Submitted:
31 August 2024
Posted:
02 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and methods
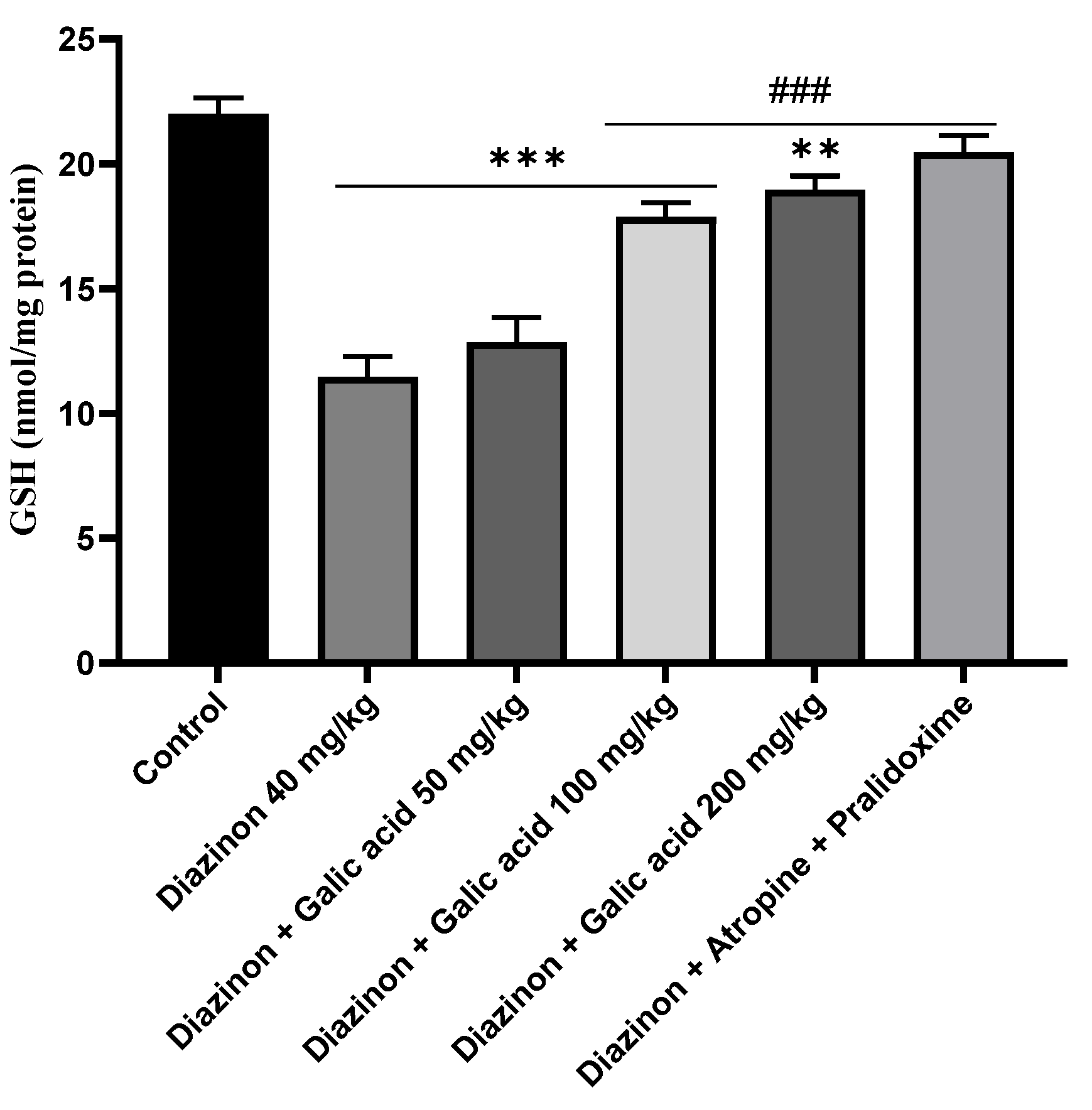
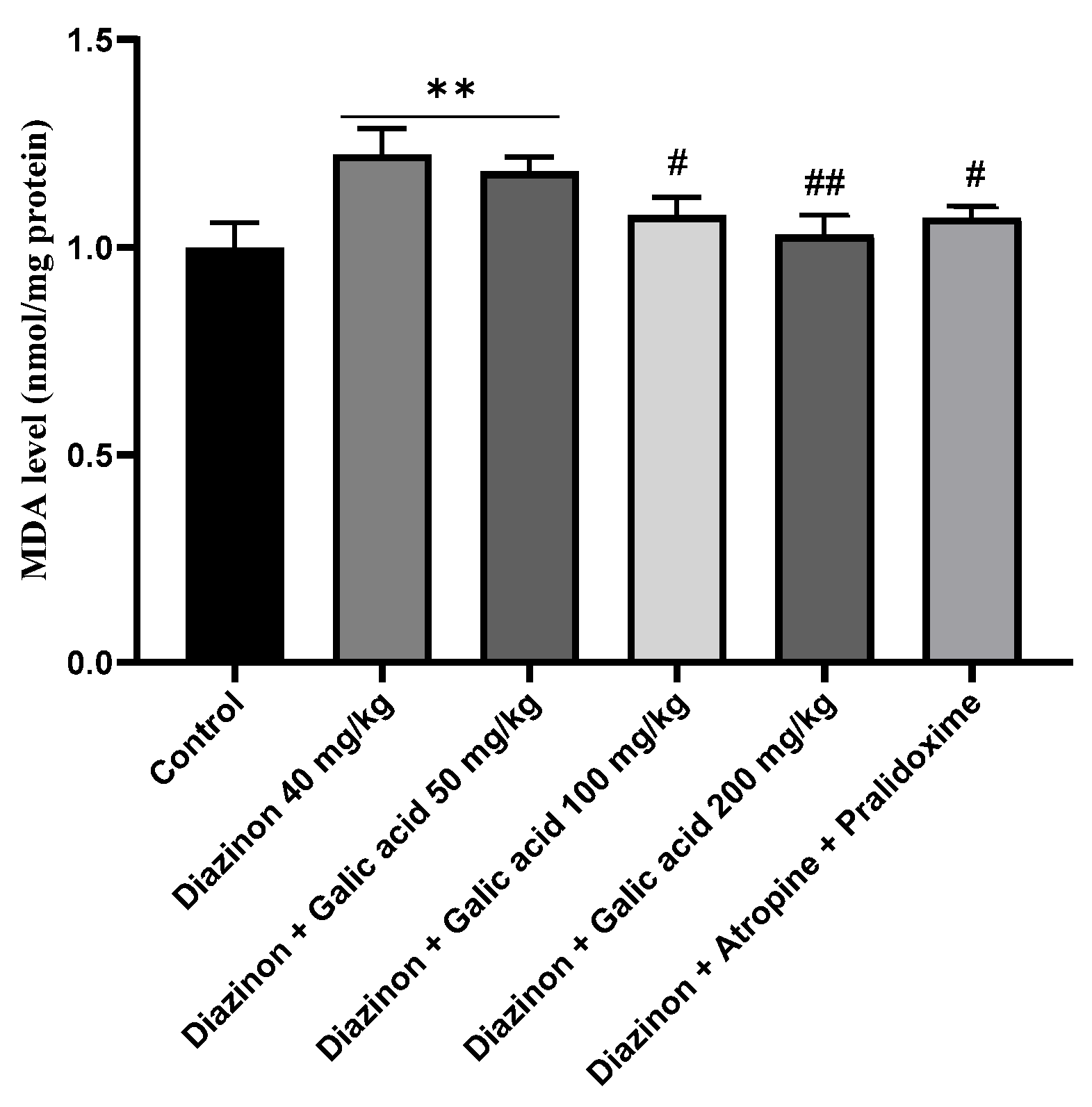
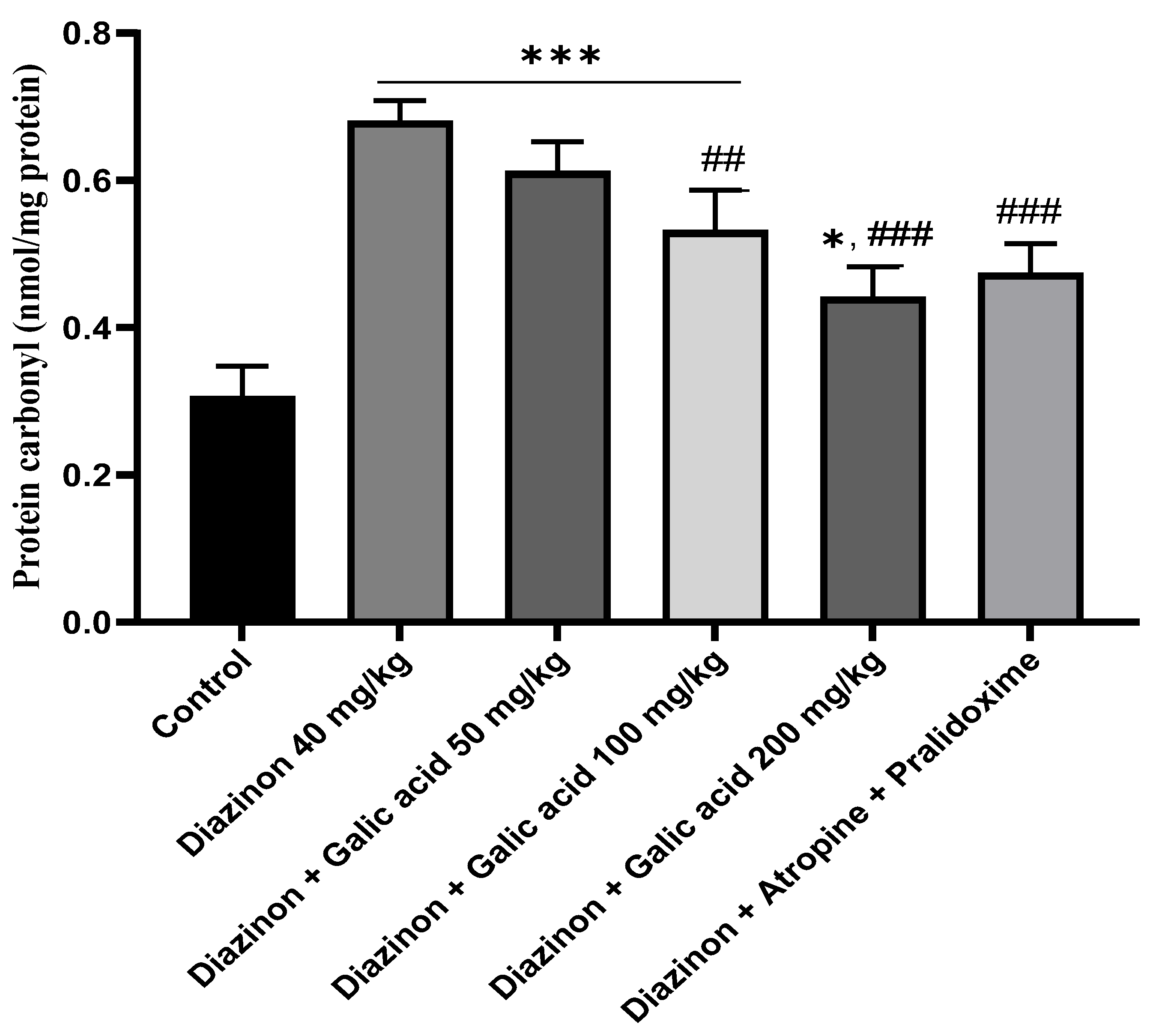
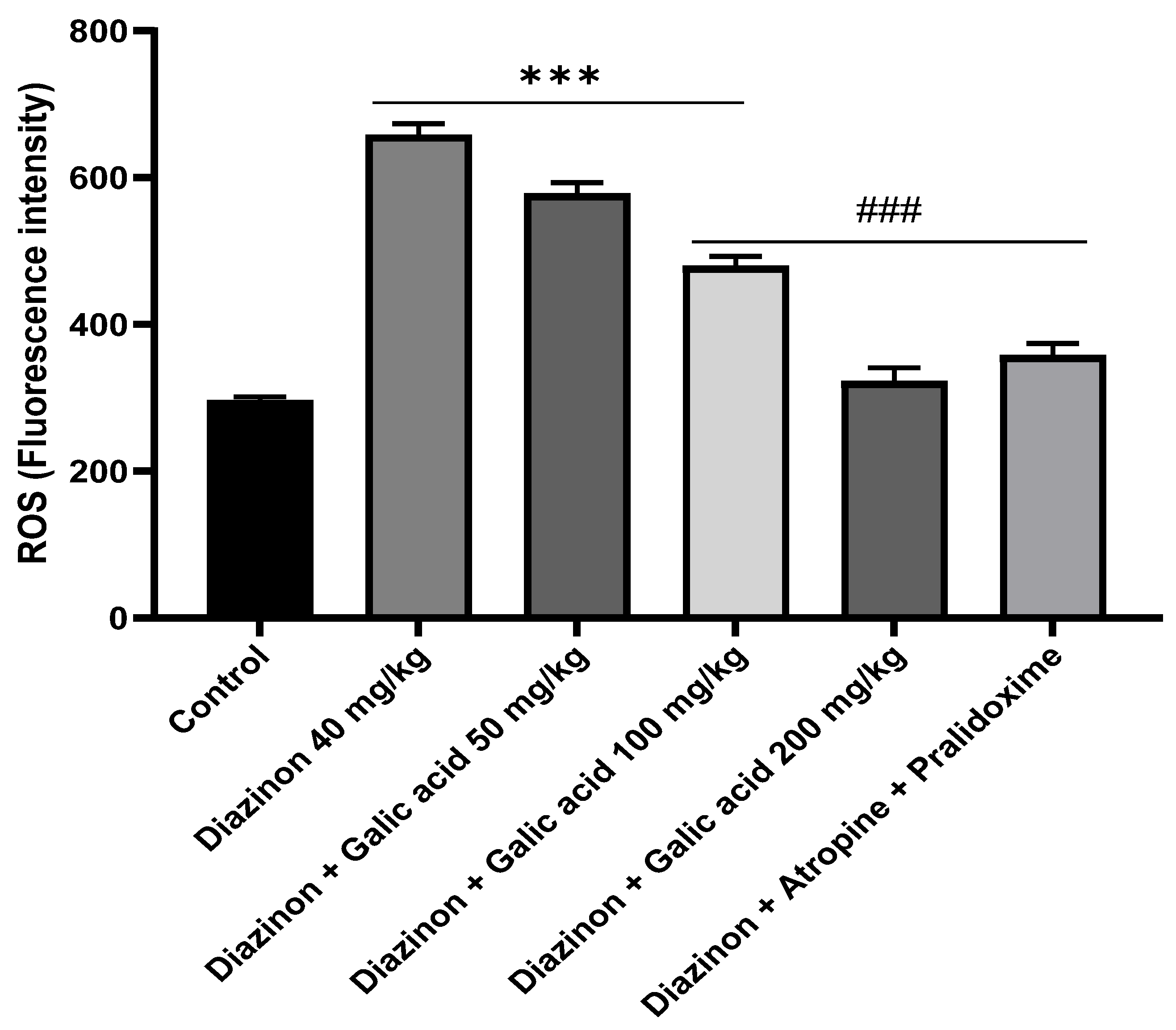
3. Results
4. Discussion
5. Conclusion
References
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jabbar ASA. Pesticide poisoning in humans. JPMA The Journal of the Pakistan Medical Association. 1992;42 10:251-5.
- Pratama, D.A.; Setiani, O.; Darundiati, Y.H. STUDI LITERATUR : PENGARUH PAPARAN PESTISIDA TERHADAP GANGGUAN KESEHATAN PETANI. J. Ris. Kesehat. Poltekkes Depkes Bdg. 2021, 13, 160–171. [Google Scholar] [CrossRef]
- Prajawahyudo, T.; Asiaka, F.K.P.; Ludang, E. Peranan keamanan pestisida di bidang pertanian bagi petani dan lingkungan. J. Socio Econ. Agric. 2022, 17, 1–9. [Google Scholar] [CrossRef]
- Pamungkas, OS. Bahaya paparan pestisida terhadap kesehatan manusia. Bioedukasi. 2017;14(1).
- Pawukir ES, Mariyono J. Hubungan Antara Penggunaan Pestisida Dan Dampak Kesehatan: Studi Kasus Di Dataran Tinggi Sumatra Barat (the Relationship Between Pesticides Use and Health Impact: a Case Study in Highlands of West Sumatera). Jurnal Manusia dan Lingkungan. 2002;9(3):126-36.
- Goel A, Aggarwal P. Pesticide poisoning. The National medical journal of India. 2022;20 4:182-91.
- Gupta RC, Mukherjee IRM, Malik JK, Doss RB, Dettbarn W-D, Milatovic D. Insecticides. Biomarkers in toxicology: Elsevier; 2019. p. 455-75.
- Araújo, M.F.; Castanheira, E.M.S.; Sousa, S.F. The Buzz on Insecticides: A Review of Uses, Molecular Structures, Targets, Adverse Effects, and Alternatives. Molecules 2023, 28, 3641. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M. The killer chemicals as controller of agriculture insect pests: The conventional insecticides. International Journal of Chemical and Biomolecular Science. 2015;1(3):141-7.
- Stehle, S.; Schulz, R. Global Insecticide Surface Water Contamination Assessment: BECT’s Contribution in the Last Five Decades. Bull. Environ. Contam. Toxicol. 2016, 96, 563–564. [Google Scholar] [CrossRef] [PubMed]
- Stehle, S.; Schulz, R. Agricultural insecticides threaten surface waters at the global scale. Proc. Natl. Acad. Sci. USA 2015, 112, 5750–5755. [Google Scholar] [CrossRef]
- Smith, J.J.; Herzig, V.; King, G.F.; Alewood, P.F. The insecticidal potential of venom peptides. Cell. Mol. Life Sci. 2013, 70, 3665–3693. [Google Scholar] [CrossRef]
- Alavanja, M.C.R.; Hofmann, J.N.; Lynch, C.F.; Hines, C.J.; Barry, K.H.; Barker, J.; Buckman, D.W.; Thomas, K.; Sandler, D.P.; Hoppin, J.A.; et al. Non-Hodgkin Lymphoma Risk and Insecticide, Fungicide and Fumigant Use in the Agricultural Health Study. PLOS ONE 2014, 9, e109332. [Google Scholar] [CrossRef]
- Baldi, I.; Gruber, A.; Rondeau, V.; Lebailly, P.; Brochard, P.; Fabrigoule, C. Neurobehavioral effects of long-term exposure to pesticides: results from the 4-year follow-up of the PHYTONER Study. Occup. Environ. Med. 2010, 68, 108–115. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, J.; Ren, J.; Hou, Y.; Han, Z.; Xiao, J.; Li, Y. Exposure Level of Neonicotinoid Insecticides in the Food Chain and the Evaluation of Their Human Health Impact and Environmental Risk: An Overview. Sustainability 2020, 12, 7523. [Google Scholar] [CrossRef]
- Viana, M.; Hughes, A.; Matthiopoulos, J.; Ranson, H.; Ferguson, H.M. Delayed mortality effects cut the malaria transmission potential of insecticide-resistant mosquitoes. Proc. Natl. Acad. Sci. 2016, 113, 8975–8980. [Google Scholar] [CrossRef]
- Chittrakul, J.; Sapbamrer, R.; Sirikul, W. Insecticide Exposure and Risk of Asthmatic Symptoms: A Systematic Review and Meta-Analysis. Toxics 2021, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Modak, S.; Ghosh, P.; Mandal, S.; Sasmal, D.; Kundu, S.; Sengupta, S.; Kanthal, S.; Sarkar, T. Organophosphate Pesticide: Environmental impact and toxicity to organisms. Int. J. Res. Agron. 2024, 7, 138–141. [Google Scholar] [CrossRef]
- Sidhu, G.K.; Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Singh, J. Toxicity, monitoring and biodegradation of organophosphate pesticides: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1135–1187. [Google Scholar] [CrossRef]
- Melo JS, editor Overview on Biosensors for Detection of Organophosphate Pesticides2017.
- Risal, P.; Lama, S.; Thapa, S.; Bhatta, R.; Karki, R.K. Cholinesterase and Liver Enzymes in Patients with Organophosphate Poisoning. J. Nobel Med Coll. 2019, 8, 33–37. [Google Scholar] [CrossRef]
- Chuang, C.-S.; Yang, K.-W.; Yen, C.-M.; Lin, C.-L.; Kao, C.-H. Risk of Seizures in Patients with Organophosphate Poisoning: A Nationwide Population-Based Study. Int. J. Environ. Res. Public Heal. 2019, 16, 3147. [Google Scholar] [CrossRef]
- Chowdhury, F.R.; Bari, S.; Alam, M.J.; Rahman, M.; Bhattacharjee, B.; Qayyum, J.A.; Mridha, S. Organophosphate poisoning presenting with muscular weakness and abdominal pain- a case report. BMC Res. Notes 2014, 7, 140–140. [Google Scholar] [CrossRef]
- Peter, J.V.; Sudarsan, T.; Moran, J. Clinical features of organophosphate poisoning: A review of different classification systems and approaches. Indian J. Crit. Care Med. 2014, 18, 735–745. [Google Scholar] [CrossRef]
- Peter, J.V.; Jerobin, J.; Nair, A.; Bennett, A.; Samuel, P.; Chrispal, A.; Abraham, O.C.; Mathews, K.P.; Fleming, J.J.; Oommen, A. Clinical profile and outcome of patients hospitalized with dimethyl and diethyl organophosphate poisoning. Clin. Toxicol. 2010, 48, 916–923. [Google Scholar] [CrossRef]
- Dündar ZD, Ergin M, Köylü R, Günaydın YK, Özer R, Cander B. Prognostic value of red cell distribution width in patients with organophosphate poisoning. 2015.
- E Ibrahim, A.; Ghantarchyan, H.; Le, T.; Bhagat, A.; Maknouni, B.; Arabian, S. A Rare Presentation of Severe Organophosphate Poisoning: A Case Report and Review of Literature. Cureus 2022, 14, e31497. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.; Abdulla, M.C.; Alungal, J. Transient Distal Renal Tubular Acidosis in Organophosphate Poisoning. Indian J. Crit. Care Med. 2017, 21, 170–171. [Google Scholar] [CrossRef] [PubMed]
- Alghafees, M.A.; Abdulmomen, A.; Eid, M.; Alhussin, G.I.; Alosaimi, M.Q.; Alduhaimi, G.S.; Albogami, M.T.; Alhelail, M. Poisoning-related emergency department visits: the experience of a Saudi high-volume toxicology center. Ann. Saudi Med. 2022, 42, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.A.; Sovocool, G.W.; Curley, A.; Ahmed, N.S.; El-Fiki, S.; El-Sebae, A.-K. Two Acute Human Poisoning Cases Resulting from Exposure to Diazinon Transformation Products in Egypt. Arch. Environ. Heal. Int. J. 1982, 37, 207–212. [Google Scholar] [CrossRef]
- Dahlgren J, Takhar H, Ruffalo C, Zwass M. Health effects of diazinon on a family. Journal of Toxicology: Clinical Toxicology. 2004;42(5):579-91.
- Liang, T.-W.; Balcer, L.J.; Solomon, D.; Messé, S.R.; Galetta, S.L. Supranuclear gaze palsy and opsoclonus after Diazinon poisoning. J. Neurol. Neurosurg. Psychiatry 2003, 74, 677–679. [Google Scholar] [CrossRef]
- Sergi, CM. Diazinon—An Insecticide. Encyclopedia of Environmental Health. 2019.
- Namba T, Nolte CT, Jackrel J, Grob D. Poisoning due to organophosphate insecticides: acute and chronic manifestations. The American journal of medicine. 1971;50(4):475-92.
- Klemmer, H.W.; Reichert, E.R.; Yauger, W.L.; Haley, T.J. Five Cases of Intentional Ingestion of 25 Percent Diazinon with Treatment and Recovery. Clin. Toxicol. 1978, 12, 435–444. [Google Scholar] [CrossRef]
- Kamha AA, Al Omary IY, Zalabany HA, Hanssens Y, Adheir FS. Organophosphate poisoning in pregnancy: a case report. Basic & clinical pharmacology & toxicology. 2005;96(5):397-8.
- Ajibade, T.O.; Oyagbemi, A.A.; Omobowale, T.O.; Asenuga, E.R.; Afolabi, J.M.; Adedapo, A.A. Original article. Mitigation of diazinon-induced cardiovascular and renal dysfunction by gallic acid. Interdiscip. Toxicol. 2016, 9, 66–77. [Google Scholar] [CrossRef]
- Anbarkeh, F.R.; Nikravesh, M.R.; Jalali, M.; Soukhtanloo, M. The protective role of alpha-lipoic acid on the appearance of fibronectin and laminin in renal tubules following diazinon exposure: An experimental immunohistochemical study. Toxicology 2020, 444, 152583. [Google Scholar] [CrossRef]
- Karimani, A.; Ramezani, N.; Goli, A.A.; Shirazi, M.H.N.; Nourani, H.; Jafari, A.M. Subchronic neurotoxicity of diazinon in albino mice: Impact of oxidative stress, AChE activity, and gene expression disturbances in the cerebral cortex and hippocampus on mood, spatial learning, and memory function. Toxicol. Rep. 2021, 8, 1280–1288. [Google Scholar] [CrossRef]
- Afshari, S.; Sarailoo, M.; Asghariazar, V.; Safarzadeh, E.; Dadkhah, M. Persistent diazinon induced neurotoxicity: The effect on inhibitory avoidance memory performance, amyloid precursor proteins, and TNF-α levels in the prefrontal cortex of rats. Hum. Exp. Toxicol. 2024, 43. [Google Scholar] [CrossRef] [PubMed]
- Reshi M, Bhat A, Kaur P, editors. The Role of Antioxidants in Human Health 2013.
- Willcox, J.K.; Ash, S.L.; Catignani, G.L. Antioxidants and Prevention of Chronic Disease. Crit. Rev. Food Sci. Nutr. 2004, 44, 275–295. [Google Scholar] [CrossRef] [PubMed]
- Hamid A, Aiyelaagbe O, Usman L, Ameen O, Lawal A. Antioxidants: Its medicinal and pharmacological applications. African Journal of pure and applied chemistry. 2010;4(8):142-51.
- Halliwell, B. Antioxidants in human health and disease. Annual review of nutrition. 1996;16(1):33-50.
- Buelga, CS. Los polifenoles y la salud. ACTA/CL: revista de la Asociación de Científicos y Tecnólogos de Alimentos de Castilla y León. 2020(70):15-6.
- Kuhnert, N. Polyphenole: Vielseitige Pflanzeninhaltsstoffe: In Garten, Industrie, Medizin und Nahrung. Chemie in unserer Zeit. 2013;47(2):80-91.
- Quiñones M, Miguel M, Aleixandre A. Los polifenoles, compuestos de origen natural con efectos saludables sobre el sistema cardiovascular. Nutrición hospitalaria. 2012;27(1):76-89.
- Achat, S. Polyphénols de l’alimentation: extraction, pouvoir antioxydant et interactions avec des ions métalliques: Université d’Avignon; Université Abderrahmane Mira-Bejaïa (Bejaïa, Algérie); 2013.
- Daglia M, Di Lorenzo A, F Nabavi S, S Talas Z, M Nabavi S. Polyphenols: well beyond the antioxidant capacity: gallic acid and related compounds as neuroprotective agents: you are what you eat! Current pharmaceutical biotechnology. 2014;15(4):362-72.
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef]
- Gao, J.; Hu, J.; Hu, D.; Yang, X. A Role of Gallic Acid in Oxidative Damage Diseases: A Comprehensive Review. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef]
- Inoue, M.; Suzuki, R.; Sakaguchi, N.; Li, Z.; Takeda, T.; Ogihara, Y.; Jiang, B.Y.; Chen, Y. Selective Induction of Cell Death in Cancer Cells by Gallic Acid. Biol. Pharm. Bull. 1995, 18, 1526–1530. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M.; Haslam, E. Phenol biosynthesis in higher plants. Gallic acid. Biochem. J. 1969, 113, 537–542. [Google Scholar] [CrossRef]
- Pengelly, A. The constituents of medicinal plants: an introduction to the chemistry and therapeutics of herbal medicine: Routledge; 2020.
- Fiuza S, Gomes C, Teixeira L, Da Cruz MG, Cordeiro M, Milhazes N, et al. Phenolic acid derivatives with potential anticancer properties––a structure–activity relationship study. Part 1: Methyl, propyl and octyl esters of caffeic and gallic acids. Bioorganic & medicinal chemistry. 2004;12(13):3581-9.
- Kaur, M.; Velmurugan, B.; Rajamanickam, S.; Agarwal, R.; Agarwal, C. Gallic Acid, an Active Constituent of Grape Seed Extract, Exhibits Anti-proliferative, Pro-apoptotic and Anti-tumorigenic Effects Against Prostate Carcinoma Xenograft Growth in Nude Mice. Pharm. Res. 2009, 26, 2133–2140. [Google Scholar] [CrossRef]
- Choubey, S.; Varughese, L.R.; Kumar, V.; Beniwal, V. Medicinal Importance of Gallic Acid and Its Ester Derivatives: a Patent Review. Pharm. Pat. Anal. 2015, 4, 305–315. [Google Scholar] [CrossRef]
- Sarjit, A.; Wang, Y.; Dykes, G.A. Antimicrobial activity of gallic acid against thermophilic Campylobacter is strain specific and associated with a loss of calcium ions. Food Microbiol. 2015, 46, 227–233. [Google Scholar] [CrossRef]
- Hsieh, S.-C.; Wu, C.-C.; Hsu, S.-L.; Yen, J.-H. Molecular mechanisms of gallic acid-induced growth inhibition, apoptosis, and necrosis in hypertrophic scar fibroblasts. Life Sci. 2017, 179, 130–138. [Google Scholar] [CrossRef]
- Goldstein, A.; Aronow, L.; Kalman, S.M. Principles of Drug Action. The Basis of Pharmacology. The Basis of Pharmacology. J. Med. Chem. 1968, 13, 337–337. [Google Scholar] [CrossRef]
- Kaur, S.; Singla, N.; Dhawan, D.K. Neuro-protective potential of quercetin during chlorpyrifos induced neurotoxicity in rats. Drug Chem. Toxicol. 2019, 42, 220–230. [Google Scholar] [CrossRef]
- Abbassy M, Belal M, Nasr H, Mansy A. Role of vitamin E and rutin as potent inducers of chlorpyrifos degradation in the blood of treated male albino rats. Journal of Environmental Toxicology and Analytical Research. 2019;1(1):1.
- Adampourezare, M.; Sistani, P.; Nemati, H.H. Protective Effect of Dorema glabrum on Induced Oxidative Stress by Diazinon in Hippocampus of Rat. Int. J. Biochem. Res. Rev. 2019, 1–7. [Google Scholar] [CrossRef]
- Bameri, B.; Shaki, F.; Ahangar, N.; Ataee, R.; Samadi, M.; Mohammadi, H. Evidence for the Involvement of the Dopaminergic System in Seizure and Oxidative Damage Induced by Tramadol. Int. J. Toxicol. 2018, 37, 164–170. [Google Scholar] [CrossRef]
- Fathi, H.; Ebrahimzadeh, M.A.; Ziar, A.; Mohammadi, H. Oxidative damage induced by retching; antiemetic and neuroprotective role of Sambucus ebulus L. Cell Biol. Toxicol. 2015, 31, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Salehi, M.; Ahmadi, S.; Asgari, A.; Abasnezhad, M.; Hajigholamali, M. The role of oxidative stress in diazinon-induced tissues toxicity in Wistar and Norway rats. Toxicol. Mech. Methods 2012, 22, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Shah MD, Iqbal M. Diazinon-induced oxidative stress and renal dysfunction in rats. Food and chemical toxicology. 2010;48(12):3345-53.
- Salehi M, Jafari M, Saleh-Moqadam M, Asgari A. The comparison of the effect of diazinon and paraoxon on biomarkers of oxidative stress in rat serum. Zahedan Journal of Research in Medical Sciences. 2012;14(3).
- Izadi F, Jafari M, Bahdoran H, Asgari A, Divsalar A, Salehi M. The role of N-acetyl cysteine on reduction of diazinon-induced oxidative stress in rat liver and kidney. Journal of Rafsanjan University of Medical Sciences. 2014;12(11):895-906.
- Sherifa, K. Hepatic and renal biochemical responses to the toxicological interaction between acetylsalicylic acid and diazinon in albino rats. 2006.
- Abdel-Daim, M.M.; Taha, R.; Ghazy, E.W.; El-Sayed, Y.S. Synergistic ameliorative effects of sesame oil and alpha-lipoic acid against subacute diazinon toxicity in rats: hematological, biochemical, and antioxidant studies. Can. J. Physiol. Pharmacol. 2016, 94, 81–88. [Google Scholar] [CrossRef]
- Aronzon, C.M.; Marino, D.J.; Ronco, A.E.; Coll, C.S.P. Differential toxicity and uptake of Diazinon on embryo-larval development of Rhinella arenarum. Chemosphere 2014, 100, 50–56. [Google Scholar] [CrossRef]
- Pizzurro, D.M.; Dao, K.; Costa, L.G. Diazinon and diazoxon impair the ability of astrocytes to foster neurite outgrowth in primary hippocampal neurons. Toxicol. Appl. Pharmacol. 2013, 274, 372–382. [Google Scholar] [CrossRef]
- Lee, B.; Park, S.M.; Jeong, S.; Kim, K.; Jeung, E.-B. Combined Exposure to Diazinon and Nicotine Exerts a Synergistic Adverse Effect In Vitro and Disrupts Brain Development and Behaviors In Vivo. Int. J. Mol. Sci. 2021, 22, 7742. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.K.; Ahmed, J.A. The Neuro-protective Role of Vitamin B12 in Diazinon Poisoned Male Wistar Rats: Histopathological and Biochemical Evaluation. Egyptian Journal of Veterinary Sciences. 2021;52, 3: International Conference of Veterinary Research Division National Research Centre, Giza, Egypt 27th-29th 21), 20 September.
- Gao B, Bian X, Chi L, Tu P, Ru H, Lu K. Editor’s Highlight: Organophosphate diazinon altered quorum sensing, cell motility, stress response, and carbohydrate metabolism of gut microbiome. Toxicological Sciences. 2017;157(2):354-64.
- Sadri S, Bahrami F, Khazaei M, Hashemi M, Asgari A. Cannabinoid receptor agonist WIN-55,212-2 protects differentiated PC12 cells from organophosphorus-induced apoptosis. International journal of toxicology. 2010;29(2):201-8.
- Slotkin, T.A.; Seidler, F.J.; Fumagalli, F. Targeting of neurotrophic factors, their receptors, and signaling pathways in the developmental neurotoxicity of organophosphates in vivo and in vitro. Brain Res. Bull. 2008, 76, 424–438. [Google Scholar] [CrossRef] [PubMed]
- Wisudanti DD, Normasari R, Hidayati T. Hepatoprotective effects of soy flour (Glycine max (L.) Merr.) supplementation in diazinon-treated Wistar rats. 2021.
- Sinaei, N.; Jafari, E.; Najafi, A.; Karami-Mohajeri, S. Hepatic Oxidative Damages and Glucose Tolerance in Diabetic Rats Exposed to Repeated Oral Doses of Diazinon. Iran. J. Toxicol. 2022, 16, 221–228. [Google Scholar] [CrossRef]
- Oruc, E. Effects of diazinon on antioxidant defense system and lipid peroxidation in the liver of Cyprinus carpio (L.). Environ. Toxicol. 2011, 26, 571–578. [Google Scholar] [CrossRef]
- Esfandiarifar A, Azarbayjani M-A, Peeri M, Jameie SB. The Effect of Resistance Training and Berberine Chloride on the Apoptosis-Related UPR Signaling Pathway in the Hippocampus of Diazinon-Poisoned Rats Running title: Resistance Training and Berberine on the Hippocampus Apoptosis.
- Attabi, M.R.A.; Hussain, S.M.; Al-Okaily, B.N. Effect of Diazinon on Reproductive System of Adult Male Mice. J. Kerbala Agric. Sci. 2017, 4, 229–242. [Google Scholar] [CrossRef]
- Ebadimanas, G.; Najafi, G. The Regulation of Testosterone Levels and Improvement of Sperm DNA Maturity and In-Vitro Fertilization Outcome by Selenium Administration in Diazinon-treated Wistar Rats. J. Kermanshah Univ. Med Sci. 2020, 24. [Google Scholar] [CrossRef]
- Ghazy, E.; Mokh, A.; Abdelhady, D.; Goda, W.; Hashem, E. The role of thymoquinone in ameliorating the hepatoxic effect of diazinon in male rats. Slov. Veter- Res. 2019, 56, 745–744. [Google Scholar] [CrossRef]
- Sonei, A.; Fazelipour, S.; Kanaani, L.; Jahromy, M.H. Protective Effects of Berberis vulgaris on Diazinon-Induced Brain Damage in Young Male Mice. Prev. Nutr. Food Sci. 2020, 25, 65–70. [Google Scholar] [CrossRef]
- Roegge, C.S.; Timofeeva, O.A.; Seidler, F.J.; Slotkin, T.A.; Levin, E.D. Developmental diazinon neurotoxicity in rats: Later effects on emotional response. Brain Res. Bull. 2007, 75, 166–172. [Google Scholar] [CrossRef]
- Slotkin, T.A.; Ryde, I.T.; Levin, E.D.; Seidler, F.J. Developmental neurotoxicity of low dose diazinon exposure of neonatal rats: Effects on serotonin systems in adolescence and adulthood. Brain Res. Bull. 2007, 75, 640–647. [Google Scholar] [CrossRef]
- Slotkin TA, Tate CA, Ryde IT, Levin ED, Seidler FJ. Organophosphate insecticides target the serotonergic system in developing rat brain regions: disparate effects of diazinon and parathion at doses spanning the threshold for cholinesterase inhibition. Environmental health perspectives. 2006;114(10):1542-6.
- Gao, B.; Bian, X.; Mahbub, R.; Lu, K. Sex-Specific Effects of Organophosphate Diazinon on the Gut Microbiome and Its Metabolic Functions. Environ. Heal. Perspect. 2017, 125, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Wang, Z.; Zheng, J.; Wang, R. Protective effects of gallic acid against spinal cord injury-induced oxidative stress. Mol. Med. Rep. 2015, 12, 3017–3024. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.T.; Farbood, Y.; Sameri, M.J.; Sarkaki, A.; Naghizadeh, B.; Rafeirad, M. Neuroprotective effects of oral gallic acid against oxidative stress induced by 6-hydroxydopamine in rats. Food Chem. 2013, 138, 1028–1033. [Google Scholar] [CrossRef]
- Jin, L.; Piao, Z.H.; Sun, S.; Liu, B.; Kim, G.R.; Seok, Y.M.; Lin, M.Q.; Ryu, Y.; Choi, S.Y.; Kee, H.J.; et al. Gallic Acid Reduces Blood Pressure and Attenuates Oxidative Stress and Cardiac Hypertrophy in Spontaneously Hypertensive Rats. Sci. Rep. 2017, 7, 15607. [Google Scholar] [CrossRef]
- de Oliveira LS, Thomé GR, Lopes TF, Reichert KP, de Oliveira JS, da Silva Pereira A, et al. Effects of gallic acid on delta–aminolevulinic dehydratase activity and in the biochemical, histological and oxidative stress parameters in the liver and kidney of diabetic rats. Biomedicine & Pharmacotherapy. 2016;84:1291-9.
- Soumya, K.; James, J.; Archana, T.M.; Dhanya, A.T.; Shahid, A.P.; Sudheesh, S. Cytotoxic and antigenotoxic properties of phenolic compound isolated from the fruit of Terminalia chebula on HeLa cell. Beni-Suef Univ. J. Basic Appl. Sci. 2019, 8, 1–6. [Google Scholar] [CrossRef]
- Yang, D.J.; Moh, S.H.; Son, D.H.; You, S.; Kinyua, A.W.; Ko, C.M.; Song, M.; Yeo, J.; Choi, Y.-H.; Kim, K.W. Gallic Acid Promotes Wound Healing in Normal and Hyperglucidic Conditions. Molecules 2016, 21, 899. [Google Scholar] [CrossRef]
- Wen, L.; Tang, L.; Zhang, M.; Wang, C.; Li, S.; Wen, Y.; Tu, H.; Tian, H.; Wei, J.; Liang, P.; et al. Gallic Acid Alleviates Visceral Pain and Depression via Inhibition of P2X7 Receptor. Int. J. Mol. Sci. 2022, 23, 6159. [Google Scholar] [CrossRef]
- Karaman, A.; Aydın, H.; Geçkinli, B.; Çetinkaya, A.; Karaman, S. DNA damage is increased in lymphocytes of patients with metabolic syndrome. Mutat. Res. Toxicol. Environ. Mutagen. 2015, 782, 30–35. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



