Submitted:
09 September 2024
Posted:
10 September 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Aim of the Study
Materials and Methods
The Study Group
Flow Cytometric Peripheral Blood (PB) Lymphocyte Immunophenotyping
- -
- CD19+ B cells, immature CD19+CD21lo, immature activated CD19+CD38loCD21lo, transitional CD19+CD38hisIgMhi, naïve CD19+CD27-sIgD+, non-switched memory CD19+CD27+sIgD+, switched memory CD19+CD27+IgD- B cells, and CD19+CD38hisIgM- plasmablasts
- -
- CD3+ T cells, CD3+CD4+ T helper cells, CD3+CD4+CD31+CD45RA+ recent thymic emigrants, naïve CD3+CD4+CD27+CD45RA+, regulatory CD3+CD4+CD25++CD27-, central memory CD3+CD4+CD27+CD45RO+, effector memory CD3+CD4+CD27-CD45RO+, terminally differentiated CD3+CD4+CD27-CD45RA+, follicular CD3+CD4+CD185+CD45RO+, and regulatory CD3+CD4+CD45RO+CD127-CD25++ T helper cells.
- -
- among CD3+CD8+ cytotoxic T cells, the following subsets were distinguished: naïve CD3+CD8+CD197+CD27+CD45RA+, central memory CD3+CD8+CD197+CD27+CD45RO+, effector memory CD3+CD8+CD197-CD27-CD45RO+, and terminally differentiated CD3+CD8+CD197-CD27-CD45RA+ cells.
Results
Discussion
Conclusions
Author Contributions
Funding support
Ethics approval
Informed consent to participate/ publication:
Availability of data and material/data transparency
Conflict of interest/Competing interest
Abbreviations
| APC | Allophycocyanin |
| AVSD | Atrioventricular septal defect |
| CoA | Coarctation of aorta |
| CVID | Common variable immunodeficiency |
| CTCF | CCCT-Binding Factor |
| CTLA-4 | Cytotoxic T Cell-Associated Protein 4 |
| DS | Down Syndrome |
| DSCR | Down Syndrome Critical Region |
| EBV | Epstein-Barr virus |
| FITC | Fluorescein isothiocyanate |
| GATA1 | Globin Transcription Factor 1 |
| IEI | Inborn errors of immunity |
| IFN | Interferon |
| IFNAR | Interferon alpha/beta receptor |
| JAK | Janus Kinase |
| LRBA | Lipopolysaccharide Responsive Beige-Like Anchor Protein |
| ML-DS | Myeloid leukemia associated with Down syndrome |
| NFAT | Nuclear factor of activated T cells |
| NF-κB | Nuclear factor kappa B |
| N6AMT1 | N(6)-Adenine Specific DNA Methyltransferase |
| PD-1 | Programmed death receptor |
| PE | Phycoerythrin |
| PerCP | Peridinin chlorophyll protein |
| PIK3CD | Phosphatidylinositol-4,5-Biphosphate 3-Kinase Catalytic Subunit Delta |
| PIK3R1 | Phosphoinositide-3-Kinase Regulatory Subunit 1 |
| PRMT2 | Protein Arginine Methyltransferase 2 |
| RBM1 | RNA-Binding Motif 1 (human spermatogenesis candidate gene) |
| RCAN1 | Regulator of Calcineurin 1 |
| SLAM | Signaling lymphocyte activation molecule |
| SOD1 | Superoxide Dismutase 1 |
| STAT4 | Signal Transducer and Activator of Transcription 4 |
| TAM | Transient abnormal myelopoiesis |
| TAPVR | Total anomalous pulmonary vein return |
| TNF | Tumor necrosis factor |
| ToF | Tetralogy of Fallot |
| U2AF1 | U2 Small Nuclear RNA Auxiliary Factor 1 |
| U2AF1L5 | Small Nuclear RNA Auxiliary Factor 1 Like 5 |
| VSD | Ventricular septal defect |
References
- De Graaf, G.; Buckley, F.; Skotko, B.G. Estimation of the number of people with Down syndrome in Europe. Eur J Human Genet 2021, 29, 402–410. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, G.; Buckley, F.; Skotko, B.G. Estimates of live births, natural losses, and elective terminations with Down syndrome in the United States. Am J Med Genet 2015, 167A, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Presson, A.P.; Partyka, G.; Jensen, K.M.; Devine, O.J.; Rasmussen, S.A.; McCabe, L.L.; et al. Current estimates of Down syndrome population prevalence in the United States. J Pediatr 2013, 163, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Asim, A.; Kumar, A.; Muthuswamy, S.; Jain, S.; Agarwal, S. Down syndrome: an insight of the disease. J Biomed Sci 2015, 22, 41. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, A.; Raja, K.; Venugopalan, M.; Chandrasekaran, B.; Kovanur Sampath, K.; Muthusamy, H.; et al. Down syndrome-a narrative review with a focus on anatomical features. Clin Anat 2016, 29, 29,568–577. [Google Scholar] [CrossRef]
- De Lausnay, M.; Ides, K.; Wojciechowski, M.; Boudewyns, A.; Verhulst, S.; Van Hoorenbeek, K. Pulmonary complications in children with Down syndrome: a scoping review. Paediatr Resp Rev 2021, 40, 65–72. [Google Scholar] [CrossRef]
- Alsubie, H.S.; Rosen, D. The evaluation and management of respiratory disease in children with Down syndrome (DS). Paediatr Resp Rev 2018, 26, 49–54. [Google Scholar] [CrossRef]
- Danopoulos, S.; Deutsch, G.H.; Dumortier, C.; Mariani, T.J.; Al Alam, D. Lung disease manifestations in Down syndrome. Am J Physiol Lung Cell Mol Physiol 2021, 321, 892–899. [Google Scholar] [CrossRef]
- Verstegen, R.H.J.; Chang, K.J.J.; Kusters, M.A.A. Clinical implications of immune-mediated diseases in children with Down syndrome. Pediatr Allergy Immunol 2020, 31, 117–123. [Google Scholar] [CrossRef]
- Szczawinska-Poplonyk, A.; Begier, K.; Dorota, A.; Dabrowska, M.; Galecka, D.; Wawrzeniak, K.; Wroblewski, K. Syndromic immunodeficiencies: a pediatrician’s perspective on selected diseases. Allergol Immunopathol 2021, 49, 117–136. [Google Scholar] [CrossRef]
- Krivega, M.; Stiefel, C.M.; Storchova, Z. Consequences of the chromosome gain: a new view on trisomy syndromes. Am J Human Genet 2022, 109, 2126–2140. [Google Scholar] [CrossRef] [PubMed]
- Chunduri, N.K.; Storchova, Z. The diverse consequences of aneuploidy. Nat Cell Biol 2019, 21, 2010–2021. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, S.E.; Skotko, B.G.; Rafii, M.S.; Strydom, A.; Pape, S.E.; Bianchi, D.W.; et al. Down syndrome. Nat Rev Dis Primers 2020, 6, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Huggard, D.; Doherty, D.G.; Molloy, E.J. Immune dysregulation in children with Down syndrome. Front Pediatr 2020, 8, 73. [Google Scholar] [CrossRef]
- Ferrari, M.; Stagi, S. Autoimmunity and genetic syndromes: a focus on Down syndrome. Genes 2021, 12, 268. [Google Scholar] [CrossRef]
- Satge D, Seidel MG, The pattern of malignancies in Down syndrome and its potential context with the immune system. Front Immunol 2018, 9, 3058. [CrossRef]
- Ram, G.; Chinen, J. Infections and ammunodeficiency in Down syndrome. Clin Exp Immunol 2011, 164, 9–16. [Google Scholar] [CrossRef]
- Piatosa, B.; Wolska-Kuśnierz, B.; Pac, M.; Siewiera, K.; Gałkowska, E.; Bernatowska, E. B cell subsets in healthy children: reference values for evaluation of B cell maturation process in peripheral blood. Cytometry B Clin Cytom 2010, 78, 372–381. [Google Scholar] [CrossRef]
- Schatorje, E.J.H.; Gemen, E.F.A.; Driessen, G.J.A.; Leuvenink, J.; van Hout, R.W.N.M.; de Vries, E. Paediatric reference values for the peripheral T cell compartment. Scand J Immunol 2012, 75, 436–444. [Google Scholar] [CrossRef]
- Huggard, D.; Worrall, A.P.; Kirkham, C.; McGrane, F.; Mandira, R.; Casey, L.; et al. Immune screening in children with Down syndrome. Acta Paediatr 2022, 111, 2025–2028. [Google Scholar] [CrossRef]
- Eissa, E.; Afifi, H.H.; Abo-Shanab, A.M.; Thomas, M.M.; Taher, M.B.; Kandil, R.; et al. Importance of TREC and KREC as molecular markers for immunological evaluation of Down syndrome children. Sci Rep 2023, 13, 15445. [Google Scholar] [CrossRef] [PubMed]
- Verstegen, R.H.J.; Kusters, M.A.A. Inborn errors of adaptive immunity in Down syndrome. J Clin Immunol 2020, 40, 791–806. [Google Scholar] [CrossRef] [PubMed]
- Verstegen, R.H.J.; Borte, S.; Bok, L.A.; van Zwieten, P.H.T.; von Dobeln, U.; Hammarstrom, L.; et al. Impact of Down syndrome on the performance of neonatal screening assays for severe primary immunodeficiency diseases. J Allergy Clin Immunol 2014, 133, 1208–1211. [Google Scholar] [CrossRef] [PubMed]
- Carsetti, R.; Valentini, D.; Marcellini, V.; Scarsella, M.; Marasco, E.; Giustini, F.; et al. Reduced numbers of switched memory B cells with high terminal differentiation potential in Down syndrome. Eur J Immunol 2015, 45, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Akkaya, M.; Kwa Kk Pierce, S.K. B cell memory: building two walls of protection against pathogens. Nat Rev Immunol 2020, 20, 229–238. [Google Scholar] [CrossRef]
- Verstegen, R.H.J.; Driessen, G.J.; Bartol, S.J.W.; van Noesel, C.J.M.; Boon, L.; van der Burg, M.; et al. Defective B cell memory in patients with Down syndrome. J Allergy Clin Immunol 2014, 134, 1346–1353. [Google Scholar] [CrossRef]
- Verstegen, R.H.J.; Kusters, M.A.A.; Gemen, E.F.A.; de Vries, E. Down syndrome B lymphocyte subpopulations, intrinsic defect or decreased T lymphocyte help. Pediatr Res 2010, 67, 563–569. [Google Scholar] [CrossRef]
- Szczawińska-Popłonyk, A.; Schwartzmann, E.; Bukowska-Olech, E.; Biernat, M.; Gattner, S.; Korobacz, T.; et al. The pediatric common variable immunodeficiency – from genetics to therapy: a review. Eur J Pediatr 2022, 181, 1371–1383. [Google Scholar] [CrossRef]
- Gensous, N.; Bacalini, M.G.; Franceschi, C.; Garagnani, P. Down syndrome, accelerated aging and immunosenescence. Semin Immunopathol 2020, 42, 635–645. [Google Scholar] [CrossRef]
- Marcovecchio, G.E.; Ferrua, F.; Fontana, E.; Beretta, S.; Genua, M.; Bortolomai, I.; et al. Premature senescence and increased oxidative stress in the thymus of Down syndrome patients. Front Immunol 2021, 12, 669893. [Google Scholar] [CrossRef]
- Campos, J.S.; Henrickson, S.E. Defining and targeting patterns of T cell dysfunction in inborn errors of immunity. Front Immunol 2022, 13, 932715. [Google Scholar] [CrossRef] [PubMed]
- Peeters, D.; Pico-Knijnenburg, I.; Wieringa, D.; Rad, M.; Cuperus, R.; Ruige, M.; et al. AKT hyperphosphorylation and T cell exhaustion in Down syndrome. Front Immunol 2022, 13, 724436. [Google Scholar] [CrossRef] [PubMed]
- Delmonte, O.M.; Castagnoli, R.; Calzoni, E.; Notarangelo, L.D. Inborn errors of immunity with immune dysregulation: from bench to bedside. Front Pediatr 2019, 7, 353. [Google Scholar] [CrossRef] [PubMed]
- Huggard, D.; Kelly, L.; Ryan, E.; McGrane, F.; Lagan, N.; Roche, E.; et al. Increased systemic inflammation in children with Down syndrome. Cytokine 2020, 127, 154938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Che, M.; Yuan, J.; Yu, Y.; Cao, C.; Qin, X.Y.; et al. Aberrations in circulating inflammatory cytokine levels in patients with Down syndrome: a meta-analysis. Oncotarget 2017, 8, 84489–84496. [Google Scholar] [CrossRef]
- Araya, P.; Waugh, K.A.; Sullivan, K.D.; Nunez, N.G.; Roselli, E.; Smith, K.P.; et al. Trisomy 21 dysregulates T cell lineages toward an autoimmunity-prone state associated with interferon hyperactivity. Proc Natl Acad Sci USA 2019, 116, 24231–24241. [Google Scholar] [CrossRef]
- Aversa, T.; Crisafulli, G.; Zirilli, G.; De Luca, F.; Gallizi, R.; Valenzise, R. Epidemiological aspects of autoimmune thyroide disease in children with Down’s syndrome. Ital J Pediatr 2018, 44, 39. [Google Scholar] [CrossRef]
- Guaraldi, F.; Rossetto Giaccherino, R.; Lanfranco, F.; Motta, G.; Gori, D.; Arvat, E.; et al. Endocrine autoimmunity in Down’s syndrome. Front Horm Res 2017, 48, 133–146. [Google Scholar] [CrossRef]
- Guild, A.; Fritch, J.; Patel, S.; Reinhardt, A.; Acquazzino, M. Hemophagocytic lymphohistiocytosis in trisomy 21: successful treatment with interferon inhibition. Pediatr Rheumatol Online J 2022, 20, 104. [Google Scholar] [CrossRef]
- Leung, C.; Su Li Simoes-E-Silva, A.C.; Arocha, L.S.; de Paiva, K.M.; Haas, P. Risk for severe illness and death among pediatric patients with Down syndrome hospitaliza for COVID-19, Brazil. Emerg Infect Dis 2023, 29, 126–35. [Google Scholar] [CrossRef]
- Pitchan Velammal, P.N.K.; Balasubramanian, S.; Ayoobkhan, F.S.; Mohan, G.V.K.; Aggarval, P.; Rabaan, A.A.; et al. COVID-19 in patients with Down syndrome: a systematic review. Immun Inflamm Dis 2024, 12, 1219. [Google Scholar] [CrossRef] [PubMed]
- Kołtan, S.; Ziętkiewicz, M.; Becht, R.; Berdej-Szczot, E.; Cienkusz, M.; Ewertowska, M.; et al. COVID-19 in unvaccinated patients with inborn errors of immunity – Polish experience. Front Immunol 2022, 13, 953700. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, J.M. Down syndrome and COVID-19: a perfect storm? Cell Rep Med 2020, 1, 100019. [Google Scholar] [CrossRef] [PubMed]
- Szczawińska-Popłonyk, A.; Jończyk-Potoczna, K.; Bręborowicz, A.; Bartkowska-Śniatkowska, A.; Figlerowicz, M. Fatal respiratory distress syndrome due to coronavirus infection in a child with severe combined immunodeficiency. Influenza Other Respir Viruses 2013, 7, 634–636. [Google Scholar] [CrossRef] [PubMed]
- Ogimi, C.; Englund, J.A.; Bradford, M.C.; Qin, X.; Boeckh, M.; Waghmare, A. Characteristics and outcomes of coronavirus infection in children: the role of viral factors and an immunocompromised state. J Pediatr Infect Dis Soc 2019, 8, 21–28. [Google Scholar] [CrossRef]
- Fitzpatrick, V.; Rivelli, A.; Chaudhari, S.; Chicoine, L.; Jia, G.; Rzhetsky, A.; et al. Prevalence of infectious diseases among 6078 individuals with Down syndrome in the United States. J Patient Cent Res Rev 2022, 9, 64–69. [Google Scholar] [CrossRef]
- Ghezzi, M.; Garancini, N.; De Santis, R.; Gianolino, L.; Zirpoli, S.; Mandelli, A.; et al. Recurrent respiratory infections in children with Down syndrome: a review. Children 2014, 11, 246. [Google Scholar] [CrossRef]
- Eijsvoogel, N.B.; Verstegen, R.H.J.; van Well, G.T.J.; van Hout, R.W.N.M.; de Vries, E. Increased rate of respiratory symptoms in children with Down syndrome: a 2-year web-based parent-reported prospective study. Eur J Pediatr 2022, 181, 4079–4089. [Google Scholar] [CrossRef]
- Notarangelo, L.D.; Bosticardo, M. Interferons in Down syndrome: when more is less. Immunity 2022, 55, 1967–1969. [Google Scholar] [CrossRef]
- Malle, L.; Bogunovic, D. Down syndrome and type I interferon: not so simple. Curr Opin Immunol 2022, 72, 196–205. [Google Scholar] [CrossRef]
- Malle, L.; Patel, R.S.; Martin-Fernandez, M.; Stewart, O.; Philippot, Q.; Buta, S.; et al. Autoimmunity in Down’s syndrome via cytokines, CD4 T cells and CD11+ B cells. Nature 2023, 615, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Biselli, J.M.; Zampieri, B.L.; Biselli-Chicote, P.M.; de Souza, J.E.S.; Burger, M.C.; da Silva, W.A., Jr.; et al. Differential microRNA expression profile in blood of children with Down syndrome suggests a role in immunological dysfunction. Human Cell 2022, 35, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Baruchel, A.; Bourquin, J.; Crispino, J.; Cuartero, S.; Hasle, H.; Hitzler, J.; et al. Down syndrome and leukemia: from basic mechanisms to clinical advances. Haematologica 2023, 108, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- Peroni, E.; Gottardi, M.; D’Antona, L.; Randi, M.L.; Rosato, A.; Coltro, G. Hematologic neoplasms associated with Down syndrome: cellular and molecular heterogeneity of the disease. Int J Mol Sci 2023, 24, 15325. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K. Recent advances in the understanding of transient abnormal myelopoiesis in Down syndrome. Pediatrics Int 2019, 61, 222–229. [Google Scholar] [CrossRef]
- Li, J.; Kalev-Zylinska, M.L. Advances in molecular characterization of myeloid proliferations associated with Down syndrome. Front Genet 2022, 13, 891214. [Google Scholar] [CrossRef]
- Hendrix, J.A.; Amon, A.; Abbeduto, L.; Agiovlasitis, S.; Alsaied, T.; Anderson, H.A.; et al. Opportunities, barriers, and recommendations in Down syndrome research. Transl Sci Rare Dis 2021, 5, 99–129. [Google Scholar] [CrossRef]
- Van Gameren-Oosterom, H.B.M.; Weijerman, M.E.; van Wieringen, H.; de Winter, J.P.; van Wermeskerken, A. Clinical practice-latest insights in optimizing the care of children with Down syndrome. Eur J Pediatr 2023, 182, 2027–2039. [Google Scholar] [CrossRef]
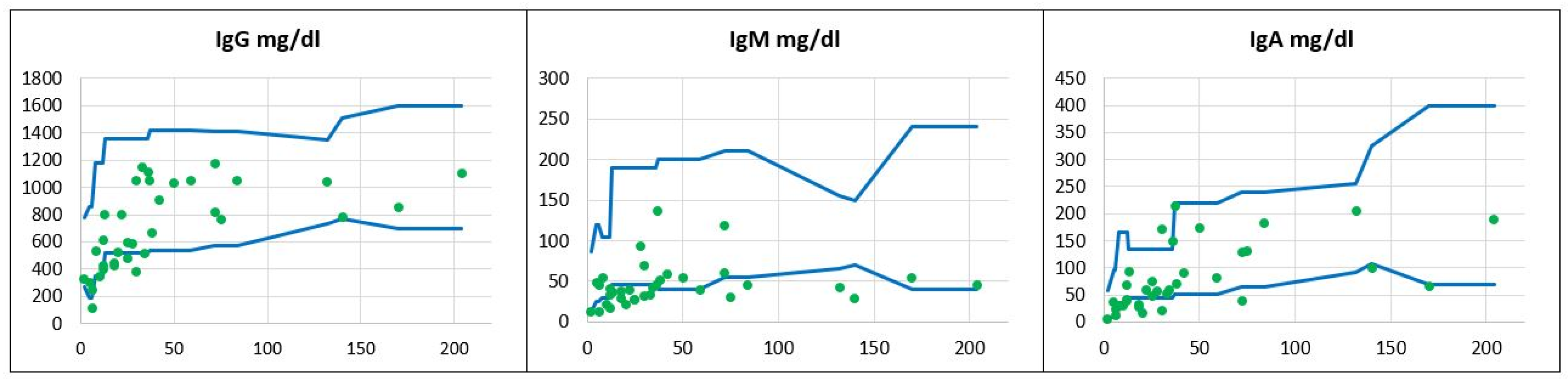
 , patients’ values
, patients’ values  .
.
 , patients’ values
, patients’ values  .
.
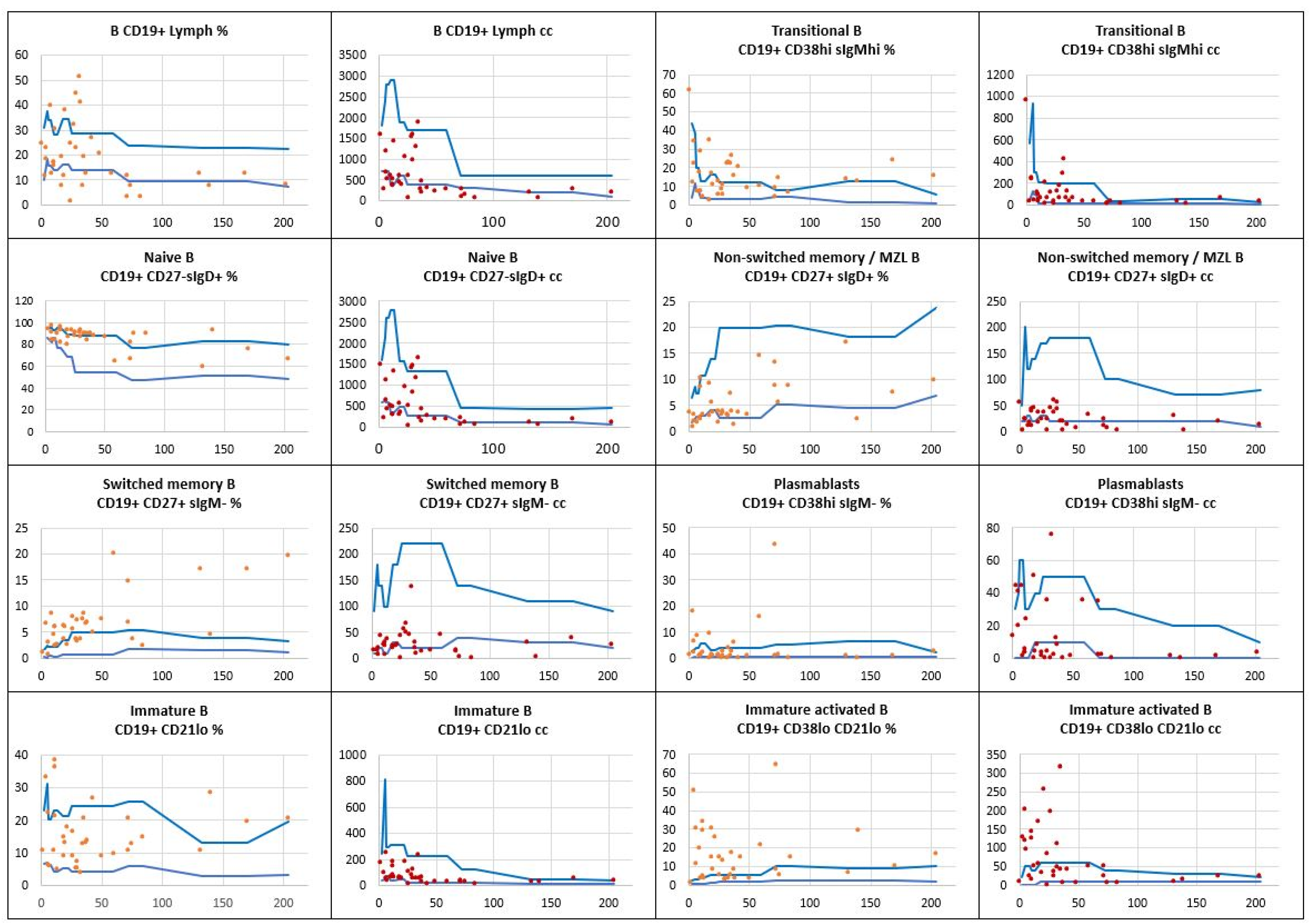
 , patients’ relative counts (%)
, patients’ relative counts (%)  , absolute numbers (cc)
, absolute numbers (cc)  .
.
 , patients’ relative counts (%)
, patients’ relative counts (%)  , absolute numbers (cc)
, absolute numbers (cc)  .
.
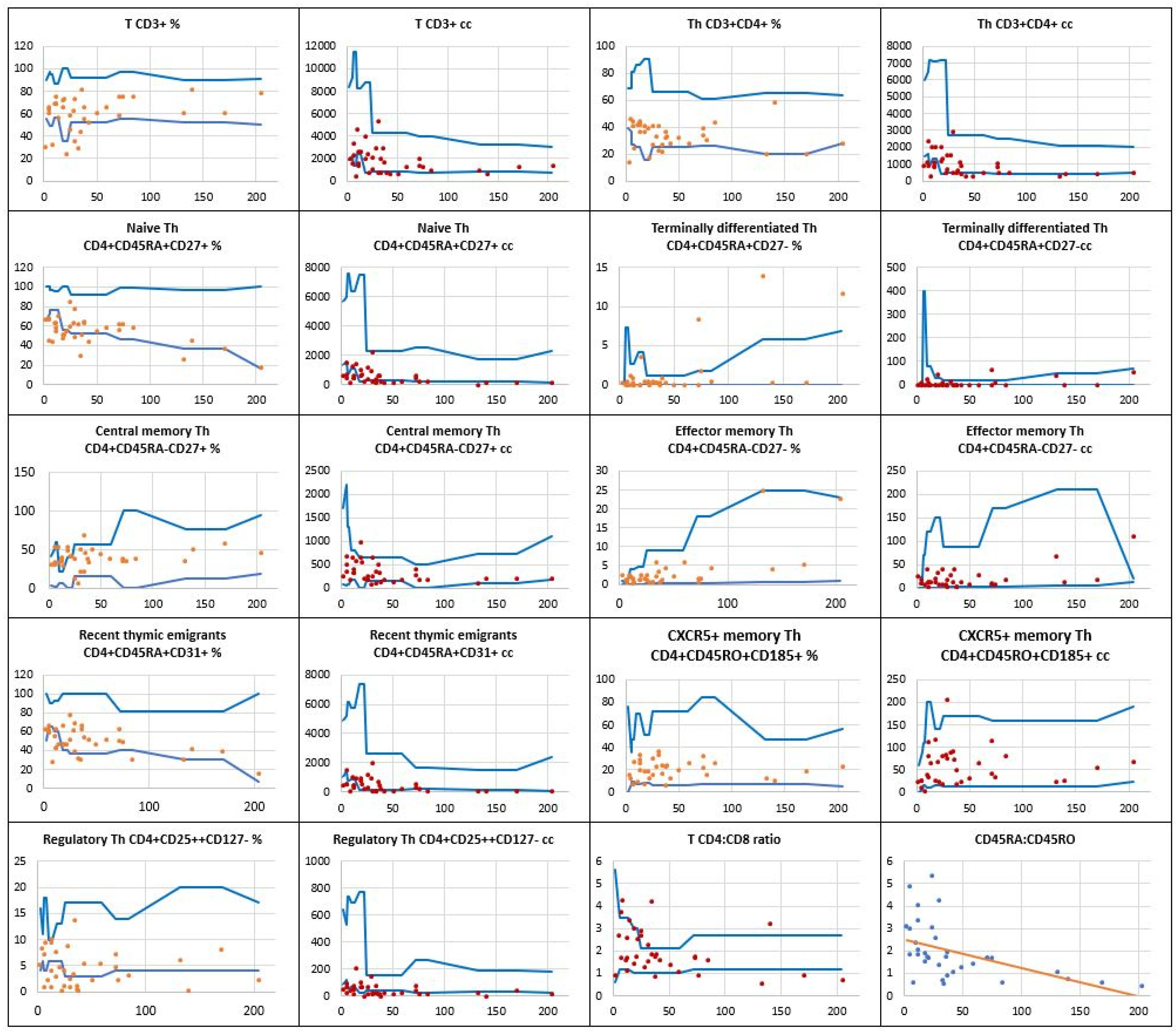
 , patients’ relative counts (%)
, patients’ relative counts (%)  , absolute numbers (cc)
, absolute numbers (cc)  .
.
 , patients’ relative counts (%)
, patients’ relative counts (%)  , absolute numbers (cc)
, absolute numbers (cc)  .
.
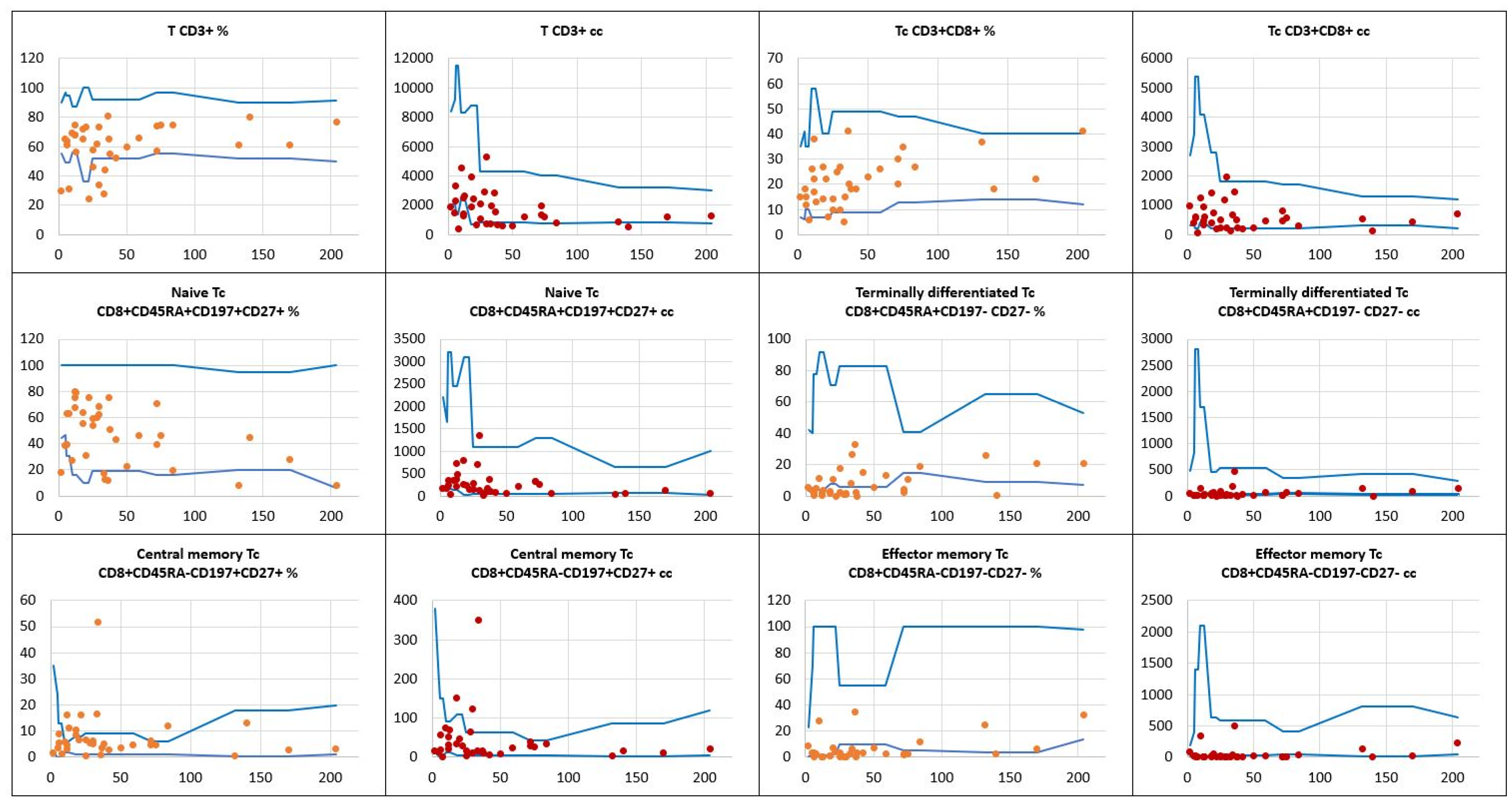
 , patients’ relative counts (%)
, patients’ relative counts (%)  , absolute numbers (cc)
, absolute numbers (cc)  .
.
 , patients’ relative counts (%)
, patients’ relative counts (%)  , absolute numbers (cc)
, absolute numbers (cc)  .
.
| Infectious etiology | |||
|---|---|---|---|
| Type of infection | Frequency | ||
| Viral |
SARS-CoV2 RSV Rhinovirus Bocavirus Adenovirus Coronavirus OC43 Influenza virus A/B Parainfluenza 3 and 4 Coronavirus HKU1 |
N=12 (34%) N=11 (31%) N=11 (31%) N=6 (17%) N=3 (9%) N=3 (9%) N=2 (6%) N=2 (6%) N=1 (3%) |
Total number of infected children N=17 (49%) |
| Bacterial |
Streptococcus pneumoniae Haemophilus influenzae Staphylococcus aureus Escherichia coli Klebsiella pneumoniae Pseudomonas aeruginosa |
N=12 (34%) N=9 (26%) N=8 (23%) N=6 (18%) N=4 (11%) N=1 (3%) |
Total number of infected children N=15 (43%) |
| Immune dysregulation disorders | |||
|---|---|---|---|
| Type of disorder | Frequency | ||
| Allergic |
Asthma Food allergy Allergic rhinitis |
N=8 (23%) N=8 (23%) N=1 (3%) |
Total N=17 (49%) |
| Autoimmune |
Thyroiditis Alopecia Celiac disease Crohn disease |
N=1 (3%) N=1 (3%) N=1 (3%) N=1 (3%) |
Total N=4 (11%) |
| Inflammatory |
Balanitis xerotica obliterans Hydradenitis suppurativa Lymphadenopathy |
N=2 (6%) N=1 (3%) N=2 (6%) |
Total N=6 (18%) |
| Hematopoietic | Transient abnormal myelopoiesis | N=4 (11%) | Total N=4 (11%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





