Submitted:
16 September 2024
Posted:
17 September 2024
You are already at the latest version
Abstract
Keywords:
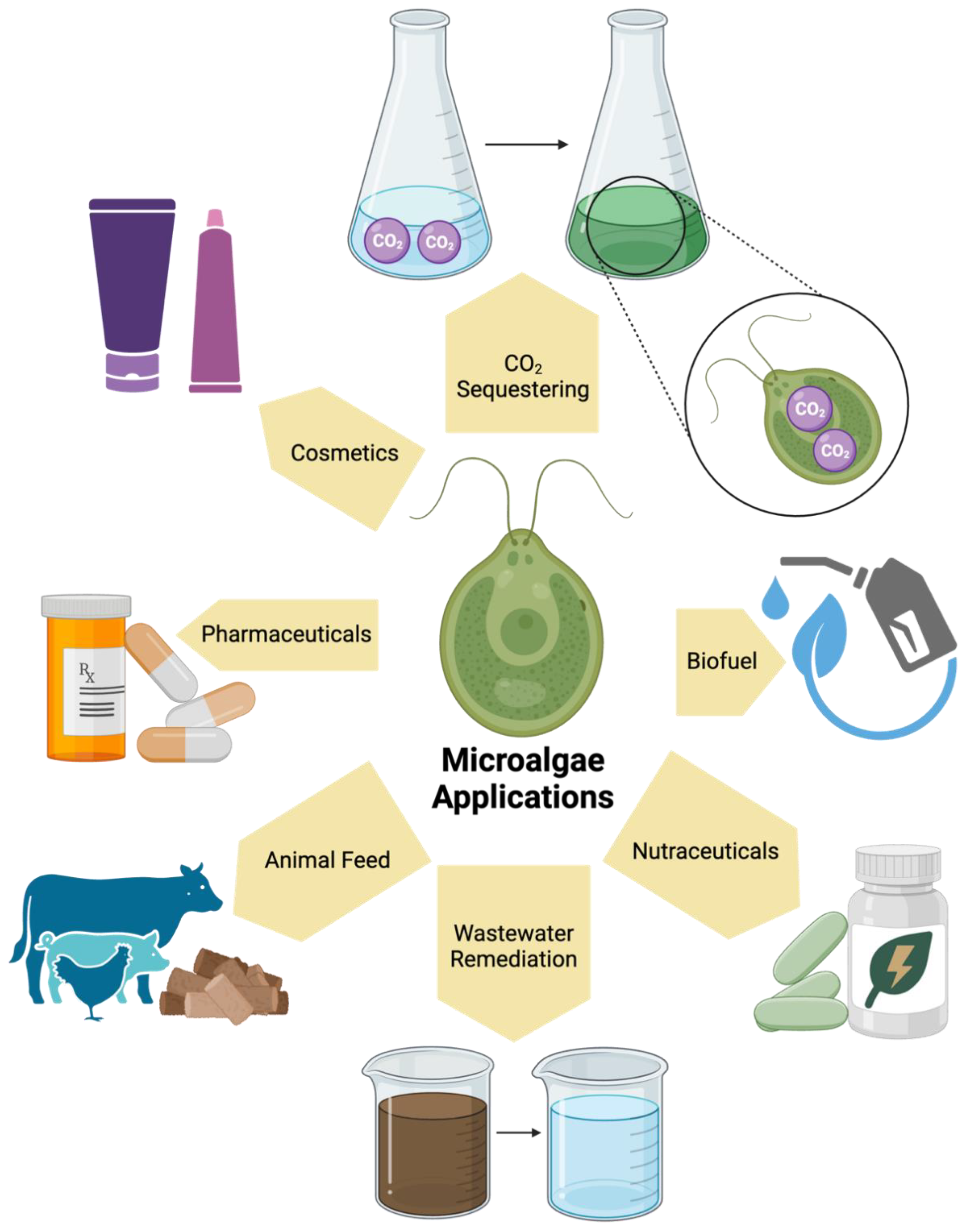
1. Introduction
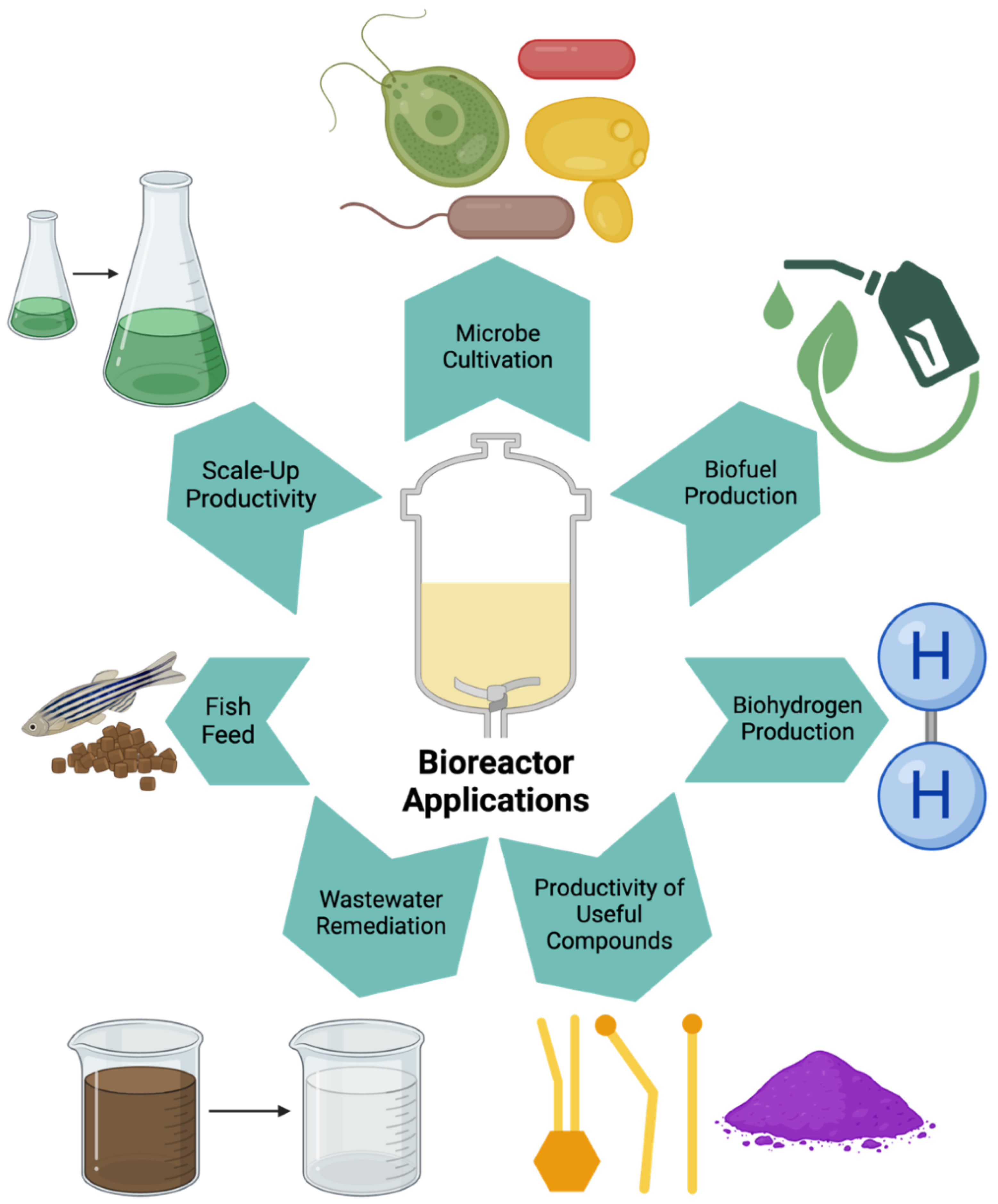
2. Types and Uses of Bioreactors
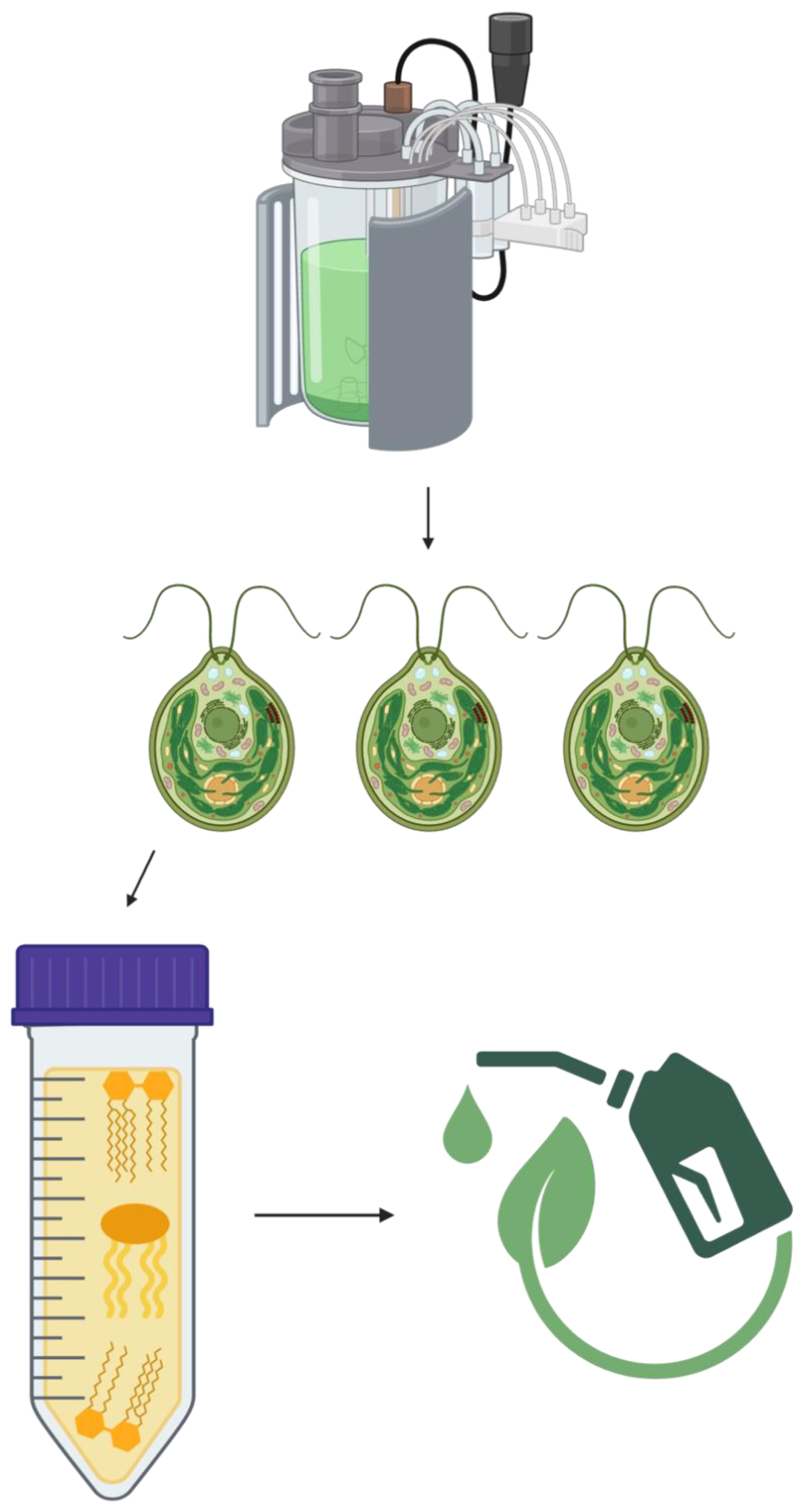
3. Photobioreactors for Energy
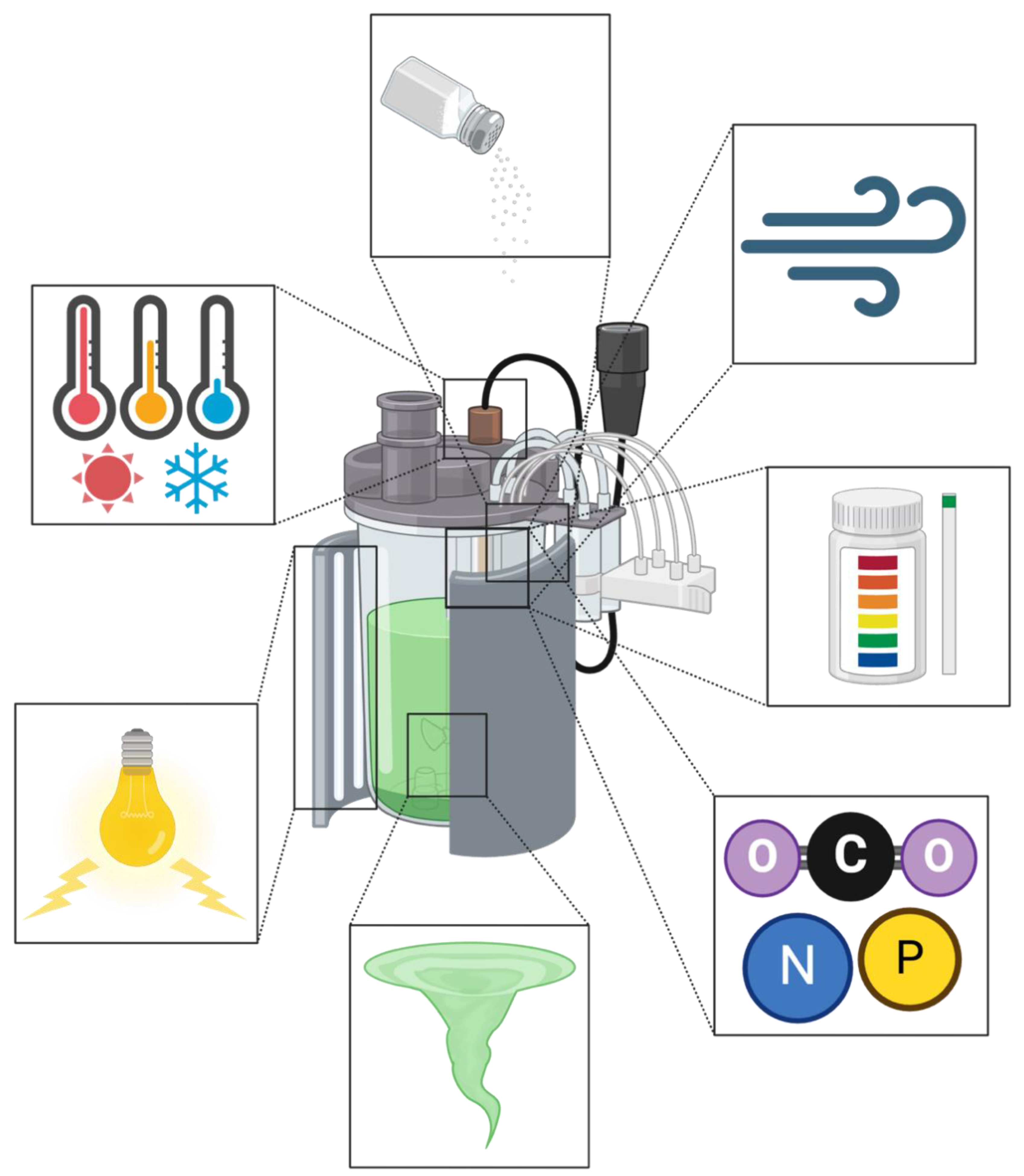
4. Incubation Factors for Photobioreactors
5. Challenges and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwon, M. H., & Yeom, S. H. (2017). Evaluation of closed photobioreactor types and operation variables for enhancing lipid productivity of Nannochloropsis sp. KMMCC 290 for biodiesel production. Biotechnology and Bioprocess Engineering, 22(5), 604–611. [CrossRef]
- Regueiras, A., Huguet, Á., Conde, T., Couto, D., Domingues, P., Domingues, M. R., Costa, A. M., Silva, J. L. da, Vasconcelos, V., & Urbatzka, R. (2022). Potential Anti-Obesity, Anti-Steatosis, and Anti-Inflammatory Properties of Extracts from the Microalgae Chlorella vulgaris and Chlorococcum amblystomatis under Different Growth Conditions. Marine Drugs, 20(1), 9. [CrossRef]
- Arunkumar, K., Nalluri, M., Anjana, K., Mohan, G., & Raja, R. (2023). Fucoxanthin as antioxidant, anti-hyaluronidase and cytotoxic agent: Potential of brown seaweeds decoction for tea supplement. Journal of Food Measurement and Characterization, 17(4), 3980–3989. [CrossRef]
- Truong, T. Q., Park, Y. J., Winarto, J., Huynh, P. K., Moon, J., Choi, Y. B., Song, D.-G., Koo, S. Y., & Kim, S. M. (2024). Understanding the Impact of Nitrogen Availability: A Limiting Factor for Enhancing Fucoxanthin Productivity in Microalgae Cultivation. Marine Drugs, 22(2), 93. [CrossRef]
- Arashiro, L. T., Josa, I., Ferrer, I., Van Hulle, S. W. H., Rousseau, D. P. L., & Garfí, M. (2022). Life cycle assessment of microalgae systems for wastewater treatment and bioproducts recovery: Natural pigments, biofertilizer and biogas. Science of The Total Environment, 847, 157615. [CrossRef]
- Abraham, J., Prigiobbe, V., Abimbola, T., & Christodoulatos, C. (2023). Integrating biological and chemical CO2 sequestration using green microalgae for bioproducts generation. Frontiers in Climate, 4. [CrossRef]
- Xin, Y., Shen, C., She, Y., Chen, H., Wang, C., Wei, L., Yoon, K., Han, D., Hu, Q., & Xu, J. (2019). Biosynthesis of Triacylglycerol Molecules with a Tailored PUFA Profile in Industrial Microalgae. Molecular Plant, 12(4), 474–488. [CrossRef]
- Matos, J., Cardoso, C. L., Falé, P., Afonso, C. M., & Bandarra, N. M. (2020). Investigation of nutraceutical potential of the microalgae Chlorella vulgaris and Arthrospira platensis. International Journal of Food Science & Technology, 55(1), 303–312. [CrossRef]
- Shimidzu, N., Goto, M., & Miki, W. (1996). Carotenoids as Singlet Oxygen Quenchers in Marine Organisms. Fisheries Science, 62(1), 134–137. [CrossRef]
- Ahmed, F., Fanning, K., Netzel, M., Turner, W., Li, Y., & Schenk, P. M. (2014). Profiling of carotenoids and antioxidant capacity of microalgae from subtropical coastal and brackish waters. Food Chemistry, 165, 300–306. [CrossRef]
- Zhang, Z., Wang, B., Hu, Q., Sommerfeld, M., Li, Y., & Han, D. (2016). A New Paradigm for Producing Astaxanthin From the Unicellular Green Alga Haematococcus pluvialis. Biotechnology and Bioengineering, 113(10), 2088–2099. [CrossRef]
- Chaudhary, A., Mishra, P., Amaz, S. A., Mahato, P. L., Das, R., Jha, R., & Mishra, B. (2023). Dietary supplementation of microalgae mitigates the negative effects of heat stress in broilers. Poultry Science, 102(10), 102958. [CrossRef]
- Sforza, E., Bertucco, A., Morosinotto, T., & Giacometti, G. M. (2012). Photobioreactors for microalgal growth and oil production with Nannochloropsis salina: From lab-scale experiments to large-scale design. Chemical Engineering Research and Design, 90(9), 1151–1158. [CrossRef]
- Manhaeghe, D., Blomme, T., Van Hulle, S. W. H., & Rousseau, D. P. L. (2020). Experimental assessment and mathematical modelling of the growth of Chlorella vulgaris under photoautotrophic, heterotrophic and mixotrophic conditions. Water Research, 184, 116152. [CrossRef]
- Al Jabri, H., Taleb, A., Touchard, R., Saadaoui, I., Goetz, V., & Pruvost, J. (2021). Cultivating Microalgae in Desert Conditions: Evaluation of the Effect of Light-Temperature Summer Conditions on the Growth and Metabolism of Nannochloropsis QU130. Applied Sciences, 11(9), 3799. [CrossRef]
- Romagnoli, F., Weerasuriya-Arachchige, A. R. P. P., Paoli, R., Feofilovs, M., & Ievina, B. (2021). Growth Kinetic Model for Microalgae Cultivation in Open Raceway Ponds: A System Dynamics Tool. Environmental and Climate Technologies, 25(1), 1317–1336. [CrossRef]
- Bartley, M. L., Boeing, W. J., Dungan, B. N., Holguin, F. O., & Schaub, T. (2014). pH effects on growth and lipid accumulation of the biofuel microalgae Nannochloropsis salina and invading organisms. Journal of Applied Phycology, 26, 1431–1437. [CrossRef]
- Qiu, R., Gao, S., Lopez, P. A., & Ogden, K. L. (2017). Effects of pH on cell growth, lipid production and CO2 addition of microalgae Chlorella sorokiniana. Algal Research, 28, 192–199. [CrossRef]
- Patel, A., Matsakas, L., Hrůzová, K., Rova, U., & Christakopoulos, P. (2019). Biosynthesis of Nutraceutical Fatty Acids by the Oleaginous Marine Microalgae Phaeodactylum tricornutum Utilizing Hydrolysates from Organosolv-Pretreated Birch and Spruce Biomass. Marine Drugs, 17(2), 119. [CrossRef]
- Aussant, J., Guihéneuf, F., & Stengel, D. B. (2018). Impact of temperature on fatty acid composition and nutritional value in eight species of microalgae. Applied Microbiology & Biotechnology, 102(12), 5279–5297. [CrossRef]
- Perin, G. Perin, G., Cimetta, E., Monetti, F., Morosinotto, T., & Bezzo, F. (2016). Novel micro-photobioreactor design and monitoring method for assessing microalgae response to light intensity. Algal Research, 19, 69–76. [CrossRef]
- Nzayisenga, J. C., Farge, X., Groll, S. L., & Sellstedt, A. (2020). Effects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnology for Biofuels, 13(1), 4. [CrossRef]
- Fawzy, M. A., El-Otify, A. M., Adam, M. S., & Moustafa, S. S. A. (2021). The impact of abiotic factors on the growth and lipid accumulation of some green microalgae for sustainable biodiesel production. Environmental Science and Pollution Research, 28(31), 42547–42561. [CrossRef]
- Gao, B., Hong, J., Chen, J., Zhang, H., Hu, R., & Zhang, C. (2023). The growth, lipid accumulation and adaptation mechanism in response to variation of temperature and nitrogen supply in psychrotrophic filamentous microalga Xanthonema hormidioides (Xanthophyceae). Biotechnology for Biofuels and Bioproducts, 16(1), 12. [CrossRef]
- Josephine, A., Kumar, T. S., Surendran, B., Rajakumar, S., Kirubagaran, R., & Dharani, G. (2022). Evaluating the effect of various environmental factors on the growth of the marine microalgae, Chlorella vulgaris. Frontiers in Marine Science, 9. [CrossRef]
- Narala, R. R., Garg, S., Sharma, K. K., Thomas-Hall, S. R., Deme, M., Li, Y., & Schenk, P. M. (2016). Comparison of Microalgae Cultivation in Photobioreactor, Open Raceway Pond, and a Two-Stage Hybrid System. Frontiers in Energy Research, 4. [CrossRef]
- Prado, L. O., Bolzani, H. R., Souza, H. H. S., Ruas, G., & Silva, G. H. R. (2023). Microalgal cultivation in open and closed systems under a tropical climate: A life cycle comparison. Journal of Cleaner Production, 422, 138631. [CrossRef]
- Naumann, T., Çebi, Z., Podola, B., & Melkonian, M. (2013). Growing microalgae as aquaculture feeds on twin-layers: A novel solid-state photobioreactor. Journal of Applied Phycology, 25(5), 1413–1420. [CrossRef]
- Magalhães, I. B., Ferreira, J., Castro, J. de S., Assis, L. R. de, & Calijuri, M. L. (2022). Agro-industrial wastewater-grown microalgae: A techno-environmental assessment of open and closed systems. Science of The Total Environment, 834, 155282. [CrossRef]
- Fernandes, B. D., Mota, A., Ferreira, A., Dragone, G., Teixeira, J. A., & Vicente, A. A. (2014). Characterization of split cylinder air-lift photobioreactors for efficient microalgae cultivation. Chemical Engineering Science, 117, 445–454. [CrossRef]
- Chen, C.-Y., Chang, Y.-H., & Chang, H.-Y. (2016). Outdoor cultivation of Chlorella vulgaris FSP-E in vertical tubular-type photobioreactors for microalgal protein production. Algal Research, 13, 264–270. [CrossRef]
- del Rio-Chanona, E. A., Liu, J., Wagner, J. L., Zhang, D., Meng, Y., Xue, S., & Shah, N. (2018). Dynamic modeling of green algae cultivation in a photobioreactor for sustainable biodiesel production. Biotechnology and Bioengineering, 115(2), 359–370. [CrossRef]
- Barros, A., Pereira, H., Campos, J., Marques, A., Varela, J., & Silva, J. (2019). Heterotrophy as a tool to overcome the long and costly autotrophic scale-up process for large scale production of microalgae. Scientific Reports, 9, 13935. [CrossRef]
- Ratomski, P., Hawrot-Paw, M., & Koniuszy, A. (2021). Utilisation of CO2 from Sodium Bicarbonate to Produce Chlorella vulgaris Biomass in Tubular Photobioreactors for Biofuel Purposes. Sustainability, 13(16), 9118. [CrossRef]
- Díaz, J. P., Inostroza, C., & Acién, F. G. (2021). Scale-up of a Fibonacci-Type Photobioreactor for the Production of Dunaliella salina. Applied Biochemistry and Biotechnology, 193(1), 188–204. [CrossRef]
- Oncel, S., & Kose, A. (2014). Comparison of tubular and panel type photobioreactors for biohydrogen production utilizing Chlamydomonas reinhardtii considering mixing time and light intensity. Bioresource Technology, 151, 265–270. [CrossRef]
- Novoveská, L., Zapata, A. K. M., Zabolotney, J. B., Atwood, M. C., & Sundstrom, E. R. (2016). Optimizing microalgae cultivation and wastewater treatment in large-scale offshore photobioreactors. Algal Research, 18, 86–94. [CrossRef]
- Fang, F., Xu, R.-Z., Huang, Y.-Q., Wang, S.-N., Zhang, L.-L., Dong, J.-Y., Xie, W.-M., Chen, X., & Cao, J.-S. (2019). Production of polyhydroxyalkanoates and enrichment of associated microbes in bioreactors fed with rice winery wastewater at various organic loading rates. Bioresource Technology, 292, 121978. [CrossRef]
- Pereira, H., Páramo, J., Silva, J., Marques, A., Barros, A., Maurício, D., Santos, T., Schulze, P., Barros, R., Gouveia, L., Barreira, L., & Varela, J. (2018). Scale-up and large-scale production of Tetraselmis sp. CTP4 (Chlorophyta) for CO2 mitigation: From an agar plate to 100-m3 industrial photobioreactors. Scientific Reports, 8, 5112. [CrossRef]
- Schädler, T., Thurn, A.-L., Brück, T., & Weuster-Botz, D. (2021). Continuous Production of Lipids with Microchloropsis salina in Open Thin-Layer Cascade Photobioreactors on a Pilot Scale. Energies, 14(2), 500. [CrossRef]
- Liu, H., Tian, Y., Zhou, Y., Kan, Y., Wu, T., Xiao, W., & Luo, Y. (2021). Multi-modular engineering of Saccharomyces cerevisiae for high-titre production of tyrosol and salidroside. Microbial Biotechnology, 14(6), 2605–2616. [CrossRef]
- Ali, H., Solsvik, J., Wagner, J. L., Zhang, D., Hellgardt, K., & Park, C. W. (2019). CFD and kinetic-based modeling to optimize the sparger design of a large-scale photobioreactor for scaling up of biofuel production. Biotechnology and Bioengineering, 116(9), 2200–2211. [CrossRef]
- Bani, A., Fernandez, F. G. A., D’Imporzano, G., Parati, K., & Adani, F. (2021). Influence of photobioreactor set-up on the survival of microalgae inoculum. Bioresource Technology, 320, 124408. [CrossRef]
- Cimini, D., Iacono, I. D., Carlino, E., Finamore, R., Restaino, O. F., Diana, P., Bedini, E., & Schiraldi, C. (2017). Engineering S. equi subsp. Zooepidemicus towards concurrent production of hyaluronic acid and chondroitin biopolymers of biomedical interest. AMB Express, 7, 61. [CrossRef]
- Bianconi, M., Ceriotti, L., Cuzzocrea, S., Esposito, E., Pressi, G., Sgaravatti, E., Bertaiola, O., Guarnerio, C., Barbieri, E., Semenzato, A., Negri, S., Commisso, M., Avesani, L., & Guzzo, F. (2020). Red Carrot Cells Cultured in vitro Are Effective, Stable, and Safe Ingredients for Skin Care, Nutraceutical, and Food Applications. Frontiers in Bioengineering and Biotechnology, 8, 575079. [CrossRef]
- Patel, A., Rova, U., Christakopoulos, P., & Matsakas, L. (2019). Simultaneous production of DHA and squalene from Aurantiochytrium sp. grown on forest biomass hydrolysates. Biotechnology for Biofuels, 12(1), 255. [CrossRef]
- Abdel-Baset, A., Matter, I. A., & Ali, M. A. (2024). Enhanced Scenedesmus obliquus Cultivation in Plastic-Type Flat Panel Photobioreactor for Biodiesel Production. Sustainability, 16, 3148. [CrossRef]
- Mousavi, S., Najafpour, G. D., & Mohammadi, M. (2018). CO2 bio-fixation and biofuel production in an air-lift photobioreactor by an isolated strain of microalgae Coelastrum sp. SM under high CO2 concentrations. Environmental Science and Pollution Research, 25(30), 30139–30150. [CrossRef]
- Leong, Y. K., Show, P. L., Lin, H. C., Chang, C. K., Loh, H.-S., Lan, J. C.-W., & Ling, T. C. (2016). Preliminary integrated economic and environmental analysis of polyhydroxyalkanoates (PHAs) biosynthesis. Bioresources and Bioprocessing, 3(1), 41. [CrossRef]
- Swanson, D., Block, R., & Mousa, S. A. (2012). Omega-3 Fatty Acids EPA and DHA: Health Benefits Throughout Life. Advances in Nutrition, 3(1), 1-7. [CrossRef]
- Bernasconi, A. A., Wiest, M. M., Lavie, C. J., Milani, R. V., & Laukkanen, J. A. (2021). Effect of Omega-3 Dosage on Cardiovascular Outcomes: An Updated Meta-Analysis and Meta-Regression of Interventional Trials. Mayo Clinic Proceedings, 96(2), 304-313. [CrossRef]
- Gupta, P. L., Lee, S.-M., & Choi H.-J. (2015). A mini review: photobioreactors for large scale algal cultivation. World Journal of Microbiology and Biotechnology, 31(9), 1409-1417. [CrossRef]
- Li, M.-J., Tong, Z.-X., Zhou, Z.-J., Huang, D., & Wang, R.-L. (2019). A numerical model coupling bubble flow, light transfer, cell motion and growth kinetics for real timescale microalgae cultivation and its applications in flat plate photobioreactors. Algal Research, 44, 101727. [CrossRef]
- Chaisutyakorn, P., Praiboon, J., & Kaewsuralikhit, C. (2018). The effect of temperature on growth and lipid and fatty acid composition on marine microalgae used for biodiesel production. Journal of Applied Phycology, 30(1), 37–45. [CrossRef]
- Feng, P., Xu, Z., Qin, L., Alam, M. A., Wang, Z., & Zhu, S. (2020). Effects of different nitrogen sources and light paths of flat plate photobioreactors on the growth and lipid accumulation of Chlorella sp. GN1 outdoors. Bioresource Technology, 301, 122762. [CrossRef]
- Putri, D. S., Sari, D. A., Marianah, Astuti, S. P., & Wangiyana, I. G. A. S. (2021). Effect of medium type, light intensity, and photoperiod on the growth rate of microalgae Chlorococcum sp. local isolate. IOP Conference Series: Earth and Environmental Science, 913, 012071. [CrossRef]
- Guzmán-Palomino, A., Aguilera-Vázquez, L., Hernández-Escoto, H., García-Vite, P. M., & Martínez-Salazar, A. L. (2023). Dynamical Simulation, Sensitivity, and Productivity Analysis of a Light-Photoacclimation Model for Microalgae-Based Carbohydrate Production in Continuous Photobioreactors. Processes, 11(7), 1866. [CrossRef]
- Gao, X., Kong, B., & Vigil, R. D. (2018). Multiphysics simulation of algal growth in an air-lift photobioreactor: Effects of fluid mixing and shear stress. Bioresource Technology, 251, 75–83. [CrossRef]
- Cheng, J., Miao, Y., Guo, W., Song, Y., Tian, J., & Zhou, J. (2018). Reduced generation time and size of carbon dioxide bubbles in a volute aerator for improving Spirulina sp. growth. Bioresource Technology, 270, 352–358. [CrossRef]
- Villaró, S., Sánchez-Zurano, A., Ciardi, M., Alarcón, F. J., Clagnan, E., Adani, F., Morillas-España, A., Álvarez, C., & Lafarga, T. (2022). Production of microalgae using pilot-scale thin-layer cascade photobioreactors: Effect of water type on biomass composition. Biomass and Bioenergy, 163, 106534. [CrossRef]
- Falinski, K. A., Timmons, M. B., Callan, C., & Laidley, C. (2018). Response of Tisochrysis lutea [Prymnesiophycidae] to aeration conditions in a bench-scale photobioreactor. Journal of Applied Phycology, 30(4), 2203–2214. [CrossRef]
- Venkata Subhash, G., Rohit, M. V., Devi, M. P., Swamy, Y. V., & Venkata Mohan, S. (2014). Temperature induced stress influence on biodiesel productivity during mixotrophic microalgae cultivation with wastewater. Bioresource Technology, 169, 789–793. [CrossRef]
- Leupold, M., Hindersin, S., Gust, G., Kerner, M., & Hanelt, D. (2013). Influence of mixing and shear stress on Chlorella vulgaris, Scenedesmus obliquus, and Chlamydomonas reinhardtii. Journal of Applied Phycology, 25(2), 485–495. [CrossRef]
- Guler, B. A., Deniz, I., Demirel, Z., Oncel, S. S., & Imamoglu, E. (2020). Computational fluid dynamics modelling of stirred tank photobioreactor for Haematococcus pluvialis production: Hydrodynamics and mixing conditions. Algal Research, 47, 101854. [CrossRef]
- Belohlav, V., Zakova, T., Jirout, T., & Kratky, L. (2020). Effect of hydrodynamics on the formation and removal of microalgal biofilm in photobioreactors. Biosystems Engineering, 200, 315–327. [CrossRef]
- Hussin, A. A., To, S. W., Sani, M. H., Amin, M. F. M., & Kamaroddin, M. F. (2021). Optimisation and growth kinetic analysis of Microalgae, Arthrospira platensis in 2-L Photobioreactors. IOP Conference Series: Earth and Environmental Science, 842, 012036. [CrossRef]
- Wang, Y., Castillo-Keller, M., Eustance, E., & Sommerfeld, M. (2017). Early detection and quantification of zooplankton grazers in algal cultures by FlowCAM. Algal Research, 21, 98–102. [CrossRef]
- Deruyck, B., Nguyen, K. H. T., Decaestecker, E., & Muylaert, K. (2019). Modeling the impact of rotifer contamination on microalgal production in open pond, photobioreactor and thin layer cultivation systems. Algal Research, 38, 101398. [CrossRef]
- Martínez, C., Pessi, B. A., & Bernard, O. (2021). Dynamics and productivity of microalgae in presence of predators. IFAC-PapersOnLine, 54(3), 673–678. [CrossRef]
- Tan, K. M., Kassim, M. A., Ng, Z. J., & Lalung, J. (2020). Isolation and characterization of novel acidophilic microalgae from abandoned mining site area for carbohydrate biosynthesis and its kinetic growth study in photobioreactor. IOP Conference Series: Materials Science and Engineering, 716, 012011. [CrossRef]
- Yuarrina, W. P., Pradana, Y. S., Budiman, A., Majid, A. I., Indarto, & Suyono, E. A. (2018). Study of cultivation and growth rate kinetic for mixed cultures of local microalgae as third generation (G-3) bioethanol feedstock in thin layer photobioreactor. Journal of Physics: Conference Series, 1022(1), 012051. [CrossRef]
- Pruvost, J., Le Gouic, B., & Cornet, J.-F. (2022). Kinetic Modeling of CO2 Biofixation by Microalgae and Optimization of Carbon Supply in Various Photobioreactor Technologies. ACS Sustainable Chemistry & Engineering, 10(38), 12826–12842. [CrossRef]
| Category of Use | Type of Bioreactor | Species Cultivated | Volume | Reference |
|---|---|---|---|---|
| Biohydrogen production | Tubular PBR | Chlamydomonas reinhardtii strain CC124 | 0.004 m3 | [36] |
| Panel PBR | 0.004 m3 | |||
| Fishmeal alternative production | Vertical tubular-type PBR | Chlorella vulgaris FSP-E | 50 L | [31] |
| Biofuel feedstock cultivation and wastewater remediation | Floating offshore PBR | Scenedesmus spp., Chlorella spp., Cryptomonas spp., Micractinium spp., Desmodesmus spp., Chlamydomonas spp., Euglena spp., Pandorina spp., Coelastrum spp., and Geitlerinema spp. | 4.18-20.91 m3 | [37] |
| Polyhydroxyalkanoate productivity in rice winery wastewater | Sequencing batch reactors | Zoogloea | 3L | [38] |
| Scale-up productivity | Fibonacci-type photobioreactor (PBR) | Dunaliella salina | 1250 L | [35] |
| Tubular PBR | Tetraselmis sp. CTP4 | 35 m3 | [39] | |
| 100 m3 | ||||
| 8 m2 thin-layer cascade PBR | Microchloropsis salina | 55 L | [40] | |
| 50 m2 thin-layer cascade PBR | 330 L | |||
| Bioreactor | Saccharomyces cerevisiae | 5 L | [41] | |
| Photobioreactor | Chlorella vulgaris | 100 m3 | [33] | |
| Pilot-scale flat-plate PBR | Chlamydomonas reinhardtii | 120 L | [42] | |
| Cultivation | Tubular reactor | Scenedesmus almeriensis | 3 m3 | [43] |
| Raceway reactor | 20 m3 | |||
| 4 m3 | ||||
| Thin-layer reactor | 1.5 m3 | |||
| Recombinant bacteria cultivation | Bioreactor | Streptococcus equi subsp. zooepidemicus | 3 L | [44] |
| Plant cell line cultivation | Bioreactor | red carrot R4G cell line | 50 L | [45] |
| Cultivation and productivity | Bioreactor | Aurantiochytrium sp. T66 | 1 L | [46] |
| Productivity | Tubular PBR | Chlorella vulgaris | 100 L | [34] |
| Fatty acid productivity | Plastic-type flat panel PBR | Scenedesmus obliquus | 5 L | [47] |
| CO2 biofixation and biofuel productivity | Air-lift PBR | Coelastrum sp. SM | 3.26 L | [48] |
| Lipid Productivity | Flat-plate PBR | Nannochloropsis sp. KMMCC 290 | 5 L | [1] |
| Bubble column PBR | ||||
| Air-lift PBR |
| Purpose | PBR Type | Challenge(s) | Reference(s) |
|---|---|---|---|
| Research | Fibonacci-type PBR | Smaller scaled versions of this design decreases illuminated surface area and increases the ratio of space to culture volume. |
[35] |
| Industry | Fibonacci-type PBR | Productivity varies with strains’ light requirements as large scale outside designs’ light source is solar. | [35] |
| Floating offshore PBR | In listed reference, this design was utilized to cultivate polyculture, thus replicability of data is uncertain. Additionally, relayed low lipid productivity rates. |
[37] | |
| Tubular PBR | For outdoor models, growth of culture is dependent on season. | [39] | |
| High lag phase/time compared to panel PBR. Higher pressure accumulation may result in lower productivity compared to panel PBR. | [36] | ||
| Vertical tubular-type PBR | This design can generate high shear stress. High aeration rate is not viable for large-scale growth. |
[31] | |
| Pilot-scale flat-plate PBR | This design can generate high shear stress. | [42] | |
| Research & Industry | Stir tank PBR | This design can generate high shear stress. | [1,52] |
| Horizontal tubular PBR | This design requires more space. Challenges also include gas transfer and heat transfer. |
[1,52] |
| Microalgae Species | Growth Factor Type | Growth Requirements |
Productivity | Reference | |
|---|---|---|---|---|---|
| Ankistrodesmus braunii | Salinity | 50 mM NaCl | After six days of cultivation, lipid content reached 34.4% dry weight | [23] | |
| Ankistrodesmus falcatus | Salinity | 100 mM NaCl | After 10 days of cultivation, lipid content reached 53% dry weight | ||
| Chaetoceros sp. FIKU035 | Temperature | 25 °C | Growth rate reached approximately 0.537 1/d | [54] | |
| Lipid productivity reached approximately 66.73 mg/L*d | |||||
| 30 °C | Biomass was about 777.93 mg/L and biomass productivity was approximately 388.97 mg/L*d | ||||
| Lipid productivity reached approximately 61.35 mg/L*d | |||||
| Chlamydomonas reinhardtii mutant | Gas Exchange | Airflow rate of 5.0-7.5 L/min | Increases biomass concentration by 18% | [42] | |
| Chlamydomonas reinhardtii strain CC124 | pH | 7.65 | Biomass productivity was approximately 31.8 mg/L*h in a tubular photobioreactor (PBR) | [36] | |
| Light Intensity | At tube: 150 µE/m2s At tank: 400 µE/m2s |
||||
| pH | 7.8 | Biohydrogen productivity was about 1.3 mL/L*h in a panel PBR | |||
| Light Intensity | 150 µE/m2s | ||||
| Chlorella sorokiniana DOE1412 | pH | 6 | Biomass productivity was approximately 0.140 g/L*day | [18] | |
| 7 | Average growth rate was approximately 0.353 g/L*day | ||||
| Chlorella sp. GN1 | Light Intensity | 5 cm light path, supplying higher light intensity than larger light paths | Lipid content was 53.5% | [55] | |
| Nitrogen Supply | 0.8 g/L urea, nitrogen concentration of approximately 3 mM | Biomass productivity rate was about 345 mg/L*d | |||
| Nitrogen Supply | Nitrogen deprived conditions: 0.01 g/L urea in growth medium | Lipid productivity was 63.5 mg/L*day | |||
| Lipid concentration comprised of 48.65% cells’ dry weight | |||||
| Phosphorous Supply | Phosphorous deprived conditions: 0.001 g/L K2HPO4*3H2O in growth medium | Lipid concentration comprised of 36.28% cells’ dry weight | |||
| Chlorella vulgaris | Light Intensity | 150 µE/m2s | After 8 days of cultivation, biomass productivity was 0.6 g/L | [22] | |
| CO2 Source | Sodium bicarbonate | Increase of lipid concentrations approximately 26% | [34] | ||
| CO2 fixation rate was about 0.925 g/L*d | |||||
| Temperature | 25 °C | Biomass reached 1.52 g/L | [25] | ||
| pH | 8.0 | ||||
| Salinity | 30 PSU | ||||
| Light | Blue light at 499-465 nm | ||||
| Chlorella vulgaris FSP-E | Nitrogen Supply | 18.6 mM urea concentration | Biomass productivity reached 268.1 mg/L/d and protein was produced at a rate of 155.4 mg/L/d | [31] | |
| Aeration Rate | 0.05 vvm | ||||
| Chlorococcum sp. | Light Intensity | 2500-3500 lux | After five days of cultivation, optimal growth rate was achieved | [56] | |
| Growth Medium | Saline water as water source for growth medium | After five days of cultivation, optimal growth rate was reached 323*104 cells/mL | |||
| Light Cycle | 24-hour light period | After nine days of cultivation, optimal growth rate was achieved | |||
| Initial Cell Density | - | Ideal cell density is dependent on growth conditions | |||
| Coelastrum sp. SM | CO2 Supply | 12% | Biomass productivity reached 0.267 g/L*d and CO2 biofixation rate was 0.302 g/L*h Lipid content was 37.91% of cell dry weight and carbohydrate content reached 58.45% of cell dry weight |
[48] | |
| Air flow | Approximately 0.06 VVM | ||||
| Light Intensity | 6900 lux | ||||
| Light Cycle | 12-hour light period, 12-hour dark period | ||||
| Desmodesmus sp. | Light Intensity data |
300 µE/m2s data |
After 8 days of cultivation, biomass productivity was 1.1 g/L | [22] | |
| After 15 days of cultivation, biomass productivity was 1.4 g/L | |||||
| After 8 days of cultivation, fatty acid content increased to 6.2% | |||||
| Dunaliella salina | Light Intensity | 600-995 μE/m2s | Biomass concentration and productivity was 0.96 g/L and 0.12 g/L*d, respectively | [35] | |
| Temperature | 18.2-22.5 °C | ||||
| pH | 7.5-8.5 | ||||
| Isochrysis galbana | Light Intensity | 350 μmol/m2s | Carbohydrate production of 48.11 gC/m3d | [57] | |
| Temperature | 14 °C | After 10 days of cultivation, docosahexaenoic acid (DHA) content was 19.55 mg/g of ash-free dry weight | [20] | ||
| After five days of cultivation, DHA productivity was 1.08 mg/L*d | |||||
| Nannochloropsis gaditana | Light Intensity | 360 µmol photons/(m2s) | Three-fold increase in growth compared to low light conditions | [21] | |
| Nannochloropsis oculata | Temperature | 20 °C | After five days of cultivation, eicosapentaenoic acid (EPA) productivity was 2.52 mg/L*d | [20] | |
| Nannochloropsis QU130 | Light Cycle | 24-hour light period at 500 µmolhν/m2s | Biomass productivity increased by 13.6%, to 33 g/m2*d | [15] | |
| Temperature | Fluctuating temperatures between 32-41 °C | ||||
| Larger cell size than continuous temperature conditions | |||||
| Nannochloropsis salina | Light Cycle | 24-hour light period | Growth rate reached 0.42 1/d | [13] | |
| Biomass concentration was about 0.77 g/L | |||||
| Gas Exchange | 1L/h of 5% CO2 supplemented air | After 10 days of cultivation, stationary phase was reached | |||
| Nitrogen Supply | Nitrogen-deprived medium with 0.075 g/L NaNO3 | Lipid concentration was 63% of cells’ dry weight | |||
| 1.5 g/L NaNO3 | Growth rate increased approximately 3.5-fold | ||||
| Gas Exchange | 5% CO2 supplemented air | ||||
| pH | 8 | Growth rate approximately 0.19 | [17] | ||
| Largest cell density after 21 days of cultivation was about 95.6*106 cells/mL | |||||
| 9 | Growth rate approximately 0.19 | ||||
| Largest cell density after 21 days of cultivation was about 92.8*106 cells/mL | |||||
| Nannochloropsis sp. FIKU036 | Temperature | 25 °C | Growth rate reached approximately 0.3311/d | [54] | |
| Biomass was about 885.35 mg/L and biomass productivity was approximately 293.05 mg/L*d | |||||
| Nannochloropsis sp. KMMCC 290 | Light Intensity | 11,600 lux | Cell concentration increased by 50%, with a final concentration of 0.51 g/L in air-lift photobioreactor (ALP) | [1] | |
| Lipid productivity increased by 47.7%, to 13.4*10-3 g/L/d in ALP | |||||
| Lipid productivity increased by 45.7%, to 18.8*10-3 g/L/d in flat-plate photobioreactor (FPP) | |||||
| Aeration Rate | 1.0 vvm or 5.0 L/min | ||||
| Cell concentration increased by 44.1%, with a final concentration of 0.49 g/L in ALP | |||||
| Lipid productivity was 13.4*10-3 g/L/d in ALP | |||||
| CO2 Feeding | 10% CO2 at 0.5 L/min for 2 hr every 12 hr intervals | Final cell concentration was 0.65 g/L for FPP | |||
| Lipid productivity reached 19.8*10-3 g/L/d for ALP and FPP | |||||
| Lipid content was 31.5% for ALP | |||||
| Phaeodactylum tricornutum | Nutrient Supply/Growth Medium | 8.82 mM nitrogen concentration in f/2 growth medium | After 10 days of cultivation, biomass concentration reached about 2.76 g/L | [4] | |
| Fucoxanthin content was around 2.18 mg/g of fresh weight | |||||
| Fucoxanthin productivity reached about 5.07 mg/L/d | |||||
| Increased fucoxanthin production to approximately 9.82 mg/L/d | |||||
| Light Intensity | 20 µmol/m2/s | ||||
| Porphyridium sp. | Air Flow | 0.16 cm/s | Dry biomass concentration reached approximately 5 g/L | [58] | |
| Scenedesmus incrassatulus | Salinity | 100 mM NaCl | After six days of cultivation, lipid content reached 37.7% dry weight | [23] | |
| Scenedesmus obliquus | Light Intensity |
150 µE/m2s | After 8 days of cultivation, biomass productivity was 0.8 g/L | [22] | |
| 300 µE/m2s | After 15 days of cultivation, biomass productivity was 1.2 g/L | ||||
| After 15 days of cultivation, fatty acid content increased to 11.6% | |||||
| Nitrogen Supply | Nitrogen source was urea | Cells’ dry biomass was composed of 40% lipids | [47] | ||
| Light Intensity | 3000 lux | ||||
| Spirulina sp. | Shear Force | Decreased bubble size (1.8 mm) and formation time (3.3 ms) in volute aerator | Average growth rate increased by 26.6% in comparison to using a strip aerator | [59] | |
| Biomass productivity increased by 50.7% in comparison to using a strip aerator | |||||
| Tetradesmus almeriensis | Nutrient Supply/Growth Medium | Freshwater with fertilizer | Biomass productivity was 30.3 g/m2*day | [60] | |
| Tetraselmis suecica FIKU032 | Temperature | 30 °C | Growth rate reached approximately 0.378 1/d | [54] | |
| Biomass was about 978.43 mg/L and biomass productivity was approximately 369.84 mg/L*d | |||||
| Tisochrysis lutea | Air Flow | 6.25 vvm | Specific net growth rate reached 3.8 L/min | [61] | |
| Xanthonema hormidioides | Temperature | 20 °C | After three days of cultivation, biomass productivity was 11.73 g/L | [24] | |
| Nitrogen Supply | 18 mM nitrogen concentration | ||||
| Temperature | 25 °C | After 18 days of cultivation, lipid content was 57.49% of cells’ dry weight | |||
| Nitrogen Supply | 3 mM nitrogen concentration | ||||
| Mixed microalgae culture sourced from the Nacharam Cheruvu in India | Temperature | 30 °C | Increase in total lipid productivity to 24.5% | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





