Submitted:
10 September 2024
Posted:
10 September 2024
You are already at the latest version
Abstract
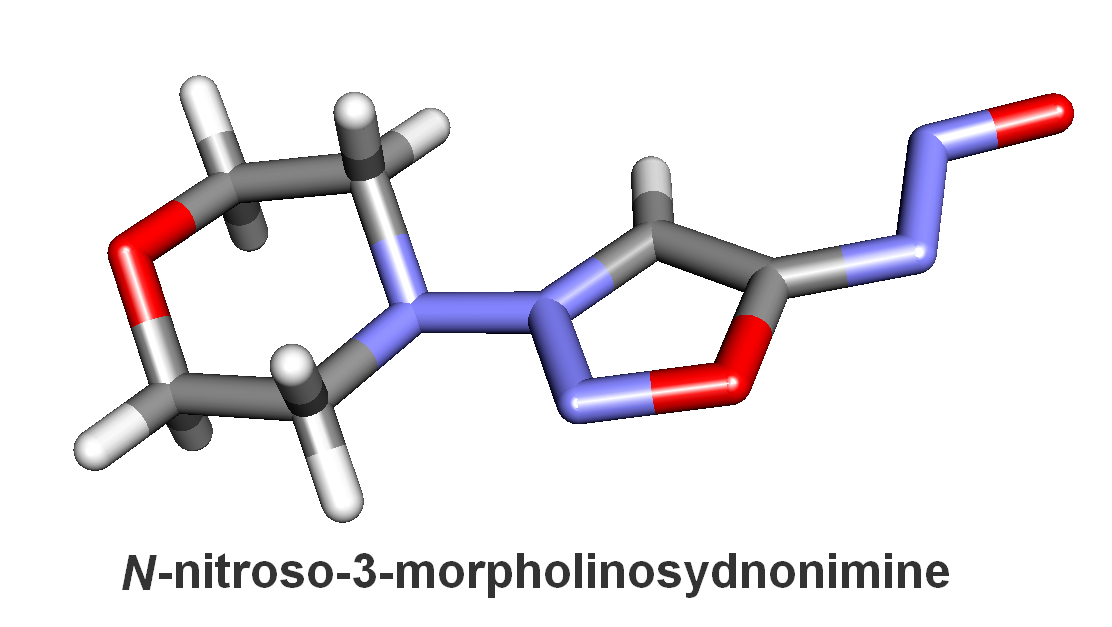
Keywords:
1. Introduction
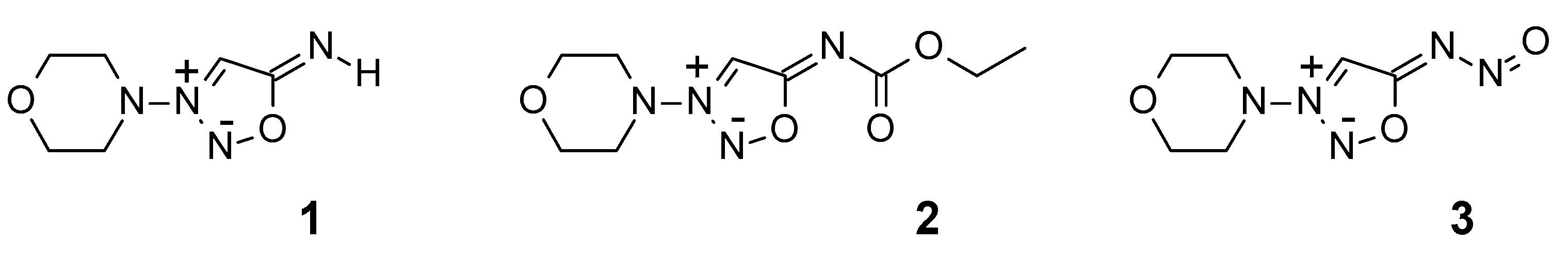
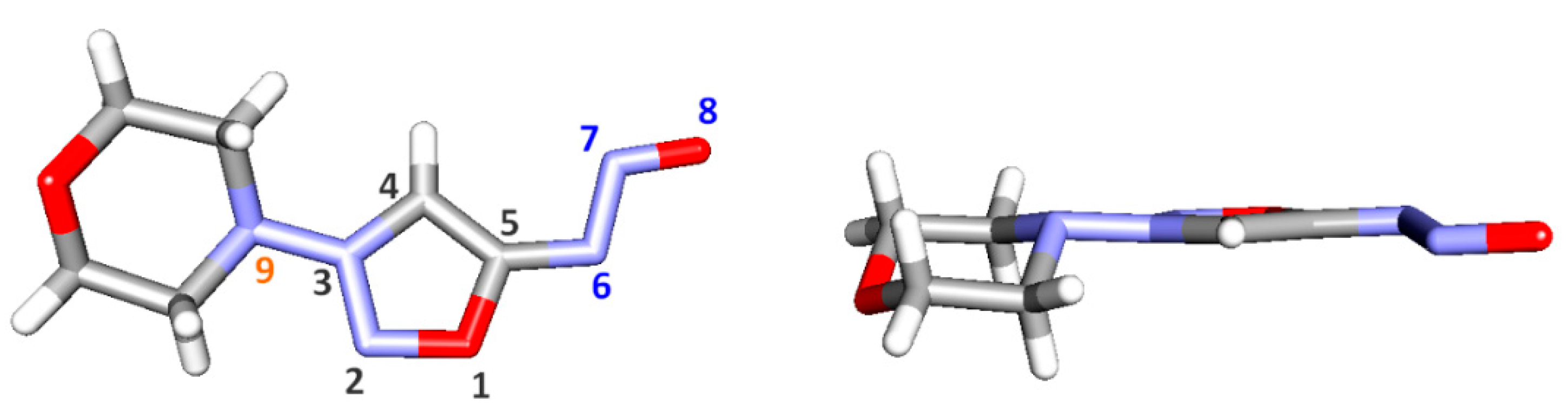
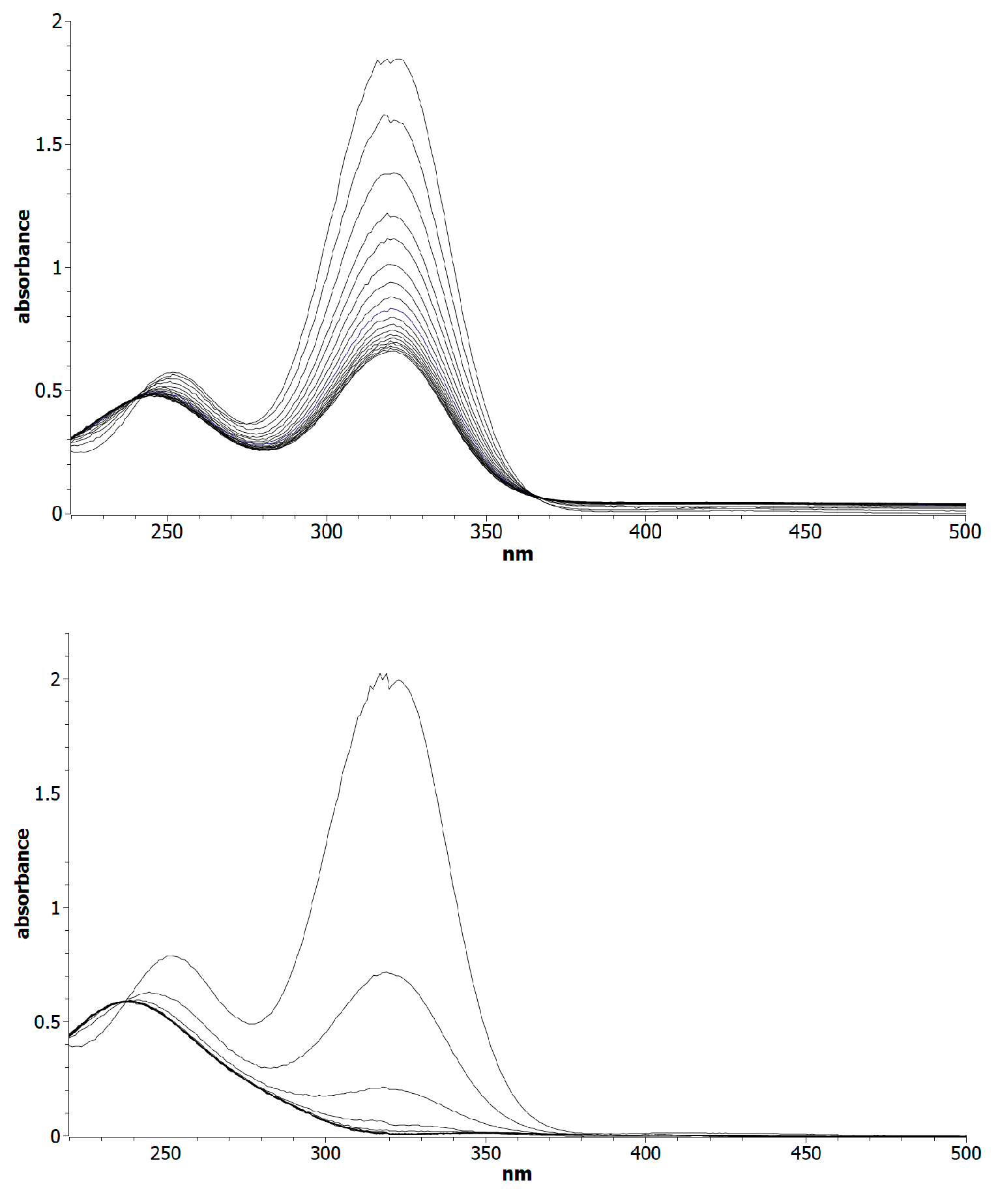
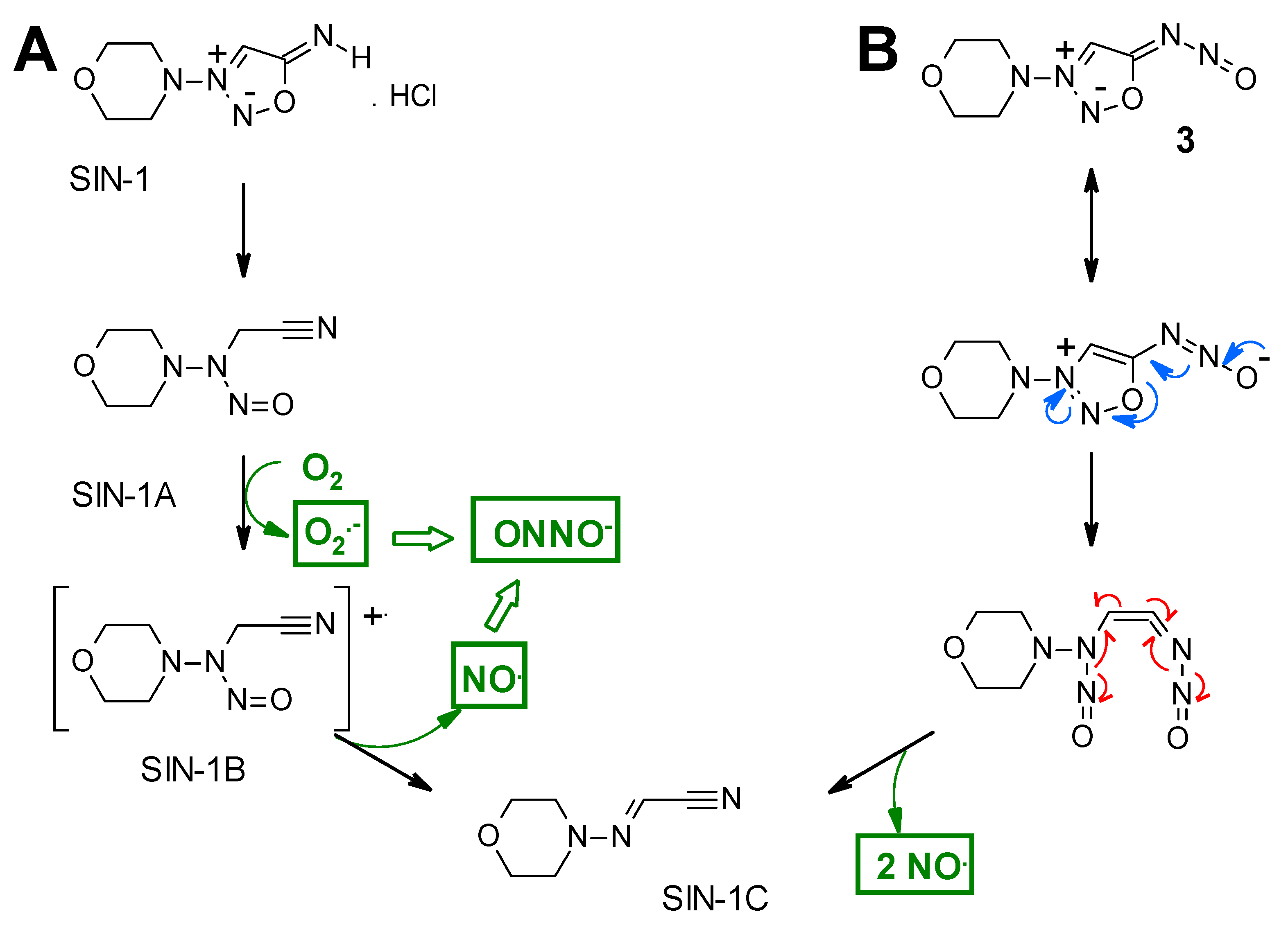
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abdualkader, A. M.; Taher, M.; Yusoff, N. I. N. Mesoionic Sydnone: A Review in their Chemical and Biological Properties. Int. J. Pharm. Pharm. Sci. 2023, 9, 1–9. [Google Scholar] [CrossRef]
- Hladíková, V.; Vánã, J. , Hanusek. [3+2]-Cycloaddition Reaction of Sydnones with Alkynes. Beilstein J. Org. Chem. 2017, 14, 1317–1348. [Google Scholar]
- Holm, P.; Kankaanranta, H.; Metsä-Ketelä, T.; Moilanen, E. Radical Releasing Properties of Nitric Oxide Donors GEA 3162, SIN-1 and S-nitroso-N-acetylpenicillamine. Eur. J. Pharmacol. 1998, 346, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Groves, J. T. Peroxynitrite: Reactive, Invasive and Enigmatic. Curr. Opin. Chem. Biol. 1999, 3, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Singh, R. J.; Hogg, N.; Joseph, J.; Konorev, E.; Kalyanaraman, B. The Peroxynitrite Generator, SIN-1, Becomes a Nitric Oxide Donor in the Presence of Electron Acceptors. Arch. Biochem. Biophys. 1998, 361, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Kawase, M.; Sakagami, H.; Motohashi, N. The chemistry of bioactive mesoionic heterocycles. Bioactive Heterocycles VII: Flavonoids and Anthocyanins in Plants, and Latest Bioactive Heterocycles II, 2009, pp. 135–152.
- Lehmann, J. Nitric oxide donors - Current Trends in Therapeutic Applications. Expert. Opin. Ther. Pat. 2000, 10, 559–574. [Google Scholar] [CrossRef]
- Rosenkranz, B.; Winkelmann, B. R.; Parnham, M. J. Clinical Pharmacokinetics of Molsidomine. Clin. Pharmacokinet. 1996, 30, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Imashiro, Y.; Kaneto, T. Studies on Mesoionic Compounds. I. Synthesis of 3-Dialkylaminosydnonimines. Chem. Pharm. Bull. 1970, 18, 128–132. [Google Scholar] [CrossRef]
- Masuda, K.; Kamiya, T.; Imashiro, Y.; Kaneko, T. Studies on Mesoionic Compounds. II. Synthesis of N-Acyl Derivatives of 3-Dialkylaminosydnonimines. Chem. Pharm. Bull. 1971, 19, 72–79. [Google Scholar] [CrossRef]
- See for example: Fun, H. -K.; Hemamalini, M.; Nithinchandra; Kalluraya, B. 2,3-Di bromo-3-(2-bromophenyl)-1-(3-phenyl sydnon-4-yl)propan-1-one. Acta Cryst. 2014, E66, o3094. [Google Scholar]
- Feelisch, M.; Ostrowski, J.; Noack, E. On the Mechanism of NO Release from Sydnonimines. J. Cardiovasc. Pharmacol. 1989, 14, S13–22. [Google Scholar] [CrossRef] [PubMed]
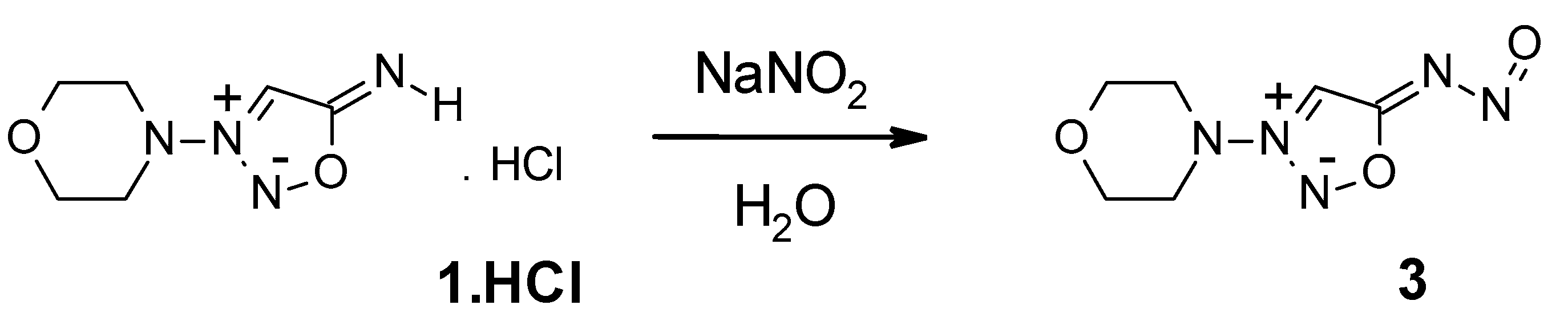
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




