Introduction
In recent years, there has been increasing interest in nanosized materials, among others in silica nanoparticles (SNPs). SNP applications’ main advantages are that these particles are chemically inert and non-toxic in a wide concentration range[
1]. Their surface can be modified with several functional groups, proteins, or enzymes[
2]. Because of these advantageous properties, SNPs are widely applied in several research fields, such as drug delivery[
3], diagnosis and therapy[
4], catalysis[
5], chemical sensing[
6] , and biotechnology[
7].
To this day, research groups focus on developing a simple method for well-size-controlled SNP synthesis with high reproducibility, where the SNP particles are highly monodisperse and sphere-shaped. Because of the complicated reaction mechanism (monomer addition growth model[
8]
,[
9] and aggregation growth model[
10]) and the laborious preparation method (centrifugation and washing, dialysis, filtering), the reproducibility and size controlling of synthesis of SNPs under the 200 nm size range are not adequate. The original Stöber[
11] reaction provides an excellent basis for SNP synthesis; Stöber and his colleagues prepared SNPs in the 50-900 nm size range in a comprehensive study; the reaction is a sol-gel synthesis, where the silanol precursors hydrolyze in an alcohol solvent, the reaction is catalyzed with ammonia. They investigated the influence of solvents and different silanol precursors on particle size and shape. Several researchers modify this method by changing the precursor concentrations[
12,
13] or reaction conditions, e.g., temperature[
14] or reaction time[
15], to synthesize SNPs with the desired size. Others work with modified Stöber reaction, the so-called seeded polymerization[
16], but in this process, the effect of reactant addition rate and the number or size of seeds highly influence the product properties, which factors make it more difficult to control the reactions, with this the properties of SNPs.
We aimed to develop a simple, well-size-controlled synthesis method with high reproducibility and product yield for SNPs under 200 nm particle size. We want to focus on only the synthesis procedure, not on the reaction mechanism; it may provide practical help to researchers who deal with the application of SNPs. We assumed that with the proper choice of tetraethyl orthosilicate (TEOS), ammonium hydroxide, or water concentration and reaction temperature, we could find relationships between the SNPs’ size and reaction conditions that may be suitable to calculate the reaction conditions for synthesizing SNPs with the desired size.
We chose the reaction conditions and reagents based on literature data and our previous observations and experiences. We worked with TEOS as a silanol precursor and ethanol as a solvent. Green and co-workers described before that if the alcohol chain length increases, the first nucleation step will be slower, and the size of the nuclei will be bigger, so the final particle size will also be bigger[
17]. Therefore, in our experiments, we worked with ethanol instead of methanol because if we would like to synthesize SNPs in a large amount, it is an important practical aspect that ethanol is cheaper and more eco-friendly. The choice of TEOS and ammonium hydroxide concentration is based on our previous experiments. We worked with 0.26 M TEOS concentration in all cases because the product yields were unsatisfactory at lower values. If the TEOS concentration is higher than 0.3 M, the SNP size is higher than 500 nm, and here, we focus on the 5-200 nm particle size range. We have also examined previously if the ammonium hydroxide concentration is lower than 0.0192 M, then at 0.26 M TEOS concentration particles do not form, presumably under this ammonium hydroxide concentration value the hydrolysis rate is very slow, only partially hydrolyzed monomers are formed. On the other hand, the particle size increases significantly if the ammonium hydroxide concentration is higher than 0.5 M. Based on these, we worked with a constant 0.26 M TEOS concentration and in the 0.29-0.097 M ammonium hydroxide concentration range.
Plumeré et al.[
18] and J. Yang et al.[
19] established that if the reaction temperature is higher, the particle size and the polydispersity index will be smaller; if the reaction temperature increases, the hydrolysis and condensation rates also increase, and at higher temperature, more secondary particles form, which leads to faster nucleation kinetics. Two research teams independently studied the formation of SNPs, and they found that because of the faster nucleation process, more nuclei are produced, so smaller and more uniform particles can be synthesized at higher reaction temperature
14,[
20]. Besides this, Park et al.[
21] and Kim et al.[
22] determined that if the temperature is higher than 55°C, the aggregation of nuclei dramatically increases, which will control the final particle size; hence, the particle size increases again. We considered these observations and experiences and varied the reaction temperature between 25 and 55°C.
The water concentration also influences the hydrolysis and nucleation rates; it enhances the aggregation of nuclei, which controls the primary particle number and size. Lindberg et al.[
23] and Zukoski et al.[
24] observed the following trend in particle size between 0.5-17 M water concentrations: the particle size decreases if the water concentration decreases under 9 M, but over this value, the particle size increases again because of the enhanced aggregation of primary particles. High water concentration is rarely used in the Stöber synthesis because its high value limits the TEOS solubility
21, which decreases the reaction yield and lengthens the reaction time. Based on these observations, we worked in the 2-5 M water concentration range.
We characterized the SNPs with dynamic light scattering (DLS) and transmission electron microscopy (TEM), as our main focus was to tailor the size and morphology of SNPs.
2. Materials and Methods
2.1. Materials
Tetraethoxysilane [TEOS], (Alfa Aesar [USA], purity 98%), absolute ethanol AnalaR Normapur, purity ≥99.8% (VWR Chemicals, Debrecen, Hungary), 28 w/w% ammonium solution AnalaR Normapur, analytical reagent (VWR Chemicals, Debrecen, Hungary), ultrapure water (membraPure Astacus Analytical with UV, VWR Chemicals, Debrecen, Hungary). TEOS was freshly distilled before each reaction.
2.2. Silica nanoparticle synthesis
The silica nanoparticles were prepared by hydrolysis of TEOS in ethanol in the presence of ammonium hydroxide as a catalyst. The concentration of TEOS was constant in each reaction, c=0.26 mol/dm3. We systematically changed the concentration of ammonia, water, or temperature as follows:
- varied ammonia concentration between 0.29-0.097 mol/dm3, at constant water concentration (5 mol/dm3) and constant temperature (25°C)
- varied water concentration between 2-5 mol/dm3 at constant ammonia concentration (0.29/0.194/0.097 mol/dm3) and constant temperature (25°C)
- varied temperature between 25-50°C at constant ammonia concentration (0.29/0.194/0.097 mol/dm3) and constant water concentration (5 mol/dm3)
The water, ethanol, and TEOS mixture were stirred at constant temperature for 15 min. Then, the reaction mixtures were sonicated for 15 min, followed by adding 10 m/m% ammonium hydroxide solution under stirring. The reaction mixtures were stirred for 24 hours at a constant temperature. For all experiments, we used vials of the same size, magnetic stirring bars of the same size and shape, and a constant stirring rate (1000 min-1). The synthesized silica nanoparticles were stored and found to be stable without aggregation and any change in size or morphology for several months in the residual reaction solution.
2.3. Characterization of silica nanoparticles
The size distribution, hydrodynamic diameter (d), and polydispersity index (PDI) were determined by DLS using a Malvern Zetasizer Nano S instrument. The size distribution was confirmed, and the morphology of silica nanoparticles was studied with TEM (JEM 1200 EX II and JEOL-1400 TEM [JEOL Ltd., Japan]). Samples for TEM experiments were prepared by drop casting “as is” samples on 400 mesh copper grids with carbon coating (Micro to Nano Ltd., Netherlands) were used.
2.4. Statistical analysis
Statistical analyses were conducted using Microsoft Excel®. Correlations between the studied parameters and the diameter of silica nanoparticles were assessed by linear regression. The coefficient of determination (R2) was determined to establish the fit of the models. Finally, confidence intervals for each model were examined to determine the accuracy of the prediction made by the models. All experimental results were performed in triplicates and are presented as mean ± SD.
3. Results
Firstly, we investigated the influence of the ammonium hydroxide concentration on the particle size if the temperature is constant, t=25°C. We determined that in the 0.29-0.097 M ammonium hydroxide concentration range, SNPs between 27.1 and 190.8 nm could be synthesized with high product yield (≥80 %) (see
Table 1, A1-A3 samples). The SNPs are highly monodisperse (PDI: 0.082-0.008) and spherical (see
Figure 1). The SNPs could be synthesized with high reproducibility, and three parallel syntheses have been performed.
We determined that by increasing the reaction temperature, the SNP particle size decreases at constant TEOS (0.26 M), water (5 M), and ammonium hydroxide (0.29/0.194/0.097 M) concentration. At different ammonium hydroxide concentrations, the SNP size decreased by the different rates. At 0.29 M ammonia concentration, the particle size is between 190.8-69.3 nm in the 25-55°C temperature range (see
Table 1, A1 and T30A-T55A samples). At lower ammonia concentration, 0.194 M, the particle sizes are between in the 30.2-99.1 nm interval (see
Table 1, A2 and T30B-T55B samples), and at 0.097 M, these values are in the 6.1-27.1 nm size range (see
Table 1, A3 and T30C-T55C samples). We performed all synthesis three times, and we found that the reproducibility of SNP synthesis is excellent; the standard deviations are very low. At each temperature and ammonium hydroxide concentration, the SNPs are highly monodisperse (PDI: 0.002-0.091) and spherical (see
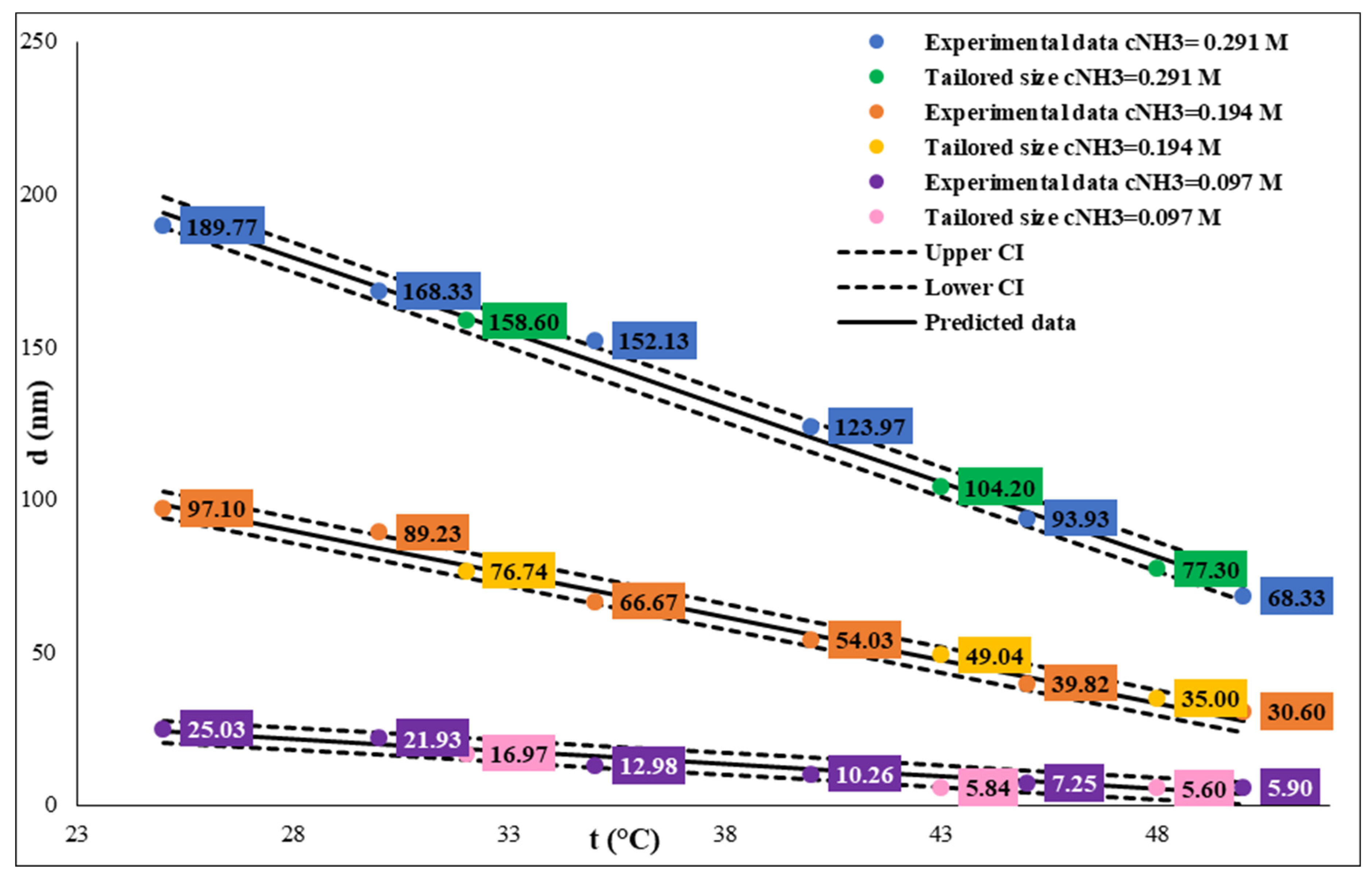
Figure 2). Higher PDI values (0.016-0.091) were measured at higher temperatures; we assumed this phenomenon might indicate the temperature heterogeneity in the reaction vessels. We found a linear correlation between particle size and reaction temperature, and we used it to calculate the reaction temperature for tailored particle size by regulating the temperature at constant TEOS and ammonia concentration (see
Figure 3). Our results show that precise temperature control makes it possible to synthesize SNPs with a desired particle size in three different size ranges at each ammonia concentration (
Figure 3 and
Table 2). These calculations in the different SNP size intervals allow for SNP size tailoring or fine-tuning. At 0.29 M ammonia concentration, we can precisely control the particle size to be between 65 and 200 nm. If we want to synthesize SNPs in the 30 and 100 nm particle size range, we should work with constant 0.194 M ammonia concentration. At 0.097 M ammonia concentration, SNPs under 30 nm size can be produced, and we can fine-tune the particle size from 6 nm to 23 nm.
Similarly to the size dependence of temperature, at different ammonium hydroxide concentrations, the SNP particle sizes decrease in different degrees: at 0.29 M ammonia concentration, the particle size is between 52.18-190.80 nm increase in the 2-5 M water concentration range (see
Table 1 A1 and W4A-W2A samples). At lower ammonia concentration, 0.194 M, the particle sizes are between in the 27.81-99.09 nm interval (see
Table 1 A2 and W4B-W2B samples), and at 0.097 M, these values are in the 6.92-27.1 nm size range (see
Table 1 A3 and W4C-W2C samples). Here, we also found a correlation between water concentration and SNP size. By decreasing the water concentration, the SNP particle size decreased at constant TEOS (0.26 M) and ammonia concentration (0.29/0.194/0.097 M) at 25°C; the relation between water concentration and SNP size can be fitted with quadratic equation (
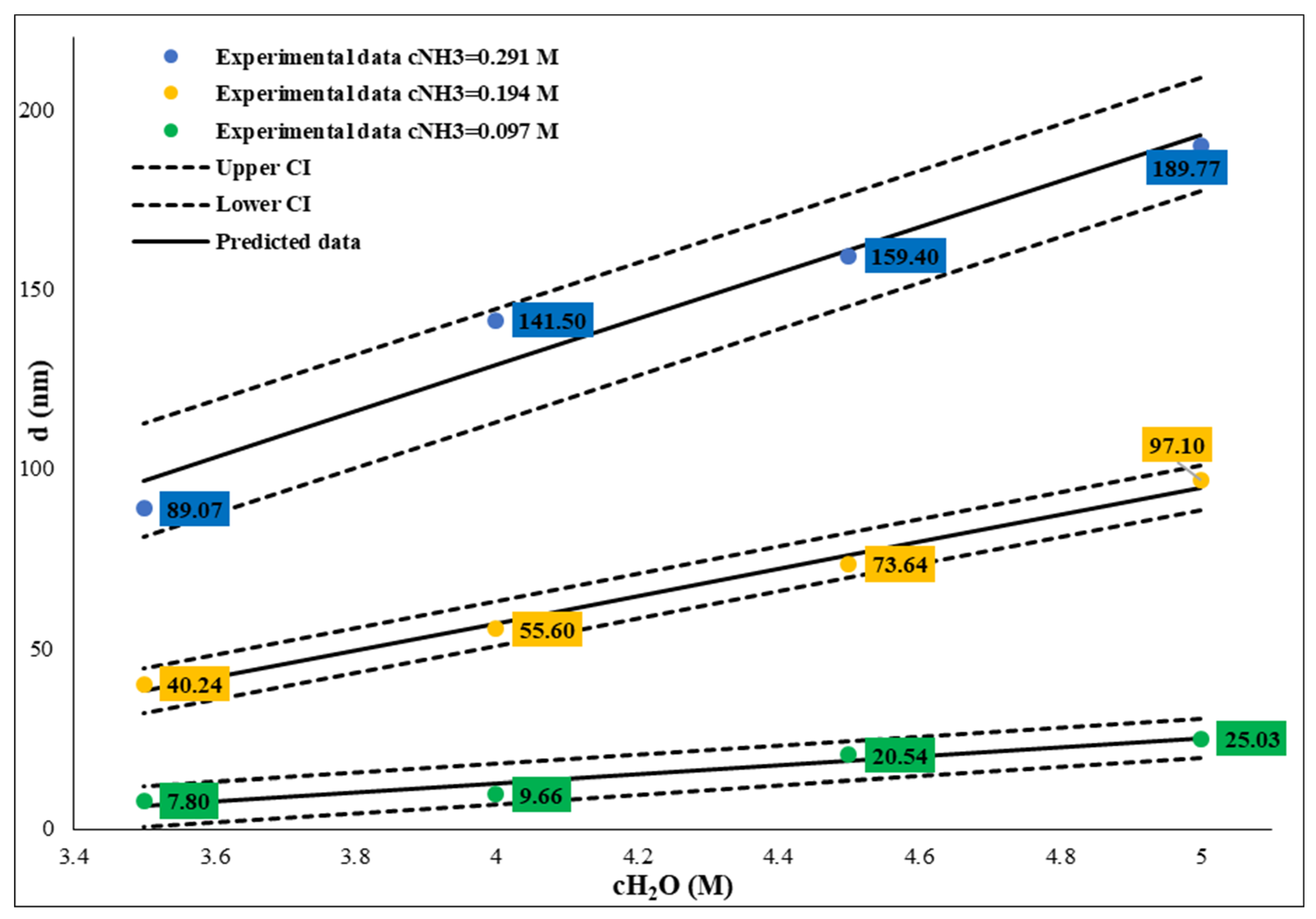
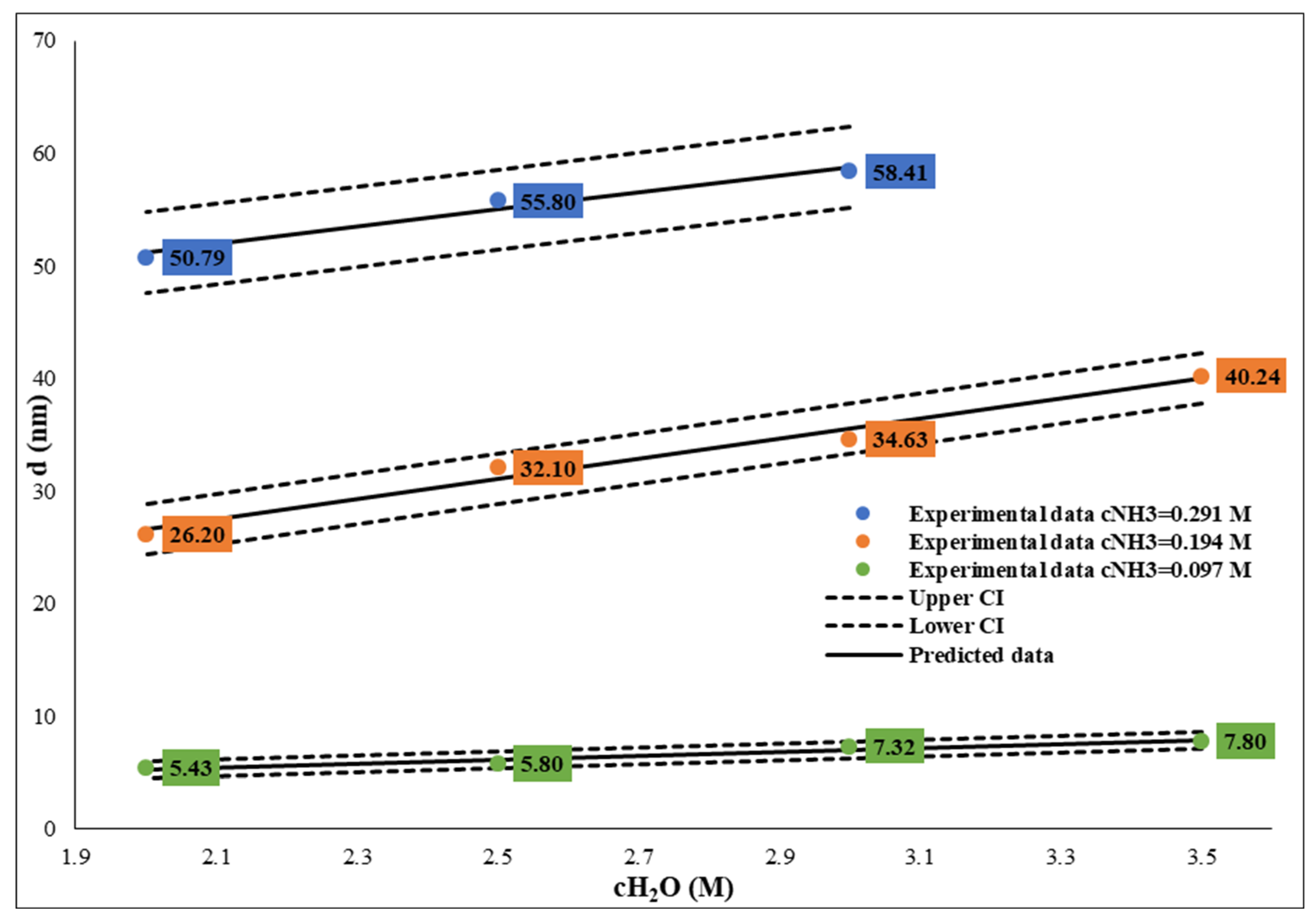
Figure 5). These relations also make it possible to calculate and tailor the SNP size. It is important to note that if the water concentration is lower than 3 M, the sphericity of SNPs decreases while polydispersity increases.
Table 1.
Reaction condition of SNP synthesis and parameters of SNPs. The TEOS concentration is constant, c=0.26 M. Three parallel synthesis have been performed.
Table 1.
Reaction condition of SNP synthesis and parameters of SNPs. The TEOS concentration is constant, c=0.26 M. Three parallel synthesis have been performed.
| Sample |
cNH3 (mol/dm3)
|
cH2O (mol/dm3) |
t (°C) |
dDLS (nm) |
PDIDLS
|
dTEM (nm) |
PDITEM
|
| A1 |
0.291 |
5 |
25 |
190.80±0.93 |
0.008 |
191.38 |
0.003 |
| A2 |
0.194 |
99.09±1.15 |
0.021 |
91.72 |
0.006 |
| A3 |
0.097 |
27.05±0.80 |
0.082 |
28.33 |
0.005 |
| T30A |
0.291 |
5 |
30 |
170.50±1.17 |
0.002 |
169.75 |
0.001 |
| T30B |
0.194 |
88.60±1.05 |
0.018 |
81.85 |
0.004 |
| T30C |
0.097 |
22.51±0.25 |
0.095 |
23.19 |
0.002 |
| T35A |
0.291 |
5 |
35 |
153.60±0.91 |
0.014 |
155.12 |
0.007 |
| T35B |
0.194 |
67.81±0.77 |
0.005 |
64.53 |
0.015 |
| T35C |
0.097 |
12.08±0.06 |
0.146 |
14.06 |
0.026 |
| T40A |
0.291 |
5 |
40 |
142.70±1.02 |
0.023 |
145.17 |
0.008 |
| T40B |
0.194 |
56.11±0.85 |
0.041 |
55.42 |
0.003 |
| T40C |
0.097 |
10.43±0.12 |
0.226 |
10.99 |
0.018 |
| T45A |
0.291 |
5 |
45 |
96.35±0.99 |
0.041 |
95.91 |
0.008 |
| T45B |
0.194 |
41.87±±0.75 |
0.048 |
42.14 |
0.007 |
| T45C |
0.097 |
7.49±1.04 |
0.467 |
6.53 |
0.019 |
| T50A |
0.291 |
5 |
50 |
69.32±0.88 |
0.016 |
67.80 |
0.012 |
| T50B |
0.194 |
30.15±0.69 |
0.455 |
31.96 |
0.031 |
| T50C |
0.097 |
6.11±0.20 |
0.455 |
8.91 |
0.074 |
| W4A |
0.291 |
4 |
25 |
142.70±3.34 |
0.041 |
146.62 |
0.004 |
| W4B |
0.194 |
58.97±1.58 |
0.043 |
60.29 |
0.002 |
| W4C |
0.097 |
9.54±1.15 |
0.263 |
20.27 |
0.008 |
| W3A |
0.291 |
3 |
25 |
58.50±2.17 |
0.061 |
54.74 |
0.010 |
| W3B |
0.194 |
34.50±2.08 |
0.095 |
33.60 |
0.011 |
| W3C |
0.097 |
7.58±1.64 |
0.190 |
15.71 |
0.006 |
| W2A |
0.291 |
2 |
25 |
52.18±3.72 |
0.059 |
50.74 |
0.013 |
| W2B |
0.194 |
27.81±8.16 |
0.073 |
28.2 |
0.013 |
| W2C |
0.097 |
6.92±±2.23 |
0.250 |
6.55 |
0.019 |
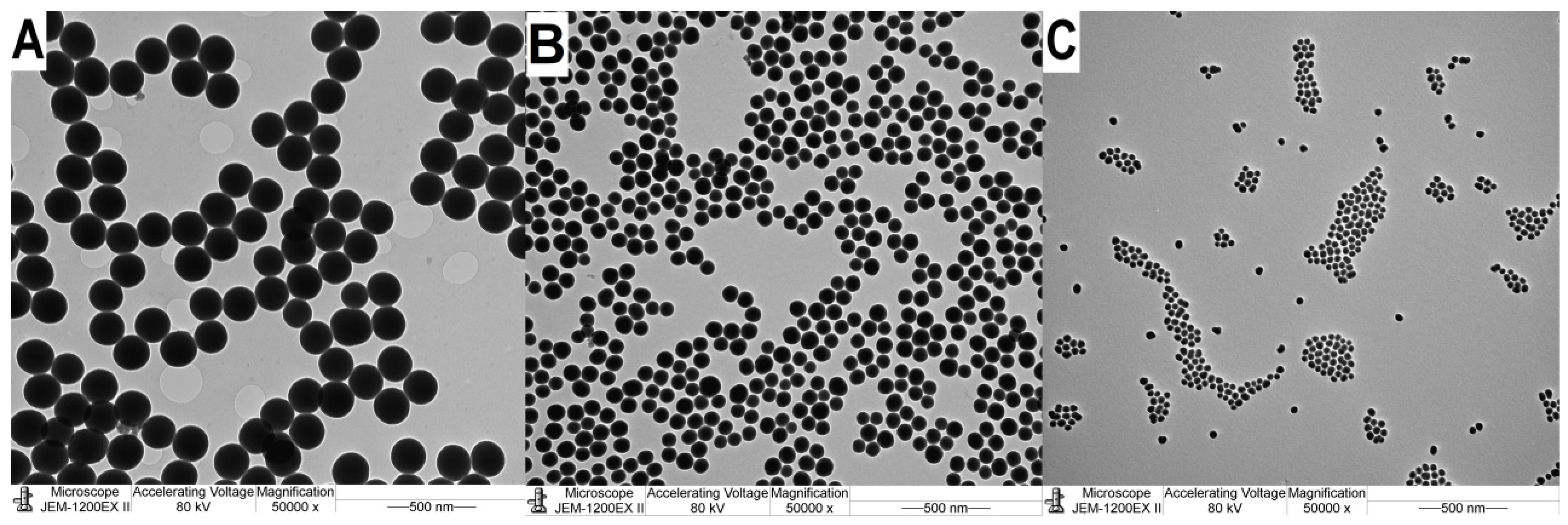
Figure 1.
TEM images of SNPs. Samples: A:A1 d=191.38 nm, B:A2 d=91.72 nm, C:A3 d=28.33 nm. The SNPs are highly monodisperse and spherical.
Figure 1.
TEM images of SNPs. Samples: A:A1 d=191.38 nm, B:A2 d=91.72 nm, C:A3 d=28.33 nm. The SNPs are highly monodisperse and spherical.
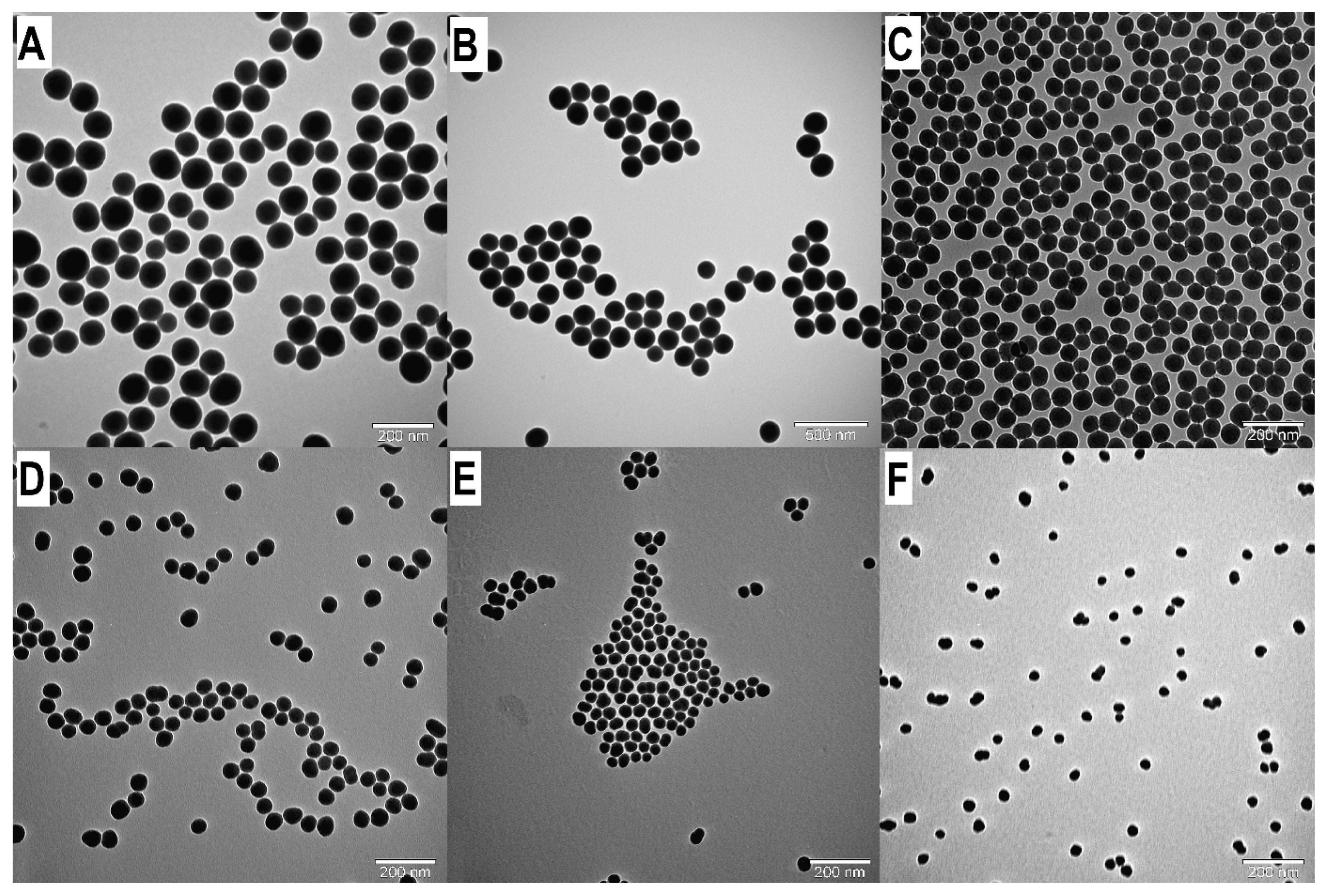
Figure 2.
TEM images of SNPs: Examination the influence of reaction temperature on the SNP size. A: T25B d=91.72 nm. B: T30B d=81.85 nm. C: T35B d=65.43 nm. D: T40B d=55.42 nm. E: T45B d=42.14 nm. F: T50B d=31.96 nm.
Figure 2.
TEM images of SNPs: Examination the influence of reaction temperature on the SNP size. A: T25B d=91.72 nm. B: T30B d=81.85 nm. C: T35B d=65.43 nm. D: T40B d=55.42 nm. E: T45B d=42.14 nm. F: T50B d=31.96 nm.
Table 2.
Tailoring SNP particle size with systematic change of reaction conditions. The tailored particle sizes are calculated from correlations between the experimental particle size and reaction temperature or water concentration. dcal value is the calculated SNP size, dDLS is the synthesised and measured SNP size.
Table 2.
Tailoring SNP particle size with systematic change of reaction conditions. The tailored particle sizes are calculated from correlations between the experimental particle size and reaction temperature or water concentration. dcal value is the calculated SNP size, dDLS is the synthesised and measured SNP size.
| Sample |
cNH3 (mol/dm3)
|
cH2O (mol/dm3) |
t (°C) |
dcal (nm) |
dDLS (nm) |
PDIDLS
|
| T32A |
0.291 |
5 |
32 |
160.92 |
158.60 |
0.024 |
| T32B |
0.194 |
79.61 |
76.70 |
0.043 |
| T32C |
0.097 |
19.04 |
17.00 |
0.143 |
| T43A |
0.291 |
5 |
43 |
106.82 |
104.20 |
0.059 |
| T43B |
0.194 |
48.35 |
49.00 |
0.065 |
| T43C |
0.097 |
9.52 |
5.80 |
0.070 |
| T48A |
0.291 |
5 |
48 |
77.3 |
57.33 |
0.004 |
| T48B |
0.194 |
35.0 |
35.00 |
0.093 |
| T48C |
0.097 |
5.19 |
5.60 |
0.245 |
| W4.5A |
0.291 |
4.5 |
25 |
158.44 |
159.40 |
0.028 |
| W4.5B |
0.194 |
78.88 |
73.64 |
0.068 |
| W4.5C |
0.097 |
18.01 |
20.54 |
0.090 |
| W3.5A |
0.291 |
3.5 |
25 |
97.99 |
89.07 |
0.026 |
| W3.5B |
0.194 |
46.24 |
40.24 |
0.051 |
| W3.5C |
0.097 |
7.51 |
7.80 |
0.176 |
| W2.5A |
0.291 |
2.5 |
25 |
58.44 |
55.78 |
0.057 |
| W2.5B |
0.194 |
30.30 |
32.09 |
0.081 |
| W2.5C |
0.097 |
5.30 |
5.82 |
0.227 |
Figure 3.
Influence of temperature on size of SNPs at different ammonium hydroxide concentration. The TEOS concentration is constant, c=0.26 M.
Figure 3.
Influence of temperature on size of SNPs at different ammonium hydroxide concentration. The TEOS concentration is constant, c=0.26 M.
Table 3.
Statistical analysis of temperature size dependence on SNP synthesis.
Table 3.
Statistical analysis of temperature size dependence on SNP synthesis.
| cNH3 (mol/dm3)
|
Linear regression |
CI |
R2
|
| 0.291 M |
y=-4.906x+316.720 |
93% |
0.9909 |
| 0.194 M |
y=-2.819x+168.630 |
95% |
0.9846 |
| 0.194 M |
y=-0.814x+44.412 |
92% |
0.9326 |
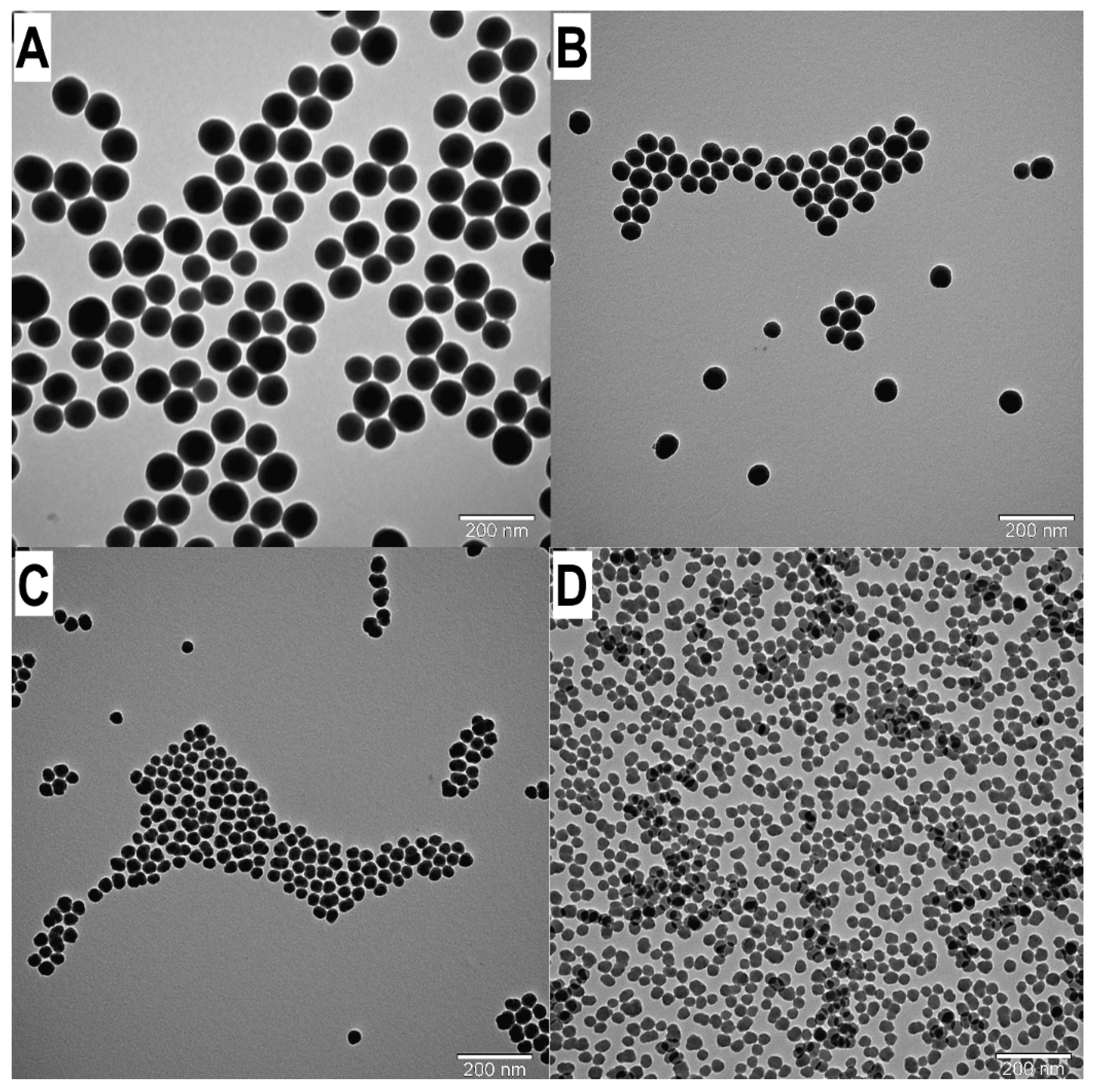
Figure 4.
TEM images of SNPs: Examination the influence of water concentration on the SNP size. A: W5B d=91.73 nm. B: W4B d=60.29 nm. C: W3B d=33.60 nm. D: W2B d=28.20 nm.
Figure 4.
TEM images of SNPs: Examination the influence of water concentration on the SNP size. A: W5B d=91.73 nm. B: W4B d=60.29 nm. C: W3B d=33.60 nm. D: W2B d=28.20 nm.
Figure 5.
Influence of water concentration between 3.5-5 mol/dm3 on size of SNPs at different ammonium hydroxide concentration. The TEOS concentration was constant, c=0.26M.
Figure 5.
Influence of water concentration between 3.5-5 mol/dm3 on size of SNPs at different ammonium hydroxide concentration. The TEOS concentration was constant, c=0.26M.
Figure 6.
Influence of water concentration between 2-3.5 mol/dm3 on size of SNPs at different ammonium hydroxide concentration. The TEOS concentration was constant, c=0.26M.
Figure 6.
Influence of water concentration between 2-3.5 mol/dm3 on size of SNPs at different ammonium hydroxide concentration. The TEOS concentration was constant, c=0.26M.
Table 4.
Statistical analysis of water concentration 3.5-5 mol/dm3 size dependence SNP synthesis.
Table 4.
Statistical analysis of water concentration 3.5-5 mol/dm3 size dependence SNP synthesis.
| cNH3 (mol/dm3)
|
Linear regression |
CI |
R2
|
| 0.291 M |
y=63.998x+127.057 |
99% |
0.9566 |
| 0.194 M |
y=37.222x+96.647 |
95% |
0.9906 |
| 0.097 M |
y=12.514+34.427 |
95% |
0.9350 |
Table 5.
Statistical analysis of water concentration 2-3.5 mol/dm3 size dependence SNP synthesis.
Table 5.
Statistical analysis of water concentration 2-3.5 mol/dm3 size dependence SNP synthesis.
| cNH3 (mol/dm3)
|
Linear regression |
CI |
R2
|
| 0.291 M |
y=7.623x+35.940 |
99% |
0.9679 |
| 0.194 M |
y=8.931x+8.730 |
95% |
0.9793 |
| 0.097 M |
y=1.726+1.840 |
95% |
0.9385 |
4. Discussion
The study focused on the controlled growth of various sizes of silica nanoparticles (SNPs) by systematically adjusting the ammonium hydroxide concentration, water concentration, and temperature. SNP synthesis of less than 200 nm was targeted using common approaches while ensuring high yields, narrow size distributions, and reproducibility in the synthesis of the nanoparticles. In this discussion, the consequences that the results may have will be appreciated; a comparison of the results obtained with what is already known from past work will be made, and finally, how the findings can be applied will be considered.
4.1. Influence of Ammonium Hydroxide Concentration
The study confirmed a strong correlation between ammonium hydroxide concentration and the size of the SNPs. Higher concentrations of ammonium hydroxide yielded larger particles, with sizes ranging from 27.1 nm to 190.8 nm within the 0.29-0.097 M concentration range at 25°C. This trend aligns with previous findings in the literature, where the hydrolysis and condensation rates are affected by the catalyst concentration, influencing the nucleation and growth stages of particle formation. The reproducibility of the results with low standard deviations demonstrates the method’s robustness, which is crucial for scaling up the production of SNPs for industrial applications. The results also suggest that fine-tuning the ammonium hydroxide concentration can effectively control particle sizes within a targeted range, offering flexibility for different use cases, such as drug delivery systems or biosensing applications requiring specific nanoparticle sizes.
4.2. Influence of Temperature
The study also established that increasing the reaction temperature results in smaller SNPs across different ammonium hydroxide concentrations. The linear correlation observed between temperature and particle size provides a predictable means to tailor SNP sizes through temperature control, allowing researchers to adjust reaction conditions to achieve desired particle dimensions. This finding corroborates earlier studies, which noted that higher temperatures accelerate nucleation rates, leading to smaller and more uniform particles. However, the study also found higher temperatures increased polydispersity, especially at temperatures above 50°C. We can assume that the temperature heterogeneity within the reaction vessel may cause uneven particle formation. Hence, while temperature control is effective for size tuning, there is a need for careful monitoring to maintain uniformity.
4.3. Influence of Water Concentration
Water concentration had a significant but complex influence on SNP size. The study demonstrated that decreasing water concentration generally resulted in smaller particles. However, at concentrations below 3 M, the particles tended to be less spherical and more polydisperse. This observation is consistent with existing research indicating that water concentration affects the aggregation of primary particles during the growth phase. The quadratic relationship between water concentration and particle size offers a more nuanced approach to size control than the linear temperature relationship. While this provides additional flexibility, it also necessitates careful optimization to avoid undesirable particle morphologies and polydispersity.
4.4. Practical Implications
The ability to precisely control SNP sizes through systematic variation of reaction conditions holds significant promise for various applications, particularly in biomedical fields. For instance, particle size can critically influence the biodistribution, cellular uptake, and clearance of nanoparticles in drug delivery. The study’s findings offer a clear framework for researchers to tailor SNPs for such applications with high reproducibility and efficiency (Stöber article). Additionally, the study’s emphasis on using eco-friendly solvents like ethanol enhances the practical utility of the method by making it more sustainable and cost-effective, especially for small and mid-scale production; this aligns with current trends in green chemistry, where minimizing environmental impact is a priority.
Author Contributions
Conceptualization, K.B., A.Sz. and B.V-H.; methodology and investigation, A.Sz. and B.V-H.; Software A.S. and Sz.P.; data curation, A.S., Sz.P, A.Sz., B.V-H..; writing—original draft preparation, A.Sz., B.V-H..; writing—review and editing, K.B., A.Sz., Sz.P and B.V-H..; visualization, A.Sz., B.V-H..; supervision, A.Sz.