Submitted:
03 September 2024
Posted:
05 September 2024
You are already at the latest version
Abstract
Keywords:
1.Introduction
2. Materials
- 1X phosphate buffered saline (PBS), pH 7.3
- 4% paraformaldehyde (PFA), diluted in 1X PBS pH 7.3
- Surgical tools: large surgical scissors, small dissection scissors, curved forceps, spatula (with microspoon), serrated blunt forceps, vaculet blood collection set (see Note 1)
- 15 mL Falcon tubes
- Vibratome
- 24-well plates
- 10X PO4 Buffer, pH 7.2-7.4: 65g NaH2PO4 x H2O, 15g NaOH, 2N HCl, distilled H2O to total volume of 500mL
- Glycerol-based storing solution: 150mL Glycerol, 150mL Ethylene Glycol, 50mL 10X PO4 Buffer pH 7.2-7.4, 150mL distilled H2O
- Rocking platform at room temperature
- Paintbrushes
- Positive charged glass microscope slides
- Mounting media (DAPI-free)
- Coverslips
- Clear nail polish
- Epifluorescence microscope
- Confocal microscope
- Image analysis software
- Fluorophore-conjugated cadaverine (see Table 1)
- 1X PBS, sterile
- Isoflurane
- Vaporizer for anesthesia (for example Somnoflow® low-flow electronic vaporizer)
- Anesthesia box
- Insulin syringes
3. Methods
3.1. Tissue preparation and imaging
- Resuspend cadaverine conjugated to a fluorophore in sterile PBS solution at a concentration of 3.3 mg/1 mL.
- Put the mouse under anesthesia with isoflurane 3% for 4 min in the anesthesia box.
- Remove animal from anesthesia chamber and retroorbitally inject 100 µL of resuspended cadaverine with an insulin syringe. Start 30 min timer (see Note 3, Note 4, Note 5).
- Place mouse back in home cage for 30 min.
- When 30 min are up, transcardially perfuse the anesthetized mouse as described in [11].
- Extract the brain from the skull and immerse it in 15 mL Falcon tube with 10 mL of 4% PFA overnight at 4ºC.
- The following day, wash the brain with filtered PBS and store the washed brain in filtered PBS (see Note 6).
- Section the brains using a vibratome in 50 µm slices. Section can be kept at 4ºC in PBS for short term storage. For long term storage, slices can be kept in 1.5 mL Eppendorf tubes in a glycerol-based storing solution at -20ºC (see Note 7).
- This can be combined with co-staining with antibodies, avoiding the use of secondary fluorophores with the same color as cadaverine.
- Using a paint brush transfer brain slices into a well of a 24-well plate with DAPI 1:1000 in PBS.
- Incubate sections in DAPI for 4-5 minutes on a rocking platform at room temperature.
- Wash sections in PBS twice for 10 minutes on a rocking platform (see Note 8).
- Check the staining on one section on a slide in a fluorescence microscope (see Note 9).
- Mount sections on positively charged glass slides. When sections are dried attach coverslip with water-based fluorescence-protective mounting media (see Note 10). Apply clear nail polish to coat the edges of the coverslip in order to seal it in place. Let it dry overnight.
- After mounting the sections, acquire a stitch image of the cortex on a confocal microscope at 10x magnification and 1024x1024 resolution. Set blending at 15%. We recommend sampling multiple slices per animal, for having an accurate estimation of expression across the entire cortex, we most often collect a total of 3-5 sections.
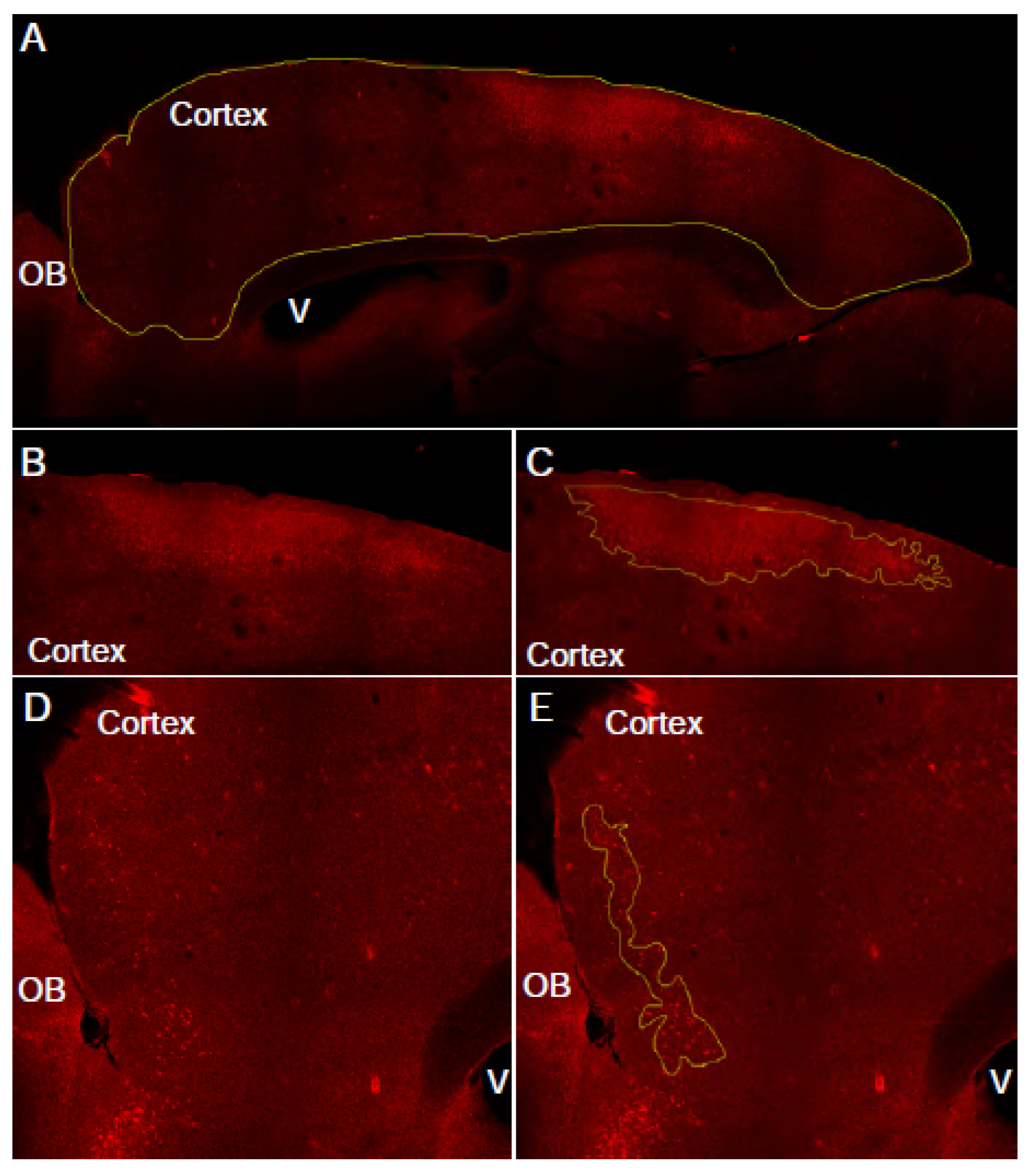
3.2. Manual assessment of cadaverine leakage in cortex (see Note 11) (Figure 2).
- In an image analysis software (for example FIJI) draw region of interest (ROI) around the entire cortex, using the corpus callosum as a boundary. The DAPI channel can also be used to determine this area.
- Measure the area of the ROI. This is the “total cortex area” for this slice.
- Draw free-hand regions of interest around the cadaverine-positive cells. Selection criteria: cadaverine positive cells are brighter than background, cadaverine positive cells are clustered in reasonably close proximity to each other, there are greater than 10 cadaverine positive cells within the ROI. Continue until all ROI areas are outlined.
- Measure the total the cortical area covered by these cadaverine positive ROIs. This is going to be the “total leakage area” for this slice.
- Repeat steps 1-4 with all of the acquired images.
- Calculate the percent of cadaverine leakage in the cortex using this equation:
3.3. Automated assessment of cadaverine leakage in cortex (Figure 3).
- This method selects leakage areas based on areas and shape. This method is more prone to omitting clusters of cadaverine positive cells, which tend to be excluded along blood vessels when using size exclusion but it reduces experimenter bias.
- Draw a freehand ROI around the cortex. Use the corpus callosum as a boundary and avoid including bright blood vessels on the edge of the slice. Measure the area of this ROI. This parameter will be “total cortex area”.
- Threshold the image such as cadaverine-positive cells are clearly highlighted. At this stage, some few blood vessels might be also selected. They will be excluded in further analysis (see Note 12).
- Make a binary of the threshold image.
- Adjust size and circularity to include only cadaverine-positive cells. Examine the generated mask and compare to original image for accuracy. Adjusting circularity helps exclude blood vessels.
- Measure the area covered by the mask. This parameter will be “total leakage area”.
- Repeat steps 1-5 in 5 slices per animal.
- Calculate the percent of cadaverine leakage in the cortex using this equation:

4. Notes
- Once tools have come in contact with PFA, they can no longer be used for in vivo procedures or with unfixed tissue and cells.
- This methodology has specifically been validated with cadaverine conjugated to Alexa Fluor 488 and 555.
- Injections into the retroorbital sinus are easier to learn and more reliable than tail vein injections. If the retroorbital injection worked well, a change of color can be observed in the mouse as the cadaverine is distributed in the blood. Urine changes color also. If the retroorbital injection is not performed well, bleeding and swelling around the eye can be observed. This can happen if the experimenter did not reach the retro-orbital sinus with the syringe.
- Ensure to not exceed the maximum volume that can be injected intravenously (≤5 ml/kg or ~125 µL in a 25g mouse). Higher amounts can lead to volume overload resulting in high blood pressure, vascular and organ damage that impact experimental results.
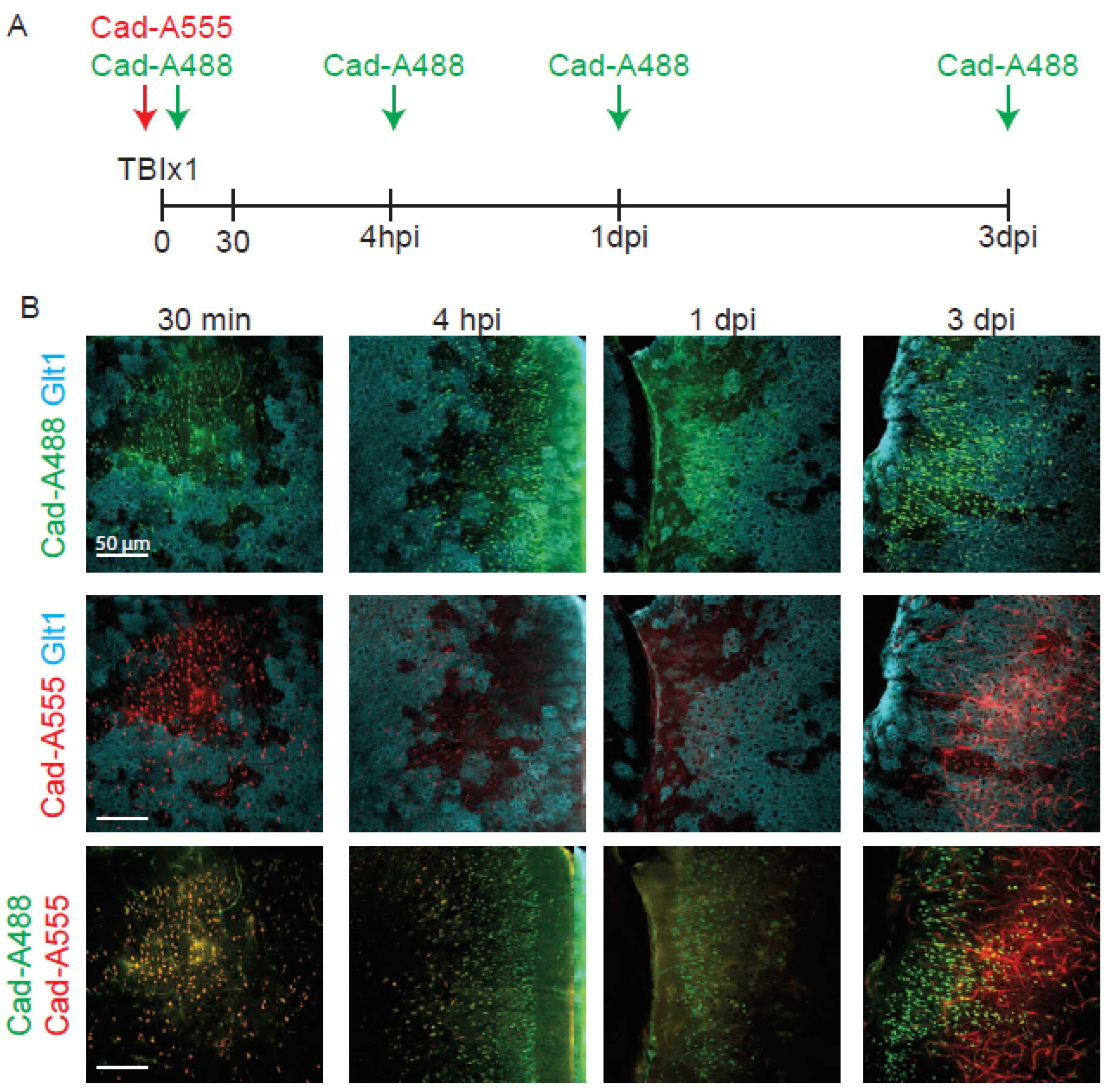
- Cadaverine can remain in the brain for longer than 30 min after injection, but the fluorescence levels decrease with time and are more difficultly distinguished after 4 hours after the injection. In Figure 4 we compared the fluorescence levels of cadaverine administered 30 min, 4 hours, 1 day and 3 days before the perfusion and best results were obtained when cadaverine was injected 30 min before the injection. Thus, cadaverine is not a great solution to label where BBB damage initially occurs when tissue fixation takes place days later.
- PBS should be filtered through a sterile 0.2 µm filter.
- If brain slices were stored in glycerol-based storing solution before starting the staining, wash the slices in PBS twice for 10 min at room temperature in 24 well plates.
- For washing steps, transfer the sections to a different well with the washing solution using a paint brush.
- If blood vessels appear fluorescent throughout the cortex (due to a suboptimal perfusion that did not flush all the dye out of the vasculature), exclude the animal from further analysis.
- Make sure that the thickness of the coverslip is compatible with the objective that will be used. Most objectives require coverslips of number 1.5, which have a thickness between 0.16 - 0.19 mm.
- Manual quantification of cadaverine leakage is fast but can be prone to bias because the cadaverine positive area that is selected is subjective to the experimenter.
- It may be appropriate to use different analysis parameters for thresholding cadaverine positive cells across slices.
Acknowledgments
References
- Ayloo, S.; Gu, C. Transcytosis at the Blood-Brain Barrier. Curr Opin Neurobiol 2019, 57, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Profaci, C.P.; Munji, R.N.; Pulido, R.S.; Daneman, R. The blood–brain barrier in health and disease: Important unanswered questions. J Exp Med 2020, 217, e20190062. [Google Scholar] [CrossRef] [PubMed]
- Saunders, N.R.; Dziegielewska, K.M.; Møllgård, K.; Habgood, M.D. Markers for blood-brain barrier integrity: how appropriate is Evans blue in the twenty-first century and what are the alternatives? Front Neurosci 2015, 9, 385. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.A. Plasma proteins have a ticket to ride the blood-brain barrier. Science Translational Medicine 2020, 12, eabd3610. [Google Scholar] [CrossRef]
- Roseth, S.; Fykse, E.M.; Fonnum, F. Uptake of L-glutamate into rat brain synaptic vesicles: effect of inhibitors that bind specifically to the glutamate transporter. J Neurochem 1995, 65, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Schürmann, B.; Wu, X.; Dietzel, I.D.; Lessmann, V. Differential modulation of AMPA receptor mediated currents by evans blue in postnatal rat hippocampal neurones. Br J Pharmacol 1997, 121, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Unraveling the mysteries of serum albumin—more than just a serum protein. Front Physiol 2014, 5. [Google Scholar] [CrossRef]
- Mills WA, Woo AM, Jiang S, et al Astrocyte plasticity in mice ensures continued endfoot coverage of cerebral blood vessels following injury and declines with age. Nat Commun 2022, 13, 1794. [CrossRef] [PubMed]
- Heithoff BP, George KK, Phares AN, et al Astrocytes are necessary for blood-brain barrier maintenance in the adult mouse brain. Glia 2021, 69, 436–472. [CrossRef] [PubMed]
- George, K.K.; Heithoff, B.P.; Shandra, O.; Robel, S. Mild Traumatic Brain Injury/Concussion Initiates an Atypical Astrocyte Response Caused by Blood-Brain Barrier Dysfunction. J Neurotrauma 2022, 39, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Marinković, P.; Godinho, L.; Misgeld, T. Generation of Tissue Sections for Screening Thy1 Mouse Lines. Cold Spring Harb Protoc 2015, 2015, pdb.prot087684. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.A.; Ryu, J.K.; Akassoglou, K. Fibrinogen in neurological diseases: mechanisms, imaging and therapeutics. Nat Rev Neurosci 2018, 19, 283–301. [Google Scholar] [CrossRef] [PubMed]

| Dyes | ||||
|---|---|---|---|---|
| Name | Manufacturer | Catalog # | RRID | Concentration |
| DAPI | ThermoFisher | D1306 | AB_2629482 | 1:1000 |
| AF555 Cadaverine |
ThermoFisher | A30677 | N/A | 0.33mg/mouse |
| AF488 Cadaverine |
ThermoFisher | A30676 | N/A | 0.33mg/mouse |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





