Submitted:
06 September 2024
Posted:
09 September 2024
You are already at the latest version
Abstract

Keywords:
1. Introduction
1.1. Melanoma
1.2. Mitochondria-Targeting Compounds
1.3. CMM Topical Treatments: Hydrogel Formulations
1.4. The Present Study
2. Results and Discussion
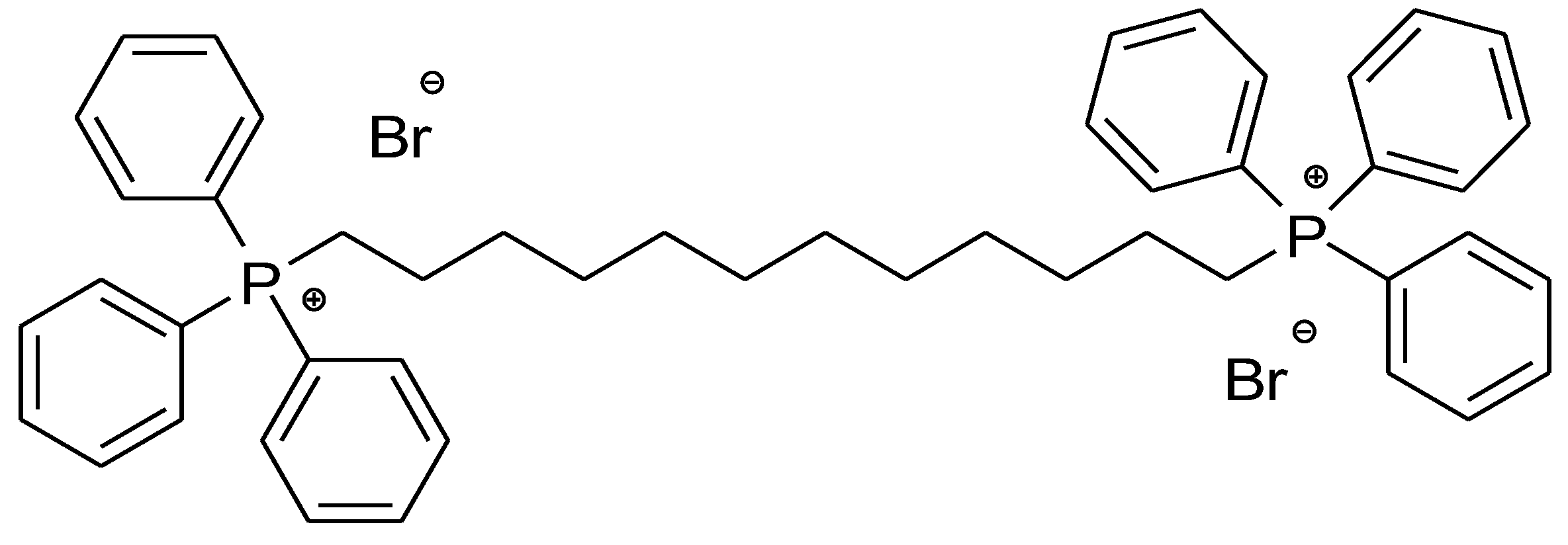
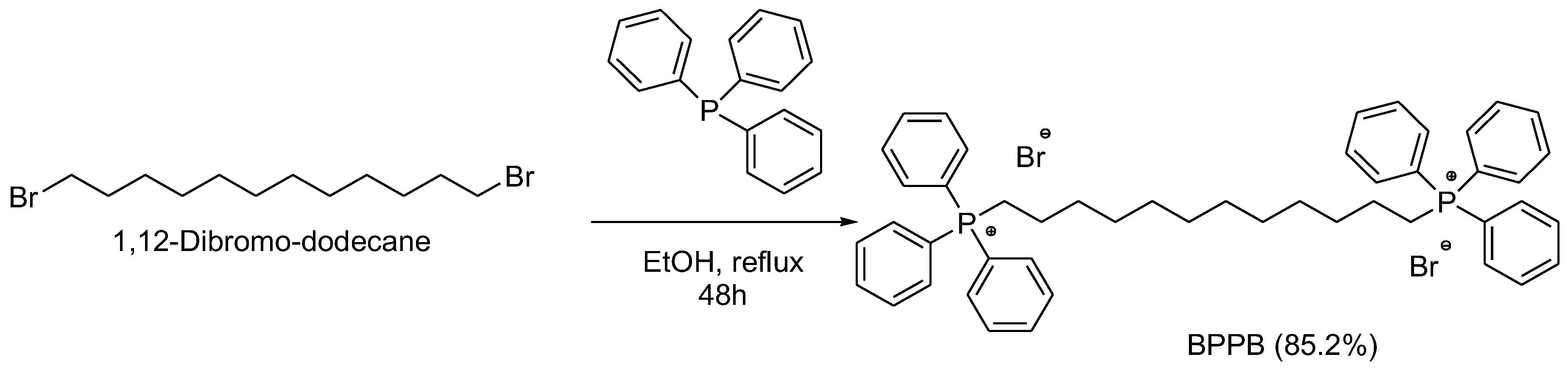
2.1. 1,1-(1,12-. dodecanediyl)bis[1,1,1]-triphenylphosphonium di-Bromide (BPPB)
BPPB Characterization
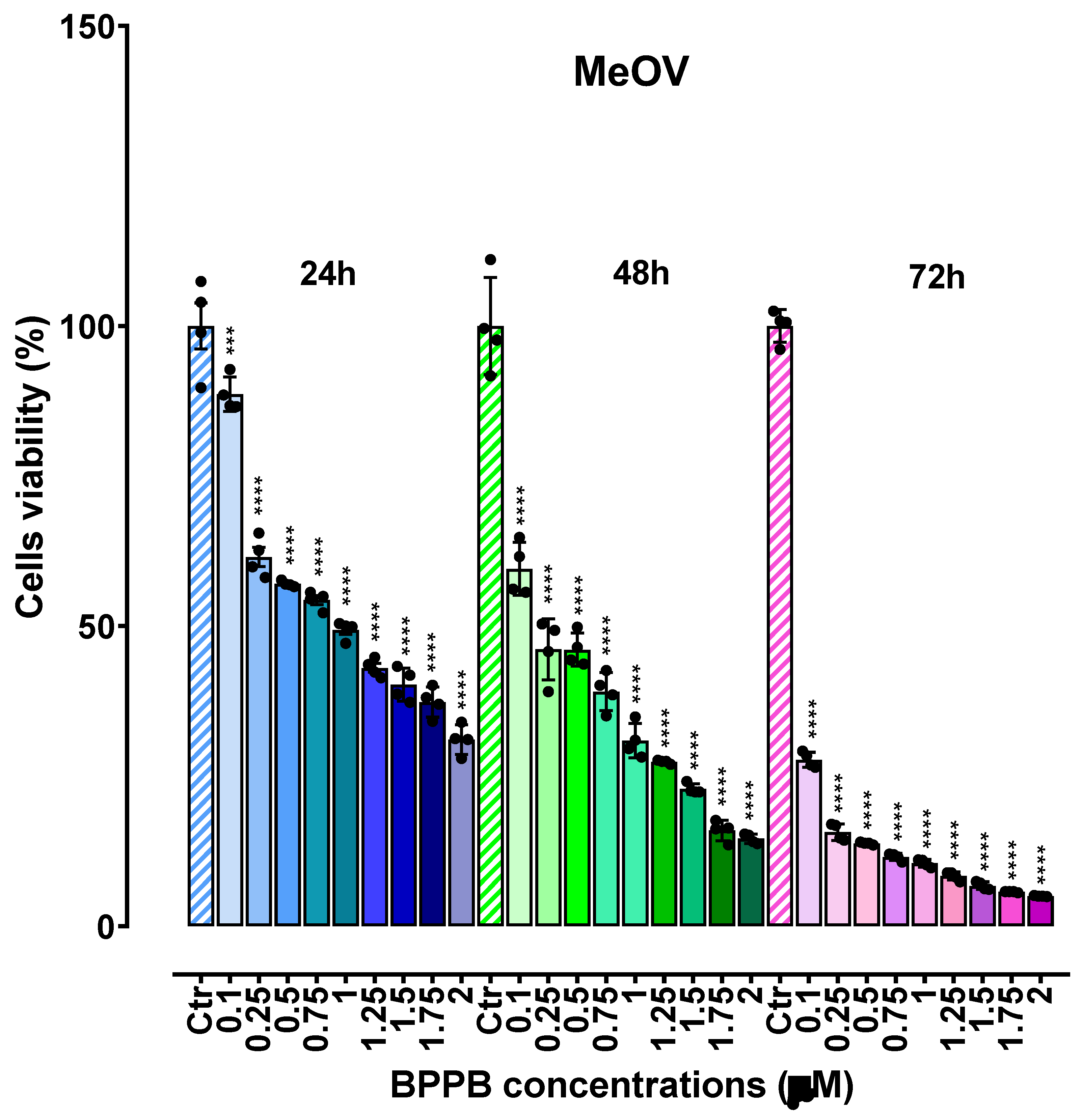
2.2. Concentration- and Time-Dependent Cytotoxic Effects of PBBP on CMM Cells
2.2.1. Concentration- and Time-Dependent Cytotoxic Effects of PBBP on CMM Cells
2.2.2. Determinations of the IC50 Values and of Selectivity of PBBP for CMM Cells vs. Not Tumoral Cells
IC50 Values
Selectivity Indices
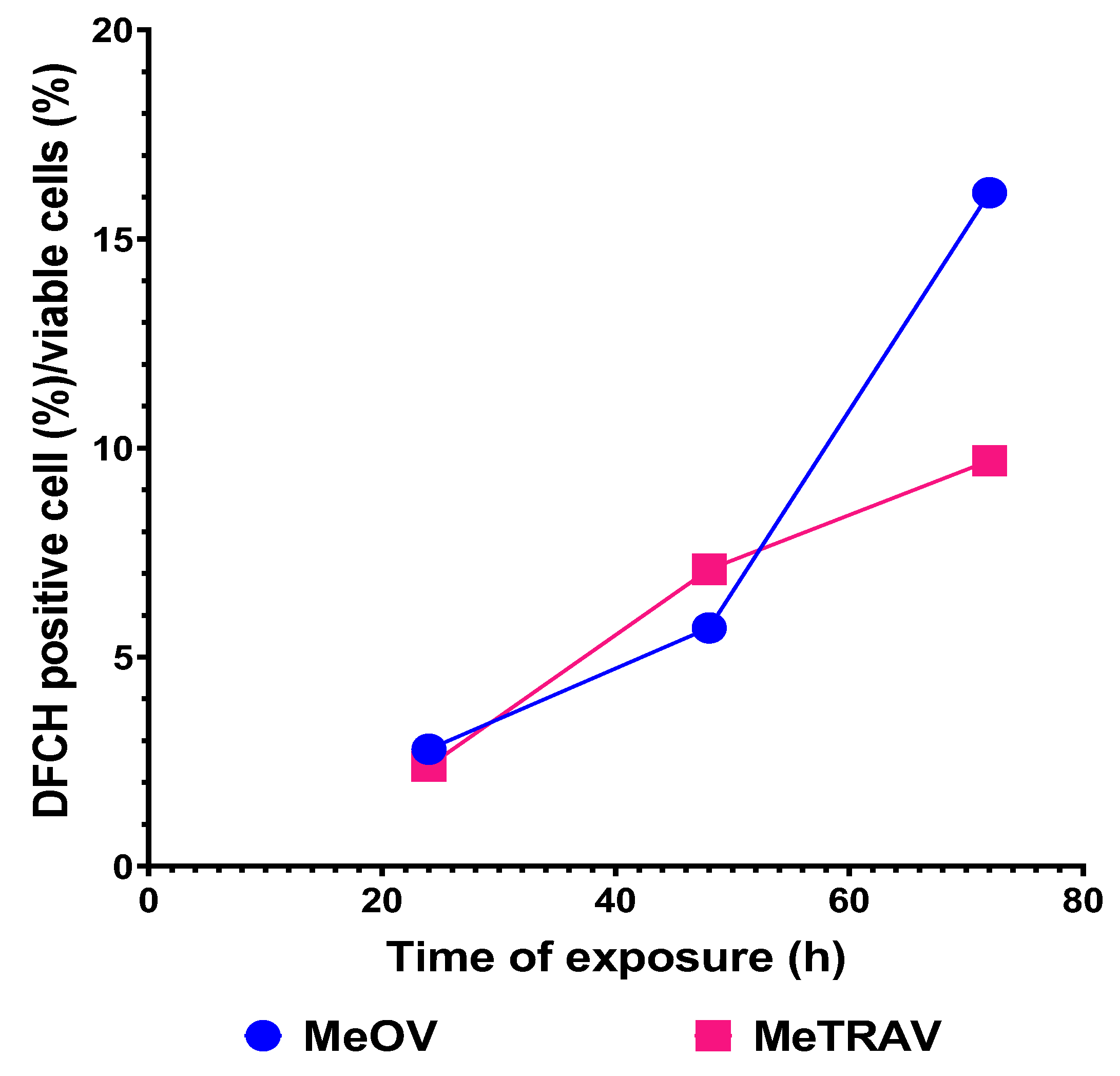
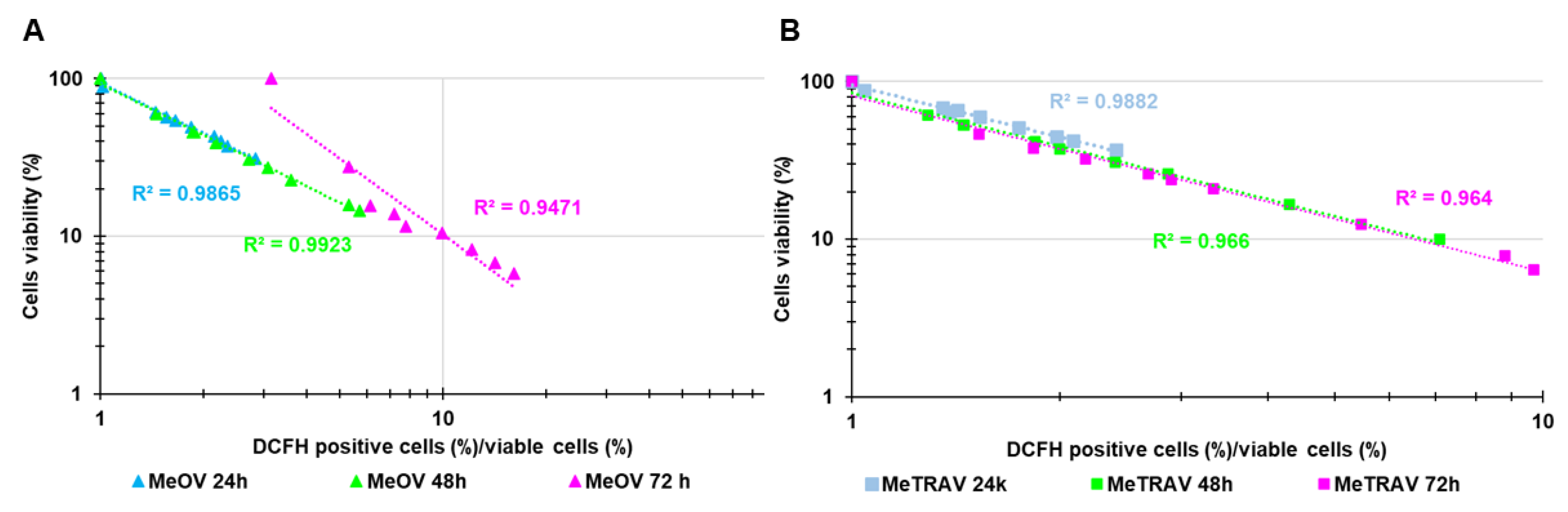
2.3. Concentration- and Time-Dependent ROS Induction by PBBP on CMM Cells
2.4. Hyaluronic Acid (HA)-Based BPPB Hydrogel (HA-BPPB-HG)
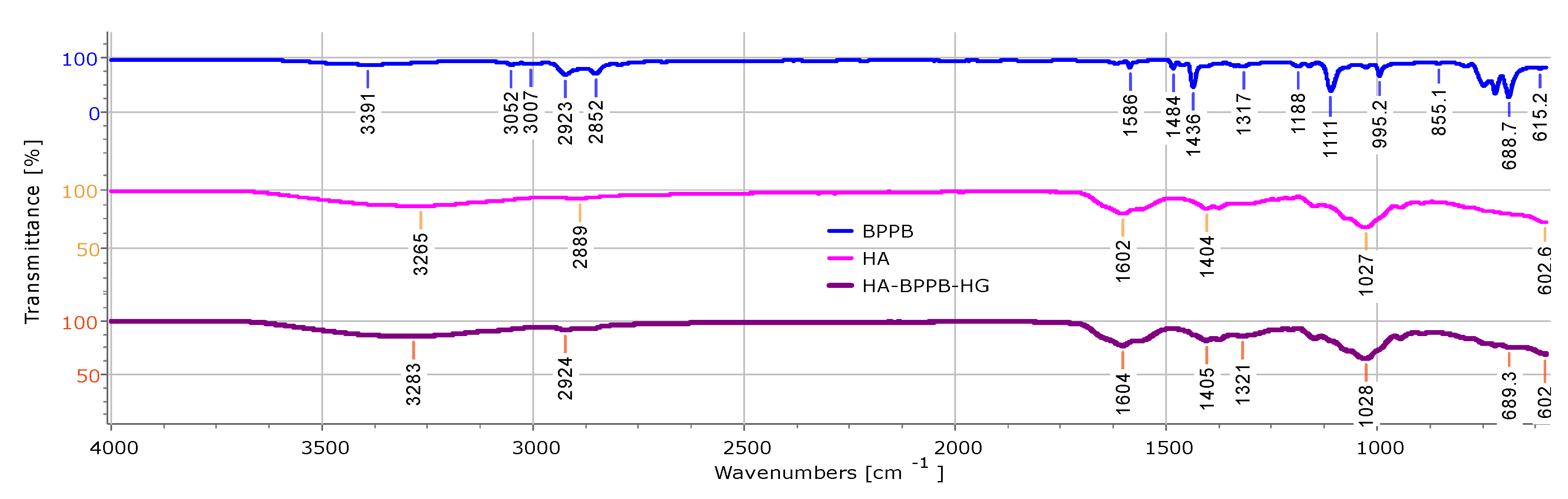
2.4.1. ATR-FTIR of HA, BPPB and HA-BPPB-HG
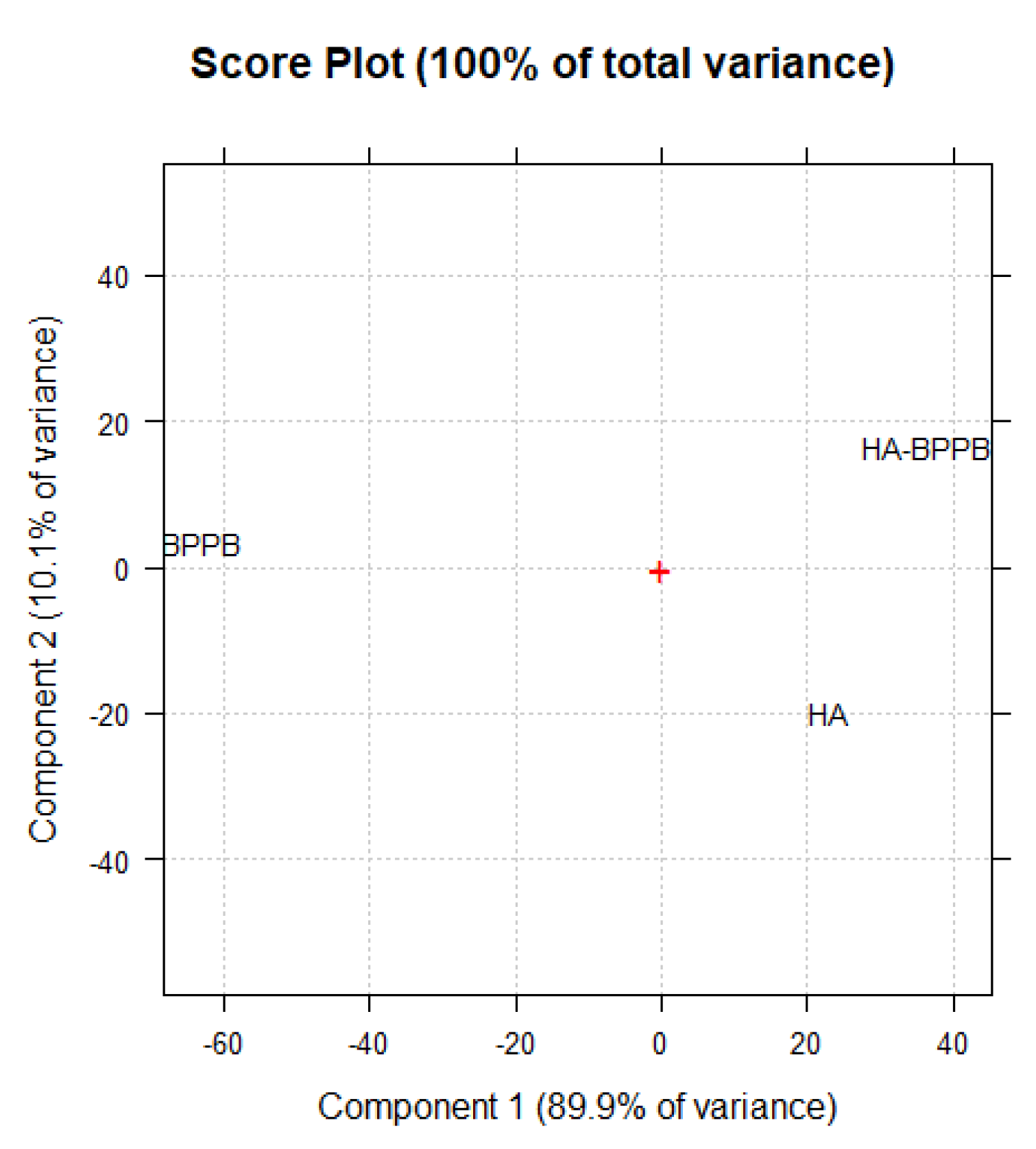
PCA Results and Discussion
2.4.2. Assessment of BPPB Content in HA-BPPB-HG
2.4.3. Scanning Electron Microscopy (SEM)
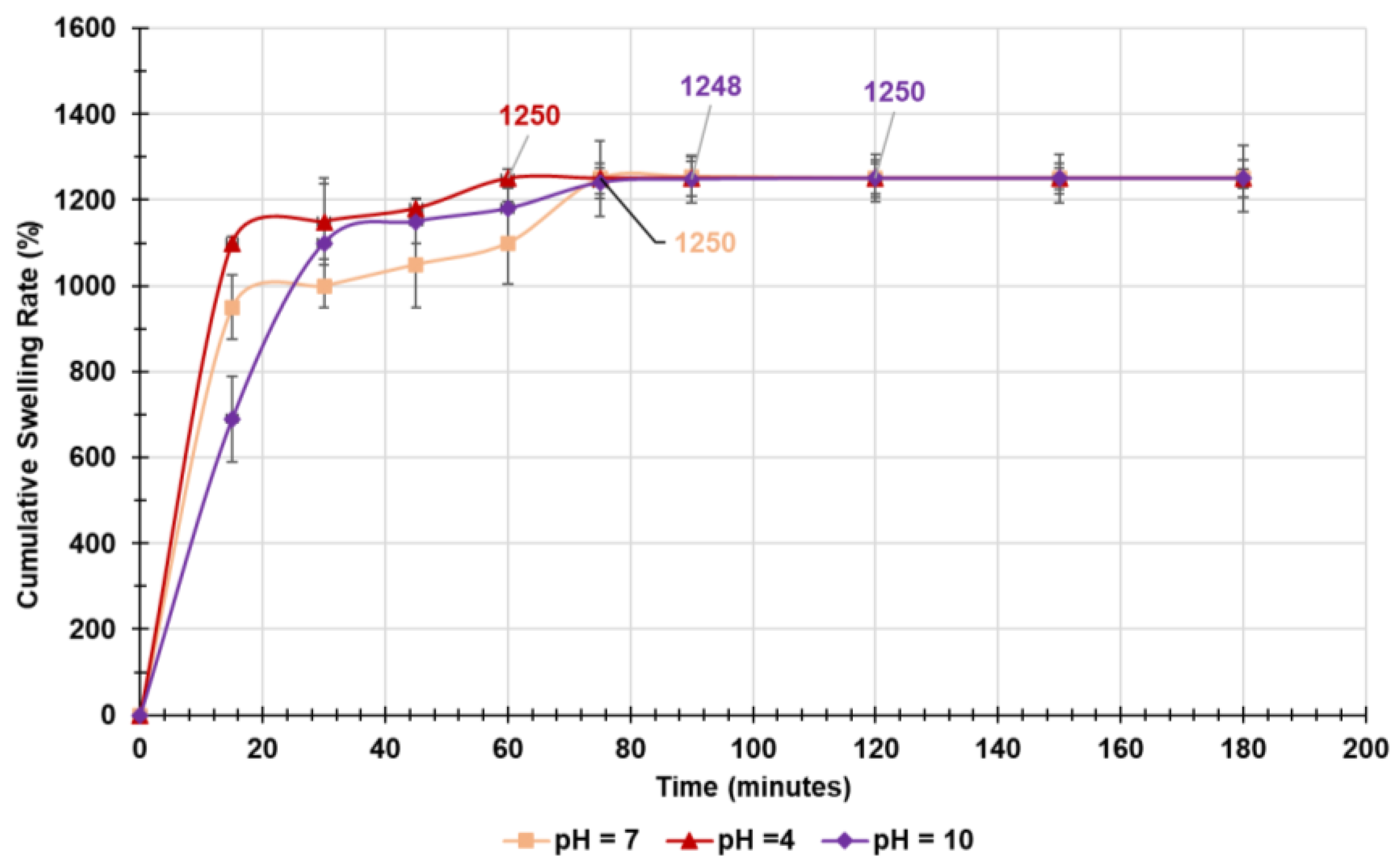
2.4.4. Equilibrium Swelling Rate
Kinetic Studies
Rheologic Considerations
2.4.5. Water Absorption Capacity (WAC (%)), Equilibrium Water Content (EWC (%)) and Porosity (%)
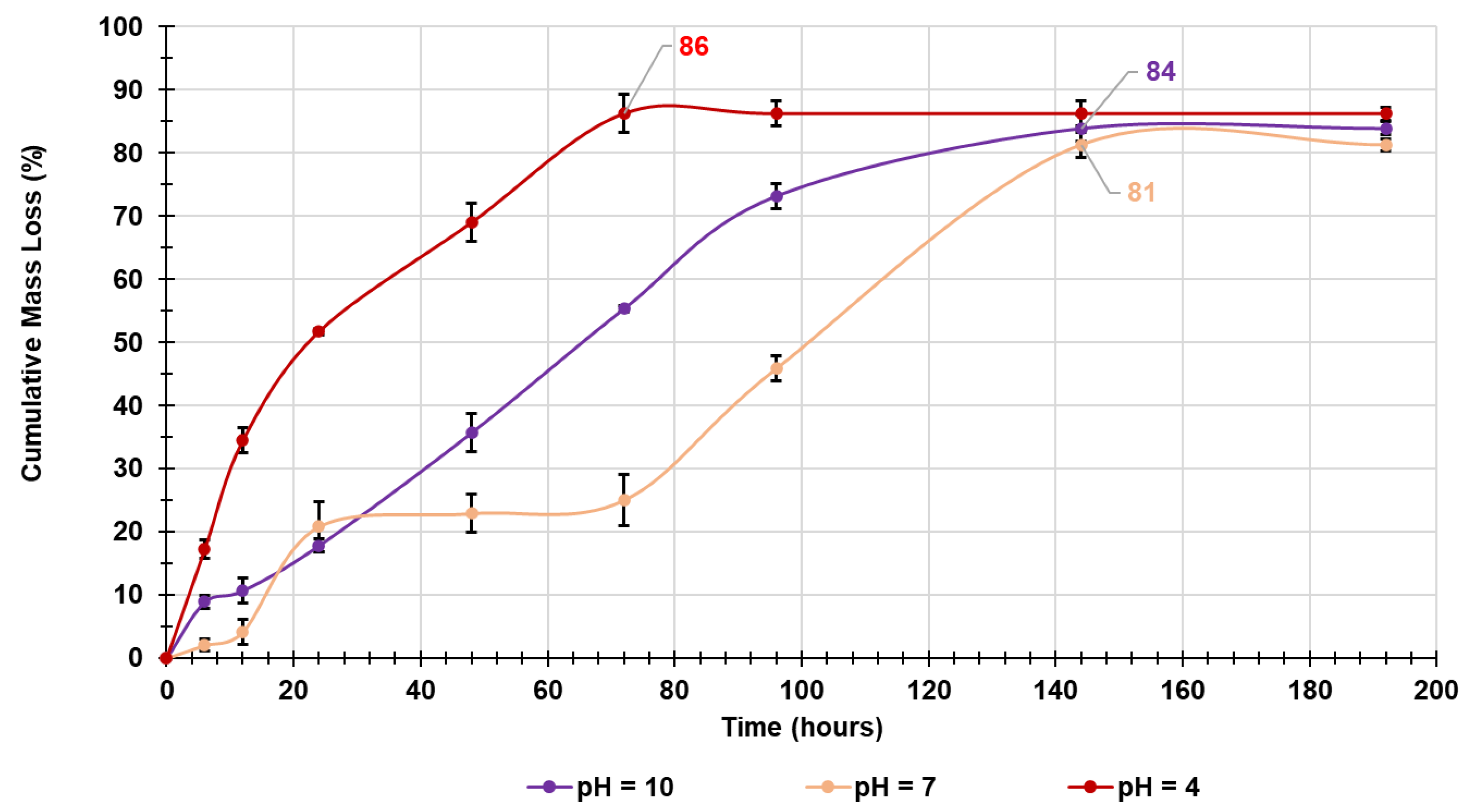
2.4.6. In Vitro Evaluation of Biodegradability of Crosslinked Gelatines Over Time by Mass Loss Experiments
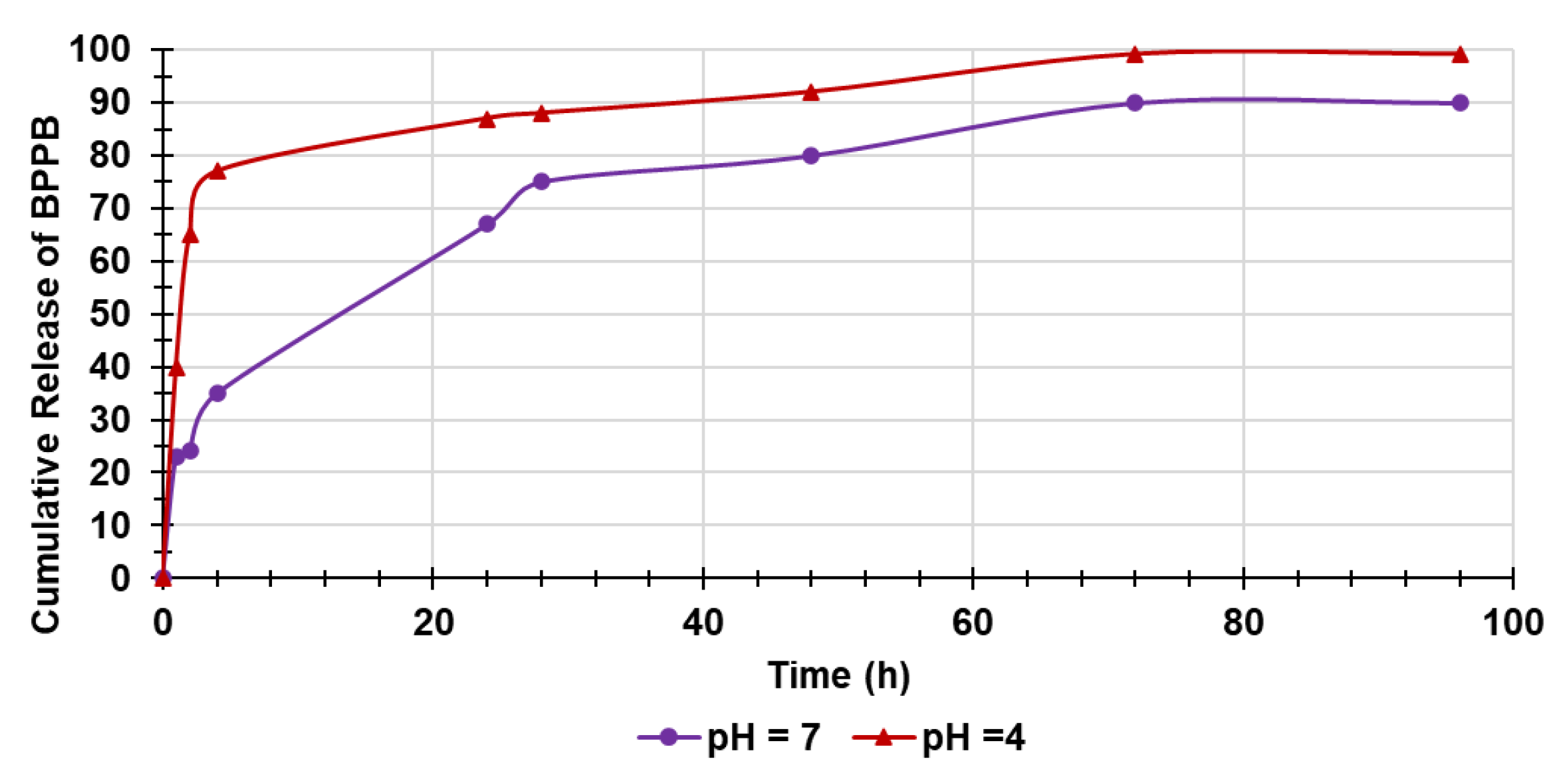
2.4.7. Evaluation of BPPB in Vitro Release Over Time
Kinetic Studies
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. BPPB Cytotoxicity Evaluation on CMM Cells
3.2.1. Cell Culture Conditions
3.2.2. Treatments
3.2.3. Cell Viability Assay
3.2.4. Detection of Hydrogen Peroxide (H2O2) Production
3.2.5. Statistical Analyses
3.3. Preparation of Hyaluronic Acid (HA)-Based BPPB Hydrogel (HA-BPPB-HG)
Reaction Work-Up and Recover of HA-BPPB-HG
3.4. Characterization of HA-BPPB-HG
3.4.1. ATR-FTIR of HA, BPPB and HA-BPPB-HG
3.4.2. Assessment of BPPB Content in HA-BPPB-HG
Drug loading (DL%) nd Encapsulation Efficincy of HA-BPPB-HG
Statistical Analysis
3.4.3. Scanning Electron Microscopy (SEM)
3.4.4. Equilibrium Swelling Rate
3.4.5. Water Absorbing Capacity (WAC (%))
3.4.6. Porosity
3.4.7. Biodegradability of HA-BPPB-HG over Time by In Vitro Mass Loss Experiments
3.4.8. Evaluation of in Vitro Releases of BPPB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boutros: A.; Croce, E.; Ferrari, M.; Gili, R.; Massaro, G.; Marconcini, R.; Arecco, L.; Tanda, E.T.; Spagnolo, F. The Treatment of Advanced Melanoma: Current Approaches and New Challenges. Crit Rev Oncol Hematol 2024, 196, 104276. [CrossRef]
- Hodi, F.S.; Chiarion -Sileni, V.; Lewis, K.D.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Survival in Advanced Melanoma for Patients Treated with Nivolumab plus Ipilimumab in CheckMate 067. Journal of Clinical Oncology 2022, 40, 9522–9522. [CrossRef]
- Garbe, C.; Eigentler, T.K.; Keilholz, U.; Hauschild, A.; Kirkwood, J.M. Systematic Review of Medical Treatment in Melanoma: Current Status and Future Prospects. Oncologist 2011, 16, 5–24. [CrossRef]
- Natarelli, N.; Aleman, S.J.; Mark, I.M.; Tran, J.T.; Kwak, S.; Botto, E.; Aflatooni, S.; Diaz, M.J.; Lipner, S.R. A Review of Current and Pipeline Drugs for Treatment of Melanoma. Pharmaceuticals 2024, 17, 214. [CrossRef]
- Akbani, R.; Akdemir, K.C.; Aksoy, B.A.; Albert, M.; Ally, A.; Amin, S.B.; Arachchi, H.; Arora, A.; Auman, J.T.; Ayala, B.; et al. Genomic Classification of Cutaneous Melanoma. Cell 2015, 161, 1681–1696. [CrossRef]
- Romano, E.; Schwartz, G.K.; Chapman, P.B.; Wolchock, J.D.; Carvajal, R.D. Treatment Implications of the Emerging Molecular Classification System for Melanoma. Lancet Oncol 2011, 12, 913–922. [CrossRef]
- Akbani, R.; Akdemir, K.C.; Aksoy, B.A.; Albert, M.; Ally, A.; Amin, S.B.; Arachchi, H.; Arora, A.; Auman, J.T.; Ayala, B.; et al. Genomic Classification of Cutaneous Melanoma. Cell 2015, 161, 1681–1696. [CrossRef]
- Long, G. V; Swetter, S.M.; Menzies, A.M.; Gershenwald, J.E.; Scolyer, R.A. Cutaneous Melanoma. The Lancet 2023, 402, 485–502. [CrossRef]
- Bradford, P.T.; Goldstein, A.M.; McMaster, M.L.; Tucker, M.A. Acral Lentiginous Melanoma. Arch Dermatol 2009, 145. [CrossRef]
- Chen, Y.A.; Teer, J.K.; Eroglu, Z.; Wu, J.-Y.; Koomen, J.M.; Karreth, F.A.; Messina, J.L.; Smalley, K.S.M. Translational Pathology, Genomics and the Development of Systemic Therapies for Acral Melanoma. Semin Cancer Biol 2020, 61, 149–157. [CrossRef]
- Arrington, J.H.; Reed, R.J.; Ichinose, H.; Krementz, E.T. Plantar Lentiginous Melanoma: A Distinctive Variant of Human Cutaneous Malignant Melanoma. Am J Surg Pathol 1977, 1, 131–143.
- Zhang, C.; Shen, H.; Yang, T.; Li, T.; Liu, X.; Wang, J.; Liao, Z.; Wei, J.; Lu, J.; Liu, H.; et al. A Single-Cell Analysis Reveals Tumor Heterogeneity and Immune Environment of Acral Melanoma. Nat Commun 2022, 13, 7250. [CrossRef]
- He, Z.; Xin, Z.; Yang, Q.; Wang, C.; Li, M.; Rao, W.; Du, Z.; Bai, J.; Guo, Z.; Ruan, X.; et al. Mapping the Single-Cell Landscape of Acral Melanoma and Analysis of the Molecular Regulatory Network of the Tumor Microenvironments. Elife 2022, 11. [CrossRef]
- Li, J.; Smalley, I.; Chen, Z.; Wu, J.-Y.; Phadke, M.S.; Teer, J.K.; Nguyen, T.; Karreth, F.A.; Koomen, J.M.; Sarnaik, A.A.; et al. Single-Cell Characterization of the Cellular Landscape of Acral Melanoma Identifies Novel Targets for Immunotherapy. Clinical Cancer Research 2022, 28, 2131–2146. [CrossRef]
- Carvalho, L.A.D.; Aguiar, F.C.; Smalley, K.S.M.; Possik, P.A. Acral Melanoma: New Insights into the Immune and Genomic Landscape. Neoplasia 2023, 46, 100947. [CrossRef]
- Hanrahan, A.J.; Chen, Z.; Rosen, N.; Solit, D.B. BRAF — a Tumour-Agnostic Drug Target with Lineage-Specific Dependencies. Nat Rev Clin Oncol 2024, 21, 224–247. [CrossRef]
- Brusnakov, M.; Golovchenko, O.; Velihina, Y.; Liavynets, O.; Zhirnov, V.; Brovarets, V. Evaluation of Anticancer Activity of 1,3-Oxazol-4-ylphosphonium Salts in Vitro. ChemMedChem 2022, 17. [CrossRef]
- Gerges, A.; Canning, U. Neuroblastoma and Its Target Therapies: A Medicinal Chemistry Review. ChemMedChem 2024, 19. [CrossRef]
- Alfei, S.; Schito, G.C.; Schito, A.M.; Zuccari, G. ..Reactive Oxygen Species (ROS)-Mediated Antibacterial Oxidative Therapies: Available Methods to Generate ROS and a Novel Option Proposal. IJMS 2024, 2024051628.
- Dryden, M. Reactive Oxygen Species: A Novel Antimicrobial. Int J Antimicrob Agents 2018, 51, 299–303. [CrossRef]
- Dryden, M. Reactive Oxygen Therapy: A Novel Therapy in Soft Tissue Infection. Curr Opin Infect Dis 2017, 30, 143–149. [CrossRef]
- Van Loenhout, J.; Peeters, M.; Bogaerts, A.; Smits, E.; Deben, C. Oxidative Stress-Inducing Anticancer Therapies: Taking a Closer Look at Their Immunomodulating Effects. Antioxidants 2020, 9, 1188. [CrossRef]
- Baldea, I.; Giurgiu, L.; Teacoe, I.D.; Olteanu, D.E.; Olteanu, F.C.; Clichici, S.; Filip, G.A. Photodynamic Therapy in Melanoma - Where Do We Stand? Curr Med Chem 2019, 25, 5540–5563. [CrossRef]
- Tanaka, M.; Kataoka, H.; Mabuchi, M.; Sakuma, S.; Takahashi, S.; Tujii, R.; Akashi, H.; Ohi, H.; Yano, S.; Morita, A.; et al. Anticancer Effects of Novel Photodynamic Therapy with Glycoconjugated Chlorin for Gastric and Colon Cancer. Anticancer Res 2011, 31, 763–769.
- Jiang, W.; Liang, M.; Lei, Q.; Li, G.; Wu, S. The Current Status of Photodynamic Therapy in Cancer Treatment. Cancers (Basel) 2023, 15, 585. [CrossRef]
- Sai, D.L.; Lee, J.; Nguyen, D.L.; Kim, Y.-P. Tailoring Photosensitive ROS for Advanced Photodynamic Therapy. Exp Mol Med 2021, 53, 495–504. [CrossRef]
- Dhanasekaran, S.; Venugopal, D.; Al-Dayan, N.; Ravinayagam, V.; Mohammed, A.A. Emerging Insights into Mitochondria-Specific Targeting and Drug Delivering Strategies: Recent Milestones and Therapeutic Implications. Saudi J Biol Sci 2020, 27, 3581–3592. [CrossRef]
- Kulkarni, C.A.; Fink, B.D.; Gibbs, B.E.; Chheda, P.R.; Wu, M.; Sivitz, W.I.; Kerns, R.J. A Novel Triphenylphosphonium Carrier to Target Mitochondria without Uncoupling Oxidative Phosphorylation. J Med Chem 2021, 64, 662–676. [CrossRef]
- Alfei, S.; Giannoni, P.; Signorello, M.G.; Torazza, C.; Zuccari, G.; Athanassopoulos, C.; Domenicotti, C.; Marengo, B. Remarkable and Selective Cytotoxicity of Synthesized Bola-Amphiphilic Nano Vesicles on Etoposide-Sensitive and Resistant Neuroblastoma Cells. Preprint 2024, 2024080465. [CrossRef]
- Cheng, X.; Feng, D.; Lv, J.; Cui, X.; Wang, Y.; Wang, Q.; Zhang, L. Application Prospects of Triphenylphosphine-Based Mitochondria-Targeted Cancer Therapy. Cancers (Basel) 2023, 15, 666. [CrossRef]
- Bacchetti, F.; Schito, A.M.; Milanese, M.; Castellaro, S.; Alfei, S. Anti Gram-Positive Bacteria Activity of Synthetic Quaternary Ammonium Lipid and Its Precursor Phosphonium Salt. Int J Mol Sci 2024, 25, 2761. [CrossRef]
- Alfei, S.; Zuccari, G.; Bacchetti, F.; Torazza, C.; Milanese, M.; Siciliano, C.; Athanassopoulos, C.M.; Piatti, G.; Schito, A.M. Synthesized Bis-Triphenyl Phosphonium-Based Nano Vesicles Have Potent and Selective Antibacterial Effects on Several Clinically Relevant Superbugs. Nanomaterials 2024, 14, 1351. [CrossRef]
- Severina, I.I.; Vyssokikh, M.Yu.; Pustovidko, A. V.; Simonyan, R.A.; Rokitskaya, T.I.; Skulachev, V.P. Effects of Lipophilic Dications on Planar Bilayer Phospholipid Membrane and Mitochondria. Biochimica et Biophysica Acta (BBA) - Bioenergetics 2007, 1767, 1164–1168. [CrossRef]
- Bachowska, B.; Kazmierczak-Baranska, J.; Cieslak, M.; Nawrot, B.; Szczęsna, D.; Skalik, J.; Bałczewski, P. High Cytotoxic Activity of Phosphonium Salts and Their Complementary Selectivity towards HeLa and K562 Cancer Cells: Identification of Tri- n -butyl- n -hexadecylphosphonium Bromide as a Highly Potent Anti-HeLa Phosphonium Salt. ChemistryOpen 2012, 1, 33–38. [CrossRef]
- Nuraje, N.; Bai, H.; Su, K. Bolaamphiphilic Molecules: Assembly and Applications. Prog Polym Sci 2013, 38, 302–343. [CrossRef]
- Ceccacci, F.; Sennato, S.; Rossi, E.; Proroga, R.; Sarti, S.; Diociaiuti, M.; Casciardi, S.; Mussi, V.; Ciogli, A.; Bordi, F.; et al. Aggregation Behaviour of Triphenylphosphonium Bolaamphiphiles. J Colloid Interface Sci 2018, 531, 451–462. [CrossRef]
- Ermolaev, V. V.; Arkhipova, D.M.; Miluykov, V.A.; Lyubina, A.P.; Amerhanova, S.K.; Kulik, N. V.; Voloshina, A.D.; Ananikov, V.P. Sterically Hindered Quaternary Phosphonium Salts (QPSs): Antimicrobial Activity and Hemolytic and Cytotoxic Properties. Int J Mol Sci 2021, 23, 86. [CrossRef]
- Lukáč, M.; Pisárčik, M.; Garajová, M.; Mrva, M.; Dušeková, A.; Vrták, A.; Horáková, R.; Horváth, B.; Devínsky, F. Synthesis, Surface Activity, and Biological Activities of Phosphonium and Metronidazole Salts. J Surfactants Deterg 2020, 23, 1025–1032. [CrossRef]
- Khasiyatullina, N.R.; Mironov, V.F.; Gumerova, S.K.; Voloshina, A.D.; Sapunova, A.S. Versatile Approach to Naphthoquinone Phosphonium Salts and Evaluation of Their Biological Activity. Mendeleev Communications 2019, 29, 435–437. [CrossRef]
- Lei, Q.; Lai, X.; Zhang, Y.; Li, Z.; Li, R.; Zhang, W.; Ao, N.; Zhang, H. PEGylated Bis-Quaternary Triphenyl-Phosphonium Tosylate Allows for Balanced Antibacterial Activity and Cytotoxicity. ACS Appl Bio Mater 2020, 3, 6400–6407. [CrossRef]
- Bergeron, K.L.; Murphy, E.L.; Majofodun, O.; Muñoz, L.D.; Williams, J.C.; Almeida, K.H. Arylphosphonium Salts Interact with DNA to Modulate Cytotoxicity. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 2009, 673, 141–148. [CrossRef]
- Millard, M.; Pathania, D.; Shabaik, Y.; Taheri, L.; Deng, J.; Neamati, N. Preclinical Evaluation of Novel Triphenylphosphonium Salts with Broad-Spectrum Activity. PLoS One 2010, 5, e13131. [CrossRef]
- Huang, H.; Wu, H.; Huang, Y.; Zhang, S.; Lam, Y.; Ao, N. Antitumor Activity and Antitumor Mechanism of Triphenylphosphonium Chitosan against Liver Carcinoma. J Mater Res 2018, 33, 2586–2597. [CrossRef]
- Arafa, K.K.; Hamzawy, M.A.; Mousa, S.A.; El-Sherbiny, I.M. Mitochondria-Targeted Alginate/Triphenylphosphonium-Grafted-Chitosan for Treatment of Hepatocellular Carcinoma. RSC Adv 2022, 12, 21690–21703. [CrossRef]
- Valizadeh, A.; Khaleghi, A.A.; Alipanah, H.; Zarenezhad, E.; Osanloo, M. Anticarcinogenic Effect of Chitosan Nanoparticles Containing Syzygium Aromaticum Essential Oil or Eugenol Toward Breast and Skin Cancer Cell Lines. Bionanoscience 2021, 11, 678–686. [CrossRef]
- Wöckel, A.; Wolters, R.; Wiegel, T.; Novopashenny, I.; Janni, W.; Kreienberg, R.; Wischnewsky, M.; Schwentner, L. The Impact of Adjuvant Radiotherapy on the Survival of Primary Breast Cancer Patients: A Retrospective Multicenter Cohort Study of 8935 Subjects. Annals of Oncology 2014, 25, 628–632. [CrossRef]
- Haider, T.; Pandey, V.; Banjare, N.; Gupta, P.N.; Soni, V. Drug Resistance in Cancer: Mechanisms and Tackling Strategies. Pharmacological Reports 2020, 72, 1125–1151. [CrossRef]
- Marzi, M.; Rostami Chijan, M.; Zarenezhad, E. Hydrogels as Promising Therapeutic Strategy for the Treatment of Skin Cancer. J Mol Struct 2022, 1262, 133014. [CrossRef]
- Chavda, H.; Patel, C. Effects of Solvent Treatments on Characteristics of Superporous Hydrogels. Ethiopian Pharmaceutical Journal 2010, 27. [CrossRef]
- Alfei, S.; Milanese, M.; Brullo, C.; Valenti, G.E.; Domenicotti, C.; Russo, E.; Marengo, B. Antiproliferative Imidazo-Pyrazole-Based Hydrogel: A Promising Approach for the Development of New Treatments for PLX-Resistant Melanoma. Pharmaceutics 2023, 15, 2425. [CrossRef]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv Drug Deliv Rev 2012, 64, 18–23. [CrossRef]
- Luo, Z.; Wang, Y.; Xu, Y.; Wang, J.; Yu, Y. Modification and Crosslinking Strategies for Hyaluronic Acid-based Hydrogel Biomaterials. Smart Medicine 2023, 2. [CrossRef]
- Maloney, F.P.; Kuklewicz, J.; Corey, R.A.; Bi, Y.; Ho, R.; Mateusiak, L.; Pardon, E.; Steyaert, J.; Stansfeld, P.J.; Zimmer, J. Structure, Substrate Recognition and Initiation of Hyaluronan Synthase. Nature 2022, 604, 195–201. [CrossRef]
- Cai, J.; Fu, J.; Li, R.; Zhang, F.; Ling, G.; Zhang, P. A Potential Carrier for Anti-Tumor Targeted Delivery-Hyaluronic Acid Nanoparticles. Carbohydr Polym 2019, 208, 356–364. [CrossRef]
- Khetan, S.; Guvendiren, M.; Legant, W.R.; Cohen, D.M.; Chen, C.S.; Burdick, J.A. Degradation-Mediated Cellular Traction Directs Stem Cell Fate in Covalently Crosslinked Three-Dimensional Hydrogels. Nat Mater 2013, 12, 458–465. [CrossRef]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic Acid and Its Biomedical Applications: A Review. Engineered Regeneration 2020, 1, 102–113. [CrossRef]
- Faivre, J.; Pigweh, A.I.; Iehl, J.; Maffert, P.; Goekjian, P.; Bourdon, F. Crosslinking Hyaluronic Acid Soft-Tissue Fillers: Current Status and Perspectives from an Industrial Point of View. Expert Rev Med Devices 2021, 18, 1175–1187. [CrossRef]
- Yang, X.; Wang, B.; Peng, D.; Nie, X.; Wang, J.; Yu, C.-Y.; Wei, H. Hyaluronic Acid-Based Injectable Hydrogels for Wound Dressing and Localized Tumor Therapy: A Review. Adv Nanobiomed Res 2022, 2. [CrossRef]
- Xu, Q.; Torres, J.E.; Hakim, M.; Babiak, P.M.; Pal, P.; Battistoni, C.M.; Nguyen, M.; Panitch, A.; Solorio, L.; Liu, J.C. Collagen- and Hyaluronic Acid-Based Hydrogels and Their Biomedical Applications. Materials Science and Engineering: R: Reports 2021, 146, 100641. [CrossRef]
- Garbarino, O.; Valenti, G.E.; Monteleone, L.; Pietra, G.; Mingari, M.C.; Benzi, A.; Bruzzone, S.; Ravera, S.; Leardi, R.; Farinini, E.; et al. PLX4032 Resistance of Patient-Derived Melanoma Cells: Crucial Role of Oxidative Metabolism. Front Oncol 2023, 13. [CrossRef]
- Smalley, K.S.M. PLX-4032, a Small-Molecule B-Raf Inhibitor for the Potential Treatment of Malignant Melanoma. Curr Opin Investig Drugs 2010, 11, 699–706.
- Luo, Z.; Wang, Y.; Xu, Y.; Wang, J.; Yu, Y. Modification and Crosslinking Strategies for Hyaluronic Acid-based Hydrogel Biomaterials. Smart Medicine 2023, 2. [CrossRef]
- Xu, X.; Jha, A.K.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X. Hyaluronic Acid-Based Hydrogels: From a Natural Polysaccharide to Complex Networks. Soft Matter 2012, 8, 3280. [CrossRef]
- Singh, S.; Rai, A.K.; Prakash Tewari, R. Recent Advancement in Hyaluronic Acid-Based Hydrogel for Biomedical Engineering Application: A Mini-Review. Mater Today Proc 2023, 78, 138–144. [CrossRef]
- Montanari, E.; Di Meo, C.; Sennato, S.; Francioso, A.; Marinelli, A.L.; Ranzo, F.; Schippa, S.; Coviello, T.; Bordi, F.; Matricardi, P. Hyaluronan-Cholesterol Nanohydrogels: Characterisation and Effectiveness in Carrying Alginate Lyase. N Biotechnol 2017, 37, 80–89. [CrossRef]
- Shi, W.; Hass, B.; Kuss, M.A.; Zhang, H.; Ryu, S.; Zhang, D.; Li, T.; Li, Y.; Duan, B. Fabrication of Versatile Dynamic Hyaluronic Acid-Based Hydrogels. Carbohydr Polym 2020, 233, 115803. [CrossRef]
- Alfei, S.; Grasso, F.; Orlandi, V.; Russo, E.; Boggia, R.; Zuccari, G. Cationic Polystyrene-Based Hydrogels as Efficient Adsorbents to Remove Methyl Orange and Fluorescein Dye Pollutants from Industrial Wastewater. Int J Mol Sci 2023, 24, 2948. [CrossRef]
- Alfei, S.; Zuccari, G.; Russo, E.; Villa, C.; Brullo, C. Hydrogel Formulations of Antibacterial Pyrazoles Using a Synthesized Polystyrene-Based Cationic Resin as a Gelling Agent. Int J Mol Sci 2023, 24, 1109. [CrossRef]
- Zafar, S.; Hanif, M.; Azeem, M.; Mahmood, K.; Gondal, S.A. Role of Crosslinkers for Synthesizing Biocompatible, Biodegradable and Mechanically Strong Hydrogels with Desired Release Profile. Polymer Bulletin 2022, 79, 9199–9219. [CrossRef]
- Alfei, S.; Zorzoli, A.; Marimpietri, D.; Zuccari, G.; Russo, E.; Caviglia, D.; Schito, A.M. A Self-Forming Hydrogel from a Bactericidal Copolymer: Synthesis, Characterization, Biological Evaluations and Perspective Applications. Int J Mol Sci 2022, 23, 15092. [CrossRef]
- Zhang, K.; Feng, W.; Jin, C. Protocol Efficiently Measuring the Swelling Rate of Hydrogels. MethodsX 2020, 7, 100779. [CrossRef]
- Gulfam, M.; Jo, S.-H.; Vu, T.T.; Ali, I.; Rizwan, A.; Joo, S.-B.; Park, S.-H.; Lim, K.T. NIR-Degradable and Biocompatible Hydrogels Derived from Hyaluronic Acid and Coumarin for Drug Delivery and Bio-Imaging. Carbohydr Polym 2023, 303, 120457. [CrossRef]
- Wu, N.; Yu, H.; Sun, M.; Li, Z.; Zhao, F.; Ao, Y.; Chen, H. Investigation on the Structure and Mechanical Properties of Highly Tunable Elastomeric Silk Fibroin Hydrogels Cross-Linked by γ-Ray Radiation. ACS Appl Bio Mater 2020, 3, 721–734. [CrossRef]
- Carbon Nanomaterials as Adsorbents for Environmental and Biological Applications; Bergmann, C.P., Machado, F.M., Eds.; Springer International Publishing: Cham, 2015; ISBN 978-3-319-18874-4.
- Alfei, S.; Orlandi, V.; Grasso, F.; Boggia, R.; Zuccari, G. Cationic Polystyrene-Based Hydrogels: Low-Cost and Regenerable Adsorbents to Electrostatically Remove Nitrites from Water. Toxics 2023, 11, 312. [CrossRef]
- Alfei, S.; Grasso, F.; Orlandi, V.; Russo, E.; Boggia, R.; Zuccari, G. Cationic Polystyrene-Based Hydrogels as Efficient Adsorbents to Remove Methyl Orange and Fluorescein Dye Pollutants from Industrial Wastewater. Int J Mol Sci 2023, 24, 2948. [CrossRef]
- Mircioiu, C.; Voicu, V.; Anuta, V.; Tudose, A.; Celia, C.; Paolino, D.; Fresta, M.; Sandulovici, R.; Mircioiu, I. Mathematical Modeling of Release Kinetics from Supramolecular Drug Delivery Systems. Pharmaceutics 2019, 11, 140. [CrossRef]
- Vrentas, J.S.; Duda, J.L. Molecular Diffusion in Polymer Solutions. AIChE Journal 1979, 25, 1–24. [CrossRef]
- Crank, J. The Mathematics of Diffusion; 2nd ed.; Clarendon Press: Oxford, 1975; ISBN 0 19 853344 6.
- Lu, T.; Li, Q.; Chen, W.; Yu, H. Composite Aerogels Based on Dialdehyde Nanocellulose and Collagen for Potential Applications as Wound Dressing and Tissue Engineering Scaffold. Compos Sci Technol 2014, 94, 132–138. [CrossRef]
- Alfei, S.; Giordani, P.; Zuccari, G. Synthesis and Physicochemical Characterization of Gelatine-Based Biodegradable Aerogel-like Composites as Possible Scaffolds for Regenerative Medicine. Int J Mol Sci 2024, 25, 5009. [CrossRef]
- Greco, I.; Varon, C.; Iorio, C.S. Synthesis and Characterization of a New Alginate-Gelatine Aerogel for Tissue Engineering. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC); IEEE, July 11 2022; pp. 3915–3918.
- Noman, M.T.; Amor, N.; Ali, A.; Petrik, S.; Coufal, R.; Adach, K.; Fijalkowski, M. Aerogels for Biomedical, Energy and Sensing Applications. Gels 2021, 7, 264. [CrossRef]
- No, Y.J.; Tarafder, S.; Reischl, B.; Ramaswamy, Y.; Dunstan, C.; Friedrich, O.; Lee, C.H.; Zreiqat, H. High-Strength Fiber-Reinforced Composite Hydrogel Scaffolds as Biosynthetic Tendon Graft Material. ACS Biomater Sci Eng 2020, 6, 1887–1898. [CrossRef]
- Freyman, T.M.; Yannas, I.V.; Gibson, L.J. Cellular Materials as Porous Scaffolds for Tissue Engineering. Prog Mater Sci 2001, 46, 273–282. [CrossRef]
- Alfei, S.; Milanese, M.; Brullo, C.; Valenti, G.E.; Domenicotti, C.; Russo, E.; Marengo, B. Antiproliferative Imidazo-Pyrazole-Based Hydrogel: A Promising Approach for the Development of New Treatments for PLX-Resistant Melanoma. Pharmaceutics 2023, 15, 2425. [CrossRef]
- Bitar, K.N.; Zakhem, E. Design Strategies of Biodegradable Scaffolds for Tissue Regeneration. Biomed Eng Comput Biol 2014, 6, BECB.S10961. [CrossRef]
- Zhang, H.; Hao, R.; Ren, X.; Yu, L.; Yang, H.; Yu, H. PEG/Lecithin–Liquid-Crystalline Composite Hydrogels for Quasi-Zero-Order Combined Release of Hydrophilic and Lipophilic Drugs. RSC Adv 2013, 3, 22927. [CrossRef]
- Michielin, O.; van Akkooi, A.C.J.; Ascierto, P.A.; Dummer, R.; Keilholz, U. Cutaneous Melanoma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Annals of Oncology 2019, 30, 1884–1901. [CrossRef]
- Li, S.; Mao, W.; Xia, L.; Wu, X.; Guo, Y.; Wang, J.; Huang, J.; Xiang, H.; Jin, L.; Fu, H.; et al. An Injectable, Self-Healing and Degradable Hydrogel Scaffold as a Functional Biocompatible Material for Tissue Engineering Applications. J Mater Sci 2023, 58, 6710–6726. [CrossRef]
- Wang, J.; Liu, C.; Shuai, Y.; Cui, X.; Nie, L. Controlled Release of Anticancer Drug Using Graphene Oxide as a Drug-Binding Effector in Konjac Glucomannan/Sodium Alginate Hydrogels. Colloids Surf B Biointerfaces 2014, 113, 223–229. [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic Modeling on Drug Release from Controlled Drug Delivery Systems. Acta Pol Pharm 2010, 67, 217–223.
- Alfei, S.; Marengo, B.; Domenicotti, C. Polyester-Based Dendrimer Nanoparticles Combined with Etoposide Have an Improved Cytotoxic and Pro-Oxidant Effect on Human Neuroblastoma Cells. Antioxidants 2020, 9, 50. [CrossRef]
- Alfei, S.; Marengo, B.; Zuccari, G.; Turrini, F.; Domenicotti, C. Dendrimer Nanodevices and Gallic Acid as Novel Strategies to Fight Chemoresistance in Neuroblastoma Cells. Nanomaterials 2020, 10, 1243. [CrossRef]
- Colla, R.; Izzotti, A.; De Ciucis, C.; Fenoglio, D.; Ravera, S.; Speciale, A.; Ricciarelli, R.; Furfaro, A.L.; Pulliero, A.; Passalacqua, M.; et al. Glutathione-Mediated Antioxidant Response and Aerobic Metabolism: Two Crucial Factors Involved in Determining the Multi-Drug Resistance of High-Risk Neuroblastoma. Oncotarget 2016, 7, 70715–70737. [CrossRef]
- Luo, Y.; Kirker, K.R.; Prestwich, G.D. Cross-Linked Hyaluronic Acid Hydrogel Films: New Biomaterials for Drug Delivery. Journal of Controlled Release 2000, 69, 169–184. [CrossRef]
- Liu, X.; Zhou, M.; Zhou, X.; Wang, L.; Liu, X. Functionalized Poly(Arylene Ether Nitrile) Porous Membrane with High Pb(II) Adsorption Performance. Polymers (Basel) 2019, 11, 1412. [CrossRef]
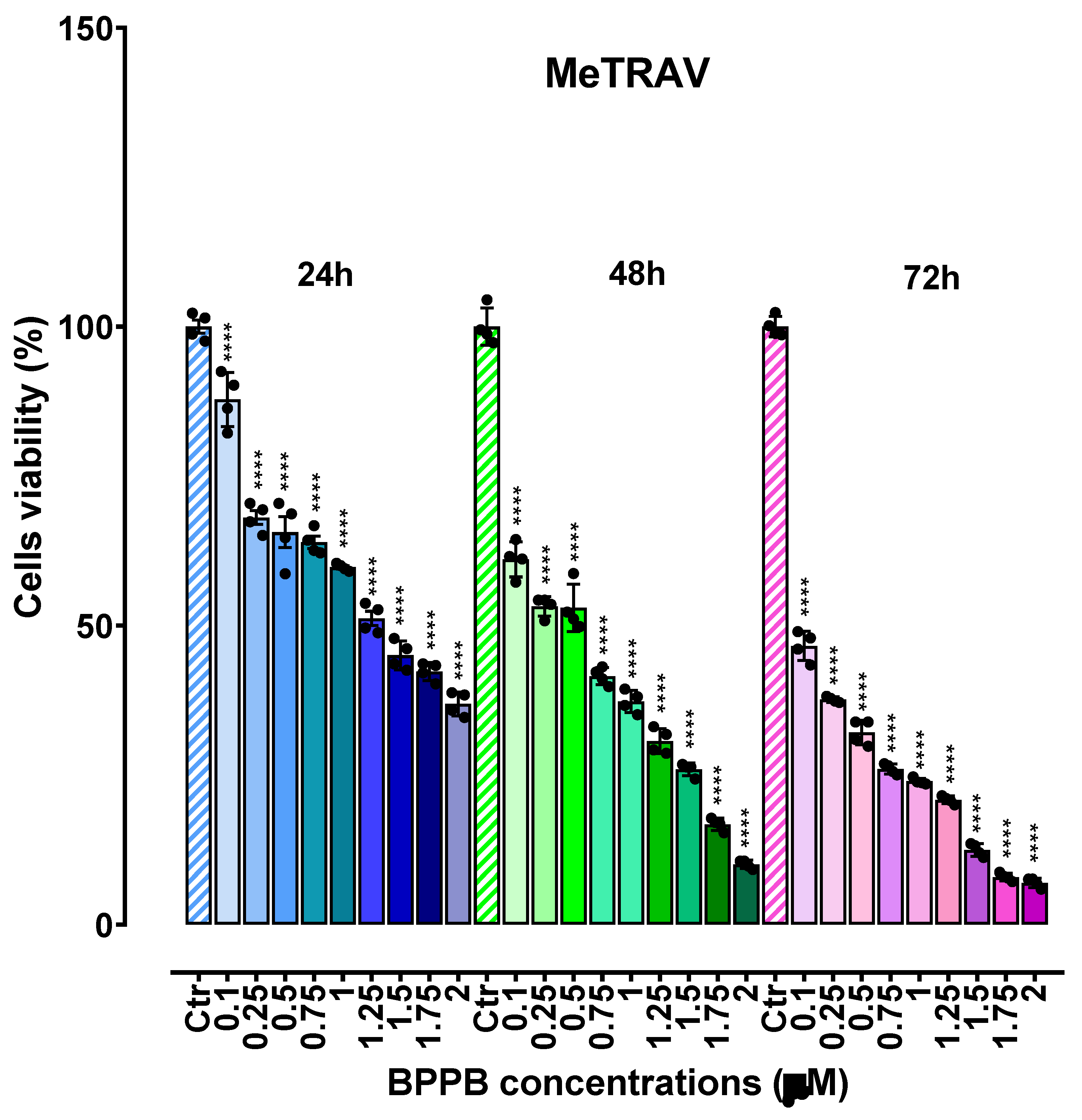

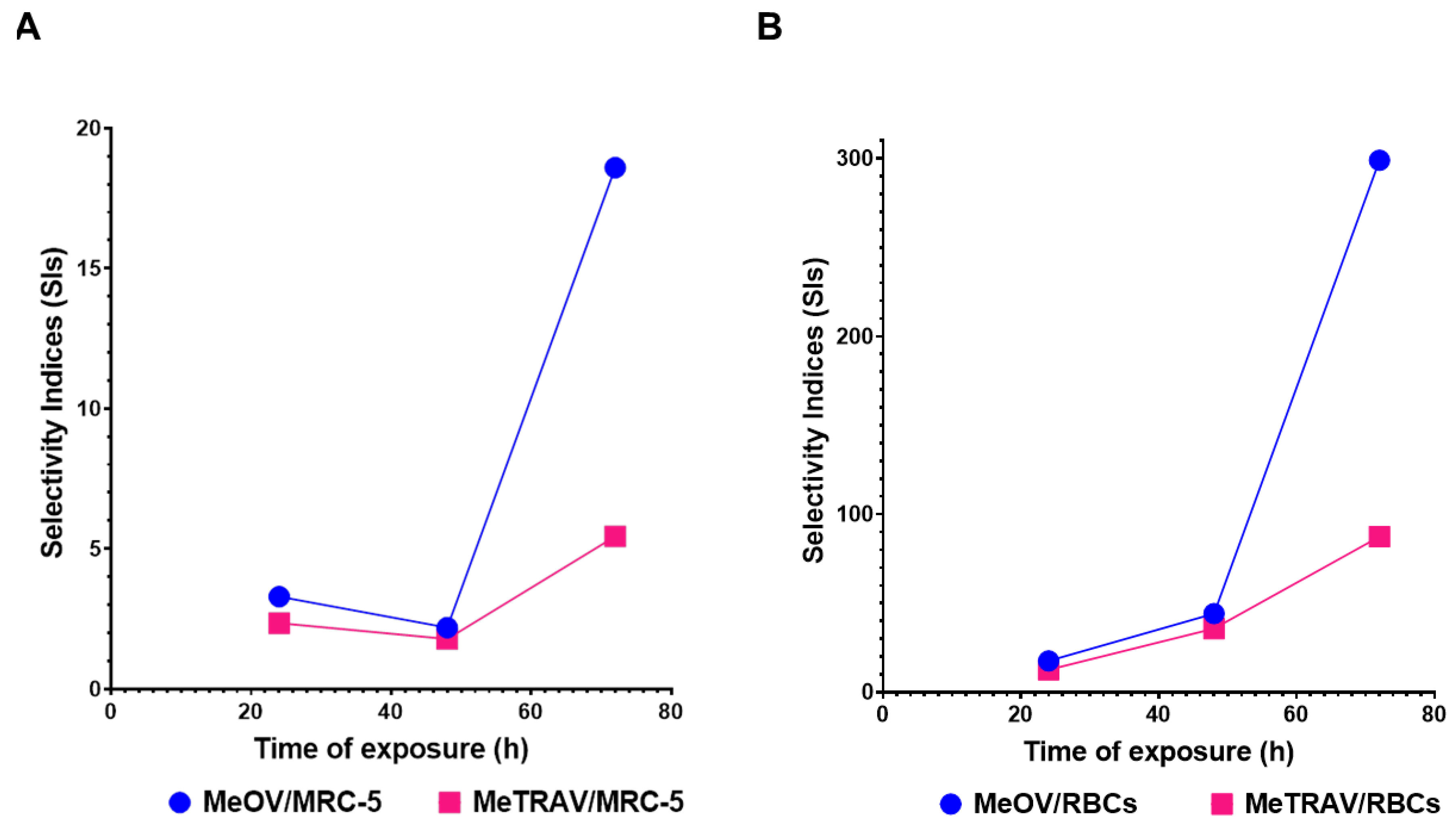
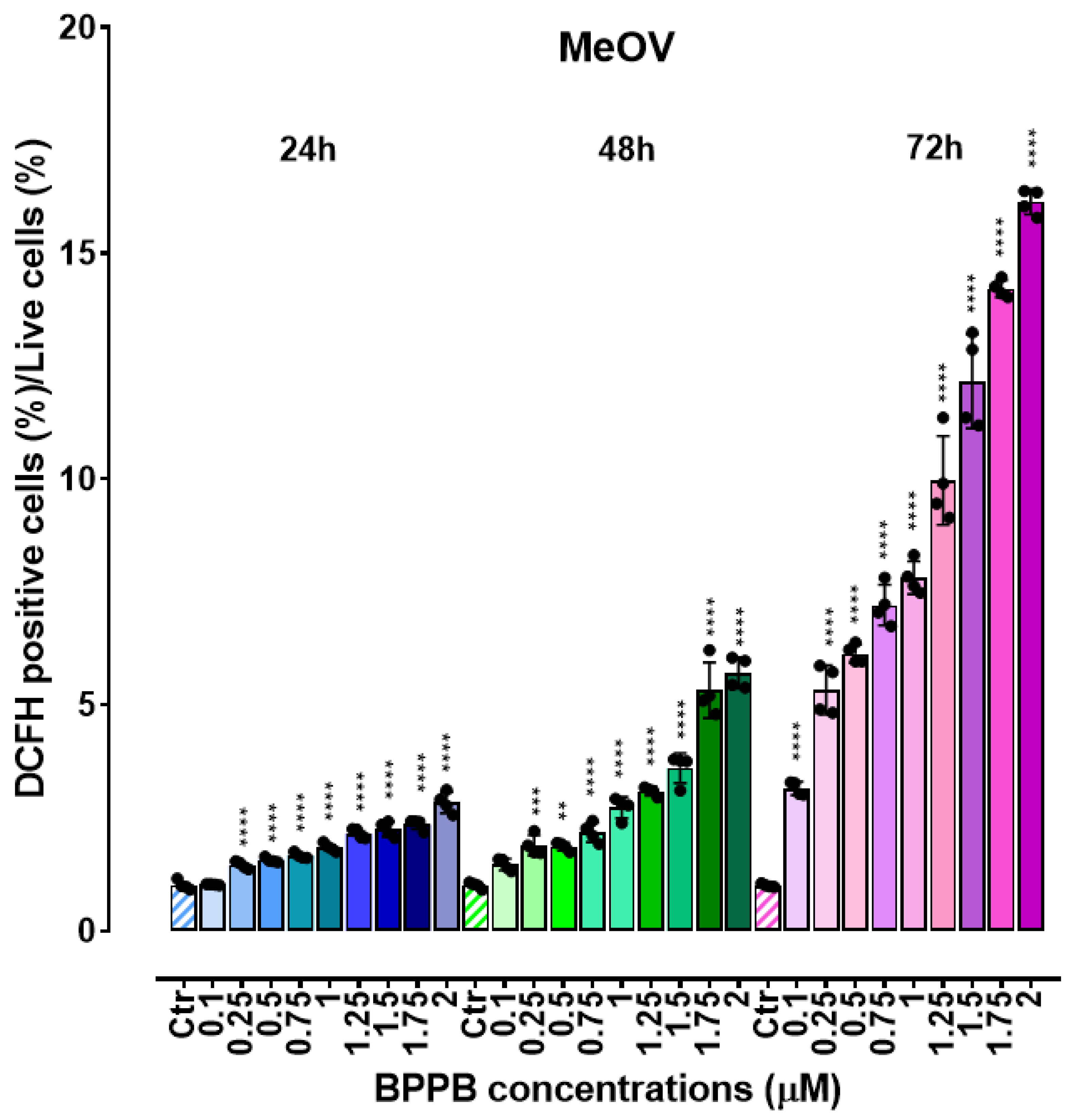
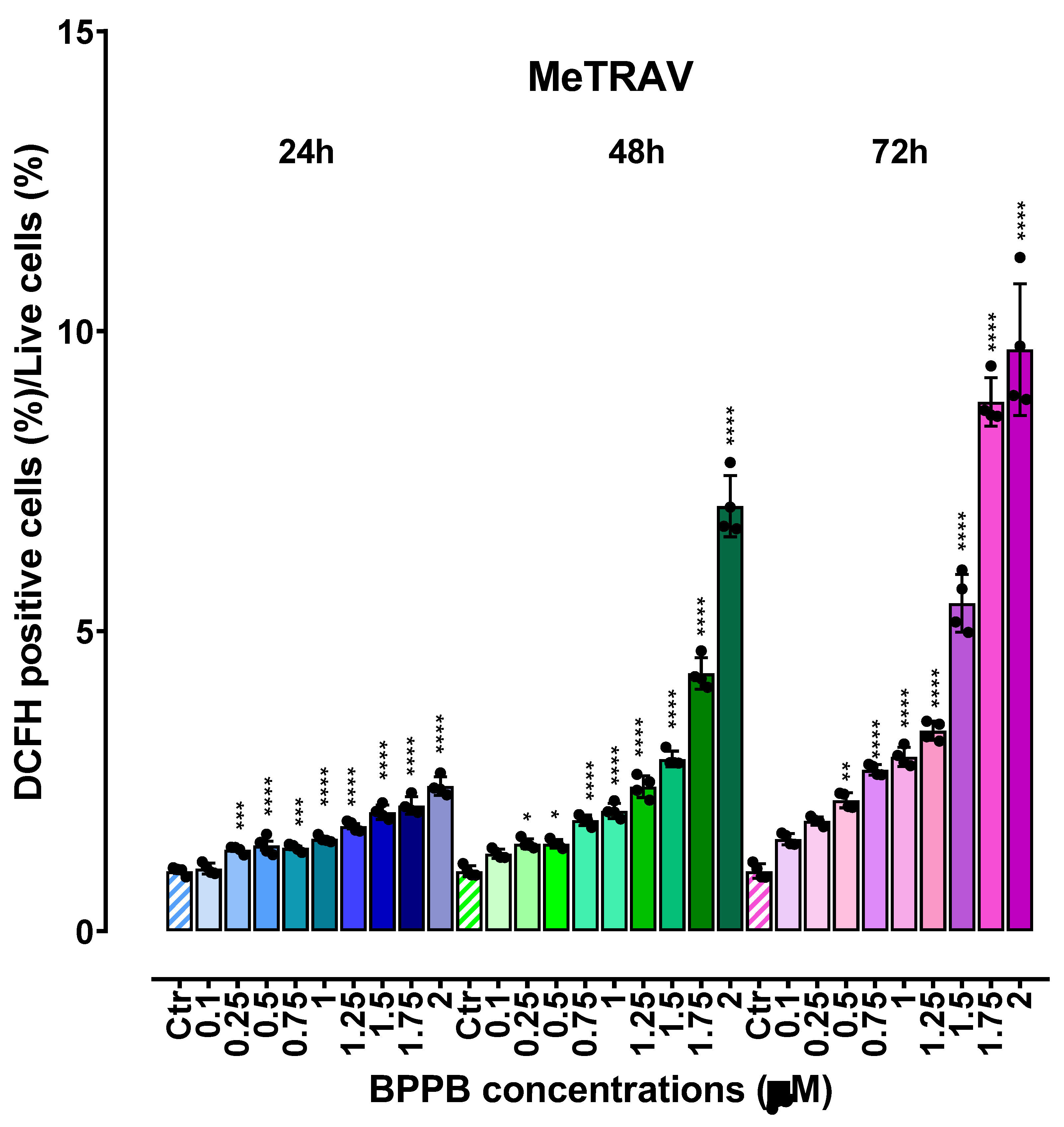

| Exposure time (hours) | IC50 MeOV (µM) | IC50 MeTRAV (µM) |
|---|---|---|
| 24 | 0.8433 ± 0.0901 | 1.1810 ± 0.1210 |
| 48 | 0.3371 ± 0.0585 | 0.4161 ± 0.0711 |
| 72 | 0.0499 ± 0.0080 | 0.1706 ± 0.0328 |
| IC50 (µM) | IC50 24 hours (µM) | IC50 48 hours (µM) | IC50 72 hours (µM) | IC50 experiment time (µM) |
|---|---|---|---|---|
| MRC-5 | 2.7740 ± 2.6655 | 0.7395 ± 0.5716 | 0.9277 ± 0.8956 | A.R. |
| Cos-7 * | 4.9100 ± 0.8100 | N.A.Q. | N.A.Q. | A.R. |
| HepG2 ** | 9.6400 ± 1.3100 | N.A.Q. | N.A.Q. | A.R. |
| RBCs | N.A.Q. | N.A.Q. | N.A.Q. | 14.92 ± 10.80 |
| MeOV | 0.8433 ± 0.0901 | 0.3371 ± 0.0585 | 0.0499 ± 0.0080 | A.R. |
| MeTRAV | 1.1810 ± 0.1210 | 0.4161 ± 0.0711 | 0.1706 ± 0.0328 | A.R. |
| Cells | SI 24 hours a | SI 48 hours a | SI 72 hours a | SI Experiment Time b |
|---|---|---|---|---|
| MRC-5 * | 3.29 | 2.19 | 18.59 | A.R. |
| MRC-5 ** | 2.35 | 1.78 | 5.44 | A.R. |
| Cos-7 * | 5.82 | D.N.A. | D.N.A. | D.N.A. |
| Cos-7 ** | 4.16 | D.N.A. | D.N.A. | D.N.A. |
| HepG2 ** | 11.43 | D.N.A. | D.N.A. | D.N.A. |
| HepG2 ** | 8.16 | D.N.A. | D.N.A. | D.N.A. |
| RBCs * | D.N.A. | D.N.A. | D.N.A. | 17.69 #, 44.26 ##, 299.00 ### |
| RBCs ** | D.N.A. | D.N.A. | D.N.A. | 12.63 #, 35.86 ##, 87.46 ### |
| Sample | WAC (%) | Porosity (%) | EWC(%) | WAC (%) * | (EDS) (%)/Q e (%) ** |
|---|---|---|---|---|---|
| WAC | 1262±72 | 98.3±2.4 | 98.6±2.4 | 1238±18 | 1250±112 |
| Abs | Sample (mg) | BPPB cargo (mg) | DL (%) | EE (%) | |
|---|---|---|---|---|---|
| 0.7852 | 1.20 | 10.00 | 82.7±2.8 | 90.8±3.0 | |
| 0.7630 | MeOH (mL) | HA used (mg) | |||
| 0.7888 | 5.00 | 2.00 | |||
| 0.7722 | mg/mL | BPPB entrapped (mg/mL) | |||
| 0.7663 | 0.24 | 0.1984±0.0066 | |||
| Mean | 0.7751 | BPPB entrapped (mg/1.2 mg) | |||
| S.D. | 0.0114 | 0.9920±0.0330 | |||
| Sample | KPSO | Q e (%)EXP | Q e (%)PSO |
|---|---|---|---|
| pH = 4 | 3.77 × 10-4 | 1250±23 | 1250 |
| pH = 7 | 1.04 × 10-4 | 1250±87 | 1250 |
| pH = 10 | 1.10 × 10-4 | 1250±55 | 1250 |
| Sample | D e (%)PSO * | D e (%)EXP | K (PSO) ** |
|---|---|---|---|
| pH = 7 | 95.2 | 90.0 | 0.0015 |
| pH = 4 | 101.0 | 99.2 | 0.0042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





