Submitted:
06 September 2024
Posted:
09 September 2024
You are already at the latest version
Abstract
Keywords:
Introduction
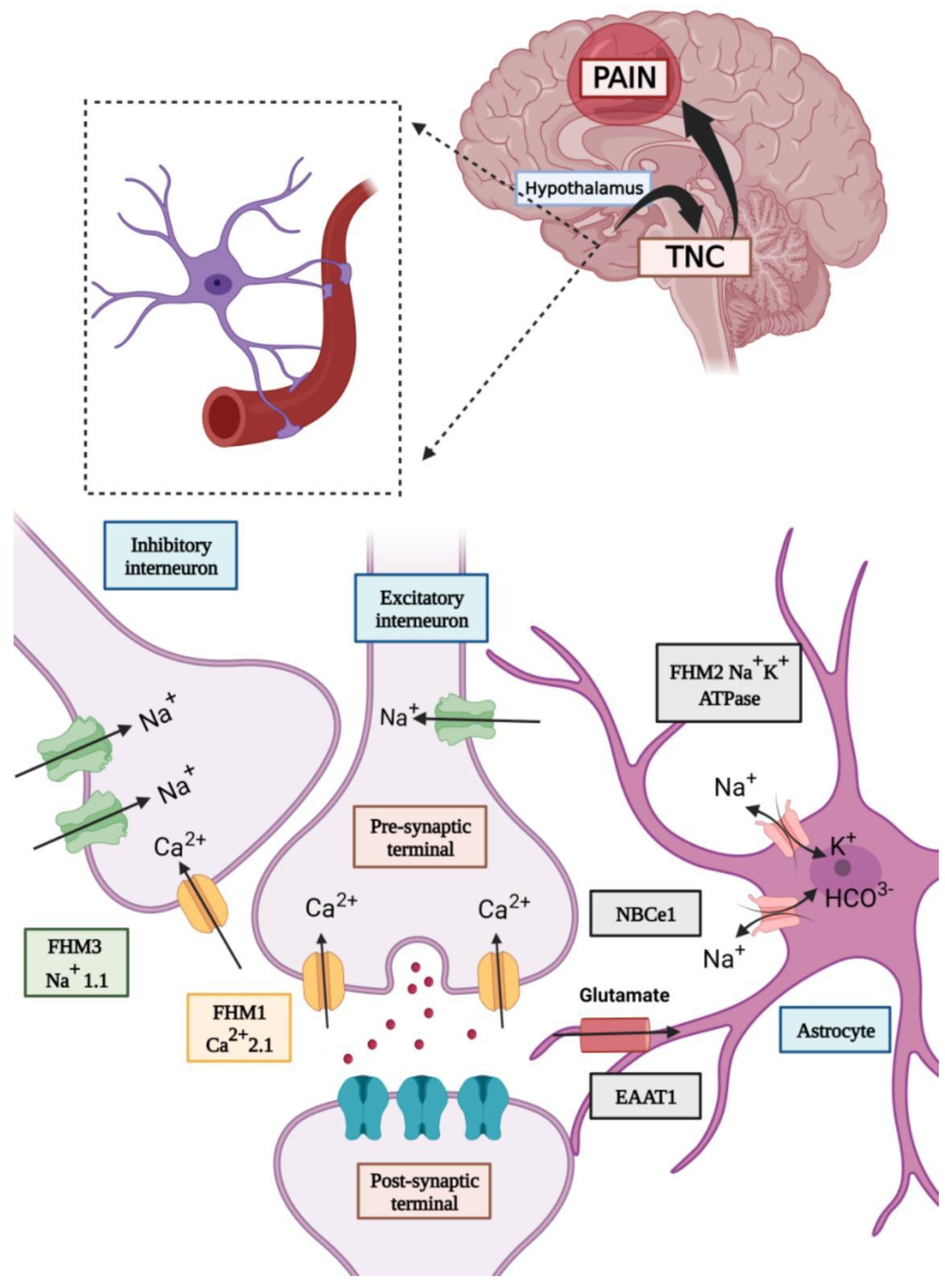
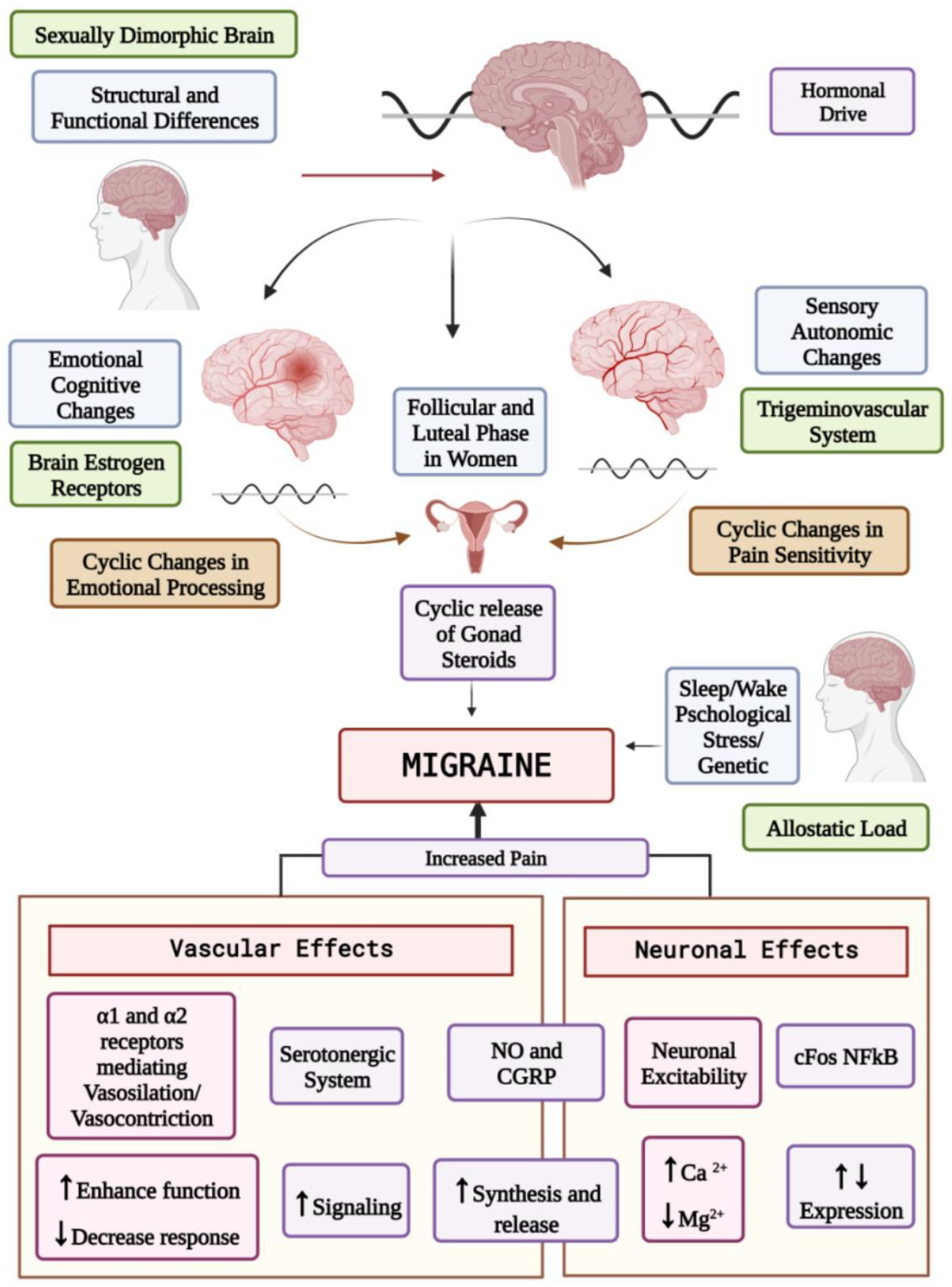
Impact of Sex Hormones in the Pathophysiology of Migraine
Physiology of the Female Hormonal Life Cycle
Effects of Sex Hormones on the Central Nervous System
Effects on Neurotransmitter Systems
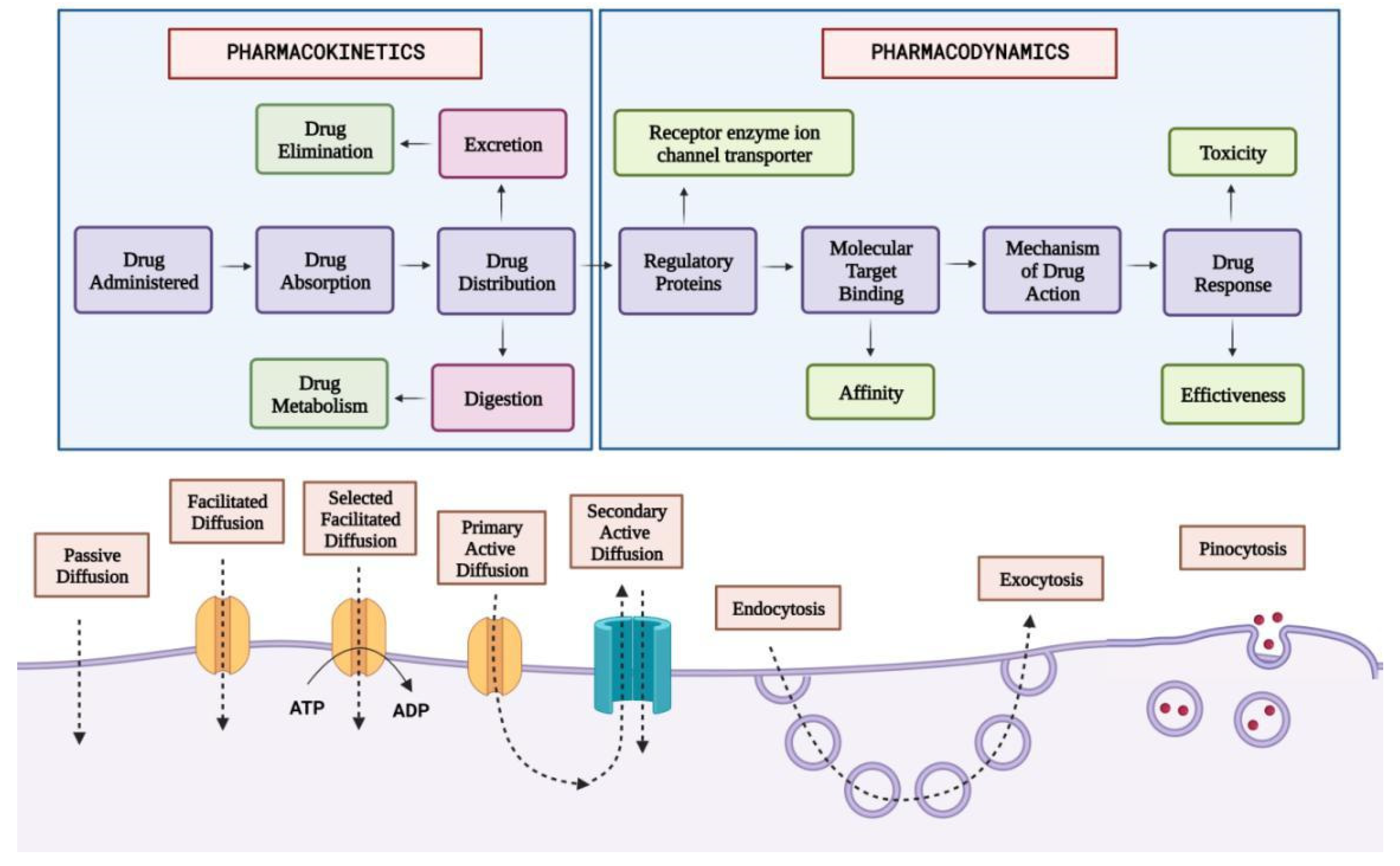
Management of Migraine
Abortive Therapy For the Management of Migraine
- Triptans: Triptans comprise a class of medications approved by the US Food and Drug Administration (FDA) as the first-line agent for treating acute migraine episodes with or without aura [101,102]. In the United States, seven triptans are available in diverse dosage formulations, including Sumatriptan, Naratriptan, Zolmitriptan, Rizatriptan, Almotriptan, frovatriptan, and Eletriptan [103]. Triptans bind to the vascular 5-HT1B receptors, leading to vasoconstriction of the cranial arteries, which dilate during a migraine attack. Triptans bind to the neurogenic and central 5-HT1D receptors and prevent the release of vasoactive neuropeptides by inhibiting trigeminal nerve activation and blocking the transmission of pain signals to the central nervous system [104]. Triptans are available in multiple dosage forms, including oral tablets, orally disintegrating tablets, nasal sprays, and SQ injections to accommodate patient preferences [103]. Patients are instructed to administer triptans at the first onset of the headache phase of a migraine attack, as their efficacy diminishes if taken during the aura phase before the onset of the headache [104]. If a patient has no response to one of the triptans after III trials, increasing the dose, switching to a different dosage of the same agent, or another triptan should be considered [105]. Triptans may cause nausea, dizziness, coronary vasoconstriction, flushing and paresthesia [106].
- Sumatriptan: Sumatriptan acts as an agonist on 5-HT1B/1D receptors by inducing vasoconstriction in the basilar artery and blood vessels within the dura mater. The drug reduces peripheral nociception either by selective cranial vasoconstriction or by affecting trigeminovascular nerves [107].
- Frovatriptan: It is the most potent triptan for its selective action on the 5HT1B receptor. It has a high affinity for 5HT1B and 5HT1D, like other triptans, and also a moderate affinity for 5HT1A and 5HT1F [108,109]. Studies have revealed the role of estrogen in mediating serotonin levels in the raphe nucleus by regulating the function of the tyrosine hydroxylase enzyme involved in the synthesis of 5HT and reducing the reuptake of this neurotransmitter. Estrogen also lowers the vasoconstriction effect of 5HT [110,111]. Frovatriptan attains twice the higher plasma levels in females as compared to males due to higher bioavailability in females [111,112,113]. It has a longer half-life and also a lower recurrence rate in the next 24 hours [114]. Naratriptan, Rizatriptan, and Zolmitriptan also exhibit similar efficacy in migraine management; however, they do not exhibit a sexually dimorphic response [115]. Frovatriptan is chiefly metabolized by CYP1A2 and is cleared by the kidney and liver making moderate failure of either organ not a limiting factor in treatment. Frovatriptan has a low risk of interactions with other drugs [116].
- Lasmiditan—Lasmiditan is a high-affinity, highly selective 5-HT1F receptor agonist that acts on the trigeminal system, where it hyperpolarizes nerve endings and reduces the release of CGRP to provide pain relief [117]. Because it is selective to 5-HT1F receptors, lasmiditan has no action on 5-HT1B/1D receptors located on cerebral blood vessels and does not involve vasoconstrictive mechanisms [118]. Lasmiditan can be used as an acute therapy for migraine in patients for whom triptans may be ineffective or contraindicated [119]. It is less likely to produce other side effects common to triptans, such as chest, neck, and throat tightness, and, therefore, may be a useful option for patients who experience these undesired phenomena [120]. The most common adverse effects associated with Lasmiditan use include dizziness, nausea, fatigue and paresthesias, which are dose-dependent [121].
- NSAIDs: Non-steroidal anti-inflammatory Drugs (NSAIDs) function by inhibiting the enzyme cyclooxygenase, which is responsible for the conversion of arachidonic acid into prostaglandins, especially E1, F2⍺, I (prostacyclin). These prostaglandins alter the excitability of afferent neurons and the sensitivity of the trigeminal nerve [122].
- CGRP Antagonist: CGRP antagonists reduce neuronal inflammation and thereby provide relief from migraine headaches [123]. Some of the CGRP antagonists that have the potential for abortive management of migraine episodes are Rimegepant, Olcegepant, and Telcagepant. Studies in healthy human beings have revealed that CGRP levels are higher in plasma in females than in males [123]. Animal experiments have revealed that in ovariectomized rats, administration of estrogen increases the level of CGRP in the arterioles. This establishes the fact that CGRP levels are higher in males than females and are particularly raised at higher levels by the intake of estrogen-containing birth control pills [124]
- Hormone Therapy: Fluctuating levels of sex steroids, both estrogen and androgen, have been found to be associated with frequent attacks of migraine. Menstrual migraine is one such condition where the fluctuation of plasma estrogen level is more drastic than in non-migraineurs [125]. This was proved in a study where intramuscular administration of estrogen during the perimenstrual period led to the postponement of migraine attacks. However, the same effect was not seen in the administration of progesterone during migraine attacks [126,127]. HRT, which is used for the management of postmenopausal symptoms, can also lead to the onset of migraine de novo or worsening of the preexisting condition. These conditions can be managed by minimizing the degree of fluctuation of the level of estrogen. This can be done by administration of gels and patch forms of sex steroids rather than their oral variants [128,129]. The association of migraine attacks is higher in surgically induced menopause than in naturally induced conditions. Furthermore, studies have shown that in male-to-female transsexuals, the use of anti-androgens to suppress male characteristics has led to an increased frequency of migraine attacks [130].
- GnRH Agonist: Recent studies have shown the role of GnRH agonists in the management of severe treatment-resistant menstrual migraine. Administration of GnRH agonists with a specific dose of estrogen and progesterone has shown relief in menstrual migraine. The role of GnRH agonists in migraine of males has not been studied yet [131].
- Prophylactic Therapy for Migraine: The main aim of prophylactic therapy is to reduce the frequency and severity of the episodes in chronic cases. There is a wide range of therapeutic options available like calcium channel blockers, antiepileptic drugs, botulinum neurotoxin, and antibodies against CGRP. Sexually dimorphic response to prophylactic management has not been identified yet. However, it is noted that women are more likely to use prescribed medications for prophylactic therapy than males [132].
Drugs commonly used for prophylactic management: Focused on Sex-based differences
Alternative treatment approach for migraine
Nutraceuticals
- 1.1
- Riboflavin (Vitamin B12)—Riboflavin is a precursor of flavin mononucleotide and flavin adenine dinucleotide. All these coenzymes are important for energy production inside mitochondria and energy-related cellular functions. During magnetic resonance spectroscopy use of Riboflavin in migraine emerged, and studies suggest that there can be mitochondrial dysfunction in the migraine brain. In Belgium the first randomized controlled trial to assess the use of Riboflavin was done in which 400 mg of the daily dose was tested in 55 adult migraine patients (with or without aura). Riboflavin showed positive results by reducing headache and attack frequency with only minor or rare adverse effects compared to placebo. AAN/AHS guidelines have given evidence B for the treatment of migraine. The recommended dose is 400 mg daily for at least three months, during which adverse effects like diarrhoea, polyuria and yellowish discolouration of urine are noticed. Still, research is needed on Riboflavin. Riboflavin Coenzyme Q10 plays a vital role in energy metabolism. In one randomized control trial, 50 migraine children and adolescents were given a dose of CoQ10 100 mg per day and compared to a placebo, and no difference or effect was seen. Further studies are needed to support the use of CoQ10 for migraine. CoQ10 is available in the market in the US, and AAN/AHS guidelines consider CoQ10 for the prevention of migraine. The recommended dose is 1-3mg/kg/day [137,138].
- 1.2
- 1.3
- Petasites hybridus or butterbur—Butterbur root is an herbal extract. Petadolex is a tablet made of butterbur root extract manufactured in Germany, and safety concerns there due to its liver toxicity issues. To date, two placebo-controlled trials have been conducted for the first time by Lipton et al., in which 50 mg and 75 mg doses reduced migraine attacks with no adverse effects. Another study conducted by Diener in which 100mg butterbur was given for 12 weeks showed promising results by reducing migraine attacks compared to placebo [137,138].
- 1.4
- 1.5
- OnabotulinumtoxinA- Botulinum toxin is used as a muscle relaxant for pain. In 2010 FDA approved botulinum toxin (Brand name –Botox) for migraine prevention based on two large trials [137].
2. Behavioural Techniques
- 2.1
- Relaxation Technique—Relaxation techniques (RT) include meditation, autogenic training and muscle relaxation. RT does not only help in relaxing muscles but also reduces stress. It also enhances self-efficacy, self-esteem and self-control. RT includes deep breathing, intense progressive muscular RT, guided imagery RT, etc. Studies suggest that RT can reduce migraine attack frequency to 41% and 43% [139].
- 2.2
- Cognitive Behavior Therapy- Cognitive behaviour therapy (CBT) is mainly used for managing stress, anxiety, depression, sleep disorders, migraine pain, etc. CBT is mainly used when medication does not positively affect patients, such as during pregnancy, history of allergy to a specific medicine or medical comorbidities. CBT showed positive results with proper pharmacological therapies. CBT helps in the self-management of migraine pain; in a recent randomized trial, 135 children and adolescents were compared based on CBT plus amitriptyline with headache education plus amitriptyline. The CBT shows a positive result by reducing headaches to 11.5 in comparison to 20 weeks. In one study, CBT reduces stress; in another study, CBT reduces stress by 4% to 12%, and one CBT study helped reduce medication frequency by 20% to 25% [141,142].
- 2.3
- Mindfulness- Mindfulness is becoming popular in the United States, but MF has a long relation with Buddhism, Hinduism and Daoism. Langer and colleagues were the first US researchers to examine the effect of MF on patients. MF-based stress reduction (MBSR) was developed by Kabat –Linn which is effective in chronic pain. Standard MBSR is an eight week two hours groups with a mindfulness retreat as a conclusive session of 6 hours. Well et al. conducted the first randomized controlled trial on 25 people with episodic migraine. After one month of MBSR, there was a reduced headache with enhanced self-efficacy. Acceptance and Commitment is the newest member of MF-based interventions. ACT is based on psychological flexibility in which the patient, day-to-day life obstacles and other life circumstances are addressed. ACT is divided into six core processes, i.e., acceptance, cognitive delusion, contact with the present, self-values and Commitment to action. In one study, patients receiving MF reduced the frequency of headaches to 50%. MF-based intervention reduces the symptoms of chronic pain, and still, further studies need to be done [140,141].
- 3.
- Surgical Techniques for Migraine- Surgical management for migraine was first attempted by Dr. Harvey Cushing, but his attempt was unsuccessful. Migraine surgery became famous in 1999 when two patients got relief from headaches after forehead rejuvenation. In this surgery, part of the globular muscles is removed around the supraorbital and supratrochlear nerves with an incision on the eyelid or endoscopically and skeletonizing the nerves. Still, research needed to be done on surgery as it is not only a brain disorder. It also involves functional and structural plasticity of both the central and peripheral nervous systems [140,141].
- 3.1
-
Complementary Alternative Medicine (CAM)Complementary Alternative Medicine (CAM) for migraine includes a variety of non‐conventional therapies, such as acupuncture, herbal supplements, chiropractic care, and relaxation techniques. While these treatments are not universally recommended, many people turn to CAM approaches to complement traditional medical treatments, seeking to reduce the frequency and severity of migraine attacks and improve their overall quality of life.
- 3.2
- Acupuncture—Acupuncture is an ancient Chinese therapy based on the theory of disease causation secondary to an energy imbalance in the body. In acupuncture, needles are interested in acupoints (specific points along the energy meridians) in the body, which releases obstructed energy, which helps bring the body to balance and disease. A meta-analysis reported that acupuncture led to a 5% reduction in headache frequency. Acupuncture needs 6-8 sessions to manage symptoms of migraine. However, Acupuncture has adverse effects ranging from minimal effects like therapy failure or change in pain intensity to severe issues like bleeding, pneumothorax, infection, and nerve injury. Patients in whom pharmacological therapies are not effective are seen opting for acupuncture [137,138,139].
- 3.3
- Homoeopathic medicine—Several individuals frequently use homoeopathic medicine for migraine attacks. Damiana is the most popular homoeopathic medicine for migraine. Ceanothus is used when a patient has a migraine due to acidity. When a patient has a frontal headache with nausea, Iris V is effective. A few more homoeopathic medicines are available for migraine treatment and management: Onosmodium, Ptelea, Robin, etc. Evidence for their use is still lacking and further research is required to understand their role in the management of migraine [139,140,141].
- 3.4
- Chiropractors- Chiropractors are the most common complementary and alternative medicine (CAM) for treating and managing migraine. Roland et al. conducted an evidence-based chiropractic study that needs to be done based on guidelines even considering type, frequency, dosage, and duration of treatment [142]. Adverse effects of chiropractic study were seen in a controlled trial conducted by Aleksander et al. in which tenderness was seen [143]. Craig et al. highlighted important questions about the therapeutic approach to chiropractic migraine management that warrant further investigation. They reported that more primary research is needed to evaluate how chiropractors approach headache and migraine management, as well as to understand the prevalence, burden, and comorbidities of migraine within chiropractic patient populations [144].
Future Directions and Conclusion
Acknowledgments
Data Availability Statement
Conflicts of Interest
Abbreviations
| TMC | Trigeminal Nucleus Caudalis |
| EAAT1 | Excitatory Amino acid transport |
| CSD | Cortical Spreading Depression |
| NE | Norepinephrine |
| CGRP | Calcitonin Gene Related Peptide |
| HRT | Hormone Replacement Therapy |
| COCs | Combined Oral Contraceptives |
| PCOS | Polycystic Ovary Syndrome |
| REM | Sleep Rapid Eye Movement Sleep |
| eNOS | endothelial Nitric Oxide Synthase |
| MAO | Monoamine Oxidase |
| NSAIDs | Non Steroidal Anti-inflammatory Drugs |
| SSRIs | Selective Serotonin Reuptake Inhibitors |
| RT | Relaxation Techniques |
| CBT | Cognitive Behaviour Therapy |
| MBSR | Mindfulness based Stress reduction |
| GON | Greater Occipital Nerve |
References
- Critchley M. Migraine: from Cappadocia to Queen Square. London: Heinemann, 1967.
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459-480. [CrossRef]
- Gori S, Lucchesi C, Baldacci F, Bonuccelli U. Preferential occurrence of attacks during night sleep and/or upon awakening negatively affects migraine clinical presentation. Funct Neurol. 2015;30(2):119-123. [CrossRef]
- Kelman L, Rains JC. Headache and sleep: examination of sleep patterns and complaints in a large clinical sample of migraineurs. Headache. 2005;45(7):904-910. [CrossRef]
- Silberstein SD. Migraine symptoms: results of a survey of self-reported migraineurs. Headache. 1995;35(7):387-396. [CrossRef]
- Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol Rev. 2017;97(2):553-622. [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1-211. [CrossRef]
- Weitzel KW, Strickland JM, Smith KM, Goode JV. Gender-specific issues in the treatment of migraine. J Gend Specif Med. 2001;4(1):64-74.
- Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41(7):646-657. [CrossRef]
- Couturier EG, Bomhof MA, Neven AK, van Duijn NP. Menstrual migraine in a representative Dutch population sample: prevalence, disability and treatment. Cephalalgia. 2003;23(4):302-308. [CrossRef]
- Granella F, Sances G, Zanferrari C, Costa A, Martignoni E, Manzoni GC. Migraine without aura and reproductive life events: a clinical epidemiological study in 1300 women. Headache. 1993;33(7):385-389. [CrossRef]
- Fillingim RB, Hastie BA, Ness TJ, Glover TL, Campbell CM, Staud R. Sex-related psychological predictors of baseline pain perception and analgesic responses to pentazocine. Biol Psychol. 2005;69(1):97-112. [CrossRef]
- Gasbarri A, Arnone B, Pompili A, et al. Emotional memory and migraine: effects of amitriptyline and sex related difference. Behav Brain Res. 2008;189(1):220-225. [CrossRef]
- Wang XP, Liu JM, Zhao YB. Migraine: sex-influenced trait model?. Med Hypotheses. 2008;71(1):14-21. [CrossRef]
- Delaruelle Z, Ivanova TA, Khan S, et al. Male and female sex hormones in primary headaches. J Headache Pain. 2018;19(1):117. Published 2018 Nov 29. [CrossRef]
- Buse DC, Loder EW, Gorman JA, et al. Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2013;53(8):1278-1299. [CrossRef]
- Robinson ME, Riley JL 3rd, Myers CD, et al. Gender role expectations of pain: relationship to sex differences in pain. J Pain. 2001;2(5):251-257. [CrossRef]
- Bolay H, Ozge A, Saginc P, et al. Gender influences headache characteristics with increasing age in migraine patients. Cephalalgia. 2015;35(9):792-800. [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629-808.
- 20. https://www.scienceofmigraine.com/pathophysiology/phases-of-migraine.
- Andersen AR, Friberg L, Olsen TS, Olesen J. Delayed Hyperemia Following Hypoperfusion in Classic Migraine: Single Photon Emission Computed Tomographic Demonstration. Arch Neurol. 1988;45(2):154–159. [CrossRef]
- Olsen, T.S. (1990), Migraine With and Without Aura: The Same Disease Due to Cerebral Vasospasm of Different Intensity. A hypothesis based on CBF studies during migraine.. Headache: The Journal of Head and Face Pain, 30: 269-272. [CrossRef]
- Ferrari MD, Haan J, Blokland JAK, et al. Cerebral Blood Flow During Migraine Attacks Without Aura and Effect of Sumatriptan. Arch Neurol. 1995;52(2):135–139. [CrossRef]
- Gazerani P, Cairns BE. Dysautonomia in the pathogenesis of migraine. Expert Rev Neurother. 2018;18:153-165.
- Lashley KS. Patterns of cerebral integration indicated by the scotomas of migraine. Arch Neurol Psychiatry. 1941;46:331–9.
- Olesen J. Cerebral and extracranial circulatory disturbances in migraine: Pathophysiological implications. Cerebrovasc Brain Metab Rev. 1991;3:1–28.
- Goadsby PJ, Giffin N, Kaube H, Afridi S. Ketamine reduces the severity of prolonged migraine aura: Support for the glutamate hypothesis of aura. Neurology. 2011;76(Suppl 4):A443.
- Moskowitz MA, Macfarlane R. Neurovascular and molecular mechanisms in migraine headaches. Cerebrovasc Brain Metab Rev. 1993 Fall;5(3):159-77. [PubMed]
- Petersen KA, Nilsson E, Olesen J, Edvinsson L. Presence and function of the calcitonin gene-related peptide receptor on rat pial arteries investigated in vitro and in vivo. Cephalalgia. 2005;25:424–32.
- Moskowitz MA, Cutrer FM. SUMATRIPTAN: A receptor-targeted treatment for migraine. Ann Rev Med. 1993;44:145–54.
- Dimitriadou V, Buzzi MG, Moskowitz MA, Theoharides TC. Trigeminal sensory fiber stimulation induces morphological changes reflecting secretion in rat dura mater mast cells. Neuroscience. 1991;44:97–112.
- Schytz HW, Birk S, Wienecke T, Kruuse C, Olesen J, Ashina M. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain. 2009 Jan;132(Pt 1):16-25. Epub 2008 Dec 3. [CrossRef] [PubMed]
- Pavlović JM, Allshouse AA, Santoro NF, Crawford SL, Thurston RC, Neal-Perry GS, Lipton RB, Derby CA. Sex hormones in women with and without migraine: Evidence of migraine-specific hormone profiles. Neurology. 2016 Jul 5;87(1):49-56. Epub 2016 Jun 1. [CrossRef] [PubMed] [PubMed Central]
- Aggarwal M, Puri V, Puri S. Effects of estrogen on the serotonergic system and calcitonin gene-related peptide in trigeminal ganglia of rats. Ann Neurosci. 2012 Oct;19(4):151-7. [CrossRef] [PubMed] [PubMed Central]
- I: de Vries Lentsch, Eloísa Rubio-Beltrán, Antoinette MaassenVanDenBrink, Changing levels of sex hormones and calcitonin gene-related peptide (CGRP) during a woman’s life, 2021; -77, 35. Simone de Vries Lentsch, Eloísa Rubio-Beltrán, Antoinette MaassenVanDenBrink, Changing levels of sex hormones and calcitonin gene-related peptide (CGRP) during a woman’s life: Implications for the efficacy and safety of novel antimigraine medications, Maturitas, Volume 145, 2021, Pages 73-77, ISSN 0378-5122. [CrossRef]
- Durham PL. Calcitonin gene-related peptide (CGRP) and migraine. Headache. 2006 Jun;46 Suppl 1(Suppl 1):S3-8. [CrossRef] [PubMed] [PubMed Central]
- 37. https://www.factsaboutfertility.org/hormonal-balance-and-the-female-brain-a-review/.
- Stephania Paredes, Santiago Cantillo, Kenneth D. Candido, Nebojsa Nick Knezevic. “An Association of Serotonin with Pain Disorders and Its Modulation by Estrogens”, International Journal of Molecular Science, 2019.
- Herbison AE, Simonian SX, Thanky NR, Bicknell RJ. Oestrogen modulation of noradrenaline neurotransmission. Novartis Found Symp. 2000;230:74-85; discussion 85-93. [CrossRef] [PubMed]
- Pavlović JM, Allshouse AA, Santoro NF, Crawford SL, Thurston RC, Neal-Perry GS, Lipton RB, Derby CA. Sex hormones in women with and without migraine: evidence of migraine-specific hormone profiles. Neurology. 2016 Jul 5;87(1):49-56.
- Güven B, Güven H, Çomoğlu S. Clinical characteristics of menstrually related and non-menstrual migraine. Acta Neurologica Belgica. 2017 Sep;117:671-6.
- Wharton W, E Gleason C, Sandra O, M Carlsson C, Asthana S. Neurobiological underpinnings of the estrogen-mood relationship. Current psychiatry reviews. 2012 Aug 1;8(3):247-56.
- Craft RM. Modulation of pain by estrogens. Pain. 2007 Nov 1;132:S3-12.
- SHOUPE D, MONTZ FJ, LOBO RA. The effects of estrogen and progestin on endogenous opioid activity in oophorectomized women. The Journal of Clinical Endocrinology & Metabolism. 1985 Jan 1;60(1):178-83.
- Villablanca AC, Hanley MR. 17β-estradiol stimulates substance P receptor gene expression. Molecular and cellular endocrinology. 1997 Dec 12;135(2):109-17.
- Iversen L. Substance P equals pain substance?. Nature. 1998 Mar 26;392(6674):334-5.
- Zhang J, Bai W, Wang W, Jiang H, Jin B, Liu Y, Liu S, Wang K, Jia J, Qin L. Mechanisms underlying alterations in norepinephrine levels in the locus coeruleus of ovariectomized rats: Modulation by estradiol valerate and black cohosh. Neuroscience. 2017 Jun 23;354:110-21.
- Stahl SM. Effects of estrogen on the central nervous system. Journal of Clinical Psychiatry. 2001 May 1;62(5):317-8.
- Kelly MJ, Qiu J, Rønnekleiv OK. Estrogen signaling in the hypothalamus. Vitamins & Hormones. 2005 Jan 1;71:123-45.
- Raffaelli B, Storch E, Overeem LH, Terhart M, Fitzek MP, Lange KS, Reuter U. Sex hormones and calcitonin gene–related peptide in women with migraine: a cross-sectional, matched cohort study. Neurology. 2023 Apr 25;100(17):e1825-35.
- Pau KY, Hess DL, Kohama S, Bao J, Pau CY, Spies HG. Oestrogen upregulates noradrenaline release in the mediobasal hypothalamus and tyrosine hydroxylase gene expression in the brainstem of ovariectomized rhesus macaques. J Neuroendocrinol. 2000 Sep;12(9):899-909. [CrossRef] [PubMed]
- Krolick KN, Zhu Q, Shi H. Effects of Estrogens on Central Nervous System Neurotransmission: Implications for Sex Differences in Mental Disorders. Prog Mol Biol Transl Sci. 2018;160:105-171. Epub 2018 Aug 28. [CrossRef] [PubMed] [PubMed Central]
- Del Rà o Juan Pablo, Alliende Marà a I., Molina Natalia, Serrano Felipe G., Molina Santiago, Vigil Pilar, TITLE=Steroid Hormones and Their Action in Women’s Brains: The Importance of Hormonal Balance , JOURNAL=Frontiers in Public Health, VOLUME=6,2018,URL=https://www.frontiersin.org/articles/10.3389/fpubh.2018.00141 ISSN=2296-2565. [CrossRef]
- Al-Hassany Linda, Haas Jennifer, Piccininni Marco, Kurth Tobias, Maassen Van Den Brink Antoinette, Rohmann Jessica L., TITLE=Giving Researchers a Headache – Sex and Gender Differences in Migraine, JOURNAL=Frontiers in Neurology, VOLUME=11, 2020, URL=https://www.frontiersin.org/articles/10.3389/fneur.2020.549038 DOI=10.3389/fneur.2020.549038, ISSN=1664-2295.
- Hehui Zhang, Xiaokai Yang, Yijun Lin, Linglong Chen, Hua Ye, The efficacy of greater occipital nerve block for the treatment of migraine: A systematic review and meta-analysis, Clinical Neurology and Neurosurgery, Volume 165, 2018, Pages 129-133, ISSN 0303-8467. [CrossRef]
- Calhoun, A.H.; Gill, N. Presenting a New, Non-Hormonally Mediated Cyclic Headache in Women: End-Menstrual Migraine. Headache 2017, 57, 17–20.
- MacGregor, E.A.; Frith, A.; Ellis, J.; Aspinall, L.; Hackshaw, A. Incidence of migraine relative to menstrual cycle phases of rising and falling estrogen. Neurology 2006, 67, 2154–2158.
- Fanciullacci M, Alessandri M, Del Rosso A. Dopamine involvement in the migraine attack. Funct Neurol. 2000;15 Suppl 3:171-81. PMID: 11200788.
- Lammers, C.-H., D’Souza, U., Qin, Z.-H., Lee, S.-H., Yajima, S. and Mouradian, M.M. (1999), regulation of striatal dopamine receptors by estrogen. Synapse, 34: 222-227. [CrossRef]
- Sarchielli P, Tognoloni M, Russo S, Vulcano MR, Feleppa M, Mala` M, Sartori M, Gallai V (1996) Variations in the platelet arginine nitric oxide pathway during the ovarian cycle in females affected by menstrual migraine. Cephalalgia 16:468–475.
- Kim C, Siscovick DS, Sidney S, Lewis CE, Kiefe CI, Koepsell TD. Oral contraceptive use and association with glucose, insulin, and diabetes in young adult women: the CARDIA study. Diabetes Care. 2002 Jun 1;25(6):1027-32.
- Lieba-Samal D, Wöber C, Frantal S, Brannath W, Schmidt K, Schrolnberger C, Wöber-Bingöl Ç, PAMINA Study Group. Headache, menstruation and combined oral contraceptives: a diary study in 184 women with migraine. European Journal of Pain. 2011 Sep 1;15(8):852-7.
- Curtis KM, Mohllajee AP, Peterson HB. Use of combined oral contraceptives among women with migraine and nonmigrainous headaches: a systematic review. Contraception. 2006 Feb 1;73(2):189-94.
- Sarahian N, Noroozzadeh M, Saei Ghare Naz M, Eskandari-Roozbahani N, Mahboobifard F, Ramezani Tehrani F. Is there any association between migraine headache and polycystic ovary syndrome (PCOS)? A review article. Molecular biology reports. 2022 Jan 1:1-9.
- Ripa P, Ornello R, Degan D, Tiseo C, Stewart J, Pistoia F, Carolei A, Sacco S. Migraine in menopausal women: a systematic review. International journal of women’s health. 2015 Aug 20:773-82.
- MacGregor EA. Migraine, menopause and hormone replacement therapy. Post reproductive health. 2018 Mar;24(1):11-8.
- Somerville BW (1972) The role of estradiol withdrawal in the etiology of menstrual migraine. Neurology 22:355–365.
- D.P. Shackelford, M.M. McConnaughey, S.G. Iams; The effects of estradiol and mestranol on alpha-adrenoceptors in select regions of the rat brain; Brain Research Bulletin; Volume 21, Issue 2, 1988; Pages 329-333,ISSN 0361-9230.
- Nattero G, Allais G, De Lorenzo C, et al. Menstrual migraine: new biochemical and psychological aspects. Headache. 1988;28(2):103-107.
- Calhoun, A.H. (2018), Understanding Menstrual Migraine. Headache: The Journal of Head and Face Pain, 58: 626-630. https://doi.org/10.1111/head.13291.
- Silberstein SD, Merriam GR. Estrogens, progestins,and headache. Neurology. 1991;41(6):786-793.
- Akcali D, Sayin A, Sara Y, Bolay H (2010) Does single cortical spreading depression elicit pain behaviour in freely moving rats? Cephalalgia 30:1195–1206.
- Granella F, Sances G, Pucci E, Nappi RE, Ghiotto N, Nappi G (2000) Migraine with aura and reproductive life events: a case control study. Cephalalgia 20:701–707.
- Aegidius K, Zwart JA, Hagen K, Stovner L (2009) The effect of pregnancy and parity on headache prevalence: the Head-HUNT study. Headache 49:851–859.
- Kvisvik EV, Stovner LJ, Helde G, Bovim G, Linde M (2011) Headache and migraine during pregnancy and puerperium: the MIGRA-study. J Headache Pain 12:443–451.
- Nattero G, Allais G, De Lorenzo C, et al. Menstrual migraine: new biochemical and psychological aspects. Headache. 1988;28(2):103-107.
- Gruber HJ, Bernecker C, Lechner A, Weiss S, Wallner-Blazek M, Meinitzer A, Höbarth G, Renner W, Fauler G, Horejsi R, Fazekas F. Increased nitric oxide stress is associated with migraine. Cephalalgia. 2010 Apr;30(4):486-92.
- Ramadan NM. The link between glutamate and migraine. CNS spectrums. 2003 Jun;8(6):446-9.
- Paredes S, Cantillo S, Candido KD, Knezevic NN. An association of serotonin with pain disorders and its modulation by estrogens. International journal of molecular sciences. 2019 Nov 15;20(22):5729.
- Vos T, Abajobir A, Abate K, Abbafati C, Abbas K, Abd-Allah F et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. 2017;390(10100):1211-1259.
- Gazerani P. Current Evidence on Potential Uses of MicroRNA Biomarkers for Migraine: From Diagnosis to Treatment. Molecular Diagnosis & Therapy. 2019;23(6):681-694.
- Vetvik K, MacGregor E. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. The Lancet Neurology. 2017;16(1):76-87.
- The International Classification of Headache Disorders—ICHD-3 [Internet]. ICHD-3. 2022 [cited 21 July 2022]. Available from: https://ichd-3.org.
- Buse D, Scher A, Dodick D, Reed M, Fanning K, Manack Adams A et al. Impact of Migraine on the Family: Perspectives of People With Migraine and Their Spouse/Domestic Partner in the CaMEO Study. Mayo Clinic Proceedings. 2016;91(5):596-611.
- Correction to Lancet Respir Med 2021; published online April 9. https://doi. org/10.1016/S2213-2600(21)00160-0. The Lancet Respiratory Medicine. 2021;9(6):e55.
- Announcements. Cephalalgia. 1999;19(10):913-913.
- Buse D, Manack A, Serrano D, Turkel C, Lipton R. Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. 2022.
- Lipton R, Bigal M, Diamond M, Freitag F, Reed M, Stewart W. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343-349.
- Evans R, Linde M. Expert Opinion: Adherence to Prophylactic Migraine Medication. Headache: The Journal of Head and Face Pain. 2009;49(7):1054-1058.
- Gracia Naya M, Santos Lasaosa S, Ríos Gómez C, Sánchez Valiente S, García Gomara M, Latorre Jiménez A et al. Factores predisponentes al abandono del tratamiento preventivo en una serie de pacientes con migraña. Revista de Neurología. 2011;53(04):201.
- Silberstein S, Holland S, Freitag F, Dodick D, Argoff C, Ashman E. Evidence-based guideline update: Pharmacologic treatment for episodic migraine prevention in adults: Table 1. Neurology. 2012;78(17):1337-1345.
- Silberstein S, Winner P, Chmiel J. Migraine Preventive Medication Reduces Resource Utilization. Headache: The Journal of Head and Face Pain. 2003;43(3):171-178.
- Loder E, Burch R, Rizzoli P. The 2012 AHS/AAN Guidelines for Prevention of Episodic Migraine: A Summary and Comparison With Other Recent Clinical Practice Guidelines. Headache: The Journal of Head and Face Pain. 2012;52(6):930-945.
- Shamliyan T, Choi J, Ramakrishnan R, Miller J, Wang S, Taylor F et al. Preventive Pharmacologic Treatments for Episodic Migraine in Adults. Journal of General Internal Medicine. 2013;28(9):1225-1237.
- Goadsby P, Sprenger T. Current practice and future directions in the prevention and acute management of migraine. The Lancet Neurology. 2010;9(3):285-298.
- van Casteren D, Couturier E, van den Brink A. Sex- and Gender-Specific Aspects of Migraine Treatment. Gender and Migraine. 2019;:31-43.
- Buse D, Loder E, Gorman J, Stewart W, Reed M, Fanning K et al. Sex Differences in the Prevalence, Symptoms, and Associated Features of Migraine, Probable Migraine and Other Severe Headache: Results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache: The Journal of Head and Face Pain. 2013;53(8):1278-1299.
- Lipton R, Manack Adams A, Buse D, Fanning K, Reed M. A Comparison of the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study and American Migraine Prevalence and Prevention (AMPP) Study: Demographics and Headache-Related Disability. Headache: The Journal of Head and Face Pain. 2016;56(8):1280-1289.
- Dodick D, Lipton R, Goadsby P, Tfelt-Hansen P, Ferrari M, Diener H et al. Predictors of Migraine Headache Recurrence: A Pooled Analysis From the Eletriptan Database. Headache: The Journal of Head and Face Pain. 2007;0(0):070629211050002.
- Cady R, Schreiber C. Sumatriptan: update and review. Expert Opin Pharmacother. 2006 Aug;7(11):1503-14. [CrossRef] [PubMed]
- Negro A, Koverech A, Martelletti P. Serotonin receptor agonists in the acute treatment of migraine: a review on their therapeutic potential. J Pain Res. 2018 Mar 8;11:515-526. [CrossRef] [PubMed] [PubMed Central]
- Antonaci F, Ghiotto N, Wu S, Pucci E, Costa A. Recent advances in migraine therapy. Springerplus. 2016 May 17;5:637. [CrossRef] [PubMed] [PubMed Central]
- Tepper SJ, Rapoport AM, Sheftell FD. Mechanisms of action of the 5-HT1B/1D receptor agonists. Arch Neurol. 2002 Jul;59(7):1084-8. [CrossRef] [PubMed]
- Olesen J, Diener HC, Schoenen J, Hettiarachchi J. No effect of eletriptan administration during the aura phase of migraine. Eur J Neurol. 2004 Oct;11(10):671-7. [CrossRef] [PubMed]
- Sinclair AJ, Sturrock A, Davies B, Matharu M. Headache management: pharmacological approaches. Pract Neurol. 2015 Dec;15(6):411-23. Epub 2015 Jul 3. [CrossRef] [PubMed] [PubMed Central]
- González-Hernández A, Marichal-Cancino BA, MaassenVanDenBrink A, Villalón CM. Side effects associated with current and prospective antimigraine pharmacotherapies. Expert Opin Drug Metab Toxicol. 2018 Jan;14(1):25-41. Epub 2017 Dec 15. [CrossRef] [PubMed]
- Brar Y, Hosseini SA, Saadabadi A. Sumatriptan. [Updated 2023 Nov 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470206/.
- Brown AM, Ho M, Thomas DR, Parsons AA. Functional effects of frovatriptan (VML 251), sumatriptan and naratriptan on human recombinant 5-HT1 and 5-HT7 receptors. Headache. 1998;38:376.
- Stewart M. The binding affinity and functional activity of eletriptan and other 5-HT_< 1B/1D> agonists at the human recombinant 5-HT_< 1B> and 5-HT_< 1D> receptors. Br J Pharmacol. 1999;127:93P.
- Gupta S, McCarson KE, Welch KM, Berman NE. Mechanisms of pain modulation by sex hormones in migraine. Headache: The Journal of Head and Face Pain. 2011 Jun;51(6):905-22.
- Aggarwal M, Puri V, Puri S. Effects of estrogen on the serotonergic system and calcitonin gene-related peptide in trigeminal ganglia of rats. Annals of neurosciences. 2012 Oct;19(4):151.
- Jhee SS, Shiovitz T, Crawford AW, Cutler NR. Pharmacokinetics and pharmacodynamics of the triptan antimigraine agents. Clinical pharmacokinetics. 2001 Mar;40(3):189-205.
- Elkind AH, Wade A, Ishkanian G. Pharmacokinetics of frovatriptan in adolescent migraineurs. The Journal of Clinical Pharmacology. 2004 Oct;44(10):1158-65.
- Negro A, Lionetto L, Casolla B, Lala N, Simmaco M, Martelletti P. Pharmacokinetic evaluation of frovatriptan. Expert Opinion on Drug Metabolism & Toxicology. 2011 Nov 1;7(11):1449-58.
- Franconi F, Finocchi C, Allais G, Omboni S, Tullo V, Campesi I, Reggiardo G, Benedetto C, Bussone G. Gender and triptan efficacy: a pooled analysis of three double-masked, randomized, crossover, multicenter, Italian studies comparing frovatriptan vs. other triptans. Neurological Sciences. 2014 May;35(1):99-105.
- Buchan P, Wade A, Ward C, Oliver SD, Stewart AJ, Freestone S. Frovatriptan: a review of drug-drug interactions. Headache. 2002 Apr;42 Suppl 2:S63-73. [CrossRef] [PubMed]
- Clemow DB, Johnson KW, Hochstetler HM, Ossipov MH, Hake AM, Blumenfeld AM. Lasmiditan mechanism of action—review of a selective 5-HT1F agonist. J Headache Pain. 2020 Jun 10;21(1):71. [CrossRef] [PubMed] [PubMed Central]
- Hou M, Xing H, Li C, Wang X, Deng D, Li J, Zhang P, Chen J. Short-term efficacy and safety of lasmiditan, a novel 5-HT1F receptor agonist, for the acute treatment of migraine: a systematic review and meta-analysis. J Headache Pain. 2020 Jun 5;21(1):66. [CrossRef] [PubMed] [PubMed Central]
- Chan C, Goadsby PJ. Recent Advances in Pharmacotherapy for Episodic Migraine. CNS Drugs. 2019 Nov;33(11):1053-1071. doi: 10.1007/s40263-019-00665-9. PMID: 31556018.
- Lupi C, Benemei S, Guerzoni S, Pellesi L, Negro A. Pharmacokinetics and pharmacodynamics of new acute treatments for migraine. Expert Opin Drug Metab Toxicol. 2019 Mar;15(3):189-198. Epub 2019 Feb 12. [CrossRef] [PubMed]
- Rizzoli PB. Emerging therapeutic options for acute migraine: focus on the potential of lasmiditan. Neuropsychiatr Dis Treat. 2014 Mar 31;10:547-52. [CrossRef] [PubMed] [PubMed Central]
- Dong XD, Svensson P, Cairns BE. The analgesic action of topical diclofenac may be mediated through peripheral NMDA receptor antagonism. PAIN®. 2009 Dec 15;147(1-3):36-45.
- Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies—successful translation from bench to clinic. Nature Reviews Neurology. 2018 Jun;14(6):338-50.
- Valdemarsson S, Edvinsson L, Hedner P, Ekman R. Hormonal influence on calcitonin gene-related peptide in man: effects of sex difference and contraceptive pills. Scandinavian Journal of Clinical and Laboratory Investigation. 1990 Jan 1;50(4):385-8.
- Gupta S, McCarson KE, Welch KM, Berman NE. Mechanisms of pain modulation by sex hormones in migraine. Headache: The Journal of Head and Face Pain. 2011 Jun;51(6):905-22.
- Pavlović JM, Allshouse AA, Santoro NF, Crawford SL, Thurston RC, Neal-Perry GS, Lipton RB, Derby CA. Sex hormones in women with and without migraine: evidence of migraine-specific hormone profiles. Neurology. 2016 Jul 5;87(1):49-56.
- MacGregor EA. Estrogen replacement and migraine. Maturitas. 2009 May 20;63(1):51-5.
- Nappi RE, Cagnacci A, Granella F et al. (2001) Course of primary headaches during hormone replacement therapy. Maturitas 38:157–163.
- Macgregor A (1999) Effects of oral and transdermal estrogen replacement on migraine. Cephalalgia 19:124–125.
- Pringsheim T, Gooren L. Migraine prevalence in male to female transsexuals on hormone therapy. Neurology. 2004 Aug 10;63(3):593-4.
- Murray SC, Muse KN. Effective treatment of severe menstrual migraine headaches with gonadotropin-releasing hormone agonist and “add-back” therapy. Fertility and sterility. 1997 Feb 1;67(2):390-3.
- Vetvik KG, MacGregor EA. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. The Lancet Neurology. 2017 Jan 1;16(1):76-87.
- Soldin OP, Chung SH, Mattison DR. Sex differences in drug disposition. Journal of Biomedicine and Biotechnology. 2011 Oct;2011.
- Costa C, Tozzi A, Rainero I, Cupini LM, Calabresi P, Ayata C, Sarchielli P. Cortical spreading depression as a target for anti-migraine agents. The journal of headache and pain. 2013 Dec;14(1):1-8.
- White HS. Molecular pharmacology of topiramate: managing seizures and preventing migraine. Headache: The Journal of Head and Face Pain. 2005 Apr;45:S48-56.
- Yerby MS, Friel PN, McCormick K, Koerner M, Van Allen M, Leavitt AM, Sells CJ, Yerby JA. Pharmacokinetics of anticonvulsants in pregnancy: alterations in plasma protein binding. Epilepsy research. 1990 Apr 1;5(3):223-8.
- Francesca Puledda and Kevin Shields Non-Pharmacological Approaches for Migraine Neurotherapeutics. 2018 Apr; 15(2): 336–345.
- .Licia Grazzi, Claudia Toppo, Domenico D’Amico Non-Pharmacological Approaches to Headaches: Non-Invasive Neuromodulation, Nutraceuticals, and Behavioral Approaches Int J Environ Res Public Health. 2021 Feb; 18(4): 1503.
- Andrea Pérez-Muñoz , Dawn C Buse , Frank Andrasik Behavioral Interventions for Migraine Neurol Clin2019 Nov;37(4):789-813. doi: 10.1016/j.ncl.2019.07.003. Epub 2019 Aug 22.
- Palak S Patel , Mia T Minen Complementary and Integrative Health Treatments for Migraine J Neuroophthalmol 2019 Sep;39(3):360-369. [CrossRef]
- A: Totonchi, Bahman Guyuron, Hossein Ansari Surgical Options for Migraine, 2021; 141. Ali Totonchi , Bahman Guyuron, Hossein Ansari Surgical Options for Migraine: An Overview Neurology India (2021) 105-109. [CrossRef]
- Bryans R, Descarreaux M, Duranleau M, Marcoux H, Potter B, Ruegg R, Shaw L, Watkin R, White E. Evidence-based guidelines for the chiropractic treatment of adults with headache. J Manipulative Physiol Ther. 2011 Jun;34(5):274-89. [CrossRef] [PubMed]
- Chaibi A, Benth JŠ, Tuchin PJ, Russell MB. Adverse events in a chiropractic spinal manipulative therapy single-blinded, placebo, randomized controlled trial for migraineurs. Musculoskelet Sci Pract. 2017 Jun;29:66-71. Epub 2017 Mar 14. Erratum in: Musculoskelet Sci Pract. 2017 Oct;31:21. [CrossRef] [PubMed]
- Craig Moore, Jon Adams, Andrew Leaver, Romy Lauche, and David Sibbritt The treatment of migraine patients within chiropractic: analysis of a nationally representative survey of 1869 chiropractors BMC Complement Altern Med. 2017; 17: 519.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




