Introduction
The cut flower industry plays a pivotal role in the global floriculture market, with roses being one of the most cherished and economically significant varieties. The aesthetic appeal and commercial value of cut roses are directly tied to their vase life, making it imperative for floriculturists and enthusiasts to explore innovative approaches to extend the longevity of these blooms. In this endeavor, the current research explores the possibility of utilizing sucrose and moringa leaf extract as natural supplements to prolong the cut flower roses. Rose, a quintessential symbol of beauty and romance, captivates hearts with its unparalleled elegance. Beyond its ornamental allure, the economic significance of roses is deeply rooted in agro-based industries, particularly cosmetics and perfumes. Hybrid Tea Roses (Rosa x hybrida L.), as integral players in the Rosaceae family, contribute substantially to the booming cut-flower market. With over 150 species and 1400 cultivars under the Rosa genus. The rose has maintained its popularity as a cut flower since ancient times, continuing to captivate researchers (Synge, 1971; Elgimabi, 2011). Nonetheless, the primary focus of rose cultivation is the production of cut flowers, a significant aspect of the floriculture industry (Butt, 2003).
Understanding the challenges posed during the postharvest phase, research by Van Doorn et al. (1997) sheds light on the phenomenon of “bent-neck” In instances where cut roses wilt and the floral stem bends just beneath the flower head. Another factor contributing to quality deterioration is the clogging of xylem vessels by bacterial or microbial buildup (Jalili Marandi, et al., 2011). This obstruction can result in inadequate water absorption and water loss (Hassan, 2005). Over the years, scholars such as (Marousky, 1969), (Gilman and Steponkus, 1972), (Parups and Chan, 1973) as well as (Kaltaler and Steponkus, 1976) have investigated the capability of sucrose solutions to prolong vase life, recognizing the significance of soluble carbohydrates as vital substrates for respiration and the opening of flowers.
However, sucrose alone presents challenges as documented by Penniston et al. (2008), who highlighted its propensity to promote bacterial proliferation, resulting in a shortened vase life. Bravdo et al. (1974) observed that lower concentrations of sucrose extend the vase life of gladiolus florets by enhancing uptake, while higher concentrations appear to hinder uptake. In the intricate world of floriculture, the cultivation of roses extends beyond the mere production of blooms, it is a delicate dance to achieve a prolonged vase life for cut flowers. As highlighted by esteemed authors like Synge (1971) and Butt (2003), the primary goal of rose cultivation is to yield high-quality cut flowers that fuel the thriving floricultural business. A critical factor in determining the success of this venture lies in the length of vase life, a parameter that directly influences the commercial value of these delicate blooms.
Understanding the physiology of cut flowers is essential for uncovering the complexities of extending vase life. Factors such as water absorption, transpiration, and microbial proliferation significantly impact the post-harvest longevity of cut roses. The role of carbohydrates, including sucrose, in providing a continuous energy source for the flowers has been extensively documented (van Doorn, et al., 2004). Additionally, the antimicrobial properties of certain plant extracts, like those from moringa leaves, have garnered attention in recent research endeavors (Anwar et al., 2007).
Literature Review
Botanical Description
The floral industry stands as a vibrant and economically significant sector, with cut roses being one of its flagship commodities. The allure of roses transcends cultural boundaries, symbolizing love, celebration, and beauty. In the global floriculture market, cut roses hold a pivotal position, contributing substantially to both local and international trade. This overview delves into the importance of cut roses in the floral industry, highlighting their economic, cultural, and aesthetic significance.
Physiology of cut flower
Understanding the physiological processes of cut flowers is crucial for comprehending the factors influencing their vase life. Studies such as those by (van Doorn et al., 2004) and (van Meeteren et al., 2000) emphasize the importance of water uptake, transpiration, and microbial growth in assessing the lifespan of cut flower roses.
Economic significance
The commercial cultivation and trade of cut roses represent a lucrative sector within the broader floral industry. According to market analyses, the global cut flower market is expected to witness continuous growth, with roses often commanding a premium price due to their high demand and symbolic value (Vijayakumar et al., 2018). Countries with favorable climates for rose cultivation, such as Colombia, Ecuador, and Kenya, have emerged as major suppliers, contributing significantly to their national economies.
Cultural symbolism
Roses have deep-rooted cultural significance and are integral to various traditions and celebrations. They are prominently featured in weddings, anniversaries, and other joyous occasions, symbolizing love, romance, and elegance. The color of the rose carries additional meanings, further enhancing its versatility in conveying emotions. This cultural symbolism contributes to the sustained demand for cut roses throughout the year, making them a staple in floral arrangements and bouquets (Davies, 2016).
Aesthetic appeal
The aesthetic appeal of cut roses is unparalleled, making them a preferred choice for both formal and informal floral arrangements. Their velvety petals, vibrant colors, and iconic fragrances make roses a timeless and classic choice for expressing sentiments and enhancing the visual appeal of various settings. The aesthetic qualities of cut roses make them indispensable in the creation of intricate bouquets, centerpieces, and other decorative elements (Van Meeteren and van Gelder, 2018).
Costumer’s demand and Market trends
Consumer preferences drive the floral industry, and cut roses consistently rank among the most sought-after flowers. Changes in consumer tastes and preferences, along with evolving market trends, influence the demand for specific rose varieties and colors. Sustainable and eco-friendly practices in flower production have gained traction, prompting the exploration of natural additives and methods to prolong the lifespan of cut roses (Armellini et al., 2020).
Sucrose as a vase life extender
Numerous researchers have explored the positive effects of sucrose on cut flower vase life. Reid and Jiang (2012) demonstrated that sucrose serves as an essential energy source, promoting turgor pressure and reducing water loss through transpiration. Additional studies by Smith et al. (2018) and Patel and Patel (2020) further underscore the beneficial impact of sucrose in enhancing the post-harvest quality of various cut flower species.
Moringa leaf extract as a natural preservative
Moringa oleifera, known for its antimicrobial properties, has gained attention as a potential natural preservative. Research by Anwar et al. (2007) and Rathi et al. (2011) highlights the antimicrobial and antioxidant attributes of moringa leaf extract, implying its possible use in prolonging the longevity of cut flower roses. However, limited studies have specifically explored its effectiveness in the context of cut roses.
The combined effect of sucrose and moringa leaf extract
While sucrose and moringa leaf extract have been studied individually, there is a notable gap in the literature regarding their combined effect on cut flower vase life. This study seeks to build on the work of previous researchers by investigating the synergistic potential of these natural additives. The approach aligns with the findings of recent studies advocating for sustainable and eco-friendly practices in the floral industry (Armellini et al., 2020).
Recent advances and emerging trends
In light of the growing interest in sustainable floriculture practices, recent research has explored innovative approaches to increase the lifespan of roses. Studies by Garcia et al. (2022) and Lee and Kim (2023) have investigated novel natural additives and protocols, indicating a broader shift towards environmentally friendly methods in the floral industry.
Research Methodology
Collection of fresh roses
The collection of roses was carried out early in the morning to aid in preserving the freshness of the flowers. Clean and sharp pruning shears were employed to harvest the flowers precisely above the node, ensuring a clean cut without crushing or tearing the stem. The cutting was performed through the stem about ¼ inches above the five leaflets at a 45-degree angle, using sharp and clean shears. After cutting the roses, the cut ends of the stems were then plunged into a container of water. Subsequently, the container of roses was placed indoors in a cool room, away from direct sunlight (Jafarpour et al., 2015).
Sucrose preparation
The amount of granulated sugar, 10g, was precisely measured and combined with 100 ml of distilled water in the vessel. Continuous stirring with a spoon until the sucrose is fully dissolved. The container was labeled with the concentration and the date it was processed. The solution was stored in a dark, dry, cool place.
Moringa leaf extract preparation
The preparation of leaf extracts from fresh moringa leaves involved collecting 200g of fresh leaves, cleaning, washing, and storing them overnight at freezing temperatures. After 24 hours, the stored leaves were crushed using a blender and sieved through cheesecloth and collected 70 ml of moringa extract out of it. The extracts were then diluted based on the requirement (Rady and Mohamed 2015).
Experimental site
The experiment was conducted in a controlled environment at room temperature at N Blessing Farm located at Sitio Dam-an Barangay Pamutan Cebu City. The room provided a stable climate, allowing for precise control of temperature, and humidity. This controlled environment was essential to minimize outside factors that could impact the lifespan of cut roses and to ensure consistency in experimental conditions (Selamawit et al. 2018). The room temperature was monitored using a home thermometer and hygrometer.
Experimental design
The study employed a Completely Randomized Design, CRD (Maxwell, S. E. 2005). It consisted of 4 treatments replicated 4 times. The experimental units, in this case, were individual cut roses. The roses were grouped based on factors such as similar initial physiological age, size, and quality. Each group was then subjected to various treatments, including sucrose, moringa leaf extract, and combinations of both. The random assignment of treatments within each group helped control for variability and ensured a more robust analysis.
Treatment and procedure
-
1.
Preparation of experimental units
-
✓
High-quality cut rose (Rosa x hybrida) was sourced from a reputable rose farm at Sodlon Cebu City. The roses were of a similar physiological age, free from physical damage.
-
2.
Treatments
-
✓
-
The experimental treatments include the following:
- a.
Control group (distilled water) - Roses were placed in a 150-glass bottle with 50 ml distilled water and observed for 8 days.
- b.
Sucrose treatment - Roses were placed in a 150ml glass bottle with 50ml solution containing a 1:10 (sugar/water) concentration of sucrose and observed for 8 days.
- c.
Moringa leaf extracts treatment - Roses were placed in a glass 150 ml glass bottle with 50 ml solution containing a 1:10 (extracts/water) concentration of moringa leaf extract and observed for 8 days.
- d.
Combined treatment - Roses were placed in a 150-glass container with 25 ml of sucrose solution and 25 ml of moringa leaf extract, prepared with a ratio of 1:10 (sucrose/moringa/water), and monitored for 8 days.
-
3.
Application of treatments
-
✓
The experimental solutions were prepared in advance, and each vase was filled with the respective treatment solution.
-
✓
Cut rose stems will be re-cut at a 45-degree angle to facilitate water uptake and immersed in the assigned treatment solution immediately after cutting.
-
4.
Monitoring and data collection
-
✓
The lifespan of each cut rose was monitored, and relevant data were recorded over 8 days. Changes in petal color were assessed using a visual quality rating (VQR). The number of leaves falling off and the number of petals dropping were counted. Stem rotting was measured in millimeters, and the flower's bent neck was recorded based on its degree of bending. The temperature and humidity of the room were monitored from early morning to late evening
-
✓
Daily measurements were taken throughout the experiment to capture any noticeable effects of the treatments on the roses.
-
5.
Statistical analysis
For statistical results, the STAR Program was used for statistical analysis. Means were examined through Analysis of Variance (Raudonius, 2017) following a Completely Randomized Design (Maxwell, S. E. 2005). This was performed to evaluate the significance of differences in vase life among the treatment groups.
Results & Discussion
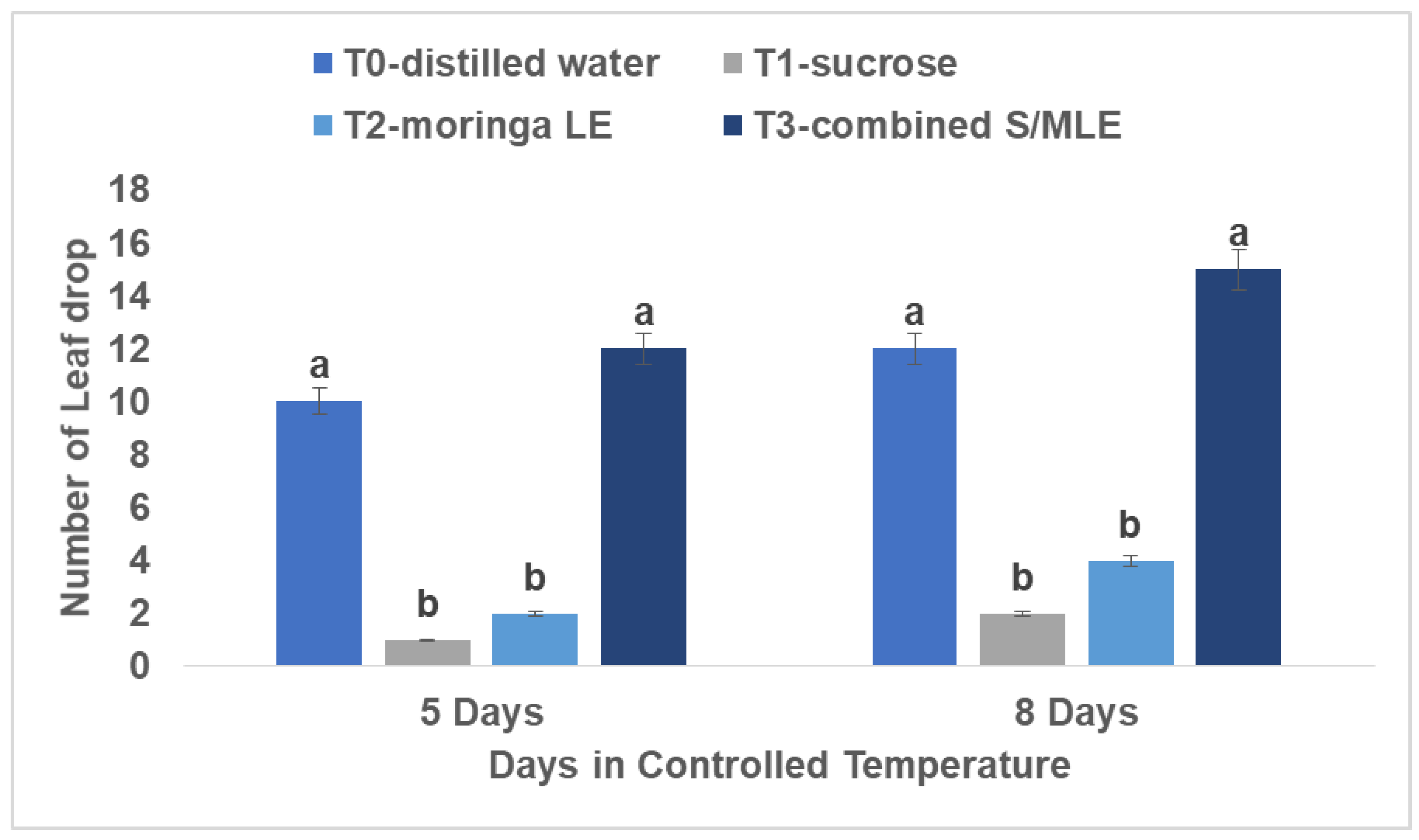
Number of leaf drop
Moringa leaf extract and sucrose individually markedly improved the cut roses' ability to retain their leaves, as evidenced in
Figure 1. The treatment groups with moringa leaf extract or sucrose applied alone exhibited the fewest dropped leaves. However, the combination of sucrose and moringa extract resulted in the highest leaf drop, particularly notable from day five onward, with a total of fifteen leaves dropped. This trend was followed by the distilled water treatment, which saw twelve leaves drop. These observations were made under controlled conditions of 22 degrees Celsius and 80% relative humidity.
Moringa leaf extract and sucrose individually improved the cut roses' ability to retain their leaves. Sucrose serves as a substrate for respiration, maintains a balanced water level, and decreases susceptibility to ethylene, consequently prolonging the lifespan of the flower (Umed and Kazuo, 2003). It was mentioned by several researchers (Basra et al., 2011; Abdalla, 2013) that the quantity of leaves enhanced when treated with moringa leaf extract. Moringa leaves are rich in zeatin, a naturally occurring cytokine. Researchers (Basra et al., 2011; Abdalla, 2013) have noted the significance of zeatin found in moringa leaves, highlighting its role as a crucial growth hormone in plants.
Application of moringa extract has been reported to enhance agronomic crop yields of coffee, soybean, and maize by 25-30%. One notable biostimulant obtained from Moringa oleifera Lam is Moringa leaf extract (MLE). MLE is useful in agriculture and belongs to the Moringaceae family (Phiri and Mbewe, 2010). Along with vital minerals, MLE contains bioactive substances such as proline, flavonoids, cytokinins (such as zeatin), ascorbic acid, phenolics, carotenoids, and vitamin A (Gopalakrishnan et al., 2016; Carillo, 2018; Hassan and Fetouh, 2019).
Expanding on the concept of utilizing amino acids to prolong the lifespan of cut roses, the study finds resonance with the findings of Pascual et al. (2020). The research highlights the role of amino acids sourced from marine waste fertilizers in promoting leaf retention, suggesting a potential avenue for enhancing the longevity of cut roses through similar mechanismsExpanding on this concept, Catubis et al. (2013) showed the positive benefits of amino acid supplementation on the development and growth of native tomatoes grown in the Philippines, underscoring the broader applicability of amino acids in horticultural practices. Thus, the integration of amino acids, as observed in both studies, presents a compelling strategy for extending the lifespan of cut flower roses, aligning with the research focus on natural additives for flower preservation.
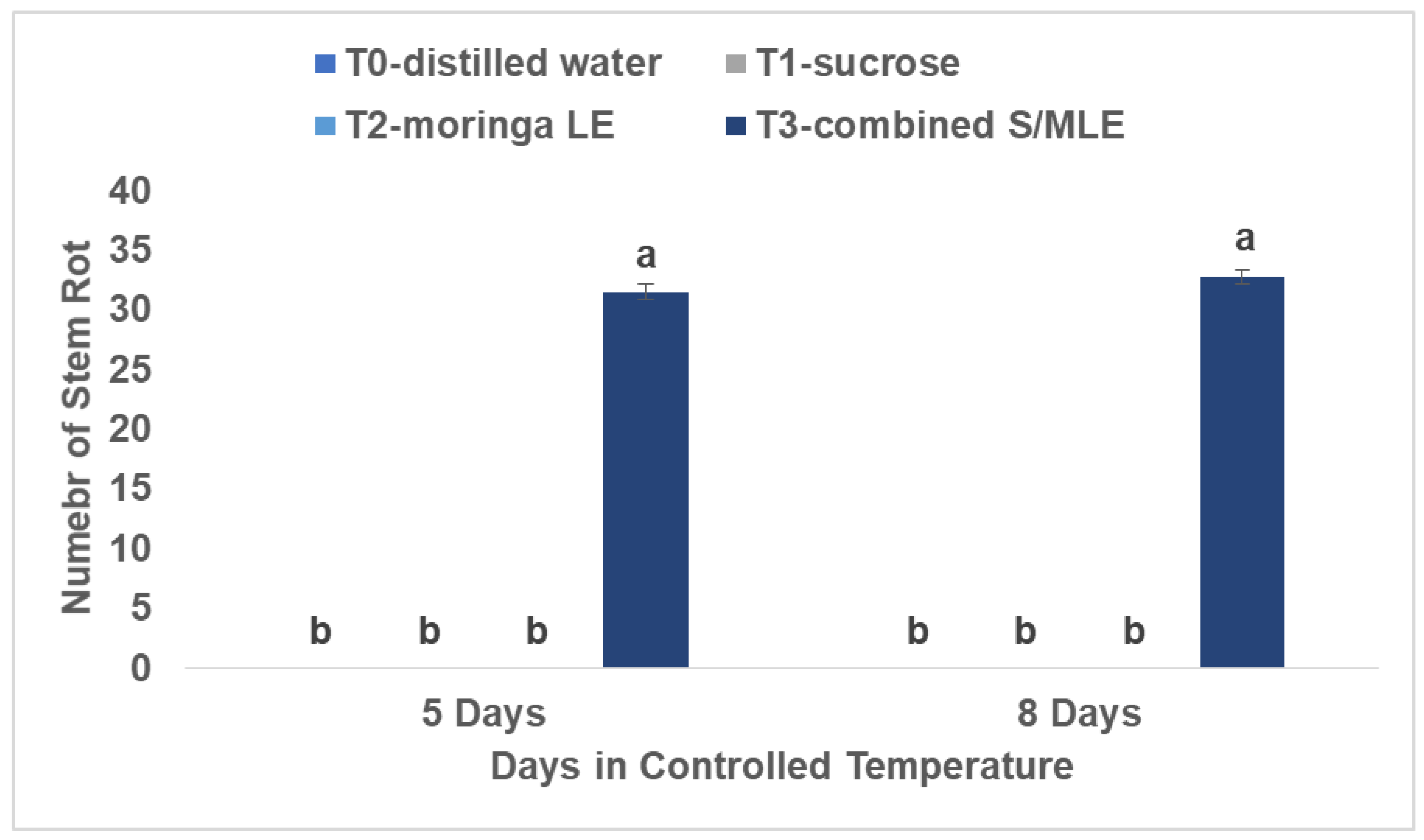
Stem rotting (mm)
The result of this study is evident in cases involving the application of combined sucrose and moringa leaf extract. Stem rot incidences were noted consistently across all four replications, with an average length of rot reaching 32.75 mm. This decay was initially observed on day five and persisted for eight days under ambient conditions of 22 °C and 80% Relative Humidity.
Stem rotting was notably observed in samples treated with combined sucrose and moringa leaf extract after 8 days, indicating a significant impact on flower quality (
Figure 2). Sucrose, a component of the treatment, is known to provide respiratory substrates, maintaining balance of water, lowering sensitivity to ethylene, and slowing down ethylene generation, thereby extending flower longevity (Umed and Kazuo, 2003). Additionally, studies by Khoshbakht (2001) have shown that carbohydrates, like sucrose, can increase the fresh and dry weight of plant tissues, which could further contribute to stem stability. However, xylem vessel blockage brought on by bacterial or microbe accumulation might result in stem rotting, leading to water uptake deficiency and loss (Jalili Marandi et al., 2011; Hassan, 2005).
Therefore, controlling microbial proliferation is crucial for maintaining the quality and lifespan of roses (Hassan et al., 2014) using moringa leaf extract as a natural additive. Biocides are frequently employed to diminish bacterial proliferation in vase water, although certain ones carry potential health and environmental hazards and could lead to toxicity in flowers (Damanupola and Joyce, 2006; Hassan and Schmidt, 2004). Despite these challenges, various approaches have been explored to explore alternative methods that are both efficient and environmentally friendly to meet the demands of the floral industry and sustainable agriculture to extend the lifespan of flowers, methods such as managing water levels, postponing senescence, decreasing microbial counts, and stimulating antioxidant mechanisms have been explored (Hassan and Ali, 2014; Hassan et al., 2014; Saeed et al., 2014).
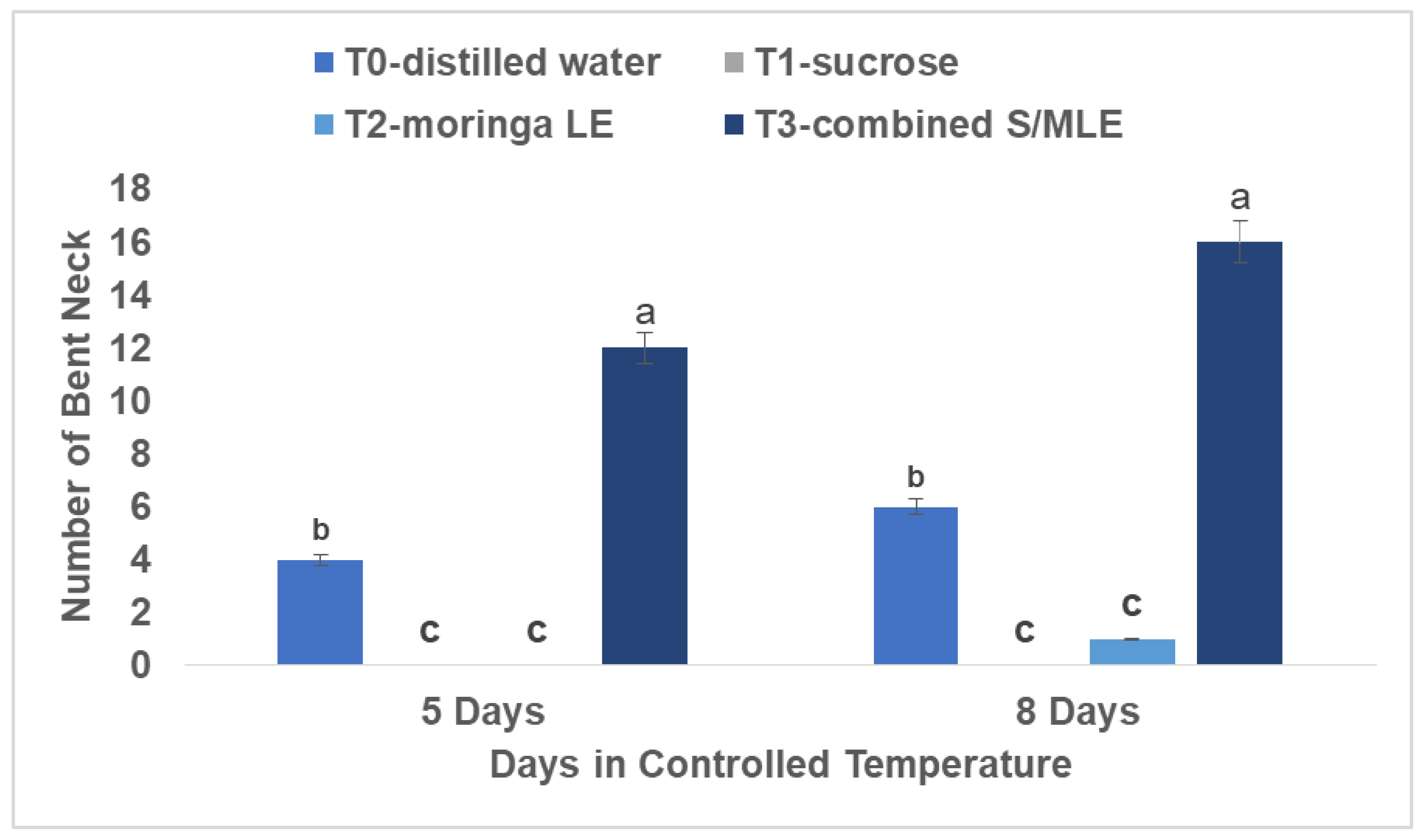
Bent neck
The bent neck was most observed in combined sucrose and moringa leaf extract, with an average of fourteen roses exhibiting a pronounced bend of 45 degrees, followed by distilled water with six roses similarly affected, while moringa leaf extracts alone displayed the least susceptibility, with only one rose exhibiting such deformity. This evaluation was performed over eight days at room temperature (22°C) and 80% relative humidity.
The combination of sucrose and moringa leaf extract observed to lead to a higher occurrence of bent neck in cut flowers can be attributed to several factors (
Figure 3). Firstly, the blockage of the xylem in the stem by either bacterial proliferation or other factors could hinder the transport of essential nutrients needed for the flower's structural integrity and health. This blockage prevents proper water and nutrient uptake, resulting in wilting and curvature of the flower stem (Van Doorn et al., 1997). Secondly, low temperatures can cause the sucrose and moringa leaf extract solution to gel or become viscous, thereby impeding the absorption of water by the flower stems. This reduced water uptake exacerbates the wilted appearance and bending observed in the flowers (Rafi and Ramezanian, 2013). The idea of vase life, which refers to the duration from the start of treatment until the occurrence of bent stems, highlights the significance of tackling elements that lead to flower decay, such as stem curvature (Rafi and Ramezanian, 2013).
The rating system used to quantify the degree of stem bending provides a standardized approach for assessing the severity of bent neck in cut flowers, facilitating comparisons across different treatments and environmental conditions (Celikel and Reid, 2002). While certain vase solutions containing sucrose have demonstrated that certain substances can extend the lifespan of severed roses. However, the problem of bacterial growth in treatments containing only sucrose underscores the importance of careful selection when choosing vase additives (Marousky, 1969; Gilman and Steponkus, 1972; Parups and Chan, 1973; Kaltaler and Steponkus, 1976). The possibility of carbohydrate shortages contributing to short vase life underscores the importance of understanding the role of nutrients like sucrose in maintaining flower health and longevity. Overall, addressing factors such as bacterial proliferation and carbohydrate availability can help mitigate issues like bent neck, enhancing the quality and longevity of cut flowers.
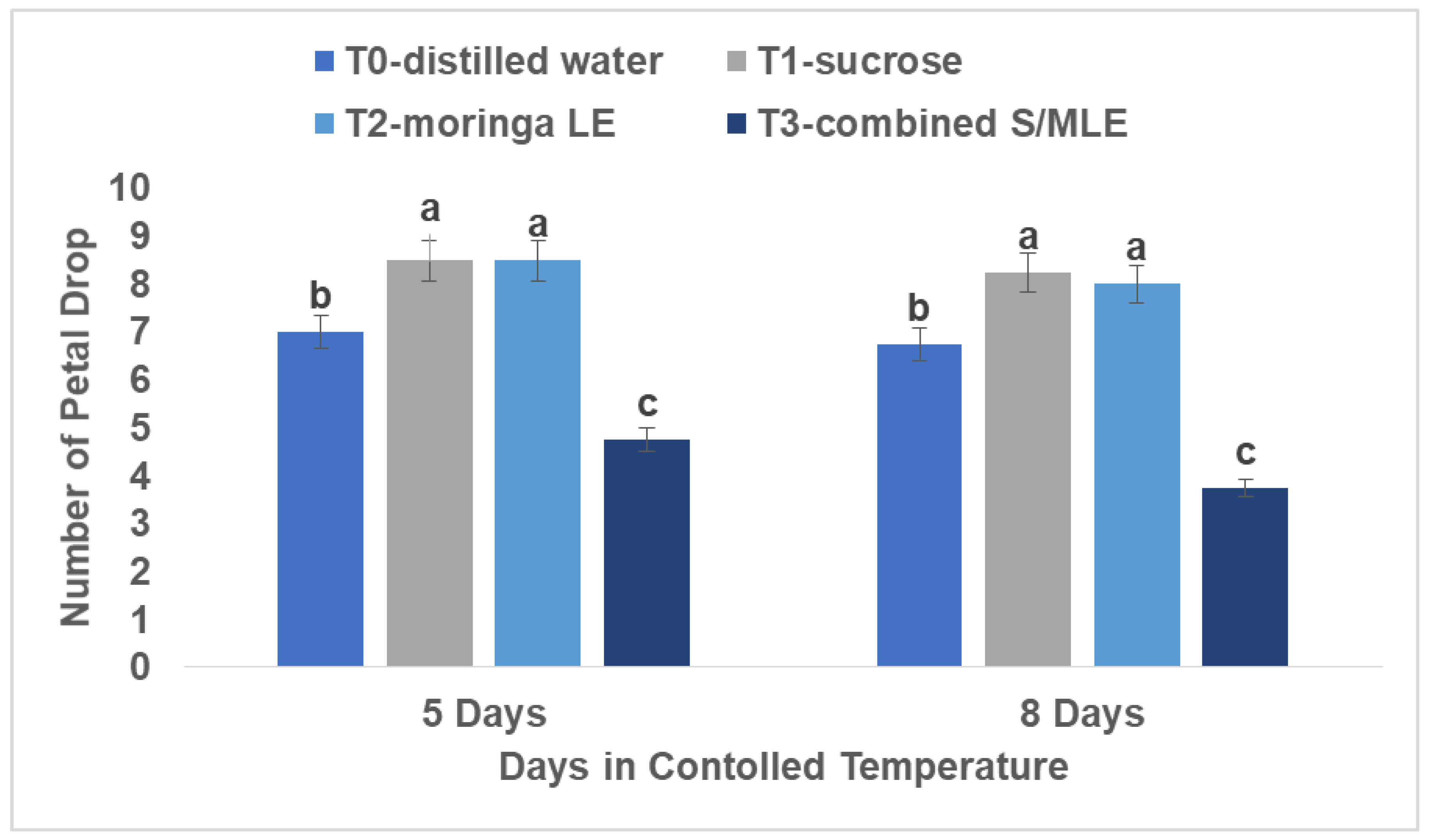
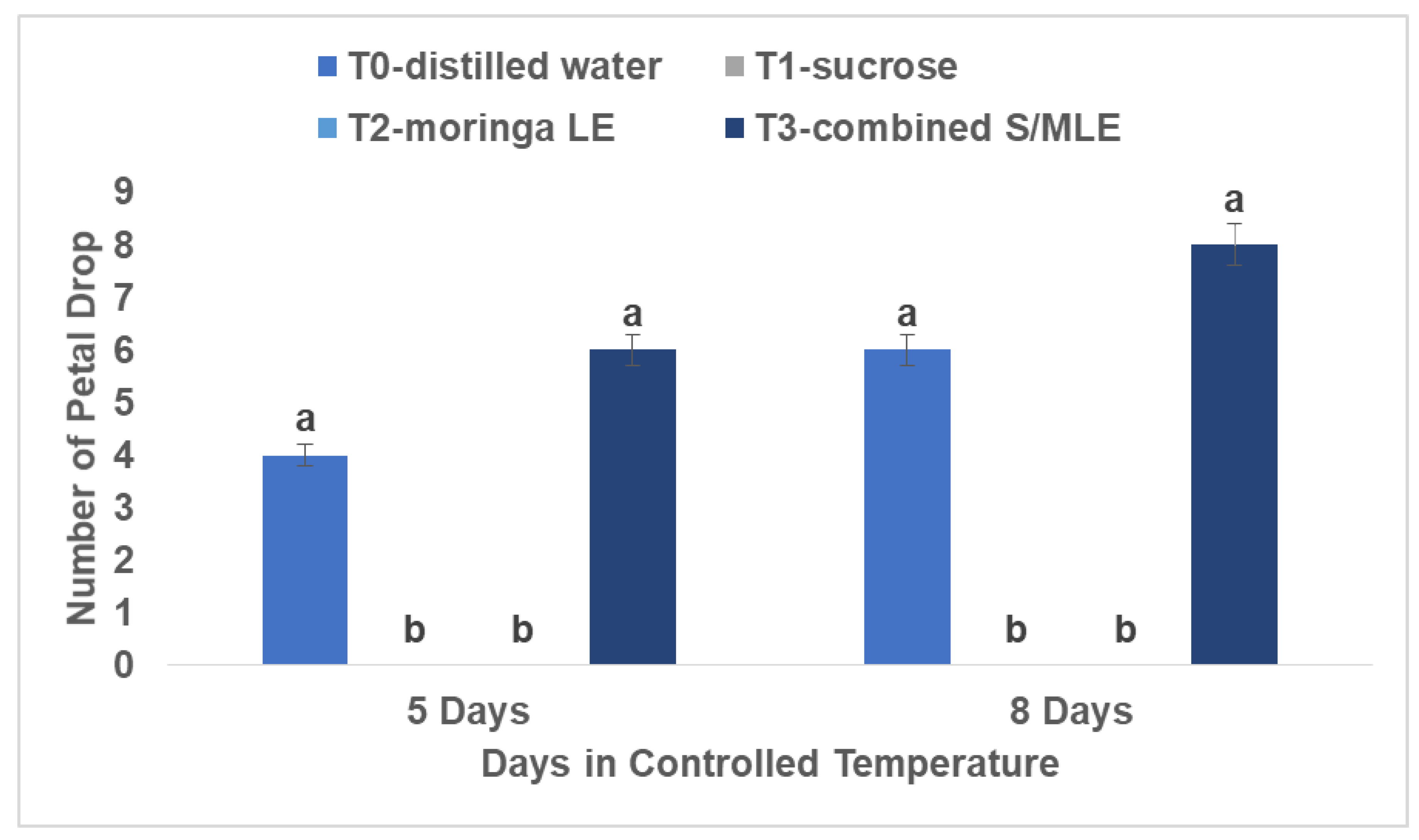
Petal Drop
Analysis of petal drop rates elucidated a similar pattern, with combined sucrose and moringa leaf extract exhibiting the highest incidence of petal loss, tallying eight petals shed, followed by distilled water with six petals shed. This evaluation was performed over eight days at room temperature (22°C) and 80% relative humidity.
The observation of increased petal dropping in cut flowers treated with a combination of sucrose and moringa leaf extract, as well as in those treated with distilled water alone, can be linked to several factors (
Figure 4). Firstly, research by Shirin and Mohsen (2011) has indicated that certain treatments, such as citric acid combined with sucrose, are more effective and safer for maintaining the quality of rose cultivars, makeup of the vase solution is pivotal in preserving flowers. Petal dropping can result from reductions in petal thickness, which decreases the overall quality of the flower in the vase (Rafi and Ramezanian, 2013). Oxidative stress is an additional element that leads to decreased flower quality during handling and storage (Ezhilmathi et al., 2007; Saeed et al., 2014). The act of cutting flowers can trigger oxidative damage, resulting in excessive production of reactive oxygen species (ROS) that harm cellular structures like nucleic acids, proteins, and membrane lipids (Reezi et al., 2009; Marandi et al., 2011; Hatamzadeh et al., 2012).
Studies have revealed that Moringa leaf extract (MLE) exhibits potent antimicrobial characteristics and can alleviate oxidative damage by boosting the performance of antioxidant enzymes that eliminate ROS, thus preserving the integrity of cellular membranes (Tesfay and Magwaza, 2017; Yasmeen et al., 2013; Ashraf et al., 2016; Aslam et al., 2016). The increased occurrence of petal dropping in cut roses treated with a combination of sucrose and moringa leaf extract, as well as in those treated with distilled water alone, contrasts with the lesser petal dropping observed in flowers treated with sucrose alone or moringa leaf extract alone. This disparity suggests that the composition of the preservation solutions plays a significant role in influencing flower quality.
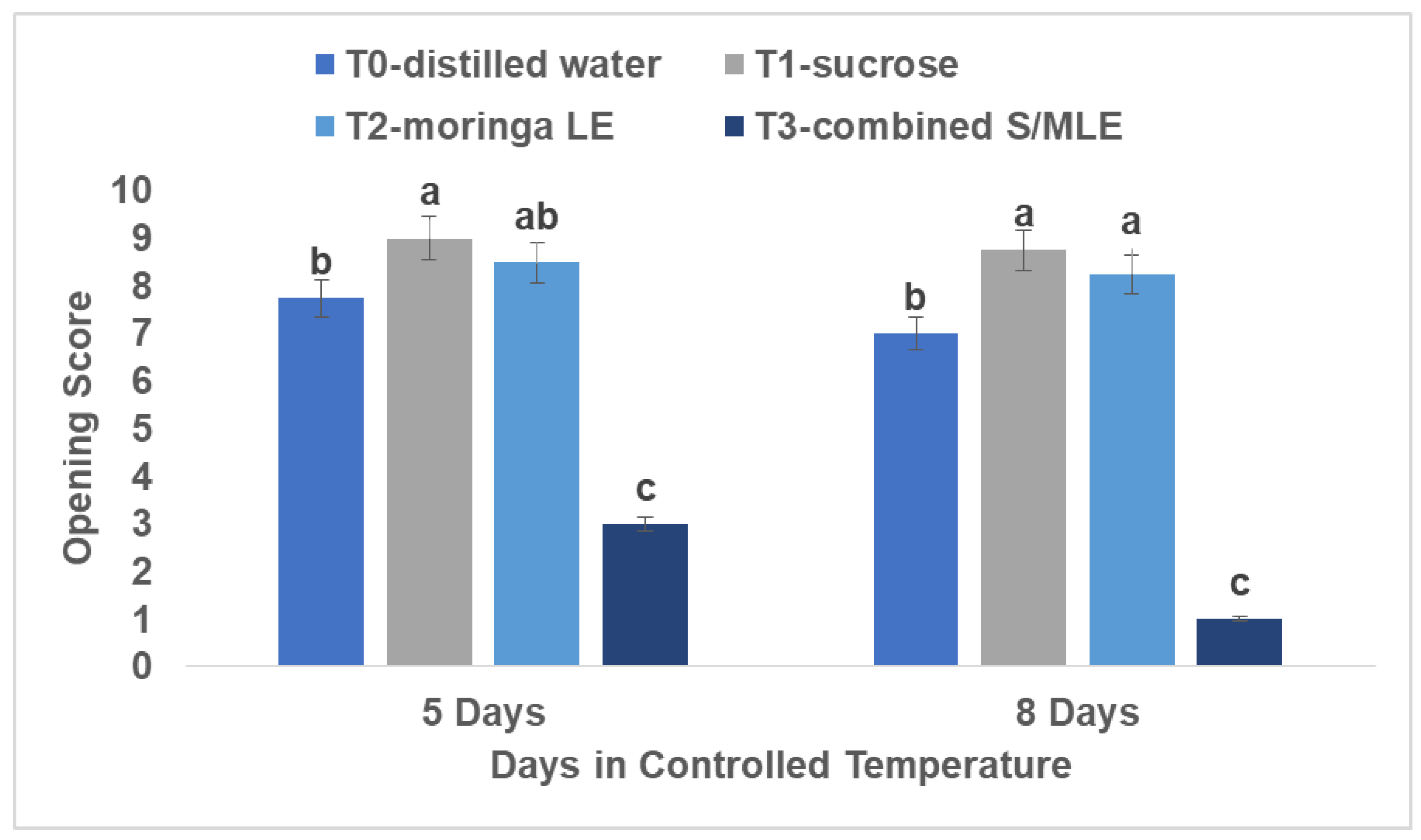
Flower opening (opening score)
Assessment of flower opening dynamics was conducted on a scale from 1 (fully open) to 9 (excellent/fresh). The combined sucrose and moringa leaf extract displayed the lowest average score of 1 (completely open), indicative of optimal flower opening, followed by distilled water with an average score of 7, denoting good opening, while sucrose alone and moringa alone garnered an average score of 8.75, reflecting an excellent and fresh presentation. This evaluation was performed over 8 days at room temperature (22°C) and 80% relative humidity.
Figure 5.
Impact of Sucrose and Moringa Leaf Extracts on the flower opening of.
Figure 5.
Impact of Sucrose and Moringa Leaf Extracts on the flower opening of.
Black Rose (Rosa x hybrida). Analysis of Variance (ANOVA) for FLOWER OPENING (opening score) of Cut Flower Rose. Distinct lowercase letters denote significant variances. Tukey HSD, with a significance level of α = 0.05, was employed.
This study is in connection with (Halevy, A. H. & Mayak, S. 1979) that the positive impact of sucrose on flower senescence is linked to their provision of substrates for respiration, structural components, and osmotic balance. This applies to cut flowers (Ichimura, K. 1998), as they lack access to food, hormones, and water post-detachment from the plant, relying solely on stored nutrients at harvest and the application of external sucrose (van Staden, J. 1995). Chamani et al. (2005) proposed a connection between uneven opening and inhibition of flower opening and decreased vase life. According to Van Doorn et al. (1991), there is a correlation between the reduction in water potential and the inhibition of corolla expansion and flower opening.
Recent studies have revealed the advantageous role of sucrose in delaying senescence in a range of cut flowers, including sweet peas (Ichimura, K. & Suto, K. 1999), delphinium (Ichimura, K., Kohata, K. & Goto, R. 2000), gentiana (Zhang, Z. M. et al., 2001), snapdragon (Ichimura, K. & Hisamatsu, T. 1999), rose Liao, L. J. et al., (2000), and oncidium Chen, W. S. et al. (2001) this is attributed to the inhibition of ethylene production or its sensitivity.
Applying sucrose externally provides vital respiratory substrates to the flower, not only prolonging vase life but also facilitating the opening of buds that would otherwise remain closed (Downs, C. G. 1988). Biocides like moringa are commonly utilized to diminish bacterial proliferation and prolong the lifespan of flowers in vase water (Hassan et al., 2004; Solgi et al., 2009; Hassan and Ali, 2014). Consequently, sucrose, often combined with biocides such as moringa leaf extract, have emerged as crucial preservatives for various cut flowers. Therefore, managing microbial growth is a crucial element in improving the longevity and quality of cut roses (Hassan et al., 2014).
Petal Color (Visual Quality Rate)
Evaluation of petal color changes, gauged via the Visual Quality Rating (VQR) system ranging from (1 as dark brown, 3 as light brown, 5 as shriveled pale red, 7 as good/fairly dark red, 9 as excellent fresh dark red), unveiled a progression wherein combined sucrose and moringa leaf extract attained the lowest average VQR score of 3.75, indicative of a light brown hue, second was observed in distilled water with a score of 6.75 denoting good/fairly dark red, third was observed in moringa leaf extract with a score of 8 representing a good/fairly dark red tone, and finally third was observed in sucrose with a score of 8.25 reflecting a fairly dark red hue. This evaluation was performed over 8 days at room temperature (22°C) and 80% relative humidity.
Figure 6.
Impact of Sucrose and Moringa Leaf Extracts on the petal color of Black Rose (Rosa x hybrida). Analysis of Variance (ANOVA) for PETAL COLOR (Visual Quality) of Cut Flower Rose. Distinct lowercase letters denote significant variances. Tukey HSD, with a significance level of α = 0.05, was employed.
Figure 6.
Impact of Sucrose and Moringa Leaf Extracts on the petal color of Black Rose (Rosa x hybrida). Analysis of Variance (ANOVA) for PETAL COLOR (Visual Quality) of Cut Flower Rose. Distinct lowercase letters denote significant variances. Tukey HSD, with a significance level of α = 0.05, was employed.
The observation that combined sucrose and moringa leaf extract scored lower in terms of petal freshness compared to sucrose alone or moringa leaf extract alone in research on extending cut flower vase life suggests a potential link between vase solutions and petal color. Sucrose is recognized for its ability to stimulate bud emergence and preserve the freshness of petals in various cut flowers, including roses, by providing essential nutrients for petal expansion (Borochov & Mayak, 1984; Han, 1992; Downs, 1988; Han, 1998; Doi & Reid, 1995; Kofranek & Halevy, 1976). Additionally, It has been discovered that sugar reduces water loss from rose petals, potentially by inducing stomatal closure, thus reducing transpiration and maintaining petal freshness (Marousky, 1969).
Moringa seed extract, as emphasized by (Elrys and Merwad, 2017), contains a wealth of phytohormones, antioxidants, and osmoprotectants, which have the potential to trigger antioxidant defense mechanisms in plants. These antioxidants and osmoprotectants in Moringa seed extract may help maintain petal color by reducing oxidative stress and preserving cellular integrity, ultimately contributing to the longevity of the flowers. Similarly, in other studies, sucrose application during initial hydration has been shown to reduce water uptake, suggesting that sugars may help maintain adequate water balance in cut flowers by minimizing water loss rather than increasing uptake (Durkin, 1979). Therefore, the effectiveness of sucrose and moringa leaf extract in preserving petal color and freshness may be attributed to its ability to support petal expansion, reduce water loss, and maintain water balance, highlighting its importance in vase solutions for cut flowers.
Conclusion & Recommendation
The study investigated the efficacy of sucrose and moringa leaf extracts as natural additives to increase the longevity of rose cut flowers, Rosa x hybrida. Through careful observation and data collection, it was evident that among the treatments tested T0 control-distilled water, T1 Sucrose, T2 Moringa leaf extract, and T3 Combined—sucrose and moringa leaf extract, the most significant improvements in vase life were observed with T1-Sucrose and T2- Moringa leaf extracts. These treatments demonstrated notable effects on delaying petal wilting, reducing stem rotting, and maintaining overall flower quality over the experimental period.
Applying sucrose and moringa leaf extract separately to cut roses significantly extends their vase life. However, using a combination of sucrose and moringa leaf extract does not have the same effect because it blocks the xylem, which is a primary reason for the reduced vase life. Additionally, low temperatures can cause the solution of sucrose and moringa leaf extract to gel or thicken, further hindering water absorption by the flower stems.
Given the results of this research, it is advisable to delve deeper into investigating the possible advantages of the combined approach, T3 - sucrose and moringa leaf extracts. While the combined treatment showed promising, it was noted that proper dilution might enhance its effectiveness. Therefore, future research should focus on optimizing the formulation and concentration of the combined treatment to maximize its benefits in prolonging the lifespan of cut-flower roses. Additionally, investigating the long-term effects of these natural additives on other flower species and exploring potential mechanisms underlying their action could provide valuable insights for the floral industry.
Author Contributions
Authors have contribution to this work.
Funding
This work received no specific grant from any funding agency.
Acknowledgment
The authors would like to thank the research advisory board.
Conflict of Interests
The authors declare that they have no conflicts of interest.
| Plate 1. The experimental site of the study |
 |
| Plate 2. Sucrose Preparation |
 |
| Plate 3. Moringa leaf extracts preparation |
 |
| Plate 4. Collection of roses |
 |
| Plate 5. Application of treatments |
 |
| Plate 6. Data Collection |
 |
| Plate 7. Black rose (Rosa x hybrida) in 8 days comparison |
 |
 |
References
- Anwar, F., S. Latif, M. Ashraf and A.H. Gilani. (2007). Moringa oleifera: a food plant with multiple bio-chemical and medicinal uses- a review. Phytother. Res., 21: 17-25. [CrossRef]
- Bravdo, B., Mayak, S. & Gravrieli, Y. (1974) Sucrose and water uptake from concentrated sucrose solutions by gladiolus shoots and the effect of these treatments on floret life. Can. J. Bot., 52, 1271–1281. [CrossRef]
- Butt, S. J. 2003. A Review on prolonging the vase life of Roses. Pakistan Rose Annual. Published by Pakistan National Rose Society, pp: 49-53.
- Elgimabi, M., 2011. Vase life extension of rose cut flowers (Rosa Hybrida) as influenced by silver nitrate and sucrose pulsing. American Journal of Agricultural and Biological Sciences, 6(1): 128-133. [CrossRef]
- Gilman, K. F. and P. L. Steponkus. 1972. Vascular blockage in cut roses. J. Amer. Soc. Hort. Sci., 97: 662-667.
- Gilman KF, Stephonkus PL (1974) Vascular blockage in cut roses. J Am Soc Hortic Sci 97:662.
- Hassan, F., (2005) Postharvest studies on some important flower crops. Ph.D. Thesis, Faculty of Horticultural Sciences, Corvinus University of Budapest, Hungary.
- Jafarpour et al (2015). Improving postharvest vase-life and quality of cut gerbera flowers using natural and chemical preservatives. Journal of Central European Agriculture, 2015, 16(2), p.199-211. [CrossRef]
- Jalili Marandi, R., Hassani, A., Abdollahi, A., Hanafi, S., (2011) Improvement of the vase life of cut gladiolus flowers by essential oils, salicylic acid and silver thiosulfate. Journal of Medicinal Plants Research, 5 (20), 5039-5043.
- Kaltaler, R. E. L. and P. L. Steponkus. 1976. Factors affecting respiration in cut roses. J. Amer. Soc. Hort. Sci., 101: 352-354.
- Kim, Y. and J. Lee (2002). Changes in lignin content, phenylalanine ammonia lyase activity, and peroxidase activity as affected by bent neck and senescence of cut rose flowers. J. Korean Soc. Hort. Sci.; 43(2):208-212.
- Marousky (1976). Control of bacteria in cut flower vase water.
- Marousky, F. J. 1969. Vascular blockage, water absorption, stomatal opening, and respiration of cut ‘Better times’ roses treated with 8-hydroxiquinoline citrate and sucrose. J. Amer. Soc. Hort. Sci., 94: 223-226.
- Maxwell, S. E. (2005). Completely randomized designs. In B. S. Everitt & D. C. Howell (Eds.), Encyclopedia of Statistics in Behavioral Science. New York: Wiley.
- Montgomery, D. C. (1999). “Experimental Design for Product and Process Design and Development.” Journal of the Royal Statistical Society D, Vol. 48, pp. 159–177. [CrossRef]
- Parups, E. V. and A. P. Chan. 1973. Extension of vase-life of cut flowers by use of isoascorbate-containing preservative solutions. J. Amer. Soc. Hort. Sci. 98: 22- 26.
- Penniston et al. (2008). Quantitative Assessment of Citric Acid in Lemon Juice, Lime Juice, and Commercially-Available Fruit Juice Products.
- Penniston. K. L., Y. N. Stephen, P. H. Ross and D. G. Assimos. 2008. Quantitative Assessment of Citric Acid in Lemon Juice, Lime Juice, and Commercially.Available Fruit Juice Products. J Endourol., 22(3): 567–570. [CrossRef]
- Reid, M.S. and Jiang, C. Z. (2012). Postharvest biology and technology of cut flowers and potted plants. In: Horticulture Reviews (Eds. Jules Janick), pp. 1-45.
- Selamawit, Z.; Melkamu, A.; Tadele, Y (2018). Pulsing preservatives to prolong vase life of cut rose flowers in Bahir Dar, Northwestern Ethiopia. International Journal of Sustainable Agricultural Research, v.5, n.4, p.54-67. [CrossRef]
- Synge, P. M. 1971. The dictionary of rose in color. 1st Edn., Madison Square Press, New York, ISBN-10: 0448025043, p: 191.
- Van Doorn, W. G., et al. (1997). "Effect of pre- and postharvest salicylic acid treatment on physio-chemical attributes in relation to vase-life of rose cut flowers.". [CrossRef]
- Van DoornW G, Sinz A,Tomassen MM. (2004). Daffodil flowers delay senescence in cut Iris flowers. Phytochemistry65,571–577. [CrossRef]
- Van Meeteren U., van Gelder A., van Ieperen W. (2005). Effect of growth conditions on post-harvest rehydration ability of cut chrysanthemum flowers. Acta Hortic., 287–296. [CrossRef]
- Van Meeteren, U., H. Van Gelder and W. Van Ieperen. 2000. Reconsideration of the use of deionized water as vase water in Postharvest experiments on cut flowers. Postharvest Biol. Tech. 18: 169-181. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).