1. Introduction
Wound healing is a complex and dynamic process involving a series of overlapping stages: hemostasis, inflammation, proliferation, and remodeling (Raziyeva et al., 2021). While acute wounds typically follow a well-arranged healing trajectory, chronic wounds, such as diabetic foot ulcers, often experience prolonged inflammatory phases and impaired healing, leading to significant morbidity and healthcare costs. Chronic wounds are a global health concern, affecting millions of people, particularly older adults, and pose a substantial burden on healthcare systems. Mesenchymal Stem Cells (MSCs) have emerged as a promising therapeutic option for enhancing wound healing due to their unique properties.
Derived from various tissues, including bone marrow, adipose tissue, and Wharton’s Jelly, MSCs are characterized by their multipotency and immunomodulatory capabilities (Laroye et al., 2019). One of the most critical aspects of MSCs’ function is their ability to secrete a wide range of bioactive molecules, including growth factors, cytokines, and chemokines, which mediate their therapeutic effects through paracrine signaling. The secretions of MSCs play crucial roles in wound healing. Growth factors such as VEGF and PDGF promote cell proliferation, migration, and angiogenesis. Cytokines, including IL-10 and TGF-β, modulate the inflammatory response and aid in tissue repair. Chemokines like SDF-1 attract necessary cells to the wound site, facilitating the repair process. These mechanisms highlight the potential of MSCs in addressing the challenges of chronic wound healing.
This review aims to provide a comprehensive summary of the current knowledge on the role of MSCs in wound healing. It focuses on how their secretions—growth factors, cytokines, and chemokines—increase the wound healing rate. The review explores the biological properties of MSCs, the mechanisms through which they enhance wound healing, and the clinical applications and challenges of MSC-based therapies for chronic wounds.
2. Materials and Methods
This review provides an overview of recent developments in using Mesenchymal Stem Cells (MSCs) for accelerating wound healing, focusing on the mechanisms involving growth factors, cytokines, and chemokines. We conducted a comprehensive search of PubMed, Scopus, and Web of Science databases using keywords such as ‘Mesenchymal Stem Cells,’ ‘Chronic Wounds,’ ‘Growth Factors,’ and ‘Regenerative Medicine.’ Studies were selected based on the following criteria: publication within the last five years, peer-reviewed status, and relevance to wound healing mechanisms involving MSCs. Articles focusing on the paracrine effects of MSCs in both in vivo and in vitro models were prioritized. In total, 200 articles were reviewed, with 50 selected for detailed analysis based on their relevance and methodological rigor. Non-peer-reviewed articles, preprints, and studies that lacked significant evidence on MSC mechanisms were excluded.
3. Chronic Wound
Chronic wounds are a significant global health concern, affecting millions of people, particularly older people, and posing a substantial burden on healthcare systems (Gupta et al., 2021). These wounds, which include pressure ulcers, diabetic foot ulcers, venous leg ulcers, and arterial ulcers, fail to progress through the normal stages of wound healing in an orderly and timely manner (Bowers & Franco, 2020). The rise in chronic wounds can be attributed to several key factors. Firstly, the aging population worldwide is a significant contributor, as elderly individuals are more prone to chronic wounds due to age-related changes in skin integrity and diminished healing capacity. Secondly, the escalating rates of diabetes and obesity globally play a crucial role, as these conditions are major risk factors for the development of chronic wounds. Additionally, the higher incidence of vascular diseases such as peripheral artery disease (PAD) and venous insufficiency adds to the burden by contributing to the formation of arterial and venous ulcers, respectively. These factors often prolong or exacerbate inflammation, highlighting the urgent need for effective treatments.
Pressure ulcers, also known as bedsores or decubitus ulcers, occur due to prolonged pressure on the skin, often affecting individuals who are bedridden or immobile (Bryam et al. et al., 2024). Diabetic foot ulcers are common in individuals with diabetes and result from a combination of neuropathy, poor circulation, and immune system deficiencies (George et al., 2017). Venous leg ulcers are caused by chronic venous insufficiency, where blood pools in the veins of the legs, leading to increased pressure and skin breakdown (McDermott et al., 2023). Arterial ulcers resulting from PAD occur due to reduced blood flow to the extremities, leading to tissue ischemia and necrosis (Zafar & Hinchliffe, 2023). The prevalence of chronic wounds is rising, primarily due to the aging population and the increasing rates of diabetes and obesity globally (Sen, 2023). Elderly individuals are more prone to chronic wounds due to age-related changes in skin integrity and diminished healing capacity. Additionally, conditions such as diabetes and obesity are major risk factors for the development of chronic wounds. The higher incidence of vascular diseases, such as PAD and venous insufficiency, further contributes to the burden of chronic wounds by forming arterial and venous ulcers, respectively (S. H. Wang et al., 2023).
3.1. Mechanisms of Chronic Wounds
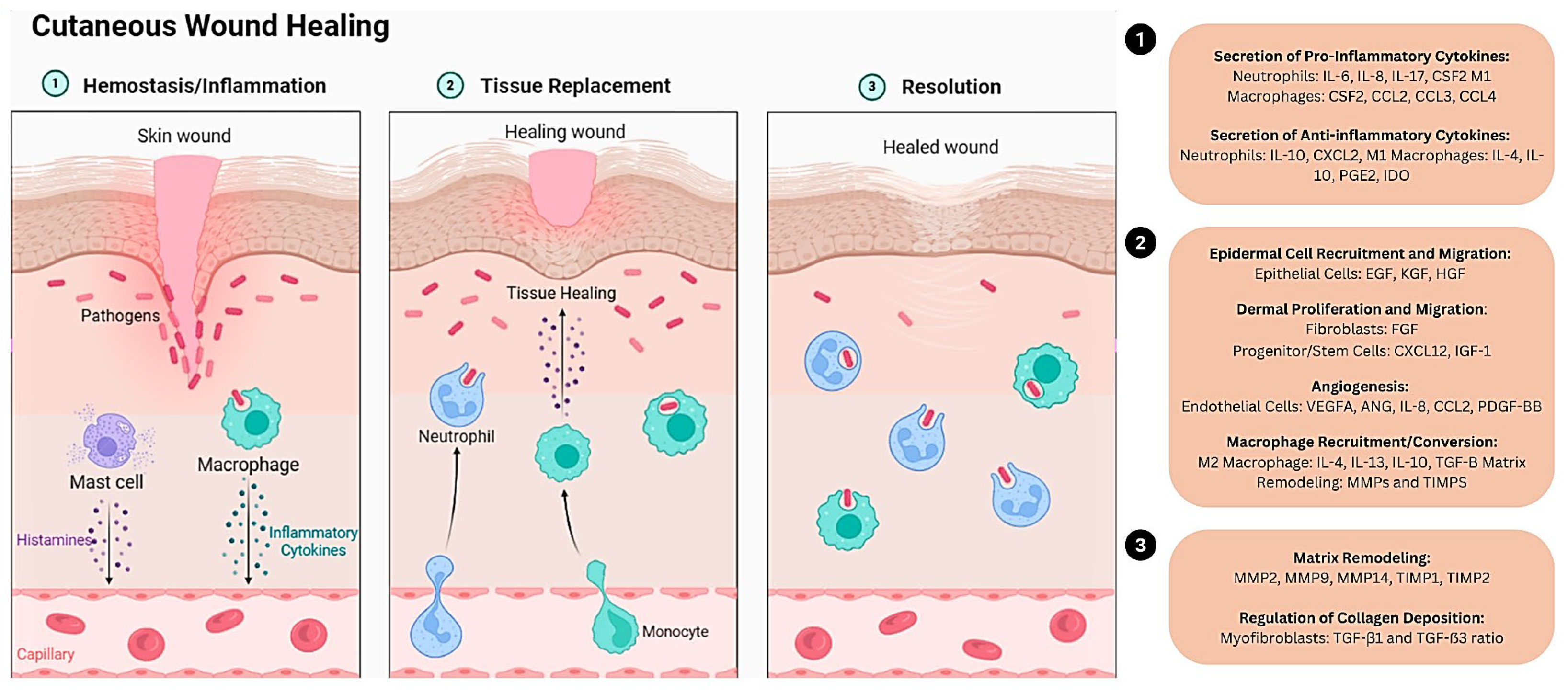
Chronic wounds significantly contribute to patient morbidity, negatively impacting their quality of life. They can cause extended hospital stays, numerous medical appointments, and elevated healthcare expenses (Darwin & Tomic-Canic, 2018). The main objective of contemporary wound care strategies is to pinpoint and mitigate factors contributing to or worsening the wound, intending to diminish inflammation and foster healing (Darwin & Tomic-Canic, 2018). Effective wound healing undergoes four critical stages: hemostasis, inflammation, proliferation, and remodeling (Patenall et al., 2024). Each phase must occur accurately and within its specific period for a wound to heal correctly. Hemostasis, the initial phase, triggers the body’s immediate response to injury, where blood vessels contract to minimize blood loss, and platelets form a clot, effectively stopping the bleeding. This stage is essential for preventing significant blood loss and commencing the healing phase (Mussbacher et al., 2019).
Following hemostasis, the body enters the inflammatory phase, typically lasting a few days. During this phase, white blood cells, particularly neutrophils and macrophages, migrate to the wound site to remove debris, bacteria, and damaged tissue. This process triggers inflammation, characterized by redness, swelling, warmth, and pain. Inflammation is a necessary response that helps to protect the wound from infection and initiate the repair process. Next, the proliferative phase is where active tissue repair and regeneration occur (Kita et al., 2024). It typically spans from about three days to three weeks after the injury. Fibroblasts, specialized cells in connective tissue, play a crucial role during this phase by producing collagen, which serves as the structural framework for new tissue growth. Additionally, new blood vessels, known as angiogenesis, form to provide oxygen and nutrients to the healing tissue. Granulation tissue, rich in blood vessels and collagen, fills the wound, promoting further tissue regeneration.
The final phase of wound healing, known as the remodeling phase, can last several months to years. During this phase, the newly formed tissue undergoes maturation and strengthening (Kita et al., 2024). Collagen fibers reorganize to increase the strength of the tissue, and excess collagen is broken down and removed. As a result, the wound contracts, becoming smaller and less noticeable. The result is a more muscular, more organized scar tissue that restores skin integrity. Several factors can influence the healing process’s speed and effectiveness, including the wound’s size and depth, the individual’s overall health, and underlying medical conditions. However, numerous variables can disrupt or hinder any phase of this process, leading to suboptimal or impaired wound recovery (
Figure 1).
Environmental factors include the surrounding conditions, which include pH, humidity, dehydration, or the patient’s lifestyle, which includes smoking, alcohol, poor diet, UV light exposure, psychological stress, and pollution (Ekingen et al., 2022; Popkin et al., 2010). Excessive wound moisture causes peeling of the wound and the surrounding area, while a lack of humidity causes wound desiccation, inhibiting wound healing (McNichol et al., 2022). Systemic variables can cause a prolonged effect on wound healing, and the symptoms include diabetes, advanced age, malnutrition, and other chronic organ diseases. The most common disease that is related to systemic factors is diabetes, and patients with a chronic wound with diabetes are usually aware of their diagnosis. They are taking medication to treat it. Diabetes is a condition where hyperglycemia occurs, also known as a high glucose level in the blood, which contributes to impaired wound closure. Hyperglycemia causes atherosclerosis, which prevents circulating nutrients from healing wounds and thus inhibits healing (N. Zhao et al., 2024). On the other hand, malnutrition disrupts the body’s natural healing process, prolongs inflammation, weakens the area of wound healing, and increases the risk of infection (Y. Zhang et al., 2021).
4. Key Factors in MSC-Mediated Wound Healing
Mesenchymal stem cells (MSCs) facilitate wound healing through a multifaceted approach involving several vital factors. These factors include growth factors, cytokines, chemokines, and extracellular matrix components, such as collagen, all playing crucial roles in the various tissue repair and regeneration stages. By secreting these bioactive molecules, MSCs create a conducive environment for cellular activities essential to wound healing, such as cell proliferation, migration, and angiogenesis. This section will delve into the specific roles and mechanisms of these critical factors, highlighting how they contribute to the overall process of MSC-mediated wound healing.
4.1. Growth Factor
A growth factor is a secreted protein or a steroid hormone, a naturally occurring substance in a cell. They can stimulate cell proliferation, heal wounds, and occasionally differentiate cellularly (Stone & Bhimji, 2018). Various growth factors involve the wound healing process and have their role. Platelet-derived growth Factor (PDGF) and Vascular Endothelial Growth Factor (VEGF) hold a role in the activation of immune cells and fibroblasts and extracellular matrix (ECM) deposition by increasing collagen synthesis, increasing tissue inhibitors of metalloproteinases (TIMP) synthesis and decrease matrix metalloproteinase (MMP) Synthesis (Y. Zhang et al., 2022). Transforming Growth Factor Beta (TGF-β) helps in fibroblast chemotaxis, activation, and ECM deposition by increasing collagen synthesis, TIMP synthesis, and decreasing MMP synthesis. They also play a role in reducing scarring by decreasing collagen and fibronectin (Deng et al., 2024). Transforming Growth Factor Alpha (TGF-α) was for keratinocyte proliferation, migration, and ECM Deposition (Bunker et al., 2021). Basic Fibroblast Growth Factor (BFGF) is involved in angiogenesis, endothelial cell activation, keratinocyte proliferation and migration, and ECM deposition (Dong et al., 2019; Son et al., 2023). Insulin-like growth Factor-1 (IGF-1) participates in keratinocyte and fibroblast proliferation, endothelial cell activation, angiogenesis, increased collagen synthesis, and ECM deposition cell metabolism (Werner, 2023; X. Zhang et al., 2024). There were a lot of other growth factors reported that help in increasing wound healing. Furthermore, various studies have reported that either MSCs or MSCs secretome/CM contain all these essential growth factors.
Studies around the globe have explored the role of Mesenchymal Stem Cells (MSCs) from various sources in wound healing, highlighting their ability to secrete key growth factors such as VEGF (Vascular Endothelial Growth Factor), TGF-β1 (Transforming Growth Factor Beta 1), FGF-2 (Fibroblast Growth Factor 2), IGF-1 (Insulin-like Growth Factor 1), and stem cell-derived factors. These MSCs surpass dermal fibroblasts in their production of wound healing essentials like VEGF-A, IGF-1, EGF (Epidermal Growth Factor), KGF (Keratinocyte Growth Factor), angiopoietin-1, and stromal-derived factor-1. Specifically, mice’s bone marrow-derived MSCs (BM-MSCs) have been shown to produce higher amounts of these factors critical for wound repair (L. Chen et al., 2008). Furthermore, exosomes, which are tiny vesicles released by MSCs, have been found to carry wound healing-promoting growth factors, including VEGF-A, FGF-2, HGF (Hepatocyte Growth Factor), and PDGF-BB (Platelet-derived Growth Factor BB) (Marofi et al., 2021). These exosomal contents underline the multifaceted role of MSCs in enhancing wound healing. A significant aspect of the MSC-mediated wound-healing process is the early upregulation of MMP-9 (Matrix Metallopeptidase 9), as Kim et al., 2011 discovered. This enzyme plays a critical role in wound healing by inducing the mobilization of VEGF, further promoting the healing process. Placenta-derived MSCs (PMSCs), as per the findings by Du et al., 2016, have been identified to secrete proangiogenic molecules such as VEGF, HGF, bFGF (basic Fibroblast Growth Factor), TGF-β, and IGF-1 at bioactive levels, contributing significantly to the angiogenesis and healing process.
Research over the years has emphasized the critical role of mesenchymal stem cells (MSCs) and their exosomes in wound healing, particularly by activating essential signaling pathways and inducing the production of key growth factors. Shabbir and colleagues in 2015 outlined that MSC exosomes activate signaling pathways such as Akt, ERK, and STAT3, which are crucial for wound healing, and boost the expression of growth factors, including hepatocyte growth factor (HGF), insulin-like growth factor 1 (IGF-1), nerve growth factor (NGF), and stromal cell-derived factor 1 (SDF1). Furthermore, in the context of diabetic wound healing, Kuo et al. (2011) demonstrated that MSCs considerably enhance the expression of epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), prolyl 4-hydroxylase, and Ki-67 compared to controls.
Bone marrow-derived MSCs (BM-MSCs) have been shown to produce higher levels of transforming growth factor-beta 1 (TGF-β1), leading to improved wound healing outcomes when compared to MSCs sourced from the spleen (MSC-Sp) or control treatments, as reported by Sherman et al. (2017). Despite this, the cost-effectiveness of using MSC-Sp suggests it is a viable option. Additionally, Wu et al. (2007) discovered that BM-MSCs facilitate wound healing by differentiating into cell types that release proangiogenic factors, notably VEGF and angiopoietin-1, focusing on their role in promoting angiogenesis. Moreover, adipose-derived stem cells have been recognized for their ability to secrete VEGF, HGF, and fibroblast growth factor 2 (FGF2), which notably enhances neovascularization and aids the healing of injured tissues, a finding corroborated by Nie et al. (2011). Building upon these discoveries, Fierro et al. (2019) have developed an innovative product to extend the duration of VEGF expression from MSCs, thereby significantly promoting angiogenesis in wound healing.
Secretomes or conditioned media (CMs) are harvested from the spent culture medium of mesenchymal stem cells (MSCs). They are rich in various growth factors, cytokines, and chemokines secreted by MSCs. Research has shown that the secretome or CM from MSCs significantly enhances wound healing, as demonstrated in several studies (Ahangar et al., 2020; Ajit & Ambika Gopalankutty, 2021; Chouw et al., 2022). Specifically, CM derived from human adipose tissue MSCs (CM-hATMSCs) holds great promise in expediting wound repair and regenerating cells. This is attributed to its composition, which includes critical growth factors like TGF-b1, TGF-b2, VEGF2, FGF, Vascular cell adhesion protein 1 (VCAM1), and EGF (Noverina et al., 2019). The presence of wound healing mediators such as VEGF, PDGF, HGF, and TGFβ1 has been confirmed in MSC-CM, further underscoring its therapeutic efficacy (Yamakawa & Hayashida, 2019). Moreover, studies have assessed gene expression levels of bFGF, hypoxia-inducible factor (HIF-1α), and SDF-1α through Reverse transcription polymerase chain reaction (RT-PCR). The findings revealed heightened gene expression in groups treated with CM and laser therapy and those receiving laser therapy alone, compared to the CM-only and control groups. Additionally, employing CM in conjunction with pulse wave photobiomodulation, either separately or combined, has been shown to accelerate wound healing processes considerably (Amini et al., 2018).
Recent advancements in regenerative medicine highlight the therapeutic potential of stem cell-derived treatments for wound healing and cartilage regeneration. Research has delved into how different sources of mesenchymal stem cells (MSCs) and their conditioned medium (MSC-CM) facilitate these processes, offering promising insights into new therapeutic methods. A notable study by Arno et al. (2014) demonstrated that the conditioned medium from Wharton’s Jelly (WJ) MSCs upregulated genes critical for wound healing processes, including re-epithelialization, neovascularization, and fibroproliferation, by enhancing the proliferation and migration of normal skin fibroblasts. This suggests that MSC-CM, enriched with growth factors from stem cells, significantly accelerates wound healing, making it a potent alternative to stem cell transplantation. Supporting this, Tamari et al. (2013) further confirmed that treatments utilizing MSCs and their conditioned medium expedite wound closure to a similar extent to controls, reinforcing the therapeutic value of growth factors derived from stem cells in wound management.
In cartilage regeneration, bone marrow aspirate concentrate (BMAC) presents a substantial advancement. (Park et al., 2023) introduced how growth factors and pluripotent stromal cells in BMAC aid in differentiating MSCs into chondrocytes, potentially fostering the development of native, hyaline-like cartilage. BMAC’s immune-regulating and anti-inflammatory properties further facilitate cartilage restoration, showing superior outcomes in cartilage recovery during high tibial osteotomy (HTO) procedures compared to treatments sans BMAC.(Pontikoglou et al., 2011)Elaborated on the role of BMAC, indicating that bone marrow mesenchymal stem cells (BM-MSCs) support the survival of long-lived plasma cells through the secretion of survival factors, which are crucial for long-term immunoglobulin secretion and represent a cornerstone of sustained immune system support.
The therapeutic potential extends to treatments of diabetic wounds as well. (Kong et al., 2013) observed that injections of human placenta-derived MSCs (hPMSCs) substantially improved microvessel formation in diabetic rat skin wounds, courtesy of the secretion of several proangiogenic molecules such as VEGF and HGF. Complementary findings by Liang et al. (2021) indicated that the conditioned medium from hPMSCs supports endothelial cell survival and enhances their migration and tube formation, attributing to increased secretion of proangiogenic proteins. Lastly, research by Liu et al. (2022) on the use of dual-crosslinked hydrogels for diabetic wounds demonstrated a notable upregulation in TGF-1 expression compared to control groups, underscoring the importance of this growth factor in transitioning from the inflammatory to the proliferative phase in wound healing. This, alongside the increased expressions of VEGF and IGF-1, showcases the hydrogel’s superior efficacy in facilitating wound re-epithelialization. The summary of the growth factor is in
Table 1.
4.2. Cytokines
Cytokines are a group of proteins secreted by cells that play a specific role in cell communication and interactions (Duque & Descoteaux, 2014). Among the cytokines secreted by mesenchymal stem cells (MSCs) is interleukin, made by one leukocyte to act on other leukocytes. Besides TGF-α, IL-6 has been reported to regulate angiogenesis, accelerating wound bed revascularization by activating VEGF and stimulating endothelial proliferation and tubing formation (Grebenciucova & VanHaerents, 2023; Xiao et al., 2020). However, the regulation of IL-6 is controlled and downregulated by the secretion of PGE2 by MSCs, which increases the secretion of IL-10 to reduce collagen formation, thereby helping minimize scar formation (Hezam et al., 2023). Liu et al. (2014) successfully detected the presence of IL-1, IL-6, and IL-10 at significantly different levels in treated burnt rats with human umbilical cord MSCs (hUC-MSCs) compared to the control group. The study showed that the rats treated with hUC-MSCs exhibited a higher rate of healing and faster wound area closure than the control group. This suggests that the presence of cytokines from the hUC-MSCs promotes the proliferation and migration of keratinocytes and fibroblasts at the wound bed and suppresses inflammatory mediators to lessen inflammation (Z. Hu et al., 2024; Walter et al., 2010). Consequently, the healing process is faster than the native healing process.
A group of researchers has suggested that MSCs regulate the endogenous host cell to promote wound healing (L. Chen et al., 2014; J. Li et al., 2024; J. Liu et al., 2020). Chen et al. (2014) found that human bone marrow MSCs (hBM-MSCs) secrete several growth factors and cytokines, including IL-6 and IL-8, in both standard and hypoxic conditions, with higher levels in hypoxic conditions. The hBM-MSCs from hypoxic conditions increased the proliferation and migration of keratinocytes and fibroblasts in a trans-well culture setting. In an in-vivo setting, the wound area of treated nude mice with hypoxic hBM-MSCs closed faster than the control group. An et al. (2015) also reported a 1.5-fold increase in IL-6 secretion in hypoxic human adipose MSCs compared to normoxic conditions. These mediators affect the resident cells’ proliferation, migration, differentiation, and functional recovery.
Exploring cytokines from various sources has provided valuable insights into their therapeutic potential. Walter et al. (2010) demonstrated that MSCs from human bone marrow express MIP-1 and RANTES, highlighting their role in modulating immune responses. Similarly, Chen et al. (2008) found that MSCs from mice bone marrow express MIP-1, further supporting the immunomodulatory capabilities of these cells. Expanding on these findings, Li et al. (2017) and Maharlooei et al. (2011) showed that MSCs from the umbilical cord and rat adipose tissue express RANTES. This suggests that MSCs from different sources can contribute to immune regulation and inflammation control. Shohara et al. (2012) also reported RANTES expression in MSCs from human umbilical cord perivascular cells, reinforcing the potential of umbilical cord-derived MSCs in therapeutic applications. Chaudhary et al. (2024) and Peshkova et al. (2023) provided additional evidence by demonstrating that MSCs from rat bone marrow express MCP-1, IL-1, and IL-2, indicating their involvement in immune modulation and inflammation. Joseph et al. (2020) further expanded the scope by examining MSCs from caprine, canine, and guinea pig bone marrow, finding IL-6 expression crucial for inflammatory response and tissue repair.
Kim et al. (2013) and Nakamura et al. (2013) highlighted the expression of IL-6 in MSCs from rat and dog bone marrow, respectively. This consistency across different species highlights the universal role of MSCs in promoting inflammation and healing. Walter et al. (2010) and Chen et al. (2014) also observed IL-6 and IL-8 expression in MSCs from human bone marrow, further validating these findings. Shohara et al. (2012) and Liu et al. (2014) explored MSCs from human umbilical cord Wharton’s jelly and umbilical cord MSCs, respectively, noting the expression of IL-10 and collagens. These studies highlight the potential of umbilical cord-derived MSCs in promoting tissue regeneration and reducing inflammation. An et al. (2015) also reported IL-10 expression in human adipose MSCs, emphasizing their anti-inflammatory properties.
Recent studies have further enriched our understanding of MSCs. Peshkova et al. (2023) demonstrated IL-6 and IL-10 expression in MSCs from the human umbilical cord, while (Ackermann et al., 2024) confirmed IL-6 expression in MSCs from human adipose tissue. Moghadam et al. (2020) and Peshkova et al. (2023) reported IL-6 expression in MSCs from human bone marrow and umbilical cord, reinforcing the importance of these cytokines in MSC-mediated healing. Peshkova et al. (2023) added to this by showing IL-6 expression in MSCs from the human placenta, highlighting the diverse MSC sources that can be used for therapeutic purposes.
MSC-conditioned medium (MSC-CM) is a treasure trove of cytokines that enhance wound healing. These factors stimulate cellular processes such as angiogenesis, cell proliferation, and migration, which are crucial for effective wound repair. Studies by Saadh et al. (2023) and Saheli et al., 2020) have shown that MSC-CM can accelerate wound closure, improve re-epithelialization, and enhance the quality of granulation tissue. This highlights the importance of cytokines in these processes, as they orchestrate a symphony of cellular activities that lead to efficient wound healing. MSC-derived exosomes, tiny vesicles packed with cytokines, growth factors, and regulatory microRNAs, have emerged as critical mediators of MSCs’ therapeutic effects on wound healing. These exosomes promote fibroblast proliferation, angiogenesis, and re-epithelialization, reducing inflammation and scar formation. Research by (D. Li et al., 2024; Y. Wang et al., 2022) demonstrates that the transfer of cytokines via exosomes modulates signaling pathways involved in wound healing, showcasing the potential of exosomes as a cell-free therapeutic approach. This innovative method of delivering cytokines opens new avenues for enhancing wound repair without requiring direct cell transplantation.
The immunomodulatory properties of MSCs are significantly enhanced by cytokines such as TGF-β, TNF-α, and IFN-γ. These cytokines can modify MSCs to increase their immunosuppressive effects, which is crucial in the local postoperative environment where MSCs and inflammatory cytokines converge. Liu et al. (2022) and Xia et al. (2023) have shown that pro-inflammatory cytokines like IFN-γ and TNF-α can amplify the secretome of MSCs, enhancing their ability to promote wound healing through mechanisms such as IL-6-dependent M2 macrophage polarization. This interaction between MSCs and cytokines creates a dynamic environment that fosters effective tissue repair. Enriched in MSC secretomes, cytokines such as IL-6 and CCL2 significantly promote macrophage migration and M2 polarization, essential for effective wound healing. (C. Liu et al., 2022) have highlighted how the presence of cytokines like TGF-β and TNF-α in the wound environment influences MSC-mediated remodeling of the extracellular matrix, which is critical for tissue repair. These cytokine-induced cellular responses ensure that the wound-healing process is rapid and results in high-quality tissue regeneration.
Pretreatment of MSCs with pro-inflammatory cytokines has enhanced their wound-healing capabilities. This approach promotes fibroblast migration and activation and improves the biological function of macrophages. Liu et al. (2022) have demonstrated that three-dimensional culture conditions of MSCs increase the levels of proangiogenic cytokines such as IL-6 and VEGFA, further enhancing the wound healing process in burn injury models. This cytokine-enhanced MSC therapy represents a promising strategy for improving wound care outcomes.
While cytokines are central to MSC-mediated wound healing, optimizing their therapeutic application remains challenging. The complexity of cytokine interactions and the variability in individual responses necessitate further research to harness their potential fully. Additionally, translating these findings from preclinical models to clinical settings requires careful consideration of cytokine dosing, delivery methods, and patient-specific factors. Despite these challenges, the strategic use of cytokines in MSC therapies holds promise for advancing wound care and improving patient outcomes.
Table 2.
Various sources of MSCs secrete cytokines.
Table 2.
Various sources of MSCs secrete cytokines.
| Investigator |
Cell Type |
Growth Factors/Cytokines |
| Anbiyaiee et al. (2024) |
MSCs |
Various cytokines and growth factors in MSC-conditioned medium (MSC-CM) |
| Stachura et al. (2023) |
MSCs |
Cytokines and growth factors in MSC-conditioned medium (MSC-CM) |
| Dehghani et al. (2024) |
MSC-derived exosomes |
Cytokines, growth factors, regulatory microRNAs |
| Moratin et al. (2024) |
MSCs |
TGF-β, TNF-α, IFN-γ, IL-6 |
| Liu et al. (2022) |
MSCs |
IL-6, CCL2, TGF-β, TNF-α, VEGFA |
| Daviran et al. (2021) |
MSCs |
TGF-β, TNF-α |
| Gangadaran et al. (2023) |
MSCs in 3D culture conditions |
Pro-angiogenic cytokines such as IL-6 and VEGFA |
| Zhang & Ann (2007) |
MSCs |
Interleukin (IL) |
| Nushke (2013) |
MSCs |
TGF-α, IL-6, PGE2, IL-10 |
| Liu et al. (2014) |
hUC-MSCs |
IL-1, IL-6, IL-10 |
| Walter et al. (2010) |
MSCs |
MIP-1, RANTES |
| Chen et al. (2008) |
MSCs from mice bone marrow |
MIP-1 |
| Li et al. (2017) |
MSCs from umbilical cord |
RANTES |
| Maharlooei et al. (2011) |
MSCs from rat adipose tissue |
RANTES |
| Shohara et al. (2012) |
MSCs from human umbilical cord perivascular cells |
RANTES |
| Jiang et al. (2018) |
MSCs from rat bone marrow |
MCP-1, IL-1, IL-2 |
| Joseph et al. (2020) |
MSCs from caprine, canine, and guinea pig bone marrow |
IL-6 |
| Nakamura et al. (2013) |
MSCs from rat bone marrow |
IL-6 |
| Kim et al. (2013) |
MSCs from dog bone marrow |
IL-6 |
| Chen et al. (2014) |
hBM-MSCs |
IL-6, IL-8 |
| Jun et al. (2014) |
MSCs |
Various cytokines |
| An et al. (2015) |
MSCs from human adipose tissue |
IL-6, IL-10 |
| Peshkova et al. (2023) |
MSCs from human umbilical cord |
IL-6, IL-10 |
| Kahrizi et al. (2023) |
MSCs from human adipose tissue |
IL-6 |
| Baldari et al. (2023) |
MSCs from human bone marrow |
IL-6 |
| Casado-Díaz et al. (2022) |
MSCs from human umbilical cord |
IL-6 |
| Kim et al. (2023) |
MSCs from human placenta |
IL-6 |
4.3. Chemokines
Chemokines play a crucial role in mesenchymal stem cell (MSC)-mediated wound healing by orchestrating various cellular processes essential for effective tissue repair. These small signaling proteins are involved in the recruitment and activation of immune cells, modulation of inflammation, and promotion of angiogenesis, all of which are vital for wound healing (Duque & Descoteaux, 2014; Kaur & Ghorai, 2022). The interplay between chemokines and MSCs enhances the therapeutic potential of MSCs in wound management, as evidenced by several studies (Cuesta-Gomez et al., 2021; Kaur & Ghorai, 2022; Wiredu Ocansey et al., 2020; Zhidu et al., 2024a). Chemokines, derived from the Greek word “kinos,” meaning movement, are signaling proteins secreted by cells that induce directed chemotaxis in nearby responsive cells. They are classified based on their behavior and structural characteristics. All share four cysteine residues in conserved locations and a mass of approximately 8-10 kilodaltons, which are key for forming their three-dimensional shape. These proteins are integral to the healing process, playing significant roles in various stages of wound repair.
One of the primary roles of chemokines in MSC-mediated wound healing is the recruitment and activation of immune cells (Bian et al., 2022). Chemokines are essential in recruiting immune cells to the wound site, which is essential for initiating the healing process. They facilitate the migration of macrophages and other immune cells, which release growth factors and cytokines to promote tissue repair. For instance, MSCs secrete chemokines such as CCL2, which enhance macrophage migration and M2 polarization, thereby improving wound healing outcomes. Studies by Peshkova et al. (2023), Planat-Benard et al. (2021), and Toya et al. (2023) have highlighted the importance of these processes in effective wound healing. In addition to recruiting immune cells, MSCs modulate the inflammatory response through chemokine signaling, crucial for preventing chronic inflammation and promoting healing. The secretion of chemokines by MSCs helps transition from the inflammatory phase to the proliferative phase of wound healing. This modulation is further enhanced when MSCs are pretreated with pro-inflammatory cytokines, leading to an amplified secretion of chemokines and improved wound healing (Liu et al., 2022). This dynamic interaction between MSCs and chemokines ensures a balanced inflammatory response, facilitating efficient tissue repair.
Chemokines also significantly promote angiogenesis, a critical process for supplying nutrients and oxygen to the healing tissue. MSCs release chemokines that stimulate the formation of new blood vessels, thereby enhancing tissue regeneration and repair. The presence of chemokines such as CXCL12 and VEGFC in the MSC secretome has significantly improved angiogenesis and wound closure. Research by Marofi et al. (2021) and Sandonà et al. (2021) highlights the importance of these chemokines in the angiogenic process, highlighting their role in effective wound healing.
MSC-conditioned medium (MSC-CM) is a rich mixture of chemokines, growth factors, and cytokines that collectively enhance wound healing. The administration of MSC-CM has been shown to accelerate wound closure, improve re-epithelialization, and enhance the quality of granulation tissue (Marofi et al., 2021; Saadh et al., 2023). The chemokines within MSC-CM are crucial for orchestrating the cellular processes necessary for effective wound repair. Studies have revealed that factors produced by bone marrow MSCs regulate endothelial lineage cells and macrophages in the wound, thus promoting wound closure. Chemotactic chemokines derived from BM-MSCs, such as MIP-1 alpha and erythropoietin, stimulate the proliferation of dermal fibroblasts and recruit inflammatory cells following chemical injury, thereby promoting wound healing and maintaining an effective immune response (L. Chen et al., 2014). The paracrine effects of MSCs, including the secretion of chemokines, have been demonstrated to reduce bacterial burden and accelerate wound healing in biofilm-infected wounds. This highlights the importance of chemokines in promoting healing and combating infections that can impede it (Gou et al., 2024). The ability of chemokines to modulate immune responses and enhance tissue repair highlights their therapeutic potential in wound management.
Despite the well-documented role of chemokines in MSC-mediated wound healing, optimizing their therapeutic potential remains challenging. The variability in chemokine expression among different MSC sources and the complexity of chemokine signaling pathways pose challenges for clinical translation (Willer et al., 2022). Further research is needed to understand how chemokines enhance MSC-mediated wound healing fully and to develop standardized protocols for their use in clinical settings.
Table 3.
Summary of research on chemokines in MSCs.
Table 3.
Summary of research on chemokines in MSCs.
| Investigator |
Cell Type |
Chemokines Involved |
Results |
| Duque & Descoteaux (2014) |
MSCs |
Wound healing |
Highlighted the general role of chemokines in wound healing |
| Kaur & Ghorai (2022) |
MSCs |
Wound healing |
Emphasized the therapeutic potential of MSCs in wound management |
| Cuesta-Gomez et al. (2021) |
MSCs |
Wound healing |
Demonstrated the interplay between chemokines and MSCs |
| Wiredu Ocansey et al. (2020) |
MSCs |
Wound healing |
Showed the enhancement of MSC therapeutic potential |
| Zhidu et al. (2024) |
MSCs |
Wound healing |
Confirmed the role of chemokines in MSC-mediated wound healing |
| Bian et al. (2022) |
MSCs |
CCL2 |
Improved macrophage migration and M2 polarization, enhancing wound healing outcomes |
| Peshkova et al. (2023) |
MSCs |
CCL2 |
Highlighted the importance of CCL2 in effective wound healing |
| Planat-Benard et al. (2021) |
MSCs |
CCL2 |
Demonstrated the role of CCL2 in macrophage migration and M2 polarization |
| Toya et al. (2023) |
MSCs |
CCL2 |
Showed the enhancement of wound healing through CCL2 |
| Liu et al. (2022) |
MSCs |
Modulating inflammation |
Demonstrated the modulation of inflammation by MSCs |
| Marofi et al. (2021) |
MSCs |
CXCL12, VEGFC |
Enhanced angiogenesis and wound closure |
| Sandonà et al. (2021) |
MSCs |
CXCL12, VEGFC |
Highlighted the role of CXCL12 and VEGFC in angiogenesis |
| Saadh et al. (2023) |
MSCs |
MSC-conditioned medium |
Accelerated wound closure and improved re-epithelialization |
| Chen et al. (2014) |
MSCs |
MIP-1 alpha, erythropoietin |
Stimulated dermal fibroblast proliferation and inflammatory cell recruitment |
| Gou et al. (2024) |
MSCs |
Reduce bacterial burden and accelerate wound healing |
Reduced bacterial burden and accelerated wound healing in biofilm-infected wounds |
| Willer et al. (2022) |
MSCs |
Chemokine expression among MSC sources |
Highlighted the challenges in optimizing therapeutic potential due to variability |
4.4. Other Factor-Collagen
Collagens are the most abundant protein in the human body. In the healing wound, collagen attracts fibroblasts and encourages new collagen deposition to the wound bed. The type, amount, and organization of collagen will change throughout the healing process, determining the tensile strength of the healed skin (Harsha & Brundha, 2020; Salleh et al., 2022). In the wound healing process, collagen type III is the first to be synthesized in the early stages, and then collagen type the dominant skin collagen will replace me. Some researchers reported the role of MSCs and MSC-CM in helping increase the wound healing rate by the collagens (Ding et al., 2023; Harsha & Brundha, 2020; Lee et al., 2016; Wei et al., 2024).
MSCs are multipotent progenitor cells initially from many types of tissue in the human body. UC-MSC has been reported to have therapeutic benefits compared to other MSCs, such as adipose tissue, bone marrow, and amniotic membrane. UC-MSC also improved cutaneous wound recovery by releasing macrophage and endothelial migration. Recent studies have shown the enhancement of treated wound healing and ease fewer scars. Furthermore, fibroblasts’ collagen type III to collagen type I ratio increased after receiving UC-MSC-CM treatment. Higher levels of collagen type III provide smaller reticular structures than collagen type 1, thus promoting less scar wound healing (Li et al., 2017).
Inversely, adipose tissue obtained from MSCs (AD-MSCs) enhanced the wound healing rate in diabetic rats but did not accelerate the length density of the vessels or the volume density of the collagen fibers. Contrary reports indicated that AD-MSCs diminished the numerical density of dermal fibroblasts. Hence, AD-MSCs may significantly increase diabetic wound healing by using other mechanisms instead of accelerating angiogenesis or collagen fibers (Y. Hu et al., 2021; Maharlooei et al., 2011). Several wound-healing mediators were detected in MSC-CM, such as collagen type I, fibronectin, SPARC, and IGFBP-7. Recent studies recommended that the trophic activity of MSC may be responsible for skin wound closure by impacting both keratinocyte migration and dermal fibroblast, as well as playing a role in the formation of extracellular matrix (L Dehghani, 2024; Yue et al., 2024). According to (Shohara et al., 2012), immunohistologic analysis showed that the HUCPVC significantly increased wound healing by enhancing collagen deposition and accumulating angiogenesis. Therefore, HUCPVC probably provides therapeutic benefits for treated wounds in neonatal babies due to their capability to differentiate into the osteogenic lineage via the paracrine mechanism. Nowadays, collagen dressing technology has emerged as a new alternative that helps stimulate new tissue growth while encouraging autolytic debridement, angiogenesis, and re-epithelialization.
5. Functions and Types of MSCs
Mesenchymal stem cells (MSCs) leverage their physiological actions to aid healing when applied externally to wounds. Despite their origin and characterization challenges, studies have consistently shown that MSCs can benefit wound healing and scar formation (S. Chen et al., 2024a; Zhidu et al., 2024; T. Zhou et al., 2021). Mesenchymal stem cells (MSCs), or stromal stem cells, can be obtained from different sources such as bone marrow, adipose tissue, Wharton’s jelly, or amniotic fluid. These versatile cells have the ability to differentiate into various cell types, including osteoblasts, chondrocytes, and adipocytes. MSCs are naturally present in the bone marrow and remain inactive until needed for the body’s healing processes. However, their quantity and effectiveness decrease with age.
Researchers have believed mesenchymal stem cells (MSCs) were exclusively found in bone marrow for many years. However, subsequent studies have unveiled a variety of sources for MSCs, one of which is Wharton’s Jelly (WJ). The MSCs derived from Wharton’s Jelly stand out as they are the youngest and most primitive form of MSCs available. Due to their youthful nature, WJ MSCs can differentiate into any required cell type within the body and exhibit a rapid multiplication rate, significantly enhancing their healing capabilities. Spees et al. (2016) and Zhuang et al. (2021) have emphasized that upon administration, MSCs are naturally inclined to migrate towards injury sites, where they engraft and differentiate into functional cells, facilitating the regeneration of damaged or diseased connective tissue. The efficacy of MSCs in differentiation and their potential in regenerative medicine could be influenced by several factors, including the source of stem cells, the conditions under which they are expanded, and their cultivation microenvironment. Aydemir et al. (2016), along with Jovic et al. (2022), highlighted the exploration of various stem cells, including skin-derived precursor cells (SKPs), epidermal stem cells (EpSCs), amnion-derived mesenchymal stem cells (AMSCs), synovium mesenchymal stem cells (SMSCs), bone marrow-derived stem cells (BMSCs), and adipose-derived stem cells (ASCs) for the treatment of chronic wounds. This research highlights the unique potential of different MSC sources in wound healing applications.
In a study by Hoang et al. (2020), the impact of MSC origin on wound healing was extensively investigated. This study focused on three specific sources of MSCs: Bone Marrow, Adipose Tissue, and Umbilical Cord. One of the crucial findings of this research was the discovery of crucial growth factors such as VEGF-A, FGF-2, HGF, and PDGF-BB within the exosomes derived from all three sources of MSCs. These exosomes were shown to induce keratinocyte and fibroblast proliferation and migration, which are essential processes in wound healing. Remarkably, the source of MSCs influenced the expression levels of these growth factors within the exosomes, with TGF-b being uniquely identified in exosomes from Umbilical Cord MSCs. Moreover, the study demonstrated that exosomes from Bone Marrow MSCs accelerated the proliferation and migration of primary dermal fibroblasts. At the same time, those from Umbilical Cord MSCs were more efficient in treating keratinocytes. Hoang et al., 2020, concluded that exosomes secreted by Bone Marrow and Umbilical Cord MSCs, under clinical conditions, exhibit significant promise for developing therapeutic products aimed at wound healing.
6. Preclinical Evidence
Various studies have highlighted the effectiveness of different sources and stem cell delivery methods in numerous animal models, particularly in diabetic wound healing. A crucial piece of research by (Javazon et al., 2007) revealed that local injections of adult murine bone marrow stromal cells into diabetic mice significantly improved re-epithelialization, granulation, and vascularization, laying the groundwork for future exploration of stem cell therapies for diabetic wounds. Building upon this foundational study, subsequent research has deepened our understanding of the therapeutic potential of stem cells in this area. Investigations conducted by previous researchers expanded these observations by employing umbilical cord-derived and bone marrow-derived stem cells in diabetic rats (Kato et al., 2015; Krasilnikova et al., 2022; Kuo et al., 2011; Lopes et al., 2018; Shrestha et al., 2013; Wan et al., 2013; Q. S. Zhao et al., 2013). These studies collectively highlighted several benefits, including enhanced angiogenesis, faster wound closure, reduced size of ulcers, accelerated epithelialization, and improved granulation tissue formation. Together, these findings emphasize the versatility and efficacy of stem cell therapies in healing diabetic wounds, illustrating a promising area of research that continues to evolve.
Research exploring using stem cells combined with various biomaterials has shown promising results in accelerating wound repair and enhancing vascularization. A few notable studies have demonstrated the efficacy of artificial skin combined with stem cells in animal models, resulting in accelerated wound healing (Badillo et al., 2007; Nourian Dehkordi et al., 2019; Przekora, 2020). Similarly, the research by (Linard et al., 2015) on the effects of bone marrow cells on radiation burns in mice highlighted the potential of stem cells to reduce inflammation in radiation-induced skin injuries. These studies highlight the viability of stem cells as a treatment option for complex wounds. Innovative delivery methods have been explored to further enhance stem cell therapeutic effects. Researchers also found that combining human umbilical cord blood-derived mesenchymal stromal cells with a hydrogel significantly improved wound healing in mice (Mebarki et al., 2021; Xie et al., 2020). A few studies built on this approach demonstrated that the local implantation of Platelet-Rich Plasma (PRP) and umbilical cord blood (UCB) cells increased the wound closure rate and angiogenesis (Tian et al., 2023; X. Wang et al., 2024; C. Zhou et al., 2024). These advancements suggest integrating stem cells with supportive materials can enhance wound healing outcomes.
Adipose-derived stem cells have been shown to hold significant promise for advancing wound healing, as evidenced by multiple studies across various models. For instance, (Riccobono et al., 2012) demonstrated the effectiveness of these cells in skin healing processes by reporting successful recovery in minipigs following the intradermal injection of adipocyte-derived stem cells, which resulted in no necrosis or unmanageable pain. Similarly, Fakiha (2022) and Ullah et al. (22024) found that the subcutaneous injection of adipose stromal cells not only sped up wound closure but also enhanced the structural integrity of the skin while decreasing inflammation in mice suffering from pressure sores. Further supporting the therapeutic potential of combining stem cell types, François et al. (2017) reported that using a mix of bone marrow cells and BMP-2 (Bone Morphogenetic Protein 2) could speed up the healing process and promote angiogenesis in rats, thereby improving outcomes in wound treatment through innovative cellular therapies.
Recent studies have significantly reinforced the evidence favoring stem cell therapy to enhance wound healing. Various investigations indicate that different sources of stem cells and their delivery methods play a crucial role in improving the healing process, particularly for diabetic animal models. In 2021, Zhao and colleagues demonstrated that the local injection of mesenchymal stem cells (MSCs) notably improved wound healing and spurred angiogenesis in diabetic mice, pointing towards the potential of MSCs in therapeutic applications. Following this, in the same year, Liang et al. found that MSCs derived from bone marrow similarly enhanced angiogenesis and promoted faster wound closure in diabetic rats, aligning with Zhao’s findings and suggesting a consistent benefit of MSC therapy across different studies (W. Liang et al., 2021; Y. Zhao et al., 2021). Expanding on the types of stem cells, Fakiha, in 2022, highlighted the effectiveness of induced pluripotent stem cells (iPSCs) in bettering wound healing and reducing inflammation in diabetic mice. This study highlights the versatility of stem cell sources in treating wounds.
Further research by Farabi and colleagues, expected to be published in 2024, explores the advantages of adipose tissue-derived mesenchymal stem cells. Their study indicates that these cells, either applied topically or incorporated into scaffolds, can alleviate pain, accelerate healing times, and enhance the cosmetic appearance post-healing in diabetic rats. In addition to traditional stem cell therapies, innovative methods involving stem cell-derived exosomes are emerging as a promising direction. In 2024, Fakouris showed that the local injection of stem cell-derived exosomes can quicken wound healing and minimize scar formation in mice. Similarly, Nouri et al. (2024) reported that combining stem cell-derived exosomes and biomaterials markedly boosts wound healing and tissue regeneration in rats. These findings open new avenues for enhancing the therapeutic efficacy of stem cell therapy by incorporating exosomes, either alone or alongside biomaterials, for wound healing.
The combined results of these studies highlight stem cell therapy’s potential to improve wound healing in various animal models. The diversity of stem cell sources, such as bone marrow, umbilical cord, adipose tissue, and induced pluripotent stem cells, alongside innovative delivery methods like hydrogel, PRP, and exosomes, highlight the promising future of regenerative medicine in wound care. Continuous research and clinical trials will be crucial in translating these findings into effective treatments for human patients.
Table 4.
Summary of Chemokines and Growth Factors in MSC-Mediated Wound Healing.
Table 4.
Summary of Chemokines and Growth Factors in MSC-Mediated Wound Healing.
| Animal Model |
Cell Source |
Delivery Method |
Outcome |
References |
| Diabetic mice |
Bone marrow stromal cells |
Local injection |
Improved reepithelialization, granulation, and vascularization |
Javazon et al., 2007 |
| Diabetic rats |
Umbilical cord-derived and bone marrow-derived stem cells |
Local injection |
Enhanced angiogenesis, faster wound closure, reduced ulcer size, accelerated epithelialization, improved granulation tissue formation |
Kato et al., 2015; Krasilnikova et al., 2022; Kuo et al., 2011; Lopes et al., 2018; Shrestha et al., 2013; Wan et al., 2013; Q. S. Zhao et al., 2013 |
| Various animal models |
Stem cells combined with artificial skin |
Topical application |
Accelerated wound healing |
Badillo et al., 2007; Nourian Dehkordi et al., 2019; Przekora, 2020 |
| Mice |
Bone marrow cells |
Local injection |
Reduced inflammation in radiation-induced skin injuries |
Linard et al., 2015 |
| Mice |
Human umbilical cord blood-derived mesenchymal stromal cells with hydrogel |
Local injection |
Improved wound healing |
Mebarki et al., 2021; Xie et al., 2020 |
| Mice |
Platelet-rich plasma (PRP) and umbilical cord blood (UCB) cells |
Local implantation |
Increased wound closure rate and angiogenesis |
Tian et al., 2023; X. Wang et al., 2024; C. Zhou et al., 2024 |
| Minipigs |
Adipocyte-derived stem cells |
Intradermal injection |
Successful recovery, no necrosis or unmanageable pain |
Riccobono et al., 2012 |
| Mice |
Adipose stromal cells |
Subcutaneous injection |
Faster wound closure enhanced structural integrity, decreased inflammation |
Fakiha, 2022; Ullah et al., 2024 |
| Rats |
Bone marrow cells and BMP-2 |
Local injection |
Faster healing, promoted angiogenesis |
François et al., 2017 |
| Diabetic mice |
Mesenchymal stem cells (MSCs) |
Local injection |
Improved wound healing, spurred angiogenesis |
W. Liang et al., 2021; Y. Zhao et al., 2021 |
| Diabetic mice |
Induced pluripotent stem cells (iPSCs) |
Local injection |
Improved wound healing, reduced inflammation |
Fakiha, 2022 |
| Diabetic rats |
Adipose tissue-derived mesenchymal stem cells |
Topical application or incorporated into scaffolds |
Alleviated pain, accelerated healing, enhanced cosmetic appearance |
Farabi et al., 2024 |
| Mice |
Stem cell-derived exosomes |
Local injection |
Quickened wound healing, minimized scar formation |
Fakouri, 2024 |
| Rats |
Stem cell-derived exosomes and biomaterials |
Local injection |
Boosted wound healing, tissue regeneration |
Nouri et al., 2024 |
7. Clinical Evidence
Various clinical trials have substantiated the efficacy of Mesenchymal Stem Cell (MSC)- based therapies in enhancing wound healing. These trials, targeting conditions such as diabetic foot ulcers, venous leg ulcers, and severe burns, collectively highlight the significant therapeutic potential of MSCs. Based on information gathered from
www.clinicaltrials.gov, they offer insights into the mechanisms through which these cells contribute to improved healing outcomes.
Several studies have explored the application of MSCs to different types of wounds. For instance, the use of allogeneic MSCs for second-degree burn wounds, as noted in NCT02104713, showed significant improvements. These include accelerated wound closure, reduced scar formation, and enhanced overall skin quality compared to standard treatments. The immunomodulatory and regenerative properties of MSCs were key in creating an optimal environment for tissue repair and regeneration in these wounds. Similarly, research into tissue repair cells (TRC) and bone marrow stem cells (BMC) for treating diabetic foot wounds, detailed in NCT01065337, revealed that expanded bone marrow stem cells significantly enhanced wound healing. This improved closure rates, reduced infections, and improved overall limb function. MSCs’ ability to promote angiogenesis and modulate the immune response was important in achieving these outcomes.
A separate study outlined in NCT04104451 confirmed the safety and preliminary efficacy of umbilical cord-lining MSCs in managing chronic diabetic foot ulcers. This study found that patients treated with umbilical cord-derived MSCs exhibited significant improvements, including faster closure and reduced ulcer size, highlighting the potential of MSCs from various sources in managing chronic wounds.
Furthermore, subsequent trials, such as NCT02619877, evaluated allogeneic adipose-derived stem cells (ALLO-ASC-DFU) combined with standard therapy for diabetic foot ulcers. Results showed significantly improved wound healing compared to standard therapy alone. The regenerative and anti-inflammatory effects of adipose-derived MSCs were key contributors to these enhanced outcomes. Additionally, a trial indicated in NCT00955669 compared autologous MSCs and mononuclear cells in treating diabetic critical limb ischemia and foot ulcers. This trial unveiled the superior efficacy of MSCs in reducing wound size and enhancing tissue regeneration, further emphasizing their potential in wound healing applications.
Further supporting the potential of MSC-based therapies, a pilot study in NCT01750749 demonstrated the beneficial effects of bone marrow-derived MSCs in treating venous ulcers. Patients experienced accelerated wound closure and improved skin integrity after BMDC implantation. Moreover, using umbilical cord-derived MSCs in a gel form for difficult-to-heal skin ulcers, as seen in NCT02685722, yielded significant enhancements in wound healing. The treatments led to faster wound closure, reduced ulcer size, and improved overall healing outcomes, underscoring the versatile and powerful therapeutic potential of MSC-based therapies in wound management.
The consistent findings across these studies (
Table 5) show that MSCs, irrespective of their source, contribute to faster wound closure, enhanced tissue regeneration, and reduced scar formation. The potential of MSCs to modulate the immune response, promote angiogenesis, and secrete bioactive molecules highlights their versatility and efficacy as therapeutic agents in wound healing, paving the way for further research and potential clinical applications in regenerative medicine and wound management.
8. Challenges and Future Perspective
One of the key hurdles in advancing mesenchymal stem cell (MSC)-based treatments from the lab to the patient involves securing a consistent and dependable source of these cells. Typically, MSCs are harvested from bone marrow or adipose tissue in preclinical settings, which demands significant effort and time. Additionally, variations among donors can affect the quality and effectiveness of the MSCs. To tackle these challenges, the scientific community is actively exploring alternative MSC sources, including umbilical cord blood, amniotic fluid, and induced pluripotent stem cells (iPSCs). Each of these sources presents its pros and cons, which need a thorough examination before being integrated into clinical trials, as emphasized by Das & Sloan, 2023; Prakash et al., 2023; and L. Wu et al., 2024.
Another significant obstacle is devising an efficient strategy for delivering MSCs to patients. While direct injection has been a conventional approach in preclinical studies, it might not be practical for treating larger patient cohorts due to logistical issues and its invasive nature. Hence, researchers are investigating other delivery mechanisms like intravenous infusion or the use of biodegradable scaffolds for encapsulation, which could potentially enhance scalability and reduce invasiveness (Bian et al., 2022; M. Li et al., 2023; Nosrati et al., 2021). However, these approaches pose challenges, including ensuring the cells’ survival, proper engraftment, and effectively targeting the required sites within the body. Additionally, as we transition from small-scale lab studies to human applications, maintaining the quality and therapeutic potential of MSCs amid large-scale production emerges as a daunting task. This scalability is impeded by the complexities surrounding the isolation and expansion of MSCs, necessitating a shift towards optimizing these procedures.
Improvement in isolating and cultivating MSCs is crucial for overcoming the barriers. This entails the development of more sophisticated methods for extracting MSCs from various sources, such as bone marrow, adipose tissue, and umbilical cord blood. Current research is delving into diverse isolation techniques, including enzymatic digestion, gradient centrifugation, and magnetic-activated cell sorting (MACS), aiming to enhance the purity and quantity of MSCs obtained (Mushahary et al., 2018; Nikfarjam et al., 2020; Yi et al., 2020). Moreover, fine-tuning the conditions under which MSCs are cultured is essential for preserving their ‘stemness’ and ability to proliferate. This involves optimizing factors like the composition of the culture medium, oxygen levels, and the substrates on which the cells are grown to foster robust MSC proliferation while retaining their therapeutic attributes, as reported by Chen et al., 2024.
The continuous exploration and adaptation of mesenchymal stem cells (MSCs) in therapeutics highlights the significance of refinement in their clinical application strategies. As Zhao et al., 2021 suggest, one important approach involves establishing standardized protocols for administering MSCs within clinical environments. The essence of standardization lies in its capacity to bring about uniformity in critical aspects such as cell dosing, delivery routes, the timing of administration, and the observation of patient responses. Establishing rigorous guidelines for cell preparation, storage, and infusion procedures can exponentially enhance the reproducibility and safety of MSC-based therapies. Moreover, standardized protocols streamline the regulatory approval processes by setting clear criteria for the assessment of MSC products’ efficacy and safety. Achieving this requires coordinated efforts from researchers, clinicians, regulatory bodies, and industry stakeholders to draft and implement consensus guidelines across varied clinical trials and treatment environments, as advocated by Jovic et al., (2022).
In parallel with standardization efforts, integrating MSCs with biomaterials emerged as a promising frontier for amplifying their therapeutic efficacy, particularly in tissue engineering applications. By enveloping MSCs in biomaterial platforms like hydrogels, scaffolds, nanoparticles, and microcarriers, researchers aim to achieve targeted delivery to injured tissues (Handral et al., 2023; Khayambashi et al., 2021; Prakash et al., 2023; L. Xuan et al., 2024). Such biomaterial-based systems safeguard the MSCs from immune rejection and bolster their retention at the target sites, thereby enhancing the cells’ paracrine signaling functions critical for tissue repair. Customizing these systems to replicate the extracellular matrix or incorporate bioactive cues pertinent to specific tissues (e.g., cartilage, bone, skin) can significantly refine the regenerative capabilities of MSC-based therapies, as highlighted by Zeng et al. (2023).
However, the path to harnessing the full potential of MSC-based therapies is not devoid of challenges. Recent investigations, including those by Chen et al. (2024), shed light on the variability of individual responses to MSC secretions. This variability plays a crucial role in influencing the effectiveness of MSC-based therapies, suggesting that therapeutic outcomes are not universally consistent (Chen et al., 2024). A patient’s age, genetic makeup, overall health, and pre-existing conditions influence MSC secretions’ impact on tissue repair and regeneration. For example, (Khunti et al., 2023) note that older patients or those with chronic conditions may experience a muted response, given the age-related alterations in their microenvironments or comorbidities impinging on MSC functionality. Likewise, genetic diversities among individuals can affect the interactions between MSC-derived factors and their target cells, leading to disparate treatment outcomes.
Acknowledging such variability highlights the urgency for personalized medicine in MSC therapy. Tailoring treatments to accommodate individual differences exemplifies a crucial step towards maximizing therapeutic efficacy while curtailing potential adverse outcomes, thus significantly improving patient care in regenerative medicine. Such personalized approaches are beneficial and indispensable for propelling the field forward and ensuring patients receive the most apt and efficacious treatments based on their unique profiles. This commitment to individualized care echoes the broader narrative within modern medicine, seeking to customize healthcare solutions better to meet patients’ diverse needs and conditions worldwide.
8.1. Complexities of Utilizing MSCs for Wound Healing
The clinical use of MSCs is accompanied by a range of complexities in their differentiation potential, paracrine effects, and immunomodulatory properties that necessitate thorough examination in modulating effective tissue repair and regeneration.
Several critical challenges must be addressed to ensure the safe and effective use of Mesenchymal Stem Cells (MSCs) in clinical settings. First, the variability in MSCs sources is a significant concern, as MSCs can be derived from various tissues such as bone marrow, adipose tissue, umbilical cord blood, and dental pulp, each presenting unique advantages and limitations in terms of cell yield, proliferation rates, and differentiation potential (Maldonado et al., 2023). Umbilical cords were often chosen as a source for mesenchymal stem cells (MSCs) due to their numerous advantages (Nagamura-Inoue, 2014; Shen et al., 2024). Harvesting MSCs from the umbilical cord avoids controversies as the cord is typically discarded post-birth. Additionally, umbilical cords provide a readily accessible and abundant source of MSCs, collected non-invasively without risk to the mother or newborn. These MSCs also demonstrate high proliferative capacity, multipotency, and strong immunomodulatory properties, making them highly valuable for regenerative medicine (Xie et al., 2020). MSCs pose a lower risk of immune rejection when transplanted, as they are less immunogenic than MSCs from other sources. These sources of MSCs can affect their genomic stability and susceptibility to senescence for consistent therapeutic outcomes and minimizing the risk of adverse effects.
Second, the scalability of cell production poses another challenge, as producing MSCs at a scale sufficient for clinical application requires overcoming substantial hurdles in cell culture techniques, including maintaining cell quality and function during expansion. Thus, ensuring the stability and viability of MSCs during storage and transport is essential for their therapeutic efficacy, necessitating the development of effective cryopreservation methods and storage protocols (Cherian et al., 2020; Fernández-Santos et al., 2022). MSCs must remain alive and functional to exert their therapeutic effects. Damaged cells during storage or transportation may lose their regenerative and immunomodulatory capabilities, rendering them ineffective for treatment.
Lastly, an effective delivery method is needed for the success of MSCs therapy. This includes determining the appropriate mode of administration, whether topical, injectable, or scaffold-based, to ensure that MSCs remain viable and functional upon delivery to the wound site. Local injection was the most frequently reported administration method for wound healing treatment, but topical application was the most frequently used method in clinical studies (Nourian Dehkordi et al., 2019). Considering the larger surface area, the method of treatment given must consider reducing blood loss and injury. However, local injection is the most precise delivery of the cells into the wound where needed in a specific dosage. Although MSCs are generally considered low immunogenicity, the potential for immune rejection or adverse immune reactions, particularly with allogeneic MSCs, remains a concern (T. Zhou et al., 2021). The risk of MSCs differentiating into unwanted cell types could lead to complications such as ectopic tissue formation, necessitating strict control over their differentiation pathways. The potential for MSCs to contribute to tumor development through direct transformation or by creating a pro-tumorigenic environment also requires continuous monitoring and investigation (X. Xuan et al., 2021). Factors such as infection, the extent of tissue damage, and the patient’s overall health and comorbidities can significantly influence therapeutic outcomes. Therefore, addressing these individual factors is essential for optimizing treatment efficacy and advancing the field of regenerative medicine.
8.2. Potential Risks Associated with MSCs Therapy
Like any medical intervention, there are potential risks associated with mesenchymal stem cell (MSC) therapy. The immunogenicity of MSCs is a critical consideration in their therapeutic application, as it can lead to immune rejection and limit their clinical utility. While MSCs are generally considered to have low immunogenicity, they are not entirely immunoprivileged, and their interaction with the immune system can vary based on several factors. MSCs exhibit low levels of major histocompatibility complex (MHC) class I and II molecules, contributing to reduced immunogenicity. However, under inflammatory conditions, such as exposure to IFN-γ and IL-1β, MSCs can upregulate these molecules, increasing their susceptibility to immune attack (Dabrowska et al., 2021; Oh et al., 2022). To minimize MSC immunogenicity, researchers are exploring various preconditioning or priming techniques that can enhance the immunomodulatory properties of MSCs while minimizing their recognition by the immune system. One promising approach involves using hypoxic conditions, which have been shown to promote a more favorable secretory profile in MSCs, enhancing their therapeutic potential while maintaining low immunogenicity (Kahrizi et al., 2023; Zhidu et al., 2024). Additionally, the application of pharmacological agents, such as dexamethasone or prostaglandin E2, has been investigated to modulate the MSCs’ behavior further and improve their efficacy in various therapeutic settings (Hezam et al., 2023). Moreover, combining these strategies with genetic modifications to enhance the expression of anti-inflammatory cytokines could lead to even greater improvements in MSC functionality and survival in hostile environments (Chehelgerdi et al., 2024; X. Chen et al., 2024).
The risk of tumorigenicity in MSC-based therapies is a major concern in both scientific and regulatory domains. MSCs are multipotent stem cells capable of differentiating into various cell types, making them valuable in regenerative medicine. However, concerns about their potential for malignant transformation have been raised (Zhuang et al., 2021). Spontaneous malignant transformation can occur during the manufacturing process, which may affect the genetic stability of MSCs. Long-term cell expansion and certain culture conditions may increase the risk of tumorigenicity-related chromosomal abnormalities. In this context, experts have suggested that slow growth and short expansion times can mitigate these risks. Stimulated malignant transformation, conversely, can arise from different mechanisms, including cell fusion, fusion proteins, and tumor microenvironment induction (Patrikoski et al., 2019; Røsland et al., 2009; Zhuang et al., 2021). Rigorous quality control measures and comprehensive genomic analyses such as karyotyping, fluorescence in situ hybridization (FISH), and comparative genomic hybridization (CGH) are essential to ensure the safety of MSC products before clinical application (L. Hu et al., 2014; Shakoori, 2017).
The transmission of infections in MSC therapy is a significant concern, particularly for immunocompromised patients undergoing these treatments. While MSCs are generally considered safe, there are potential risks associated with viral infections, especially from viruses that can remain latent or carry tumorigenic potential. Studies have shown that MSCs can be vulnerable to viral infections in vitro, particularly from the herpes and hepatitis families. However, these infections typically result in minimal viral gene expression and are nonproductive (Rocha et al., 2021; Taechangam et al., 2022). Furthermore, MSCs derived from Wharton’s jelly have been found to contain low levels of viral DNA from polyomaviruses, raising concerns about tumorigenic risks, especially in immunocompromised individuals (Davies et al., 2017; Drobiova et al., 2023). This highlights the need for international consensus guidelines and standardized testing protocols to detect rare viruses in cellular products, with the goal of establishing minimal release criteria worldwide and maintaining high safety standards in cellular therapy. Ensuring adherence to national and international guidelines, including compliance with Good Manufacturing Practices (GMP) and other relevant standards, is crucial for meeting safety requirements in MSC production and application.
8.3. The Multifactorial Nature of Chronic Wound Healing: Beyond Paracrine Signalling
The argument that paracrine signaling via growth factors, cytokines, and chemokines may not be the sole determining factor in chronic wound healing is reasonable. While these signaling molecules play crucial roles in regulating cellular responses and locally facilitating tissue repair, they are part of a broader, more complex network of factors determining wound healing. (Vasalou et al., 2023). Environmental factors such as oxygen tension, mechanical stress, and the presence of pathogens can significantly impact the healing process. For instance, hypoxia or inadequate oxygen supply can prevent cellular proliferation and function, leading to impaired wound healing. Additionally, chronic mechanical stress or repeated trauma can worsen wound conditions and disrupt normal healing progression. (Kimmel et al., 2016).
Systemic factors also play an important role in wound healing. These include metabolic status, nutritional deficiencies, and comorbidities like diabetes or cardiovascular disease. Systemic conditions can alter immune function, blood supply, and cellular metabolism, all of which contribute to the efficiency and quality of the healing process. For example, hyperglycemia associated with diabetes can impair leukocyte function and collagen synthesis, thus delaying wound repair. (Guo & DiPietro, 2010). Therefore, while paracrine signaling through growth factors, cytokines, and chemokines is essential for coordinating local cellular responses and facilitating wound healing, an extensive understanding of chronic wound healing must also consider these additional environmental and systemic factors. Integrating these appearances is important for developing effective therapeutic strategies to enhance wound healing outcomes. (Kolimi et al., 2022).
9. Conclusion
This comprehensive review highlights the significant potential of mesenchymal stem cells (MSCs) in accelerating wound repair through their multifaceted role in secreting growth factors, cytokines, chemokines, and extracellular matrix components. The paracrine effects of MSCs create an optimal environment for tissue regeneration by promoting cell proliferation, migration, angiogenesis, and modulating inflammation. Preclinical and clinical evidence demonstrates the efficacy of MSCs from various sources in enhancing wound healing outcomes, particularly for chronic and difficult-to-treat wounds. However, several challenges remain in translating MSC-based therapies to widespread clinical use. These include optimizing cell sources, delivery methods, and dosing regimens; addressing potential risks such as tumorigenicity and immune rejection; and developing standardized cell preparation and administration protocols. Additionally, the variability in patient responses and the complex interplay between local and systemic factors in wound healing necessitate a personalized approach to MSC therapy. Future research should focus on elucidating the molecular mechanisms underlying MSC-mediated wound healing, developing innovative delivery systems to enhance cell survival and function, and conducting large-scale randomized clinical trials to establish safety and efficacy. Integrating MSCs with advanced biomaterials and exploring combination therapies may further enhance their therapeutic potential. As our understanding of MSC biology and wound healing mechanisms continues to evolve, MSC-based treatments hold promises for transforming wound care and improving outcomes for patients with chronic wounds. While MSCs offer a promising approach to wound healing, a holistic strategy that considers both cellular therapies and the broader wound environment is essential for developing effective treatments. Continued research and interdisciplinary collaboration will be crucial in overcoming current limitations and realizing the full potential of MSCs in regenerative medicine and wound care.
Author Contributions
Conceptualization, Abdul Ghani N.I. and Nurhidayatul S.O.; methodology, Abdul Ghani N.I. and Nurhidayatul S.O.; validation, Shamsul B.S. and Chua K.H.; investigation and resources, Abdul Ghani N.I., Nurhidayatul S.O., Khairul A.K., Hasmad H.N., Afifa N.T., Helmi B.S., Aida Juliana A.J., Farisha M.N. and Balqish M.R.; writing—original draft preparation, Abdul Ghani N.I., Nurhidayatul S.O., Khairul A.K., Hasmad H.N., Afifa N.T., Helmi B.S., Aida Juliana A.J., Farisha M.N. and Balqish M.R.; writing—review and editing, Shamsul B.S. and Chua K.H.; supervision, Shamsul B.S., Chua K.H. and BiiChau T; project administration, Shamsul B.S., Chua K.H. and BiiChau T; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We want to extend our thanks to our colleagues for their valuable discussions and helpful feedback, which significantly improved the quality of this review. We are also grateful to Supergenic Life Science Sdn Bhd and Hope Life Science Sdn Bhd for their provision of essential resources and support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ackermann, J., Arndt, L., Fröba, J., Lindhorst, A., Glaß, M., Kirstein, M., Hobusch, C., Wunderlich, F. T., Braune, J., & Gericke, M. (2024). IL-6 signaling drives self-renewal and alternative activation of adipose tissue macrophages. Frontiers in Immunology, 15. [CrossRef]
- Ahangar, P., Mills, S. J., & Cowin, A. J. (2020). Mesenchymal stem cell secretome as an emerging cell-free alternative for improving wound repair. In International Journal of Molecular Sciences (Vol. 21, Issue 19). [CrossRef]
- Ajit, A., & Ambika Gopalankutty, I. (2021). Adipose-derived stem cell secretome as a cell-free product for cutaneous wound healing. In 3 Biotech (Vol. 11, Issue 9). [CrossRef]
- Amini, A., Pouriran, R., Abdollahifar, M. A., Abbaszadeh, H. A., Ghoreishi, S. K., Chien, S., & Bayat, M. (2018). Stereological and molecular studies on the combined effects of photobiomodulation and human bone marrow mesenchymal stem cell conditioned medium on wound healing in diabetic rats. Journal of Photochemistry and Photobiology B: Biology, 182(January), 42–51. [CrossRef]
- An, H. Y., Shin, H. S., Choi, J. S., Kim, H. J., Lim, J. Y., & Kim, Y. M. (2015). Adipose mesenchymal stem cell secretome modulated in hypoxia for remodeling of radiation-induced salivary gland damage. PLoS ONE, 10(11), 1–17. [CrossRef]
- Arno, A. I., Amini-Nik, S., Blit, P. H., Al-Shehab, M., Belo, C., Herer, E., Tien, H., & Jeschke, M. G. (2014). Human Wharton’s jelly mesenchymal stem cells promote skin wound healing through paracrine signaling. Available online: http://stemcellres.
- Aydemir, I., Öztürk, Ş., Kılıçaslan Sönmez, P., & Tuğlu, M. İ. (2016). Mesenchymal stem cells in skin wound healing. Anatomy, 10(3), 228–234. https://doi.org/10.2399/ana.16.043. [CrossRef]
- Badillo, A. T., Redden, R. A., Zhang, L., Doolin, E. J., & Liechty, K. W. (2007). Treatment of diabetic wounds with fetal murine mesenchymal stromal cells enhances wound closure. Cell and Tissue Research, 329(2). [CrossRef]
- Bian, D., Wu, Y., Song, G., Azizi, R., & Zamani, A. (2022). The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: a comprehensive review. In Stem Cell Research and Therapy (Vol. 13, Issue 1). BioMed Central Ltd. [CrossRef]
- Bowers, S., & Franco, E. (2020). Chronic Wounds: Evaluation and Management. American Family Physician, 101(3), 159–166. Available online: http://www.ncbi.nlm.nih.gov/pubmed/32003952.
- Bryam Esteban Coello García, Regiane Vita Maximiniano, Jessica Vanessa Sibri Lazo, Marcos Patricio Saguay Cabrera, Karla Lucia Jaramillo Carrasco, Francisco Javier Maldonado Rodríguez, Daniel Esteban Maldonado Barzallo, Gladys Fabiola Zamora Zamora, Cecibel Carolina Mogrovejo Zúñiga, & Estefanny Dayana Villafuerte Ruiz. (2024). OVERVIEW OF PRESSURE ULCERS: PATHOPHYSIOLOGY, EPIDEMIOLOGY, RISK FACTORS, PRESENTATION AND TREATMENT. EPRA International Journal of Multidisciplinary Research (IJMR), 24–30. [CrossRef]
- Bunker, E. N., Wheeler, G. E., Chapnick, D. A., & Liu, X. (2021). Suppression of α-catenin and adherens junctions enhances epithelial cell proliferation and motility via TACE-mediated TGF-α autocrine/paracrine signaling. Molecular Biology of the Cell, 32(4). [CrossRef]
- Chaudhary, J. K., Ahamad, N., & Rath, P. C. (2024). Mesenchymal stem cells (MSCs) from the mouse bone marrow show differential expression of interferon regulatory factors IRF-1 and IRF-2. Molecular Biology Reports, 51(1). [CrossRef]
- Chehelgerdi, M., Chehelgerdi, M., Khorramian-Ghahfarokhi, M., Shafieizadeh, M., Mahmoudi, E., Eskandari, F., Rashidi, M., Arshi, A., & Mokhtari-Farsani, A. (2024). Comprehensive review of CRISPR-based gene editing: mechanisms, challenges, and applications in cancer therapy. In Molecular Cancer (Vol. 23, Issue 1). BioMed Central Ltd. [CrossRef]
- Chen, L., Tredget, E. E., Wu, P. Y. G., Wu, Y., & Wu, Y. (2008). Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE, 3(4). [CrossRef]
- Chen, L., Xu, Y., Zhao, J., Zhang, Z., Yang, R., Xie, J., Liu, X., & Qi, S. (2014). Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS ONE, 9(4). [CrossRef]
- Chen, S., Liang, B., & Xu, J. (2024a). Unveiling heterogeneity in MSCs: exploring marker-based strategies for defining MSC subpopulations. In Journal of Translational Medicine (Vol. 22, Issue 1). BioMed Central Ltd. [CrossRef]
- Chen, S., Liang, B., & Xu, J. (2024b). Unveiling heterogeneity in MSCs: exploring marker-based strategies for defining MSC subpopulations. In Journal of Translational Medicine (Vol. 22, Issue 1). BioMed Central Ltd. [CrossRef]
- Chen, X., Zhong, S., Zhan, Y., & Zhang, X. (2024). CRISPR–Cas9 applications in T cells and adoptive T cell therapies. In Cellular and Molecular Biology Letters (Vol. 29, Issue 1). BioMed Central Ltd. [CrossRef]
- Cherian, D. S., Bhuvan, T., Meagher, L., & Heng, T. S. P. (2020). Biological Considerations in Scaling Up Therapeutic Cell Manufacturing. In Frontiers in Pharmacology (Vol. 11). [CrossRef]
- Chouw, A., Facicilia, G., Sartika, C. R., Faried, A., & Milanda, T. (2022). Factors Influencing the Therapeutic Potential of the MSC-derived Secretome. In Regenerative Engineering and Translational Medicine (Vol. 8, Issue 3). [CrossRef]
- Cuesta-Gomez, N., Graham, G. J., & Campbell, J. D. M. (2021). Chemokines and their receptors: predictors of the therapeutic potential of mesenchymal stromal cells. In Journal of Translational Medicine (Vol. 19, Issue 1). [CrossRef]
- Dabrowska, S., Andrzejewska, A., Janowski, M., & Lukomska, B. (2021). Immunomodulatory and Regenerative Effects of Mesenchymal Stem Cells and Extracellular Vesicles: Therapeutic Outlook for Inflammatory and Degenerative Diseases. In Frontiers in Immunology (Vol. 11). [CrossRef]
- Darwin, E., & Tomic-Canic, M. (2018). Healing Chronic Wounds: Current Challenges and Potential Solutions. In Current Dermatology Reports (Vol. 7, Issue 4). [CrossRef]
- Das, M., & Sloan, A. J. (2023). Stem cell sources from human biological waste material: a role for the umbilical cord and dental pulp stem cells for regenerative medicine. In Human Cell (Vol. 36, Issue 4). [CrossRef]
- Davies, J. E., Walker, J. T., & Keating, A. (2017). Concise Review: Wharton’s Jelly: The Rich, but Enigmatic, Source of Mesenchymal Stromal Cells. Stem Cells Translational Medicine, 6(7). [CrossRef]
- Deng, Z., Fan, T., Xiao, C., Tian, H., Zheng, Y., Li, C., & He, J. (2024). TGF-β signaling in health, disease, and therapeutics. In Signal Transduction and Targeted Therapy (Vol. 9, Issue 1). [CrossRef]
- Ding, J. Y., Chen, M. J., Wu, L. F., Shu, G. F., Fang, S. J., Li, Z. Y., Chu, X. R., Li, X. K., Wang, Z. G., & Ji, J. S. (2023). Mesenchymal stem cell-derived extracellular vesicles in skin wound healing: roles, opportunities and challenges. In Military Medical Research (Vol. 10, Issue 1). [CrossRef]
- Dong, X., Lu, X., Kingston, K., Brewer, E., Juliar, B. A., Kripfgans, O. D., Fowlkes, J. B., Franceschi, R. T., Putnam, A. J., Liu, Z., & Fabiilli, M. L. (2019). Controlled delivery of basic fibroblast growth factor (bFGF) using acoustic droplet vaporization stimulates endothelial network formation. Acta Biomaterialia, 97. [CrossRef]
- Drobiova, H., Sindhu, S., Ahmad, R., Haddad, D., Al-Mulla, F., & Al Madhoun, A. (2023). Wharton’s jelly mesenchymal stem cells: a concise review of their secretome and prospective clinical applications. In Frontiers in Cell and Developmental Biology (Vol. 11). [CrossRef]
- Du, W. J., Chi, Y., Yang, Z. X., Li, Z. J., Cui, J. J., Song, B. Q., Li, X., Yang, S. G., Han, Z. B., & Han, Z. C. (2016). Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta. Stem Cell Research and Therapy, 7(1). [CrossRef]
- Duque, G. A., & Descoteaux, A. (2014). Macrophage cytokines: Involvement in immunity and infectious diseases. In Frontiers in Immunology (Vol. 5, Issue OCT). [CrossRef]
- Ekingen, T., Sob, C., Hartmann, C., Rühli, F. J., Matthes, K. L., Staub, K., & Bender, N. (2022). Associations between hydration status, body composition, sociodemographic and lifestyle factors in the general population: a cross-sectional study. BMC Public Health, 22(1). [CrossRef]
- Fakiha, K.(2022). Adipose stromal vascular fraction: a promising treatment for severe burn injury. In Human Cell (Vol. 35, Issue 5). [CrossRef]
- Fakouri, A., Razavi, Z. S., Mohammed, A. T., Hussein, A. H. A., Afkhami, H., & Hooshiar, M. H. (2024). Applications of mesenchymal stem cell-exosome components in wound infection healing: new insights. In Burns and Trauma (Vol. 12). Oxford University Press. [CrossRef]
- Farabi, B., Roster, K., Hirani, R., Tepper, K., Atak, M. F., & Safai, B. (2024). The Efficacy of Stem Cells in Wound Healing: A Systematic Review. In International Journal of Molecular Sciences (Vol. 25, Issue 5). [CrossRef]
- Fernández-Santos, M. E., Garcia-Arranz, M., Andreu, E. J., García-Hernández, A. M., López-Parra, M., Villarón, E., Sepúlveda, P., Fernández-Avilés, F., García-Olmo, D., Prosper, F., Sánchez-Guijo, F., Moraleda, J. M., & Zapata, A. G. (2022). Optimization of Mesenchymal Stromal Cell (MSC) Manufacturing Processes for a Better Therapeutic Outcome. In Frontiers in Immunology (Vol. 13). [CrossRef]
- Fierro, F., Magner, N., Beegle, J., Dahlenburg, H., White, J. L., Zhou, P., Pepper, K., Fury, B., Coleal-bergum, D. P., Bauer, G., Annett, G., Pifer, C., & Nolta, J. (2019). HHS Public Access. 59(Suppl 1), 893–897. [CrossRef]
- François, S., Eder, V. V., Belmokhtar, K., Machet, M. C., Douay, L., Gorin, N. C., Benderitter, M., & Alain, C. A. (2017). Synergistic effect of human Bone Morphogenic Protein-2 and Mesenchymal Stromal Cells on chronic wounds through hypoxia-inducible factor-1 α induction. Scientific Reports, 7(1). [CrossRef]
- Gou, Y., Hu, L., Liao, X., He, J., & Liu, F. (2024). Advances of antimicrobial dressings loaded with antimicrobial agents in infected wounds. In Frontiers in Bioengineering and Biotechnology (Vol. 12). Frontiers Media SA. [CrossRef]
- Grebenciucova, E., & VanHaerents, S. (2023). Interleukin 6: at the interface of human health and disease. In Frontiers in Immunology (Vol. 14). [CrossRef]
- Guo, S., & DiPietro, L. A. (2010). 20. Factors Affecting Wound Healing. Journal of Dental Research, 89(3). [CrossRef]
- Gupta, S., Sagar, S., Kisaka, T., Tripathi, S., Gupta, S., Care, I., Delhi, N., Sagar, S., Delhi, N., Jaypee, G. M., & Kisaka, T. (2021). Chronic wounds: magnitude, socioeconomic burden and consequences. Wounds Asia, 4(1).
- Handral, H. K., Wyrobnik, T. A., & Lam, A. T. L. (2023). Emerging Trends in Biodegradable Microcarriers for Therapeutic Applications. In Polymers (Vol. 15, Issue 6). [CrossRef]
- Harsha, L., & Brundha, M. P. (2020). Role of collagen in wound healing. In Drug Invention Today (Vol. 13, Issue 1).
- Hezam, K., Wang, C., Fu, E., Zhou, M., Liu, Y., Wang, H., Zhu, L., Han, Z., Han, Z. C., Chang, Y., & Li, Z. (2023). Superior protective effects of PGE2 priming mesenchymal stem cells against LPS-induced acute lung injury (ALI) through macrophage immunomodulation. Stem Cell Research and Therapy, 14(1). [CrossRef]
- Hoang, D. H., Nguyen, T. D., Nguyen, H. P., Nguyen, X. H., Do, P. T. X., Dang, V. D., Dam, P. T. M., Bui, H. T. H., Trinh, M. Q., Vu, D. M., Hoang, N. T. M., Thanh, L. N., & Than, U. T. T. (2020). Differential Wound Healing Capacity of Mesenchymal Stem Cell-Derived Exosomes Originated From Bone Marrow, Adipose Tissue and Umbilical Cord Under Serum- and Xeno-Free Condition. Frontiers in Molecular Biosciences, 7(June). [CrossRef]
- Hu, L., Ru, K., Zhang, L., Huang, Y., Zhu, X., Liu, H., Zetterberg, A., Cheng, T., & Miao, W. (2014). Fluorescence in situ hybridization (FISH): An increasingly demanded tool for biomarker research and personalized medicine. In Biomarker Research (Vol. 2, Issue 1). [CrossRef]
- Hu, Y., Tao, R., Chen, L., Xiong, Y., Xue, H., Hu, L., Yan, C., Xie, X., Lin, Z., Panayi, A. C., Mi, B., & Liu, G. (2021). Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis. Journal of Nanobiotechnology, 19(1). [CrossRef]
- Hu, Z., Li, D., Wu, S., Pei, K., Fu, Z., Yang, Y., Huang, Y., Yang, J., Liu, C., Hu, J., Cai, C., & Liao, Y. (2024). Unveiling the functional heterogeneity of cytokine-primed human umbilical cord mesenchymal stem cells through single-cell RNA sequencing. Cell and Bioscience, 14(1). [CrossRef]
- Javazon, E. H., Keswani, S. G., Badillo, A. T., Crombleholme, T. M., Zoltick, P. W., Radu, A. P., Kozin, E. D., Beggs, K., Malik, A. A., & Flake, A. W. (2007). Enhanced epithelial gap closure and increased angiogenesis in wounds of diabetic mice treated with adult murine bone marrow stromal progenitor cells. Wound Repair and Regeneration, 15(3), 350–359. [CrossRef]
- Joseph, A., Baiju, I., Bhat, I. A., Pandey, S., Bharti, M., Verma, M., Pratap Singh, A., Ansari, M. M., Chandra, V., Saikumar, G., Amarpal, & Taru Sharma, G. (2020). Mesenchymal stem cell-conditioned media: A novel alternative of stem cell therapy for quality wound healing. Journal of Cellular Physiology, 235(7–8), 5555–5569. [CrossRef]
- Jovic, D., Yu, Y., Wang, D., Wang, K., Li, H., Xu, F., Liu, C., Liu, J., & Luo, Y. (2022). A Brief Overview of Global Trends in MSC-Based Cell Therapy. In Stem Cell Reviews and Reports (Vol. 18, Issue 5). [CrossRef]
- Kahrizi, M. S., Mousavi, E., Khosravi, A., Rahnama, S., Salehi, A., Nasrabadi, N., Ebrahimzadeh, F., & Jamali, S. (2023). Recent advances in pre-conditioned mesenchymal stem/stromal cell (MSCs) therapy in organ failure; a comprehensive review of preclinical studies. In Stem Cell Research and Therapy (Vol. 14, Issue 1). [CrossRef]
- Kato, Y., Iwata, T., Morikawa, S., Yamato, M., Okano, T., & Uchigata, Y. (2015). Allogeneic Transplantation of an Adipose-Derived Stem Cell Sheet Combined With Artificial Skin Accelerates Wound Healing in a Rat Wound Model of Type 2 Diabetes and Obesity. Diabetes, 64(8), 2723–2734. [CrossRef]
- Kaur, H., & Ghorai, S. M. (2022). Role of Cytokines as Immunomodulators. In Immunomodulators and Human Health. [CrossRef]
- Khayambashi, P., Iyer, J., Pillai, S., Upadhyay, A., Zhang, Y., & Tran, S. D. (2021). Hydrogel encapsulation of mesenchymal stem cells and their derived exosomes for tissue engineering. In International Journal of Molecular Sciences (Vol. 22, Issue 2). [CrossRef]
- Khunti, K., Sathanapally, H., & Mountain, P. (2023). Multiple long term conditions, multimorbidity, and co-morbidities: We should reconsider the terminology we use. In BMJ. [CrossRef]
- Kim, C. H., Lee, J. H., Won, J. H., & Cho, M. K. (2011). Mesenchymal stem cells improve wound healing in vivo via early activation of matrix metalloproteinase-9 and vascular endothelial growth factor. Journal of Korean Medical Science, 26(6), 726–733. [CrossRef]
- Kim, J. W., Lee, J. H., Lyoo, Y. S., Jung, D. I., & Park, H. M. (2013). The effects of topical mesenchymal stem cell transplantation in canine experimental cutaneous wounds. Veterinary Dermatology, 24(2). [CrossRef]
- Kimmel, H. M., Grant, A., & Ditata, J. (2016). The presence of oxygen in wound healing. In Wounds (Vol. 28, Issue 8).
- Kita, A., Yamamoto, S., Saito, Y., & Chikenji, T. S. (2024). Cellular senescence and wound healing in aged and diabetic skin. In Frontiers in Physiology (Vol. 15). [CrossRef]
- Kolimi, P., Narala, S., Nyavanandi, D., Youssef, A. A. A., & Dudhipala, N. (2022). Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. In Cells (Vol. 11, Issue 15). [CrossRef]
- Kong, P., Xie, X., Li, F., Liu, Y., & Lu, Y. (2013). Placenta mesenchymal stem cell accelerates wound healing by enhancing angiogenesis in diabetic Goto-Kakizaki (GK) rats. Biochemical and Biophysical Research Communications, 438(2), 410–419. [CrossRef]
- Krasilnikova, O. A., Baranovskii, D. S., Lyundup, A. V., Shegay, P. V., Kaprin, A. D., & Klabukov, I. D. (2022). Stem and Somatic Cell Monotherapy for the Treatment of Diabetic Foot Ulcers: Review of Clinical Studies and Mechanisms of Action. In Stem Cell Reviews and Reports (Vol. 18, Issue 6). [CrossRef]
- Kuo, Y. R., Wang, C. T., Cheng, J. T., Wang, F. S., Chiang, Y. C., & Wang, C. J. (2011). Bone marrow-derived mesenchymal stem cells enhanced diabetic wound healing through recruitment of tissue regeneration in a rat model of streptozotocin-induced diabetes. Plastic and Reconstructive Surgery, 128(4), 872–880. [CrossRef]
- L Dehghani, I. O. F. S. A. S. (2024). The Therapeutic Potential of Human Umbilical Cord Mesenchymal Stromal Cells Derived Exosomes for Wound Healing: Harnessing Exosomes as a Cell-free Therapy. Journal of Stem Cells and Regenerative Medicine. [CrossRef]
- Laroye, C., Boufenzer, A., Jolly, L., Cunat, L., Alauzet, C., Merlin, J. L., Yguel, C., Bensoussan, D., Reppel, L., & Gibot, S. (2019). Bone marrow vs Wharton’s jelly mesenchymal stem cells in experimental sepsis: A comparative study. Stem Cell Research and Therapy, 10(1). [CrossRef]
- Lee, D. E., Ayoub, N., & Agrawal, D. K. (2016). Mesenchymal stem cells and cutaneous wound healing: Novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Research and Therapy, 7(1), 1–8. [CrossRef]
- Li, D., Li, D., Wang, Z., Li, J., Shahzad, K. A., Wang, Y., & Tan, F. (2024). Signaling pathways activated and regulated by stem cell-derived exosome therapy. In Cell and Bioscience (Vol. 14, Issue 1). BioMed Central Ltd. [CrossRef]
- Li, J., Liu, Y., Zhang, R., Yang, Q., Xiong, W., He, Y., & Ye, Q. (2024). Insights into the role of mesenchymal stem cells in cutaneous medical aesthetics: from basics to clinics. In Stem Cell Research and Therapy (Vol. 15, Issue 1). BioMed Central Ltd. [CrossRef]
- Li, M., Luan, F., Zhao, Y., Hao, H., Liu, J., Dong, L., Fu, X., & Han, W. (2017). Mesenchymal stem cell-conditioned medium accelerates wound healing with fewer scars. International Wound Journal, 14(1), 64–73. [CrossRef]
- Li, M., Xia, W., Khoong, Y. M., Huang, L., Huang, X., Liang, H., Zhao, Y., Mao, J., Yu, H., & Zan, T. (2023). Smart and versatile biomaterials for cutaneous wound healing. In Biomaterials Research (Vol. 27, Issue 1). [CrossRef]
- Liang, W., Chen, X., Zhang, S., Fang, J., Chen, M., Xu, Y., & Chen, X. (2021). Mesenchymal stem cells as a double-edged sword in tumor growth: focusing on MSC-derived cytokines. In Cellular and Molecular Biology Letters (Vol. 26, Issue 1). [CrossRef]
- Liang, X., Lin, F., Ding, Y., Zhang, Y., Li, M., Zhou, X., Meng, Q., Ma, X., Wei, L., Fan, H., & Liu, Z. (2021). Conditioned medium from induced pluripotent stem cell-derived mesenchymal stem cells accelerates cutaneous wound healing through enhanced angiogenesis. Stem Cell Research and Therapy, 12(1). [CrossRef]
- Linard, C., Tissedre, F., Busson, E., Holler, V., Leclerc, T., Strup-Perrot, C., Couty, L., L’homme, B., Benderitter, M., Lafont, A., Lataillade, J. J., & Coulomb, B. (2015). Therapeutic Potential of Gingival Fibroblasts for Cutaneous Radiation Syndrome: Comparison to Bone Marrow-Mesenchymal Stem Cell Grafts. Stem Cells and Development, 24(10), 1182–1193. [CrossRef]
- Liu, C., Xu, Y., Lu, Y., Du, P., Li, X., Wang, C., Guo, P., Diao, L., & Lu, G. (2022). Mesenchymal stromal cells pretreated with proinflammatory cytokines enhance skin wound healing via IL-6-dependent M2 polarization. Stem Cell Research and Therapy, 13(1). [CrossRef]
- Liu, J., Qiu, X., Lv, Y., Zheng, C., Dong, Y., Dou, G., Zhu, B., Liu, A., Wang, W., Zhou, J., Liu, S., Liu, S., Gao, B., & Jin, Y. (2020). Apoptotic bodies derived from mesenchymal stem cells promote cutaneous wound healing via regulating the functions of macrophages. Stem Cell Research and Therapy, 11(1). [CrossRef]
- Liu, J., Qu, M., Wang, C., Xue, Y., Huang, H., Chen, Q., Sun, W., Zhou, X., Xu, G., & Jiang, X. (2022). A Dual-Cross-Linked Hydrogel Patch for Promoting Diabetic Wound Healing. Small, 18(17). [CrossRef]
- Liu, L., Yu, Y., Hou, Y., Chai, J., Duan, H., Chu, W., Zhang, H., Hu, Q., & Du, J. (2014). Human umbilical cord mesenchymal stem cells transplantation promotes cutaneous wound healing of severe burned rats. PLoS ONE, 9(2). [CrossRef]
- Lopes, L., Setia, O., Aurshina, A., Liu, S., Hu, H., Isaji, T., Liu, H., Wang, T., Ono, S., Guo, X., Yatsula, B., Guo, J., Gu, Y., Navarro, T., & Dardik, A. (2018). Stem cell therapy for diabetic foot ulcers: A review of preclinical and clinical research. In Stem Cell Research and Therapy (Vol. 9, Issue 1). [CrossRef]
- Maharlooei, M. K., Bagheri, M., Solhjou, Z., Jahromi, B. M., Akrami, M., Rohani, L., Monabati, A., Noorafshan, A., & Omrani, G. R. (2011). Adipose tissue derived mesenchymal stem cell (AD-MSC) promotes skin wound healing in diabetic rats. Diabetes Research and Clinical Practice, 93(2), 228–234. [CrossRef]
- Maldonado, V. V., Patel, N. H., Smith, E. E., Barnes, C. L., Gustafson, M. P., Rao, R. R., & Samsonraj, R. M. (2023). Clinical utility of mesenchymal stem/stromal cells in regenerative medicine and cellular therapy. In Journal of Biological Engineering (Vol. 17, Issue 1). [CrossRef]
- Marofi, F., Kozlitina, I. A., Margiana, R., Bahramali, M., Suksatan, W., Abdelbasset, W. K., Chupradit, S., Nasimi, M., & Maashi, M. S. (2021). MSCs and their exosomes: a rapidly evolving approach in the context of cutaneous wounds therapy. In Stem Cell Research and Therapy (Vol. 12, Issue 1). BioMed Central Ltd. [CrossRef]
- McDermott, K., Fang, M., Boulton, A. J. M., Selvin, E., & Hicks, C. W. (2023). Etiology, Epidemiology, and Disparities in the Burden of Diabetic Foot Ulcers. In Diabetes Care (Vol. 46, Issue 1). [CrossRef]
- McNichol, L., Bliss, D. Z., & Gray, M. (2022). Moisture-Associated Skin Damage: Expanding Practice Based on the Newest ICD-10-CM Codes for Irritant Contact Dermatitis Associated with Digestive Secretions and Fecal or Urinary Effluent from an Abdominal Stoma or Enterocutaneous Fistula. Journal of Wound, Ostomy and Continence Nursing, 49(3). [CrossRef]
- Mebarki, M., Abadie, C., Larghero, J., & Cras, A. (2021). Human umbilical cord-derived mesenchymal stem/stromal cells: a promising candidate for the development of advanced therapy medicinal products. In Stem Cell Research and Therapy (Vol. 12, Issue 1). BioMed Central Ltd. [CrossRef]
- Moghadam, M., Tokhanbigli, S., Baghaei, K., Farivar, S., Asadzadeh Aghdaei, H., & Zali, M. R. (2020). Gene expression profile of immunoregulatory cytokines secreted from bone marrow and adipose derived human mesenchymal stem cells in early and late passages. Molecular Biology Reports, 47(3). [CrossRef]
- Mushahary, D., Spittler, A., Kasper, C., Weber, V., & Charwat, V. (2018). Isolation, cultivation, and characterization of human mesenchymal stem cells. In Cytometry Part A (Vol. 93, Issue 1, pp. 19–31). Wiley-Liss Inc. [CrossRef]
- Mussbacher, M., Kral-Pointner, J. B., Salzmann, M., Schrottmaier, W. C., & Assinger, A. (2019). Mechanisms of Hemostasis: Contributions of Platelets, Coagulation Factors, and the Vessel Wall. [CrossRef]
- Nagamura-Inoue, T.(2014). Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World Journal of Stem Cells, 6(2), 195. [CrossRef]
- Nakamura, Y., Ishikawa, H., Kawai, K., Tabata, Y., & Suzuki, S. (2013). Enhanced wound healing by topical administration of mesenchymal stem cells transfected with stromal cell-derived factor-1. Biomaterials, 34(37), 9393–9400. [CrossRef]
- Nie, C., Yang, D., Xu, J., Si, Z., Jin, X., & Zhang, J. (2011). Locally administered Adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplantation, 20(2), 205–216. [CrossRef]
- Nikfarjam, S., Rezaie, J., Zolbanin, N. M., & Jafari, R. (2020). Mesenchymal stem cell derived-exosomes: a modern approach in translational medicine. In Journal of Translational Medicine (Vol. 18, Issue 1). [CrossRef]
- Nosrati, H., Aramideh Khouy, R., Nosrati, A., Khodaei, M., Banitalebi-Dehkordi, M., Ashrafi-Dehkordi, K., Sanami, S., & Alizadeh, Z. (2021). Nanocomposite scaffolds for accelerating chronic wound healing by enhancing angiogenesis. In Journal of Nanobiotechnology (Vol. 19, Issue 1). [CrossRef]
- Nouri, S., Shokraneh, S., Fatehi Shalamzari, P., Ahmed, M. H., Radi, U. K., Idan, A. H., Ebrahimi, M. J., Moafi, M., & Gholizadeh, N. (2024). Application of Mesenchymal Stem Cells and Exosome alone or Combination Therapy as a Treatment Strategy for Wound Healing. Cell Biochemistry and Biophysics, 1–14. [CrossRef]
- Nourian Dehkordi, A., Mirahmadi Babaheydari, F., Chehelgerdi, M., & Raeisi Dehkordi, S. (2019). Skin tissue engineering: Wound healing based on stem-cell-based therapeutic strategies. In Stem Cell Research and Therapy (Vol. 10, Issue 1). [CrossRef]
- Noverina, R., Widowati, W., Ayuningtyas, W., Kurniawan, D., Afifah, E., Laksmitawati, D. R., Rinendyaputri, R., Rilianawati, R., Faried, A., Bachtiar, I., & Wirakusumah, F. F. (2019). Growth factors profile in conditioned medium human adipose tissue-derived mesenchymal stem cells (CM-hATMSCs). Clinical Nutrition Experimental, 24, 34–44. [CrossRef]
- Oh, J. Y., Kim, H., Lee, H. J., Lee, K., Barreda, H., Kim, H. J., Shin, E., Bae, E. H., Kaur, G., Zhang, Y., Kim, E., Lee, J. Y., & Lee, R. H. (2022). MHC Class I Enables MSCs to Evade NK-Cell-Mediated Cytotoxicity and Exert Immunosuppressive Activity. Stem Cells, 40(9). [CrossRef]
- Park, W., Wei, S., Kim, B. S., Kim, B., Bae, S. J., Chae, Y. C., Ryu, D., & Ha, K. T. (2023). Diversity and complexity of cell death: a historical review. In Experimental and Molecular Medicine (Vol. 55, Issue 8). [CrossRef]
- Patenall, B. L., Carter, K. A., & Ramsey, M. R. (2024). Kick-Starting Wound Healing: A Review of Pro-Healing Drugs. In International Journal of Molecular Sciences (Vol. 25, Issue 2). [CrossRef]
- Patrikoski, M., Rajala, K., & Miettinen, S. (2019). Safety, efficacy, and regulation of mesenchymal stromal/stem cells. In Tissue Engineering in Oral and Maxillofacial Surgery. [CrossRef]
- Peshkova, M., Korneev, A., Suleimanov, S., Vlasova, I. I., Svistunov, A., Kosheleva, N., & Timashev, P. (2023). MSCs’ conditioned media cytokine and growth factor profiles and their impact on macrophage polarization. Stem Cell Research and Therapy, 14(1). [CrossRef]
- Planat-Benard, V., Varin, A., & Casteilla, L. (2021). MSCs and Inflammatory Cells Crosstalk in Regenerative Medicine: Concerted Actions for Optimized Resolution Driven by Energy Metabolism. In Frontiers in Immunology (Vol. 12). [CrossRef]
- Pontikoglou, C., Deschaseaux, F., Sensebé, L., & Papadaki, H. A. (2011). Bone Marrow Mesenchymal Stem Cells: Biological Properties and Their Role in Hematopoiesis and Hematopoietic Stem Cell Transplantation. In Stem Cell Reviews and Reports (Vol. 7, Issue 3). [CrossRef]
- Popkin, B. M., D’Anci, K. E., & Rosenberg, I. H. (2010). Water, hydration, and health. In Nutrition Reviews (Vol. 68, Issue 8). [CrossRef]
- Prakash, N., Kim, J., Jeon, J., Kim, S., Arai, Y., Bello, A. B., Park, H., & Lee, S. H. (2023). Progress and emerging techniques for biomaterial-based derivation of mesenchymal stem cells (MSCs) from pluripotent stem cells (PSCs). In Biomaterials Research (Vol. 27, Issue 1). [CrossRef]
- Przekora, A.(2020). A concise review on tissue engineered artificial skin grafts for chronic wound treatment: Can we reconstruct functional skin tissue in vitro? Cells, 9(7). [CrossRef]
- Raziyeva, K., Kim, Y., Zharkinbekov, Z., Kassymbek, K., Jimi, S., & Saparov, A. (2021). Immunology of acute and chronic wound healing. In Biomolecules (Vol. 11, Issue 5). [CrossRef]
- Riccobono, D., Agay, D., Scherthan, H., Forcheron, F., Vivier, M., Ballester, B., Meineke, V., & Drouet, M. (2012). Application of adipocyte-derived stem cells in treatment of cutaneous radiation syndrome. Health Physics, 103(2), 120–126. [CrossRef]
- Rocha, J. L. M., de Oliveira, W. C. F., Noronha, N. C., dos Santos, N. C. D., Covas, D. T., Picanço-Castro, V., Swiech, K., & Malmegrim, K. C. R. (2021). Mesenchymal Stromal Cells in Viral Infections: Implications for COVID-19. In Stem Cell Reviews and Reports (Vol. 17, Issue 1). [CrossRef]
- Røsland, G. V., Svendsen, A., Torsvik, A., Sobala, E., McCormack, E., Immervoll, H., Mysliwietz, J., Tonn, J. C., Goldbrunner, R., Lønning, P. E., Bjerkvig, R., & Schichor, C. (2009). Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Research, 69(13). [CrossRef]
- Saadh, M. J., Ramírez-Coronel, A. A., Saini, R. S., Arias-Gonzáles, J. L., Amin, A. H., Gavilán, J. C. O., & Sârbu, I. (2023). Advances in mesenchymal stem/stromal cell-based therapy and their extracellular vesicles for skin wound healing. In Human Cell (Vol. 36, Issue 4). [CrossRef]
- Saheli, M., Bayat, M., Ganji, R., Hendudari, F., Kheirjou, R., Pakzad, M., Najar, B., & Piryaei, A. (2020). Human mesenchymal stem cells-conditioned medium improves diabetic wound healing mainly through modulating fibroblast behaviors. Archives of Dermatological Research, 312(5), 325–336. [CrossRef]
- Salleh, F., Amid, A., & Nordin, N. F. H. (2022). In Vitro Study on Collagen Application in Wound Healing: A Systematic Review. IIUM Medical Journal Malaysia, 21(4). [CrossRef]
- Sandonà, M., Di Pietro, L., Esposito, F., Ventura, A., Silini, A. R., Parolini, O., & Saccone, V. (2021). Mesenchymal Stromal Cells and Their Secretome: New Therapeutic Perspectives for Skeletal Muscle Regeneration. In Frontiers in Bioengineering and Biotechnology (Vol. 9). [CrossRef]
- Sen, C. K.(2023). Human Wound and Its Burden: Updated 2022 Compendium of Estimates. In Advances in Wound Care (Vol. 12, Issue 12, pp. 657–670). Mary Ann Liebert Inc. [CrossRef]
- Shakoori, A. R.(2017). Fluorescence In Situ hybridization (FISH) and its applications. In Chromosome Structure and Aberrations. [CrossRef]
- Shen, R., Lu, Y., Cai, C., Wang, Z., Zhao, J., Wu, Y., Zhang, Y., & Yang, Y. (2024). Research progress and prospects of benefit-risk assessment methods for umbilical cord mesenchymal stem cell transplantation in the clinical treatment of spinal cord injury. In Stem Cell Research and Therapy (Vol. 15, Issue 1). BioMed Central Ltd. [CrossRef]
- Sherman, A. B., Gilger, B. C., Berglund, A. K., & Schnabel, L. V. (2017). Effect of bone marrow-derived mesenchymal stem cells and stem cell supernatant on equine corneal wound healing in vitro. Stem Cell Research and Therapy, 8(1), 1–10. [CrossRef]
- Shohara, R., Yamamoto, A., Takikawa, S., Iwase, A., Hibi, H., Kikkawa, F., & Ueda, M. (2012). Mesenchymal stromal cells of human umbilical cord Wharton’s jelly accelerate wound healing by paracrine mechanisms. Cytotherapy, 14(10), 1171–1181. [CrossRef]
- Shrestha, C., Zhao, L., Chen, K., He, H., & Mo, Z. (2013). Enhanced healing of diabetic wounds by subcutaneous administration of human umbilical cord derived stem cells and their conditioned media. International Journal of Endocrinology, 2013. [CrossRef]
- Son, B., Kim, M., Won, H., Jung, A., Kim, J., Koo, Y., Lee, N. K., Baek, S. H., Han, U., Park, C. G., Shin, H., Gweon, B., Joo, J., & Park, H. H. (2023). Secured delivery of basic fibroblast growth factor using human serum albumin-based protein nanoparticles for enhanced wound healing and regeneration. Journal of Nanobiotechnology, 21(1). [CrossRef]
- Spees, J. L., Lee, R. H., & Gregory, C. A. (2016). Mechanisms of mesenchymal stem/stromal cell function. In Stem Cell Research and Therapy (Vol. 7, Issue 1). [CrossRef]
- Stone, W. L., & Bhimji, S. S. (2018). Physiology, growth factor. StatPearls [Internet].
- Taechangam, N., Kol, A., Arzi, B., & Borjesson, D. L. (2022). Multipotent Stromal Cells and Viral Interaction: Current Implications for Therapy. In Stem Cell Reviews and Reports (Vol. 18, Issue 1). [CrossRef]
- Tamari, M., Nishino, Y., Yamamoto, N., & Ueda, M. (2013). Acceleration of Wound Healing with Stem Cell–Derived Growth Factors. The International Journal of Oral & Maxillofacial Implants, 28(6), e369–e375. [CrossRef]
- Tian, J., Li, X. J., Ma, Y., Mai, Z., Yang, Y., Luo, M., Xu, W., Chen, K., Chen, X., Tang, J., Cheng, B., & Cui, X. (2023). Correlation of bioactive components of platelet rich plasma derived from human female adult peripheral blood and umbilical cord blood with age. Scientific Reports, 13(1). [CrossRef]
- Toya, M., Zhang, N., Tsubosaka, M., Kushioka, J., Gao, Q., Li, X., Chow, S. K. H., & Goodman, S. B. (2023). CCL2 promotes osteogenesis by facilitating macrophage migration during acute inflammation. Frontiers in Cell and Developmental Biology, 11. [CrossRef]
- Ullah, M. M., Collett, J. A., Monroe, J. C., Traktuev, D., Coleman, M., March, K. L., & Basile, D. P. (2024). Subcutaneous injection of adipose stromal cell-secretome improves renal function and reduces inflammation in established acute kidney injury. Stem Cell Research and Therapy, 15(1). [CrossRef]
- Vasalou, V., Kotidis, E., Tatsis, D., Boulogeorgou, K., Grivas, I., Koliakos, G., Cheva, A., Ioannidis, O., Tsingotjidou, A., & Angelopoulos, S. (2023). The Effects of Tissue Healing Factors in Wound Repair Involving Absorbable Meshes: A Narrative Review. In Journal of Clinical Medicine (Vol. 12, Issue 17). [CrossRef]
- Walter, M. N. M., Wright, K. T., Fuller, H. R., MacNeil, S., & Johnson, W. E. B. (2010). Mesenchymal stem cell-conditioned medium accelerates skin wound healing: An in vitro study of fibroblast and keratinocyte scratch assays. Experimental Cell Research, 316(7), 1271–1281. [CrossRef]
- Wan, J., Xia, L., Liang, W., Liu, Y., & Cai, Q. (2013). Transplantation of bone marrow-derived mesenchymal stem cells promotes delayed wound healing in diabetic rats. Journal of Diabetes Research, 2013. [CrossRef]
- Wang, S. H., Shyu, V. B. H., Chiu, W. K., Huang, R. W., Lai, B. R., & Tsai, C. H. (2023). An Overview of Clinical Examinations in the Evaluation and Assessment of Arterial and Venous Insufficiency Wounds. In Diagnostics (Vol. 13, Issue 15). [CrossRef]
- Wang, X., Li, J., Lu, W., Gao, F., Zhang, S., & Li, J. (2024). Therapeutic roles of platelet-rich plasma to restore female reproductive and endocrine dysfunction. In Frontiers in Endocrinology (Vol. 15). Frontiers Media SA. [CrossRef]
- Wang, Y., Zhu, J., Chen, J., Xu, R., Groth, T., Wan, H., & Zhou, G. (2022). The Signaling Pathways Induced by Exosomes in Promoting Diabetic Wound Healing: A Mini-Review. In Current issues in molecular biology (Vol. 44, Issue 10, pp. 4960–4976). NLM (Medline). [CrossRef]
- Wei, Q., Liu, M., Li, S., Shi, S., Du, F., Peng, H., Zeng, D., Deng, Q., Pan, S., Zhang, J., & Yu, S. (2024). The composite biomatrix SC/CM improved the therapeutic effects of xenogeneic MSC on wound healing in immune-competent mice via immune niche reprogramming. Journal of Materials Science. [CrossRef]
- Werner, H.(2023). The IGF1 Signaling Pathway: From Basic Concepts to Therapeutic Opportunities. In International Journal of Molecular Sciences (Vol. 24, Issue 19). [CrossRef]
- Willer, H., Spohn, G., Morgenroth, K., Thielemann, C., Elvers-Hornung, S., Bugert, P., Delorme, B., Giesen, M., Schmitz-Rixen, T., Seifried, E., Pfarrer, C., Schäfer, R., & Bieback, K. (2022). Pooled human bone marrow-derived mesenchymal stromal cells with defined trophic factors cargo promote dermal wound healing in diabetic rats by improved vascularization and dynamic recruitment of M2-like macrophages. Frontiers in Immunology, 13. [CrossRef]
- Wiredu Ocansey, D. K., Pei, B., Yan, Y., Qian, H., Zhang, X., Xu, W., & Mao, F. (2020). Improved therapeutics of modified mesenchymal stem cells: An update. In Journal of Translational Medicine (Vol. 18, Issue 1). [CrossRef]
- Wu, L., Lu, J., Lan, T., Zhang, D., Xu, H., Kang, Z., Peng, F., & Wang, J. (2024). Stem cell therapies: a new era in the treatment of multiple sclerosis. In Frontiers in Neurology (Vol. 15). Frontiers Media SA. [CrossRef]
- Wu, Y., Chen, L., Scott, P. G., & Tredget, E. E. (2007). Mesenchymal Stem Cells Enhance Wound Healing Through Differentiation and Angiogenesis. Stem Cells, 25(10), 2648–2659. [CrossRef]
- Xia, T., Fu, S., Yang, R., Yang, K., Lei, W., Yang, Y., Zhang, Q., Zhao, Y., Yu, J., Yu, L., & Zhang, T. (2023). Advances in the study of macrophage polarization in inflammatory immune skin diseases. In Journal of Inflammation (United Kingdom) (Vol. 20, Issue 1). [CrossRef]
- Xiao, T., Yan, Z., Xiao, S., & Xia, Y. (2020). Proinflammatory cytokines regulate epidermal stem cells in wound epithelialization. In Stem Cell Research and Therapy (Vol. 11, Issue 1). [CrossRef]
- Xie, Q., Liu, R., Jiang, J., Peng, J., Yang, C., Zhang, W., Wang, S., & Song, J. (2020). What is the impact of human umbilical cord mesenchymal stem cell transplantation on clinical treatment? In Stem Cell Research and Therapy (Vol. 11, Issue 1). [CrossRef]
- Xuan, L., Hou, Y., Liang, L., Wu, J., Fan, K., Lian, L., Qiu, J., Miao, Y., Ravanbakhsh, H., Xu, M., & Tang, G. (2024). Microgels for Cell Delivery in Tissue Engineering and Regenerative Medicine. In Nano-Micro Letters (Vol. 16, Issue 1). Springer Science and Business Media B.V. [CrossRef]
- Xuan, X., Tian, C., Zhao, M., Sun, Y., & Huang, C. (2021). Mesenchymal stem cells in cancer progression and anticancer therapeutic resistance. In Cancer Cell International (Vol. 21, Issue 1). [CrossRef]
- Yamakawa, S., & Hayashida, K. (2019). Advances in surgical applications of growth factors for wound healing. In Burns and Trauma (Vol. 7). [CrossRef]
- Yi, X., Chen, F., Liu, F., Peng, Q., Li, Y., Li, S., Du, J., Gao, Y., & Wang, Y. (2020). Comparative separation methods and biological characteristics of human placental and umbilical cord mesenchymal stem cells in serum-free culture conditions. Stem Cell Research and Therapy, 11(1). [CrossRef]
- Yue, G., Li, Y., Liu, Z., Yu, S., Cao, Y., & Wang, X. (2024). Efficacy of MSC-derived small extracellular vesicles in treating type II diabetic cutaneous wounds: a systematic review and meta-analysis of animal models. Frontiers in Endocrinology, 15. [CrossRef]
- Zafar, A.Bin, & Hinchliffe, R. J. (2023). Macrovascular complications: Peripheral artery disease. In BIDE’s Diabetes Desk Book: For Healthcare Professionals. [CrossRef]
- Zhang, X., Hu, F., Li, J., Chen, L., Mao, Y. fei, Li, Q. bo, Nie, C. yao, Lin, C., & Xiao, J. (2024). IGF-1 inhibits inflammation and accelerates angiogenesis via Ras/PI3K/IKK/NF-κB signaling pathways to promote wound healing. European Journal of Pharmaceutical Sciences, 200. [CrossRef]
- Zhang, Y., Ideguchi, H., Aoyagi, H., Yamashiro, K., Yamamoto, T., Nishibori, M., & Takashiba, S. (2021). Malnutrition delayed wound healing after tooth extraction by HMGB1-related prolonged inflammation. International Immunopharmacology, 96. [CrossRef]
- Zhang, Y., Manouchehri Doulabi, E., Herre, M., Cedervall, J., Qiao, Q., Miao, Z., Hamidi, A., Hellman, L., Kamali-Moghaddam, M., & Olsson, A. K. (2022). Platelet-Derived PDGFB Promotes Recruitment of Cancer-Associated Fibroblasts, Deposition of Extracellular Matrix and Tgfβ Signaling in the Tumor Microenvironment. Cancers, 14(8). [CrossRef]
- Zhao, N., Yu, X., Zhu, X., Song, Y., Gao, F., Yu, B., & Qu, A. (2024). Diabetes Mellitus to Accelerated Atherosclerosis: Shared Cellular and Molecular Mechanisms in Glucose and Lipid Metabolism. In Journal of Cardiovascular Translational Research (Vol. 17, Issue 1). [CrossRef]
- Zhao, Q. S., Xia, N., Zhao, N., Li, M., Bi, C. L., Zhu, Q., Qiao, G. F., & Cheng, Z. F. (2013). Localization of human mesenchymal stem cells from umbilical cord blood and their role in repair of diabetic foot ulcers in rats. International Journal of Biological Sciences, 10(1), 80–89. [CrossRef]
- Zhao, Y., Wang, M., Liang, F., & Li, J. (2021). Recent strategies for enhancing the therapeutic efficacy of stem cells in wound healing. In Stem Cell Research and Therapy (Vol. 12, Issue 1). [CrossRef]
- Zhidu, S., Ying, T., Rui, J., & Chao, Z. (2024a). Translational potential of mesenchymal stem cells in regenerative therapies for human diseases: challenges and opportunities. In Stem Cell Research and Therapy (Vol. 15, Issue 1). BioMed Central Ltd. [CrossRef]
- Zhidu, S., Ying, T., Rui, J., & Chao, Z. (2024b). Translational potential of mesenchymal stem cells in regenerative therapies for human diseases: challenges and opportunities. In Stem Cell Research and Therapy (Vol. 15, Issue 1). BioMed Central Ltd. [CrossRef]
- Zhidu, S., Ying, T., Rui, J., & Chao, Z. (2024c). Translational potential of mesenchymal stem cells in regenerative therapies for human diseases: challenges and opportunities. In Stem Cell Research and Therapy (Vol. 15, Issue 1). BioMed Central Ltd. [CrossRef]
- Zhou, C., Jiao, L., Qiao, X., Zhang, W., Chen, S., Yang, C., & Meng, M. (2024). Combined treatment of umbilical cord Wharton’s jelly-derived mesenchymal stem cells and platelet-rich plasma for a surgical patient with hospital-acquired pressure ulcer: a case report and literature review. Frontiers in Bioengineering and Biotechnology, 12. [CrossRef]
- Zhou, T., Yuan, Z., Weng, J., Pei, D., Du, X., He, C., & Lai, P. (2021). Challenges and advances in clinical applications of mesenchymal stromal cells. In Journal of Hematology and Oncology (Vol. 14, Issue 1). [CrossRef]
- Zhuang, W. Z., Lin, Y. H., Su, L. J., Wu, M. S., Jeng, H. Y., Chang, H. C., Huang, Y. H., & Ling, T. Y. (2021). Mesenchymal stem/stromal cell-based therapy: mechanism, systemic safety and biodistribution for precision clinical applications. In Journal of Biomedical Science (Vol. 28, Issue 1). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).