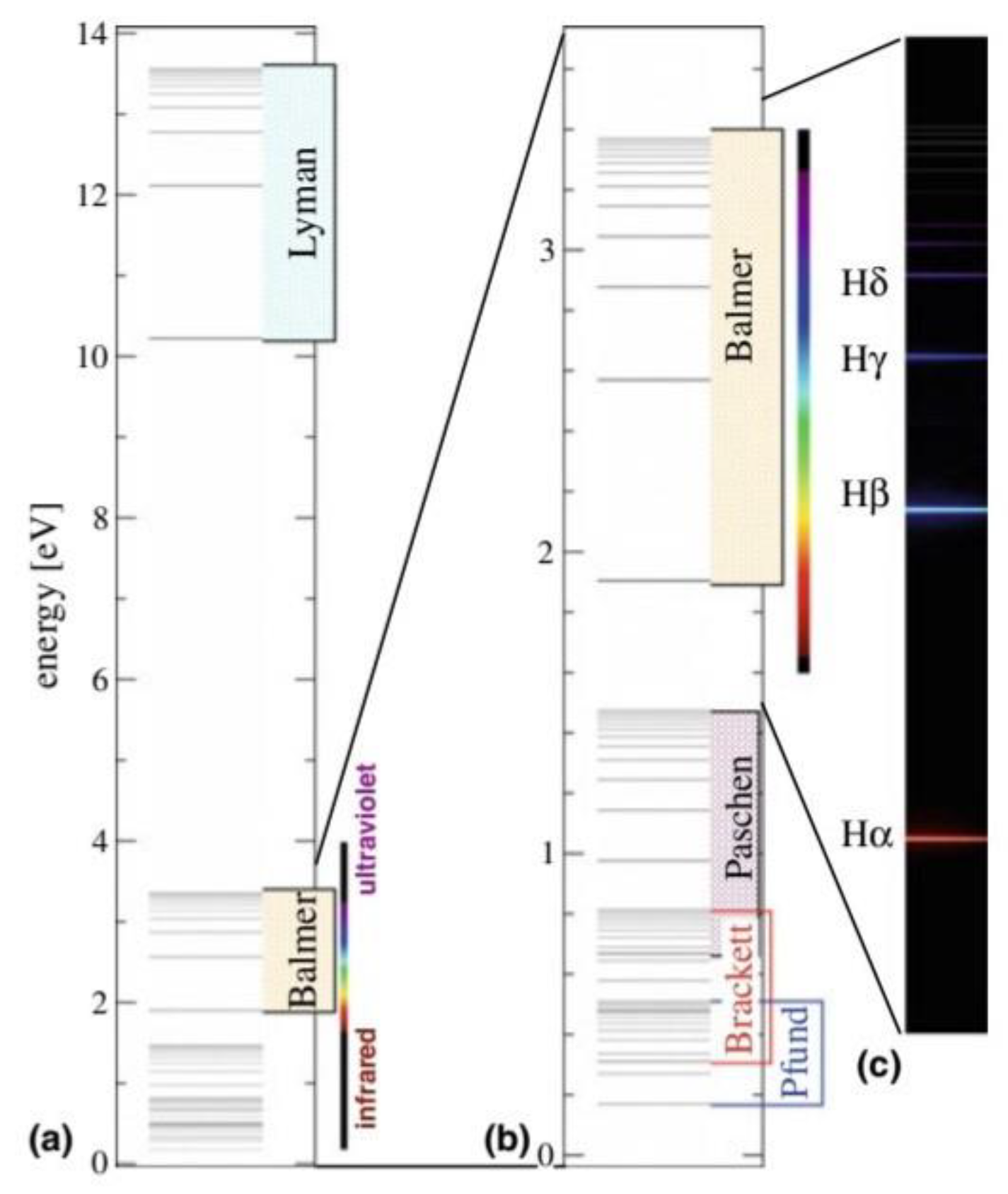
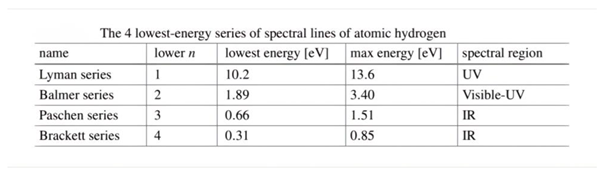
7. Results and Discussion
| Theoretical value (My work) Experimental value | |||||
| Spectral Line | Energy | λ | λ | ||
| λ(n’=2, n=1) | 10.204269824 | eV | 121.50227162 | nm | 121.5 nm |
| λ(n’=3, n=1) | 12.093949421 | eV | 102.51754168 | nm | 102.5 nm |
| λ(n’=4, n=1) | 12.75533728 | eV | 97.201817292 | nm | 97.20 nm |
| λ(n’=3, n=2) | 1.8896795971 | eV | 656.1122667 | nm | 656.1 nm |
| λ(n’=4, n=2) | 2.5510674561 | eV | 486.00908641 | nm | 486.0 nm |
| λ(n’=4, n=3) | 0.66138785898 eV | 1874.6064763 | nm | 1874.6 nm | |
5. Conclusions
References
- Svidzinsky, A. Scully, M. Bohr’s molecular model, a century later. Physics T. 2014, 67, 33-39. [CrossRef]
- Udema, I. I. Renaissance of Bohr’s model via derived alternative equation. American J. Mod. Phys 2017, 6, 23-31. [CrossRef]
- Nanni, L. The hydrogen atom: A review on the birth of modern quantum mechanics. Physics 2015. [CrossRef]
- Jordan, R. B. Principles of Inorganic Chemistry. Springer N., 2024; pp. 1--18. [CrossRef]
- Manini, N. Introduction to the physics of matter: basic atomic, molecular, and solid-state physics. Springer N., 2020; pp. 11--16. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




