Submitted:
10 October 2024
Posted:
12 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drug Preparation
2.3. SNS Denervation Procedure
2.4. 4V Cannulations for Acute Injections in Rats
2.5. 4V Cannulations for Chronic Infusions in Rats
2.6. 4V Cannulations for Chronic Infusions in Female C57BL/6J and DBA/2J Mice
2.7. Implantation of Temperature Transponders Underneath IBAT
2.8. Acute IP or 4V Injections and Measurements of TIBAT
2.9. Body Composition
2.10. Tissue Collection for NE Content Measurements
2.11. Norepinephrine (NE) Content Measurements (Biochemical Confirmation of IBAT Denervation Procedure)
2.12. Study Protocols
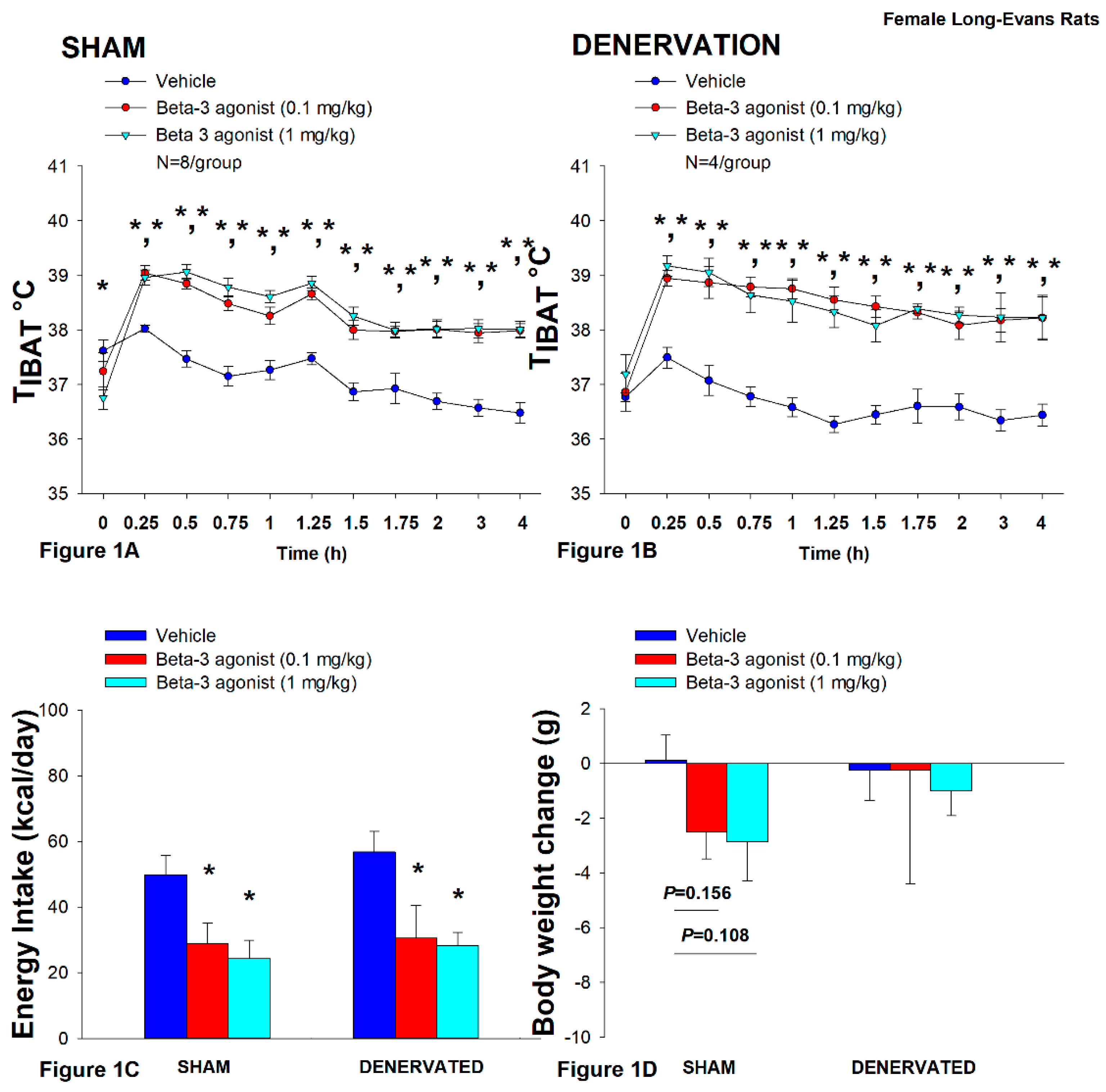
2.12.1. Study 1: Determine If Surgical Denervation of IBAT Changes the Ability of the β-3R Agonist, CL 316243, to Increase TIBAT in DIO Rats
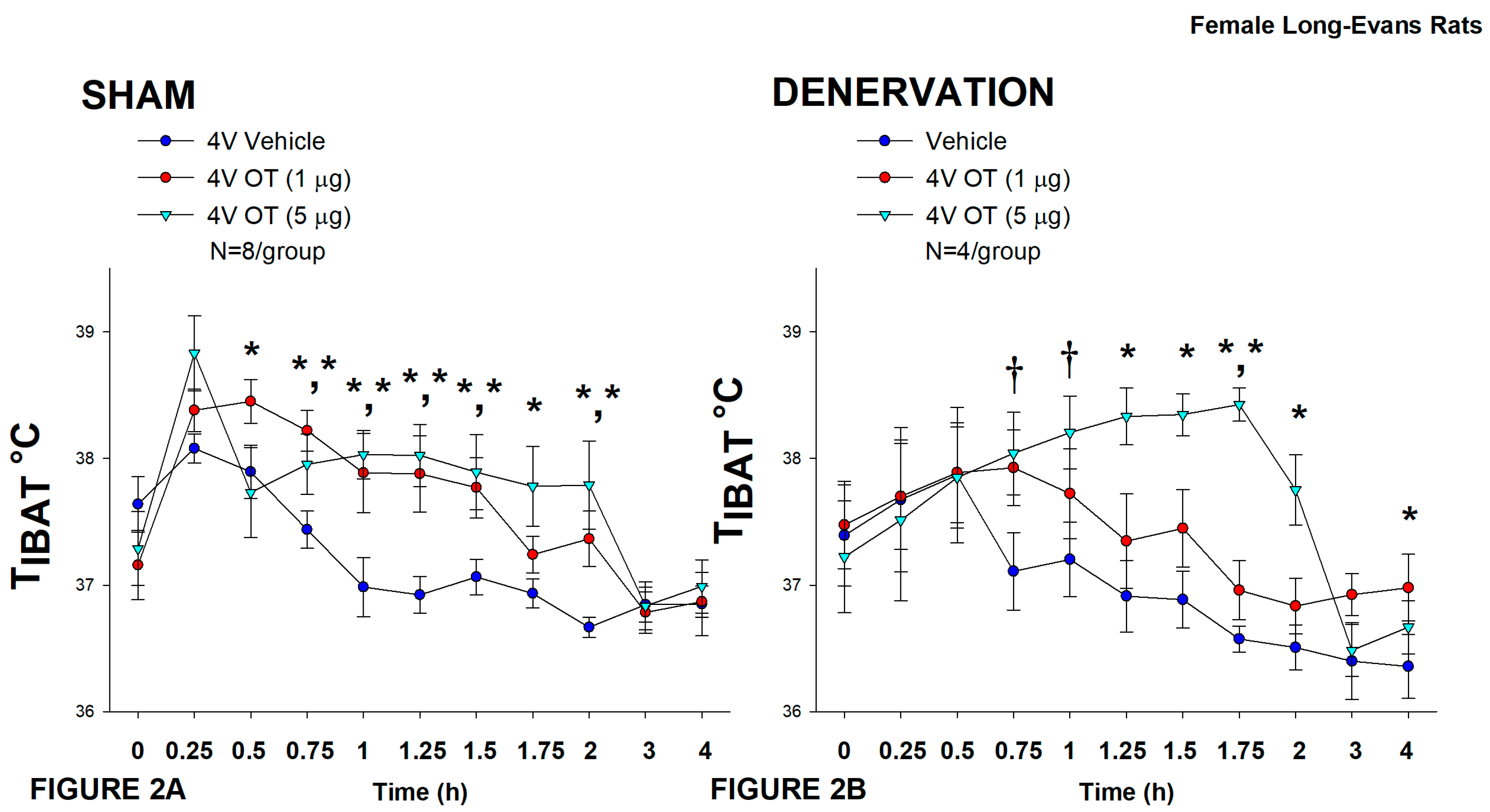
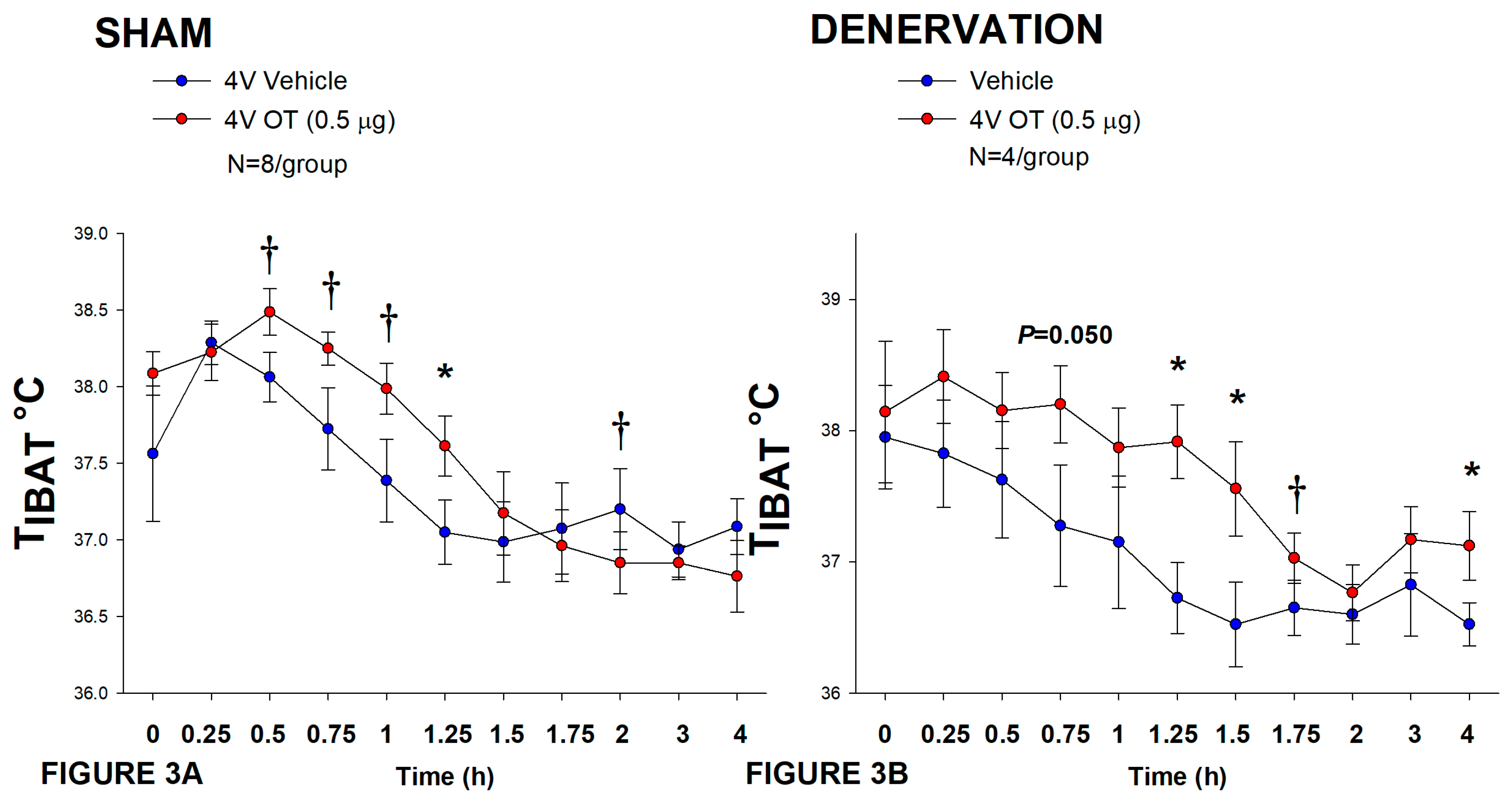
2.12.2. Study 2: Determine the Extent to which OT-Induced Activation of Sympathetic Outflow to IBAT Contributes to Its Ability to Increase TIBAT in DIO Rats
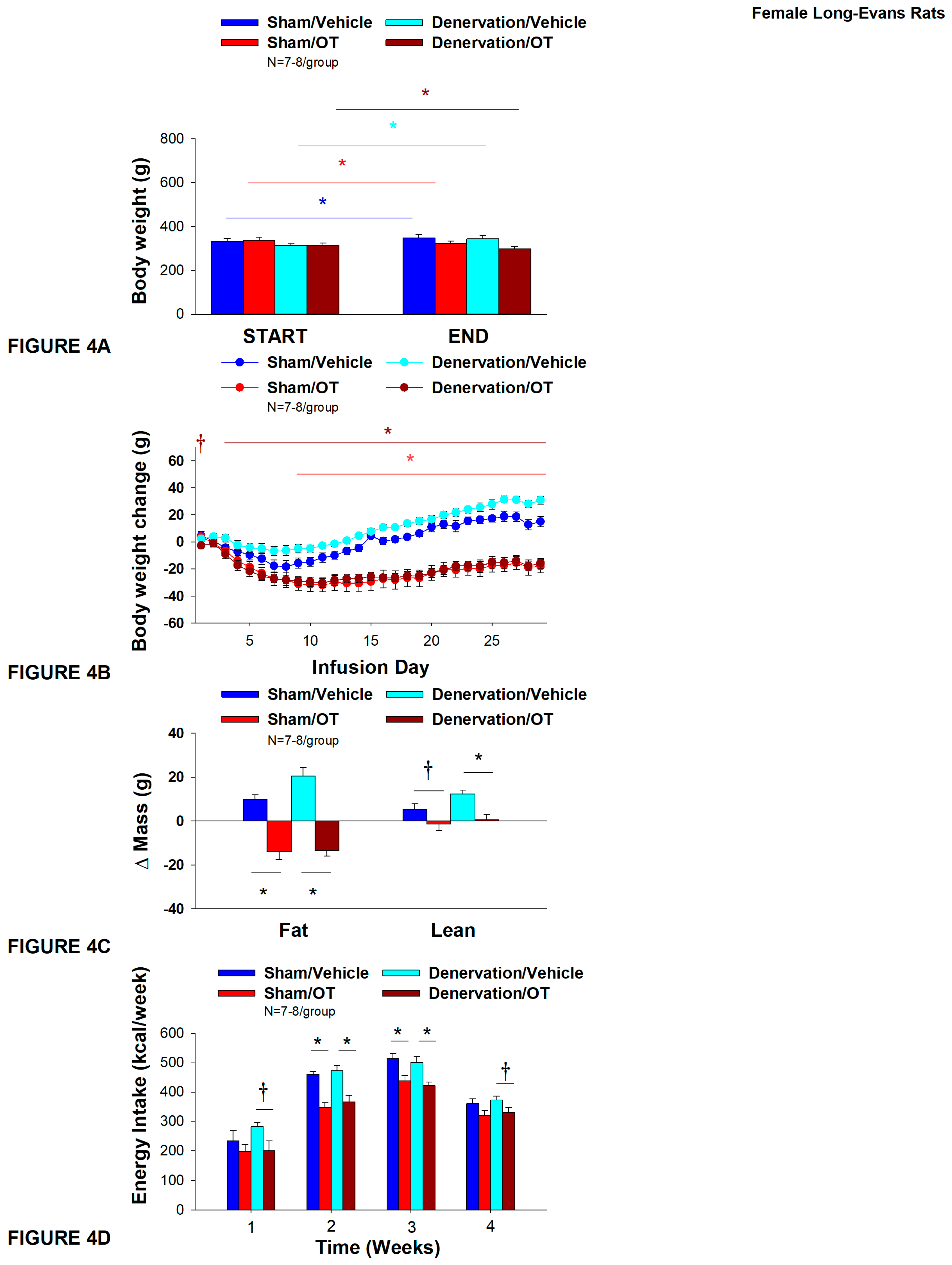
2.12.3. Study 3A: Determine the Extent to Which OT-Induced Activation of Sympathetic Outflow to IBAT Contributes to Its Ability to Reduce Weight Gain in Female HFD-Fed Rats
2.12.4. Study 3B: Determine the Extent to Which 4V OT Impacts Thermogenic Gene Expression in IBAT and IWAT in Female HFD-Fed Rats
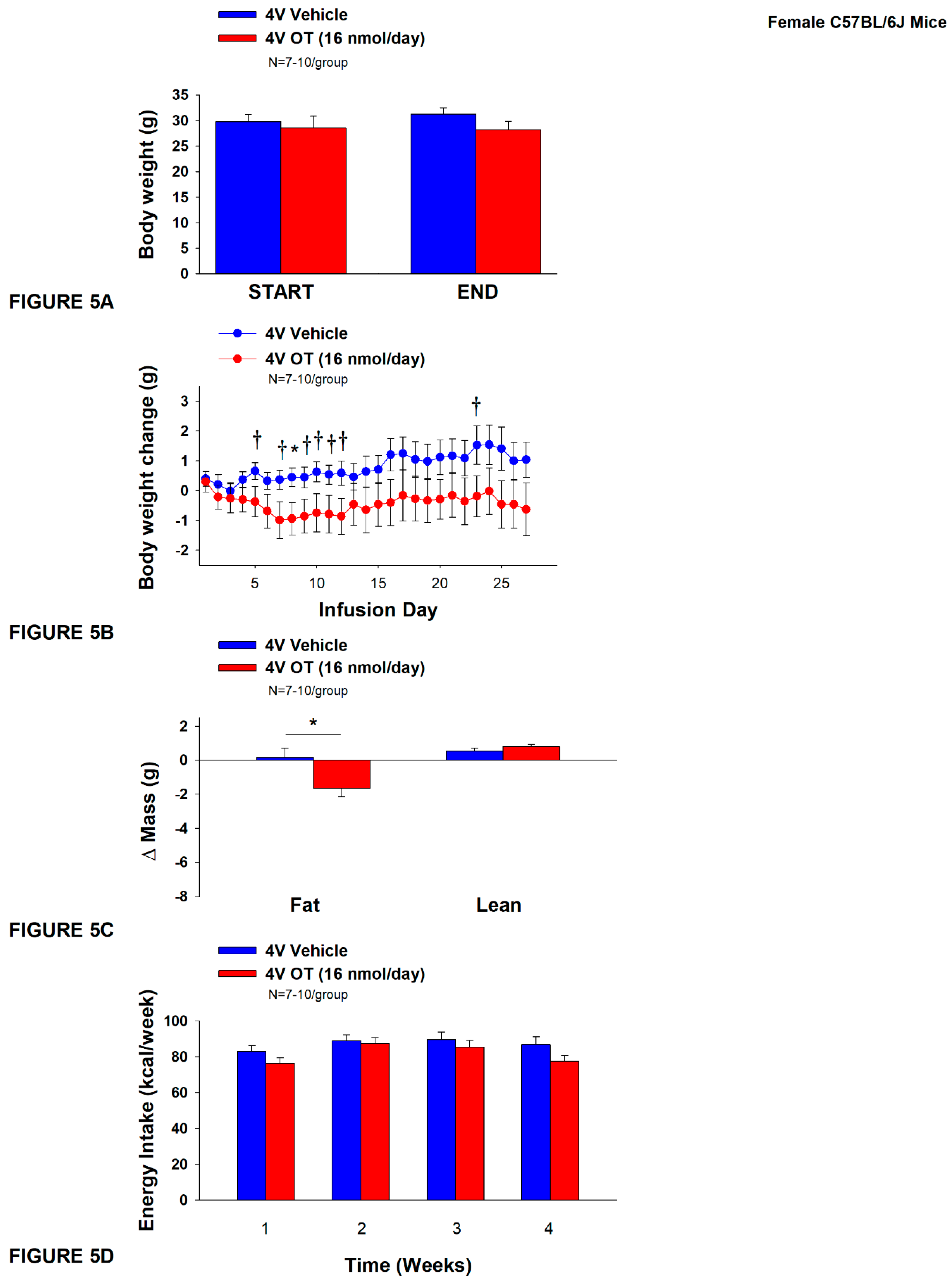
2.12.5. Study 4A: Determine the Effects of Chronic 4V OT Treatment on Body Weight, Adiposity and Energy Intake in Female HFD-Fed C57BL/6J Mice
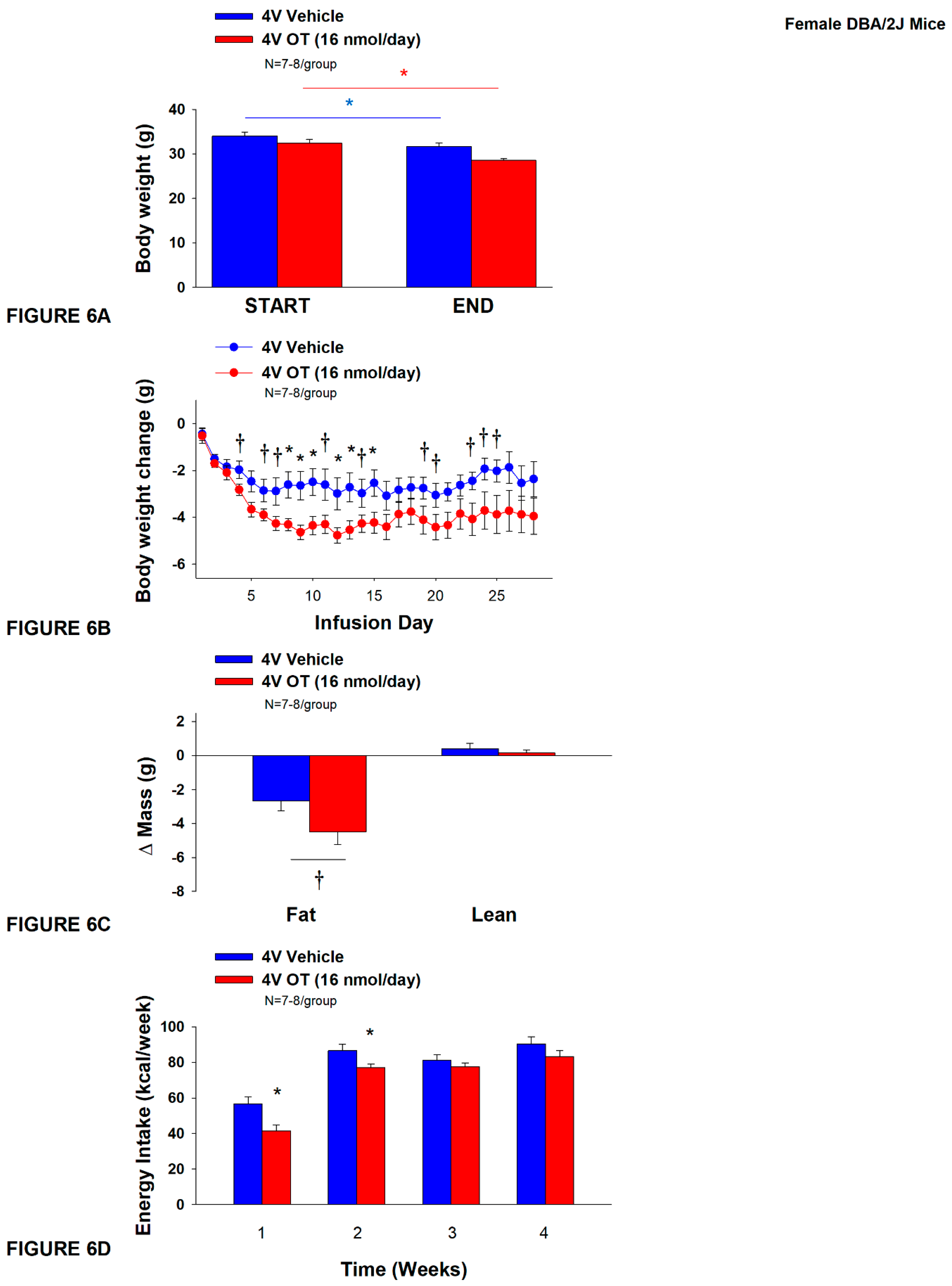
2.12.6. Study 4B: Determine the Effects of Chronic 4V OT Treatment on Body Weight, Adiposity and Energy Intake in Female DIO DBA2J Mice
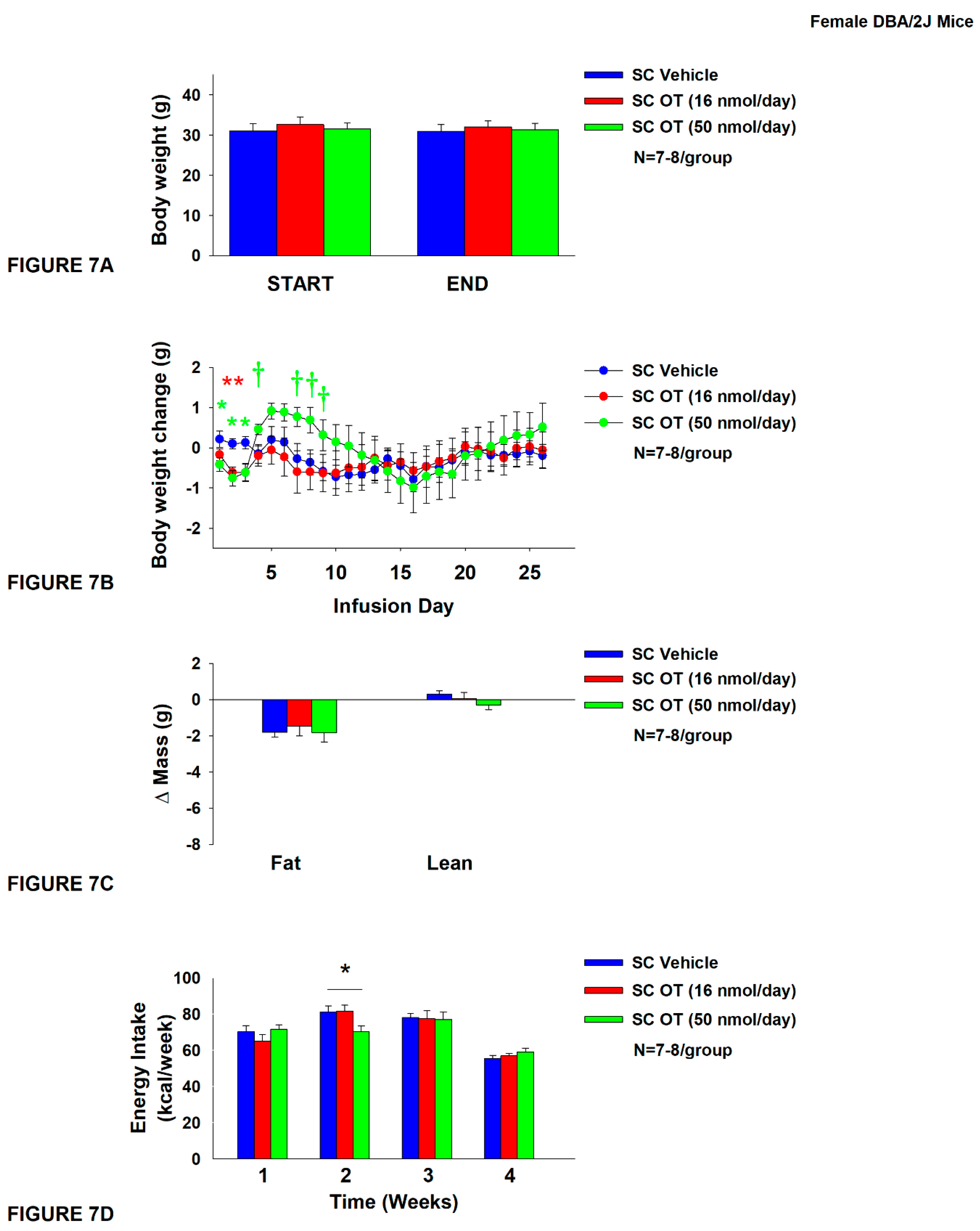
2.12.7. Study 5: Determine the Effects of Chronic Systemic OT Treatment (16 and 50 nmol/day) on Body Weight, Adiposity and Energy Intake in Female DIO DBA/2J Mice
2.13. Blood Collection
2.14. Plasma Hormone Measurements
2.15. Blood Glucose and Lipid Measurements
2.16. Adipose Tissue Processing for Adipocyte Size
2.17. Adipocyte Size Analysis
2.18. Tissue Collection for Quantitative Real-Time PCR (qPCR)
2.19. qPCR
2.20. Statistical Analyses
3. Results
3.1. Study 1: Determine If Surgical Denervation of IBAT Changes the Ability of the Beta 3-Adrenergic Receptor (β3-AR) Agonist, CL 316243, to Increase TIBAT in Female HFD-Fed Rats
3.1.1. Energy Intake
3.1.2. Body Weight
3.2. Study 2: Determine the Extent to Which OT-Induced Activation of Sympathetic Outflow to IBAT Contributes to Its Ability to Increase TIBAT in Female HFD-Fed Rats
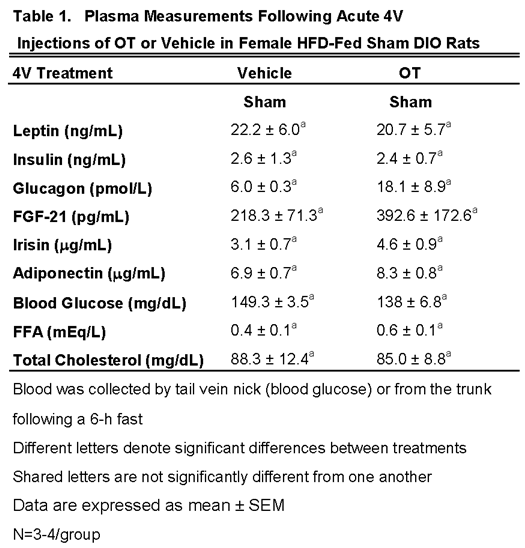
3.2.1. Plasma Hormone Concentrations
3.3. Study 3A: Determine the Extent to Which OT-Induced Activation of Sympathetic Outflow to IBAT Contributes to Its Ability to Impact Body Weight in Female HFD-Fed Rats
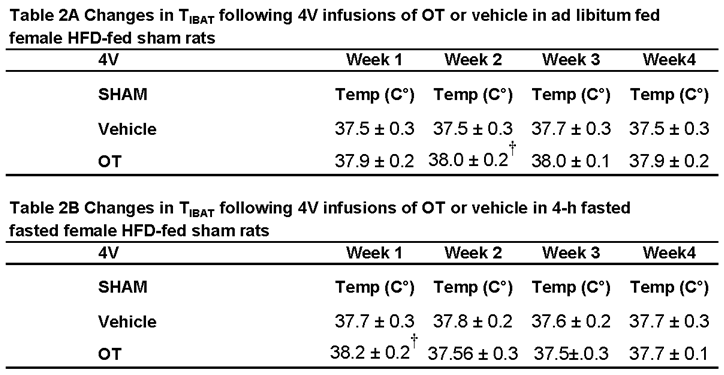
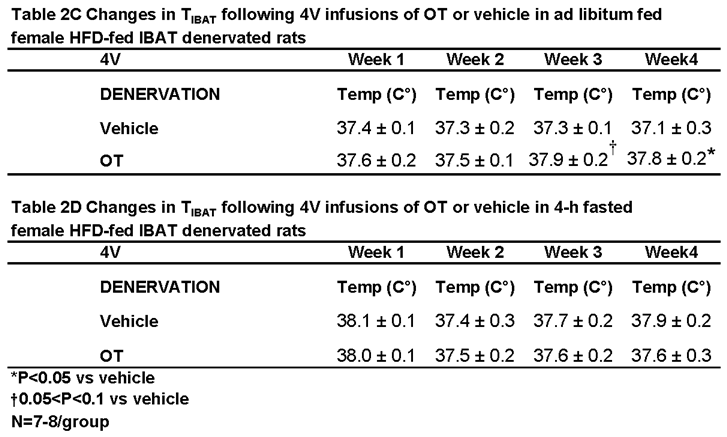
3.3.1. TIBAT
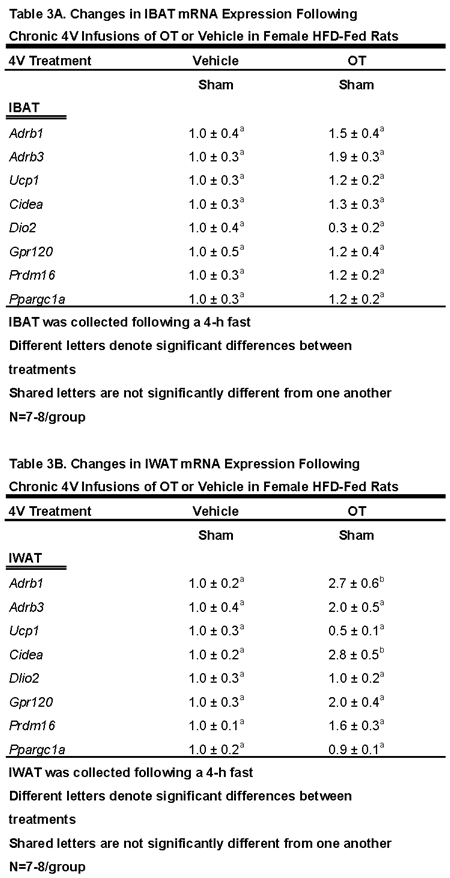
3.4. Study 3B: Determine the Extent to Which 4V OT Impacts Thermogenic Gene Expression in IBAT and IWAT in Female HFD-Fed Rats
3.4.2. IBAT:
3.4.2. IWAT
3.5. Study 4A: Determine the Effects of Chronic 4V OT Treatment (16 nmol/day) on Body Weight, Adiposity and Energy Intake in Female DIO C57BL/6J Mice
3.5.1. TIBAT
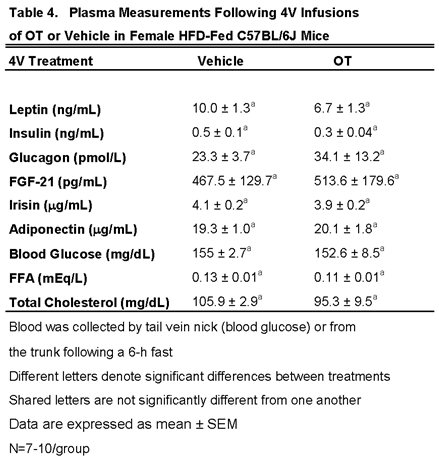
3.5.2. Plasma Hormone Concentrations
3.6. Study 4B: Determine the Effects of Chronic 4V OT Treatment (16 nmol/day) on Body Weight, Adiposity and Energy Intake in Female DIO DBA/2J Mice
3.6.1. TIBAT
3.6.2. Plasma Hormone Concentrations
3.7. Study 5: Determine the Effects of Chronic Systemic OT Treatment (16 and 50 nmol/day) on Body Weight, Adiposity and Energy Intake in Female DIO DBA/2J Mice
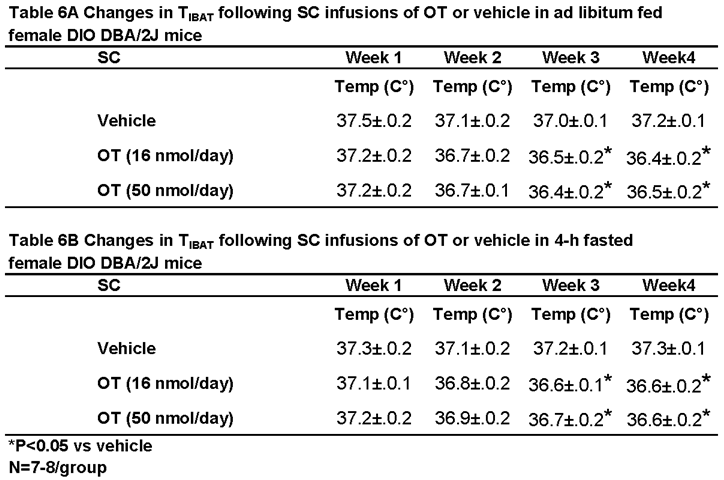
3.7.1. TIBAT
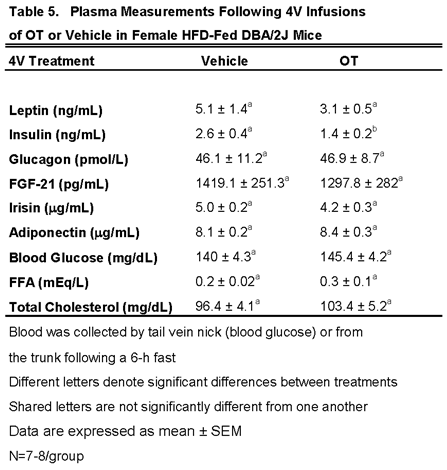
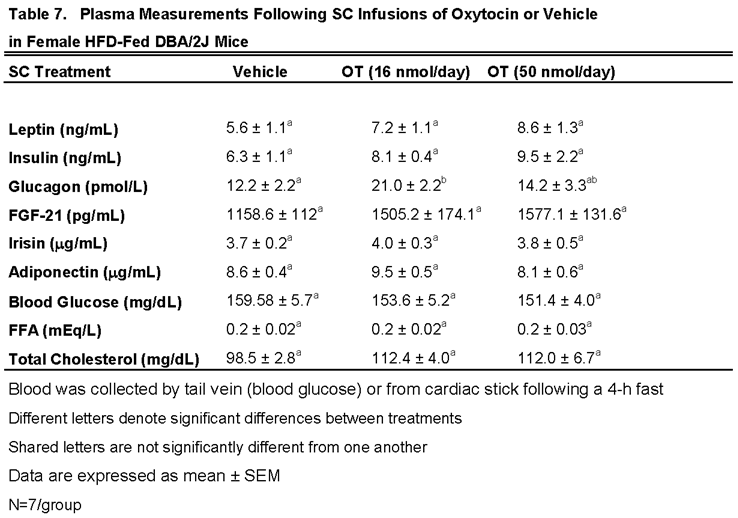
3.7.2. Plasma Hormone Concentrations
4. Discussion
Funding
Acknowledgments
Disclosures
References
- Kosfeld, M.; Heinrichs, M.; Zak, P.J.; Fischbacher, U.; Fehr, E. Oxytocin increases trust in humans. Nature 2005, 435, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Striepens, N.; Kendrick, K.M.; Maier, W.; Hurlemann, R. Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Frontiers in neuroendocrinology 2011, 32, 426–450. [Google Scholar] [CrossRef] [PubMed]
- Gimpl, G.; Fahrenholz, F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev 2001, 81, 629–683. [Google Scholar] [CrossRef] [PubMed]
- Veening, J.G.; de Jong, T.R.; Waldinger, M.D.; Korte, S.M.; Olivier, B. The role of oxytocin in male and female reproductive behavior. European journal of pharmacology 2015, 753, 209–228. [Google Scholar] [CrossRef]
- Blevins, J.E.; Baskin, D.G. Translational and therapeutic potential of oxytocin as an anti-obesity strategy: Insights from rodents, nonhuman primates and humans. Physiol Behav, 2015. [Google Scholar] [CrossRef]
- Lawson, E.A. The effects of oxytocin on eating behaviour and metabolism in humans. Nature reviews. Endocrinology 2017, 13, 700–709. [Google Scholar] [CrossRef]
- Lawson, E.A.; Olszewski, P.K.; Weller, A.; Blevins, J.E. The role of oxytocin in regulation of appetitive behaviour, body weight and glucose homeostasis. J Neuroendocrinol 2020, 32, e12805. [Google Scholar] [CrossRef]
- McCormack, S.E.; Blevins, J.E.; Lawson, E.A. Metabolic Effects of Oxytocin. Endocrine reviews 2020, 41. [Google Scholar] [CrossRef]
- Kublaoui, B.M.; Gemelli, T.; Tolson, K.P.; Wang, Y.; Zinn, A.R. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol Endocrinol 2008, 22, 1723–1734. [Google Scholar] [CrossRef]
- Liu, C.M.; Davis, E.A.; Suarez, A.N.; Wood, R.I.; Noble, E.E.; Kanoski, S.E. Sex Differences and Estrous Influences on Oxytocin Control of Food Intake. Neuroscience 2020, 447, 63–73. [Google Scholar] [CrossRef]
- Altirriba, J.; Poher, A.L.; Rohner-Jeanrenaud, F. Chronic Oxytocin Administration as a Treatment Against Impaired Leptin Signaling or Leptin Resistance in Obesity. Frontiers in endocrinology 2015, 6, 119. [Google Scholar] [CrossRef]
- Deblon, N.; Veyrat-Durebex, C.; Bourgoin, L.; Caillon, A.; Bussier, A.L.; Petrosino, S.; Piscitelli, F.; Legros, J.J.; Geenen, V.; Foti, M.; et al. Mechanisms of the anti-obesity effects of oxytocin in diet-induced obese rats. PloS one 2011, 6, e25565. [Google Scholar] [CrossRef]
- Morton, G.J.; Thatcher, B.S.; Reidelberger, R.D.; Ogimoto, K.; Wolden-Hanson, T.; Baskin, D.G.; Schwartz, M.W.; Blevins, J.E. Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. Am J Physiol-Endoc M 2012, 302, E134–E144. [Google Scholar] [CrossRef]
- Blevins, J.E.; Graham, J.L.; Morton, G.J.; Bales, K.L.; Schwartz, M.W.; Baskin, D.G.; Havel, P.J. Chronic oxytocin administration inhibits food intake, increases energy expenditure, and produces weight loss in fructose-fed obese rhesus monkeys. Am J Physiol Regul Integr Comp Physiol 2015, 308, R431–438. [Google Scholar] [CrossRef]
- Noble, E.E.; Billington, C.J.; Kotz, C.M.; Wang, C. Oxytocin in the ventromedial hypothalamic nucleus reduces feeding and acutely increases energy expenditure. Am J Physiol Regul Integr Comp Physiol 2014, 307, R737–745. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, H.; Zhang, H.; Dean, C.; Wu, Q.; Li, J.; Guariglia, S.; Meng, Q.; Cai, D. Neuropeptide exocytosis involving synaptotagmin-4 and oxytocin in hypothalamic programming of body weight and energy balance. Neuron 2011, 69, 523–535. [Google Scholar] [CrossRef]
- Zhang, G.; Cai, D. Circadian intervention of obesity development via resting-stage feeding manipulation or oxytocin treatment. Am J Physiol Endocrinol Metab 2011, 301, E1004–1012. [Google Scholar] [CrossRef]
- Yi, K.J.; So, K.H.; Hata, Y.; Suzuki, Y.; Kato, D.; Watanabe, K.; Aso, H.; Kasahara, Y.; Nishimori, K.; Chen, C.; et al. The regulation of oxytocin receptor gene expression during adipogenesis. J Neuroendocrinol 2015, 27, 335–342. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Morrison, S.F.; Madden, C.J.; Tupone, D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab 2014, 19, 741–756. [Google Scholar] [CrossRef]
- Kotz, C.M.; Perez-Leighton, C.E.; Teske, J.A.; Billington, C.J. Spontaneous Physical Activity Defends Against Obesity. Current obesity reports 2017, 6, 362–370. [Google Scholar] [CrossRef]
- Periasamy, M.; Herrera, J.L.; Reis, F.C.G. Skeletal Muscle Thermogenesis and Its Role in Whole Body Energy Metabolism. Diabetes Metab J 2017, 41, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Conte, E.; Romano, A.; De Bellis, M.; de Ceglia, M.; Rosaria Carratu, M.; Gaetani, S.; Maqoud, F.; Tricarico, D.; Camerino, C. Oxtr/TRPV1 expression and acclimation of skeletal muscle to cold-stress in male mice. The Journal of endocrinology 2021, 249, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Elabd, C.; Cousin, W.; Upadhyayula, P.; Chen, R.Y.; Chooljian, M.S.; Li, J.; Kung, S.; Jiang, K.P.; Conboy, I.M. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nature communications 2014, 5, 4082. [Google Scholar] [CrossRef]
- Espinoza, S.E.; Lee, J.L.; Wang, C.P.; Ganapathy, V.; MacCarthy, D.; Pascucci, C.; Musi, N.; Volpi, E. Intranasal Oxytocin Improves Lean Muscle Mass and Lowers LDL Cholesterol in Older Adults with Sarcopenic Obesity: A Pilot Randomized Controlled Trial. J Am Med Dir Assoc, 2021. [Google Scholar] [CrossRef]
- Stanic, S.; Bardova, K.; Janovska, P.; Rossmeisl, M.; Kopecky, J.; Zouhar, P. Prolonged FGF21 treatment increases energy expenditure and induces weight loss in obese mice independently of UCP1 and adrenergic signaling. Biochemical pharmacology 2024, 221, 116042. [Google Scholar] [CrossRef]
- Clemmensen, C.; Jall, S.; Kleinert, M.; Quarta, C.; Gruber, T.; Reber, J.; Sachs, S.; Fischer, K.; Feuchtinger, A.; Karlas, A.; et al. Publisher Correction: Coordinated targeting of cold and nicotinic receptors synergistically improves obesity and type 2 diabetes. Nature communications 2018, 9, 4975. [Google Scholar] [CrossRef]
- Haynes, W.G.; Morgan, D.A.; Walsh, S.A.; Mark, A.L.; Sivitz, W.I. Receptor-mediated regional sympathetic nerve activation by leptin. The Journal of clinical investigation 1997, 100, 270–278. [Google Scholar] [CrossRef]
- Hernandez, A.; Obregon, M.J. Triiodothyronine amplifies the adrenergic stimulation of uncoupling protein expression in rat brown adipocytes. Am J Physiol Endocrinol Metab 2000, 278, E769–777. [Google Scholar] [CrossRef]
- Li, Y.; Schnabl, K.; Gabler, S.M.; Willershauser, M.; Reber, J.; Karlas, A.; Laurila, S.; Lahesmaa, M.; M, U.D.; Bast-Habersbrunner, A.; et al. Secretin-Activated Brown Fat Mediates Prandial Thermogenesis to Induce Satiation. Cell 2018, 175, 1561–1574. [Google Scholar] [CrossRef]
- Laurila, S.; Sun, L.; Lahesmaa, M.; Schnabl, K.; Laitinen, K.; Klen, R.; Li, Y.; Balaz, M.; Wolfrum, C.; Steiger, K.; et al. Secretin activates brown fat and induces satiation. Nat Metab 2021, 3, 798–809. [Google Scholar] [CrossRef]
- Cereijo, R.; Villarroya, J.; Villarroya, F. Non-sympathetic control of brown adipose tissue. International Journal of Obesity Supplments 2015, 5, S40–S44. [Google Scholar] [CrossRef]
- Lopez, M.; Alvarez, C.V.; Nogueiras, R.; Dieguez, C. Energy balance regulation by thyroid hormones at central level. Trends Mol Med 2013, 19, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Roberts, Z.S.; Wolden-Hanson, T.H.; Matsen, M.E.; Ryu, V.; Vaughan, C.H.; Graham, J.L.; Havel, P.J.; Chukri, D.W.; Schwartz, M.W.; Morton, G.J.; et al. Chronic Hindbrain Administration of Oxytocin is Sufficient to Elicit Weight Loss in Diet-Induced Obese Rats. Am J Physiol Regul Integr Comp Physiol, 2017. [Google Scholar] [CrossRef]
- Edwards, M.M.; Nguyen, H.K.; Dodson, A.D.; Herbertson, A.J.; Wolden-Hanson, T.; Wietecha, T.; Honeycutt, M.K.; Slattery, J.D.; O'Brien, K.D.; Graham, J.L.; et al. Sympathetic innervation of interscapular brown adipose tissue is not a predominant mediator of oxytocin-elicited reductions of body weight and adiposity in male diet-induced obese mice. Frontiers in endocrinology 2024, 15. [Google Scholar] [CrossRef]
- Ong, Z.Y.; Bongiorno, D.M.; Hernando, M.A.; Grill, H.J. Effects of Endogenous Oxytocin Receptor Signaling in Nucleus Tractus Solitarius on Satiation-Mediated Feeding and Thermogenic Control in Male Rats. Endocrinology 2017, 158, 2826–2836. [Google Scholar] [CrossRef]
- Sutton, A.K.; Pei, H.; Burnett, K.H.; Myers, M.G., Jr.; Rhodes, C.J.; Olson, D.P. Control of food intake and energy expenditure by Nos1 neurons of the paraventricular hypothalamus. J Neurosci 2014, 34, 15306–15318. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, R.; Wu, R.; Gu, Y.; Lu, Y. The effects of oxytocin to rectify metabolic dysfunction in obese mice are associated with increased thermogenesis. Mol Cell Endocrinol 2020, 514, 110903. [Google Scholar] [CrossRef]
- Kasahara, Y.; Sato, K.; Takayanagi, Y.; Mizukami, H.; Ozawa, K.; Hidema, S.; So, K.H.; Kawada, T.; Inoue, N.; Ikeda, I.; et al. Oxytocin receptor in the hypothalamus is sufficient to rescue normal thermoregulatory function in male oxytocin receptor knockout mice. Endocrinology 2013, 154, 4305–4315. [Google Scholar] [CrossRef]
- Kasahara, Y.; Tateishi, Y.; Hiraoka, Y.; Otsuka, A.; Mizukami, H.; Ozawa, K.; Sato, K.; Hidema, S.; Nishimori, K. Role of the Oxytocin Receptor Expressed in the Rostral Medullary Raphe in Thermoregulation During Cold Conditions. Frontiers in endocrinology 2015, 6, 180. [Google Scholar] [CrossRef]
- Harshaw, C.; Leffel, J.K.; Alberts, J.R. Oxytocin and the warm outer glow: Thermoregulatory deficits cause huddling abnormalities in oxytocin-deficient mouse pups. Hormones and behavior 2018, 98, 145–158. [Google Scholar] [CrossRef]
- Xi, D.; Long, C.; Lai, M.; Casella, A.; O'Lear, L.; Kublaoui, B.; Roizen, J.D. Ablation of Oxytocin Neurons Causes a Deficit in Cold Stress Response. Journal of the Endocrine Society 2017, 1, 1041–1055. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, Y.; Zhu, Y.; Sutton, A.K.; Zhao, R.; Lowell, B.B.; Olson, D.P.; Tong, Q. An obligate role of oxytocin neurons in diet induced energy expenditure. PloS one 2012, 7, e45167. [Google Scholar] [CrossRef]
- Camerino, C. Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obesity 2009, 17, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, Y.; Kasahara, Y.; Onaka, T.; Takahashi, N.; Kawada, T.; Nishimori, K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport 2008, 19, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Anekonda, V.T.; Thompson, B.W.; Ho, J.M.; Roberts, Z.S.; Edwards, M.M.; Nguyen, H.K.; Dodson, A.D.; Wolden-Hanson, T.; Chukri, D.W.; Herbertson, A.J.; et al. Hindbrain Administration of Oxytocin Reduces Food Intake, Weight Gain and Activates Catecholamine Neurons in the Hindbrain Nucleus of the Solitary Tract in Rats. J Clin Med 2021, 10. [Google Scholar] [CrossRef]
- Blevins, J.E.; Schwartz, M.W.; Baskin, D.G. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol 2004, 287, R87–96. [Google Scholar] [CrossRef]
- Blevins, J.E.; Thompson, B.W.; Anekonda, V.T.; Ho, J.M.; Graham, J.L.; Roberts, Z.S.; Hwang, B.H.; Ogimoto, K.; Wolden-hanson, T.H.; Nelson, J.O.; et al. Chronic CNS oxytocin signaling preferentially induces fat loss in high fat diet-fed rats by enhancing satiety responses and increasing lipid utilization. Am J Physiol-Reg I 2016.
- Morton, G.J.; Matsen, M.E.; Bracy, D.P.; Meek, T.H.; Nguyen, H.T.; Stefanovski, D.; Bergman, R.N.; Wasserman, D.H.; Schwartz, M.W. FGF19 action in the brain induces insulin-independent glucose lowering. The Journal of clinical investigation 2013, 123, 4799–4808. [Google Scholar] [CrossRef]
- The rat brain in stereotaxic coordinates. , 6th ed.Paxinos, G., Watson, C., Eds.; Academic Press: Burlington, 2007.
- Edwards, M.M.; Nguyen, H.K.; Herbertson, A.J.; Dodson, A.D.; Wietecha, T.; Wolden-Hanson, T.; Graham, J.L.; O'Brien, K.D.; Havel, P.J.; Blevins, J.E. Chronic Hindbrain Administration of Oxytocin Elicits Weight Loss in Male Diet-Induced Obese Mice. Am J Physiol Regul Integr Comp Physiol, 1152. [Google Scholar] [CrossRef]
- Dorfman, M.D.; Krull, J.E.; Douglass, J.D.; Fasnacht, R.; Lara-Lince, F.; Meek, T.H.; Shi, X.; Damian, V.; Nguyen, H.T.; Matsen, M.E.; et al. Sex differences in microglial CX3CR1 signalling determine obesity susceptibility in mice. Nature communications 2017, 8, 14556. [Google Scholar] [CrossRef]
- The mouse brain in stereotaxic coordinates. , 2nd ed.Paxinos, G., Franklin, K.B.J., Eds.; Academic Press: San Diego, CA, 2001.
- Edwards, M.M.; Nguyen, H.K.; Dodson, A.D.; Herbertson, A.J.; Wietecha, T.A.; Wolden-Hanson, T.; Graham, J.L.; Honeycutt, M.K.; Slattery, J.D.; O'Brien, K.D.; et al. Effects of combined oxytocin and beta-3 receptor agonist (CL 316243) treatment on body weight and adiposity in male diet-induced obese rats. Frontiers in physiology 2021. [Google Scholar] [CrossRef]
- Brito, M.N.; Brito, N.A.; Baro, D.J.; Song, C.K.; Bartness, T.J. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology 2007, 148, 5339–5347. [Google Scholar] [CrossRef]
- Vaughan, C.H.; Shrestha, Y.B.; Bartness, T.J. Characterization of a novel melanocortin receptor-containing node in the SNS outflow circuitry to brown adipose tissue involved in thermogenesis. Brain Res 2011, 1411, 17–27. [Google Scholar] [CrossRef]
- Depocas, F.; Foster, D.O.; Zaror-Behrens, G.; Lacelle, S.; Nadeau, B. Recovery of function in sympathetic nerves of interscapular brown adipose tissue of rats treated with 6-hydroxydopamine. Canadian journal of physiology and pharmacology 1984, 62, 1327–1332. [Google Scholar] [CrossRef]
- Wang, L.Y.; Murphy, R.R.; Hanscom, B.; Li, G.; Millard, S.P.; Petrie, E.C.; Galasko, D.R.; Sikkema, C.; Raskind, M.A.; Wilkinson, C.W.; et al. Cerebrospinal fluid norepinephrine and cognition in subjects across the adult age span. Neurobiology of aging 2013, 34, 2287–2292. [Google Scholar] [CrossRef]
- Williams, D.L.; Bowers, R.R.; Bartness, T.J.; Kaplan, J.M.; Grill, H.J. Brainstem melanocortin 3/4 receptor stimulation increases uncoupling protein gene expression in brown fat. Endocrinology 2003, 144, 4692–4697. [Google Scholar] [CrossRef]
- Product Data-DIO Series Diets. in: R.D. Inc., (Ed.), pp. 3.
- Suarez, J.; Rivera, P.; Arrabal, S.; Crespillo, A.; Serrano, A.; Baixeras, E.; Pavon, F.J.; Cifuentes, M.; Nogueiras, R.; Ballesteros, J.; et al. Oleoylethanolamide enhances beta-adrenergic-mediated thermogenesis and white-to-brown adipocyte phenotype in epididymal white adipose tissue in rat. Disease models & mechanisms 2014, 7, 129–141. [Google Scholar] [CrossRef]
- De Boer, S.F.; Van der Gugten, J. Daily Variations in Plasma Noradrenaline, Adrenaline and Corticosterone Concentrations in Rats Physiol Behav 1987, 40, 323-328.
- Davidovic, V.; Petrovic, V.M. Diurnal variations in the catecholamine content in rat tissues Effects of exogenous noradrenaline. Archives Iniernationales de PhysioIogie et de Biochimie 1981, 89, 457–460. [Google Scholar] [CrossRef]
- Bremer, A.A.; Stanhope, K.L.; Graham, J.L.; Cummings, B.P.; Wang, W.; Saville, B.R.; Havel, P.J. Fructose-fed rhesus monkeys: a nonhuman primate model of insulin resistance, metabolic syndrome, and type 2 diabetes. Clinical and translational science 2011, 4, 243–252. [Google Scholar] [CrossRef]
- Blevins, J.E.; Moralejo, D.H.; Wolden-Hanson, T.H.; Thatcher, B.S.; Ho, J.M.; Kaiyala, K.J.; Matsumoto, K. Alterations in activity and energy expenditure contribute to lean phenotype in Fischer 344 rats lacking the cholecystokinin-1 receptor gene. Am J Physiol Regul Integr Comp Physiol 2012, 303, R1231–1240. [Google Scholar] [CrossRef]
- Cummings, B.P.; Digitale, E.K.; Stanhope, K.L.; Graham, J.L.; Baskin, D.G.; Reed, B.J.; Sweet, I.R.; Griffen, S.C.; Havel, P.J. Development and characterization of a novel rat model of type 2 diabetes mellitus: the UC Davis type 2 diabetes mellitus UCD-T2DM rat. Am J Physiol Regul Integr Comp Physiol 2008, 295, R1782–1793. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rinaman, L.; Rothe, E.E. GLP-1 receptor signaling contributes to anorexigenic effect of centrally administered oxytocin in rats. Am J Physiol Regul Integr Comp Physiol 2002, 283, R99–106. [Google Scholar] [CrossRef]
- Tortoriello, D.V.; McMinn, J.; Chua, S.C. Dietary-induced obesity and hypothalamic infertility in female DBA/2J mice. Endocrinology 2004, 145, 1238–1247. [Google Scholar] [CrossRef]
- Totten, M.S.; Wallace, C.W.; Pierce, D.M.; Fordahl, S.C.; Erikson, K.M. The impact of a high-fat diet on physical activity and dopamine neurochemistry in the striatum is sex and strain dependent in C57BL/6J and DBA/2J mice. Nutritional neuroscience 2022, 25, 2601–2615. [Google Scholar] [CrossRef] [PubMed]
- Susulic, V.S.; Frederich, R.C.; Lawitts, J.; Tozzo, E.; Kahn, B.B.; Harper, M.E.; Himms-Hagen, J.; Flier, J.S.; Lowell, B.B. Targeted disruption of the beta 3-adrenergic receptor gene. The Journal of biological chemistry 1995, 270, 29483–29492. [Google Scholar] [CrossRef]
- Rothwell, N.J.; Stock, M.J.; Sudera, D.K. Beta-adrenoreceptors in rat brown adipose tissue: proportions of beta 1- and beta 2-subtypes. Am J Physiol 1985, 248, E397–402. [Google Scholar] [CrossRef]
- Levin, B.E.; Sullivan, A.C. Beta-1 receptor is the predominant beta-adrenoreceptor on rat brown adipose tissue. The Journal of pharmacology and experimental therapeutics 1986, 236, 681–688. [Google Scholar]
- Ueta, C.B.; Fernandes, G.W.; Capelo, L.P.; Fonseca, T.L.; Maculan, F.D.; Gouveia, C.H.; Brum, P.C.; Christoffolete, M.A.; Aoki, M.S.; Lancellotti, C.L.; et al. beta(1) Adrenergic receptor is key to cold- and diet-induced thermogenesis in mice. The Journal of endocrinology 2012, 214, 359–365. [Google Scholar] [CrossRef]
- Atgie, C.; D'Allaire, F.; Bukowiecki, L.J. Role of beta1- and beta3-adrenoceptors in the regulation of lipolysis and thermogenesis in rat brown adipocytes. Am J Physiol 1997, 273, C1136–1142. [Google Scholar] [CrossRef]
- Straat, M.E.; Hoekx, C.A.; van Velden, F.H.P.; Pereira Arias-Bouda, L.M.; Dumont, L.; Blondin, D.P.; Boon, M.R.; Martinez-Tellez, B.; Rensen, P.C.N. Stimulation of the beta-2-adrenergic receptor with salbutamol activates human brown adipose tissue. Cell Rep Med 2023, 4, 100942. [Google Scholar] [CrossRef]
- Blondin, D.P.; Nielsen, S.; Kuipers, E.N.; Severinsen, M.C.; Jensen, V.H.; Miard, S.; Jespersen, N.Z.; Kooijman, S.; Boon, M.R.; Fortin, M.; et al. Human Brown Adipocyte Thermogenesis Is Driven by beta2-AR Stimulation. Cell Metab 2020, 32, 287–300. [Google Scholar] [CrossRef]
- Ishida, Y.; Matsushita, M.; Yoneshiro, T.; Saito, M.; Fuse, S.; Hamaoka, T.; Kuroiwa, M.; Tanaka, R.; Kurosawa, Y.; Nishimura, T.; et al. Genetic evidence for involvement of beta2-adrenergic receptor in brown adipose tissue thermogenesis in humans. Int J Obes (Lond) 2024, 48, 1110–1117. [Google Scholar] [CrossRef]
- Tate, K.M.; Briend-Sutren, M.M.; Emorine, L.J.; Delavier-Klutchko, C.; Marullo, S.; Strosberg, A.D. Expression of three human beta-adrenergic-receptor subtypes in transfected Chinese hamster ovary cells. European journal of biochemistry / FEBS 1991, 196, 357–361. [Google Scholar] [CrossRef]
- Reed, N.; Fain, J.N. Stimulation of respiration in brown fat cells by epinephrine, dibutyryl-3',5'-adenosine monophosphate, and m-chloro(carbonyl cyanide)phenylhydrazone. The Journal of biological chemistry 1968, 243, 2843–2848. [Google Scholar] [CrossRef]
- Strack, A.M.; Sawyer, W.B.; Platt, K.B.; Loewy, A.D. CNS cell groups regulating the sympathetic outflow to adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus. Brain Res 1989, 491, 274–296. [Google Scholar] [CrossRef] [PubMed]
- Dum, R.P.; Levinthal, D.J.; Strick, P.L. The mind-body problem: Circuits that link the cerebral cortex to the adrenal medulla. Proc Natl Acad Sci U S A 2019, 116, 26321–26328. [Google Scholar] [CrossRef]
- de Jong, J.M.; Larsson, O.; Cannon, B.; Nedergaard, J. A stringent validation of mouse adipose tissue identity markers. Am J Physiol Endocrinol Metab 2015, 308, E1085–1105. [Google Scholar] [CrossRef]
- Kalinovich, A.V.; de Jong, J.M.; Cannon, B.; Nedergaard, J. UCP1 in adipose tissues: two steps to full browning. Biochimie 2017, 134, 127–137. [Google Scholar] [CrossRef]
- Nguyen, N.L.; Barr, C.L.; Ryu, V.; Cao, Q.; Xue, B.; Bartness, T.J. Separate and shared sympathetic outflow to white and brown fat coordinately regulate thermoregulation and beige adipocyte recruitment. Am J Physiol Regul Integr Comp Physiol, 2016. [Google Scholar] [CrossRef]
- Doslikova, B.; Tchir, D.; McKinty, A.; Zhu, X.; Marks, D.L.; Baracos, V.E.; Colmers, W.F. Convergent neuronal projections from paraventricular nucleus, parabrachial nucleus, and brainstem onto gastrocnemius muscle, white and brown adipose tissue in male rats. J Comp Neurol 2019, 527, 2826–2842. [Google Scholar] [CrossRef]
- Shi, H.; Bartness, T.J. Neurochemical phenotype of sympathetic nervous system outflow from brain to white fat. Brain research bulletin 2001, 54, 375–385. [Google Scholar] [CrossRef]
- Rinaman, L. Oxytocinergic inputs to the nucleus of the solitary tract and dorsal motor nucleus of the vagus in neonatal rats. J Comp Neurol 1998, 399, 101–109. [Google Scholar] [CrossRef]
- Sawchenko, P.E.; Swanson, L.W. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol 1982, 205, 260–272. [Google Scholar] [CrossRef]
- Bi, S. Dorsomedial hypothalamic NPY modulation of adiposity and thermogenesis. Physiol Behav 2013, 121, 56–60. [Google Scholar] [CrossRef]
- Sakamoto, T.; Sugimoto, S.; Uekita, T. Effects of intraperitoneal and intracerebroventricular injections of oxytocin on social and emotional behaviors in pubertal male mice. Physiol Behav 2019, 212, 112701. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.W.; Schlein, C.; Cannon, B.; Heeren, J.; Nedergaard, J. Intact innervation is essential for diet-induced recruitment of brown adipose tissue. Am J Physiol Endocrinol Metab 2019, 316, E487–E503. [Google Scholar] [CrossRef] [PubMed]
- Bal, N.C.; Maurya, S.K.; Singh, S.; Wehrens, X.H.; Periasamy, M. Increased Reliance on Muscle-based Thermogenesis upon Acute Minimization of Brown Adipose Tissue Function. The Journal of biological chemistry 2016, 291, 17247–17257. [Google Scholar] [CrossRef]
- Bartness, T.J.; Vaughan, C.H.; Song, C.K. Sympathetic and sensory innervation of brown adipose tissue. Int J Obes (Lond) 2010, 34 Suppl 1, S36–42. [Google Scholar] [CrossRef]
- Hicks, C.; Ramos, L.; Reekie, T.; Misagh, G.H.; Narlawar, R.; Kassiou, M.; McGregor, I.S. Body temperature and cardiac changes induced by peripherally administered oxytocin, vasopressin and the non-peptide oxytocin receptor agonist WAY 267,464: a biotelemetry study in rats. British journal of pharmacology 2014, 171, 2868–2887. [Google Scholar] [CrossRef]
- Kohli, S.; King, M.V.; Williams, S.; Edwards, A.; Ballard, T.M.; Steward, L.J.; Alberati, D.; Fone, K.C.F. Oxytocin attenuates phencyclidine hyperactivity and increases social interaction and nucleus accumben dopamine release in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2019, 44, 295–305. [Google Scholar] [CrossRef]
- Maejima, Y.; Aoyama, M.; Sakamoto, K.; Jojima, T.; Aso, Y.; Takasu, K.; Takenosihita, S.; Shimomura, K. Impact of sex, fat distribution and initial body weight on oxytocin's body weight regulation. Scientific reports 2017, 7, 8599. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





